Abstract
Mycobacterium ulcerans is the causative agent of Buruli ulcer (BU). This nontuberculous mycobacterial infection has been reported in 34 countries worldwide. In Australia, the majority of cases of BU have been recorded in coastal Victoria and the Mossman-Daintree areas of north Queensland. Mosquitoes have been postulated as a vector of M. ulcerans in Victoria, however the specific mode of transmission of this disease is still far from being well understood. In the current study, we trapped and analysed 16,900 (allocated to 845 pools) mosquitoes and 296 March flies from the endemic areas of north Queensland to examine for the presence of M. ulcerans DNA by polymerase chain reaction. Seven of 845 pools of mosquitoes were positive on screening using the IS2404 PCR target (maximum likelihood estimate 0.4/1,000). M. ulcerans DNA was detected from one pool of mosquitoes from which all three PCR targets: IS2404, IS2606 and the ketoreductase B domain of mycolactone polyketide synthase gene were detected. None of the March fly samples were positive for the presence of M. ulcerans DNA.
Author summary
The causative agent of Buruli ulcer is Mycobacterium ulcerans. This destructive skin disease is characterized by extensive and painless necrosis of skin and underlying tissues usually on extremities of body due to production of toxin named mycolactone. The disease is prevalent in Africa and coastal Australia. The exact mode of transmission and potential environmental reservoir for the pathogen still remain obscure. Aquatic and biting insects have been identified as potential niche in transmission and maintenance of pathogen in the environment. In this study we screened mosquitoes and march flies captured from endemic areas of northern Queensland for the presence of M. ulcerans DNA. We found seven pools of mosquito out of 845 pools positive for IS2404. In only one of the seven samples were the additional targets IS2606 and KR detected. None of the March fly samples were positive. The results could indicate a low burden of the bacteria in the environment coinciding with a comparatively low number of human cases of M. ulcerans infection seen during the trapping period of the study.
Introduction
Buruli ulcer (BU), also known regionally as Daintree ulcer in north Queensland, Australia or Bairnsdale ulcer in Victoria, Australia, is an emerging disease of skin and underlying tissue, with a potential to lead to permanent disability, particularly if treatment is inadequate or delayed. The causative agent of this disease, M. ulcerans secretes a polyketide exotoxin, mycolactone, the production of which requires expression of a series of contiguous genes on the large pMUM001 plasmid. This exotoxin is the main virulence determinant of the bacteria [1]. The outbreaks of BU have been consistently linked with wetland or coastal regions [2]. Environmental samples such as water, aquatic plants, soil at endemic areas has been found PCR-positive for M. ulcerans DNA [3, 4]. Insects such as mosquitoes and aquatic bugs has been proposed as a vital ecological niche for the maintenance of pathogen in environment [5, 6].The detection of M. ulcerans DNA in insects does not prove their ability to transmit M. ulcerans but could indicate potential to act as either biological or mechanical vector. A study conducted by Marsollier and his colleagues provided evidence of the presence of M. ulcerans DNA in the salivary gland of wild caught Naucoridae (aquatic bug). They successfully isolated the pathogen by culture from the salivary glands of aquatic bugs and suggested aquatic insects as having an important ecological niche in the maintenance of the organism in the environment. They were also able to demonstrate transmission to mice in a laboratory environment [6]. Similarly, a study conducted by Wallace et al. provided evidence of the ability of mosquitoes to act as a mechanical vector of M. ulcerans [7]. Studies conducted in endemic areas of Africa suggest that conducting farming activities close to rivers [8] and swimming in rivers located in endemic areas [9] are risk factors for exposure to M. ulcerans.
In Australia, foci of BU infection have been found in tropical north Queensland [10, 11], the Capricorn Coast region of central Queensland [10], the Northern Territory [12] and temperate coastal Victoria [5]. In Queensland, Australia, cases of Daintree ulcer have been reported primarily in Douglas Shire, exclusively in the vicinity of Wonga, Miallo and Daintree [10, 11]. A few cases has also been reported from Capricorn Coast region of central Queensland [10]. The Douglas Shire covers an area of 2,445 sq. Kms and the total population is around 11,000. A majority of the population (around 70%) reside in Port Douglas and Mossman. Thus, the Daintree ulcer endemic areas in north Queensland is sparsely populated. There has been a significant decrease in human cases of BU in north Queensland, since a large outbreak in 2011–2012, when more than 60 cases were reported. This outbreak occurred after prolonged and heavy rainfall in 2010–2011 [11]. The average reported rate over fifteen years period from 2002–2016 was 0.2 cases/100,000 population per year [13].
Victorian researchers detected the presence of M. ulcerans DNA in five different species of mosquito during a BU outbreak in an endemic area of Victoria, Australia. They demonstrated the absence of M. ulcerans in a neighboring area, where BU did not occur [5]. Together, the evidence was proposed to support a link with mosquitoes in the ecology of BU in Victoria [5, 14]. More recently, a small study conducted in the BU endemic region of north Queensland, found that of 35 insect/insects pools, one sample of an individual mosquito and one pool of two mosquitoes were positive for IS2404. The IS2404 positive mosquito pool contained DNA of a closely related M. ulcerans subspecies that had a low copy number for IS2606 which does not commonly cause disease in human. The individual mosquito had insufficient DNA for detection of the additional gene targets. The study highlighted a need to examine a larger sample size to gauge the significance of the role of mosquito in ecology of BU in Northern Queensland [15]. An additional suggestion proposed by the local population (including people with a history of BU) was that March flies (Tabanidae) might have a role in transmission. We therefore aimed, in this study to capture and screen mosquitoes and March flies for the presence of M. ulcerans DNA in the BU endemic area of Northern Queensland.
Materials and methods
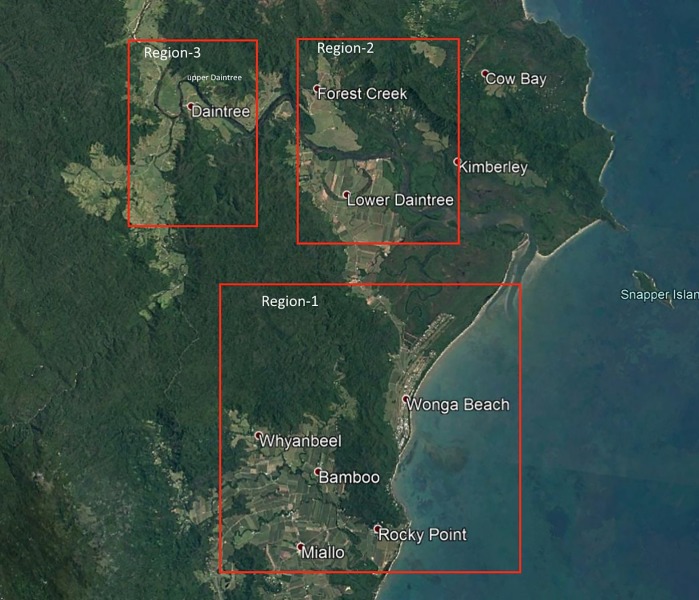
Selection of the study site was based on GIS mapping of human cases of BU in Northern Queensland [16]. We divided the endemic area of northern Queensland into three regions: Region-1: extending from Miallo to lower Daintree including Wonga/Wonga Beach area, Region-2: Forest Creek area and Region-3: Upper Daintree area (Fig 1).
Fig 1. BU endemic areas of Northern Queensland, Australia and Mosquito trapping regions.
This figure was created using base layer obtained from https://landsatlook.usgs.gov/.
Trapping of mosquitoes
Mosquitoes were captured using a model 512 “CDC miniature light trap” (John W. Hock Company, Gainesville Florida USA) baited with 1 kg of dry ice as the source of CO2. This trap is the most reliable, efficient and portable device for trapping mosquitoes and sand flies [17]. This trap consists of an electric light and fan just over the collection container and is operated by a 12V battery. A two liter insulated container was used to hold dry ice and a pipe was attached to release CO2 over the trap to attract mosquitoes (Fig 2). Thirty overnight trapping sessions were conducted starting from September 2016 through to February 2018, with at least 4 CDC traps placed within a 1 kilometer radius of each-other. Of the 30 trapping sessions, 14 were conducted at eight different sites within region-1, nine at six different sites within region-2 and seven at five different sites of region-3 (Fig 1). Traps were placed at different sites after obtaining permission to access properties from the owners and selection of sites were based on history of BU cases in humans in nearby households. Geographical Information System (GIS) coordinates of each trap was recorded. On each occasion, traps were set before dusk and checked for mosquitoes after dawn the next morning. After each occasion of trapping, catches were transported to the Mosquito Research Facility, Australian Institute of Tropical Health and Medicine (AITHM), James Cook University, Cairns, Australia where they were counted, sorted and pooled by genus, with each pool containing ≤ 20 mosquitoes of same genus and collected from the same site. The key of Russell was used to identify the genus of mosquitoes trapped [18].
Fig 2. CDC miniature light trap baited with dry ice.
Trapping of march flies
Several attempts were made to trap march flies from endemic areas with an investigator wearing dark clothes to attract them, or with the use of an insect net sprayed with insecticide. These attempts occurred from February 2016 through September 2016. The yield from these attempts were very low. A request was made to residents of region-1 through the local State School to collect march flies. This effort was successful and large numbers of March flies of genus Tabanus were collected by the local community. The addresses of properties from which March flies were collected were recorded. Sampling of March flies was restricted to region-1.
Molecular analyses
The molecular analyses were performed using the protocol available on given link: dx.doi.org/10.17504/protocols.io.vqbe5sn.
Screening of mosquitoes and march flies for MU DNA by PCR
DNA was extracted from each pools of ≤ 20 mosquitoes of the same genus by using the FastPrep Instrument (MP Biomedicals, Solon, OH, USA) as per manufacturer’s instruction with FastDNA Kit (MP Biomedicals). Using the same instrument, DNA from individual March fly was extracted with FastDNA Spin Kit (MP Biomedicals). One sterile water sample in each batch of extractions was used as a negative control to identify the possible contamination during the process of extraction of DNA. Extracted DNA was stored at -20 oC. The extracted DNA samples were screened for the presence of M. ulcerans DNA by using a semi-quantitative real-time PCR adapted from a method for the detection of M. ulcerans DNA from environmental samples [19]. To rule-out the possibility of contamination, three negative controls (double deionized water, MilliQ) and three positive controls (purified M. ulcerans DNA obtained from Victorian Infectious Disease Reference Laboratory) were used during qPCR assay run. All of the extracted DNA samples were initially screened for the M. ulcerans insertion sequence (IS) element IS2404. Samples positive for IS2404 were re-analyzed by a second real-time PCR for the detection of two additional regions in the genome of M. ulcerans: IS2606 and ketoreductase B domain (KR). This screening process has been validated by Fyfe et al. to differentiate M. ulcerans from other mycolactone producing mycobacteria (MPM) [19]. They suggested that the difference in real-time PCR cycle thresholds (Ct) between IS2606 and IS2404 (ΔCt [IS2606 –IS2404]) allows for the differentiation of M. ulcerans strains commonly causing disease in human from other MPM (which are also considered members of the species M. ulcerans) that contain IS2404 but which have fewer copy numbers of IS2606. Samples containing all three independent DNA sequences and with expected Ct values were considered positive for M. ulcerans DNA. The software recommended by Centers of Disease Control and Prevention (Atlanta, GA, USA) was used to calculate the maximum likelihood estimate (MLE) per 1,000 mosquitoes tested (bias corrected MLE) [20].
Accession numbers
The Genebank accession number of nucleotide sequence on M. ulcerans gene IS2404, IS2606 and KR have been allocated as BX649209, BX649209 and BX649209 respectively.
Results
Screening of mosquitoes
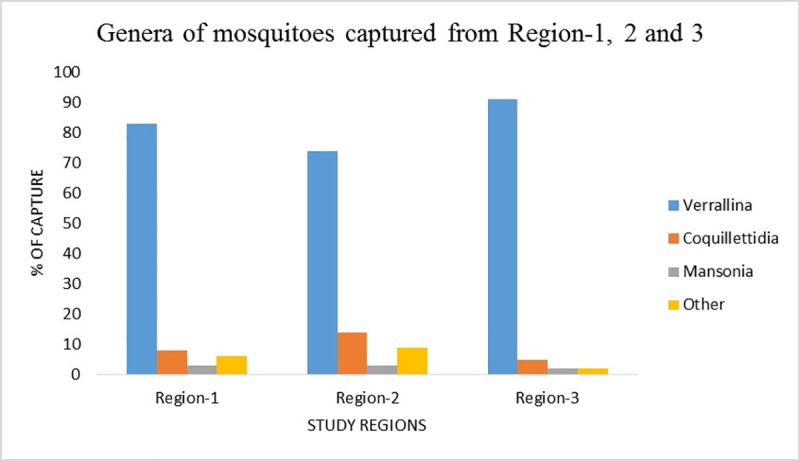
A total of 16,900 mosquitoes were captured over the course of the study from 30 occasions of trapping at three different regions of northern Queensland. Total mosquitoes captured from region-1, region-2 and region-3 were 7880, 5100, and 3920, respectively. The majority of captured mosquitos belonged to the Verrallina genus (specifically Verrallina lineata) 82%, followed by Coquillettidia (9%) and Mansonia (3%). The remaining 6% consisting seven other genera that were classified as “other” for screening. See Fig 3 below.
Fig 3. Genera of mosquitoes captured from three different regions: Region-1 comprising 83% of Verrallina sp., 8% of Coquillettidia sp., 3% of Mansonia sp. and 6% of others; Region-2 comprising 74% of Verrallina sp., 14% of Coquillettidia sp., 3% of Mansonia sp. and 9% of others and Region-3 comprising 91% of Verrallina sp., 5% of Coquillettidia sp., 2% of Mansonia sp. and 2% of others of total catches.
Of a total of 16,900 mosquitoes screened (845 pools), seven pools were positive for IS2404. Three of those seven pools were Verrallina sp. from region-1, two pools were Coquillettidia sp. one each from capture region-1 and 3 and the remaining two pools were Mansonia sp. from region-1. Of the seven pools positive for IS2404, two pools had a high cycle threshold (Ct) values for IS2404 and did not contain sufficient amount of DNA to detect IS2606 and KR. IS2606 was not detected from four pools, despite of having desired Ct values for IS2404. All three targets were detected from remaining pool (Table 1). Thirty pools of mosquitoes which were negative for IS2404 were tested for IS2606 and KR. None of them were positive for these probes signifying the dependent nature of existence of IS 2606 and KR with IS2404. Similar findings were reported during the Victorian outbreak [5]. The bias corrected MLE value for all mosquitoes collected from study site (region-1, region-2 and region-3) was 0.06 M. ulcerans PCR-positive mosquitoes per 1,000 tested (95% confidence interval, 0.00–0.29). Only Region-1 had M. ulcerans PCR-positive mosquitoes and calculated MLE value was 0.13 (95% confidence interval,0.01–0.61)/1,000 mosquitoes tested.
Table 1. Ct values on qPCR analysisScreening of march flies.
| Samples | Species | Location and collection | qPCR analysis | |||
|---|---|---|---|---|---|---|
| IS2404 | IS2606 | IS2404-IS2606 | KR | |||
| Mosquito Pool-1 | Verrallina sp. | Region-1; Feb 2017 | 31.1 | 32.9 | 1.8 | 27.6 |
| Mosquito Pool-2 | Verrallina sp. | Region-1; March 2017 | 31.3 | ND | ND | ND |
| Mosquito Pool-3 | Verrallina sp. | Region-1;Aug 2017 | 36.1 | ND | ND | ND |
| Mosquito Pool-4 | Coquillettidia sp | Region-1; Feb 2017 | 31.2 | ND | ND | ND |
| Mosquito Pool-5 | Coquillettidia sp | Region-3; Sep 2017 | 30.4 | ND | ND | ND |
| Mosquito Pool-6 | Mansonia sp. | Region-1; Feb 2017 | 32.6 | ND | ND | ND |
| Mosquito Pool-7 | Mansonia sp. | Region-1; Aug 2017 | 38.2 | ND | ND | ND |
DNA extracts of 296 March flies were screened for IS2404. None of the samples were positive for this probe. Twenty-four randomly selected IS2404 negative samples were tested for IS2606 and KR and none were positive.
Discussion
Mosquitoes serve as important biological vectors for a variety of pathogens. The movement of pathogens from the gastro-intestinal tract after ingestion to the salivary glands for subsequent transmission is well documented for many diseases. However, this phenomenon has not been demonstrated for M. ulcerans. A study conducted by Wallace and colleagues (2010) provided evidence on the maintenance of M. ulcerans throughout larval development without further passage of the organisms into pupa or adult mosquitoes [21]. They concluded that mosquitoes were an unlikely biological vector of M. ulcerans. Wallace et al (2017) subsequently provided evidence of mechanical transmission of M. ulcerans via anthropogenic skin puncture or mosquito bites [7].
For mechanical transmission, insect vectors such as mosquitoes must acquire the pathogen either from the environment or an infected host. For this to occur efficiently, the organism must be abundantly present in the environment. A survey in Victoria, Australia has confirmed a strong correlation between mosquitoes found to test positive for carrying M. ulcerans DNA and the number of human cases of BU occurring [5, 22]. The group found a significantly higher number of mosquitoes screened positive for M. ulcerans DNA during an intense outbreak of BU in endemic areas, in comparison to areas with a lower incidence of human cases.
The number of human cases of BU has decreased in Northern Queensland, Australia since the largest recorded outbreak in 2011 (> 60 cases). The majority of the cases during the 2011 outbreak were from Wonga and the Wonga beach area, referred as region-1 in the study by Steffen and Freeborn (2018) [23]. Out of 394 pools collected in region 1, six pools were positive for IS2404 DNA in this study. Interestingly, three pools mosquitoes of these positive pools were trapped in the backyard of a property in Wonga Beach area (region-1) where two human cases of BU were confirmed in 2017. All other pools of mosquitoes and march flies collected from that properties negative for M. ulcerans DNA.
As shown in the result, seven pools of mosquitoes were positive for IS2404. However, all three targets with expected Ct value were detected from only one of these seven pools. Samples that were positive for only IS2404 were not considered further.
In north Queensland, the Daintree River arises in mountainous rainforest region around the town of Mossman and flows into the sea at Cape Tribulation. The wet season starts normally from November/December and continues up to April, and the dry season starts from May and continues up to October/November. Outbreaks of human cases of BU in north Queensland have been linked with heavy rainfall and flooding. This survey was conducted from September 2016 through to February 2018, when dryer environmental conditions prevailed. Out of seven M. ulcerans DNA positive pools of mosquitoes, five were collected in wet season and two were collected in dry season. A majority of cases of Daintree ulcer are reported after rainy season ends [13]. The estimated mean incubation period of Daintree ulcer is 4.5 months [24]. Thus, it is more likely that the transmission occurs in the wet season which justifies the detection of M. ulcerans DNA from the pools of mosquitoes that were captured in wet season in this study.
In a separate study conducted in North Queensland, Australia, one sample of a single mosquito and one pool of two mosquitoes was found positive for IS2404.[15]. However, it must be noted that this study was conducted soon after 2011 which raises the possibility that sampling should occur as close as possible in time to when transmission is thought to be occurring.
M. ulcerans is an environmental pathogen and detection of M. ulcerans DNA positive mosquitoes may only be an indicator for the presence of the organism in the environment. A significant decrease in human cases of BU in Northern Queensland in recent years could be due to a lower load of bacteria in the environment. This may explain the low detection of M. ulcerans DNA positive mosquitoes and March fly populations in the study sites. However, the detection of M. ulcerans DNA even in a single pool of mosquitoes from the endemic areas of Northern Queensland is significant, as it corroborates findings in Victoria where five different species of mosquitoes captured from BU-endemic regions during human outbreaks were positive for M. ulcerans.
Our detection of M. ulcerans DNA in mosquitoes in Northern Queensland does support the earlier report from Victoria in Australia [5]. The Victorian study provides evidence for high detection rates of M. ulcerans positive mosquitoes if captured during peak times of outbreaks. Our study found that it is less likely to find M. ulcerans positive mosquitoes if they are trapped from areas where human incidence of BU is currently low. We hypothesize that mosquitoes and perhaps other biting insects, such as March flies may have a significant role in the ecology and transmission of M. ulcerans in endemic areas during outbreaks and that the level of detection of M. ulcerans positive mosquitoes in the environment could be an indicator for disease outbreaks.
Conclusions
Our study confirms the presence of M. ulcerans DNA in the mosquitoes samples captured from the BU-endemic regions of North Queensland, Australia. Lower detection of M. ulcerans positive mosquitoes in BU-endemic areas in North Queensland may partially explain low endemicity of the disease.
Acknowledgments
We thank laboratory staff of Mosquito Research Facility, AITHM for their technical advice trapping of mosquitoes and designing blood feeding experiments and Janet Fyfe from Mycobacterium Reference Laboratory at VIDRL for her technical advice in analyzing samples. We are grateful to Wonga Beach State School for their assistance in collection of March flies. We thank Hendrik Weimar and local community for their continuous support and assistance in arranging access to the sites for setting traps.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
AS and WJHM received FAr north Queensland Hospital Foundation Grant (https://www.fnqhf.org.au/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.George KM, Chatterjee D, Gunawardana G, Welty D, Hayman J, Lee R, et al. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science. 1999;283(5403):854–7. . [DOI] [PubMed] [Google Scholar]
- 2.Williamson HR, Benbow ME, Nguyen KD, Beachboard DC, Kimbirauskas RK, McIntosh MD, et al. Distribution of Mycobacterium ulcerans in buruli ulcer endemic and non-endemic aquatic sites in Ghana. PLoS Negl Trop Dis. 2008;2(3):e205 Epub 2008/03/28. 10.1371/journal.pntd.0000205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fyfe JA, Lavender CJ, Handasyde KA, Legione AR, O'Brien CR, Stinear TP, et al. A major role for mammals in the ecology of Mycobacterium ulcerans. PLoS neglected tropical diseases. 2010;4(8):e791 Epub 2010/08/14. 10.1371/journal.pntd.0000791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandelannoote K, Durnez L, Amissah D, Gryseels S, Dodoo A, Yeboah S, et al. Application of real-time PCR in Ghana, a Buruli ulcer-endemic country, confirms the presence of Mycobacterium ulcerans in the environment. FEMS microbiology letters. 2010;304(2):191–4. Epub 2010/02/12. 10.1111/j.1574-6968.2010.01902.x . [DOI] [PubMed] [Google Scholar]
- 5.Johnson PD, Azuolas J, Lavender CJ, Wishart E, Stinear TP, Hayman JA, et al. Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerging infectious diseases. 2007;13(11):1653–60. Epub 2008/01/26. 10.3201/eid1311.061369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsollier L, Robert R, Aubry J, Saint Andre JP, Kouakou H, Legras P, et al. Aquatic insects as a vector for Mycobacterium ulcerans. Applied and environmental microbiology. 2002;68(9):4623–8. Epub 2002/08/30. 10.1128/AEM.68.9.4623-4628.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace JR, Mangas KM, Porter JL, Marcsisin R, Pidot SJ, Howden B, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis. 2017;11(4):e0005553 Epub 2017/04/15. 10.1371/journal.pntd.0005553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marston BJ, Diallo MO, Horsburgh CR Jr., Diomande I, Saki MZ, Kanga JM, et al. Emergence of Buruli ulcer disease in the Daloa region of Cote d'Ivoire. The American journal of tropical medicine and hygiene. 1995;52(3):219–24. Epub 1995/03/01. . [DOI] [PubMed] [Google Scholar]
- 9.Aiga H, Amano T, Cairncross S, Adomako J, Nanas OK, Coleman S. Assessing water-related risk factors for Buruli ulcer: a case-control study in Ghana. The American journal of tropical medicine and hygiene. 2004;71(4):387–92. Epub 2004/11/02. . [PubMed] [Google Scholar]
- 10.Francis G, Whitby M, Woods M. Mycobacterium ulcerans infection: a rediscovered focus in the Capricorn Coast region of central Queensland. The Medical journal of Australia. 2006;185(3):179–80. Epub 2006/08/09. . [DOI] [PubMed] [Google Scholar]
- 11.Steffen CM, Smith M, McBride WJ. Mycobacterium ulcerans infection in North Queensland: the 'Daintree ulcer'. ANZ journal of surgery. 2010;80(10):732–6. Epub 2010/11/03. 10.1111/j.1445-2197.2010.05338.x . [DOI] [PubMed] [Google Scholar]
- 12.Radford AJ. Mycobacterium ulcerans in Australia. Australian and New Zealand journal of medicine. 1975;5(2):162–9. Epub 1975/04/01. . [DOI] [PubMed] [Google Scholar]
- 13.Queensland Health. Mycobacterium ulcerans in Queensland, 2002–2016. 2017.
- 14.Quek TY, Athan E, Henry MJ, Pasco JA, Redden-Hoare J, Hughes A, et al. Risk factors for Mycobacterium ulcerans infection, southeastern Australia. Emerging infectious diseases. 2007;13(11):1661–6. Epub 2008/01/26. 10.3201/eid1311.061206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roltgen K, Pluschke G, Johnson PDR, Fyfe J. Mycobacterium ulcerans DNA in Bandicoot Excreta in Buruli Ulcer-Endemic Area, Northern Queensland, Australia. Emerging infectious diseases. 2017;23(12):2042–5. Epub 2017/11/18. 10.3201/eid2312.170780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steffen CM, Freeborn H. Mycobacterium ulcerans in the Daintree 2009–2015 and the mini-epidemic of 2011. ANZ J Surg. 2018;88(4):E289–E93. 10.1111/ans.13817 [DOI] [PubMed] [Google Scholar]
- 17.Sriwichai P, Karl S, Samung Y, Sumruayphol S, Kiattibutr K, Payakkapol A, et al. Evaluation of CDC light traps for mosquito surveillance in a malaria endemic area on the Thai-Myanmar border. Parasites & vectors. 2015;8:636 Epub 2015/12/17. 10.1186/s13071-015-1225-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell RC. A colour photo atlas of mosquitoes of southeastern Australia. 1996 [cited 2015 September]. Available from: http://medent.usyd.edu.au/mosqkey/mosquito_key.htm#8a.
- 19.Fyfe JA, Lavender CJ, Johnson PD, Globan M, Sievers A, Azuolas J, et al. Development and application of two multiplex real-time PCR assays for the detection of Mycobacterium ulcerans in clinical and environmental samples. Applied and environmental microbiology. 2007;73(15):4733–40. Epub 2007/05/29. 10.1128/AEM.02971-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biggerstaff B. Software for mosquito surveillance. Atlanta: Centers for Disease Control and Prevention; [cited 2018 October 14]. Available from: https://www.cdc.gov/westnile/resourcepages/mosqSurvSoft.html.
- 21.Wallace JR, Gordon MC, Hartsell L, Mosi L, Benbow ME, Merritt RW, et al. Interaction of Mycobacterium ulcerans with mosquito species: implications for transmission and trophic relationships. Appl Environ Microbiol. 2010;76(18):6215–22. Epub 2010/08/03. 10.1128/AEM.00340-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavender CJ, Fyfe JAM, Azuolas J, Brown K, Evans RN, Ray LR, et al. Risk of Buruli ulcer and detection of Mycobacterium ulcerans in mosquitoes in southeastern Australia. PLoS neglected tropical diseases. 2011;5(9):e1305–e. 10.1371/journal.pntd.0001305 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steffen CM, Freeborn H. Mycobacterium ulcerans in the Daintree 2009–2015 and the mini‐epidemic of 2011. ANZ journal of surgery. 2018;88(4):E289–E93. 10.1111/ans.13817 [DOI] [PubMed] [Google Scholar]
- 24.Trubiano JA, Lavender CJ, Fyfe JA, Bittmann S, Johnson PD. The incubation period of Buruli ulcer (Mycobacterium ulcerans infection). PLoS neglected tropical diseases. 2013;7(10):e2463 Epub 2013/10/08. 10.1371/journal.pntd.0002463 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.