Abstract
Purpose of review:
To review recent advances in the imaging of hypertensive heart disease (HHD) with an emphasis on developments in the imaging of diffuse myocardial fibrosis using cardiac magnetic resonance (CMR).
Recent findings:
HHD results from long-standing hypertension and is characterized by the development of left ventricular hypertrophy and diffuse interstitial fibrosis. Diffuse fibrosis traditionally required endomyocardial biopsy to diagnose, but recent developments using T1 mapping in CMR allow for noninvasive assessment. Studies using T1 mapping have shown an increase in extracellular volume fraction (ECV) in patients with HHD compared to normal controls, suggesting ECV can be used as a noninvasive marker for fibrosis in HHD. In addition to T1 mapping, other recent advances in HHD imaging include improvements in three-dimensional echocardiography, allowing for accurate real-time volumetric measurements, and the use of speckle tracking echocardiography to detect subclinical systolic dysfunction.
Summary:
Measurement of ECV using T1 mapping in CMR can be used as a noninvasive marker of diffuse myocardial fibrosis in HHD. While further studies are needed to validate this approach with larger patient cohorts, ECV can potentially be used to both monitor disease progression and assess therapeutic interventions in HHD.
Keywords: Hypertensive heart disease, Cardiac magnetic resonance, T1 mapping, Three-dimensional echocardiography, Speckle tracking echocardiography
Introduction
Arterial hypertension is extremely common, affecting one of three adults in the United States, and nearly two of three adults over the age of 60 [1]. It is associated with significant morbidity and mortality, including increased risk of myocardial infarction, stroke, and heart failure [1]. Hypertensive heart disease (HHD) results from long-standing hypertension and is characterized by the development of structural remodeling of the myocardium, including development of left ventricular hypertrophy (LVH) and diffuse interstitial fibrosis [2]. This structural remodeling in HHD has significant clinical consequences, leading to diastolic, and eventually systolic, dysfunction [3].
LVH (Figure 1AB) develops as an adaptive response to increased afterload and is in part due to cardiomyocyte hypertrophy [2]. The development of LVH has been shown to be a predictor of morbidity and mortality in patients with hypertension [4,5]. Antihypertensive therapy leading to LVH regression has been shown to improve diastolic dysfunction [6] and cardiovascular outcomes [7].
Figure 1:
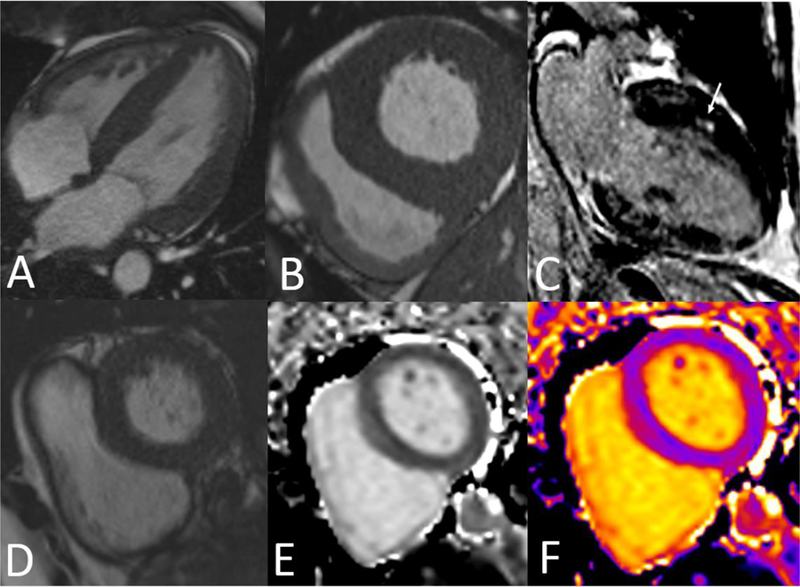
CMR images of (A) Four chamber SSFP showing severe concentric LVH. (B) Basal short axis SSFP showing severe concentric LVH. (C) Two chamber view depicting anterior midwall LGE (arrow) in patient with HHD. (D) SSFP basal short axis image. (E) Pre-contrast T1 map in black & white. (F) T1 color map
In addition to causing cardiomyocyte hypertrophy, hypertension has been shown to also lead to diffuse interstitial fibrosis, which has been demonstrated both on autopsy [8] and biopsy studies [9,10]. Myocardial fibrosis in hypertension has been discovered to cause an increase in ventricular arrhythmias [11] and worsening diastolic dysfunction [12]. Treatment of hypertension with lisinopril or losartan can cause regression of myocardial fibrosis and improvement in diastolic dysfunction [12,13].
Given the significant morbidity and mortality seen in hypertensive heart disease, and considering improved outcomes with treatment, early recognition of these manifestations of HHD through noninvasive imaging is critical. LVH and diastolic dysfunction have traditionally been diagnosed on two-dimensional echocardiography, although cardiac magnetic resonance (CMR) is considered the gold standard for ventricular function and mass assessment as it does not require geometric assumptions and provides excellent delineation of endocardial/epicardial borders [14]. Three dimensional echocardiography has also been shown to be an accurate modality for measuring cardiac volumes and function [15]. A recent advance in echocardiography has been the use of speckle tracking, which can be used to detect subclinical systolic dysfunction in HHD [16]. Another recent advance in HHD is the imaging of diffuse myocardial fibrosis, which traditionally required endomyocardial biopsy to diagnose but can now be assessed noninvasively using T1 mapping in CMR [17].
This article will review these recent developments in noninvasive imaging in hypertensive heart disease with an emphasis on imaging of diffuse myocardial fibrosis using CMR.
Myocardial Fibrosis
Pathology
In normal myocardium, myocytes account for roughly one-third of all cells, while the remaining two-thirds of cells include endothelial and vascular smooth muscle cells and fibroblasts in interstitial/perivascular spaces [18]. The noncellular components of the myocardium include the intramyocardial vasculature and the extracellular matrix, which is a network of proteins and polysaccharides that provides support to the surrounding cells [2]. Hypertension affects both the cellular and noncellular components of the myocardium. In addition to cardiomyocyte hypertrophy, hypertensive heart disease is characterized by deposition of fibrous tissue in the extracellular matrix in both sides of the heart over time [19]. The fibrous tissue deposited in the heart primarily consists of type I fibrillar collagen. Fibrillar collagen has a similar tensile strength to steel, and when deposited in myocardium causes increased tissue stiffness [20].
Clinical consequences and treatment
As type I fibrillar collagen is deposited in the interstitium of the myocardium in patients with hypertension, the initial consequence is an increase in tissue stiffness leading to decreased rate of myocardial relaxation and diastolic dysfunction. However, as collagen continues to accumulate, systolic dysfunction also occurs due to loss of ability to translate contraction of cardiomyocytes into myocardial force [2]. In a study looking at the histopathological factors related to diastolic dysfunction, percentage of fibrosis was identified in multiple regression analysis as the most significant predictor of diastolic dysfunction [21]. In another study in which the type I collagen deposition was quantified via endomyocardial biopsies in hypertensive patients, the amount of collagen tissue was found to be inversely correlated with the ejection fraction, suggesting myocardial fibrosis is involved in the development of systolic heart failure in hypertensive patients [22].
In regards to therapeutic interventions, Brilla et al evaluated the use of lisinopril vs hydrochlorothiazide in the treatment of hypertension, and compared the amount of myocardial fibrosis on endomyocardial biopsy at baseline and after six months of treatment. They found that lisinopril caused a significant decrease in the collagen volume fraction on biopsy, and this correlated with improvement in LV diastolic function [13]. Diez et al evaluated losartan in the treatment of hypertensive heart disease and found that in patients with severe fibrosis, both the amount of fibrosis on biopsy and LV stiffness on echo improved following 12 months of losartan [12].
Diagnosis
Given the clinical consequences of myocardial fibrosis in hypertension, and considering the potential for reversal of fibrosis with appropriate treatment, the need for accurate diagnosis of myocardial fibrosis is apparent. While endomyocardial biopsy is the gold standard for diagnosis, it is an invasive procedure associated with potential complications, including hematoma, right bundle branch block, arrythmias, and tricuspid regurgitation, with an overall complication rate up to 6% [23]. To avoid these risks, noninvasive cardiac imaging has been explored to diagnose fibrosis. Mizuno et al examined the use of echocardiography with integrated backscatter analysis towards this end. They found excellent correlation between the amount of fibrosis seen on endomyocardial biopsy and the predicted amount using integrated backscatter in patients with good image quality. In patients with poor image quality, the correlation was weaker, though was enhanced with the use of tissue harmonic imaging [24].
While myocardial characterization using echo is dependent on image quality, CMR has proven to be a more effective modality for imaging of myocardial fibrosis [25]. Late gadolinium enhancement (LGE) has traditionally been used to identify areas of replacement fibrosis. This technique involves T1-weighted myocardial imaging 10–15 minutes after the administration of gadolinium, which collects in the extracellular space. In areas of replacement fibrosis, there is expansion of the extracellular space leading to a larger volume of distribution for gadolinium, in addition to altering the kinetics such that it takes longer for gadolinium to both collect and disperse from the extracellular space. As a result, areas of fibrosis will retain gadolinium and show hyperenhancement on LGE imaging (Figure 1C) [26]. In a study looking at LGE in various forms of LVH, Rudolph et al identified LGE in 50% of their subjects with hypertensive heart disease; no specific pattern of LGE was seen [27]. Moreo et al found a significant correlation between extent of fibrosis by LGE and degree of diastolic dysfunction [28].
A limitation of LGE imaging is that it is a qualitative technique, dependent on the difference in signal intensity between normal and fibrotic myocardium. In hypertensive heart disease, myocardial fibrosis is often diffuse, so without normal myocardium to provide a difference in signal intensity, often no areas of LGE will be seen [29]. To overcome this limitation of LGE imaging, T1 mapping in CMR has been developed as a quantitative technique to identify diffuse myocardial fibrosis.
T1 mapping: background
T1 is the longitudinal relaxation time constant and represents a fundamental tissue property. A T1 map is a two dimensional color-coded display of the calculated T1 values for each pixel in the image (Figure 1DEF) [30]. While native (pre-contrast) T1 values reflect the signal from the intracellular and extracellular compartments, post-contrast T1 primarily reflects the signal from the extracellular compartment. As a result, the difference between post and pre contrast T1 can be used to calculate the extracellular volume fraction (ECV) according to the following equation [31]:
Calculated ECV represents the fraction of myocardial volume consisting of extracellular space [31]. One study of ECV in healthy volunteers found an average ECV of 0.28 ± 0.03, but was found to vary with age with an average ECV of 0.25 ± 0.02 for patients under 40 years old and an average ECV of 0.31 ± 0.02 for patients over 60 years old [32].
T1 Mapping in HHD
ECV as calculated using CMR has been shown to correlate well with histologic collagen vascular fraction [33]. Since hypertension leads to diffuse myocardial fibrosis through deposition of collagen into the extracellular matrix, ECV would be expected to increase in hypertensive heart disease. T1 mapping could therefore be used to identify diffuse myocardial fibrosis in hypertensive patients noninvasively [31]. Initial animal studies in mice showed that increased ECV as measured using T1 mapping can be used to detect myocardial fibrosis in hypertension. Coelho-Filho et al used a murine model for hypertension induced by l-N(G)-nitroarginine methyl ester (L-NAME). Following 7 weeks of L-NAME, mice were found to have significant increase in histologic connective tissue fraction (8.5 ± 1.6% vs 2.6 ± 0.6%, p<0.001), which correlated with significant elevations in ECV as measured by CMR (0.42 ± 0.08 vs 0.25 ± 0.03) [34]. In another study, Coelho-Filho et al compared ECV in mice with untreated hypertension due to L-NAME to those with hypertension from L-NAME but treated with spironolactone. They found significant reduction in histologic connective tissue fraction (2.7 ± 0.8% vs 8.5 ± 1.6%, p <0.001) in the group treated with spironolactone, which correlated with the ECV values measured on CMR (0.25 ± 0.03 vs 0.43 ± 0.09, p<0.001). Interestingly, mice that were treated with spironolactone had similar ECV values to the placebo group (0.25 ± 0.03 vs 0.26 ± 0.03) [35].
In the first human study to demonstrate the use of ECV to detect fibrosis in HHD, Kuruvilla et al calculated ECV using T1 mapping in 20 subjects with hypertension and LVH, 23 subjects with hypertension and no LVH, and 22 control subjects. Subjects with hypertension and LVH were found to have significant increase in ECV compared to hypertensive subjects without LVH (0.29 ± 0.03 vs 0.27 ± 0.02, p <0.05) or normotensive controls (0.29 ± 0.03 vs 0.26 ± 0.02, p<0.05). In addition, they found that increased ECV correlated with reduced regional systolic function as measured using strain imaging [36].
In another study, Treibel et al also demonstrated an increased ECV in hypertensive patients with LVH. Their study cohort included 50 healthy volunteers and 40 subjects with hypertension, including 14 with LVH. Similar to Kuruvilla et al, they found no difference in ECV between control patients and hypertensive patients without LVH, but a significant difference between control subjects and patients with hypertension and LVH (0.28 ±0.03 vs 0.26 ± 0.02, p <0.001) [37].
Rodrigues et al evaluated ECV across hypertensive heart disease LV phenotypes. They examined differences in ECV in 88 patients divided into four groups: 1. Normal indexed LV mass (LVM) and LVM to volume ratio (M/V), 2. Concentric remodeling: defined as normal LVM but elevated M/V, 3. Concentric LV hypertrophy: defined as elevated LVM with normal indexed end-diastolic volume (EDV), and 4. Eccentric LV hypertrophy: defined as elevated LVM and EDV. Similar to the findings of Kuruvilla et al [36] and Treibel et al [37], they found a significant difference in ECV in hypertensive patients with LVH (both concentric and eccentric) compared to those without LVH (including those with concentric remodeling, which showed no increase in ECV). But by further subdividing the hypertensive patients by phenotype, they found that patients with eccentric LVH were associated with the highest ECV values, as well as the lowest systolic function [38].
In addition to detecting diffuse fibrosis in HHD, T1 mapping can be used to distinguish etiologies of LVH. Cardiac amyloid is associated with significantly higher ECV values than HHD, with one study reporting an average ECV of 0.47 ± 0.07 in amyloid [39]. Hinojar et al studied whether HHD could be distinguished from HCM using T1 mapping in 95 patients with HCM, 69 patients with HTN, and 23 patients with positive gene mutation but no clinical evidence of HCM. Both native T1 and ECV were found to be significantly elevated in HCM compared to HTN (septal ECV 0.31 ± 0.06 in HCM compared to 0.24 ± 0.04 in HTN, p <0.0001). Septal native T1 was found to be the most significant discriminator between HCM and HTN, and was also able to distinguish genotype positive/phenotype negative HCM patients from control patients [40].
Limitations of T1 mapping in HHD
While ECV measured using CMR has been shown to estimate diffuse myocardial fibrosis, it is important to recognize the limitations of the technique. One is that the increase in ECV between normal subjects and hypertensive patients with LVH is small: 0.29 ± 0.03 vs 0.27 ± 0.02 in Kuruvilla et al [36] and 0.28 ± 0.03 vs 0.26 ± 0.02 in Treibel et al [37]. The subtle difference between normal patients and hypertensive patients with LVH may limit the clinical applicability of T1 mapping in diagnosing diffuse myocardial fibrosis in any single patient.
Another limitation is that other pathologies beside fibrosis can cause an increase in ECV. A recent study by Lurz et al examined the presence of myocardial inflammation on ECV. In this study, 107 patients with clinically suspected inflammatory cardiomyopathy underwent endomyocardial biopsy and CMR with T1 mapping. ECV was found to be significantly higher in patients found to have inflammation on biopsy versus those without inflammation (0.37 ± 0.06% versus 0.33 ± 0.08%, p=0.02). While ECV correlated with fibrosis percentage on biopsy in patients without inflammation (r=0.72, p<0.0001), it did not correlate with fibrosis percentage when inflammation was present (r=0.24, p=0.06) [41].
Other advances in HHD imaging
3D echocardiography
Another recent advance in imaging of hypertensive heart disease has been improvements in 3D echocardiography (3DE). When first introduced, 3DE required labor intensive off-line reconstructions to calculate cardiac volumes. However, with advancements in technology, volumetric imaging can now be done in real-time [15]. The primary advantages over 2D echo are that 3DE does not rely on geometric assumptions and can avoid foreshortened views when determining volumes [15]. In a meta-analysis that examined 95 studies comparing 3DE to CMR (the gold standard for evaluation of myocardial volumes and function), 3DE showed excellent accuracy in measuring EF (difference between 3DE and CMR: −0.13%, p=0.41) though was found to underestimate LV volumes [42]. For patients with HHD, 3DE offers an inexpensive, bedside alternative to CMR for obtaining accurate measurements of LV size and function.
Speckle tracking echocardiography
Another advancement in echocardiography that is relevant to HHD is the development of speckle tracking echocardiography (STE). STE is used to measure both global and regional strain, defined as degree of displacement of a region over the cardiac cycle, through tracking acoustic markers generated by the effect of ultrasound on the myocardium [43]. STE has shown to be sensitive for the detection of subclinical disease, including hypertensive heart disease [44]. In a recent study by Ayoub et al, 60 hypertensive patients with preserved EF were compared to 30 control subjects using two-dimensional speckle tracking. They found no significant difference between the two groups in regard to EF, but the hypertensive group had significantly lower global longitudinal strain, suggesting STE can detect subclinical systolic dysfunction in hypertensive patients with preserved EF [16]. This correlates with prior studies that have shown strain abnormalities in hypertensive heart disease using myocardial tagging in CMR [45,46]. Kouzu and colleagues examined longitudinal, circumferential, and radial strain subdivided by LV geometry in patients with hypertension. They found that longitudinal strain is significantly reduced in patients with hypertrophy vs control subjects (concentric −15.1 ± 4.0%, eccentric −15.9 ± 4.4% vs control −18.9±3.3%; p<0.05), while hypertensive patients with normal LV geometry had a significant increase in radial strain compared to control subjects (53.8 ±19.4% vs 40.3 ±15.1%, p<0.05) [47]. Sun et al examined 120 HTN patients with STE and found that longitudinal strain was significantly decreased in the hypertensive patients, but circumferential strain and LV twist were increased. This suggests that circumferential strain/LV twist enhancement may be a compensatory mechanism to maintain ejection fraction in hypertensive patients in the setting of decreased longitudinal strain [48].
It has been hypothesized that the decreased regional systolic function in hypertensive patients is related to myocardial fibrosis. Kang et al examined 56 patients with untreated HTN with normal ejection fraction and compared to 20 age-matched control. All patients underwent an echocardiogram with 2D speckle tracking imaging and serum measurement of tissue inhibitor of matrix metalloproteinase (TIMP-1), which is a proteinase involved in the degradation of type I collagen. TIMP-1 has been demonstrated to be a potential marker of myocardial fibrosis, with studies showing increased serum TIMP-1 levels in hypertensive patients correlated with LV diastolic dysfunction [49]. Kang et al found a significant decrease in longitudinal strain in the hypertensive patients (−20.4 ± 3.0% vs −22.1 ± 2.2%, p=0.03). In addition, there was a significant increase in the serum level of log TIMP-1 in the hypertensive patients (3.6 ± 0.6 vs 3.0 ± 0.5, p< .001), with correlation between serum log TIMP-1 and longitudinal strain (r=0.405, p=0.15) [50].
STE has also been used to distinguish LVH etiologies. In one study, 34 patients with hypertensive LVH were compared with 56 patients with HCM, 27 professional athletes with LVH, and 12 control subjects. Patients with HCM were found to have significant decrease in global longitudinal strain (−11.1 ± 4.2 in HCM compared to −17.8 ± 3.1 in hypertensive LVH) [51].
Conclusions
Hypertensive heart disease develops in long-standing hypertension and is characterized by structural remodeling including left ventricular hypertrophy and diffuse myocardial fibrosis. Early diagnosis and treatment is essential, as regression of LVH and myocardial fibrosis have been demonstrated with treatment. Recent advances in the imaging of HHD include improvements in three-dimensional echocardiography in measuring volumes/function and the use of speckle tracking to detect subclinical systolic dysfunction in HHD and to distinguish LVH etiologies. Another important development has been the use of T1 mapping in CMR to calculate ECV and diagnose diffuse myocardial fibrosis. Several studies have demonstrated increased ECV in hypertensive patients with LVH. Future research in this area should include larger studies comparing ECV in normal subjects with HHD patients and examining prognostic implications of increased ECV. Future studies can potentially use ECV as a marker for diffuse fibrosis to both monitor disease progression in HHD and assess the success of therapeutic interventions.
References
Papers of particular interest, published recently, have been highlighted as
● Of importance
●● Of major importance
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics—2016 Update. Circulation 2016;133. [DOI] [PubMed] [Google Scholar]
- 2.Díez J, González A, López B, Querejeta R. Mechanisms of disease: pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat Clin Pract Cardiovasc Med 2005;2:209–16. [DOI] [PubMed] [Google Scholar]
- 3.Georgiopoulou VV, Kalogeropoulos AP, Raggi P, Butler J. Prevention, Diagnosis, and Treatment of Hypertensive Heart Disease. Cardiol Clin 2010;28:675–91. [DOI] [PubMed] [Google Scholar]
- 4.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic Implications of Echocardiographically Determined Left Ventricular Mass in the Framingham Heart Study. N Engl J Med 1990;322:1561–6. [DOI] [PubMed] [Google Scholar]
- 5.Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertens 2000;35:580–6. [DOI] [PubMed] [Google Scholar]
- 6.Wachtell K, Bella JN, Rokkedal J, Palmieri V, Papademetriou V, Dahlöf B, et al. Change in diastolic left ventricular filling after one year of antihypertensive treatment: The Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) Study. Circulation 2002;105:1071–6. [DOI] [PubMed] [Google Scholar]
- 7.Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, et al. Regression of Electrocardiographic Left Ventricular Hypertrophy During Antihypertensive Treatment and the Prediction of Major Cardiovascular Events. JAMA 2004;292:2343. [DOI] [PubMed] [Google Scholar]
- 8.Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens 1998;16:1031–41. [DOI] [PubMed] [Google Scholar]
- 9.Querejeta R, Varo N, López B, Larman M, Artiñano E, Etayo JC, et al. Serum carboxy-terminal propeptide of procollagen type I is a marker of myocardial fibrosis in hypertensive heart disease. Circulation 2000;101:1729–35. [DOI] [PubMed] [Google Scholar]
- 10.Mundhenke M, Schwartzkopff B, Strauer BE. Structural analysis of arteriolar and myocardial remodelling in the subendocardial region of patients with hypertensive heart disease and hypertrophic cardiomyopathy. Virchows Arch 1997;431:265–73. [DOI] [PubMed] [Google Scholar]
- 11.McLenachan JM, Dargie HJ. Ventricular arrhythmias in hypertensive left ventricular hypertrophy. Relationship to coronary artery disease, left ventricular dysfunction, and myocardial fibrosis. Am J Hypertens 1990;3:735–40. [DOI] [PubMed] [Google Scholar]
- 12.Díez J, Querejeta R, López B, González A, Larman M, Martínez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation 2002;105:2512–7. [DOI] [PubMed] [Google Scholar]
- 13.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation 2000;102:1388–93. [DOI] [PubMed] [Google Scholar]
- 14.Alfakih K, Reid S, Jones T, Sivananthan M. Assessment of ventricular function and mass by cardiac magnetic resonance imaging. Eur Radiol 2004;14:1813–22. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Mor-Avi V, Sugeng L, Nieman PS, Sahn DJ. Three-Dimensional Echocardiography. J Am Coll Cardiol 2006;48:2053–69. [DOI] [PubMed] [Google Scholar]
- 16.●Ayoub AM, Keddeas VW, Ali YA, El Okl RA. Subclinical LV Dysfunction Detection Using Speckle Tracking Echocardiography in Hypertensive Patients with Preserved LV Ejection Fraction. Clin Med Insights Cardiol 2016;10:85–90.This study demonstrates the utility of speckle tracking echocardiography in imaging of HHD, as it can be used to detect subclinical systolic function in these patients.
- 17.Mavrogeni S, Katsi V, Vartela V, Noutsias M, Markousis-Mavrogenis G, Kolovou G, et al. The emerging role of Cardiovascular Magnetic Resonance in the evaluation of hypertensive heart disease. BMC Cardiovasc Disord 2017;17:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber KT. Fibrosis and hypertensive heart disease. Curr Opin Cardiol 2000;15:264–72. [DOI] [PubMed] [Google Scholar]
- 19.Shahbaz AU, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC, Mcgee JE, et al. Fibrosis in hypertensive heart disease: molecular pathways and cardioprotective strategies. J Hypertens 2010; 28 (Suppl 1):S25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber KT. Are myocardial fibrosis and diastolic dysfunction reversible in hypertensive heart disease? Congest Heart Fail 11:322–4; quiz 325 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Ohsato K, Shimizu M, Sugihara N, Konishi K, Takeda R. Histopathological factors related to diastolic function in myocardial hypertrophy. Jpn Circ J 1992;56:325–33. [DOI] [PubMed] [Google Scholar]
- 22.Querejeta R, López B, González A, Sánchez E, Larman M, Martínez Ubago JL, et al. Increased Collagen Type I Synthesis in Patients With Heart Failure of Hypertensive Origin. Circulation 2004;110:1263–8. [DOI] [PubMed] [Google Scholar]
- 23.From AM, Maleszewski JJ, Rihal CS. Current status of endomyocardial biopsy. Mayo Clin Proc 2011;86:1095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizuno R, Fujimoto S, Saito Y, Nakamura S. Non-invasive quantitation of myocardial fibrosis using combined tissue harmonic imaging and integrated backscatter analysis in dilated cardiomyopathy. Cardiology 2007;108:11–7. [DOI] [PubMed] [Google Scholar]
- 25.Raman SV The Hypertensive Heart. J Am Coll Cardiol 2010;55:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordin S, Dancy L, Moon JC, Sado DM. Clinical applications of multiparametric CMR in left ventricular hypertrophy. Int J Cardiovasc Imaging 2018;34:577–85. [DOI] [PubMed] [Google Scholar]
- 27.Rudolph A, Abdel-Aty H, Bohl S, Boyé P, Zagrosek A, Dietz R, et al. Noninvasive Detection of Fibrosis Applying Contrast-Enhanced Cardiac Magnetic Resonance in Different Forms of Left Ventricular Hypertrophy. J Am Coll Cardiol 2009;53:284–91. [DOI] [PubMed] [Google Scholar]
- 28.Moreo A, Ambrosio G, De Chiara B, Pu M, Tran T, Mauri F, et al. Influence of myocardial fibrosis on left ventricular diastolic function: noninvasive assessment by cardiac magnetic resonance and echo. Circ Cardiovasc Imaging 2009;2:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janardhanan R, Kramer CM. Imaging in hypertensive heart disease. Expert Rev Cardiovasc Ther 2011;9:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radenkovic D, Weingärtner S, Ricketts L, Moon JC, Captur G. T1 mapping in cardiac MRI. Heart Fail Rev 2017;22:415–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.●●Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M. T1 Mapping. JACC Cardiovasc Imaging 2016;9:67–81.Comprehensive review of T1 mapping techniques and clinical applications
- 32.Neilan TG, Coelho-Filho OR, Shah RV, Abbasi SA, Heydari B, Watanabe E, et al. Myocardial Extracellular Volume Fraction From T1 Measurements in Healthy Volunteers and Mice. JACC Cardiovasc Imaging 2013;6:672–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller CA, Naish JH, Bishop P, Coutts G, Clark D, Zhao S, et al. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging 2013;6:373–83. [DOI] [PubMed] [Google Scholar]
- 34.Coelho-Filho OR, Shah RV, Mitchell R, Neilan TG, Moreno H, Simonson B, et al. Quantification of cardiomyocyte hypertrophy by cardiac magnetic resonance: implications for early cardiac remodeling. Circulation 2013;128:1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coelho-Filho OR, Shah RV, Neilan TG, Mitchell R, Moreno H, Kwong R, et al. Cardiac magnetic resonance assessment of interstitial myocardial fibrosis and cardiomyocyte hypertrophy in hypertensive mice treated with spironolactone. J Am Heart Assoc 2014;3:e000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.●●Kuruvilla S, Janardhanan R, Antkowiak P, Keeley EC, Adenaw N, Brooks J, et al. Increased extracellular volume and altered mechanics are associated with LVH in hypertensive heart disease, not hypertension alone. JACC Cardiovasc Imaging 2015; 8(2):172–180.First study to demonstrate the use of ECV to detect fibrosis in HHD patients
- 37.Treibel TA, Zemrak F, Sado DM, Banypersad SM, White SK, Maestrini V, et al. Extracellular volume quantification in isolated hypertension - changes at the detectable limits? J Cardiovasc Magn Reson 2015;17:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigues JCL, Amadu AM, Dastidar AG, Szantho GV, Lyen SM, Godsave C, et al. Comprehensive characterisation of hypertensive heart disease left ventricular phenotypes. Heart 2016;102:1671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sado DM, Flett AS, Banypersad SM, White SK, Maestrini V, Quarta G, et al. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart 2012;98:1436–41. [DOI] [PubMed] [Google Scholar]
- 40.●Hinojar R, Varma N, Child N, Goodman B, Jabbour A, Yu CY, et al. T1 Mapping in Discrimination of Hypertrophic Phenotypes: Hypertensive Heart Disease and Hypertrophic Cardiomyopathy: Findings from the International T1 Multicenter Cardiovascular Magnetic Resonance Study. Circ Cardiovasc Imaging 2015; 8:e003285.This study demonstrated that T1 mapping can be used to distinguish HHD from HCM
- 41.●Lurz JA, Luecke C, Lang D, Besler C, Rommel K-P, Klingel K, et al. CMR–Derived Extracellular Volume Fraction as a Marker for Myocardial Fibrosis. JACC Cardiovasc Imaging 2018;11:38–45.This study illustrates an important limitation of the use of T1 mapping in HHD, as ECV can be affected by myocardial inflammation
- 42.Shimada YJ, Shiota T. A Meta-Analysis and Investigation for the Source of Bias of Left Ventricular Volumes and Function by Three-Dimensional Echocardiography in Comparison With Magnetic Resonance Imaging. Am J Cardiol 2011;107:126–38. [DOI] [PubMed] [Google Scholar]
- 43.Lee J-H, Park J-H. Role of echocardiography in clinical hypertension. Clin Hypertens 2015;21:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali SI, Li Y, Adam M, Xie M. Evaluation of Left Ventricular Systolic Function and Mass in Primary Hypertensive Patients by Echocardiography. J Ultrasound Med 2018; [DOI] [PubMed]
- 45.Palmon LC, Reichek N, Yeon SB, Clark NR, Brownson D, Hoffman E, et al. Intramural myocardial shortening in hypertensive left ventricular hypertrophy with normal pump function. Circulation 1994;89:122–31. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed MI, Desai RV., Gaddam KK, Venkatesh BA, Agarwal S, Inusah S, et al. Relation of Torsion and Myocardial Strains to LV Ejection Fraction in Hypertension. JACC Cardiovasc Imaging 2012;5:273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kouzu H, Yuda S, Muranaka A, Doi T, Yamamoto H, Shimoshige S, et al. Left ventricular hypertrophy causes different changes in longitudinal, radial, and circumferential mechanics in patients with hypertension: a two-dimensional speckle tracking study. J Am Soc Echocardiogr 2011;24:192–9. [DOI] [PubMed] [Google Scholar]
- 48.Sun JP, Xu T, Yang Y, Yang XS, Shang Q, Li Y, et al. Layer-specific quantification of myocardial deformation may disclose the subclinical systolic dysfunction and the mechanism of preserved ejection fraction in patients with hypertension. Int J Cardiol 2016;219:172–6. [DOI] [PubMed] [Google Scholar]
- 49.Lindsay MM, Maxwell P, Dunn FG. TIMP-1: a marker of left ventricular diastolic dysfunction and fibrosis in hypertension. Hypertension 2002;40:136–41. [DOI] [PubMed] [Google Scholar]
- 50.Kang S-J, Lim H-S, Choi B-J, Choi S-Y, Hwang G-S, Yoon M-H, et al. Longitudinal strain and torsion assessed by two-dimensional speckle tracking correlate with the serum level of tissue inhibitor of matrix metalloproteinase-1, a marker of myocardial fibrosis, in patients with hypertension. J Am Soc Echocardiogr 2008;21:907–11. 4 [DOI] [PubMed] [Google Scholar]
- 51.Afonso L, Kondur A, Simegn M, Niraj A, Hari P, Kaur R, et al. Two-dimensional strain profiles in patients with physiological and pathological hypertrophy and preserved left ventricular systolic function: a comparative analyses. BMJ Open 2012;2:e001390. [DOI] [PMC free article] [PubMed] [Google Scholar]


