Abstract
Background:
Animal studies suggest polybrominated diphenyl ethers (PBDEs) may be obesogens. However, epidemiologic studies investigating childhood exposure to PBDEs and adiposity are limited, with several reporting an inverse association.
Objectives:
To investigate associations between repeated childhood PBDE concentrations and adiposity measures at age 8 years.
Methods:
We examined 206 children from the Health Outcomes and Measures of the Environment Study, a birth cohort in Cincinnati, OH (2003-2006). Serum PBDEs were measured at ages 1, 2, 3, 5, and 8 years. We used multiple imputation to estimate missing PBDE concentrations. At 8 years, we measured weight, height, waist circumference, and body fat percentage. We used multiple informant models to estimate age-specific associations between PBDEs and adiposity measures.
Results:
We observed significant inverse associations between BDE-153 with all adiposity measures that became increasingly stronger with later childhood measurements. A 10-fold increase in BDE-153 at ages 1 and 8 years was associated with 2% (95% CI −3.9, −0.1) and 7% (95% CI −9.1, −4.7) lower body fat, respectively. No statistically significant associations were found with BDE-28, −47, −99, or −100. Child sex modified some associations; inverse associations between BDE-153 and body fat were stronger among boys, while positive and null associations were noted among girls.
Conclusions:
Childhood BDE-153 concentrations were inversely associated with adiposity measures and these associations became stronger as BDE-153 measurements were more proximal to adiposity measures. Inverse associations could be attributed to reverse causality arising from greater storage of PBDEs in adipose tissue of children with higher adiposity.
Keywords: Polybrominated diphenyl ethers (PBDEs), flame retardants, endocrine disruptors, postnatal, childhood, weight, adiposity, body mass index, children, epidemiology
1. Introduction
From 1999 to 2016, the prevalence of overweight and obesity increased among U.S. children, especially among young children (Skinner et al. 2018). Obesity is multifaceted and complex, with contributions from genetic, lifestyle, and environmental factors. The prevalence of obesity, which has reached epidemic proportions cannot be fully explained by the classical risk factors. Endocrine disrupting chemicals have been leading candidates for environmental factors that may play a role as “obesogens” that could potentially interfere with metabolism and adipocyte function to increase the risk of childhood obesity (Nappi et al. 2016).
Polybrominated diphenyl ethers (PBDEs) are flame retardants that were incorporated into household furnishings and electronics from the 1970s to early 2000s. Beginning in 2004, they were phased out of use in the U.S. because of increasing concerns about their persistence and developmental toxicity. PBDEs are identified neurotoxicants and have been reported to adversely affect intellectual abilities and behavior (Herbstman and Mall 2014; Lam et al. 2017; Vuong et al. 2017b). However, their potential role in the risk of obesity remains uncertain. Infancy and childhood exposures are of particular concern given that higher concentrations have been reported among these age groups compared to adults (Schecter et al. 2005; Toms et al. 2008; Toms et al. 2009). Animal studies have reported increased weight gain with higher PBDE exposures occurring during the perinatal via oral gavage or intravenous injections (Bondy et al. 2013; Fernie et al. 2006; Suvorov et al. 2009) and postnatal period via oral dose (Dufault et al. 2005; Gee and Moser 2008). Further, toxicological studies have shown that PBDEs interfere with adipogenic pathways, increase adipocyte differentiation, and alter glucose homeostasis (Auwerx 1999; Bastos Sales et al. 2013; Hoppe and Carey 2007; Kamstra et al. 2014).
Epidemiological studies have primarily reported an inverse relationship between childhood PBDE exposures with adiposity measures in children and adolescents, with some indication of potential sexual dimorphism (Darrow et al. 2017; Erkin-Cakmak et al. 2015; Hoffman et al. 2016; Leijs et al. 2017; Windham et al. 2010). Given the limited number of epidemiological studies that have investigated postnatal PBDEs and the lack of studies that have prospectively investigated PBDEs during childhood and adiposity measures, a majority were cross-sectional studies, we examined whether child serum PBDEs measured repeatedly from 1-8 years were associated with adiposity measures at age 8 years.
2. Methods
2.1. Study participants and design
We employed the Health Outcomes and Measures of the Environment (HOME) Study, an ongoing prospective pregnancy and birth cohort (2003-2006, Cincinnati, Ohio, USA), to test whether postnatal PBDEs are associated with increased childhood adiposity. Details on the HOME Study have been described by Braun et al. (2017). Briefly, we enrolled 468 pregnant women at 16±3 weeks who provided informed consent. The participants were from nine collaborating obstetric practices and were ≥18 years, living in a residential home that was built before 1978, and were not HIV positive or taking medications for seizures, thyroid disorders, or chemotherapy/radiation treatment. Of the enrollees, 66 dropped out prior to delivery, 9 delivered sets of twins, and 3 had stillbirths. From the 390 women who delivered liveborn singletons, 206 (53%) were eligible for the present study based on available PBDE measurements during childhood (at any of the 5 follow-up visits) and anthropometry at 8 years. This study was approved by the institutional review board (IRB) at the Cincinnati Children’s Hospital Medical Center (CCHMC); the Centers for Disease Control and Prevention (CDC) and collaborating institutions relied on CCHMC’s IRB as the IRB of record.
2.2. Assessment of childhood PBDEs
We measured serum PBDE concentrations at the CDC using gas chromatography/isotope dilution high-resolution mass spectrometry from blood samples collected at ages 1, 2, 3, 5, and 8 years (Jones et al. 2012; Sjodin et al. 2004). Details regarding quality assurance and limit of detection (LOD) were previously described (Vuong et al. 2017a). We focused on congeners −28, −47, −99, −100, and −153 (as well as their sum: ∑PBDEs), because detection frequencies at ages 1, 2, 3, 5, and 8 years were 96-100%, 86-100%, 82-100%, 66-100%, and 50-100%, respectively (see Supplemental Table S1). PBDE concentrations <LOD were replaced with LOD/√2 (Hornung and Reed 1990). Total serum lipids are based on triglycerides and total cholesterol that were measured using standard enzymatic methods (Phillips et al. 1989), which were used in the lipid adjustment of PBDEs as ng/g lipid. While 206 children had at least one measurement of PBDEs during childhood, only 86 (42%), 69 (33%), and 69 (33%) children had available PBDE concentrations at ages 1, 2, and 3 years, respectively, due to limited serum availability, compared to 140 (68%) at 5 years and 190 (92%) at 8 years. We used multiple imputation with the Markov Chain Monte Carlo method to estimate missing PBDE concentrations for children who had at least one PBDE measurement during childhood. These procedures have been detailed previously for the 100 generated imputed datasets (Vuong et al. 2017c; Vuong et al. 2017d).
2.3. Child anthropometry
We measured height (to the nearest 0.1 cm), weight (to the nearest 0.01 kg), waist circumference (measured as cm), and body fat percentage in triplicate at age 8 years. We used an Ayrton Stadiometer Model S100 to measure children’s standing height without shoes, and with his/her heels against the wall. We used a ScaleTronix Pediatric Scale Model 4802 to measure weight of children clothed in their undergarments. We measured waist circumference using a plastic measuring tape that was placed around the abdomen at the level of the iliac crest. We measured body fat percentage with bioelectrical impedance analysis using the Tanita children’s body fat monitor (Arlington Heights, IL). We calculated age- and sex-specific weight and BMI z-scores using US references from the National Center for Health Statistics (Kuczmarski et al. 2000).
2.4. Statistical analyses
We used multiple informant models to estimate age-specific βs and 95% confidence intervals (CIs) for associations of repeated log10-transformed PBDE concentrations (BDE−28, −47, −99, −100, −153, and ∑PBDEs) during childhood with adiposity measures at age 8 years for each of the 100 imputed datasets. Multiple informant models, which are non-standard versions of generalized estimating equations, allowed us to estimate associations for each of the repeated PBDE measurements and test whether these associations differ from one another and identify potential windows of susceptibility to PBDEs (Sanchez et al. 2011). We calculated average βs and 95% CIs from the 100 results from the imputed datasets; these are presented as age-specific estimates, because several interaction terms (PBDEs×age) were p<0.10 (Beunckens et al. 2008; Shen and Chen 2013).
We examined non-linear exposure-response using separate generalized additive models for PBDEs at 8 years and adiposity measures using the original, non-imputed data. We explored effect measure modification by sex to investigate whether associations between PBDEs and adiposity measures are influenced by sex. To do this, we included a 3-way interaction term between PBDEs×age×sex (p<0.10) and all possible 2-way interaction terms. All models were adjusted for the following based on a review of the literature (categorized as displayed in Table 1): maternal age, race/ethnicity, household income, maternal smoking status and alcohol consumption, maternal depression (Beck et al. 1996), vitamin intake during pregnancy, marital status, whether the child was breastfed, child age, and sedentary measures, including amount of time spent outside and watching TV. We additionally adjusted for maternal prepregnancy weight (for weight z-score outcome), maternal prepregnancy BMI (for outcomes BMI z-score, waist circumference, and body fat percentage), and child sex (for outcomes waist circumference and body fat percentage) depending on the outcome.
Table 1.
Child serum concentrations of ∑PBDEs (ng/g lipid) and child adiposity at 8 years by maternal and child characteristics, HOME Studya
| ∑PBDEs 2 years |
∑PBDEs 8 years |
BMI z-score |
Weight z-score |
Body Fat Percentage |
Waist Circumference |
||||
|---|---|---|---|---|---|---|---|---|---|
| n | GM(GSD) | GM(GSD) | Mean±SD | Mean±SD | n | Mean±SD | n | Mean±SD | |
| Maternal age, yearsb,c | |||||||||
| <25 | 58 | 169.6(2.0) | 39.3(2.2) | 0.5±1.1 | 0.5±1.2 | 57 | 22.2±7.9 | 56 | 60.9±10.5 |
| 25-34 | 117 | 129.4(2.5) | 49.0(2.1) | 0.4±0.9 | 0.4±1.1 | 116 | 20.5±5.6 | 116 | 60.8±7.2 |
| ≥35 | 31 | 68.4(2.3) | 34.2(2.3) | 0.2±0.9 | 0.3±1.2 | 31 | 20.5±6.8 | 31 | 61.1±10.5 |
| Race/ethnicityb,d,e,f | |||||||||
| Non-Hispanic White | 122 | 99.0(2.3) | 44.0(2.2) | 0.2±0.9 | 0.2±1.0 | 120 | 19.4±5.0 | 120 | 60.4±7.2 |
| Non-Hispanic Black and Others | 84 | 182.0(2.5) | 43.1(2.2) | 0.7±1.1 | 0.7±1.3 | 84 | 23.2±7.7 | 83 | 61.5±10.6 |
| Maternal Educationb,d,f | |||||||||
| High school or less | 56 | 190.1(2.3) | 41.9(2.1) | 0.7±0.9 | 0.6±1.1 | 55 | 23.2±6.6 | 55 | 61.9±9.7 |
| Some college/2 yr degree | 58 | 165.7(2.4) | 49.3(2.2) | 0.1±1.1 | 0.2±1.2 | 58 | 19.7±7.1 | 57 | 58.9±8.6 |
| Bachelor’s | 55 | 83.1(2.3) | 40.5(2.3) | 0.4±0.9 | 0.5±1.1 | 55 | 21.0±5.6 | 55 | 62.2±8.9 |
| Graduate or professional | 37 | 85.0(2.0) | 42.7(2.0) | 0.3±0.8 | 0.3±0.9 | 36 | 19.6±5.9 | 36 | 60.4±6.8 |
| Family Incomeb,f | |||||||||
| <$40,000 | 88 | 195.8(2.3) | 45.8(2.2) | 0.6±1.1 | 0.6±1.2 | 88 | 22.4±7.8 | 87 | 61.3±10.4 |
| $40,000-$79,999 | 65 | 122.0(2.3) | 44.3(2.2) | 0.3±0.9 | 0.4±1.1 | 64 | 20.0±5.4 | 64 | 61.0±8.3 |
| ≥$80,000 | 53 | 64.8(2.0) | 39.4(2.2) | 0.2±0.8 | 0.1±0.9 | 52 | 19.8±4.9 | 52 | 60.0±5.8 |
| Maternal Depressionb,d,f,g | |||||||||
| Minimal/mild | 184 | 120.1(2.4) | 44.6(2.1) | 0.3±1.0 | 0.4±1.1 | 183 | 20.6±6.3 | 182 | 60.5±8.5 |
| Moderate/severe | 20 | 204.1(3.1) | 36.0(2.7) | 0.8±1.1 | 0.8±1.2 | 19 | 25.1±7.3 | 19 | 65.2±10.2 |
| Maternal Alcohol Consumption | |||||||||
| Never | 114 | 141.2(2.4) | 44.3(2.2) | 0.4±1.0 | 0.3±1.2 | 114 | 21.1±6.9 | 114 | 60.7±9.5 |
| <1 per month | 64 | 110.9(2.4) | 42.2(2.0) | 0.3±0.9 | 0.3±1.0 | 63 | 20.1±5.0 | 63 | 60.0±6.0 |
| ≥1 per month | 28 | 111.9(2.7) | 44.2(2.3) | 0.6±1.0 | 0.8±0.9 | 27 | 22.3±7.6 | 26 | 63.7±10.7 |
| Maternal Smokingb | |||||||||
| None | 170 | 117.8(2.5) | 44.8(2.2) | 0.3±1.0 | 0.4±1.1 | 169 | 20.5±6.2 | 168 | 60.4±8.1 |
| ETS | 19 | 180.7(1.8) | 48.1(2.2) | 0.5±1.2 | 0.4±1.4 | 19 | 23.1±7.9 | 19 | 61.4±9.0 |
| Active | 17 | 179.3(2.5) | 29.7(2.0) | 0.9±0.9 | 0.8±1.2 | 16 | 23.4±7.3 | 16 | 64.4±13.5 |
| Maternal Vitamin Useb,f | |||||||||
| Daily | 158 | 116.4(2.5) | 43.0(2.2) | 0.3±0.9 | 0.3±1.1 | 157 | 20.3±5.5 | 157 | 60.3±7.3 |
| <Daily | 34 | 146.7(2.3) | 48.8(2.2) | 0.6±1.1 | 0.7±1.4 | 33 | 23.3±8.7 | 33 | 63.7±13.9 |
| Never | 14 | 235.8(2.4) | 38.8(2.0) | 0.6±1.3 | 0.7±1.2 | 14 | 22.9±9.1 | 13 | 60.1±7.9 |
| Prepregnancy BMIb,d,e,f,g | |||||||||
| Underweight/Normal | 103 | 106.7(2.5) | 42.5(2.2) | 0.1±1.0 | 0.1±1.1 | 101 | 19.7±5.6 | 101 | 59.3±7.1 |
| Overweight | 55 | 145.9(2.5) | 47.3(2.2) | 0.3±0.9 | 0.4±1.0 | 55 | 20.0±5.0 | 54 | 59.5±5.4 |
| Obese | 48 | 156.7(2.3) | 42.0(2.0) | 1.0±1.0 | 1.1±1.1 | 48 | 24.7±8.4 | 48 | 65.7±12.6 |
| Marital statusb,d,f | |||||||||
| Married/living with partner | 150 | 99.1(2.2) | 43.2(2.1) | 0.3±0.9 | 0.3±1.1 | 148 | 20.2±5.7 | 147 | 60.4±8.1 |
| Not married, living alone | 56 | 245.8(2.3) | 44.6(2.3) | 0.7±1.1 | 0.7±1.2 | 56 | 23.1±7.9 | 56 | 62.0±10.3 |
| Breastfedb,f,g | |||||||||
| No | 41 | 196.7(2.8) | 39.7(2.7) | 0.5±1.2 | 0.6±1.4 | 41 | 23.0±8.4 | 41 | 63.3±12.3 |
| Yes | 164 | 113.8(2.3) | 44.7(2.1) | 0.3±0.9 | 0.4±1.0 | 162 | 20.5±5.8 | 161 | 60.3±7.5 |
| Child Sexf,g | |||||||||
| Male | 92 | 129.6(2.2) | 44.0(2.3) | 0.3±1.0 | 0.4±1.1 | 92 | 18.6±4.6 | 92 | 59.4±7.9 |
| Female | 114 | 124.7(2.7) | 43.3(2.1) | 0.5±1.0 | 0.4±1.2 | 112 | 22.9±7.1 | 111 | 62.1±9.3 |
Abbreviations: GM, geometric mean; GSD, geometric standard deviation; SD, standard deviation.
Frequencies may not add to the total number of participants because of missing values.
p< 0.05 for comparisons of PBDE concentrations or adiposity measures within maternal or child characteristics
∑PBDEs at 2 years
∑PBDEs at 8 years
BMI
Weight
Body fat percentage
Waist circumference (two-sided p values using ANOVA or t-test)
2.5. Sensitivity analyses
We conducted several sensitivity analyses. First, all models were re-examined using the original, non-imputed data. Second, we adjusted for maternal intake of fruits and vegetables during pregnancy as a proxy for children’s dietary intake. Third, we eliminated maternal anthropometric measures from the models. Fourth, we used two additional lipid adjustment methods for PBDEs (as pg/g serum or ng/g lipid) that both had serum lipids as a covariate in the model as recommended by O’Brien et al. (2016). We adjusted for gestational PBDEs since we previously reported inverse associations with adiposity measures (Vuong et al. 2015). Lastly, we excluded children with high body fat percentages ≥30% (n=19) and children with low body fat percentages ≤14% (n=13) to determine whether outliers influenced our results.
3. Results
3.1. Study participants
The geometric mean (GM) of ∑PBDEs in the HOME Study children was highest at age 2 years and lowest at age 8 years, with concentrations of 130.5(2.5) and 43.6(2.2) ng/g lipid, respectively (see Supplemental Table SI). Concentrations of ∑PBDEs at age 2 years were significantly higher in children whose mothers were younger, non-Hispanic Black or other racial and ethnic groups, had lower education, moderately/severely depressed, actively or passively exposed to tobacco smoke, did not take daily vitamin supplementation, obese, and not married or living alone (Table 1). Children who were from households with annual incomes <$40,000 and were not breastfed were also more likely to have higher ∑PBDEs at 2 years. We observed lower BMI z-scores and body fat percentages in children that had mothers who were non-Hispanic white and who more than a high school education. Children’s BMI z-scores and body fat percentages were higher among mothers that were moderately/severely depressed, not married/living alone, and who were obese. Children who had a smaller waist circumferences were more likely to be males and breastfed during infancy; they were also more likely to have mothers with minimal/mild depression who were not obese.
3.2. PBDEs and childhood measures of adiposity
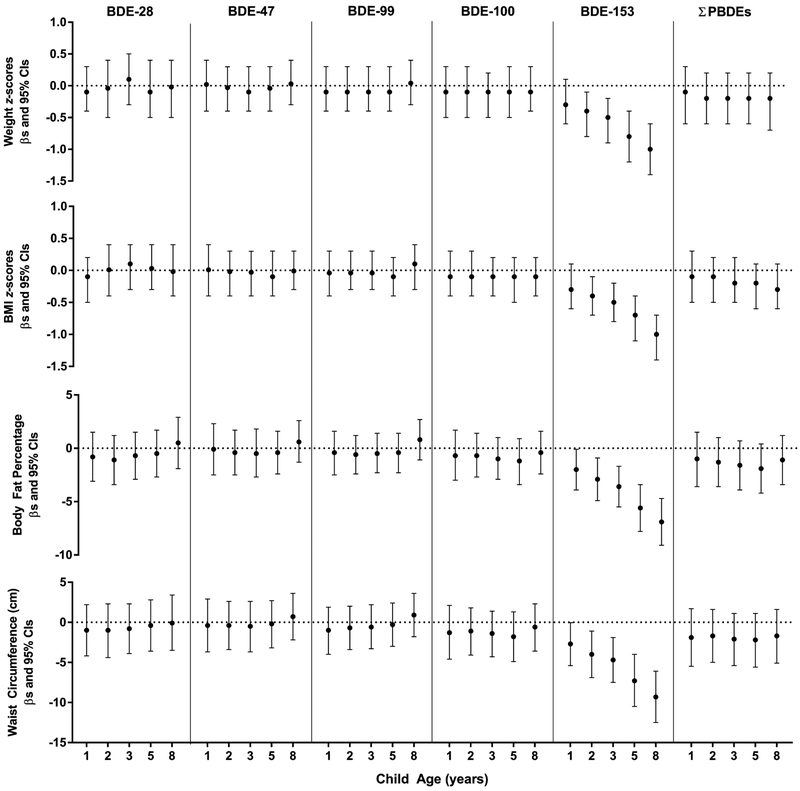
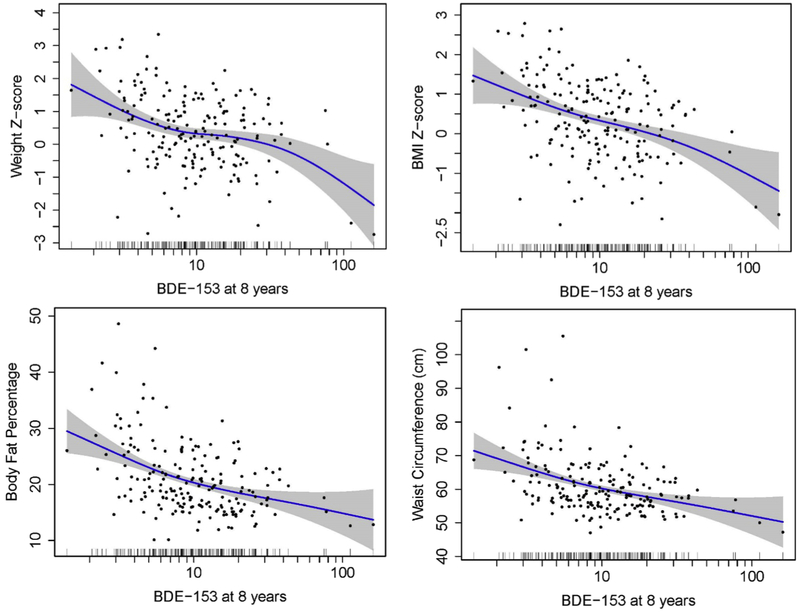
We observed lower weight and BMI z-scores at higher concentrations of BDE-153 at ages 2-8 years, with increasingly larger decrements noted with concentrations more proximal to age 8 years (Figure 1). For example, a 10-fold increase in BDE-153 at age 2 years was associated with 0.4 (95% CI −0.7, −0.1) lower BMI z-scores, while a decrease of 1.0 (95% CI −1.4, −0.7) was noted with concurrent BDE-153 concentrations. Childhood BDE-153 was inversely associated with body fat percentage. Ten-fold concentration increases of BDE-153 at ages 1, 2, 3, 5, and 8 years were associated with lower body fat percentages of 2.0% (95% CI −3.9, −0.1), 2.9% (95% CI −4.9, −0.9), 3.6% (95% CI −5.5, −1.7), 5.6% (95% CI −7.8, −3.4), and 6.9% (95% CI −9.1, −4.7), respectively. We observed similar inverse associations between childhood BDE-153 and waist circumference. In particular, a 10-fold increase in BDE-153 at ages 2, 5, and 8 were associated with a decrease of 4.0 cm (95% CI −6.9, 1.1), 7.3 cm (95% CI −10.5, −4.0), and 9.3 cm (95% CI −12.5, −6.1), respectively. In generalized additive models with only exposure at age 8 years, childhood BDE-153 concentrations was inversely associated with all adiposity measures (Figure 2). In contrast, childhood BDE-28, −47, −99, and −100 were not associated with measures of adiposity at age 8 years.
Figure 1.
Estimated differences and 95% CIs in adiposity measures at age 8 years by 10-fold increases in child serum concentrations of polybrominated diphenyl ethers (ng/g lipid), HOME Study. All models were adjusted for maternal age, race/ethnicity, household income, maternal smoking status, maternal alcohol consumption, maternal depression, maternal vitamin use, marital status, whether the child was breastfed, child age, time spent watching television, time playing video games, and time playing outside. Select models were additionally adjusted for maternal pre-pregnancy weight (weight z-score). BMI (BMI z-score, body fat percentage, waist circumference), and child sex (body fat percentage, waist circumference).
Figure 2.
Concurrent serum concentrations of BDE-153 (ng/g lipid) and adiposity measures at 8 years with generalized additive model curve fitting using original, non-imputed data. Solid lines represent the natural cubic spline of the adjusted associations, and dotted lines represent 95% CIs. Distribution of BDE-153 is illustrated by vertical bars on the log10-transformed x-axis. All models were adjusted for maternal age, race/ethnicity, household income, maternal smoking status, maternal alcohol consumption, maternal depression, maternal vitamin use, marital status, whether the child was breastfed, child age, time spent watching television, time playing video games, and time playing outside. Select models were additionally adjusted for maternal pre-pregnancy weight (weight z-score), BMI (BMI z-score, body fat percentage, waist circumference), and child sex (body fat percentage, waist circumference).
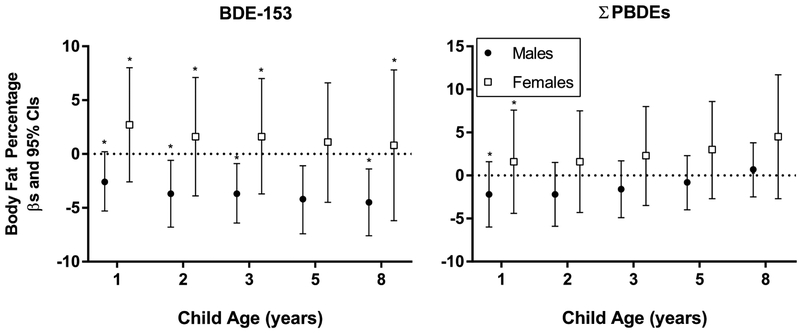
3.3. Child sex differences
Child sex modified associations between childhood BDE-153 concentrations and body fat percentages (Figure 3). A 10-fold increase in BDE-153 at 8 years was associated with a decreased body fat percentage of 4.5% (95% CI −7.6, −1.4) in males, while nonsignificant associations were noted in females (β=0.8%, 95% CI −6.2, 7.8) (pBDE-153*sex*8years=0.023), but effect measure modification was not present at age 5 years. Similar associations were noted between males and females with BDE-153 concentrations at ages 1-3 years. Child sex did not modify associations between childhood PBDEs and z-scores for weight or BMI, or waist circumference (results not shown). However, we observed similar patterns of statistically significant inverse associations between BDE=153 and z-scores for weight and BMI and waist circumference in males, but null associations in females (see Supplemental Table S2).
Figure 3.
Estimated differences and 95% CIs in body fat percentage at age 8 years by 10-fold increases in child serum concentrations of BDE-153 and ∑PBDEs (ng/g lipid) by child sex, HOME Study. All models were adjusted for maternal age, race/ethnicity, household income, maternal smoking status, maternal alcohol consumption, maternal depression, maternal pre-pregnancy BMI, vitamin use, marital status, child sex, whether the child was breastfed, child age, time spent watching television, time playing video games, and time playing outside. Asterisks denote statistically significant interaction terms between PBDEs, sex, and age (p<0.100).
3.4. Sensitivity analyses
The associations between childhood PBDEs and adiposity measures using the original, non-imputed data yielded similar significant inverse associations between BDE-153 and adiposity measures, although several associations between earlier windows of exposure (1-3 years) were only marginally significant (see Supplemental Table S3). Adjusting for lipids as a covariate or lipid-standardizing PBDE concentrations resulted in similar conclusions as when we used the original, non-imputed data (results not shown). In addition, we observed a significant inverse association between BDE-100 at age 3 years and body fat percentage. Additional adjustment for maternal intake of fruits and vegetables during pregnancy and prenatal PBDEs did not materially affect our result, nor did the removal of maternal adiposity measures from the models (results not shown). Lastly, removing children with body fat percentages ≥30% yielded similar conclusions for BDE-153, although statistically significant inverse associations were mainly observed with concentrations at 5 and 8 years (see Supplemental Table S4). Similar observations were noted when we excluded children with body fat percentages ≤14%, with mainly statistically inverse associations observed between BDE-153 at ages 5 and 8 years and z-scores for weight and BMI and BDE-153 at ages 2, 3, 5, and 8 years and body fat percentage and waist circumference (see Supplemental Table S5). Statistically significant effect modification by sex was noted between BDE-153 associations at ages 1, 2, and 5 years and body fat percentage; in particular, a 10-fold increase in concurrent BDE-153 concentration was associated with a decrement of 3.9% in body fat (95% CI −7.4, −0.5) in males, but null associations in females (β=1.0%, 95% CI −6.6, 8.7) (results not shown).
4. Discussion
Findings from the present study do not support the hypothesis that postnatal PBDEs are associated with increased childhood adiposity. We observed a pattern of inverse associations between repeated childhood serum BDE-153 concentrations with weight and BMI z-scores, body fat percentage, and waist circumference, which became stronger with BDE-153 measures more proximal to child adiposity assessment.
Epidemiological studies have generally reported inverse relationships between postnatal PBDEs and measures of adiposity in children. Among girls aged 6-8 years from the Breast Cancer and the Environment Research Centers (BCERC) Study, the means of PBDE congeners −47, −100, −153, and −154 were significantly lower among those with higher BMI (Windham et al. 2010). Darrow et al. reported low BMI z-score was also a significant predictor of higher BDE-153 concentrations among children aged 1-5 years (2017). The Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) Study also reported an inverse association between concurrent serum concentrations of BDE-153 and BMI z-score and waist circumference at age 7 years, with a monotonic decrease in both adiposity measures with increasing quartiles of BDE-153 concentrations (Erkin-Cakmak et al. 2015). In contrast, no relationship was noted between concurrent PBDEs and BMI among adolescents from a cohort in the Netherlands (Leijs et al. 2017).
Our study corroborates prior reports of postnatal PBDEs and childhood adiposity, and is congruent with previous findings from the HOME Study examining PBDEs during gestation. Previously, prenatal BDE-153 was reported to be inversely associated with adiposity measures in the HOME Study (Vuong et al. 2016). However, concentrations of prenatal PBDEs from the CHAMACOS Study had null and positive associations with BMI, respectively (Erkin-Cakmak et al. 2015).
The lipophilic nature of PBDEs and their pharmokinetic properties may have led to the observed results through reverse causation. It has been postulated that lipophilic compounds, including PBDEs and organochlorines, would be lower in concentrations for individuals with more adipose tissue, because the total storage compartment of adipose tissue would in effect render the serum concentrations to be “diluted” (Chevrier 2013; Glynn et al. 2003). Previously, individuals who experienced weight loss had significantly higher plasma and tissue concentrations of organochlorines (Chevrier et al. 2000; Imbeault et al. 2001; Kim et al. 2011; Walford et al. 1999).
There is no evidence from this study that postnatal PBDEs, aside from BDE-153, are associated with altered adiposity outcomes in childhood. BDE-153 has been uniquely identified as having an inverse association with adiposity measures in three other U.S. cohorts (Darrow et al. 2017; Erkin-Cakmak et al. 2015; Hoffman et al. 2016) and in NHANES (Lim et al. 2008); no relationship was noted with other congeners. BDE-153 has a higher fat deposition, considerably longer half-life, and is the more difficult to metabolize and excrete than BDE-47, −99, and −100 (Geyer et al. 2004; Staskal et al. 2006). In addition, Malarvannan et al. (2018) reported a significant increase in serum BDE-153 concentrations after weight loss among 94 Belgian obese adolescents even though a non-significant trend was observed with BDE-47 and −100, suggesting possible congener-specific variability in the pharmacokinetic processes controlling metabolism and storage.
If the true association between BDE-153 and adiposity measures is indeed an inverse one, the mechanisms involved in reduction of adipose tissue is as yet unknown. Animal studies on PBDEs’ role as potential obesogens are not conclusive, with some reporting weight gain (Bondy et al. 2013; Dufault et al. 2005; Fernie et al. 2006; Gee and Moser 2008; Suvorov et al. 2009) while others have reported either weight loss or no association (Daubie et al. 2011; Ta et al. 2011; Talsness et al. 2008). Because PBDEs are structurally similar to PCBs, they may also disrupt the GH/IGF-1 axis to delay growth (Ahmed 2013).
We found that sex modified the relationship between BDE-153 and body fat percentage, with significant reductions in body fat percentage in males, but null associations in females. No other study has reported significant sex interactions between postnatal PBDEs and childhood adiposity. However, Hoffman et al. (2016) noted similar inverse associations between PBDEs (except for BDE-153) and weight-for-height z-scores in males and positive associations among females upon stratification, but PBDEs were measured in breastmilk. For gestational PBDEs and childhood adiposity, the CHAMACOS Study found sex modified the relationship between PBDEs and BMI, with positive associations among males and inverse associations in females (Erkin-Cakmak et al. 2015). It is unclear whether the relationship between postnatal PBDEs and childhood adiposity is sexually dimorphic given the limited number of studies to examine this and the disparate findings thus far. It is plausible that PBDEs have sexually dimorphic biological processes since they are agonists of estrogen receptors and antagonists of androgen receptors (Meerts et al. 2001; Stoker et al. 2005). The hypothalamic-pituitary-gonadal (HPG) axis is active during the first months of infancy and reactivates at the onset of puberty in a gender-specific pattern (Chellakooty et al. 2003). Prepubertal children are sensitive to sex steroid actions, because normal endogenous sex steroid levels are low (Aksglaede et al. 2006). Small variations result in shifts in total hormone activity and may subsequently result in phenotypic effects as sex steroids have fat mobilizing properties. Given the sex steroid environment and receptor distribution in adipose tissue are distinct between males and females, it is conceivable that PBDEs’ mechanistic actions regarding adiposity vary by sex (Grun and Blumberg 2007). It is unclear what biological mechanisms are involved in potential sex differences in the reverse causality hypothesis between childhood PBDEs and measures of adiposity.
However, trajectories of fat and lean body mass vary by age and sex, with a divergence in the gain of fat mass between the sexes around age 5 (Weber et al. 2012). Higher gains in fat mass among females could result in lower PBDE concentrations as PBDEs would have more adipose tissue to partition in to. Further, the increase in lean body mass observed in males could also contribute to the higher concentrations of PBDEs. Thus, the findings of effect modification by sex between PBDEs and body fat percentage may be partially explained by the trajectories of fat and lean body mass between sexes rather than sexual dimorphism.
There were several strengths of our study. First, we employed a longitudinal design, which allowed for the use of repeated measures of postnatal PBDEs from infancy to prepubescence. Second, we adjusted for an extensive array of covariates, including sociodemographic factors, child sedentary measures, maternal depression, maternal prepregnancy BMI and weight, whether the child was breastfed, and prenatal PBDEs. Third, we used imputed PBDE concentrations to increase the precision of our estimates and conclusions were similar when we examined the original, non-imputed data. Finally, we modeled PBDEs using different lipid adjustment methods.
This study also had some limitations. First, we lacked data on dietary intake. However, we adjusted for maternal intake of fruits and vegetables during pregnancy as a proxy for dietary intake of children in a sensitivity analysis and found no difference in our results. Second, we did not have information on body fat distribution at earlier ages, which prevented us from analyzing it as both an outcome and an intermediate for body fat percentage at age 8 years. Third, our findings may not be generalizable to the US population. HOME Study children at age 8 years had a prevalence of overweight and obesity of 17% and 2%, respectively, compared to 33% and 19% among children ages 6-8 years in NHANES 2015-2016 (Skinner et al. 2018). Selection bias is also a concern as children who were excluded due to missing information (n=184) were statistically more likely than those included (n=206) to have mothers who were married or living with a partner and were more likely to have mothers with blood lead levels that were higher (0.78 ug/dL versus 0.69 ug/dL) (see Supplemental Table S6). However, they were similar in several maternal and child characteristics, including maternal prepregnancy BMI, age, race/ethnicity, income, education, and child sex.
5. Conclusions
We found significant inverse associations of childhood BDE-153 concentrations with z-scores for weight and BMI, body fat percentage, and waist circumference at ages 8 years. Our findings are consistent with other epidemiological studies identifying inverse associations between childhood BDE-153 and adiposity. Based on PBDEs’ physiochemical properties, reverse causality may have resulted in the observed inverse associations.
Supplementary Material
Highlights.
Findings do not support postnatal PBDEs are associated with increased adiposity
BDE-28, −47, −99, −100, and ∑PBDEs were not associated with adiposity at 8 years
There was a pattern of inverse associations between BDE-153 and adiposity measures
Child sex modified BDE-153 associations, with decreases in body fat % in males, but not in females
Reverse causality may have resulted in inverse associations
Acknowledgements:
This work was supported by grants from the National Institute of Environmental Health Sciences and the US Environmental Protection Agency (NIEHS P01 ES11261, R01 ES020349, R01 ES025214, R01 ES014575, T32ES010957, P30ES006096; EPA P01 R829389). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services. The authors declare no competing financial interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed RG. 2013. Early weaning pcb 95 exposure alters the neonatal endocrine system: Thyroid adipokine dysfunction. J Endocrinol 219:205–215. [DOI] [PubMed] [Google Scholar]
- Aksglaede L, Juul A, Leffers H, Skakkebaek NE, Andersson AM. 2006. The sensitivity of the child to sex steroids: Possible impact of exogenous estrogens. Hum Reprod Update 12:341–349. [DOI] [PubMed] [Google Scholar]
- Auwerx J. 1999. Ppargamma, the ultimate thrifty gene. Diabetologia 42:1033–1049. [DOI] [PubMed] [Google Scholar]
- Bastos Sales L, Kamstra JH, Cenijn PH, van Rijt LS, Hamers T, Legler J. 2013. Effects of endocrine disrupting chemicals on in vitro global DNA methylation and adipocyte differentiation. Toxicol In Vitro 27:1634–1643. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. 1996. Beck depression inventory 2nd ed. San Antonio, TX:Psychological Corporation. [Google Scholar]
- Beunckens C, Sotto C, Molenberghs G. 2008. A simulation study comparing weighted estimating equations with multiple imputation based estimating equations for longitudinal binary data. Comput Stat Data An 52:1533–1548. [Google Scholar]
- Bondy GS, Lefebvre DE, Aziz S, Cherry W, Coady L, Maclellan E, et al. 2013. Toxicologic and immunologic effects of perinatal exposure to the brominated diphenyl ether (bde) mixture de-71 in the sprague-dawley rat. Environ Toxicol 28:215–228. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, et al. 2017. Cohort profile: The health outcomes and measures of the environment (home) study. Int J Epidemiol 46:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellakooty M, Schmidt IM, Haavisto AM, Boisen KA, Damgaard IN, Mau C, et al. 2003. Inhibin a, inhibin b, follicle-stimulating hormone, luteinizing hormone, estradiol, and sex hormone-binding globulin levels in 473 healthy infant girls. J Clin Endocrinol Metab 88:3515–3520. [DOI] [PubMed] [Google Scholar]
- Chevrier J, Dewailly E, Ayotte P, Mauriege P, Despres JP, Tremblay A. 2000. Body weight loss increases plasma and adipose tissue concentrations of potentially toxic pollutants in obese individuals. Int J Obes Relat Metab Disord 24:1272–1278. [DOI] [PubMed] [Google Scholar]
- Chevrier J. 2013. Invited commentary: Maternal plasma polybrominated diphenyl ethers and thyroid hormones--challenges and opportunities. Am J Epidemiol 178:714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow LA, Jacobson MH, Preston EV, Lee GE, Panuwet P, Hunter RE Jr., et al. 2017. Predictors of serum polybrominated diphenyl ether (pbde) concentrations among children aged 1–5 years. Environ Sci Technol 51:645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubie S, Bisson JF, Lalonde R, Schroeder H, Rychen G. 2011. Neurobehavioral and physiological effects of low doses of polybrominated diphenyl ether (pbde)-99 in male adult rats. Toxicol Lett 204:57–63. [DOI] [PubMed] [Google Scholar]
- Dufault C, Poles G, Driscoll LL. 2005. Brief postnatal pbde exposure alters learning and the cholinergic modulation of attention in rats. Toxicol Sci 88:172–180. [DOI] [PubMed] [Google Scholar]
- Erkin-Cakmak A, Harley KG, Chevrier J, Bradman A, Kogut K, Huen K, et al. 2015. And childhood polybrominated diphenyl ether exposures and body mass at age 7 years: The chamacos study. Environ Health Perspect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie KJ, Laird Shutt J, Ritchie IJ, Letcher RJ, Drouillard K, Bird DM. 2006. Changes in the growth, but not the survival, of american kestrels (falco sparverius) exposed to environmentally relevant polybrominated diphenyl ethers. J Toxicol Environ Health A 69:1541–1554. [DOI] [PubMed] [Google Scholar]
- Gee JR, Moser VC. 2008. Acute postnatal exposure to brominated diphenylether 47 delays neuromotor ontogeny and alters motor activity in mice. Neurotoxicol Teratol 30:79–87. [DOI] [PubMed] [Google Scholar]
- Geyer HJ, Schramm K, Darnerud PO, Aune M, Feicht EA, Fried KW, et al. 2004. Terminal elimination half-lives of the brominated flame retardants tbbpa, hbcd, and lower brominated pbdes in humans. Organohalogen Compd 66. [Google Scholar]
- Glynn AW, Granath F, Aune M, Atuma S, Darnerud PO, Bjerselius R, et al. 2003. Organochlorines in swedish women: Determinants of serum concentrations. Environ Health Perspect 111:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun F, Blumberg B. 2007. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev Endocr Metab Disord 8:161–171. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Mall JK. 2014. Developmental exposure to polybrominated diphenyl ethers and neurodevelopment. Curr Environ Health Rep 1:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Mendez M, Siega-Riz AM, Herring AH, Sjodin A, Daniels JL. 2016. Lactational exposure to polybrominated diphenyl ethers and its relation to early childhood anthropometric measurements. Environ Health Perspect 124:1656–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe AA, Carey GB. 2007. Polybrominated diphenyl ethers as endocrine disruptors of adipocyte metabolism. Obesity (Silver Spring) 15:2942–2950. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene 5:46–51. [Google Scholar]
- Imbeault P, Chevrier J, Dewailly E, Ayotte P, Despres JP, Tremblay A, et al. 2001. Increase in plasma pollutant levels in response to weight loss in humans is related to in vitro subcutaneous adipocyte basal lipolysis. Int J Obes Relat Metab Disord 25:1585–1591. [DOI] [PubMed] [Google Scholar]
- Jones R, Edenfield E, Anderson S, Zhang Y, Sjodin A. 2012. Semi-automated extraction and cleanup method for measuring persistent organic pollutants in human serum. Organohalogen Compd 74:97–98. [Google Scholar]
- Kamstra JH, Hruba E, Blumberg B, Janesick A, Mandrup S, Hamers T, et al. 2014. Transcriptional and epigenetic mechanisms underlying enhanced in vitro adipocyte differentiation by the brominated flame retardant bde-47. Environ Sci Technol 48:4110–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Marchand P, Henegar C, Antignac JP, Alili R, Poitou C, et al. 2011. Fate and complex pathogenic effects of dioxins and polychlorinated biphenyls in obese subjects before and after drastic weight loss. Environ Health Perspect 119:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. 2000. Cdc growth charts: United states. Adv Data:1–27. [PubMed] [Google Scholar]
- Lam J, Lanphear BP, Bellinger D, Axelrad DA, McPartland J, Sutton P, et al. 2017. Developmental pbde exposure and iq/adhd in childhood: A systematic review and meta-analysis. Environ Health Perspect 125:086001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijs MM, Koppe JG, Vulsma T, Olie K, van Aalderen WMC, de Voogt P, et al. 2017. Alterations in the programming of energy metabolism in adolescents with background exposure to dioxins, dl-pcbs and pbdes. PLoS One 12:e0184006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JS, Lee DH, Jacobs DR Jr. 2008. Association of brominated flame retardants with diabetes and metabolic syndrome in the u.S. Population, 2003–2004. Diabetes Care 31:1802–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarvannan G, Van Hoorenbeeck K, Deguchtenaere A, Verhulst SL, Dirinck E, Van Gaal L, et al. 2018. Dynamics of persistent organic pollutants in obese adolescents during weight loss. Environ Int 110:80–87. [DOI] [PubMed] [Google Scholar]
- Meerts IA, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, et al. 2001. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated pdbes, and polybrominated bisphenol a compounds. Environ Health Perspect 109:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi F, Barrea L, Di Somma C, Savanelli MC, Muscogiuri G, Orio F, et al. 2016. Endocrine aspects of environmental "obesogen" pollutants. Int J Environ Res Public Health 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien KM, Upson K, Cook NR, Weinberg CR. 2016. Environmental chemicals in urine and blood: Improving methods for creatinine and lipid adjustment. Environ Health Perspect 124:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT Jr., Henderson LO, Needham LL. 1989. Chlorinated hydrocarbon levels in human serum: Effects of fasting and feeding. Arch Environ Contam Toxicol 18:495–500. [DOI] [PubMed] [Google Scholar]
- Sanchez BN, Hu H, Litman HJ, Tellez-Rojo MM. 2011. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect 119:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Papke O, Tung KC, Joseph J, Harris TR, Dahlgren J. 2005. Polybrominated diphenyl ether flame retardants in the u.S. Population: Current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J Occup Environ Med 47:199–211. [DOI] [PubMed] [Google Scholar]
- Shen CW, Chen YH. 2013. Model selection of generalized estimating equations with multiply imputed longitudinal data. Biom J 55:899–911. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Jones RS, Lapeza CR, Focant JF, McGahee EE 3rd, Patterson DG Jr. 2004. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal Chem 76:1921–1927. [DOI] [PubMed] [Google Scholar]
- Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. 2018. Prevalence of obesity and severe obesity in us children, 1999–2016. Pediatrics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskal DF, Hakk H, Bauer D, Diliberto JJ, Birnbaum LS. 2006. Toxicokinetics of polybrominated diphenyl ether congeners 47, 99, 100, and 153 in mice. Toxicol Sci 94:28–37. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Cooper RL, Lambright CS, Wilson VS, Furr J, Gray LE. 2005. In vivo and in vitro anti-androgenic effects of de-71, a commercial polybrominated diphenyl ether (pbde) mixture. Toxicol Appl Pharmacol 207:78–88. [DOI] [PubMed] [Google Scholar]
- Suvorov A, Battista MC, Takser L. 2009. Perinatal exposure to low-dose 2,2',4,4'-tetrabromodiphenyl ether affects growth in rat offspring: What is the role of igf-1? Toxicology 260:126–131. [DOI] [PubMed] [Google Scholar]
- Ta TA, Koenig CM, Golub MS, Pessah IN, Qi L, Aronov PA, et al. 2011. Bioaccumulation and behavioral effects of 2,2',4,4'-tetrabromodiphenyl ether (bde-47) in perinatally exposed mice. Neurotoxicol Teratol 33:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talsness CE, Kuriyama SN, Sterner-Kock A, Schnitker P, Grande SW, Shakibaei M, et al. 2008. In utero and lactational exposures to low doses of polybrominated diphenyl ether-47 alter the reproductive system and thyroid gland of female rat offspring. Environ Health Perspect 116:308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms LM, Harden F, Paepke O, Hobson P, Ryan JJ, Mueller JF. 2008. Higher accumulation of polybrominated diphenyl ethers in infants than in adults. Environ Sci Technol 42:7510–7515. [DOI] [PubMed] [Google Scholar]
- Toms LM, Sjodin A, Harden F, Hobson P, Jones R, Edenfield E, et al. 2009. Serum polybrominated diphenyl ether (pbde) levels are higher in children (2–5 years of age) than in infants and adults. Environ Health Perspect 117:1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Webster GM, Romano ME, Braun JM, Zoeller RT, Hoofnagle AN, et al. 2015. Maternal polybrominated diphenyl ether (pbde) exposure and thyroid hormones in maternal and cord sera: The home study, cincinnati, USA. Environ Health Perspect 123:1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Braun JM, Sjodin A, Webster GM, Yolton K, Lanphear BP, et al. 2016. Prenatal polybrominated diphenyl ether exposure and body mass index in children up to 8 years of age. Environ Health Perspect 124:1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Braun JM, Yolton K, Xie C, Webster GM, Sjodin A, et al. 2017a. Prenatal and postnatal polybrominated diphenyl ether exposure and visual spatial abilities in children. Environ Res 153:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Dietrich KN, Braun JM, Lanphear BP, Chen A. 2017b. Exposure to polybrominated diphenyl ethers (pbdes) and child behavior: Current findings and future directions. Horm Behav. [DOI] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Poston KL, Xie C, Webster GM, Sjodin A, et al. 2017c. Prenatal and postnatal polybrominated diphenyl ether (pbde) exposure and measures of inattention and impulsivity in children. Neurotoxicol Teratol 64:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Xie C, Webster GM, Sjodin A, Braun JM, et al. 2017d. Childhood polybrominated diphenyl ether (pbde) exposure and neurobehavior in children at 8 years. Environ Res 158:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford RL, Mock D, MacCallum T, Laseter JL. 1999. Physiologic changes in humans subjected to severe, selective calorie restriction for two years in biosphere 2: Health, aging, and toxicological perspectives. Toxicol Sci 52:61–65. [PubMed] [Google Scholar]
- Weber DR, Leonard MB, Zemel BS. 2012. Body composition analysis in the pediatric population. Pediatr Endocrinol Rev 10:130–139. [PMC free article] [PubMed] [Google Scholar]
- Windham GC, Pinney SM, Sjodin A, Lum R, Jones RS, Needham LL, et al. 2010. Body burdens of brominated flame retardants and other persistent organo-halogenated compounds and their descriptors in us girls. Environ Res 110:251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.