Supplemental digital content is available in the text.
Key Words: pancreatic cancer, adult, magnetic resonance imaging, medication therapy management, phantoms, imaging, image processing, computer-assisted
Abstract
Objectives
The aim of this study was to test the feasibility of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) with concurrent perfusion phantom for monitoring therapeutic response in patients with pancreatic ductal adenocarcinoma (PDAC).
Materials and Methods
A prospective pilot study was conducted with 8 patients (7 men and 1 woman) aged 46 to 78 years (mean age, 66 years). Participants had either locally advanced (n = 7) or metastatic (n = 1) PDAC, and had 2 DCE-MRI examinations: one before and one 8 ± 1 weeks after starting first-line chemotherapy. A small triplicate perfusion phantom was imaged with each patient, serving as an internal reference for accurate quantitative image analysis. Tumor perfusion was measured with Ktrans using extended Tofts model before and after phantom-based data correction. Results are presented as mean ± SD and 95% confidence intervals (CIs). Statistical difference was evaluated with 1-way analysis of variance.
Results
Tumor-size change of responding group (n = 4) was −12% ± 4% at 8 weeks of therapy, while that of nonresponding group (n = 4) was 18% ± 15% (P = 0.0100). Before phantom-based data correction, the Ktrans change of responding tumors was 69% ± 23% (95% CI, 32% to 106%) at 8 weeks, whereas that of nonresponding tumors was −1% ± 41% (95% CI, −65% to 64%) (P = 0.0247). After correction, the data variation in each group was significantly reduced; the Ktrans change of responding tumors was 73% ± 6% (95% CI, 64% to 82%) compared with nonresponding tumors of −0% ± 5% (95% CI, −7% to 8%) (P < 0.0001).
Conclusions
Quantitative DCE-MRI measured the significant perfusion increase of PDAC tumors responding favorably to chemotherapy, with decreased variability after correction using a perfusion phantom.
Pancreatic ductal adenocarcinoma (PDAC) typically has a very poor prognosis; PDAC is the fourth leading cause of cancer death, and the 5-year survival rate of PDAC is only 8%.1 Currently, there are 2 preferred first-line therapeutic options for patients with advanced PDAC, nab-paclitaxel with gemcitabine2 and FOLFIRINOX (combination of fluoropyrimidine, leucovorin, irinotecan, and oxaliplatin).3 Overall response rates of these regimens are comparable (20%~30%), and there are no robust data favoring one over the other. With the recognition that PDAC is a biologically heterogeneous disease, a more precise or personalized approach would require novel individual tumor biomarker-based determinants. Identification of biomarkers that have potential to predict which patients are more or less likely to have favorable response or prognosticate survival is an unmet need for patients with PDAC. Choosing a more effective therapy upfront is imperative, especially for patients with locally advanced PDAC, as an effective therapy can downstage the tumor, improve curative resection rates, and potentially eradicate micrometastatic disease. In contrast, a noneffective therapy may result in disease progression.
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) has potential as a noninvasive tool for early evaluation of PDAC response to treatment. Pancreatic ductal adenocarcinoma is typically hypoperfused due to significant tumor sclerosis creating elevated interstitial pressure, which compresses tumor feeding vessels.4,5 An effective therapy induces acute necrosis, leading to reduced interstitial pressure and consequentially increased perfusion.6 As DCE-MRI can measure tissue perfusion by monitoring the dynamic change of MRI contrast agents introduced intravenously,7–10 it may detect perfusion increase in responding PDAC during early stages of therapy.
However, variability in DCE-MRI measurement across different time11–13 and scanners14 remains a major concern that restricts the clinical application of quantitative DCE-MRI. Intrascanner variability over time leads to errors in therapy monitoring, whereas interscanner variability impedes comparing quantitative data among institutes to establish standard value for accurate diagnosis. To minimize MRI scanner-dependent variation, one approach is to use an external phantom with known contrast concentration, as the pattern and extent of the variation can be detected using the phantom value measured by the scanner. As DCE-MRI measurement varies across time, it would be ideal for a small phantom to be imaged concurrently in the bore of a standard MRI scanner with a patient to ensure the accuracy of human data in real time.
A static phantom composed of multiple units with different contrast concentrations was originally proposed to reduce variation in DCE-MRI measurement.15 However, there are several concerns with this approach. First, the movement of contrast agents in vivo causes additional signal reduction not replicated by a static phantom. Second, most B1 mapping techniques are T1 dependent, thus the flip angles (FAs) of static phantom components with high contrast concentrations (low T1 values) may not be accurately calculated, especially in high-field MRI.16–19 Third, a static phantom only contains a limited number of components, which may lead to significant fitting errors. Thus a perfusion phantom that dynamically changes contrast concentration, replicating human tissue, would be a more reliable tool.
A point-of-care perfusion phantom was recently developed for accurate and reproducible DCE-MRI measurement across different scanners and time.20 This phantom is compact enough to be imaged with a human subject and large enough not to suffer from partial volume effect. This phantom was tested in abdominal DCE-MRI of healthy volunteers, and significant improvement in data reproducibility across 2 different 3 T MRI scanners was demonstrated.
We hypothesize that quantitative DCE-MRI–based analysis with this phantom can identify early therapeutic response in PDAC. This manuscript describes our recent success of testing this hypothesis in a single-center pilot clinical study.
MATERIALS AND METHODS
Patients
This study was approved by our institutional review board, and followed the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice guidelines. All patients signed informed consent forms fully disclosing the investigational nature of the study before enrollment. The health insurance portability and accountability act was strictly observed. We prospectively recruited 8 patients with newly diagnosed PDAC. Patients with safety contraindications to MRI examination (determined by standard clinical screening), and those on hemodialysis or with acute/chronic renal failure were excluded from the recruitment. Table 1 summarizes the clinical information of the 8 participants. Seven participants were white men (mean age, 65; age range, 46–78 years), and 1 participant was an African American woman (aged 66 years). Seven patients had locally advanced pancreatic cancer (LAPC) as defined by the National Comprehensive Cancer Network, whereas 1 patient had 4 small liver metastases (<5 mm) on presentation. Multiphasic intravenously contrast-enhanced standard-of-care CT was obtained in all participants before and at 8 ± 1 and 18 ± 3 weeks after therapy initiation. Tumor boundary was determined on the CT images by a board-certified radiologist with over 20 years of experience in gastrointestinal radiology and blinded to therapy and clinical findings. Before therapy initiation, the mean tumor long axis was 39 ± 15 mm (mean ± SD). Dynamic contrast-enhanced magnetic resonance imaging was obtained before and at 8 ± 1 weeks after therapy initiation. The detailed therapeutic modality and schedule (dosage and timing) were within the discretion of our oncologists, but first-line therapy was composed only of chemotherapeutic agents. Tumor response was assessed by RECIST 1.1 criteria. During the 18 weeks of therapy, 2 patients partially responded (PR), 4 patients had progressive disease (PD), and 2 patients had stable disease (SD). In this manuscript, the SD was denoted as SD−, because the tumor size was decreased, but the extent was not sufficiently large to be classified as PR. Both PR and SD− are categorized as responding group in this manuscript.
TABLE 1.
Clinical Information of 8 Participants
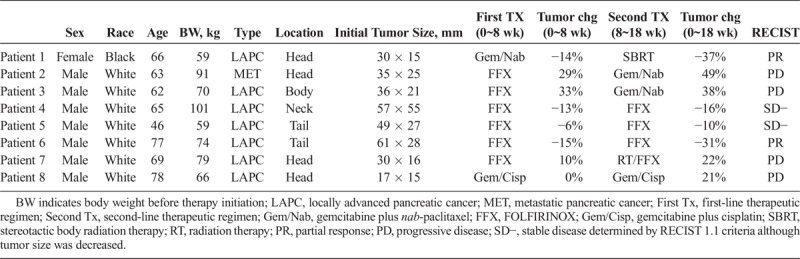
Perfusion Phantom
Figure 1A shows a photograph of the perfusion phantom. The phantom was designed using computer-aided design software, SolidWorks (Dassault Systemes American Corp, Waltham, MA), and 3D printed using a Stratasys Objet30 Pro (Eden Prairie, MN) with VeroClear material. The detailed performance mechanism of this device was described in the previous manuscript.20 In brief, the phantom is composed of top and bottom chambers separated by a semipermeable membrane with pore size of 12~14 kD (SpectrumLabs, Rancho Dominguez, CA). Before use, both chambers are filled with degassed and deionized water. After starting DCE-MRI, a contrast agent (gadoteridol; 100 mM) is infused to the top chamber at a constant rate (0.06 mL/s; 3 mL) using a syringe pump (NE-1600; New Era Pump Systems, Inc, Farmingdale, NY), displacing the water in the top chamber. Then the contrast agent within the top chamber diffuses to the bottom chamber. The contrast concentration change in the bottom chamber was linear for 10 minutes (0.11 mM per minute), when independently measured using liquid chromatography with tandem mass spectrometry detection.21
FIGURE 1.
Point-of-care perfusion phantom for quantitative DCE-MRI of the abdomen. A, Photograph of a perfusion phantom, when a penny is used as a size reference. Contrast agent is transferred to the phantom via a plastic tube, as indicated with an arrow. B, Photograph of a phantom package with dimensions, containing 3 perfusion phantom components at the center (P1–P3) surrounded by thermal insulation material (polyurethane foam). C, Illustration of the phantom package located under a patient.
Figure 1B is a photograph of the phantom package with dimensions containing the triplicate perfusion phantom (P1–P3). Employing multiple phantom components not only increases the signal-to-noise measurement ratio, but also allows detection of any functional error should it occur in one of them. All phantom components were surrounded by thermal insulation material (polyurethane foam) to reduce heat transfer from the patient.
Figure 1C shows the schematic of a patient lying on the phantom package. Two small leather cushions were placed near the phantom package to match its thickness, and a fiberglass cushion was placed between it and the patient for additional thermal insulation and patient comfort. A torso phased array coil was placed around the patient and the phantom package.
Clinical MRI Protocol
All participants were asked to refrain from eating solid food or caffeinated drinks for at least 4 hours before imaging. Two 3 T MRI scanners (GE Signa and SIEMENS Prisma) were used, and scanner selection was primarily chosen by availability. The GE scanner (scanner A) was used for 6 patients (patients 3–8), and the SIEMENS scanner (scanner B) was used for 1 patient (patient 1). One patient (patient 2) was imaged in the GE scanner before therapy, but subsequently the SIEMENS scanner was used. Each scanner was equipped with a dual-channel transmit radiofrequency coil for improved B1 field homogeneity. Before imaging, B0/B1 shimming was conducted, and B1 mapping was followed using vendor's software.22,23 In both scanners, a 3D fast spoiled gradient echo sequence was used for DCE-MRI. The imaging parameters of 2 MRI scanners were selected to most closely match each other as follows: in scanner A (field of view, 400 × 360 mm; frequency/phase encoding, 192/160; matrix size, 256 × 230; slice number, 12; thickness/gap, 5/0 mm; FA, 15 degrees; TR/TE, 3.8/2.1 milliseconds; NEX, 1; SENSE factor, 2; and temporal resolution, 2.91 seconds) and in scanner B (field of view, 400 × 320 mm; frequency/phase encoding, 192/156; matrix size, 384 × 312; slice number, 10; thickness/gap, 5/0 mm; FA, 15 degrees; TR/TE, 4.9/2.5 milliseconds; NEX, 1; SENSE factor, 2; and temporal resolution, 2.34 seconds). Dynamic contrast-enhanced magnetic resonance imaging was applied for 9 minutes, whereas gadoteridol (0.1 mmol/kg) was injected intravenously 30 seconds after starting DCE-MRI and followed by saline flush (20 mL) at a constant rate (2 mL/s). Gadoteridol (100 mM) was infused to the perfusion phantom 15 seconds after starting DCE-MRI at a constant rate (0.06 mL/s; 3 mL). Before DCE-MRI, T1-weighted images with various FAs (2, 5, and 10 degrees) were obtained for T1 mapping.24 T1-weighted imaging was conducted in a free-breathing mode for 30 seconds, and the images obtained at expiration phase was automatically selected and averaged per each FA. Dynamic contrast-enhanced magnetic resonance imaging was also conducted in a free-breathing mode, and images acquired at expiration phase were automatically selected and coregistered.25
Image Processing
Image processing was accomplished in 8 steps. First, DCE-MRI images were coregistered using expiration-phase B-spline method.25 Second, the local variation of FA was assessed by B1 mapping.22,23 Third, T1 maps were obtained using the various FA approach.24 Fourth, contrast maps were obtained using the method of Bokacheva et al.26 During T1 and contrast mapping, the FA variation over the field of view was corrected using B1 maps. Fifth, a look-up table (LUT) correlating the reference contrast enhancement curve (CEC) (obtained by liquid chromatography with tandem mass spectrometry detection) with the measured ones (by MRI) was created. Sixth, the arterial input function (AIF) was determined from the abdominal aorta as described in a previous manuscript.27 Hematocrit (HCT) was measured for each patient every 1 to 2 weeks during therapy as a standard of care, and used to retrieve plasma input function (PIF) from AIF using equation, PIF = AIF/(1-HCT). Seventh, the contrast maps and PIF were corrected using the LUT (see “Appendix B” of the previous manuscript for details20). Eighth, the map of Ktrans, volume transfer constant, was created using the extended Tofts model.28 Ktrans values in each tumor region were averaged. Image processing was implemented using a laboratory-made software package based on LabVIEW (National Instruments Co, Austin, TX), which was validated using digital reference objects created by Dr Daniel Barboriak (Duke University, Durham, NC) and Quantitative Imaging Biomarker Alliance.29
Statistical Analysis
Data are presented as means ± SD and 95% confidence intervals (CIs). Statistical difference was evaluated with 1-way analysis of variance,30 and considered significant when P value was less than 0.01. The repeatability of CEC of perfusion phantom was determined by intraclass correlation coefficient.31 All statistical analyses were implemented using SAS, version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
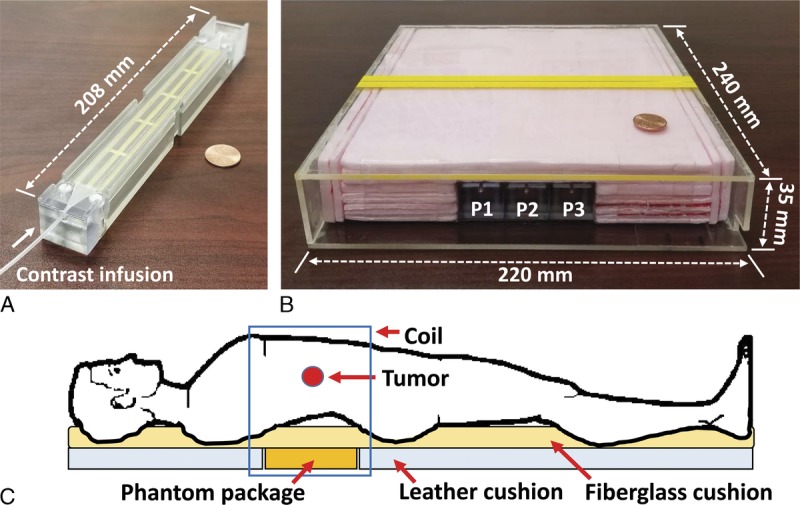
For a 12-month period, DCE-MRI was applied for 8 PDAC patients before and at 8 weeks after starting first-line chemotherapy, and the tumor Ktrans change of each patient was analyzed. Figure 2A shows representative DCE-MRI images from a patient with an LAPC in the pancreas neck indicated by the white arrow in each subfigure, before (baseline) and at 2 and 8 minutes after contrast injection. Three phantom components (P1–P3) imaged together with the patient are demarcated with white rectangles in the first subfigure. Two different gray scales were applied (0~700 for patient images; 0~3000 for phantom images) to present both images in high contrast. To date, no phantom-driven artifacts interfering with participant anatomy or pathology visualization have been observed. Figure 2B shows the contrast maps of the 3 perfusion phantoms at 2, 5, and 8 minutes after contrast injection. Figure 2C shows the CECs of the perfusion phantoms, along with the mean value. The CEC repeatability was larger than 0.99 in all measurements. No participants reported additional pain or discomfort induced by the phantom.
FIGURE 2.
DCE-MRI images with the perfusion phantom. A, Representative DCE-MRI images of a patient having a tumor in the pancreas neck before (baseline) and at 2 and 8 minutes after contrast injection. The tumor and phantom regions are indicated with arrows and white rectangles, respectively. B, Contrast maps of 3 phantom components (P1–P3) at 2, 5, and 8 minutes after contrast injection. C, Contrast enhancement curves (CECs) of the 3 phantom components (P1–P3) and the mean. Intraclass correlation coefficient (ICC) of 3 CECs was higher than 0.99.
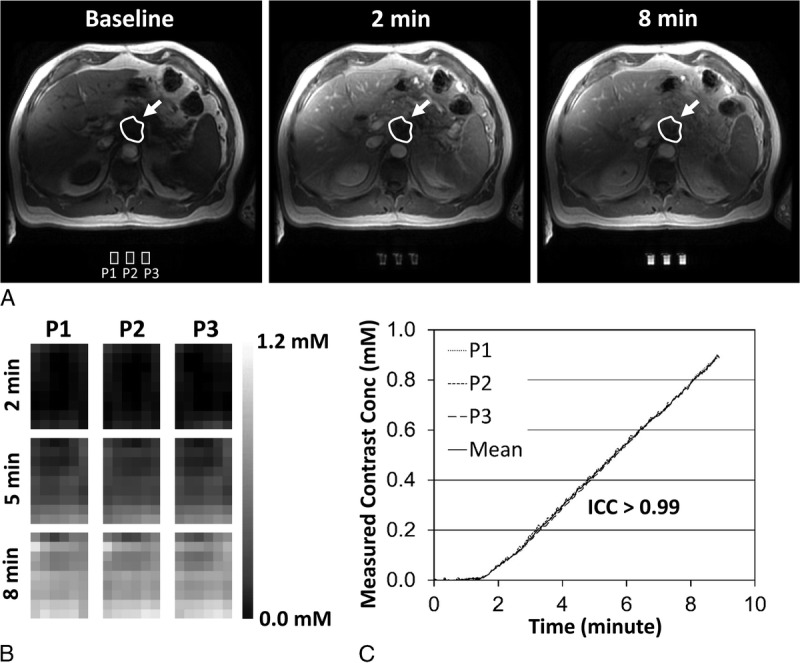
Figure 3 shows graphical LUTs obtained from the perfusion phantom on each imaging day at the center slice in either scanner A or scanner B. The LUT amplitude varied widely across time and scanners. Of note, the amplitudes of 2 LUTs obtained on the same day were approximately 20% different (Fig. 3A, “December 11, 2017” data sets). Furthermore, the LUT variation was observed across image slices as well. Figure 4A presents the Ktrans maps of a patient at the first, sixth, and 11th image slices before (upper row) and after data correction (lower row) using LUTs. Figures 4B and 4C show the Ktrans values in the paravertebral muscle and liver, respectively, over 12 slices before and after data correction. The magnitude and pattern of data variation over the image slices were different at each acquisition time.
FIGURE 3.
Graphical look-up tables (LUTs). A and B, Graphical LUTs at the center image slice on each imaging day in either (A) scanner A or (B) scanner B.
FIGURE 4.
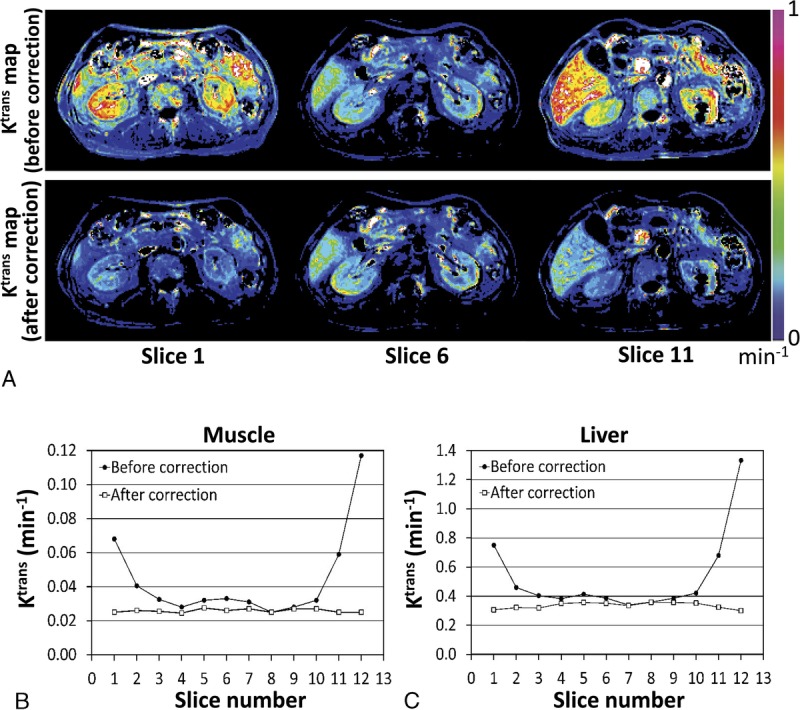
Ktrans variation across different image slices. A, Ktrans maps of a patient at the first, sixth, and 11th image slices before (upper row) and after data correction (lower row). B and C, Ktrans values over 12 image slices before (black circles) and after data correction (white squares) averaged in the (B) paravertebral muscle or (C) liver of the patient in Figure 4A.
Figure 5A shows the Ktrans maps of representative responding and nonresponding patients after phantom-based data correction (see Supplementary Figure, Supplemental Digital Content 1, which presents the Ktrans maps of all 8 patients, http://links.lww.com/RLI/A398). The baseline tumor Ktrans of all participants was 0.081 ± 0.035 min−1 (95% CI, 0.025 to 0.137 min−1) after data correction without statistical difference between responding and nonresponding groups (P = 0.6233). Figure 5B summarizes the tumor Ktrans change (%) before and after data correction. Before data correction, the Ktrans change of responding tumors was 69% ± 23% (95% CI, 32%–106%), whereas that of nonresponding tumors was −1% ± 41% (95% CI, −65%–64%) (P = 0.0247). After correction, the data variation was significantly reduced; the Ktrans change of responding tumors was 73% ± 6% (95% CI, 64% to 82%), and that of nonresponding tumors of −0% ± 5% (95% CI, −7% to 8%) (P < 0.0001). For comparison, the size changes of the responding and nonresponding tumors were − 12% ± 4% and 18% ± 15%, respectively, during the same period (P = 0.0100). The tumor Ktrans values of all 8 participants over 8 weeks of first-line chemotherapy before and after phantom-based data correction are presented in the Supplemental Digital Content 2, http://links.lww.com/RLI/A399.
FIGURE 5.
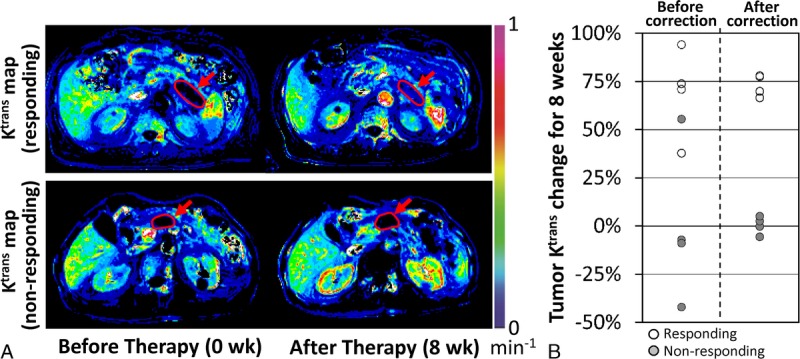
Tumor Ktrans change during 8 weeks of chemotherapy. A, Ktrans maps of 2 representative patients responding (patient 6) or nonresponding (patient 3) to therapy after phantom-based data correction. Tumor region is indicated with an arrow in each subfigure. B, Tumor Ktrans change (%) for 8 weeks before and after data correction, when responding and nonresponding tumors are indicated with hallow and gray circles, respectively.
DISCUSSION
To our knowledge, this is the first investigator-initiated clinical study in PDAC showing that the increase in tumor perfusion correlates significantly with chemotherapeutic response, consistent with previous preclinical data.6 Although the sample size was small, the statistical power to detect the difference in tumor Ktrans change between responding and nonresponding groups was 100% at type I error level of 0.0001 after perfusion-phantom–based data correction. Dynamic contrast-enhanced magnetic resonance imaging was considered to have potential for detecting the altered perfusion in PDAC lesions after an effective therapy, but the measurement variation was the major concern, preventing DCE-MRI to be used as a standard-of-care tool for PDAC therapeutic response assessment. The perfusion phantom used in this study allowed significant reduction of variation in quantitative Ktrans across time, scanners, and image slices. Because the mean Ktrans difference between the responding and nonresponding tumors was 73% at 8 weeks after starting therapy, this technology may be used in the future to detect PDAC responses even earlier (eg, 4 weeks). If so, one possible clinical application of this method is to guide neoadjuvant therapy. The clinical impact of neoadjuvant therapy for PDAC is controversial and an ongoing research area.32 The primary rationale of neoadjuvant therapy for locally advanced PDAC is to improve the selection of patients for whom resection may not offer a survival advantage by excluding those who rapidly progress to metastasis during preoperative therapy. Second, the neoadjuvant systemic therapy would facilitate the treatment of the possible micrometastases. Thus it is intuitive that early administration of appropriate neoadjuvant therapy can lead to optimal survival outcomes for locally advanced PDAC patients. For resectable PDAC patients, this proposed method applied in the neoadjuvant setting would enable early determination of whether the therapy should be continued to facilitate margin-negative resection or whether immediate surgical operation should commence before tumor size change.
One limitation of quantitative DCE-MRI–based therapy-monitoring strategy is that the positive therapeutic efficacy can be evaluated only when therapy is tumoricidal. In theory, if an effective therapy kills tumor cells, the interstitial pressure is reduced, and then intratumoral perfusion is increased.6 This may not hold true for agents with a tumoristatic response. For instance, the tumor of patient 8 (see Table 1) in this study was not enlarged for 8 weeks, and the tumor Ktrans did not increase during the same period either. In contrast, the tumor size of patient 5 decreased only 6%, but the tumor Ktrans increased 67%.
Another concern of this method is that the tumor perfusion is not only influenced by tumor cellularity, but also by extracellular matrix. Thus, if a therapy regimen contains an agent manipulating the extracellular matrix, data interpretation may be complicated. For example, Akisik et al33 demonstrated the perfusion of PDAC was significantly reduced after a dual combination therapy including sorafenib, a kinase inhibitor having antiangiogenic effect. Thus, the change of DCE-MRI parameters following different therapeutic modalities including immune therapy or irradiation would need to be further investigated in future studies.
In conclusion, the perfusion phantom used in this study is portable, inexpensive, easily used, and allowed reduction in variance of quantitative Ktrans measurement in patients with PDAC. It has potential to enhance precision for standard-of-care early therapeutic monitoring. However, this phantom will need to be tested, optimized, and validated in a larger multi-institutional study before being accepted for routine clinical use.
ACKNOWLEDGMENTS
Authors thank Mr Lael Gore, Mr Kevin McClure, and Mr Douglas for assistance with patient imaging and Ms April Riddle for patient recruitment. Authors also thank Dr Daniel Barboriak (Duke University) to provide the digital reference objects. All experiments complied will current regulatory requirements (including ethics requirements) and laws of the United States of America.
Footnotes
Grant support: Research Initiative Pilot Award from the Department of Radiology at UAB, NIH 5P30CA013148, and Department of Commerce Regional Innovation Strategies Program Cluster Grant to Southern Research ED17HDQ0200016.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.investigativeradiology.com).
REFERENCES
- 1. Cancer Facts & Figures 2018. American Cancer Society.
- 2.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 4.Danet IM, Semelka RC, Nagase LL, et al. Liver metastases from pancreatic adenocarcinoma: MR imaging characteristics. J Magn Reson Imaging. 2003;18:181–188. [DOI] [PubMed] [Google Scholar]
- 5.Sofuni A, Iijima H, Moriyasu F, et al. Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. J Gastroenterol. 2005;40:518–525. [DOI] [PubMed] [Google Scholar]
- 6.Kim H, Samuel S, Lopez-Casas P, et al. SPARC-independent delivery of Nab-Paclitaxel without depleting tumor stroma in patient-derived pancreatic cancer xenografts. Mol Cancer Ther. 2016;15:680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes SL, Whisenant JG, Loveless ME, et al. Practical dynamic contrast enhanced MRI in small animal models of cancer: data acquisition, data analysis, and interpretation. Pharmaceutics. 2012;4:442–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang CC, Yan Z, Giddabasappa A, et al. Comparison of dynamic contrast-enhanced MR, ultrasound and optical imaging modalities to evaluate the antiangiogenic effect of PF-03084014 and sunitinib. Cancer Med. 2014;3:462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craciunescu OI, Blackwell KL, Jones EL, et al. DCE-MRI parameters have potential to predict response of locally advanced breast cancer patients to neoadjuvant chemotherapy and hyperthermia: a pilot study. Int J Hyperthermia. 2009;25:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H, Folks KD, Guo L, et al. Early therapy evaluation of combined cetuximab and irinotecan in orthotopic pancreatic tumor xenografts by dynamic contrast-enhanced magnetic resonance imaging. Mol Imaging. 2011;10:153–167. [PMC free article] [PubMed] [Google Scholar]
- 11.Eikefjord E, Andersen E, Hodneland E, et al. Dynamic contrast-enhanced MRI measurement of renal function in healthy participants. Acta Radiol. 2017;58:748–757. [DOI] [PubMed] [Google Scholar]
- 12.Rata M, Collins DJ, Darcy J, et al. Assessment of repeatability and treatment response in early phase clinical trials using DCE-MRI: comparison of parametric analysis using MR- and CT-derived arterial input functions. Eur Radiol. 2016;26:1991–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waterton JC, Ho M, Nordenmark LH, et al. Repeatability and response to therapy of dynamic contrast-enhanced magnetic resonance imaging biomarkers in rheumatoid arthritis in a large multicentre trial setting. Eur Radiol. 2017;27:3662–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H. Variability in quantitative DCE-MRI: sources and solutions. J Nat Sci. 2018;4:e484. [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson EF, Gupta SN, A RM, et al. QIBA DCE-MRI technical committee update: phantom studies and first DCEMRI profile. Chicago, IL: Proceedings of the 96th Scientific Assembly and Annual Meeting of the Radiological Society of North America; 2010. [Google Scholar]
- 16.Cunningham CH, Pauly JM, Nayak KS. Saturated double-angle method for rapid B1+ mapping. Magn Reson Med. 2006;55:1326–1333. [DOI] [PubMed] [Google Scholar]
- 17.Choi N, Lee J, Kim MO, et al. A modified multi-echo AFI for simultaneous B1(+) magnitude and phase mapping. Magn Reson Imaging. 2014;32:314–320. [DOI] [PubMed] [Google Scholar]
- 18.Morrell GR. A phase-sensitive method of flip angle mapping. Magn Reson Med. 2008;60:889–894. [DOI] [PubMed] [Google Scholar]
- 19.Jiru F, Klose U. Fast 3D radiofrequency field mapping using echo-planar imaging. Magn Reson Med. 2006;56:1375–1379. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, Mousa M, Schexnailder P, et al. Portable perfusion phantom for quantitative DCE-MRI of the abdomen. Med Phys. 2017;44:5198–5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia J, Keiser M, Nassif A, et al. A LC-MS/MS method to evaluate the hepatic uptake of the liver-specific magnetic resonance imaging contrast agent gadoxetate (Gd-EOB-DTPA) in vitro and in humans. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;891–892:20–26. [DOI] [PubMed] [Google Scholar]
- 22.Sacolick LI, Wiesinger F, Hancu I, et al. B1 mapping by Bloch-Siegert shift. Magn Reson Med. 2010;63:1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung S, Kim D, Breton E, et al. Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout. Magn Reson Med. 2010;64:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liberman G, Louzoun Y, Ben Bashat D. T1 mapping using variable flip angle SPGR data with flip angle correction. J Magn Reson Imaging. 2014;40:171–180. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Tielbeek JAW, Caan MWA, et al. Expiration-phase template-based motion correction of free-breathing abdominal dynamic contrast enhanced MRI. IEEE Trans Biomed Eng. 2015;62:1215–1225. [DOI] [PubMed] [Google Scholar]
- 26.Bokacheva L, Rusinek H, Chen Q, et al. Quantitative determination of Gd-DTPA concentration in T1-weighted MR renography studies. Magn Reson Med. 2007;57:1012–1018. [DOI] [PubMed] [Google Scholar]
- 27.Kim H. Modification of population based arterial input function to incorporate individual variation. Magn Reson Imaging. 2018;45:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. [DOI] [PubMed] [Google Scholar]
- 29.Barboriak DP. 2014; Pages. Accessed at: https://scholars.duke.edu/display/gra211722.
- 30.Neter J, Kutner MH, Nachtsheim JC, et al. Applied Linear Statistical Models. 4th ed Columbus, OH: The McGraw-Hill Companies, Inc; 1996. [Google Scholar]
- 31.Bartlett JW, Frost C. Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstet Gynecol. 2008;31:466–475. [DOI] [PubMed] [Google Scholar]
- 32.Zhan HX, Xu JW, Wu D, et al. Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies. Cancer Med. 2017;6:1201–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akisik MF, Sandrasegaran K, Bu G, et al. Pancreatic cancer: utility of dynamic contrast-enhanced MR imaging in assessment of antiangiogenic therapy. Radiology. 2010;256:441–449. [DOI] [PubMed] [Google Scholar]


