Abstract
Background
Psoriasis is a chronic skin disease that affects approximately two per cent of the general population. Plaque psoriasis is the most common form: it usually appears as raised, red patches of inflamed skin, covered with silvery white scales. The patches often occur in a symmetrical pattern. Guttate psoriasis is a particular form of psoriasis with widespread, small erythematosquamous lesions. Streptococcal infection is suspected to be a triggering factor for the onset of guttate psoriasis, and flare‐up of chronic plaque psoriasis. The previous Cochrane Review on this topic was published in 2000; it required an update because antistreptococcal treatment continues to be used to treat psoriasis, especially for the acute form of guttate psoriasis.
Objectives
To assess the effects of antistreptococcal interventions for guttate and chronic plaque psoriasis.
Search methods
We searched Cochrane Skin Specialised Register, Cochrane Register of Studies Online, CENTRAL, MEDLINE, Embase, LILACS, and five trials registers (January 2019). We checked the reference lists of included and excluded studies and searched conference proceedings from the American Academy of Dermatology, Society for Investigative Dermatology, and European Academy of Dermatology and Venereology.
Selection criteria
We considered randomised controlled trials (RCTs) assessing antistreptococcal interventions (tonsillectomy or systemic antibiotic treatment) in people with clinically diagnosed acute guttate and chronic plaque psoriasis compared with placebo, no intervention, or each other.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Primary outcome measures were: 1) time‐to‐resolution; achieving clear or almost clear skin (Physician Global Assessment (PGA) 0 or 1 or Psoriasis Area and Severity Index (PASI) 90 or 100); 2) proportion of participants with adverse effects and severe adverse effects. Secondary outcomes were: 1) proportion of participants achieving clear or almost clear skin; 2) proportion of participants achieving PASI 75 or PGA 1 to 2; 3) risk of having at least one relapse at long‐term follow‐up. Short‐term assessment was defined as within eight weeks of the start of treatment; long‐term was at least one year after the start of treatment.
Main results
We included five trials (162 randomised participants); three were conducted in a hospital dermatology department. One study declared funding by a pharmaceutical company. Participants' ages ranged from 12 to 77 years; only two participants were younger than 15 years. Mean PASI score at baseline varied from 5.7 (i.e. mild) to 23 (i.e. severe) in four studies. Twenty‐three of 162 participants had streptococcus‐positive throat swab culture. We did not perform a meta‐analysis due to heterogeneity of participants' characteristics and interventions.
None of the trials measured our efficacy primary outcome, time‐to‐resolution, or the secondary outcome, risk of having at least one relapse at long‐term follow‐up.
We rated the quality of the results as very low‐quality evidence, due to high risk of bias (absence of blinding of participants and caregivers, and high risk of outcome reporting bias) and imprecision (single study data with a low number of events). Hence, we are very uncertain about the results presented.
Guttate psoriasis
One three‐armed trial (N = 43) assessed penicillin (50,000 international units (IU)/kg/day in three doses) versus erythromycin (250 mg four times per day) versus no treatment (treatment for 14 days, with six‐week follow‐up from start of treatment). Adverse events and the proportion of participants achieving clear or almost clear skin were not measured.
One trial (N = 20) assessed penicillin (1.6 MU (million units) intramuscularly once a day) versus no treatment (six weeks of treatment, with eight‐week follow‐up from start of treatment). At six‐week (short‐term) follow‐up, no adverse events were observed in either group, and there was no statistically significant difference between the two groups in the proportion of participants with clear or almost clear skin (risk ratio (RR) 2.00, 95% confidence interval (CI) 0.68 to 5.85).
One trial (N = 20) assessed rifampicin (300 mg twice daily) versus placebo (14‐day treatment duration; six‐week follow‐up from start of treatment); none of the review outcomes were measured.
These trials did not measure the proportion of participants achieving PASI 75 or PGA 1 to 2.
Chronic plaque psoriasis
One trial (N = 50) assessed long‐term azithromycin treatment (500 mg daily dose) versus vitamin C. Adverse events were reported in the azithromycin group (10 out of 30 had nausea and mild abdominal upset), but not in the vitamin C group. The proportion of participants who achieved clear or almost clear skin was not measured. In the azithromycin group, 18/30 versus 0/20 participants in the vitamin C group reached PASI 75 at the end of 48 weeks of treatment (RR 25.06, 95% CI 1.60 to 393.59).
One trial (N = 29) assessed tonsillectomy versus no treatment, with 24‐month follow‐up after surgery. One participant in the tonsillectomy group had minor bleeding. At eight‐week follow‐up, 1/15 in the tonsillectomy group, and 0/14 in the no treatment group achieved PASI 90; and 3/15 participants in the tonsillectomy group, and 0/14 in the no treatment group achieved PASI 75 (RR 6.56, 95% CI 0.37 to 116.7).
Authors' conclusions
We found only five trials (N = 162), which assessed the effects of five comparisons (systemic antibiotic treatment (penicillin, azithromycin) or tonsillectomy). Two comparisons (erythromycin compared to no treatment, and rifampicin compared to placebo) did not measure any of the outcomes of interest. There was very low‐quality evidence for the outcomes that were measured, Therefore, we are uncertain of both the efficacy and safety of antistreptococcal interventions for guttate and chronic plaque psoriasis.
The included trials were at unclear or high risk of bias and involved only a small number of unrepresentative participants, with limited measurement of our outcomes of interest. The studies did not allow investigation into the influence of Streptococcal infection, and a key intervention (amoxicillin) was not assessed.
Further trials assessing the efficacy and tolerance of penicillin V or amoxicillin are needed in children and young adults with guttate psoriasis.
Plain language summary
Does treating Streptococcal throat infection help improve psoriasis?
Review question
We wanted to find out how well treatments for infections caused by the Streptococcus bacteria worked, and how safe they were, when compared with no treatment, placebo (an identical but inactive treatment), or each other, in people with acute guttate or chronic plaque psoriasis.
Background
Chronic plaque psoriasis is a long‐term condition that causes patches of red, flaky skin, covered with scales (called plaques); it is the most frequent form of psoriasis, and is more common in adults.
Guttate psoriasis is characterised by smaller lesions, and is more common in children and young people. Some studies have suggested that guttate psoriasis occurs in less than 30% of people with psoriasis.
The cause of psoriasis is unknown, but Streptococcal infection may trigger guttate psoriasis or flare‐ups of chronic plaque psoriasis.
Tonsillectomy may prevent or reduce the severity of throat infections, and limit the Streptococcus reservoir. Antibiotics work by destroying the bacteria that appear to trigger psoriasis.
Study characteristics
The evidence is current to January 2019.
We included five studies (162 participants); three were conducted in hospital dermatology departments. Participants were 12 to 77 years old (100 males; 62 females). One study was funded by a pharmaceutical company. The severity of the condition ranged from mild to severe. Streptococcus bacteria were found in the throats of 14% of people.
We classed outcomes measured within eight weeks of the start of treatment as short‐term, and those measured at least one year after the start of treatment as long‐term. The antibiotic trials in guttate psoriasis patients were all short‐term in duration; the antibiotic trial in chronic plaque psoriasis was 48 weeks long.
Three studies included participants with guttate psoriasis, and assessed the short‐term effects of antibiotics: penicillin (20 participants), or erythromycin compared to no treatment (43 participants), and rifampicin compared to placebo (20 participants).
Two studies included participants with chronic plaque psoriasis. One study assessed azithromycin (antibiotic) versus vitamin C at 48 weeks (50 participants); one assessed tonsillectomy versus no intervention at eight weeks and 24 months (29 participants).
Key results
These results are backed by very low‐quality evidence, so we are not certain of their accuracy. Each result is based on only one study.
No studies measured our main outcome of interest, the time taken for the skin to be clear or almost clear of lesions, or the risk of relapsing at least once during long‐term follow‐up.
No side effects were seen when penicillin was compared with no treatment in people with guttate psoriasis. Side effects were not measured for the comparisons of rifampicin versus placebo, or erythromycin versus no treatment.
In participants with chronic plaque psoriasis, one trial assessed azithromycin versus vitamin C, and 10 participants in the azithromycin group complained of nausea or mild stomach upset. A trial of tonsillectomy versus no treatment reported one case of minor bleeding in the tonsillectomy group.
Two studies in participants with chronic plaque psoriasis measured the number of participants achieving a 75% reduction on the Psoriasis Area and Severity Index (PASI 75). In one, 18/30 participants in the azithromycin group reached PASI 75 versus none in the vitamin C group. In the other, 3/15 in the tonsillectomy group reached PASI 75 versus none in the no treatment group. The guttate psoriasis trials did not assess this outcome.
We are uncertain whether the number of participants with guttate psoriasis achieving clear or almost clear skin differs between those given penicillin and those receiving no treatment. Only one participant with chronic plaque psoriasis achieved almost clear skin in the tonsillectomy group compared to none in the no treatment group. The other three trials did not measure this outcome.
Quality of the evidence
Many of our main outcomes were not assessed. Those that were assessed were based on very low‐quality evidence, meaning we are not sure of their accuracy. The studies were very small, and had a high risk of bias because participants and trial assessors were aware of treatment allocation. More studies are needed to see if antibiotic treatment of Streptococcal infection shortens the duration of acute guttate psoriasis, stopping it from turning into a long‐term condition (chronic plaque psoriasis).
Summary of findings
Background
For an explanation of terms we used in this review, please see Table 6.
1. Glossary of terms used.
| Term | Explanation |
| Acute pharyngitis | Inflammation of the mucous membrane of the pharynx; sore throat |
| Adenotonsillectomy | Surgical removing of tonsils and adenoids |
| Anaphylactic shock | Allergic reaction characterised by swelling, collapse, and respiratory distress |
| Antistreptolysin O titre | Blood test to measure antibodies against streptolysin O, a substance produced by group A Streptococcus bacteria |
| Beta‐haemolytic streptococcus | Pathogenic Streptococci, anaerobic bacteria, gram positive, often arranged in a chain |
| Chronic tonsillitis | Constant or recurrent infection of tonsils |
| Cytokines | Broad category of small proteins that are important in cell signalling. They are released by cells, and affect the behaviour of other cells and sometimes the releasing cell itself |
| Cutaneous lymphocyte–associated antigen (CLA) | The CLA is a fucose‐containing carbohydrate that is attached to P‐selectin glycoprotein ligand‐1 on T cells. CLA is expressed on the surface of most T cells recovered from skin, and on about 5% to 10% of circulating CD8+ T cells |
| Dendritic cells | A subtype of white blood cells |
| Epidermal | Related to the outer layer of the skin |
| Epidermal hyperplasia | Abnormal increase in the number of normal cells in the epidermis of the skin, which increases its volume |
| Erythematous | Redness of the skin |
| Erythematosquamous | Redness and covered with scales |
| Hyperkeratosis | Thickening of the cornea |
| Histological | Related to the examination of a piece of tissue, with a microscope |
| Homozygous | Having identical pairs of genes for any given pair of hereditary characteristics |
| Heterozygous | Having dissimilar pairs of genes for any given pair of hereditary characteristics |
| Interleukin 17 (IL‐17) | A pro‐inflammatory cytokine |
| Immune‐mediated disease | Group of conditions or diseases that lack a definitive etiology, but that are characterised by common inflammatory pathways leading to inflammation, and which may result from, or be triggered by, a dysregulation of the normal immune response |
| Keratinocytes | The most important cell type in the epidermis, the most superficial layer of the skin |
| Macrolide antibiotics | A specific family of antibiotics |
| Neutrophils | A subtype of white blood cells |
| Psoriasis Area Severity Index (PASI) | Index used to express the severity of psoriasis. It combines the severity (erythema or redness, induration, and desquamation) and percentage of the affected area |
| Physician Global Assessment (PGA) | Average assessment of all psoriatic lesions based on erythema (redness), scale, and induration |
| Pathogens | Micro‐organisms responsible for infection (such as virus or bacteria) |
| Pathophysiology, physiopathological | Convergence of pathology with physiology. Pathophysiology seeks to explain the physiological processes or mechanisms whereby a condition develops and progresses |
| Ribonucleic acid (RNA) | Polymeric molecule. It is involved in a range of biological roles in coding, decoding, regulation, and expression of genes |
| Serology/serologic | Refers to the diagnostic identification of antibodies in the serum |
| Stevens‐Johnson syndrome | Allergic reaction to a drug characterised by mucous membrane and skin epidermal necrolysis and involving less than 10% of the entire body surface |
| Toxic epidermal necrolysis | Allergic reaction to a drug characterised by mucous membrane and skin epidermal necrolysis and involving more than 30% of the entire body surface |
| T lymphocytes, T cells | A subtype of a white blood cell |
Description of the condition
Psoriasis is a common, chronic inflammatory disorder that primarily affects the skin, and sometimes the joints. Guttate psoriasis is a particular form of psoriasis, characterised by a distinct clinical presentation with widespread small (0.5 to 1.5 cm) erythematosquamous lesions, located mainly on the trunk. It has an acute onset and a possibility of spontaneous resolution. Plaque psoriasis is the most common form of psoriasis. It typically appears as raised and well‐demarcated red areas of inflamed skin covered with silvery white scales; it often shows a symmetrical distribution on the body. Antibiotics or tonsillectomy have been proposed as treatments for guttate psoriasis or flares of chronic plaque psoriasis; these treatments were introduced on the basis of the suspected relationship between infections with beta‐haemolytic Streptococci (which can cause a throat infection, and more rarely perianal Streptococcal dermatitis) and acute manifestations of psoriasis (Telfer 1992).
Epidemiology
Psoriasis affects approximately two per cent of the general population, with equal distribution between the sexes (Parisi 2013). There are several forms of psoriasis, and different clinical presentations can be observed in the same person, either simultaneously or over time. Among these, chronic plaque psoriasis (psoriasis vulgaris) accounts for 90% of cases (Griffiths 2007). The prevalence of guttate psoriasis has not been clearly reported, but several studies cite a prevalence of less than 30% among people with psoriasis (Kundakci 2002; Kwon 2012; Rigopoulos 2010; Valenzuela 2011).
Clinical presentation and natural history
Chronic plaque psoriasis presents as erythematous plaques, which have a precise outline. They have a scaly surface. The plaques are usually located on the elbows, the knees, and the scalp, but the nails, hands, feet, and trunk are also frequently affected sites. Plaque size can vary from a minimal area to coverage of the entire body (Wolff 2009). Plaque psoriasis is a chronic disease with severity fluctuation over time. It is more common in adults than in children, with an increasing incidence with age up to around 40 years, and a second peak at around 50 to 59 years (Parisi 2013).
Guttate psoriasis is an eruptive form of psoriasis, with small lesions and a greater tendency toward spontaneous resolution. It typically appears in young adults and children with no previous history of psoriasis, where it is referred to as 'acute guttate psoriasis' (Mercy 2013). Sometimes, it occurs in people who already have chronic plaque psoriasis, where it is called a 'guttate flare of chronic psoriasis' (Chalmers 2001). Although guttate psoriasis is usually considered a form of psoriasis with a better prognosis, few studies have evaluated its long‐term clinical course.
Based on limited data, guttate psoriasis is considered a form that resolves spontaneously after a few months (Ko 2010), from which one‐ to two‐thirds of participants will subsequently develop a form of chronic plaque psoriasis (Ko 2010; Martin 1996; Williams 1976). There is little information on the rate at which people develop a chronic form of psoriasis after experiencing a first episode of acute guttate psoriasis. Rates of chronic plaque psoriasis after a first episode of guttate psoriasis vary in different studies: Martin 1996 found 33% of people developed it within 10 years after a first episode of guttate psoriasis; Ko 2010 found 36% developed chronic plaque psoriasis within six years; and Williams 1976 found 68% developed chronic plaque psoriasis within one year. The risk of recurrence of guttate psoriasis after a first resolved episode is also unknown. Mean time to disease clearance was 3.9 ± 2.4 months (Ko 2010).
The diagnosis of cutaneous psoriasis is clinical. A skin biopsy can be used to confirm the diagnosis in difficult cases.
Pathophysiology
The pathogenesis of psoriasis is still not fully known. An inflammatory immune response involving T lymphocytes, dendritic cells, neutrophils, and keratinocytes leads to a rapid turnover of skin renewal and histological inflammatory infiltrate, characteristic of psoriasis (Newman 2008).
Psoriasis occurs in people with a genetic predisposition (Elder 2010). Factors that can exacerbate it include skin trauma, smoking, alcohol, emotional stress, or drugs (Berth‐Jones 2005). Pathogens, notably beta‐haemolytic Streptococci, are considered to be triggering factors (Picciani 2013). A nested case‐control study on a database from the United Kingdom examined exposure to systemic antibacterial prescriptions and infections within two years prior to a diagnosis of psoriasis in children with newly diagnosed psoriasis (N = 845) compared with age‐ and sex‐matched controls (N = 8450). Infections of skin (adjusted odds ratio (aOR) 1.5, 95% confidence interval (CI) 1.2 to 1.7) and other sites (aOR 1.3, 95% CI 1.1 to 1.6) were associated with newly diagnosed psoriasis in children (Horton 2016). The link between acute guttate psoriasis and flares of chronic plaque psoriasis and Streptococcal infection is suspected but not proven.
Infections with beta‐haemolytic Streptococci, which can cause a throat infection, and more rarely, perianal Streptococcal dermatitis (Ledoux 2009), may lead to acute guttate psoriasis, and are also suspected to cause flares of chronic plaque psoriasis (Gudjonsson 2003). (Where we refer to Streptococcal or Streptococcus in this section, we specifically mean beta‐haemolytic Streptococci.) In one study, streptococci were isolated from the throats of 97% of people with guttate psoriasis (Tervaert 1970), while in two earlier studies, serologic evidence of recent Streptococcal infection was found in 56% and 85% of people (Norrlind 1955; Whyte 1964). In a prospective study, 58% of people with acute guttate psoriasis versus 26% of people with guttate exacerbations of chronic psoriasis had serologic evidence for recent Streptococcal infection (Telfer 1992). A relationship between Streptococci and chronic plaque psoriasis was also proposed in a prospective study, in which people with psoriasis reported a sore throat 10 times more often than controls in the same household, and Streptococcal throat infections could cause exacerbation of chronic plaque psoriasis (Gudjonsson 2003). However, as serological evidence is no longer considered a relevant test, the strength of the conclusions of these studies became weaker (Shulman 2012). In addition to these studies that highlighted an association without established causality, in vitro studies put forward the superantigen theory to explain the interaction between psoriasis and infection (Abe 1991; Kotzin 1993; Leung 1995; Valdimarsson 1997). Some bacteria are superantigens, which means that they are able to trigger multiple immune reactions, leading to the stimulation of different T cells and activation of cytokines (Kotzin 1993). A recent study showed the direct involvement of Streptococcal infection in pathological mechanisms of psoriasis, such as interleukin 17 (IL‐17) production and epidermal cell activation: Streptococcal throat extracts (isolated from the throats of participants with psoriatic), added to the cultures of epidermal cells, which were obtained by skin biopsy of psoriatic lesions, led to the activation of circulating psoriatic cutaneous lymphocyte–associated antigen memory T cells (Ferran 2013).
Description of the intervention
Antistreptococcal interventions include tonsillectomy or antibiotic treatment. These antistreptococcal treatments are not recommended in current guidelines; however, these guidelines addressed only chronic plaque psoriasis in adulthood, except the NICE guideline, in which phototherapy is recommended for acute guttate psoriasis (NICE 2017).
Tonsillectomy
Tonsillectomy is a surgical procedure during which the tonsils are removed; it is carried out under general anaesthesia. Recurrent acute pharyngitis and chronic tonsillitis are the most common reasons for tonsillectomy in adults. A Cochrane Review showed a modest benefit of tonsillectomy or adenotonsillectomy in the treatment of recurrent acute tonsillitis in children (Burton 2009). Most published studies refer to a paediatric population, and the small amount of information available about adult sore throat and the effect of tonsillectomy suggests that surgery is beneficial, but the evidence for this is not robust (Laing 1991; Paradise 1984; SIGN 2010). Tonsillectomy is associated with morbidity that includes possible hospitalisation, the risk of anaesthesia, and prolonged throat pain; there are also financial costs to consider (SIGN 2010). Bleeding during or after surgery is not uncommon, and there may be other complications (Baugh 2011).
Antibiotics
Antibiotics are recommended for acute sore throat, or for those with chronic recurrent sore throat. Treatment for people with Group A Streptococcal (GAS) pharyngitis, recommended by the Infectious Diseases Society of America (IDSA), is 10 days of: phenoxymethylpenicillin (penicillin V) 250 mg four times daily or 500 mg twice daily; or amoxicillin 50 mg/kg once daily (maximum of 1000 mg) or 25 mg/kg (maximum of 500 mg) twice daily (Shulman 2012; Tanz 2007).
For the treatment of GAS, few antibiotic regimens have been evaluated prospectively in randomised controlled trials, and in the literature, there are a few studies about the eradication of Streptococcal transmission (Shulman 2012). The most common adverse effects of antibiotics are gastrointestinal disorders. However, rare serious adverse effects, which are sometimes life‐threatening, such as a bacterial overgrowth (pseudomembranous colitis by overgrowth of Clostridium difficile bacteria) or allergic reactions (anaphylactic shock, Stevens‐Johnson syndrome, or toxic epidermal necrolysis), are also described. At the population level, the use of antibiotics must be limited because of the emergence of bacterial resistance to antibiotics (Baugh 2011; Burton 2009; Shulman 2012; SIGN 2010).
Treatments recommended for chronic plaque psoriasis are topical therapy (topical corticosteroids or vitamin D analogues, or both), phototherapy, and systemic therapy (non‐biological or biological). In current practice, some physicians prescribe antibiotics for acute guttate psoriasis in a context of pre‐existing or concomitant infection, mainly pharyngitis, as first‐line treatment, or in addition to recommended treatments. However, surveys carried out in different countries, and studies of the prescribing patterns of dermatologists, general practitioners, or paediatricians for children with psoriasis, did not mention the use of antibiotic therapy (Augustin 2013; De Jager 2009; Mahe 2018; Vogel 2012).
How the intervention might work
It is thought that by eradicating Streptococcus, antistreptococcal interventions will stop the superantigen activity of Streptoccocus, which is suspected of triggering the immune reactions responsible for psoriasis, and so may improve or clear an acute flare of guttate psoriasis, and decrease or inhibit flares of chronic plaque psoriasis.
Removing the tonsils, which are a site of Streptococcus infection and a Streptococcus reservoir, may prevent throat infections, reduce the severity of throat infections, or limit the Streptococcus reservoir (Burton 2009).
Why it is important to do this review
Despite the fact that guidelines on psoriasis no longer recommend the use of antistreptococcal interventions (SIGN 2010), antibiotics continue to be perceived as a good treatment option, especially for the acute form of guttate psoriasis. This is because epidemiological and in vitro studies report arguments in favour of a link between psoriasis flare and Streptococcal infection. As the previous Cochrane Review is old, it is important to conduct a new assessment of the available evidence on the efficacy and safety of antistreptococcal interventions (Owen 2000). We chose to do this by drafting a new protocol because we changed the objectives and outcomes of the original review (Dupire 2015).
Objectives
To assess the effects of antistreptococcal interventions for guttate and chronic plaque psoriasis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), including multi‐arm trials.
Types of participants
We included participants with clinically diagnosed acute guttate or chronic plaque psoriasis. In cases where studies only include a subset of relevant participants, we only included the study if the characteristics of participants and results were provided separately, or could be obtained through contact with authors.
Types of interventions
We considered any antistreptococcal antibiotic therapies, or tonsillectomy compared with placebo or no intervention, or comparisons between each other.
Types of outcome measures
Primary outcomes
Time to resolution (time between inclusion and resolution), where resolution was defined as participants achieving clear or almost clear skin (Physician Global Assessment (PGA) 0 or 1; or Psoriasis Area and Severity Index (PASI) 90, which refers to at least 90% reduction, or PASI 100, which refers to 100% reduction in the PASI score)
Proportion of participants with adverse effects, and severe adverse effects
Secondary outcomes
Proportion of participants achieving clear or almost clear skin (PGA 0 or 1 or PASI 90 or 100)
Proportion of participants achieving PASI 75 or PGA 1 to 2
Risk of having at least one relapse at long‐term follow‐up.
Timing of outcomes
By short‐term, we mean within eight weeks of the start of treatment, and by long‐term, we mean at least one year after the start of treatment.
Search methods for identification of studies
We aimed to identify all relevant RCTs, regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
The Cochrane Skin Information Specialist searched the following databases up to 23 January 2019, using strategies based on the draft strategy for MEDLINE in our published protocol (Dupire 2015):
the Cochrane Skin Specialised Register, using the search strategy in Appendix 1;
the Cochrane Infectious Diseases Specialised Register and the Cochrane Sexually Transmitted Infections Specialised Register via the Cochrane Register of Studies Online (CRSO), using the strategy in Appendix 2;
the Cochrane Central Register of Controlled Trials (CENTRAL) 2019, Issue 1, in the Cochrane Library, using the strategy in Appendix 3;
MEDLINE Ovid (from 1946), using the strategy in Appendix 4;
Embase Ovid (from 1974), using the strategy in Appendix 5; and
LILACS (Latin American and Caribbean Health Science Information database, from 1982), using the strategy in Appendix 6.
Trials registers
We searched the following trials registers, up to 24 January 2019, with the search term 'psoriasis':
the ISRCTN registry (www.isrctn.com);
ClinicalTrials.gov (www.clinicaltrials.gov);
the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au);
the World Health Organization International Clinical Trials Registry Platform (ICTRP (apps.who.int/trialsearch/)); and
the EU Clinical Trials Register (www.clinicaltrialsregister.eu).
Searching other resources
References from included studies
We checked the bibliographies of included and excluded studies for further references to relevant trials.
Contacting prominent authors in the field
We attempted to contact authors of included trials, in order to identify additional published or unpublished data.
Handsearching
We searched the following conference proceedings for years not included in the Cochrane Skin Specialised Register:
American Academy of Dermatology (AAD) for 2008 and 2009, and from 2012 to 2016;
Society for Investigative Dermatology (SID) from 2008 to 2016; and
European Academy of Dermatology and Venereology (EADV) from 2008 to 2014.
Adverse effects
We did not perform a separate search for adverse effects of the target intervention. However, we examined data on adverse effects from the included studies we identified.
Data collection and analysis
We included 'Summary of findings' tables in our review for all comparisons, which we created with GRADEpro GDT software (GRADEpro GDT). In these, we summarised all of our primary and secondary outcomes (see section 12.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)).
Some parts of the methods section of this review uses text that was originally published in another Cochrane protocol (Le Cleach 2011).
Selection of studies
Two review authors (GD and CD) independently examined each title and abstract to exclude obviously irrelevant reports; they then independently examined the full text of potentially relevant articles to determine eligibility. These review authors discussed any disagreements with a third author (LLC) to reach consensus. We contacted study authors for clarification when necessary. We listed excluded studies, and document the primary reason for exclusion.
Data extraction and management
Two review authors (GD and CD) independently extracted the data from published and unpublished reports, using a standardised form. The team piloted this data extraction form on a set of included trials. A third author (LLC) was involved to resolve any disagreements on data extraction between the two review authors. We extracted from each included trial: study design, inclusion and exclusion criteria, baseline characteristics of the total number of participants randomised to each intervention, description of interventions and outcomes. We extracted these data to populate the 'Characteristics of included studies' tables. One review author (GD) checked and entered data into Review Manager 5 computer software (Review Manager 2014). We contacted the authors of the studies to provide missing data when required.
Assessment of risk of bias in included studies
Two review authors (GD and CD) independently used Cochrane's 'Risk of bias' tool to assess the risk of bias of each of our included studies. They discussed disagreements with a third author (LLC) to reach consensus. We determined the risk of bias as 'low', 'high', or 'unclear' for each of the following domains, according to the general principles in section 8.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011):
Selection bias
Was the allocation sequence adequately generated? We considered randomisation adequate if the allocation sequence was generated from a table of random numbers or by computer. We considered it inadequate if sequences could be related to prognosis. We considered it unclear if it was stated that the trial was randomised, but the method was not described.
Was allocation adequately concealed? We deemed allocation concealment adequate if the report stated that it was undertaken by means of sequentially pre‐numbered, sealed, opaque envelopes, or by a centralised system. We considered a double‐blind double‐dummy process at low risk of bias, even if the method of allocation concealment was not described.
Performance and detection bias
Was knowledge of the allocated intervention adequately prevented during the study? We evaluated the risk of bias separately for personnel and participants, outcome assessors, and each outcome. In trials that compared pharmaceutical interventions (antibiotics) with placebo, if the presentation of the interventions was the same, we considered the blinding adequate, even in cases where there was no precise description of the blinding procedure.
Attrition bias
Were incomplete outcome data adequately addressed? We examined if there was an imbalance across intervention groups in numbers or reasons for missing data, the types of measures undertaken to handle missing data, and whether the analysis was carried out on an intention‐to‐treat basis. We assessed the use of strategies to handle missing data.
Reporting bias
Are reports of the study free of suggestion of selective outcome reporting? We evaluated whether each outcome was measured, analysed, and reported. We compared outcomes specified in study protocols (if available e.g. on trial registers), and in the methods sections, with outcomes presented in the results.
Other bias
We did not fulfil the 'other risk of bias' item, as we did not highlight particular circumstances leading to other risks of bias from particular trial designs, contamination between the experimental and control groups, or particular clinical settings.
Measures of treatment effect
For each pair‐wise comparison and each dichotomous outcome, we used risk ratios (RR) with 95% confidence intervals (CI) as a measure of treatment effect.
For time‐to‐event outcomes, we had planned to combine estimates of log hazard ratios and standard errors obtained from results of Cox proportional hazards regression models, using the generic inverse‐variance method; however, no time‐to‐event outcomes were available in the included trials.
Unit of analysis issues
The primary unit of analysis was the participant. We included only the first phase of cross‐over studies because of the risk of carry‐over bias and the unpredictable evolution of psoriasis, which may have an effect in subsequent phases.
Dealing with missing data
We extracted the number of randomised and analysed participants from each included trial. We requested missing data from trial authors or sponsors, by email. For missing data, we used simple imputation methods. We assumed that all missing data were either events or non‐events (Higgins 2011).
Assessment of heterogeneity
We had planned to assess statistical heterogeneity by visual inspection of the forest plots and by calculating I² statistics. We had planned to interpret the I² statistic value according to the following thresholds (section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)):
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity; and
75% to 100% represents considerable heterogeneity
Assessment of reporting biases
To address publication bias, we had planned to draw contour‐enhanced funnel plots for each meta‐analysis if 10 or more studies had contributed data to our outcomes (Egger 1997). We did not draw funnel plots because of the insufficient number of included studies.
Data synthesis
We had planned to undertake meta‐analyses only if we judged participants, interventions, comparisons, and outcomes to be sufficiently similar. In cases of heterogeneity, we had planned to use a random‐effects meta‐analysis to combine studies. In cases of multi‐arm trials, we had planned to combine groups to create a single pair‐wise comparison. We had not planned to combine studies in a meta‐analysis if the value of the I² statistic exceeded 75%, because this represents considerable heterogeneity. It was not possible to combine any data because of the small number of trials, and the absence of multiple trials comparing the same intervention and measuring our planned outcomes.
Subgroup analysis and investigation of heterogeneity
We had planned to investigate the influence of the presence, absence, or unknown status of an active Streptococcal infection at the time of treatment onset, via subgroup analyses. According to the IDSA guidelines, cultures or rapid antigen detection tests (RADT) are recommended rather than antibody titers that only become positive several weeks after the end of infection. We considered the results of these tests for subgroup analyses (Shulman 2012).
We also planned to investigate the following two characteristics:
guttate versus plaque psoriasis
tonsillectomy versus antibiotic therapy.
In the absence of meta‐analyses, we did not carry out any subgroup analyses.
Sensitivity analysis
For studies at higher risk of bias, we had planned to conduct sensitivity analyses. We had planned to perform sensitivity analysis to assess how sensitive the results were to reasonable changes in the assumptions we may have made with regard to missing data. In the absence of meta‐analysis, we did not carry out any sensitivity analyses.
Results
Description of studies
Results of the search
The searches of the electronic databases retrieved 414 records. The searches of other sources (e.g. handsearching conference proceedings, the Federal Drug Administration (FDA) reviews and the World Heatlh Organization (WHO) International Clinical Trials Registry Platform) identified one additional trial. After duplicate records were removed, we had 413 records.
We excluded 389 records based on titles and abstracts. We examined the full text of the remaining 24 citations. We excluded 13 studies reported in 16 papers; one was excluded based on the abstract as no full text could be obtained; see Characteristics of excluded studies (Masood 1997). We did not identify any relevant ongoing studies. We classified one study as awaiting classification; see Characteristics of studies awaiting classification. We included five trials, reported in seven papers; see Characteristics of included studies.
For a further description of our screening process, see the study flow diagram (Figure 1).
1.
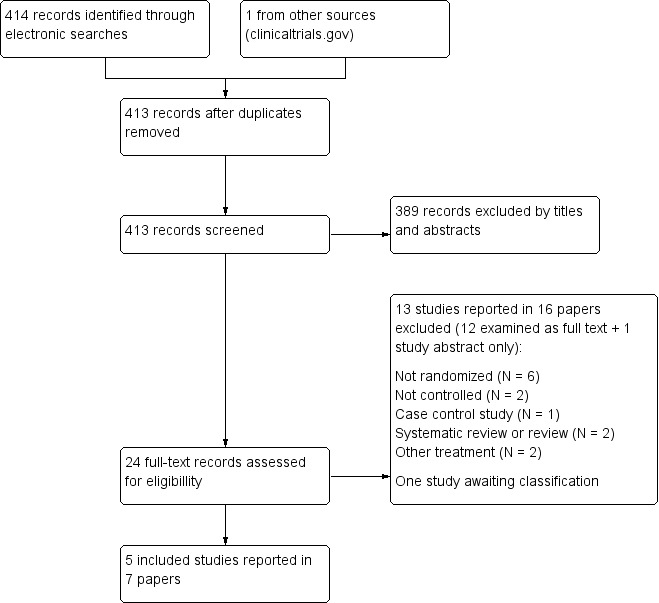
Flow diagram describing the searches and screening of studies
We obtained additional information via email for one trial (Thorleifsdottir 2012).
Included studies
We included five studies, involving 162 randomised participants; see the 'Characteristics of included studies' tables.
Trial design
All of the trials were parallel‐arm trials: four trials assessed two arms and one trial included three arms (Dogan 2008). Three were single‐centre trials (Caca‐Biljanovska 2002; Dogan 2008; Thorleifsdottir 2012), one was a multicentric trial (two centres (Saxena 2010)), and the information was not reported in one (Vincent 1992). Trials were carried out in Turkey (Dogan 2008), the Republic of Macedonia (Caca‐Biljanovska 2002), Iceland (Thorleifsdottir 2012), India (Saxena 2010), and for one, the information was not provided (Vincent 1992). Total duration of follow‐up was six weeks (Dogan 2008; Vincent 1992) to eight weeks (Caca‐Biljanovska 2002), for the three trials assessing guttate psoriasis; and 48 weeks (Saxena 2010) to two years (Thorleifsdottir 2012), for two trials assessing chronic plaque psoriasis.
One trial declared pharmaceuticals funding (Vincent 1992), one institutional funding (Thorleifsdottir 2012), one no funding (Saxena 2010); there was no information about funding for the other two trials. The setting described in three trials was a dermatology hospital department (Caca‐Biljanovska 2002; Dogan 2008; Saxena 2010); the setting of the other two trials was not reported.
Participants
The mean number of participants randomised per trial was 32 (minimum 20 to maximum 50). A total of 92 participants were included in intervention arms, and 70 in placebo or no treatment arms.
Participants' age varied from 12 to 77 years.
In total, 62 participants were female and 100 were male; one trial included only men (Dogan 2008).
In three trials, all participants had guttate psoriasis (Caca‐Biljanovska 2002; Dogan 2008; Vincent 1992); in two trials, all participants had chronic plaque psoriasis (Saxena 2010; Thorleifsdottir 2012).
Mean Psoriasis Area and Severity Index (PASI) score at baseline was 5.7, 10.6, 11.9, and 23 in Caca‐Biljanovska 2002; Dogan 2008; Saxena 2010; Thorleifsdottir 2012, respectively, corresponding to mild, moderate, and severe psoriasis. The PASI score was not provided in Vincent 1992.
Four trials took throat swabs (Caca‐Biljanovska 2002; Dogan 2008; Saxena 2010; Vincent 1992). All cultures of nose and throat swabs were negative in Caca‐Biljanovska 2002; 8/43 (17%) were positive for β‐haemolytic streptococcus in Dogan 2008, 1/20 participants were positive for β‐haemolytic Streptococcal in Vincent 1992, and 14/29 were positive for streptococcus in Thorleifsdottir 2012 (additional information provided by the author by email). In one trial, the authors reported the results of the throat swab culture as equivocal (Saxena 2010).
Interventions
Systemic antibiotic therapy
Four trials assessed systemic antibiotic therapy; penicillin (1.6 MU (million units) given intermuscularly (IM) once a day for six weeks was compared to no treatment (Caca‐Biljanovska 2002), oral azithromycin (500 mg daily dose, given orally for four days with a gap of 10 days, repeated 24 times) was compared to vitamin C (Saxena 2010), and oral rifampicin (300 mg twice daily given for 14 days) was compared to placebo (Vincent 1992). Oral erythromycin (250 mg four times a day) was compared to oral phenoxymethylpenicillin (50,000 IU/kg/d in three doses), and to no treatment for 14 days (Dogan 2008).
All of the participants in two trials received a co‐intervention, namely topical betamethasone dipropionate 0.05% cream and phototherapy in Caca‐Biljanovska 2002, and penicillin V or erythromycin 250 mg four times a day for 14 days in Vincent 1992.
The total duration of each trial was as follows: 8 weeks (Caca‐Biljanovska 2002), 48 weeks (Saxena 2010), and 6 weeks (Dogan 2008; Vincent 1992).
Tonsillectomy
Tonsillectomy was compared to no intervention in one two‐year long trial (Thorleifsdottir 2012). Topical or systemic psoriasis treatment was allowed, if needed by the participant.
Outcomes
Our primary outcome, time‐to‐achieve clear or almost clear status, was not reported in any included study. Adverse events were not reported in two trials (Dogan 2008; Vincent 1992), reported in only one group in another (Saxena 2010), and obtained through email contact with the author for one trial (Thorleifsdottir 2012); see Table 7 for details on study author contact.
2. Authors contact.
| Author | Date contacted | Reply |
| Dr Dogan (Dogan 2008) |
6 October 2012; 17 October 2012 | No answer |
| Dr Saxena (Saxena 2010) |
29 March 2016; 18 April 2016 | No answer |
| Dr V’lckova‐Laskoska (Caca‐Biljanovska 2002) |
29 March 2016; 18 April 2016 | No answer |
| Dr Vladimarsson (Thorleifsdottir 2012) |
29 March 2016; 18 April 2016; 21 April 2016 |
1. Did you have primary or secondary outcomes?
2. Could you give me more information concerning the results?
3. Did you have patients with secondary effects ?
4. Concerning the relapse of participants:
4. How did you perform the sequence generation (table, computer)? 5. How did you perform the allocation concealment? Reply:
6. What was the setting of the study ?
7. Did you perform throat swab before/during your study? If yes, what was the result? Reply:
8. Concerning the proportion of participants having at least one relapse at long‐term follow‐up (at least one year after the start of treatment) after randomisation: did you follow the control group or only the tonsillectomy Group?
|
| Dr Vincent (Vincent 1992) |
No email address found | ‐ |
Concerning our secondary outcomes, two of the five included trials reported the proportion of participants achieving clear or almost clear skin (Physician Global Assessment (PGA) 0 or 1, or PASI 90 or 100 (Caca‐Biljanovska 2002; Thorleifsdottir 2012)), and the proportion of participants achieving PASI 75 or PGA 1 to 2 (Saxena 2010; Thorleifsdottir 2012). One reported partially on the risk of having at least one relapse at long‐term follow‐up (Thorleifsdottir 2012).
The primary outcome chosen by the study authors was not stated in four of the five trials, and was uncertain in one trial. One trial provided no information in the main publication, but we were able to obtain more information by contacting the study author, and by reading a secondary report on the study. The study author stated that the primary outcome was a reduction in the PASI score, with a coinciding decrease in the blood frequency of T lymphocytes, which recognise auto‐antigens in the skin. However, in a secondary publication, the authors stated that the primary endpoints were defined as clinically significant changes in HRQoL (health‐related quality of life), assessed by the Psoriasis Disability Index (PDI) and the Psoriasis Life Stress Inventory (PLSI), at 12 and 24 months (Thorleifsdottir 2012).
PASI was assessed in all trials, reported as the decrease of mean PASI scores from baseline in each group, or rate of PASI reduction, or rate of participants achieving PASI 75. Physician clinical assessment was assessed on a scale from 0 to 4, or 0 to 5 in two trials. No numerical results for efficacy assessment were available for one trial (Vincent 1992), and were presented only in figures, with no numerical data, for another (Thorleifsdottir 2012).
Excluded studies
Reasons for exclusion were described in the 'Characteristics of excluded studies' tables. We excluded 16 reports corresponding to 13 studies, for one of which, we were unable to obtain more than an abstract. The main reason for exclusion was absence of randomisation, which was true in seven of the excluded studies.
Studies awaiting classification
One study, registered in clinicaltrials.gov in 2007, comparing bicillin L‐A to placebo in participants with chronic plaque psoriasis, was terminated in 2015 because there were "not enough enrollees to obtain". We were not able to find a report of the results or to contact the lead author, so we considered it to be awaiting classification (NCT00427609).
Ongoing study
We found no ongoing studies.
Risk of bias in included studies
We summarised 'Risk of bias' assessments in Figure 2 and Figure 3. All trials were at high risk of bias in at least two domains. Figure 2 presents our judgements about each risk of bias item, presented as percentages across all included studies. Figure 3 presents each risk of bias item for each included study.
2.
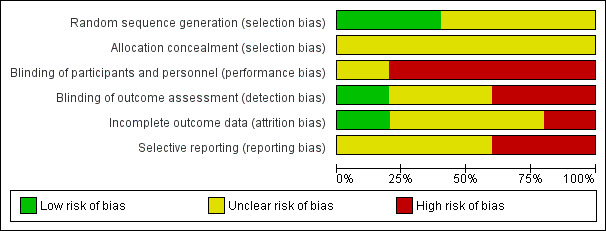
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
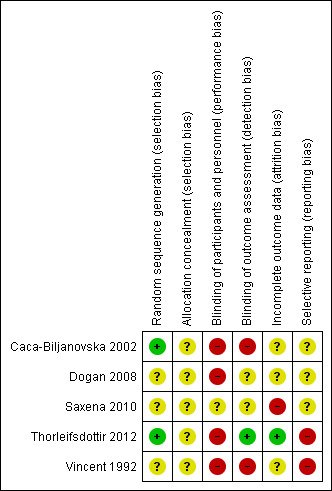
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Random sequence generation
Randomisation was never described as centralised. The report for three trials did not contain the description of the process of allocation sequence generation, and we considered them at unclear risk of bias for generation of sequence generation. In Saxena 2010, there was an important difference in PASI evaluation scores at baseline between groups that could be interpreted as a failure of the randomisation process. Sequence generation was reported, and was adequate for two others (Caca‐Biljanovska 2002; Thorleifsdottir 2012).
Allocation concealment
Methods to guarantee allocation concealment were not reported in any of the five trials. Thus, we considered all trials at unclear risk of bias for allocation concealment.
Blinding
Performance bias
In four trials, participants and caregivers were presented as not blinded, and we considered them at high risk of bias. One trial was described as single blind, however, they did not clearly explain who was supposed to be blind, the participant or the assessor, and the comparator was not a real placebo, but a vitamin C tablet, so we considered it was at unclear risk of bias for performance bias (Saxena 2010).
Detection bias
As all outcomes are subjective, absence of blinding was considered to be a high risk of bias. For two trials, the assessor was not blinded, and we considered them at high risk of detection bias (Caca‐Biljanovska 2002; Vincent 1992). In two trials, the assessor was described as blinded; however as participants were not blinded, and no information was provided regarding specific measures undertaken to avoid communication on treatment between assessor, caregiver, and participants, we considered these trials at unclear risk of detection bias. We only considered one trial at low risk (Thorleifsdottir 2012).
Incomplete outcome data
We considered this item at unclear risk of bias for three trials, as no information on the number of withdrawals or the methods used to manage missing data was provided (Caca‐Biljanovska 2002; Dogan 2008; Vincent 1992). We considered Saxena 2010 at high risk of bias, as an intention‐to‐treat analysis was not performed, and there was an unbalanced number of withdrawal between the two groups. One trial specified that no participant withdrew, and we considered it at low risk (Thorleifsdottir 2012).
Selective reporting
None of the included trials were registered in a trial registry. Four trials did not state the primary outcome. Among these, one did not report all the outcomes described in the methods section, and we considered it at high risk of bias for selective outcome reporting (Vincent 1992). We considered the three others at unclear risk of bias. For one study, the primary outcome was not stated in the main publication, and the primary outcome stated by the author in an email and the secondary publication were different, so we rated this trial at high risk of bias (Thorleifsdottir 2012).
Other potential sources of bias
None identified.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Penicillin compared to no treatment for guttate psoriasis.
| Penicillin compared to no treatment for guttate psoriasis | ||||||
|
Patient or population: guttate psoriasis Setting: inpatients in department of dermatology Intervention: penicillin Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with Penicillin | |||||
| Time‐to‐resolution: achieving PASI 90 to 100 or PGA 0 to 1 | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Proportion of participants with adverse events and severe adverse events Follow‐up: 6 weeks | See comment | See comment | ‐ | 20 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 1 | It was specified that no adverse events were observed in either group. |
| Proportion of participants achieving clear or almost clear skin (PASI 90 to 100 or PGA 0 to 1) Follow‐up: 6 weeks | 300 per 1000 | 600 per 1000 (204 to 1000) |
RR 2.00 (0.68 to 5.85) |
20 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 | Study authors state that no difference was observed between baseline and 6 weeks for psoriasis severity (PASI). |
| Proportion of participants achieving PASI 75 or PGA 1 to 2 | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured in either trial. PASI assessment results were only presented as mean PASI at 6 weeks. No difference was observed between the two groups in either trial. |
| Risk of having at least one relapse at long‐term follow‐up | ‐ | ‐ | ‐ | ‐ | ‐ | No long‐term follow‐up |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality/certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality/certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality/certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality/certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by three levels to very low certainty. Evidence was downgraded by two levels due to risk of bias. The study was at high risk of bias as the study was not blinded. The study was at unclear risk of bias for selective outcome reporting (no primary outcome stated). Evidence was downgraded further by one level due to imprecision: the study included few participants (N = 20).
2Downgraded by three levels to very low certainty. Evidence was downgraded by two levels due to risk of bias. The study was at high risk of bias as the study was not blinded and clinical assessment of psoriasis severity was subjective. The study was at unclear risk of bias for selective outcome reporting (no primary outcome stated). Evidence was downgraded further by one level due to imprecision: the study included few participants (N = 20).
Summary of findings 2. Erythromycin compared to no treatment for guttate psoriasis.
| Erythromycin compared to no treatment for guttate psoriasis | ||||||
|
Patient or population: guttate psoriasis Setting: inpatients in department of dermatology Intervention: erythromycin Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with erythromycin | |||||
| Time‐to‐resolution: achieving PASI 90 to 100 or PGA 0 to 1 | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Proportion of participants with adverse events and severe adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Proportion of participants achieving clear or almost clear skin (PASI 90 to 100 or PGA 0 to 1) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Proportion of participants achieving PASI 75 or PGA 1 to 2 | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured Results were only reported as mean PASI at baseline and at 6 weeks. No difference was found between groups for mean PASI at 6 weeks. |
| Risk of having at least one relapse at long‐term follow‐up | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality/certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality/certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality/certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality/certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
Summary of findings 3. Azithromycin compared to vitamin C for chronic plaque psoriasis.
| Azithromycin compared to vitamin C for chronic plaque psoriasis | ||||||
|
Patient or population: chronic plaque psoriasis
Setting: outpatients of department of dermatology Intervention: azithromycin Comparison: vitamin C | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with vitamin C | Risk with azithromycin | |||||
| Time‐to‐resolution: achieving PASI 90 to 100 or PGA 0 to 1 | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Proportion of participants with adverse events and severe adverse events Follow‐up: 48 weeks |
See comment | See comment | ‐ | 50 (1 RCT) | ‐ | Nausea and mild abdominal upset were reported in 10/30 in the azithromycin group. There was no mention of the presence or absence of adverse events in the Vitamin C group. |
| Proportion of participants achieving clear or almost clear skin (PASI 90 to 100 or PGA 0 to 1) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Proportion of participants achieving PASI 75 or PGA 1 to 2 Follow‐up: 48 weeks | See comment | See comment | RR 25.06 (1.60 to 393.59) |
50 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | 18/30 participants in the azithromycin group versus 0/20 in the vitamin C group. |
| Risk of having at least one relapse at long‐term follow‐up | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality/certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality/certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality/certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality/certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by three levels to very low certainty: 2 levels due to unclear risk of bias for selective reporting (any primary outcome was specified) and high risk of bias for incomplete outcome data, and a further one level due to imprecision (one small study, wide confidence interval)
Summary of findings 4. Rifampicin compared to placebo for guttate psoriasis.
| Rifampicin compared to placebo for guttate psoriasis | ||||||
|
Patient or population: guttate psoriasis Setting: not reported Intervention: rifampicin Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with rifampicin | |||||
| Time‐to‐resolution: achieving PASI 90 to 100 or PGA 0 to 1 | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Proportion of participants with adverse events and severe adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Proportion of participants achieving clear or almost clear skin (PASI 90 to 100 or PGA 0 to 1) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured However, no change in psoriasis severity was observed in any group at 14 days and 6 weeks. |
| Proportion of participants achieving PASI 75 or PGA 1 to 2 | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Risk of having at least one relapse at long‐term follow‐up | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality/certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality/certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality/certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality/certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
Summary of findings 5. Tonsillectomy compared to no intervention for chronic plaque psoriasis.
| Tonsillectomy compared to no intervention for guttate or chronic plaque psoriasis | ||||||
|
Patient or population: chronic plaque psoriasis Setting: not reported Intervention: tonsillectomy Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with tonsillectomy | |||||
| Time‐to‐resolution: achieving PASI 90 to 100 or PGA 0 to 1 | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Proportion of participants with adverse events and severe adverse events Follow‐up: 8 weeks | See comment | See comment | RR 2.81 (0.12 to 63.83) |
29 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | One participant had minor bleeding in the tonsillectomy group. No adverse events in the no treatment group. |
| Proportion of participants achieving clear or almost clear skin (PASI 90 to 100 or PGA 0 to 1) Follow‐up: 8 weeks | See comment | See comment | RR 2.81 (0.12 to 63.83) |
29 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | No events in the control group. |
| Proportion of participants achieving PASI 75 or PGA 1 to 2 Follow‐up: 8 weeks | See comment | See comment | RR 6.56 (0.37 to 116.7) |
29 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | No events in the control group. |
| Risk of having at least one relapse at long‐term follow‐up | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality/certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality/certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality/certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality/certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by three levels to very low certainty: two levels due to risk of bias (participant and caregiver were not blinded; subjective outcome) and one further level due to imprecision (only one study, small number of participants)
We could not pool any data in this review because of heterogeneity of:
intervention: different class of antibiotics: penicillin (Caca‐Biljanovska 2002; Dogan 2008), macrolides – erythromycin (Dogan 2008), and azithromycin (Saxena 2010), and rifampicin Vincent 1992); or surgery (Thorleifsdottir 2012);
comparator: no treatment (Caca‐Biljanovska 2002; Dogan 2008; Thorleifsdottir 2012;), vitamin C (Saxena 2010), or placebo (Vincent 1992); and
participants: plaques psoriasis (Saxena 2010; Thorleifsdottir 2012), or guttate psoriasis (Caca‐Biljanovska 2002; Dogan 2008; Vincent 1992).
(1) Penicillin versus no treatment
(Table 1)
Two trials compared penicillin (penicillin 1.6 MU (million units) intramuscularly once daily for 14 days (Caca‐Biljanovska 2002), and oral benzathine phenoxymethylpenicillin (penicillin V) 50,000 UI/kg/d for 14 days (Dogan 2008)) to no treatment in guttate psoriasis.
In Caca‐Biljanovska 2002, participants of both groups received a co‐intervention of ultraviolet B (UVB) and betamethasone.
Primary outcomes
1. Time‐to‐resolution (time between inclusion and resolution), where resolution was defined as participants achieving clear or almost clear skin (Physician Global Assessment (PGA) 0 or 1, or Psoriasis Area and Severity Index (PASI) 90 or 100)
This outcome was not reported.
2. Proportion of participants with adverse effects and severe adverse effects
In one trial, no adverse events were observed in either group (Caca‐Biljanovska 2002).
In the other trial, adverse events were not reported (Dogan 2008).
Secondary outcomes
1. Proportion of participants achieving clear or almost clear skin (PGA 0 or 1 or PASI 90 or 100)
In Caca‐Biljanovska 2002, there were six events of clearance in the penicillin group, and three events in the no treatment group at six weeks. There was no statistically significant difference between the two groups (relative risk (RR) 2.00, 95% confidence interval (CI) 0.68 to 5.85; 20 participants; Analysis 1.1).
1.1. Analysis.

Comparison 1 Penicillin versus no treatment in guttate psoriasis, Outcome 1 Proportion of participants achieving clear or almost clear skin (PASI 90 or 100 or PGA 0 or 1).
The proportion of participants achieving clear or almost clear skin was not reported in the other trial (Dogan 2008). No difference was observed for mean PASI score between baseline and six weeks in each group (Dogan 2008).
2. Proportion of participants achieving PASI 75 or PGA 1 to 2
Results were reported as mean PASI score at baseline and at six weeks in both trials, and not as proportion of participants achieving PASI 75 or PGA 1 to 2. No difference was found between groups for mean PASI at six weeks in both trials (Caca‐Biljanovska 2002; Dogan 2008).
3. Risk of having at least one relapse at long‐term follow‐up
Long‐term follow‐up was not reported.
(2) Erythromycin versus no treatment
(Table 2)
One trial compared oral erythromycin 1 g per day for 14 days to no treatment, in guttate psoriasis (Dogan 2008)
Primary outcomes
1. Time‐to‐resolution (time between inclusion and resolution), where resolution was defined as participants achieving clear or almost clear skin (Physician Global Assessment (PGA) 0 or 1, or Psoriasis Area and Severity Index (PASI) 90 or 100)
This outcome was not reported.
2. Proportion of participants with adverse effects and severe adverse effects
Adverse events were not reported.
Secondary outcomes
1. Proportion of participants achieving clear or almost clear skin (PGA 0 or 1, or PASI 90 or 100)
This outcome was not reported.
2. Proportion of participants achieving PASI 75 or PGA 1 to 2
Results were reported as a mean PASI score at baseline and at six weeks, and not as proportion of participants achieving PASI 75 or PGA 1 to 2. No difference was found between groups for mean PASI at six weeks (Dogan 2008).
3. Risk of having at least one relapse at long‐term follow‐up
Long‐term follow‐up was not reported.
(3) Azithromycin versus vitamin C
(Table 3)
One trial compared oral azithromycin 500 mg/d for four consecutive days every 14 days over 48 weeks in participants with chronic plaque psoriasis (Saxena 2010).
Primary outcomes
1. Time‐to‐resolution (time between inclusion and resolution), where resolution was defined as participants achieving clear or almost clear skin (Physician Global Assessment (PGA) 0 or 1, or Psoriasis Area and Severity Index (PASI) 90 or 100)
This outcome was not reported.
2. Proportion of participants with adverse effects and severe adverse effects
Adverse events (nausea and mild abdominal upset) were reported in 10/30 in the azithromycin group. There was no mention of the presence or absence of adverse events in the Vitamin C group.
Secondary outcomes
1. Proportion of participants achieving clear or almost clear skin (PGA 0 or 1 or PASI 90 or 100)
This outcome was not reported.
2. Proportion of participants achieving PASI 75 or PGA 1 to 2
At 48 weeks, 60% (18/30) of participants in the azithromycin group versus 0/20 in the vitamin C group reached PASI 75 (RR 25.06, 95% CI 1.60 to 393.59; 50 participants; Analysis 2.1).
2.1. Analysis.
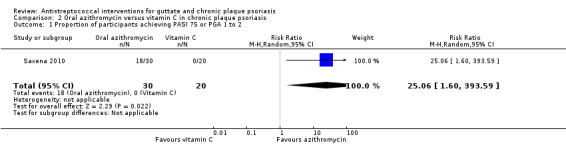
Comparison 2 Oral azithromycin versus vitamin C in chronic plaque psoriasis, Outcome 1 Proportion of participants achieving PASI 75 or PGA 1 to 2.
3. Risk of having at least one relapse at long‐term follow‐up
This outcome was not reported.
(4) Rifampicin versus placebo
(Table 4)
One trial compared oral rifampicin 600 mg/d versus placebo for five days in participants with guttate psoriasis (Vincent 1992). participants of both groups received oral penicillin V 50,000 IU/kg/d or oral erythromycin 1 g/d for 14 days as a co‐intervention.
Primary outcomes
1. Time‐to‐resolution (time between inclusion and resolution), where resolution was defined as participants achieving clear or almost clear skin (Physician Global Assessment (PGA) 0 or 1, or Psoriasis Area and Severity Index (PASI) 90 or 100)
This outcome was not reported.
2. Proportion of participants with adverse effects and severe adverse effects
Adverse events were not reported.
Secondary outcomes
1. Proportion of participants achieving clear or almost clear skin (PGA 0 or 1 or PASI 90 or 100)
This outcome was not reported. However, no change in psoriasis severity was observed in either group at 14 days and six weeks.
2. Proportion of participants achieving PASI 75 or PGA 1 to 2
This outcome was not reported.
3. Risk of having at least one relapse at long‐term follow‐up
Long‐term follow‐up was not reported.
(5) Penicillin versus erythromycin
One trial compared oral benzathine phenoxymethylpenicillin (penicillin V) 50,000 IU/kg/d to oral erythromycin 1 gr/d for 14 days in guttate psoriasis (Dogan 2008).
Primary outcomes
1. Time‐to‐resolution (time between inclusion and resolution), where resolution was defined as participants achieving clear or almost clear skin (Physician Global Assessment (PGA) 0 or 1, or Psoriasis Area and Severity Index (PASI) 90 or 100)
This outcome was not reported.
2. Proportion of participants with adverse effects and severe adverse effects
Adverse events were not reported.
Secondary outcomes
1. Proportion of participants achieving clear or almost clear skin (PGA 0 or 1 or PASI 90 or 100)
This outcome was not reported.
2. Proportion of participants achieving PASI 75 or PGA 1 to 2
Results were reported as mean PASI scores at baseline and at six weeks, and not as the proportion of participants achieving PASI 75 or PGA 1 to 2. No difference was found between groups for mean PASI at six weeks (Dogan 2008).
3. Risk of having at least one relapse at long‐term follow‐up
Long‐term follow‐up was not reported.
(6) Tonsillectomy versus no treatment
(Table 5)
One trial compared tonsillectomy to no treatment in participants with chronic plaque psoriasis (Thorleifsdottir 2012). Other treatments (topical or systemic) for psoriasis were allowed during the trial.
Primary outcomes
1. Time‐to‐resolution (time between inclusion and resolution) where resolution was defined as participants achieving clear or almost clear skin (Physician Global Assessment (PGA) 0 or 1 or Psoriasis Area and Severity Index (PASI) 90 or 100)
This outcome was not reported.
2. Proportion of participants with adverse effects and severe adverse effects
One participant had an adverse event (minor bleeding) in the tonsillectomy group (RR 2.81, 95% CI 0.12 to 63.83; 29 participants; Analysis 3.1).
3.1. Analysis.
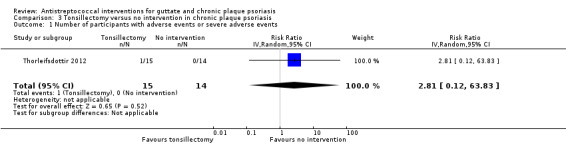
Comparison 3 Tonsillectomy versus no intervention in chronic plaque psoriasis, Outcome 1 Number of participants with adverse events or severe adverse events.
Secondary outcomes
1. Proportion of participants achieving clear or almost clear skin (PGA 0 or 1 or PASI 90 or 100)
In the tonsillectomy arm, 1/15 (7%) participants achieved PASI 90 after eight weeks; there were none in the no intervention arm (RR 2.81, 95% CI 0.12 to 63.83; 29 participants; Analysis 3.2).
3.2. Analysis.
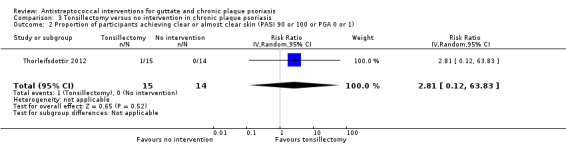
Comparison 3 Tonsillectomy versus no intervention in chronic plaque psoriasis, Outcome 2 Proportion of participants achieving clear or almost clear skin (PASI 90 or 100 or PGA 0 or 1).
2. Proportion of participants achieving PASI 75 or PGA 1 to 2
Three of fifteen (20%) participants in the tonsillectomy group, and none in the no treatment group achieved PASI 75 after eight weeks (RR 6.56, 95% CI 0.37 to 116.70; 29 participants; Analysis 3.3).
3.3. Analysis.
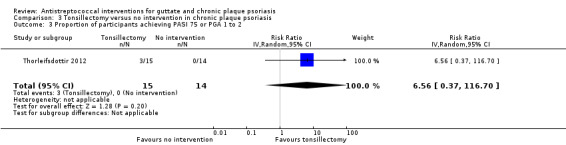
Comparison 3 Tonsillectomy versus no intervention in chronic plaque psoriasis, Outcome 3 Proportion of participants achieving PASI 75 or PGA 1 to 2.
3. Risk of having at least one relapse at long‐term follow‐up
Authors reported that five participants in the tonsillectomy arm had a recurrence of psoriasis lesions in the winter, without providing any more precise information.
Discussion
Summary of main results
We included five trials: four assessed antibiotic treatment (penicillin, a macrolide (either erythromycin or azithromycin), or rifampicin). Three examined participants with guttate psoriasis (Table 1; Table 2; Table 4); and one included participants with chronic plaque psoriasis (Table 3). One trial assessed the effects of tonsillectomy in participants with chronic plaque psoriasis (Table 5).
We performed no meta‐analyses due to heterogeneity of participants' characteristics (guttate or plaque psoriasis) and interventions. No studies reported our efficacy primary outcome of time‐to‐resolution (time between inclusion and resolution), where resolution was defined as participants achieving clear or almost clear skin (Physician Global Assessment (PGA) 0 or 1, or Psoriasis Area and Severity Index (PASI) 90 or 100. None of the studies measured the risk of having at least one relapse at long‐term follow‐up, either.
We rated the certainty of evidence as very low for all reported outcomes, because the five included trials were at high risk of bias, the results were imprecise, and the trials involved a small number of participants, with almost no children. Therefore, we are uncertain of the accuracy of the results that we report, and the efficacy and safety of antistreptococcal interventions.
Adverse events were not measured in the two trials that compared rifampicin against placebo, and erythromycin or penicillin against no treatment. In the three remaining trials, adverse events were reported in those taking azithromycin (nausea and mild abdominal upset), but there was no information provided about adverse events in those given vitamin C (50 participants randomised, measured at 48 weeks); there was only one minor adverse event (minor bleeding) reported in participants who had a tonsillectomy, and none reported in those given no treatment (29 participants randomised, measured at eight weeks); and in the trial comparing penicillin with no treatment, no adverse events were seen in either group (20 participants randomised, measured at six weeks).
The proportion of participants achieving clear or almost clear skin (Physician Global Assessment (PGA) 0 or 1, or Psoriasis Area and Severity Index (PASI) 90 or 100) was only reported in one trial on guttate psoriasis, in which penicillin was compared to no treatment (20 participants randomised). We are uncertain if the number of participants with guttate psoriasis achieving clear or almost clear skin differed between groups at six weeks. Similarly, PASI 90 was measured in only one chronic plaque psoriasis trial, which compared tonsillectomy with no intervention (29 participants randomised): only one participant in the tonsillectomy group achieved PASI 90, measured after eight weeks. Three trials, which compared rifampicin with placebo, erythromycin or penicillin with no treatment, and azithromycin with vitamin C, did not measure this outcome.
The proportion of participants achieving PASI 75 or PGA 1 to 2 was not measured in any guttate psoriasis trial, so there were no results for rifampicin versus placebo, and penicillin or erythromycin versus no treatment. However, the number of participants reaching PASI 75 was measured in both chronic plaque psoriasis trials. PASI 75 was achieved at 48 weeks in just over half of the participants (18/30) in the azithromycin group versus none in the group taking vitamin C (50 participants randomised). Three out of 15 participants in the tonsillectomy group achieved PASI 75 compared with none in the no treatment group after eight weeks (29 participants randomised).
Overall completeness and applicability of evidence
The evidence is incomplete for antistreptococcal interventions for guttate psoriasis. We found three trials assessing systemic antibiotic treatment in 83 participants with guttate psoriasis.
The objective of treatment is to shorten the time‐to‐resolution of guttate psoriasis, and to reduce the number of people with long‐term chronic plaque psoriasis. Thus, we considered time‐to‐resolution as a primary outcome of interest for efficacy, where resolution was defined as participants achieving clear or almost clear skin within eight weeks of the start of treatment. This outcome was not assessed or reported in the three guttate psoriasis trials. We also considered the risk of having at least one relapse at long‐term follow‐up as an outcome of interest. It was not reported in any of the three trials, which lasted only six to eight weeks. Adverse events were not reported at all in two trials, and very briefly described in the third.
The evidence is also incomplete for antistreptococcal interventions for chronic plaque psoriasis. We found two trials involving 79 participants; one assessed long‐term antibiotic treatment (azithromycin for 48 weeks from baseline), and one trial assessed the effects of tonsillectomy. They did not report on time‐to‐resolution, or risk of at least one relapse at long‐term follow‐up. One trial reported on adverse events in the treated group only, the other trial very briefly described adverse events.
The mean age of the participants was 26 years (a minimum age of 12 years to a maximum of 77 years); only two participants were under 15 years old, yet guttate psoriasis is described as more frequent in children and young adults (Mercy 2013). The included population was heterogeneous in term of severity; participants had mild to severe psoriasis, as attested by baseline PASI scores.
More men than women were included (100 men/62 women). Future trials should try to recruit equally from both sexes, since the burden of psoriasis is shared roughly equally by both sexes (GHDx 2018).
The link between proven infection and effect of antistreptococcal treatment was something we hoped to investigate via subgroup analyses, regarding the presence, absence, or unknown status of active Streptococcal infection at the time of treatment onset, but it was not possible, due to our inability to meta‐analyse. However, we noted that in the three trials concerning guttate psoriasis, only 10% of the participants were found to have a positive throat swab culture. The baseline results for culture of swab were reported as equivocal in one trial on chronic plaque psoriasis, and only half of participants in both groups were colonised by Streptococci in the other. In total, Streptococcus bacteria were found in the throats of only 23 people (about 14%).
The choice of antibiotic is questionable, as two comparisons assessed penicillin, and two comparisons assessed rifampicin and erythromycin, while current guidelines recommend using penicillin V or amoxicillin to treat Streptococcal pharyngitis (Shulman 2012). Amoxicillin was not assessed by any of the included studies.
Quality of the evidence
The results for all outcomes measured in this review were based on very low‐certainty evidence.
We downgraded the evidence, and rated it as very low for the three comparisons reporting at least one of our outcomes. There was a high or unclear risk of bias in the included studies for:
blinding of participants, caregivers, or assessors;
selective outcome reporting (no primary outcome specified);
incomplete outcome data.
We also downgraded the evidence for imprecision for all comparisons, because the included studies had a small number of participants, which resulted in wide confidence intervals.
The small number of included studies and heterogeneity of participants and interventions did not allow assessment of publication bias, or meta‐analysis.
Potential biases in the review process
We attempted to conduct a comprehensive search for studies, but we did not succeed in obtaining results of a study comparing Bicillin LA to placebo, which was registered in 2007, and listed as terminated because there were not enough enrollees to obtain a valid conclusion (NCT00427609). When there were missing data from results, or information that prevented us from assessing risk of bias, we contacted the authors of those trials to request additional information; however, we did not receive any reply from several trialists.
We found a trial comparing a topical antibiotic (polymyxin B) to vehicle in plaque psoriasis (Stutz 1996). We decided not to include this study, because it was not in line with the physiopathological hypothesis linking infection and psoriasis. As finding a trial assessing a topical antibiotic was not anticipated, the protocol did not specify initially that only systemic antibiotics were considered. We added this point in the section Differences between protocol and review.
Agreements and disagreements with other studies or reviews
The previous version of this Cochrane Review assessed the same question, concluding that no evidence was available about the efficacy of antistreptococcal intervention in cutaneous psoriasis (Owen 2000). They included only one trial, which was also included in this review (Vincent 1992). We included four additional trials, published after 2000. Our conclusion was different from the conclusion of the systematic review Rachakonda 2015, which concluded that "tonsillectomy may be a potential option for people with recalcitrant psoriasis associated with episodes of tonsillitis". Rachakonda 2015 considered including all types of study designs (clinical cases to randomised controlled trials) that assessed the efficacy of tonsillectomy, and included one randomised controlled trial, also included in our review (Thorleifsdottir 2012); one retrospective study; four prospective observational studies; seven case reports; and seven case series. They did not perform a formal assessment of risk of bias.
The antibiotics assessed by the studies in our review are not in accordance with national recommendations (Shulman 2012). Furthermore, the indication for tonsillectomy does not aline with current guidelines that do not recommend tonsillectomy solely to reduce the frequency of Group A Streptococcal pharyngitis (Shulman 2012).
Authors' conclusions
Implications for practice.
We do not have sufficient evidence to determine the effects of antistreptococcal interventions for guttate and chronic plaque psoriasis. The evidence we found for systemic antibiotic treatment in participants with guttate psoriasis, and for systemic antibiotic treatment and tonsillectomy in participants with chronic plaque psoriasis, was of very low quality. Thus, we cannot be certain of the accuracy of the results found.
The one study awaiting classification may alter the conclusions of the review once assessed.
A number of our outcomes of interest were either not addressed, or were addressed inadequately. The study populations were small in number, and not reflective of those with guttate psoriasis or chronic plaque psoriasis. We could only include a small number of trials and those we did include, were at high or unclear risk of bias for reasons, such as lack of blinding and outcome reporting issues. The treatments assessed did not include those regarded in the literature as important, and finally, our included studies did not facilitate investigation of active Streptococcal infection at the time of treatment.
Implications for research.
A relationship between Streptococcal infection and guttate psoriasis onset is suspected, although it is not based on high‐quality evidence.
Further, well‐designed, randomised trials assessing antibiotic treatment are needed for guttate psoriasis, and we suggest the following PICO.
Participants
Guttate psoriasis is more prevalent in young adults and children, so this population should be included in future trials of guttate psoriasis. Inclusion criteria should include Streptococcal infection, diagnosed by swabbing the throat and testing for Group A Streptococcal (GAS) pharyngitis, using a rapid antigen detection test (RADT), culture, or both. There is a need for validated tools to assess the severity of guttate psoriasis in children and young adults.
Intervention
Penicillin V or amoxicillin, according to recommendations for the treatment of Streptococcal pharyngitis.
Comparator
Options for the comparator include topical steroids, a combination of topical steroids and vitamin D analogues, or phototherapy, and placebo.
Outcomes
In the absence of a core outcome set available for guttate psoriasis, we suggest four important outcomes. The most relevant primary outcome for a form of psoriasis that resolves in a few weeks is time‐to‐resolution (time between inclusion and resolution), where resolution is defined as participants achieving clear or almost clear skin. Long‐term follow‐up (one year) of the rate of developing a chronic form of psoriasis is another important outcome, since preventing chronic psoriasis is a goal of treatment. Quality of life and adverse effects should also be included as outcomes.
To avoid high risk of bias, design of these future trials should ensure blinding of participants, personnel, and outcome assessors, as outcomes are subjective. To avoid imprecision, future studies should include a sample size calculation to ensure the study is adequately powered. They should also ensure they follow the CONSORT guideline for clinical trials, to improve the quality of research, reducing risk of bias, and guide decision making (Moher 2010).
More evidence on the relationship between flares of chronic plaque psoriasis and infection is needed prior to further interventional trials assessing antistreptococcal intervention in this form of psoriasis.
Acknowledgements
R Chateau, for his contribution to the glossary, for a better understanding for the lay audience. Dr Thorleifsdottir and Dr Tsankov for sending us additional information.
Cochrane Skin editorial base would like to thank the following people who commented on this review: our Cochrane Dermatology Editor, Sue Jessop; our Statistical Editor, Ben Carter; our Methodological Editor, Ching‐Chi Chi; and Robert Chalmers and Ruth Murphy, who were the clinical referees. We would also like to thank Vicki Pennick for copy‐editing the review.
Appendices
Appendix 1. Skin Group Specialised Register (CRS) search strategy
psoria* and (tonsil* or streptococc* or antibiotic*)
Appendix 2. Cochrane Register of Studies Online (CRSO) search strategy
(psoria* and (tonsil* or streptococc* or antibiotic*)):TI,AB,KY
Appendix 3. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor: [Psoriasis] explode all trees #2 psoria*:ti,ab,kw #3 #1 or #2 #4 streptococc*:ti,ab,kw #5 MeSH descriptor: [Streptococcal Infections] explode all trees #6 MeSH descriptor: [Streptococcus] explode all trees #7 MeSH descriptor: [Streptococcal Vaccines] explode all trees #8 tonsillectomy:ti,ab,kw #9 MeSH descriptor: [Tonsillectomy] explode all trees #10 antibiotic*:ti,ab,kw #11 MeSH descriptor: [Anti‐Bacterial Agents] explode all trees #12 {or #4‐#11} #13 #3 and #12
Appendix 4. MEDLINE Ovid search strategy
1. psoria$.ti,ab. or exp PSORIASIS/ 2. Streptococcal Infections/ 3. exp Streptococcus/ 4. exp Streptococcal Vaccines/ 5. streptococc$.ti,ab. 6. tonsillectomy.ti,ab. 7. Tonsillectomy/ 8. exp Anti‐Bacterial Agents/ 9. antibiotic$.ti,ab. 10. or/2‐9 11. randomized controlled trial.pt. 12. controlled clinical trial.pt. 13. randomized.ab. 14. placebo.ab. 15. clinical trials as topic.sh. 16. randomly.ab. 17. trial.ti. 18. 11 or 12 or 13 or 14 or 15 or 16 or 17 19. exp animals/ not humans.sh. 20. 18 not 19 21. 1 and 10 and 20
[Lines 11‐20: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)]
Appendix 5. Embase Ovid search strategy
1. exp PSORIASIS/ 2. psoria$.ti,ab. 3. 1 or 2 4. exp Streptococcus infection/ 5. exp Streptococcus/ 6. exp Streptococcus vaccine/ 7. streptococc$.ti,ab. 8. tonsillectomy.ti,ab. 9. exp tonsillectomy/ 10. exp antibiotic agent/ 11. antibiotic$.ti,ab. 12. or/4‐11 13. crossover procedure.sh. 14. double‐blind procedure.sh. 15. single‐blind procedure.sh. 16. (crossover$ or cross over$).tw. 17. placebo$.tw. 18. (doubl$ adj blind$).tw. 19. allocat$.tw. 20. trial.ti. 21. randomized controlled trial.sh. 22. random$.tw. 23. or/13‐22 24. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 25. human/ or normal human/ 26. 24 and 25 27. 24 not 26 28. 23 not 27 29. 3 and 12 and 28
Appendix 6. LILACS search strategy
psoria$ and (tonsil$ or streptococc$ or antibiotic$)
In LILACS we searched using the terms above and the Controlled clinical trials topic‐specific query filter.
Data and analyses
Comparison 1. Penicillin versus no treatment in guttate psoriasis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants achieving clear or almost clear skin (PASI 90 or 100 or PGA 0 or 1) | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.68, 5.85] |
Comparison 2. Oral azithromycin versus vitamin C in chronic plaque psoriasis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants achieving PASI 75 or PGA 1 to 2 | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 25.06 [1.60, 393.59] |
Comparison 3. Tonsillectomy versus no intervention in chronic plaque psoriasis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with adverse events or severe adverse events | 1 | 29 | Risk Ratio (IV, Random, 95% CI) | 2.81 [0.12, 63.83] |
| 2 Proportion of participants achieving clear or almost clear skin (PASI 90 or 100 or PGA 0 or 1) | 1 | 29 | Risk Ratio (IV, Random, 95% CI) | 2.81 [0.12, 63.83] |
| 3 Proportion of participants achieving PASI 75 or PGA 1 to 2 | 1 | 29 | Risk Ratio (IV, Random, 95% CI) | 6.56 [0.37, 116.70] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Caca‐Biljanovska 2002.
| Methods | Randomised, open‐label, parallel group, controlled trial One centre in Macedonia Period of inclusion: February 2000 to January 2002 |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Baseline data: Randomised: penicillin (N = 10), no treatment (N = 10)
Withdrawal: information not available |
|
| Interventions |
Duration of period of treatment: 6 weeks Follow‐up after treatment: 2 weeks Intervention 1: penicillin 1.6 106u, IM once a day for two weeks Intervention 2: none Co‐intervention 1: topical therapy with betamethasone dipropionate 0.05% cream, once daily after the phototherapy Co‐intervention 2: phototherapy 4 times a week over 6 weeks The two co‐interventions were given to all participants in both groups |
|
| Outcomes | Primary outcome: not stated At 6 weeks, change in clinical condition in comparison with baseline: "0 = worse; 1 = no change; 2 = moderate improvement (partial reduction in scaling, erythema, infiltration, or some combination); 3 = considerable improvement (significant reduction in all three variables); and 4 = clearing" PASI was assessed after 2, 4, 6, and 8 weeks of treatment |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly allocated by ClinStat software (www.sghms.ac.uk/depts/phs/staff/jmb/jmbsoft.htm)" Comment: random sequence generation performed by computer with a suitable software |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" Comment: the trial was described as open‐label by the authors; participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" Comment: the trial was described as open‐label by the authors; outcome assessors were not blinded and the outcomes were subjective |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of randomly assigned participants: 20
Number of analysed participants: 20 Comment: there was no information on missing data and how they were handled |
| Selective reporting (reporting bias) | Unclear risk | Comment: no primary outcome stated, protocol not available; no protocol found in clinical registries |
Dogan 2008.
| Methods | Randomised, single‐blind, parallel group, controlled trial One centre; Istanbul,Turkey Period of inclusion: 2001 to 2005 |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
During the treatment and follow‐up period
Baseline data: Randomised: erythromycin (N = 14), phenoxymethylpenicillin (N = 14), no treatment (N = 15)
Withdrawal: information not available |
|
| Interventions |
Duration of period treatment: 14 days Follow‐up after treatment: 4 weeks Intervention 1: no treatment Intervention 2: oral erythromycin 250 mg 4 times a day Intervention 3: oral benzathine phenoxymethylpenicillin 50 000 IU/kg/d, in 3 doses Co‐intervention(s): none During the treatment and follow‐up period: no topical and systemic treatments, including phototherapy |
|
| Outcomes | Primary outcome: not stated Mean PASI scores 14 d (end of treatment) and 6 weeks (once in 2 weeks with PASI scores) |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" Comment: method used to generate the allocation sequence was not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "PASI scorings were performed by the same physician, who was blind to the groups, before and after the treatment" Comment: no double dummy; one no treatment group, hospitalised participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "PASI scorings were performed by the same physician, who was blind to the groups, before and after the treatment" Comment: hospitalised patients; no description of measures taken to guarantee no communication between caregivers, participants, and assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of randomly assigned participants: 43 Comment: the number of analysed participants is uncertain, and there is no information on missing data and how they were handled |
| Selective reporting (reporting bias) | Unclear risk | Comment: no primary or secondary outcome stated in the methods section; no registration of the trial was found |
Saxena 2010.
| Methods | Randomised, single‐blind, parallel group, controlled trial Two centres in Jaipur, India Period of inclusion: not stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
During the treatment and follow‐up period:
Baseline data Randomised: azithromycin (N = 30), vitamin C tablets (N = 20); Analysed: azithromycin (28), vitamin C tablets (15)
Withdraw: azithromycin (n = 2, one at 24 weeks, and one at 36 weeks), vitamin C tablets (n = 5) |
|
| Interventions |
Duration of period treatment: 48 weeks Follow‐up after treatment: 0 weeks Intervention 1: oral azithromycin 500 mg once a day for 4 days, with a gap of 10 days (total of 24 such courses) Intervention 2: vitamin C tablets, the same dosage, form Co‐intervention: none |
|
| Outcomes |
Primary outcome: not stated "The disease severity was evaluated using a clinical scoring system i.e. a system translating a clinical judgement into a scale – the Psoriasis Area and Severity Index (PASI). Based on PASI score, clinical assessments of redness, scaling, and thickness of lesions were done in all the participants and response to treatment was graded as nil (< 10%), mild (11 to 30%), moderate (31 to 60%), good (61 to 90%), and excellent (91 to 100%) improvement" Time to evaluate: at 12‐week intervals, up to 48 weeks from baseline |
|
| Notes | Funding: "Financial support: none" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated" Comment: no description on the method used; 30 versus 20 randomised participants per group |
| Allocation concealment (selection bias) | Unclear risk | Comment: no centralised randomisation, information, and treatment with distinct appearance |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "single‐blind" "our patients neither guessed nor enquired about the tablet, as only chewable forms of Vitamin C 500 mg are routinely prescribed and/or available" Comment: not really a placebo but vitamin C, and it is probable that the taste is recognisable The trial is described as single‐blind, however, it was not clearly explained who was supposed to be blinded, participant or assessor |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "single‐blind" "The patients were crossed for scoring, and the same patient was always scored by the same clinician" Comment: The trial is described as single‐blind, however, it was not clearly explained who was supposed to be blinded, participant or assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of randomly assigned participants: 50 (30 to 20) Number of analysed participants: azithromycin 29, vitamin C 15 Number of lost to follow‐up: unbalanced between groups and reason not stated azithromycin 2, vitamin C 5 Comment: 25% of randomised participants were lost to follow‐up in the vitamin C group; no reasons were provided |
| Selective reporting (reporting bias) | Unclear risk | Comment: no primary outcome presented in the method; trial not found in clinical registries |
Thorleifsdottir 2012.
| Methods | Randomised, single‐blind, parallel group, controlled trial Number of centres: 1 centre in Reykjavik, Iceland Period of inclusion: not stated |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
During the treatment and follow‐up period:
Baseline data: Randomised: tonsillectomy (N = 15); no treatment (N = 14)
At baseline: 8/15 of the TX Group were colonised by Streptococci and 6/14 of the controls (additional information provided by the author by email) Withdraw: none |
|
| Interventions |
Duration of period treatment: surgical intervention Follow‐up after treatment: 2 years Intervention 1: tonsillectomy Intervention 2: no treatment The participants were off treatment, including antibiotics, for at least 4 wk before they entered the study and for 2 months thereafter; participants could have anti‐psoriasis treatment during follow‐up if needed |
|
| Outcomes |
Primary outcome: not stated in primary publication, complementary information provided by the author by email when requested:
Secondary outcomes:
Primary outcome stated in a secondary publication: primary endpoints were defined as clinically significant changes in quality of life, assessed by PDI (and PLSI) at 12 and 24 months |
|
| Notes | Funding: the Icelandic Research Fund, the Icelandic Research Fund for Graduate Students, the University of Iceland Research Fund, and the Science Fund of the National University Hospital in Iceland. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "simple randomisation in a 1:1 ratio. A numerical code was used to identify patients and their specimens" Comment: information provided by author after email contact |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The study's supervisor was responsible for the randomisation and only he had access to the numerical code." Comment: It is not clear if supervisor was aware of the group of randomisation of next participant |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participant not blinded (surgery versus no intervention) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The clinical evaluation was observer‐blinded with regard to tonsillectomy". "All included patients had strict instructions not to reveal their tonsil status to the investigator who did all the assessments." Comment: information provided by author after email contact information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of randomly assigned participants: 29 Number of analysed participants: control: 14, tonsillectomy: 15 Number lost to follow‐up: 0 Comment: it was clearly specified that no participant was lost to follow‐up; all participants randomised were included in the analysis |
| Selective reporting (reporting bias) | High risk | Comment: No primary or secondary outcomes stated in the methods section of the published report. Primary outcome stated by the authors (through mail upon request) was different from primary outcome stated in a secondary publication. |
Vincent 1992.
| Methods | Randomised, placebo, parallel group, controlled trial Number of centres and country: not stated Period of inclusion: November 1987 to June 1989 |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
During the treatment and follow‐up period:
Baseline data: Randomised: oral rifampicin (N = 9), placebo (N = 11)
|
|
| Interventions |
Duration of period treatment: 14 days Follow‐up after treatment: 4 weeks Intervention 1: oral rifampicin 300 mg twice daily for 5 days (d 10 to 14) Intervention 2: placebo twice daily for 5 days (d 10 to 14) Co‐intervention: Penicillin V or erythromycin 250 mg four times a day for 14 days |
|
| Outcomes |
Primary outcome: Not stated "PASI method at 3 and 6 weeks after the treatment, and repeat culture and Streptococcal serologic tests were done" |
|
| Notes | Funding: Merell Dow Co (Pharmaceuticals) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" Comment: method used to generate the allocation sequence was not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the method used to guarantee allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no description about blinding, but rifampicin produces red urine |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no description about blinding, but it was possible to obtain from information on the colour of the participant’s urine |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of randomly assigned participants: N = 20 Number of analysed participants: not stated Number lost to follow‐up: no information Comment: no information provided on number lost to follow‐up, and number of participants analysed |
| Selective reporting (reporting bias) | High risk | Comment: primary outcome not stated Outcomes presented in the protocol were not reported in the results; trial not found in clinical registries |
PASI: Psoriasis area and severity index d: day PDI: Psoriasis Disability Index PLSI: Psoriasis Life Stress Inventory ASLO: anti‐streptolysin O antibody
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Falagas 2008 | Systematic review and meta‐analysis |
| Forstrom 1974 | Not controlled |
| Gudjonsson 2003 | No intervention, not randomised |
| Komine 2000 | Not randomised |
| Kurwa 1973 | Not randomised |
| Masood 1997 | Only able to obtain abstract. We assumed this study was uncontrolled. The authors claimed that in 200 unselected psoriasis participants treated with phenoxymethylpenicillin, excellent responses were observed in guttate psoriasis (80% complete clearance), good responses in acute exacerbation of chronic psoriasis (50% clearance), and poor in chronic plaque psoriasis (10% clearance). |
| Nyfors 1973 | Not randomised |
| Owen 2001 | Review |
| Rappersberger 1996 | Other treatment (topical immunosuppressive treatment) |
| Raza 2007 | Not randomised |
| Rosenberg 1986 | Case control study |
| Stutz 1996 | Topical antibiotic |
| Tsankov 2011 | Not randomised; this trial compared rifampicin to placebo in participants with guttate psoriasis; in one intervention group, participants were Streptococcus positive, and in the second intervention group, Streptococcus negative. There were up to 82 participants in the intervention group and only 10 in the placebo group. |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00427609.
| Methods | Randomised controlled, double‐blind Parallel assignment Location: University of Tennessee Health Science Center, Memphis, Tennessee, USA |
| Participants |
Exclusion Criteria:
|
| Interventions | Bicillin L‐A administered intramuscularly in a dose of 2.4 million units every three weeks versus placebo The duration of intervention administration was not indicated |
| Outcomes | A reduction of an individual's PASI by 75% after five treatments of the active drug (Bicillin L‐A). To demonstrate benefit comparable to the currently available biologicals, the response rate of Bicillin L‐A must be at least 40% (time Frame: one year) No more precision on outcome time points |
| Notes | Unpublished trial: It was stated in 'recruitment status' that the trial was terminated (not enough enrollees to obtain a valid conclusion) on 21 April 2015 |
Differences between protocol and review
We specified in the methods section, criteria for considering studies, participants: In cases of studies including only a subset of relevant participants, we planned to include the studies only if the characteristics of patients and results were provided separately, or obtained through contact with authors.
We specified in the methods section, criteria for considering studies for this review, types of interventions, that we would consider systemic antibiotics. We considered it obvious to consider only systemic antibiotics; however, we forgot to specify it.
We decided to assess all of our outcomes, except 'Risk of having at least one relapse at long‐term follow‐up' at both short‐term and long‐term follow‐up, to ensure consistency of timing across outcomes.
We deleted from the methods section, dealing with missing data: "We will also synthesise data as analysed in each trial (complete cases)." This was because we only reported numerical data in intention‐to‐treat analysis with imputation.
We added to the methods section, assessing risk of bias, other bias. We did not anticipated any specific other bias for this review.
We deleted: "For the older trials, the PASI 75 will probably not be available. We will calculate it based on the percentage reduction in PASI when this information is available" because it was not possible to perform it.
Contributions of authors
GD and LLC were the contact people with the editorial base. GD and LLC co‐ordinated contributions from the co‐authors and wrote the final draft of the review. GD, CD, and LLC screened papers against eligibility criteria. GD obtained data on ongoing and unpublished studies. GD, CD, and LLC appraised the quality (risk of bias) of papers. GD, CD, and LLC extracted data for the review and sought additional information about papers. GD and LLC entered data into RevMan 5. GD and LLC analysed and interpreted data. LLC worked on the methods sections. LLC drafted the clinical sections of the background and responded to the clinical comments of the referees. LLC responded to the methodology and statistics comments of the referees. CH was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes were relevant to consumers. LLC is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
No sources of support supplied
External sources
Association Recommandations en Dermatologie (aRED), France.
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Gwendy Dupire: nothing to declare. Catherine Droitcourt: nothing to declare. Carolyn Hughes: nothing to declare. Laurence Le Cleach: nothing to declare.
Ruth Murphy (clinical referee): "I have taken part in an advisory board with Novartis about disease prevention in Psoriasis in October 2017."
New
References
References to studies included in this review
Caca‐Biljanovska 2002 {published data only}
- Caca‐Biljanovska NG, V'lckova‐Laskoska MT. Management of guttate and generalized psoriasis vulgaris: prospective randomized study. Croatian Medical Journal 2002;43(6):707‐12. [CENTRAL: CN‐00412128; PUBMED: 12476481] [PubMed] [Google Scholar]
Dogan 2008 {published data only}
- Dogan B, Karabudak O, Harmanyeri Y. Antistreptococcal treatment of guttate psoriasis: a controlled study. International Journal of Dermatology 2008;47(9):950‐2. [CENTRAL: CN‐00665439; PUBMED: 18937661] [DOI] [PubMed] [Google Scholar]
Saxena 2010 {published data only}
- Saxena VN, Dogra J. Long‐term oral azithromycin in chronic plaque psoriasis: a controlled trial. European Journal of Dermatology 2010;20(3):329‐33. [CENTRAL: CN‐00750715; PUBMED: 20299307] [DOI] [PubMed] [Google Scholar]
Thorleifsdottir 2012 {published and unpublished data}
- Thorleifsdottir RH, Sigurdardottir SL, Sigurgeirsson B, Olafsson JH, Sigurdsson MI, Petersen H, et al. Improvement of psoriasis after tonsillectomy is associated with a decrease in the frequency of circulating T cells that recognize streptococcal determinants and homologous skin determinants. Journal of Immunology 2012;188(10):5160‐5. [CENTRAL: CN‐00969900; PUBMED: 22491250] [DOI] [PubMed] [Google Scholar]
- Thorleifsdottir RH, Sigurdardottir SL, Sigurgeirsson B, Olafsson JH, Sigurdsson MI, Petersen H, et al. Patient‐reported outcomes and clinical response in patients with moderate‐to‐severe plaque psoriasis treated with tonsillectomy: a randomized controlled trial. Acta Dermato‐Venereologica 2017;97(3):340‐5. [CENTRAL: CN‐01336830; PUBMED: 27819714] [DOI] [PubMed] [Google Scholar]
- Valdimarsson H, Thorleifsdottir RH, Sigurdardottir SL, Olafsson JH, Sigurgeirsson B, Petersen H. The impact of tonsillectomy on patients with chronic plaque psoriasis. European Journal of Immunology 2009;39(S1):S311. [CENTRAL: CN‐00784461; DOI: 10.1002/eji.200990152] [DOI] [Google Scholar]
Vincent 1992 {published data only}
- Vincent F, Ross JB, Dalton M, Wort AJ. A therapeutic trial of the use of penicillin V or erythromycin with or without rifampin in the treatment of psoriasis. Journal of the American Academy of Dermatology 1992;26(3 Pt 2):458‐61. [CENTRAL: CN‐00083299; PUBMED: 1564153] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Falagas 2008 {published data only}
- Falagas ME, Vouloumanou EK, Matthaiou DK, Kapaskelis AM, Karageorgopoulos DE. Effectiveness and safety of short‐course vs long‐course antibiotic therapy for group a beta hemolytic streptococcal tonsillopharyngitis: a meta‐analysis of randomized trials. Mayo Clinic Proceedings 2008;83(8):880‐9. [PUBMED: 18674472] [PubMed] [Google Scholar]
Forstrom 1974 {published data only}
- Forstrom L, Lassus A. Fucidic acid and psoriasis. Annals of clinical research 1974;6(3):165‐6. [CENTRAL: CN‐00010569; PUBMED: 4844128] [PubMed] [Google Scholar]
Gudjonsson 2003 {published data only}
- Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, Kristinsson KG, Valdimarsson H. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective study. British Journal of Dermatology 2003;149(3):530‐4. [CENTRAL: CN‐00457920; PUBMED: 14510985] [DOI] [PubMed] [Google Scholar]
Komine 2000 {published data only}
- Komine M, Tamaki K. An open trial of oral macrolide treatment for psoriasis vulgaris. Journal of Dermatology 2000;27(8):508‐12. [PUBMED: 10989574] [DOI] [PubMed] [Google Scholar]
Kurwa 1973 {published data only}
- Kurwa A, Abdel‐Aziz AH. Preliminary clinical trial of fusidic acid in psoriasis. British Journal of Clinical Practice 1973;27(3):92‐4. [PUBMED: 4580210] [PubMed] [Google Scholar]
Masood 1997 {published data only}
- Masood Q, Manzoor S. Treatment of psoriasis with penicillin. JK Practitioner 1997;4(3):178‐9. [EMBASE: 1997226265] [Google Scholar]
Nyfors 1973 {published data only}
- Nyfors A. Fucidin in psoriasis. A double‐blind study of twenty psoriatics over two periods of four weeks each. Dermatologica 1973;146(5):281‐4. [CENTRAL: CN‐00009169; PUBMED: 4583782] [PubMed] [Google Scholar]
Owen 2001 {published data only}
- Owen CM, Chalmers RJ, O'Sullivan T, Griffiths CE. A systematic review of antistreptococcal interventions for guttate and chronic plaque psoriasis. British Journal of Dermatology 2001;145(6):886‐90. [PUBMED: 11899140] [DOI] [PubMed] [Google Scholar]
Rappersberger 1996 {published data only}
- Rappersberger K, Meingassner JG, Fialla R, Fodinger D, Sterniczky B, Rauch S, et al. Clearing of psoriasis by a novel immunosuppressive macrolide. Journal of Investigative Dermatology 1996;106(4):701‐10. [CENTRAL: CN‐00123674; PUBMED: 8618008] [DOI] [PubMed] [Google Scholar]
Raza 2007 {published data only}
- Raza N, Usman M, Hameed A. Chronic plaque psoriasis: streptococcus pyogenes throat carriage rate and therapeutic response to oral antibiotics in comparison with oral methotrexate. Journal of the College of Physicians and Surgeons – Pakistan: JCPSP 2007;17(12):717‐20. [CENTRAL: CN‐01093552; PUBMED: 18182134] [PubMed] [Google Scholar]
Rosenberg 1986 {published data only}
- Rosenberg EW, Noah PW, Zanolli MD, Skinner RB Jr, Bond MJ, Crutcher N. Use of rifampin with penicillin and erythromycin in the treatment of psoriasis. Preliminary report. Journal of the American Academy of Dermatology 1986;14(5 Pt 1):761‐4. [CENTRAL: CN‐00366543; PUBMED: 3086388] [DOI] [PubMed] [Google Scholar]
Stutz 1996 {published data only}
- Stutz JA, Ellis CN, Kang S. Failure of topical polymyxin B to improve mild plaque psoriasis. Archives of Dermatology 1996;132(2):231. [CENTRAL: CN‐00124927; PUBMED: 8629837] [DOI] [PubMed] [Google Scholar]
Tsankov 2011 {published data only}
- Grozdev I, Kazandjieva J, Tsankov N. New insights of rifampicin in psoriasis. Journal of the European Academy of Dermatology and Venereology: JEADV 2010;24(Suppl 4):11‐2. [EMBASE: 70238697] [Google Scholar]
- Tsankov N, Grozdev I. Rifampicin in the treatment of psoriasis. Journal of the European Academy of Dermatology and Venereology: JEADV 2009;23(1):93‐5. [PUBMED: 18384550] [DOI] [PubMed] [Google Scholar]
- Tsankov N, Grozdev I. Rifampicin – a mild immunosuppressive agent for psoriasis. Journal of Dermatological Treatment 2011;22(2):62‐4. [PUBMED: 20653488] [DOI] [PubMed] [Google Scholar]
- Tsankov N, Grozdev I, Kkzandjieva J. Old drug – new indication. Rifampicin in psoriasis. Journal of Dermatological Treatment 2006;17(1):18‐23. [CENTRAL: CN‐00555057; PUBMED: 16467019] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00427609 {unpublished data only}
- NCT00427609. Bicillin L‐A vs placebo for the treatment of chronic, plaque‐type psoriasis unresponsive to topical medications. clinicaltrials.gov/ct2/show/NCT00427609 (first received 25 January 2007).
Additional references
Abe 1991
- Abe J, Forrester J, Nakahara T, Lafferty JA, Kotzin BL, Leung DY. Selective stimulation of human T cells with streptococcal erythrogenic toxins A and B. Journal of Immunology 1991;146(11):3747‐50. [MEDLINE: ] [PubMed] [Google Scholar]
Augustin 2013
- Augustin M, Reich K, Glaeske G, Kampfe S, Radtke MA, Gerdau‐Heitmann C, et al. Drug supply for children with psoriasis in Germany. Journal der Deutschen Dermatologischen Gesellschaft – Journal of the German Society of Dermatology: JDDG 2013;11(8):751‐5. [PUBMED: 23718227] [DOI] [PubMed] [Google Scholar]
Baugh 2011
- Baugh RF, Archer SM, Mitchell RB, Rosenfeld RM, Amin R, Burns JJ, et al. Clinical practice guideline: tonsillectomy in children. Otolaryngology – Head & Neck surgery 2011;144(1 Suppl):S1‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berth‐Jones 2005
- Berth‐Jones J. Psoriasis. Medicine 2005;33(1):50‐5. [Google Scholar]
Burton 2009
- Burton MJ, Glasziou PP. Tonsillectomy or adeno‐tonsillectomy versus non‐surgical treatment for chronic/recurrent acute tonsillitis. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD001802.pub2] [DOI] [PubMed] [Google Scholar]
Chalmers 2001
- Chalmers RJ, O'Sullivan T, Owen CM, Griffiths CE. A systematic review of treatments for guttate psoriasis. British Journal of Dermatology 2001;145(6):891‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Jager 2009
- Jager ME, Kerkhof PC, Jong EM, Seyger MM. Epidemiology and prescribed treatments in childhood psoriasis: a survey among medical professionals. Journal of Dermatological Treatment 2009;20(5):254‐8. [PUBMED: 19418330] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elder 2010
- Elder JT, Bruce AT, Gudjonsson JE, Johnston A, Stuart PE, Tejasvi T, et al. Molecular dissection of psoriasis: integrating genetics and biology. Journal of Investigative Dermatology 2010;130(5):1213‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ferran 2013
- Ferran M, Galvan AB, Rincon C, Romeu ER, Sacrista M, Barboza E, et al. Streptococcus induces circulating CLA(+) memory T‐cell‐dependent epidermal cell activation in psoriasis. Journal of Investigative Dermatology 2013;133(4):999‐1007. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GHDx 2018
- Global Health Data Exchange (GHDx). GBD Results Tool. ghdx.healthdata.org/gbd‐results‐tool (accessed 22 January 2019).
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 10 April 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Griffiths 2007
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007;370(9583):263‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Horton 2016
- Horton DB, Scott FI, Haynes K, Putt ME, Rose CD, Lewis JD, et al. Antibiotic exposure, infection, and the development of pediatric psoriasis: a nested case‐control study. JAMA Dermatology 2016;152(2):191‐9. [PUBMED: 26560335] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ko 2010
- Ko HC, Jwa SW, Song M, Kim MB, Kwon KS. Clinical course of guttate psoriasis: long‐term follow‐up study. Journal of Dermatology 2010;37(10):894‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kotzin 1993
- Kotzin BL, Leung DY, Kappler J, Marrack P. Superantigens and their potential role in human disease. Advances in Immunology 1993;54:99‐166. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kundakci 2002
- Kundakci N, Tursen U, Babiker MO, Gurgey E. The evaluation of the sociodemographic and clinical features of Turkish psoriasis patients. International Journal of Dermatology 2002;41(4):220‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kwon 2012
- Kwon HH, Na SJ, Jo SJ, Youn Jl. Epidemiology and clinical features of pediatric psoriasis in tertiary referral psoriasis clinic. Journal of Dermatology 2012;39(3):260‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Laing 1991
- Laing MR, McKerrow WS. Adult tonsillectomy. Clinical Otolaryngology and Allied Sciences 1991;16(1):21‐4. [PUBMED: 2032352] [DOI] [PubMed] [Google Scholar]
Le Cleach 2011
- Cleach L, Trinquart L, Lebrun‐Vignes B, Giraudeau B, Chosidow O. Oral antiviral therapy for prevention of genital herpes outbreaks in immunocompetent and nonpregnant patients. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD009036] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ledoux 2009
- Ledoux M, Chazerain V, Saiag P, Mahe E. Streptococcal perianal dermatitis and guttate psoriasis [Anite streptococcique et psoriasis en gouttes]. Annales de Dermatologie et de Venereologie 2009;136(1):37‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leung 1995
- Leung DY, Travers JB, Giorno R, Norris DA, Skinner R, Aelion J, et al. Evidence for a streptococcal superantigen‐driven process in acute guttate psoriasis. Journal of Clinical Investigation 1995;96(5):2106‐12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahe 2018
- Mahe E, Bursztejn AC, Phan A, Corgibet F, Beauchet A. Management of childhood psoriasis in France. A national survey among general practitioners, pediatricians, and dermatologists. Dermatologic Therapy 2018;31(1):1‐8. [PUBMED: 29193624] [DOI] [PubMed] [Google Scholar]
Martin 1996
- Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis?. Archives of Dermatology 1996;132(6):717‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mercy 2013
- Mercy K, Kwasny M, Cordoro KM, Menter A, Tom WL, Korman N, et al. Clinical manifestations of pediatric psoriasis: results of a multicenter study in the United States. Pediatric Dermatology 2013;30(4):424‐8. [PUBMED: 23360462] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel groups randomised trials. Journal of Clinical Epidemiology 2010;63(8):834‐40. [PUBMED: 20346629] [DOI] [PubMed] [Google Scholar]
Newman 2008
- Newman MD, Weinberg JM. The pathophysiology of psoriasis. In: Weinberg JM editor(s). Treatment of Psoriasis. Basel: Birkhauser, 2008:11‐21. [ISBN 978‐3‐7643‐7722‐9] [Google Scholar]
NICE 2017
- National Institute for Health Care and Excellence. Psoriasis: assessment and management. www.nice.org.uk/guidance/cg153 (accessed prior to 19 February 2019).
Norrlind 1955
- Norrlind R. The significance of infections in the origination of psoriasis. Acta Rheumatologica Scandinavica 1955;1(2):135‐44. [PUBMED: 13248712] [PubMed] [Google Scholar]
Paradise 1984
- Paradise JL, Bluestone CD, Bachman RZ, Colborn DK, Bernard BS, Taylor FH, et al. Efficacy of tonsillectomy for recurrent throat infection in severely affected children. Results of parallel randomized and nonrandomized clinical trials. New England Journal of Medicine 1984;310(11):674‐83. [PUBMED: 6700642] [DOI] [PubMed] [Google Scholar]
Parisi 2013
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. The Journal of Investigative Dermatology 2013;133(2):377‐85. [PUBMED: 23014338] [DOI] [PubMed] [Google Scholar]
Picciani 2013
- Picciani BL, Michalski‐Santos B, Carneiro S, Sampaio AL, Avelleira JC, Azulay DR, et al. Oral candidiasis in patients with psoriasis: correlation of oral examination and cytopathological evaluation with psoriasis disease severity and treatment. Journal of the American Academy of Dermatology 2013;68(6):986‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rachakonda 2015
- Rachakonda TD, Dhillon JS, Florek AG, Armstrong AW. Effect of tonsillectomy on psoriasis: a systematic review. Journal of the American Academy of Dermatology 2015;72(2):261‐75. [PUBMED: 25455609] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rigopoulos 2010
- Rigopoulos D, Gregoriou S, Katrinaki A, Korfitis C, Larios G, Stamou C, et al. Characteristics of psoriasis in Greece: an epidemiological study of a population in a sunny Mediterranean climate. European Journal of Dermatology 2010;20(2):189‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shulman 2012
- Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2012;55(10):1279‐82. [PUBMED: 23091044] [DOI] [PubMed] [Google Scholar]
SIGN 2010
- Scottish Intercollegiate Guidelines Network. Management of sore throat and indications for tonsillectomy. Guideline No 117, April 2010. www.sign.ac.uk/guidelines/fulltext/117/index.html (accessed 8 May 2014).
Tanz 2007
- Tanz RR, Shulman ST. Chronic pharyngeal carriage of group A streptococci. Pediatric Infectious Disease Journal 2007;26(2):175‐6. [PUBMED: 17259882] [DOI] [PubMed] [Google Scholar]
Telfer 1992
- Telfer NR, Chalmers RJ, Whale K, Colman G. The role of streptococcal infection in the initiation of guttate psoriasis. Archives of Dermatology 1992;128(1):39‐42. [MEDLINE: ] [PubMed] [Google Scholar]
Tervaert 1970
- Tervaert WC, Esseveld H. A study of the incidence of haemolytic streptococci in the throat in patients with psoriasis vulgaris, with reference to their role in the pathogenesis of this disease. Dermatologica 1970;140(5):282‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Valdimarsson 1997
- Valdimarsson H, Sigmundsdottir H, Jonsdottir I. Is psoriasis induced by streptococcal superantigens and maintained by M‐protein‐specific T cells that cross‐react with keratin?. Clinical & Experimental Immunology 1997;107(Suppl 1):21‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Valenzuela 2011
- Valenzuela F, Silva P, Valdes MP, Papp K. Epidemiology and quality of life of patients with psoriasis in Chile. Actas Dermo‐Sifiliograficas 2011;102(10):810‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vogel 2012
- Vogel SA, Yentzer B, Davis SA, Feldman SR, Cordoro KM. Trends in pediatric psoriasis outpatient health care delivery in the United States. Archives of Dermatology 2012;148(1):66‐71. [PUBMED: 21931013] [DOI] [PubMed] [Google Scholar]
Whyte 1964
- Whyte HJ, Baughman RD. Acute guttate psoriasis and streptococcal infection. Archives of Dermatology 1964;89:350‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Williams 1976
- Williams RC, McKenzie AW, Roger JH, Joysey VC. HL‐A antigens in patients with guttate psoriasis. British Journal of Dermatology 1976;95(2):163‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wolff 2009
- Wolff K, Johnson RA, Fitzpatrick TB. Psoriasis. In: Wolff K, Johnson RA editor(s). Fitzpatrick's Color Atlas & Synopsis of Clinical Dermatology. 6th Edition. New York: McGraw‐Hill Medical, 2009:53‐7. [ISBN 978‐0‐07‐159975‐7] [Google Scholar]
References to other published versions of this review
Dupire 2015
- Dupire G, Droitcourt C, Ferneiny M, Hughes C, Katsahian S, Cleach L. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD011571] [DOI] [PMC free article] [PubMed] [Google Scholar]
Owen 2000
- Owen CM, Chalmers RJ, O'Sullivan T, Griffiths CE. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD001976] [DOI] [PubMed] [Google Scholar]


