Abstract
The Parties to the Montreal Protocol are informed by three Panels of experts. One of these is the Environmental Effects Assessment Panel (EEAP), which deals with two focal issues. The first focus is the effects of UV radiation on human health, animals, plants, biogeochemistry, air quality, and materials. The second focus is on interactions between UV radiation and global climate change and how these may affect humans and the environment. When considering the effects of climate change, it has become clear that processes resulting in changes in stratospheric ozone are more complex than previously believed. As a result of this, human health and environmental issues will be longer-lasting and more regionally variable. Like the other Panels, the EEAP produces a detailed report every four years; the most recent was published as a series of seven papers in 2015 (Photochem. Photobiol. Sci., 2015, 14, 1–184). In the years in between, the EEAP produces less detailed and shorter Progress Reports of the relevant scientific findings. The most recent of these was for 2015 (Photochem. Photobiol. Sci.,2016, 15, 141–147). The present Progress Report for 2016 assesses some of the highlights and new insights with regard to the interactive nature of the direct and indirect effects of UV radiation, atmospheric processes, and climate change. The more detailed Quadrennial Assessment will be made available in 2018.
1. Ozone-climate interactions and effects on solar UV radiation at the Earth’s surface
1.1. Observations and model calculations taken together indicate that signs of recovery of the Antarctic ozone loss have now emerged for the month of September. However, variability due to natural causes, such as volcanic eruptions, precludes similar conclusions for later months when the UV radiation is higher and of greater biological relevance
Ozone depleting substances have been controlled by the Montreal Protocol. Therefore increases in Antarctic ozone1 and decreases in UV radiation are expected in response to this historic agreement. Volcanic eruptions episodically interfere with recovery. For example, in 2015, close to record high levels of UV radiation were observed at the South Pole late in the spring (Fig.1), which were partly caused by enhanced ozone losses due to the eruption of the Calbuco volcano in Chile.
Fig. 1.
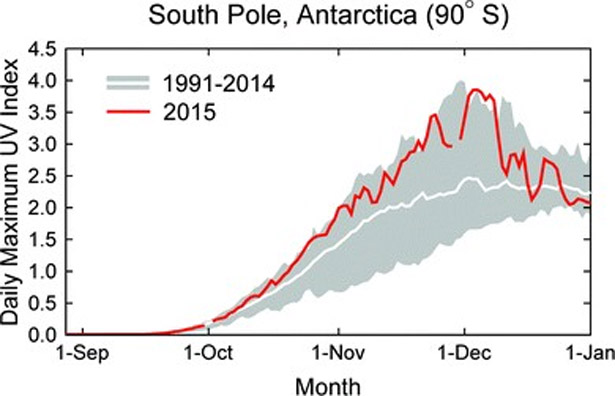
Daily maximum UV Index measured at the South Pole in 2015 (red line) compared with the average (white line) and the lowest and highest values (grey shading) of observations performed between 1990 and 2014. Measurements between the second half of October 2015 to mid-December 2015 were close to the upper limit of historical observations. These large values can be attributed to the deep ozone ‘hole’ of 2015, which was well centered over the South Pole. The figure is adapted from r ef. 2 and updated with data from November and December 2015.
1.2. As concentrations of ozone depleting substances (ODS) decrease over the next decades, greenhouse gases (GHGs) will become the dominant driver of changes in stratospheric ozone
UV radiation will decrease in the middle and high latitudes relative to the historical period 1955–1975 but the direction of change in the tropics depends on the emission scenario. Model simulations have quantified changes of ozone and UV radiation for the period of 2075–2095 relative to the historical period of 1955–1975 under different emissions scenarios.3 This study suggests that stratospheric ozone will increase substantially above its pre ozone ‘hole’ levels (also known as “super recovery”) at all latitudes, if emissions of carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) continue unabated (according to ‡RCP8.5.4) Consequently, the UV Index (UVI) would decrease substantially at all latitudes. The decreases of UVI at Northern mid-latitudes would range between ca. 5 and ca. 15%, with the largest decreases expected in the winter months, thus limiting UV radiation available for vitamin D production during winter even further (see section 2). Decreases at Southern mid-latitudes are somewhat smaller. In the tropics, the decreases would be smallest and range between 0 and 7%. These projections are greatly dependent on the emission scenario. For example, if actual emissions of CO2, CH4 and N2O could be aggressively reduced to the RCP2.6 scenario, UVI would increase by up to 5% at all latitudes, except in the spring at high latitudes. In the Arctic spring, decreases of up to 5% are predicted, while in the Antarctic spring the remaining halocarbons continue to deplete polar ozone and increase surface UV exposure by up to 20%. Note that these projections only considered changes in ozone and did not take into account changes in clouds, aerosols or surface albedo. According to Bais et al.,5 changes in UV radiation due to these factors are expected to be of similar magnitude to those related to changes in ozone.
In another study,6 simulations with a chemistry-climate model forced by three different emissions scenarios (RCP4.5, RCP6.0, and RCP8.5) suggest that total ozone columns in the tropics will be lower by the end of the 21st century compared to the 1960s in all scenarios with the largest decrease in the RCP6.0 scenario. For the RCP6.0 scenario the concomitant increase in DNA-weighted UV irradiance reaches 15% in specific tropical regions (e.g., in South America, southern Asia and over large parts of the Pacific Ocean).
1.3. There is increased confidence that stratospheric ozone depletion is a major driver of climate change in the Southern Hemisphere
For the first time, a climate model that included interactive chemistry to describe the evolution of stratospheric ozone has been used to assess the influences of the depletion of ozone in the Antarctic ozone ‘hole’ on the Southern Ocean circulation and Antarctic sea ice.7 This model predicts stronger cooling of the lower stratosphere and accelerated circumpolar westerly winds in Antarctica during November–January than models with prescribed chemistry that have been used in the past. The stronger trends in surface wind-stress predicted by this model result in larger increases of the Southern Ocean meridional overturning circulation, leading to year-round stronger ocean warming near the surface and enhanced decreases in Antarctic sea ice, which is consistent with previous studies discussed in a previous assessment.8 The results of the model have been verified by comparison with historical observations of the extent of sea ice over the 1990–2010 period.
Ozone depletion in Antarctica was shown to explain more than half of the observed long-term changes in austral subtropical precipitation between 1979 and 2013, while increasing GHGs have a weaker role.9 This finding emerged from a statistical modelling approach (maximum covariance analysis) that was used to quantify the relative contribution of different climate forcings, including ozone depletion, changes in the sea surface temperature of the equatorial Pacific, and increasing GHGs.
1.4. Stratospheric ozone depletion and increasing greenhouse gases cause changes in the tropical atmospheric circulation, resulting in a poleward shift of the boundaries of climatic zones
A modelling study has shown that the observed poleward expansion of the Hadley§ circulation is caused mainly by anthropogenic forcings, such as increasing GHGs and stratospheric ozone depletion, rather than by natural forcings.10 It was found that the effect of ozone depletion is dominant in the austral spring and summer for the southern cell, but less intense for the northern cell in the boreal spring. The continued expansion of the Hadley cell expected by the unabated increase of GHGs will be slowed down by ozone recovery. This modification of the Hadley cell will modify the boundaries of the climatic zones, leading to expansion of subtropical dry zones to higher latitudes, and affect terrestrial and aquatic ecosystems (see sections 3 and 4).
1.5. The Montreal Protocol has been beneficial not only for stratospheric ozone and surface UV radiation, but also in mitigating the adverse effects of intensifying tropical cyclones
Most ozone-depleting substances (ODS) controlled by the Montreal Protocol also are greenhouse gases and therefore the decrease in their concentrations in the atmosphere since the late 1990s11 have prevented additional warming of the sea surface, which is an important factor in determining the intensity of tropical cyclones. A modeling study12 has shown that, without the Montreal Protocol, the intensity of tropical cyclones would have been three times as large by the year 2065. Cyclone-induced damage rises rapidly with increasing cyclone intensity, and global economic savings due to implementation of the Montreal Protocol will likely be in the range of tens to hundreds of billions of US dollars.13
1.6. In the last two decades, changes in solar UV radiation in northern midlatitudes have been mainly controlled by clouds and aerosols rather than by ozone
A study of a 20-year record (1994–2014) of spectrally resolved UV irradiance in Thessaloniki, Greece,14 revealed increases in annual mean UV irradiance of 2–6% per decade. In the summer, the increases are larger (7–9% per decade) and are caused mainly by decreasing cloudiness. During the last decade of the record (since the mid-2000s) UV-B and UV-A irradiance have been stable, or slightly decreasing due mainly to effects of aerosols and clouds, which masked the effects of the very small changes in ozone. Ozone effects are mainly manifested in the short-term (year-to-year) variability of UV irradiance. These results are consistent with results for other locations discussed previously.8
1.7. Satellite-derived estimates of UV irradiance offer high spatial coverage and are increasingly used for informing the public (e.g., via cell phone applications). However, these estimates are often positively or negatively biased compared to ground-based measurements particularly in the presence of clouds, high concentrations of aerosols, and snow or ice
Estimates of UV irradiance from satellites are derived by combining measurements of reflected radiation with model calculations. Their accuracy is therefore often limited by incomplete information of the model input parameters, such as aerosols, clouds and, at some locations, surface reflectivity. Irradiance estimates are generally less accurate for UV-B than UV-A regions of the spectrum. For example, the irradiance inferred from the Ozone Monitoring Instrument (OMI) onboard NASA’s Aura satellite exceeded clear-sky ground-based measurements at Thessaloniki, Greece, by up to 14% at 305 nm and up to 10% at 310 nm. In contrast, at 324 nm and 380 nm, the OMI data underestimated the UV irradiance by less than 5%.15 These wavelength-dependent biases indicate that the spectral absorption and scattering properties of aerosols may not be correctly addressed by the satellite data processor. At the Observatoire de Haute Provence (OHP), located in a pristine mountainous region of southeast France, UV data from OMI and the Global Ozone Monitoring Experiment (GOME-2) overestimate the clear-sky noon-time UV Index (UVI) by 6% and 2%, respectively. At Saint-Denis (SDR), located on La Réunion Island in the Indian Ocean, both OMI and GOME-2 observations are biased high by 4% relative to ground-based observations. These small biases generally increase for all-sky conditions and are 9% at OHP and 11% at SDR.16 The results of the above studies are only recent examples; differences between satellite and ground-based instruments can be smaller or larger at other locations.8
Recent field experiments indicated that when soot, volcanic sand, and glacial silt are deposited on snow surfaces, they sink within minutes into the snow. For reflected radiation measured by satellite radiometers at nadir viewing directions (i.e., vertically below the satellite) the surface appears darker, but for larger viewing angles it appears brighter, almost as for natural snow.17 These discrepancies in the estimated reflectivity may affect the accuracy of satellite-derived spectral irradiance data over snow-covered regions. Ground-based measurements of UV radiation therefore continue to be valuable.
1.8. Calculations of risk-benefit thresholds from exposure to UV radiation may require revision
Risk-benefit assessments from exposures to UV radiation are usually based on the action spectra for erythema (sun-burning) and production of vitamin D.18 As has been noted in recent reports,5,19 the currently-used CIE action spectrum for pre-vitamin D3 production in human skin20 may not be correct and may also change as a function of exposure. A recent paper discussing chemical modelling of the complex reaction pathways involved in skin chemistry predicts an initial action spectrum that is similar to the CIE action spectrum, but is displaced to shorter wavelengths.21 Such a displacement would be more consistent with the observation that little vitamin D is produced at latitudes poleward of 40° in winter.22 Furthermore, this work provides evidence that the shape of the vitamin D action spectrum changes as a function of exposure to UV radiation and will become negative at wavelengths between 315 and 330 nm after exposures of only a few SED. A negative action spectrum means that vitamin D is destroyed rather than produced after absorption of photons in this wavelength range. If true, this would have important implications for people who are confined indoors behind glass windows, which transmit only UV-A, but not UV-B radiation. The study by van Dijk et al.21 also highlighted large differences in the absolute amounts of vitamin D derived by the various action spectra for vitamin D that are currently proposed in the literature.
2. Ozone, ultraviolet radiation and health: an assessment of the latest research
2.1. Exposure to UV radiation has both risks and benefits for human health
For any individual, there is likely to be an optimal level of exposure, but this is highly variable and difficult to define. There are both direct and indirect effects on health. Direct adverse effects include skin cancers, cataracts, and reactivation of some viral infections. The best defined direct benefit is the synthesis of vitamin D. Indirect effects include those resulting from changes in food quality (see section 3) and disinfection of surface waters used for drinking (see section 4).
2.2. Warmer temperatures in the future will alter how much time people spend outdoors. Greater time spent outdoors may increase exposure to UV radiation and change the balance of risks and benefits for human health
A recent Australian study showed that as the ambient temperature increased, people living in warmer climates tended to spend less time outdoors, while those living in cooler climates spent more time outdoors.23 Depending on whether effective sun protection is used, increasing time outdoors increases exposure to both UV-A and UV-B radiation. Research on the health risks of stratospheric ozone depletion has focused on UV-B radiation as the cause of DNA damage and skin cancers. New research suggests that UV-A irradiation inhibits the repair of DNA damage through a number of pathways,24–26 and fosters local invasion of tumour cells.27,28 These processes, together with immune suppression caused by both UV-A and UV-B radiation,29 are likely to enhance the development and spread of skin cancers. During the course of the 21st century, predicted changes in ambient UV radiation resulting from latitude-dependent variations in stratospheric ozone and climate-induced changes in clouds will alter the balance of risks and benefits for human health. For example, under a global warming future, the relationship between ambient UV radiation and incidence of skin cancer30 will be modified because of climate-associated changes in sun exposure behaviour.
2.3. The overall incidence of cutaneous malignant melanoma and non-melanoma skin cancer (now called keratinocyte cancer) continues to increase in most countries for which data are available, but is decreasing in several countries in younger age groups
Skin cancer is the most common cancer in many regions where the population is predominantly fair-skinned. Changes in incidence of cutaneous malignant melanoma (CMM) vary between countries or regions. Incidence has increased in all age groups in Nordic and northern European countries (for example, by over 4% per year in Denmark from 1985 to 2012), with particularly steep increases in the elderly (70+ years).31–38 In southern European countries, overall incidence of CMM has also increased,39–42 but in some regions (for example, Catalonia) is stable (30–34 year olds) or has decreased (20–29 year olds) in younger age groups.42 This pattern of increasing overall incidence but decreasing incidence in younger age groups (<20 years) is also apparent in the USA and New Zealand.43–45 In Australia, after taking account of the changing population structure toward an older population (i.e., age-standardised rates), overall incidence of CMM has decreased by 0.7% per year since 2005.45 Incidence of CMM has also decreased (by 3% per year) in Israel,46 but has increased in Iran.47 In South Africans with fair skin, incidence of CMM is high and increasing.48
Keratinocyte cancers (KCs) include basal cell carcinomas (BCC) and squamous cell carcinomas (SCC). These arise from the most common cells in the epidermis of the skin, called keratinocytes (Fig. 2). Age-standardised incidence rates of KC are continuing to rise around the world.46,49–53 Increases may be greater in women than in men.50,51,53 In some locations, such as Canada49 and northern California,50 the rates are stabilising in younger cohorts.
Fig. 2.
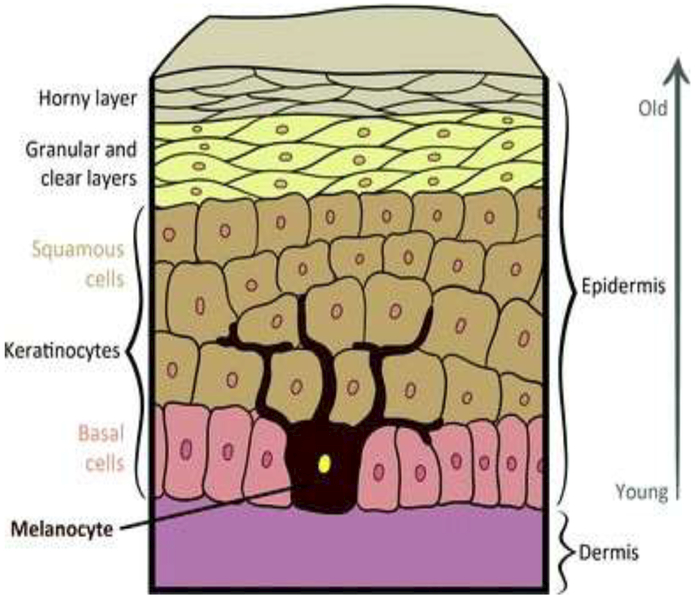
A schematic diagram of the superficial layers of human skin. Epidermal cells originate in the deeper layers and move toward the surface as they age, with new cells constantly being produced below them. The horny layer consists of dead keratinocytes that are shed and replaced from below.
Reductions in skin cancer incidence in younger age groups are probably due to a combination of effective sun protection programs and a more indoors lifestyle. However, the influence of increasing numbers of darker skinned migrants, who are at lower risk, to countries with high skin cancer incidence, cannot be discounted.42 Persistent increasing incidence in older age groups likely reflects high sun exposure in earlier life. It is concerning that the new data available for northern Europe show that incidence of CMM and KC in all age groups is continuing to increase.
2.4. Due to its high incidence, skin cancer and particularly keratinocyte cancer, result in a considerable economic burden
The lifetime cost of the 15 000 new cases of skin cancer diagnosed in New South Wales (NSW), Australia, in 2010 was estimated to be AUD 536 million (ca USD 400 million at current exchange rates) or AUD 3514 per incident case (ca USD 2500); direct costs for management of the skin cancer accounted for 72% of this total.54 Although the cost per CMM was much higher than the cost per KC (AUD 44 279 vs. 2459; ca. USD 34 000 vs. 1800), KC accounted for 68% of total costs due to its higher incidence. In South Africa the cost per lesion was much lower than in Australia (ca USD 150) but skin cancer still constituted a significant economic burden, with an estimated annual cost of USD 15.7 million.55 Costs are also high in countries where skin cancer incidence is lower. In the USA, the average number of adults treated for skin cancer was 4.9 million in 2007–2011 (an increase from 3.4 million in 2002–2006), at an annual total cost of USD 8.1 billion (up from 3.6 billion in 2002–2006). This increase by 126% is much greater than the increase of 25% seen for all other cancers over the same time period.56
Models suggest that investment in skin cancer prevention campaigns, particularly in areas where there is an extremely high incidence, have an economic benefit. An analysis of the NSW situation found a cost-to-benefit ratio of 3.85, indicating that for every $1 invested in prevention there would be an economic return of $3.85.57
2.5. Skin cancer is becoming recognised by a range of countries as an occupational disease. Implications include a responsibility by workplaces to provide adequate sun protection and the possibility that workers can claim compensation for their skin cancer
In a pooled analysis of data from Brazil and Italy, occupational sun exposure was associated with a marked increase in risk of CMM.58 Other studies show a link with KCs but not CMM.59 Outdoor workers are typically exposed to a dose of UV radiation that is 2–3 times higher than that of indoor workers who spend less time outdoors. There is an associated increase in KCs; for example, in one study, outdoor workers had a 43% higher risk of BCC and a two-fold higher risk of SCC than the general population.60 In a study in Northern Greece, farmers not only developed more BCC than workers in other occupations, but these occurred at a younger age and were 6 times more likely to be of an aggressive subtype.61 Several studies have shown increased incidence of CMM in airline pilots and cabin crew. In a recent meta-analysis of these studies, there was a two-fold increased risk among pilots.62 However, the similar increased risk among cabin crew suggests that this is unlikely to be an effect of UV radiation entering the cockpit, and other explanations, such as travel to sunny locations, need to be considered.
2.6. Sunscreen provides effective protection against sunburn and may decrease UV-induced skin cancers
Research shows that sunscreen provides protection from DNA damage and sunburn following exposure to UV radiation (reviewed in ref. 63), see Fig 3. This suggests that sunscreens should protect against skin cancer. Regular use of sunscreen was associated with a lower number of nevi (moles), a marker of melanoma risk, in children in one study from Catalonia, Spain,64 but a higher prevalence of multiple nevi (>50) was found in adults in a large study from Finland.65 The greater number of nevi in adults is likely due to increased sunburns and sunbathing vacations among sunscreen users, as shown in the Norwegian Women and Cancer Study.66 However, among sunscreen users, those who used a sunscreen with a Sun Protection Factor (SPF) of 15 or higher at least once were less likely to develop CMM than those consistently using sunscreen with an SPF of <15. The study reported that incidence of CMM could be reduced by 18% with regular use of sunscreen of SPF ≥15 by women aged 40–75 years.66 Indeed, the only randomised trial testing sunscreen use for the prevention of skin cancer showed that daily sunscreen use reduced the risk of SCC and melanoma.67,68 On the basis of the results from this study, it was estimated that regular use of sunscreen in Australia could prevent around 9% of SCC (n = 14 190 tumours in 2008) and 14% of CMM (n = 1730 tumours).69 However, a recent systematic review highlighted some limitations of this trial and observed that there was, as yet, insufficient high quality evidence to conclusively show that use of sunscreen prevented skin cancers.70
Fig. 3.
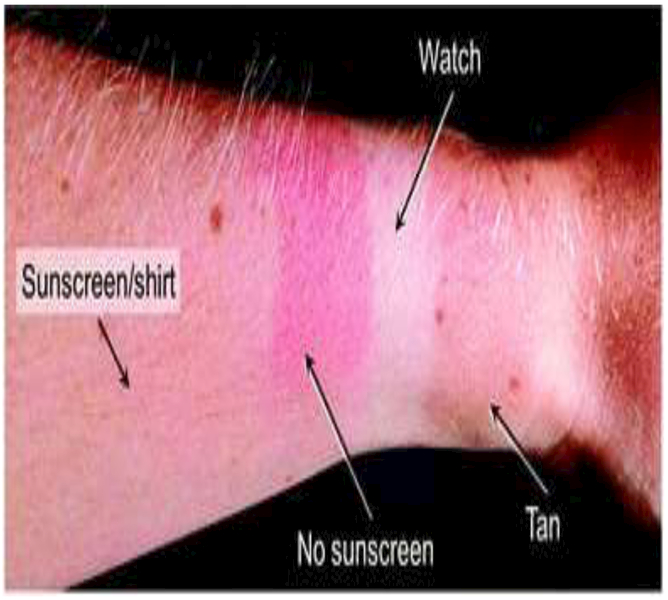
The figure shows the complete protection afforded by a watch strap, incomplete protection on the arm from a combination of clothing and sunscreen, sunburn where a patch of skin was exposed by movement of clothing and not covered with sunscreen, tanning on the unprotected skin of the hand.
2.7. Despite strong public health programs providing guidance about sun protection in many countries and research showing high levels of knowledge and a positive attitude toward sun protection, compliance remains low, and risky sun exposure behaviour and a preference for a tan are common
Teenagers are particularly resistant to messages to protect their skin against the sun. Qualitative research suggests that the desire for a tan outweighs concerns about future risks of photoageing and skin cancer.71 In Hungary, 74% of 12–19 year olds had experienced at least one serious sunburn, 5% purposely sunbathed daily, and 10% did not use any form of sun protection.72 In Ireland, which has the highest incidence of CMM in Europe, nearly 50% of a sample of Cork university students reported deliberate tanning in the previous summer.73 Parents use sun protection measures more commonly for their children than for themselves.74 A systematic review of the evidence showed that a high proportion of people diagnosed with CMM reported subsequent sunbathing (up to two-thirds at least once since diagnosis), sunburns (60% at least once in a 3-year period) and indoor tanning (up to a quarter of survivors) and did not practice skin self-examination.75 In children of CMM survivors, 28% had been sunburnt at least once in the previous 6 months.76 Sun exposure in childhood may be particularly important to later life risk of CMM and BCC. Health gains through decreasing incidence of these cancers in younger age groups that are currently being seen in many countries (see section 2.3) are likely to be reversed without continued investment in sun protection programs targeting sun exposure in childhood, adolescence, and high-risk groups.
2.8. Immunosuppression following solid organ or stem cell transplantation greatly increases the risk of all forms of skin cancer, particularly squamous cell carcinoma
There was a nearly 8-fold increase in KC and a two-fold increase in invasive CMM following heart transplantation.77 CMM and lip cancers were more common in recipients of hematopoietic stem cell transplantations compared to the general population.78,79 The location of the skin cancers points to UV radiation as the primary cause, coupled with drug-induced post-transplantation immunosuppression80 and effects on repair of DNA damage.81
2.9. UV-induced immune suppression has both positive and negative consequences for a range of diseases, including autoimmune disease and reactivation of viruses
Exposure to UV radiation results in suppression of immune responses that may have beneficial effects for disorders such as for autoimmune disease (e.g., multiple sclerosis) and allergy, but cause harm through allowing the development of skin cancer and reactivation of viral infections. A recent study from Perth, Western Australia, reported that the number of cases of shingles (caused by reactivation of herpes zoster) notified to the register of infectious diseases increased with increasing ambient levels of UV radiation.82 This confirms similar findings published previously, from Poland,83 Korea84 and Taiwan.85 Another human herpes virus, HHV8, is a necessary, but not sufficient cause of Kaposi sarcoma. In a cohort of American male veterans infected with HIV (prior to the availability of antiretroviral therapy) the risk of Kaposi sarcoma was increased in men with a diagnosis of KC (a marker of high exposure to UV radiation) and in those living at locations with high ambient UV radiation.86 The net benefit or harm of UV-induced immune suppression is not yet clear; further studies, particularly around the size of any benefits for autoimmune and allergic diseases, will better define the balance of risks and benefits.
2.10. Exposure of the eye to sunlight has both adverse and beneficial effects on the eye
A gradient of increasing incidence of conjunctival melanomas with closer location to the Equator, and the presence of UV-signature mutations in these tumours, strongly suggest that UV radiation is a major cause.87 Melanomas of the eye are rare and conjunctival melanoma accounts for only 5% of all ocular melanomas. The more common uveal melanoma that involves structures deeper in the eye is unlikely to be directly UV-induced.88
Cataracts are the leading cause of blindness worldwide. In developed countries cataract surgery is widely accessible so that cataract-related vision loss is uncommon.89 Nevertheless, socio-economic and, to some extent, geographical (e.g., urban vs. rural) differences in access to effective surgery can lead to disparities in cataract-associated loss of vision.89 Cataracts are a major contributor to vision loss in Africa.48 Exposure to UV radiation is a major cause of cataracts, especially cortical and posterior sub-capsular cataracts. In a recent study, there was a much higher prevalence of cataracts, particularly cortical cataracts, and with a younger age of onset, in a high altitude (higher UV-B radiation), compared to a low altitude, region of China.90 Furthermore, there was a positive correlation between cataract disability-adjusted life years and levels of ambient erythemal (sunburning) UV radiation in China.91
Myopia (short-sightedness) affects over 80% of young adults in many East and Southeast Asian countries. In other countries there has been a rapid increase in the prevalence of the condition, with around half of young adults in the USA and Europe now affected.92 Several studies have found that children who spend more time outdoors have a lower risk of developing myopia. In research recently reported, two large trials in China involving primary school children (aged 6–11 years) showed that interventions over 1–3 years to increase the time spent outdoors while at school were associated with a significant reduction in the incidence of myopia.93,94 It is not yet clear what element of “time outdoors” provides the protective effect. Several studies show an increased risk of myopia in association with vitamin D deficiency.95,96 Alternatively, exposure to UV radiation or the shorter wavelengths (blue) of visible light (at the much higher level experienced outdoors compared to indoors) may protect against the development of myopia by slowing the axial growth of the eye.97 Childhood exposure may be particularly important for both the risks and benefits; health messages should encourage children to have regular time outdoors, but also protect the eyes from high levels of UV radiation using hats, shade and sunglasses.
2.11. Exposure to UV radiation may have benefits through both vitamin D and non-vitamin D pathways, but any benefits will need to be balanced against the known risks
The commonly accepted marker of vitamin D status is the concentration of 25-hydroxyvitamin D (25(OH)D) in serum or plasma. In 2011, after a comprehensive systematic review of the literature, the United States Institute of Medicine concluded that a 25(OH)D concentration of 50 nmol L−1 is sufficient to optimise the bone health of most people. They further concluded that there was insufficient evidence of a causal association between low 25(OH)D concentration and non-bone health outcomes.98 While some groups argue for a higher cut-off for sufficient concentrations of 25(OH)D,99 a recent study showed that a 25(OH)D concentration of ca. 30 nmol L−1 was suffcient to optimise bone mineral density and a range of markers of muscle strength and function in middle-aged women.100
Recent exposure to UV radiation is commonly a major determinant of 25(OH)D concentration,101 although a recent systematic review showed that, in regions with negligible exposure to UV radiation, it is possible for adults to maintain 25(OH)D levels >50 nmol L−1 for several months. The authors speculated that this could have been due to preceding exposure to UV radiation and storage of vitamin D and then delayed release during periods of low ambient UV radiation.102 Nevertheless, while the serum or plasma 25(OH)D concentration is a marker of vitamin D status, in many regions it is equally a marker of recent exposure to UV radiation. To separate out a specific causal effect of vitamin D on a health outcome, vitamin D supplementation studies are required.
New evidence suggests a possible causal association between high vitamin D status and reduced risk and/or severity of asthma. Low maternal 25(OH)D concentration was associated with an increased risk of wheeze in the offspring103 and in a meta-analysis, low concentrations of 25(OH)D in early childhood were associated with an increased risk of persistent asthma (comparing lowest vs. highest category reported in each study)104 A meta-analysis found that vitamin D supplementation reduced the rate of asthma exacerbations requiring hospitalisation or treatment with systemic corticosteroids.105 These results indicate a specific beneficial effect of vitamin D, but do not provide guidance about the optimal concentration of 25(OH)D to reduce risk or severity of asthma.
Exposure to the sun may have effects that are not mediated by vitamin D106 A recent study showed that irradiation with UV-A reduced blood pressure temporarily, possibly through release of nitric oxide stores in the skin (reviewed in ref.107). In a study in southern Sweden, adults reporting a habit of intentional sun exposure had a lower risk of cardiovascular disease (CVD) and non-cancer/non-CVD death than those who avoided sun exposure.108 Compared to the highest sun exposure group, life expectancy in sun-avoiders was reduced by 0.6–2.1 years; avoidance of sun exposure was a risk factor for death of similar magnitude to smoking in this study.108Whether this association can be attributed to vitamin D, other UV-induced pathways, or differences in unmeasured lifestyle factors (e.g., exercise) between people with high and low sun exposure, cannot be determined.
The weight of risk vs. benefit for sun exposure depends on the size of the effect and the proportion that can be attributed to low/high exposure to UV radiation as well as the total burden of UV-related health outcomes. While there is some confidence in the burden of disease that can be attributed to overexposure to UV radiation,69 the range of diseases caused by low sun exposure and the size of any potential risks are unclear.
2.12. The prevalence of vitamin D deficiency varies around the world, with some evidence that it is related to latitude
It is difficult to compare the prevalence of vitamin D deficiency between countries or over time due to the historical inaccuracy and imprecision in the measurement of concentrations of 25(OH)D in blood. The development of standardised protocols and rigorous quality assurance schemes are now improving measurement and these have been used in a number of national health surveys (see Fig. 4). The overall prevalence of vitamin D deficiency (<50 nmol L−1) across a range of European countries was 40%, with 13% moderately to severely vitamin D-deficient (<30 nmol L−1).109
Fig. 4.
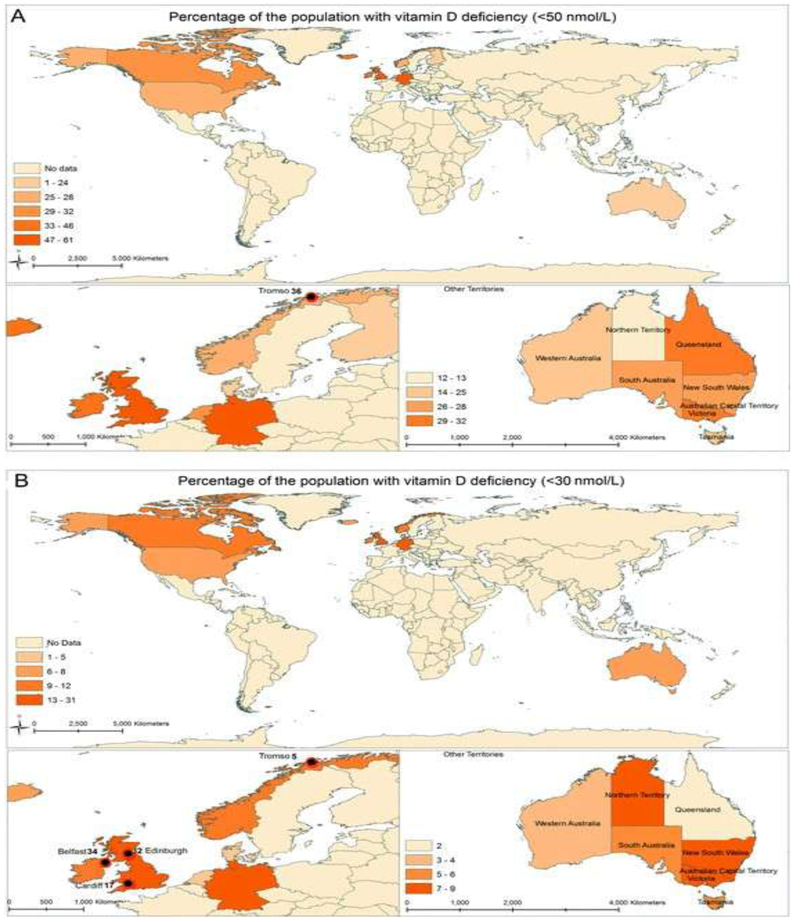
Distribution map of the proportion of population who have A), vitamin D deficiency (<50 nmol L−1) and B), severe vitamin D deficiency (<30 nmol L−1), with restriction to population-based samples and a vitamin D assay that is standardised to the Vitamin D Standardisation Program.
In the United States, standardised concentrations of 25(OH)D from the National Health and Nutrition Surveys (NHANES) show no change in the mean value from 1998 to 2006 but an increase of 5 nmol L−1 from 2007 to 2010 which is partly due to vitamin D supplementation. The prevalence of vitamin D deficiency (<50 nmol L−1) was 30% from 1988–1994 and 26% in 2009–2010. Less than 7% had moderate to severe vitamin D deficiency.110 In the Australian National Health Survey, 24% of Australian adults had 25(OH)D <50 nmol L−1 and 7% <30 nmol L−1.111 There was some evidence of a latitude gradient, although this is likely to be somewhat masked by more vitamin D supplementation in states with lower ambient UV radiation (10% in the most southern state compared with 2% in the most northerly state).111
Studies from other areas have not used standardised measurements and thus need to be interpreted with caution. Nevertheless, there is evidence of widespread deficiency in many parts of the world.112 Dark-skinned migrants to developed countries commonly have a high prevalence of vitamin D deficiency.111,113
2.13. Maximising vitamin D status while minimising DNA damage during sun exposure can be achieved by several exposures per week to low doses of UV radiation
A study from New Zealand showed that the greatest increase in levels of 25(OH)D in serum over 8 weeks occurred with sun exposure (measured using personal UV sensors) equivalent to less than half of a sunburning dose to the whole body each week.114 Higher exposures resulted in only small additional increases. However, a recent study showed that there was a high level of individual variability in the achieved 25(OH)D concentration for a given dose of UV irradiation.115 In Manchester, UK (53°North), repeated doses of UV radiation sufficient to increase 25(OH)D levels from 36 to 54 nmol L−1 in people with fair skin did result in DNA damage to skin cells, but this was at least partially cleared by natural repair processes by 24 hours after the last exposure. The level of DNA damage at the completion of the 6-week course of irradiation in the Manchester study was similar to that caused by a single exposure, suggesting DNA damage does not accumulate following exposure to repeated non-sunburning doses of UV radiation.116 Recent research shows that there are many mutations in cancer-related genes in the sun-exposed skin of older adults – a comparable mutation load to that of many internal cancers (e.g., breast, uterus). However, these exist largely without causing clinical disease, due to efficient DNA repair and containment processes.117
Concentrations of 25(OH)D are increased in proportion to the amount of skin exposed to the sun,101 particularly at lower levels of UV-B irradiation.118 These results suggest that regular, low exposures to UV radiation can increase/maintain 25(OH)D levels while minimising DNA damage. While sunscreens can be designed that maximise UV-B transmission for vitamin D production and provide excellent protection from UV-A irradiation,119 greater DNA damage also will result from the increased UV-B transmission so use of these sunscreens is not currently advised.
3. Potential effects of current and future changes in stratospheric ozone, UV radiation and accelerated climate change on terrestrial ecosystems
3.1. Large ozone-driven changes in climate in the Southern Hemisphere have occurred over the past 3–4 decades and these climate changes are continuing to influence ecosystems in a variety of ways
Ozone depletion has played a major role in driving changes in temperature across certain parts of Antarctica and has also been implicated in changes in precipitation patterns across the Southern Hemisphere and into Asia120,121 (see also section 1). Consistent with ozone-driven changes in temperature, plant growth rates and carbon storage in moss beds have increased over the past several decades at various locations on the Antarctic Peninsula and nearby islands.122 The seasonality of precipitation along with the magnitude of diurnal changes in temperature are the dominant factors influencing the distribution of high elevation woodlands of Polylepis tarapacana (Rosaceae), a species of high conservation value in the South American Altiplano. Models predict that, by the end of this century, there will be significant (up to 56%) reduction in the potential habitat of this species due to increases in aridity. These findings add to the increasing evidence indicating pervasive and far-reaching effects of ozone-driven climate change on terrestrial ecosystems.
3.2. The increasing pressures of multiple environmental stress factors together with changes in plant exposure to UV radiation continue to highlight the potential interactions of the different stressors
The balance between initiation of severe stress reactions and stimulation of normal regulatory pathways for growth and development has significant implications for plant growth and plant yield.121 Severe stress may occur from plant exposure to increased UV radiation together with environmental conditions such as extremes of temperature and drought. Although mechanisms differ, generation of potentially damaging reactive oxygen species (ROS, viz., hydrogen peroxide, hydroxyl radicals, superoxide radicals, and singlet oxygen) is a shared consequence of all these stressors including ground level ozone. With increased ROS, there may be increased damage and reduced plant vigour.123-126 The effectiveness of antioxidant defense systems that aid in removing ROS is dependent not only on the level of stress but also on crop cultivar.127 Likewise, selection of certain crop breeding lines with greater antioxidant capacity128 can improve the tolerance of agricultural crops to UV radiation, especially in areas of high UV radiation and other stressful conditions.
3.3. Research in the ways in which plants sense and respond to UV radiation using multiple molecular mechanisms, has increased our fundamental understanding of the impacts of UV radiation and other stressors
Studies of plant perception of UV radiation show that, although many plant regulatory responses to UV-B radiation can be effected through the specific UV-B photoreceptor (UVR8), other photoreceptors are also likely to be involved in the wide range of plant response to multiple environmental conditions.125,129,130 Exposure to UV-B radiation causes changes that are mediated by UVR8 and which affect plant growth and development. These processes include metabolic changes, regulation of plant development, and plant acclimation and stress tolerance to UV radiation131-134 and responses to other stressors such as plant pathogens.135 The clarification of the molecular mechanisms by which plants perceive and respond to UV radiation enhances our ability to increase crop yield and control pests through management and breeding practices.136
3.4. Certain male and female plants of the same species respond differently to elevated UV-B radiation with potential consequences for changes in population composition and diversity
The response of many dioecious (male and female reproductive structures that occur in different individuals) trees and shrubs to enhanced UV-B radiation depends on whether they are male or female. In some species males are more sensitive, in others sensitivity is more pronounced in females. For example, in white mulberry, Morus alba, female plants show more negative effects in their morphology, physiology, biomass allocation and leaf structure than do males under enhanced UV-B radiation. This is likely due to the greater requirement of female plants for resources for reproductive development.137 Enhanced UV-B radiation tends to decrease biomass and leaf thickness in male plants of the dark leaved willow (Salix myrsinifolia), which, along with the UV-induced increase in leaf phenolics in females, suggests that the females have greater tolerance to UV-B radiation compared to males.138
In a field trial with European aspen, Populus tremula, the female plants exhibited higher emission rates of volatile compounds (e.g., isoprene), an indication of stress response. In addition, there was greater compositional variability in their emissions under UV-B radiation than that in male plants.139 UV-B radiation also increases production of tremulacin, an herbivore defense-compound that is abundant in aspen seedlings.140 Concentration of this compound was increased by 4 and 11% under ambient and elevated temperature, respectively, but only in female seedlings, which grew taller than males.141 This change in chemistry may increase fitness of females to resist herbivores when exposed to enhanced levels of UV-B radiation. However, in some instances, male plants show a greater tolerance to increased UV-B radiation than do females, as evidenced in another poplar, Populus cathayana.142
The varying tolerance to UV-B radiation by male and female plants has implications for their population distribution and competitiveness with other species, which may alter plant diversity depending on whether male or female plants show greater or lesser tolerance to high levels of UV-B radiation.
3.5. The distribution of plants is being altered by climate change such that the plants are being exposed to a unique set of environmental conditions together with UV radiation
Studies examining the response of plants along natural gradients of UV radiation are providing new insights into the mechanisms by which migrating species may acclimatise or evolve to these changes in UV radiation.
Many plant species are spreading to higher elevations and/or latitudes in response to climate change and this movement is expected to continue well into the future.143 Some evidence suggests that introduced species display higher migration potentials than native species, at least along elevation gradients.144 Whether there are differences in UV acclimation or adaptation potentials between native vs. introduced species is unknown, although a study by Wang et al.145 indicates differences in UV sensitivity between introduced rather than native populations of Chinese tallow tree (Triadica sebifera). However, in this case, introduced populations were more sensitive to UV-B radiation than native populations.
It is generally accepted that plant species or populations which have evolved in environments differing in exposure to UV radiation (e.g., high vs. low elevations; high vs. low latitudes) often exhibit differential sensitivities to UV radiation, although the mechanisms responsible for these differences are not fully understood. Certain studies have shown that UV-screening within a species varies along elevation gradients (e.g., ref. 146). A study by Wang et al.147 on high vs. low elevation populations and species of rockcress (Arabidopsis) further suggests that this differential sensitivity to UV radiation is due, in part, to population differences in DNA damage and repair. Interactive effects of factors related to climate change may influence tolerance to UV radiation along elevation gradients, since increasing temperature can also reduce the levels of UV-absorbing compounds (e.g., ref.148).
3.6. Many plants can sense and respond to rapid (minutes to hours) fluctuations in UV radiation in ways that enhance their levels of UV protection
These changes have implications for the timing of plant defense and the use of UV radiation to improve food plant quality and vigour in controlled environments. In nature, plants experience substantial variation in exposure to UV radiation over time scales ranging from seconds to days as a result of seasonal and diurnal rhythms in solar elevation,5 shifting cloud cover,149 and gaps in plant canopies.150 Whereas considerable attention has been given to understanding plant responses to changes in average UV radiation conditions that occur as a result of ozone depletion (ref.121,151 and references therein), far less is known about responses of plants to rapid fluctuations under solar UV radiation.
There is increasing evidence that plants can adjust their UV screening levels (Fig. 5A) over a growing season (e.g., ref. 152), from one day to the next,153 over the course of a single day,154 and in response to rapid changes in cloud cover.155 A recent survey of 37 species growing in different locations has shown that the diurnal adjustment in UV screening is widespread among plants, although it varies substantially among species (Fig. 5B).156
Fig. 5.
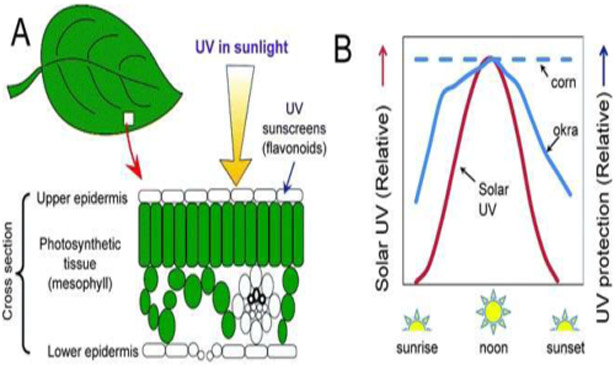
The location of UV sunscreens in plant leaves and the diurnal changes in UV sunscreen protection. A. shows a cross-section of a leaf of a typical broad-leaved plant illustrating the arrangement of major cells and tissues and the location of UV sunscreens (flavonoid pigments) in epidermal tissue. B. shows diurnal changes in solar UV radiation reaching the ground under a typical clear sky and the response of a plant species that adjusts its UV protection over the day (okra) and one that does not (corn).
The changes in UV screening over the day are rapid (within minutes), reversible, and are linked to changes in the levels and types of UV sunscreens (flavonoids and related phenolics155). How plants achieve these rapid adjustments in UV radiation protection is not entirely clear, since the induction and accumulation of UV-absorbing compounds and resultant changes in UV screening typically occur over time frames (days) that are considerably longer than these rapid changes.157 Plants are known to exhibit diurnal changes in gene expression, metabolites and the activities of key enzymes involved in the synthesis of UV-absorbing compounds158-160 but whether a linkage exists between these molecular changes and rapid adjustments in UV-screening is unknown.
The finding that plants can adjust their UV sunscreens over the day has important implications for the timing of plant responses to other abiotic and biotic stresses (e.g., drought and herbivory) that can vary in severity over the course of a day, (e.g., ref. 161), and which often employ similar suites of secondary compounds for both defense and UV radiation protection.136 The existence of a temporally dynamic UV protection system in plants also has practical consequences for how plant UV radiation research is conducted162 and the culturing of plants in controlled environments when UV-B (and UV-A) radiation from artificial sources is employed to enhance food plant quality and vigour.163,164
3.7. The combination of UV radiation and constraints from climate change, such as drought and increasing temperatures, have the potential to change crop yield and food quality
Increasing concentrations of carbon dioxide (CO2) and high temperatures have an accelerating effect on ripening of some crops, which can result in an imbalance between certain plant pigments (anthocyanins) and sugar content, leading to a reduction in anthocyanins.165 In grape berries, where anthocyanins confer colour, taste, and also function as antioxidants and in UV-protection,166 there are indications that UV radiation stimulates production of anthocyanin, which improves the anthocyanin to sugar ratios.165 The extent of these effects will likely be variable and dependent on location, type of plant crop, capacity for acclimation, duration of the stress conditions, and the influence of the interacting factors with ambient levels of solar UV radiation.157,167-170
3.8. Effects of increasing carbon dioxide may ameliorate potentially negative combined effects of UV-B radiation and drought
A recent study found that elevated levels of CO2 could have a beneficial effect on response of plants to drought.170.Carbon dioxide and water vapour exchange occurs through plant openings, the stomata, and studies find that UV-B radiation causes stomata to close (e.g., ref. 171-173). However, several studies also show that UV-B radiation stimulates the opening of stomata, (e.g., ref. 174 and 175). At the same time, an interactive effect can occur between drought and UV-B radiation (e.g., ref. 176), while elevated CO2 may ameliorate observed negative effects from both UV-B radiation and drought.177 In contrast, UV-B radiation may counteract the accelerating effect of carbon dioxide on ripening of crops such as grape berries,178 which, for certain crop quality traits, is seen as a positive effect of UV-B radiation.
Simultaneous, naturally occurring environmental factors increase the challenge of reliably predicting the overall impact of changes by UV-B radiation on natural ecosystems or agricultural, horticultural, or silvicultural productivity.
4. Interactive effects of UV radiation and climate change on aquatic ecosystems
New data on the effects of radiation on aquatic ecosystems and how these systems respond to extreme events demonstrate the important role of UV radiation for food security and ecosystem services, including altering water quality, fishery productivity, effects of contaminants such as microplastics and synthetic sunscreens, and the potential for solar disinfection of parasites and pathogens.
4.1. Increases in terrestrial dissolved organic matter are decreasing exposure to UV radiation in many aquatic ecosystems. This widespread phenomenon known as “browning” is orders of magnitude more important in regulating exposure to UV radiation than are changes in stratospheric ozone, and is altering the structure and function of inland and coastal aquatic ecosystems in fundamental ways
Browning has reduced the UV transparency of many inland waters in North America and Europe because the dissolved organic matter (DOM) selectively absorbs shorter UV wavelengths of sunlight (Fig. 6). Browning is caused primarily by recovery from anthropogenic acidification related to clean air legislation in the 1990s combined with increases in annual precipitation related to climate change. Recovery from acid deposition increases the solubility of DOM in soil by reducing the ionic strength of solutions, thus reducing the coagulation of DOM.180 The implications for UV exposure have now been demonstrated using a unique data set on browning in two lakes in northeastern Pennsylvania, USA, where the depth to which 1% of subsurface UV radiation penetrates has decreased by as much as five-fold (from over 10 m to about 2 m, Fig. 7),181 far exceeding the effects of ozone depletion. In addition to reducing UV transparency of inland and coastal marine waters, browning alters carbon cycling (see section 5), decreases water transparency to visible light, and is associated with increases in stratification that deprive bottom water layers of oxygen and make them a poor habitat for fish and other aquatic organisms.181 Effects of browning on fish and their zooplankton food can be negative,181-183 neutral184 or positive182,185 depending on the depth and initial DOM concentration of the system.181 Thus, browning is an important consideration for commercial fisheries. While reduction in exposure to UV radiation is not the only effect of browning, experimental evidence suggests that this reduction is likely to play an important role in the spatial and temporal distribution of zooplankton, which are the key link in aquatic systems between phytoplankton and fish (see sections 4.4 to 4.7). Further insights into how other browning-related effects on aquatic ecosystems (e.g., acidity, visible radiation, nutrients) alter food web and ecosystem responses to UV radiation will be enhanced where research uses a multiple stressor approach rather than studying single stresses.
Fig. 6.
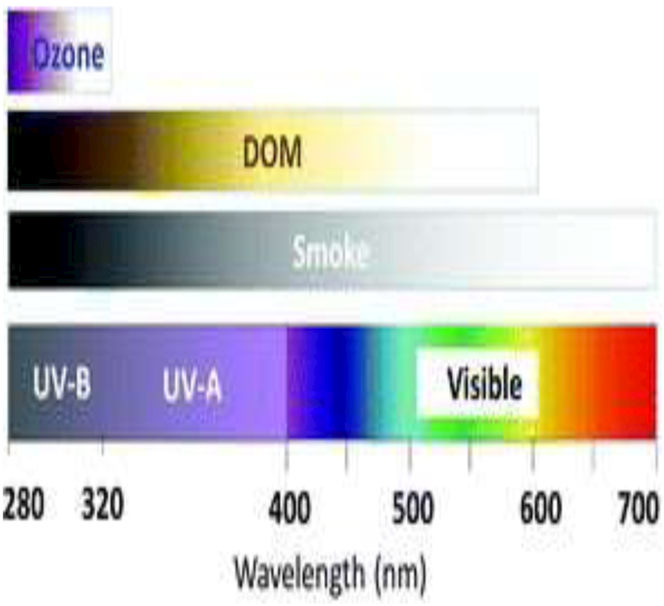
Similar to ozone in the atmosphere, dissolved organic matter (DOM) in aquatic ecosystems selectively absorbs UV radiation. The selectivity of absorption by DOM is not as strong as that of ozone, but stronger than that by smoke from wildfires. Adapted from Williamson et al. 2016.179
Fig. 7.
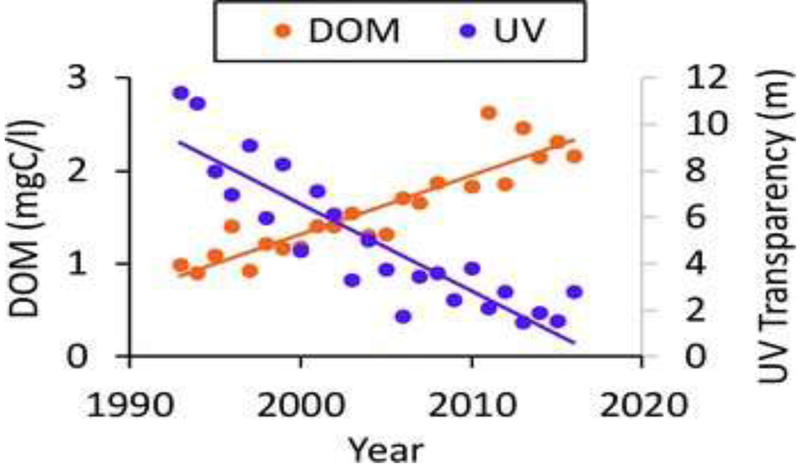
Increases in dissolved organic matter (DOM) and decreases in UV transparency (depth to which 1% of subsurface 320 nm UV penetrates) related to browning in Lake Giles, Pennsylvania, USA, in recent decades. Adapted from Williamson et al. 2015.181
Recent evidence shows that browning is now a widespread phenomenon in lakes and near-shore marine environments. Browning in high latitude lakes is linked to increasing vegetation, longer growing season, and thawing of permafrost that is itself generating many new lake basins and wetlands.186,187 Large subtropical and tropical rivers can double their release of DOM into coastal oceans during rainy periods.188-190 The effects on UV penetration are, however, restricted primarily to near-shore191 waters and quickly decline with increasing salinity and degradation of DOM offshore.192 In contrast, Antarctic coastal waters that receive only limited quantities of river discharge and associated terrestrial DOM inputs are among the most transparent coastal marine ecosystems.193 The lack of data on UV radiation in aquatic ecosystems that are undergoing browning is a key knowledge gap. If this gap can be filled, it would help clarify the role of UV radiation in these changing conditions in aquatic ecosystems. Also, the implications for water quality, fishery productivity, and the potential for solar disinfection of parasites and pathogens important for food security and human health would become clearer.
4.2. Extreme weather alters exposure to UV radiation in aquatic ecosystems
The Earth is becoming warmer and wetter, and both temperature and precipitation components of climate change can alter the UV transparency of inland waters.179 Drought can increase water transparency (see section 5.4), while sustained heavy precipitation or extreme storm events can reduce water transparency to UV radiation and visible light (Fig. 8). In California, patterns of increasing drought have been associated with increases in the UV transparency of Lake Tahoe as well as increases in the severity and frequency of wildfires.179 Smoke from wildfires can reduce incident UV radiation by 8–10%,194 or more, and reduce the ratio of UV radiation : visible light (PAR, photosynthetically active radiation, 400–700 nm) by almost half (Fig. 9).179 This stimulates a shallower distribution of zooplankton grazers in highly transparent lakes influenced by smoke, with important consequences for aquatic food webs and ecosystem services (see sections 4.7 to 4.12).
Fig. 8.
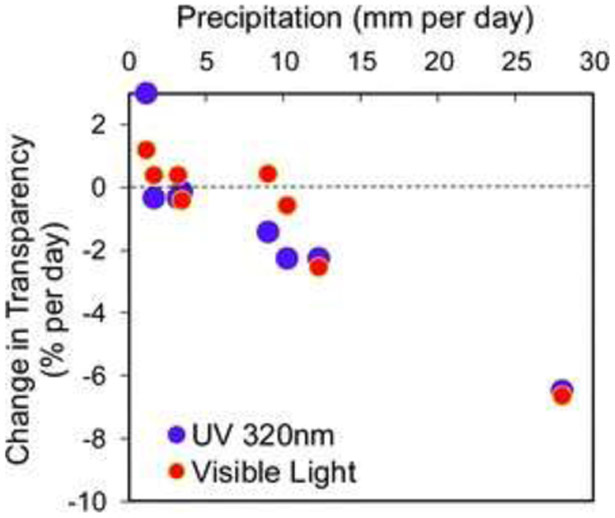
Water transparency to UV radiation and visible light decreases substantially following storm event periods with higher precipitation, but increases following periods of low precipitation during a similar time of year. Data are from Lake Giles, Pennsylvania, USA. Adapted from Williamson et al. 2016.179
Fig. 9.
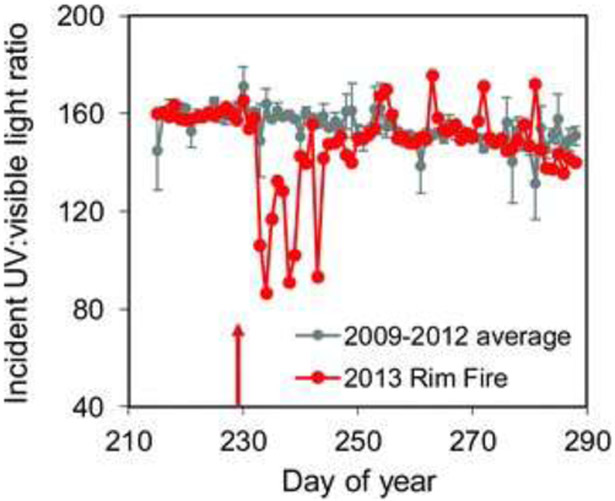
The incident UV: visible light ratio at Lake Tahoe decreased by almost half during the 2013 Rim Fire in California. These changes in incident UV: visible light can alter the vertical distribution of zooplankton in the lake (Urmy et al. 2016).195 Adapted from Williamson et al. 2016.179
Several studies have investigated the effects of extreme precipitation events on water clarity more generally.196-198 Although they do not report changes in UV transparency, the important role of DOM in regulating water transparency, as well as the selective absorption of UV radiation by DOM, makes it likely that these broader changes observed in water clarity are also paralleled by changes in UV exposure of aquatic ecosystems. A critical knowledge gap is the lack of good data on the UV transparency of aquatic ecosystems.
4.3. Climate-induced changes in glaciers, ice cover, and vertical water circulation are altering exposure to UV radiation in aquatic ecosystems by increasing the seasonal exposure of organisms to UV radiation and altering the vertical distribution of these organisms
The physical state of water – ice vs. liquid and how it moves horizontally and vertically – affects the exposure of organisms to UV radiation. Consequently, the extent of adverse effects of UV radiation in aquatic ecosystems will vary. Accelerated climate change is reducing the duration of ice cover in inland and coastal waters,199 thereby increasing seasonal exposure to solar UV radiation and visible light (Fig. 10).200 Glacial retreat is increasing inputs of melt-water discharge and glacial particles into downstream lakes, reducing the penetration of UV radiation.201,202
Fig. 10.

Spring 2012 ice break-up in the Arctic Ocean along the coast of Greenland. Trends of earlier ice break-up and shorter periods of ice cover result in earlier exposure to UV radiation and longer growing seasons for these aquatic ecosystems. Photo credit: Samuel Hylander.
Most aquatic organisms are plankton, passively carried by the water. Exposure of plankton to UV radiation partly depends on how deep and quickly they are circulated vertically near the surface of the water where UV radiation is highest. This circulation determines whether an organism receives a few minutes, several hours or no UV radiation each day. Climate change and the effects of ozone depletion on atmospheric circulation are expected to have regionally variable effects on the depth of ocean mixing203 and ocean circulation (see section 1.3 and ref. 120), which will either increase or decrease UV exposure of plankton. In the Southern hemisphere, shifts in the latitudinal position of atmospheric circulation cells due to ozone depletion over the South pole also change the position of ocean currents with consequences for distribution of marine species distributions and subtidal marine communities. Cetina-Heredia et al204 reported that recent intensification of the East Australia Current (EAC) influenced the dispersal patterns of the eastern rock lobster, such that this species range was ultimately shifted southward by ca. 270 km. The predicted incursions of warm, nutrient-poor water from the EAC along eastern Tasmania have also increased in strength, duration, and frequency of the incursions which has contributed to regional declines in the extent of giant kelp beds, marked changes in the distribution of nearshore fish, and allowed northern warmer-water species to colonise Tasmanian coastal waters.205 Declines in growth rates in Brazilian corals since the 1970s have been linked to increasing temperatures of the sea surface, which were correlated with ozone depletion.206
4.4. Anthropogenic activity is causing water temperature, dissolved carbon dioxide, nutrient loading, and stratification to all increase in aquatic ecosystems
The interactive effects of these changes can either worsen or ameliorate the negative effects of UV radiation on bacteria, phytoplankton (small suspended algae) and seaweed (e.g., kelp). Recent studies of the interactions between the effects of climate change and those of UV radiation in aquatic organisms confirm and extend previous results over the last decade.8,207 High temperatures of water usually enhance the ability of organisms to repair UV radiation damage, thus reducing negative effects as long as the increase in temperature does not put an organism near its upper tolerance limit.208–210 On the other hand, increased thermal stratification in lakes179,211 is reducing nutrient supply to phytoplankton; as is already known for the open ocean,203,249 this usually increases sensitivity of phytoplankton to UV radiation.212 Less straightforward are the interactive effects of increased CO2 and associated acidification of seawater,213,214 and nutrient loading (e.g., from urban and agricultural runoff), which can either worsen or ameliorate effects of UV radiation on phytoplankton and bacteria depending on the conditions and species involved.215–217 These new studies add to an emerging understanding that the effects of UV radiation interact with multiple anthropogenic changes in the aquatic environment and these changes affect how phytoplankton and bacteria acquire and store essential elements (C, N, P). These organismal responses can result in an increase or decrease in sensitivity to UV radiation through changes in the capacity for repair and photoprotection. Similarly, after acclimation, low UV radiation, typical of the Arctic, can have little effect on endemic seaweeds (kelps) under normal conditions, but can actually increase kelp growth under CO2 enrichment.218
4.5. New evidence suggests that, despite the presence of UV protective mechanisms, tropical zooplankton as well as mid-latitude fish, amphibians, and aquatic insects are sensitive to the negative effects of UV radiation with potential implications for fish stocks
Zooplankton are key components of the diet of juvenile fish and have been shown to be sensitive to UV radiation. Even in tropical regions with naturally high UV radiation such as the Red Sea,219 where organisms are expected to be more tolerant to UV radiation, they display high UV sensitivity. Furthermore, Won et al.220 demonstrated that zooplankton exposed to UV radiation in laboratory experiments allocate energy to DNA repair at the cost of growth and reproduction.
New information has added to our understanding of how the negative effects of UV radiation on zooplankton can be counteracted by increasing concentrations of UV defense compounds such as carotenoids, melanin, and mycosporine-like amino acids.200,221–223 Mycosporine-like amino acids can be transferred through the food web from phytoplankton to zooplankton,200 thus conferring tolerance to UV radiation to the latter organisms. Adequate vitamin D3 in the phytoplankton food source also enhances UV-tolerance of certain zooplankton.224 Furthermore, several zooplankton species can sense and escape UV radiation by migrating to deeper waters.225,226 This migration transports nutrients and carbon vertically within the water column and changes nutrient availability for phytoplankton as well as the availability of zooplankton as food for fish.
Evidence continues to accumulate that UV radiation is a direct stress factor for fish, amphibians, and their aquatic insect food resources. For example, juveniles of a common reef fish show elevated respiration and lower feeding rates when exposed to UV radiation under laboratory conditions.227 However, at times the UV radiation can confer resistance to anthropogenic environmental stressors. For instance, exposure to UV radiation increases the resistance of spotted salamanders to the formulated herbicide containing glyphosate.228 Under laboratory conditions, aquatic insects such as damselfly larvae increase their UV-protective melanin content but display impaired growth under UV radiation,229 although enhanced melanin production in these larvae was also associated with a reduced immune response in adults.229 These responses of zooplankton and aquatic insects – critical components in aquatic food webs – to UV radiation may be important for the growth, survival, and reproduction of fish and amphibians, including commercially important species. In some instances, exposure to UV radiation increases tolerance to contaminants, although the mechanisms for this still need to be elucidated.
4.6. The response of organisms such as fish, zooplankton, and other crustaceans, to UV radiation is influenced by multiple environmental factors
Recent studies taking into account interactive effects between UV radiation and other stressors of environmental change have shown new responses in both invertebrates and vertebrates. The effects depend on the species, and the magnitude of the stressors, their timing and type. Earlier ice-off (ice thaw) has been observed in many lakes and in the ocean199 and this will expose plankton to higher UV radiation stress in spring. Calanus, the most important group of zooplankton to support North Atlantic fisheries, accumulates photoprotective compounds from their diet in synchrony with the breaking up of the ice (ice-out)200 but it is not known if they can change the timing of this UV protection if the ice-off continues to be earlier in the future. In other systems, pigments that could protect zooplankton from UV radiation make them more susceptible to fish predation due to these pigments increasing their visibility to these visual predators. A trade-off between UV-protection and risk of being consumed leads to the extent of pigmentation being reduced in the presence of fish odour within two weeks.230 Although the mechanism by which this happens is not known, it may involve changes in either the production or accumulation of pigments of different types.230
4.7. Tolerance of predators to UV radiation as well as the nutrient content of foods can change the response of organisms in aquatic food webs to UV radiation
The geographic distribution of UV-sensitive predatory invertebrates in lakes expands when increased DOM provides a refuge from damage by UV radiation. Consequently, the resulting increased predation by these more protected invertebrates leads to elimination of populations of their main prey, fairy shrimp.231 This demonstrates the key role of UV transparency for predators and their prey.
In coastal marine systems, higher quality food with more nutrients can increase tolerance of amphipods (shrimp-like crustaceans) to UV radiation.232 It has also been demonstrated that amphipods obtain UV-absorbing compounds from their macroalgae (seaweeds) food, when exposed to UV radiation.233 Reductions in exposure to UV radiation and the nutrient content of food can thus alter the fundamental nature of consumer-resource interactions and lead to the reduction or elimination of key invertebrate species in aquatic ecosystems.
4.8. Changes in UV radiation can alter the behaviour of keystone zooplankton species
Solar UV radiation is an important behavioural cue in many aquatic organisms for orientation, communication, and mate-selection.234 Zooplankton exhibit strong behavioural responses to UV radiation that can have profound ecological consequences because they are perhaps the most abundant animals on Earth. They also play a fundamental role in water quality by consuming algae, and are the single most important food component for juvenile fish. The strong behavioural responses to UV radiation may thus alter aquatic food webs and fishery productivity. Two recent in situ studies demonstrated distinct behavioural avoidance of natural solar UV radiation in highly transparent lakes.235,236 Daytime depth distribution was not related to visible light, thermal structure, or the presence of fish, but instead deepened with increased UV transparency and depth of food resources.236 In another recent study that examined the behavioural response of nine species of freshwater calanoid copepods (one of the most abundant types of zooplankton in lakes and oceans) from 15 populations, these copepods displayed not only the anticipated avoidance of the damaging UV radiation in natural sunlight, but also attraction.237 Attraction was predominant in animals from more UV-transparent systems, suggesting that UV radiation is used as a cue for habitat selection, potentially allowing these copepods to utilize food resources in surface waters and avoid overlap with less UV-tolerant competitors or predators.237 Instantaneous UV-avoidance may also be species-specific where some species seek refuge in deeper waters when exposed to UV radiation, whereas other species have little or no behavioural response to UV radiation.226 These less behaviourally responsive groups likely rely on other adaptations to avoid damage from UV radiation.226 Furthermore, a recent study has demonstrated that stomatopod crustaceans (mantis shrimp) have selective UV filters in their eyes allowing them to utilize UV photoreceptors in well-lit surface waters.238
Smoke from drought-related wildfires and biomass burning as well as increased input of UV-absorbing DOM may reduce underwater exposure to UV radiation (see sections 4.1 and 4.2), leading to a shallower depth distribution of zooplankton in highly transparent lakes.195 These observations are consistent with the proposition that transparency regulates the relative importance of UV radiation vs. visual predation risk as factors driving vertical migration of zooplankton in lakes.239 Hence, even though UV radiation is harmful to zooplankton and other invertebrates if exposure is high, UV radiation is also a natural environmental cue affecting orientation and vertical distribution in clear-water systems. In addition to changes in quality of water, the critical importance of zooplankton in aquatic food webs means that these changes in behaviour in response to UV radiation have important implications for fishery productivity. Responses to UV radiation alter the depth distribution of zooplankton and thus their vertical overlap in the water column with their algal food resources as well as fish predators.
4.9. New models of inhibition of productivity of phytoplankton enable estimates of effects of UV radiation at an oceanic scale. Initial estimates of productivity for the Pacific Ocean are about 20% lower than predictions that ignore inhibition by UV and visible radiation
Decades of studies have demonstrated that near-surface inhibition of phytoplankton photosynthesis by ambient UV radiation and, to some extent, excessive visible radiation, occurs in most marine and freshwater systems.240,241 New research is providing critical information that is needed to generalise these observations to ocean-scale models in order to estimate the importance of the effects of UV radiation on the oceanic carbon budget. Particularly important are the new descriptions of the effectiveness of specific wavelengths for inhibition by UV radiation of the key primary producers in open ocean systems, using Biological Weighting Functions (BWFs). These primary producers are the smallest planktonic algae, called picophytoplankton.242,243 Based on these BWFs, an initial study found that model estimates of total primary productivity in the Pacific Ocean (including the whole water column) are ca. 20% lower than estimates that ignore inhibition effects.242 Predictions show the strongest inhibition by UV-A and visible radiation in the near-surface zone where UV-B radiation is present, thus model estimates of the absolute effects of even an “ozone-hole” scale enhancement of UV-B radiation are minimal (<2% throughout the water column).244
Global estimates of the distribution of phytoplankton in aquatic environments will also be improved by better monitoring through satellite remote sensing,245 which will be augmented by the new Sentinel 3 mission in which a triad of platforms will be launched over a four-year period (2016– 2020). Taken together, these advances will improve our ability to estimate how marine productivity is affected, at present and in the future, by the interaction between ocean environmental conditions (such as temperature, mixing depth, ocean acidification, pollutants, and transparency) and UV radiation sensitivity of phytoplankton.
4.10. Warmer water temperatures and decreasing water transparency to UV radiation are increasing suitable habitats for many waterborne pathogens
Consequently, this may increase or decrease their infectivity, with implications for human health. Many waterborne pathogens and parasites such as Cholera, Giardia, and Cryptosporidium cause severe gastrointestinal and other health problems, but infectivity is generally reduced when pathogens are exposed to solar UV radiation (both UV-B and UV-A radiation.246). Increased water temperature247,248 and decreased transparency to UV radiation – both due to accelerated climate change249 (see also 4.1 and 4.2), are increasing the infection potential and amount of habitat available for these pathogens and parasites. However, reductions in transparency to UV radiation caused by DOM or other dissolved and particulate substances may lead to an increase in mosquito-borne diseases such as malaria and Zika. Aquatic mosquito larvae (Aedes aegypti) can be controlled by certain fungi, but these fungi are themselves sensitive to UV-B radiation.250 Therefore, the effectiveness of these fungal control agents will likely increase in environments with reduced UV radiation transparency.251
4.11. Compounds in sunscreens used by humans to reduce damage by UV radiation are contaminants of growing concern that have many detrimental impacts on aquatic ecosystems
Like many pharmaceuticals and personal care products, substantial quantities of sunscreen compounds enter aquatic ecosystems around the planet.252 These compounds are found in areas with large human populations and frequent beach use as well as remote regions such as the Arctic.253–255 Sunscreens also are found in the urine of humans256 and can be present in high concentrations in wastewater effluent, but can be removed via existing treatment practices and exposure to solar UV radiation in surface waters.257,258 UV radiation-induced degradation can be faster in freshwater ecosystems than in marine ecosystems due to higher concentrations of natural sensitisers in lake water, possibly reactive nitrogen compounds, which speed up degradation in freshwaters.259
Anthropogenic sunscreen compounds have been detected in many aquatic organisms such as mussels and fish.260 Some of these compounds can transfer from mother to offspring in aquatic mammals such as Amazon river dolphins261 and can bioaccumulate in aquatic food webs.262 These compounds have a wide range of negative ecological effects.263 For example, some compounds, such as the common UV sunscreen compound, oxybenzone, are endocrine disruptors in both vertebrates and invertebrates.264 Laboratory experiments with these compounds have shown that, at concentrations higher than those in the natural environment, the sex ratio of freshwater fish can be skewed.265 Other compounds are toxic to phytoplankton such as diatoms266 and midges264 and can facilitate coral bleaching.255 Thus, widespread application of synthetic sunscreens to reduce damage by UV radiation could be having many unintended negative effects in aquatic ecosystems.
4.12. Degradation of plastic debris by UV radiation increases the concentration of microplastics in the environment, with potentially substantial negative ecological consequences
Microplastics, which are commonly defined as plastic particles smaller than 5 mm in size, are a contaminant of growing environmental concern.267 Exposure to UV radiation contributes to fragmentation of plastic debris in the environment, thereby generating microplastic fragments and contributing to their degradation (see section 7.8). This UV-induced fragmentation increases with warming temperature.268 Climate change and UV radiation therefore may interact to stimulate increasing quantities of microplastic fragments, especially in eastern Asia and the tropics where future increases in UV radiation are projected (see section 1). Studies show that microplastics are already widespread in both inland269 and marine aquatic ecosystems.270
Microplastics may have major detrimental environmental impacts on aquatic ecosystems through the release of toxic compounds or by inhibition of biological processes following ingestion. Exposure to UV radiation contributes to the release into the oceans of toxic compounds from plastics including bisphenol A, phthalates, citrates, and Irgafos® 168 phosphate.271 Many passive filter-feeders, such as mussels, ingest microplastics.272–274 However, little is known about the ecological impacts of microplastics in aquatic ecosystems beyond single species or laboratory studies.274,275
4.13. UV radiation and dissolved organic matter influence the types and quantities of toxic byproducts formed during chlorine disinfection of drinking water
Disinfection byproducts are regulated by many governments because, at high concentrations, they are hazardous to human and animal health and can cause cancer.276 Exposure to solar UV radiation of water prior to treatment can reduce formation of disinfection byproducts.277 DOM that strongly absorbs UV radiation produces more disinfection byproducts.278-280 Furthermore, concentrations of disinfection byproducts can be high in reclaimed water because reclaimed water often contains more DOM compared with non-reclaimed water.281 UV radiation also breaks down contaminants such as the commonly prescribed diabetes drug metformin,282 which forms potentially harmful disinfection byproducts in water treatment283 and ends up in many aquatic ecosystems.284
5. Interactive effects of solar UV radiation and climate change on biogeochemical cycles in the environment
5.1. Climate change affects UV-induced biogeochemical cycles
The term “biogeochemical cycles” refers to chemical and/or biological transformations of natural and man-made substances (e.g., carbon, halogen compounds, and contaminants) in terrestrial and aquatic ecosystems. Biogeochemistry also includes the transfer of substances across environmental boundaries. Fig. 11 shows schematically how interactions between solar UV radiation and climate change affect processes or flows within and between terrestrial and aquatic ecosystems. A key aspect of this conceptual framework is movement of natural organic compounds from terrestrial to aquatic ecosystems. This transfer is enhanced by heavy precipitation events (e.g., storms), and permafrost thawing, and may be reduced by droughts. Increased frequency of heavy precipitation events, thawing of permafrost soils, and droughts are likely caused by anthropogenic climate change. Droughts enable wildfires, which in turn increase the likelihood of thawing of permafrost soils. Important implications of thawing of the permafrost include release of carbon dioxide (CO2) and methane (CH4) into the atmosphere (see section 5.5) and also land erosion as the permafrost melts.
fig. 11.
Interactive effects of solar UV radiation and climate change on processes and flows within and between terrestrial and aquatic ecosystems. The processes or flows indicated by arrows are discussed in sections 5.2–5.5 and 5.7. Key to symbols: black arrows indicate linkages between environmental factors: + shows an increase in a process or flow, – a decrease in a process or flow. Dashed arrows indicate direct effects of solar UV radiation on decomposer organisms. Grey arrows indicate the flow of carbon within ecosystems. Blue arrows indicate the flow of carbon from terrestrial to aquatic ecosystems. Green arrows refer to the process of “priming” (see sections 5.2, 5.3, and 5.5). Red arrows indicate the production of carbon dioxide in terrestrial and aquatic ecosystems. POM and DOM stand for particulate and dissolved organic matter, respectively.
5.2. Solar radiation, including UV radiation, increases the rate of breakdown of dead plant material, and may be an important regulator of carbon storage in the majority of terrestrial ecosystems, even where litter is exposed to sunlight during only a small fraction of the year
An understanding of the extent to which solar radiation drives the breakdown (decomposition) of dead plant material (known as plant litter) is essential for quantifying how ongoing changes in solar radiation (see section 1.2) influence carbon storage and cycling in terrestrial ecosystems. Decomposition of plant litter via UV-induced processes has been shown to occur in arid-land ecosystems.285,286 However, new studies suggest that exposure to solar radiation can stimulate the breakdown of plant litter in a range of ecosystem and plant litter types.287–289 For example, Mediterranean grassland experiments, where UV289–291 or total solar radiation has been selectively filtered out292,293 have shown that exposure to sunlight stimulates microbial activity in plant litter (discussed in section 5.3). In addition, breakdown of plant litter is substantially reduced when UV radiation is reduced due to changes in vegetation cover from tree planting,294 or when plant litter mixes with soil.154,295 The decomposition of plant litter is not only affected by solar radiation but also by other environmental factors such as water availability, and varies with time on annual, seasonal or even daily time frames.286,288,292
5.3. In terrestrial ecosystems, exposure to solar radiation, including UV radiation but particularly blue-green light, alters the chemical composition of dead plant material in ways that accelerate its breakdown by decomposer organisms
The decomposition of plant litter is a critical process for nutrient cycling and carbon storage in terrestrial ecosystems. Exposure to solar radiation can substantially increase the rate of subsequent breakdown of plant litter by decomposer organisms. Recent research has demonstrated that this “priming” effect (see Fig.11) results from solar radiation breaking down lignin, which makes sugars more available for microbial degradation.287,296 Additionally, it is also now clear that stimulation of the breakdown of plant litter results from exposure to both UV radiation and particularly blue-green radiation.287,296 While the effectiveness of this process, and the relative effects of UV and visible wavelengths vary among plant species,287 the identification of the mechanism is an important step forward in our understanding of how solar radiation influences carbon cycling in terrestrial ecosystems.
5.4. Droughts and wildfires interact with solar UV radiation to affect carbon cycling
Evidence is accumulating that the intensity and frequency of droughts is increasing in some parts of the Earth as a result of climate change.297 In the Southern Hemisphere, droughts are additionally caused by the interactive effects of depletion of Antarctic stratospheric ozone and climate change120 (see section 1.3). Droughts and wildfires interact with UV-induced carbon cycling in various ways. For example, droughts may reduce the flux of coloured dissolved organic matter (CDOM) into inland and coastal waters, which results in increased transparency of water bodies to solar UV radiation179 (see section 4.2).
Wildfires are important sources of CO2 and other greenhouse gases, such as methane, to the atmosphere.8,298 A new study299 has shown that boreal forest fires can transform soil organic matter (SOM) into compounds with greater UV-absorbing capability, compared to the pre-fire SOM, particularly at high fire temperature (>600–700 °C).299 This enhancement in UV absorption by SOM may increase the fraction of SOM that is subject to UV-induced decomposition in watersheds impacted by wildfires.300 Hence wildfires can be direct and indirect sources of CO2 to the atmosphere, where the indirect source is due to the transformation of SOM into compounds with a higher UV absorption.300 Furthermore, wildfires enhance the likelihood that permafrost soils will thaw and collapse.301 For example, up to 0.5 m of thaw settlement was observed after recent fires in Alaska, causing impoundment of water and further thawing of permafrost301 (see section 5.5).
5.5. Solar UV radiation increases the biological availability of terrestrial dissolved organic matter from thawing permafrost soils
Permafrost soils store approximately twice as much carbon than is presently contained in the atmosphere.302 Global warming results in the thawing of permafrost soils and the release of terrestrial dissolved organic matter (tDOM) into surface waters, where it is subjected to UV-induced and biological degradation to produce CO2.303–306 Owing to its high photoreactivity, tDOM from recent thawing of permafrost soils readily undergoes UV-induced degradation.306,307 Exposure to solar UV radiation generally decreases the photoreactivity of permafrost tDOM but increases its bioavailability.305,308 After exposure to solar UV radiation, Arctic permafrost tDOM was found to be >40% more labile to bacteria, compared with the same tDOM kept in the dark.308 An exponential increase in biotic tDOM degradation over time following permafrost thawing has been observed in Arctic surface waters309 and is due to the UV-induced production of bioavailable compounds from tDOM.308 Therefore, the increase in the bioavailability of tDOM by solar UV radiation (“priming”) enhances the release of CO2 from aquatic ecosystems (see Fig.11), similar to the effects of priming in terrestrial ecosystems (see also section 5.3).
5.6. Climate change enhances the UV-induced and biological production of precursors of reactive species that participate in stratospheric ozone depletion
A new study310 has shown that low-lying tidal freshwater swamps may be important sources of chloroform (CHCl3) and bromoform (CHBr3) to the atmosphere. The reason is that sea-level rise and saltwater intrusion due to climate change bring halide ions (e.g., chloride ions (Cl−)) inland, where they react with tDOM in UV-induced processes to produce halocarbons.310 Thus climate change could enhance the UV-induced production and emissions of halocarbons that participate in stratospheric ozone depletion. Such an effect would represent a positive feedback on solar UV radiation.
Climate change may also affect the biological production of halocarbons in aquatic ecosystems.311–313 The biological formation of short-lived bromocarbons such as bromoform (CHBr3) in seawater involves phytoplankton and dissolved organic matter (DOM).311 The rate of formation of CHBr3 depends on the chemical composition of DOM;311 humic acid facilitates the biological production of CHBr3.311 “Browning” of coastal and estuarine environments179 (see section 4.1) could enhance the biological production of short-lived bromocarbons. Tegtmeier and coworkers313 estimated that, at present, ozone depletion potential (ODP)-weighted emissions of CHBr3 from the global ocean amount to 9% of ODP-weighted anthropogenic emissions of all long-lived ozone-depleting halocarbons.
5.7. Changes in solar UV radiation, whatever their cause (e.g., ozone super-recovery or not) will affect how much carbon dioxide is taken up or released by terrestrial and aquatic ecosystems, but the sign and magnitude cannot currently be defined
Some scenarios predict super-recovery of stratospheric ozone in the extratropical parts of the Earth in the second half of this century, which would result in substantial decreases in solar UV-B radiation314 (see section 1.2). As a consequence, rates and the extent of UV-induced processes would decrease and this decrease would have multiple effects across various areas, including biogeochemical cycles, but also air quality and health. For example, reduced rates of UV-induced degradation of natural organic matter would result in less CO2 production in terrestrial and aquatic ecosystems. At present, the consequences of possible future changes in solar UV radiation for biogeochemical cycles can only be described in a qualitative way.
5.8. Stratospheric ozone super-recovery could lead to increased concentrations of terrestrially derived particulate organic matter in the ocean by reducing its UV-induced conversion to dissolved organic matter
Some of the plant-derived material coming from terrestrial ecosystems is transported to the ocean as terrestrially derived particulate organic matter (tPOM) where most of it will be consumed by organisms but some will sink into the sediments or break down into DOM. Current research has indicated that light-induced conversion of tPOM to DOM (photodissolution) is primarily driven by the UV-B part of solar radiation that is sensitive to changes in atmospheric ozone315–318 (see Fig. 11). Lower rates of UV-B-induced transformation of tPOM to DOM would lead to increased participation of tPOM in other aquatic processes including uptake into aquatic food webs and the transfer of tPOM into bottom sediments. The net result could be increased carbon storage in bottom sediments with reduced release of carbon dioxide to the atmosphere.
5.9. The rates and nature of UV-induced biogeochemical cycling of chemical and biological contaminants may undergo changes by the latter part of the 21st century
The photodegradation of contaminants in aquatic ecosystems involves both direct and sensitised photoreactions.319 Possible future reductions in UV irradiance due to ozone recovery and reduced UV penetration into aquatic environments would result in increased persistence of contaminants associated mainly with decreased direct photodegradation rates. Direct photodegradation results from the absorption of solar radiation by the contaminant itself. For many organic contaminants such as pesticides and “emerging chemicals”, e.g., pharmaceuticals, direct photodegradation is caused primarily by the shortest wavelength component of sunlight in the UV-B range (280–315 nm). Sensitised photodegradation is initiated through light absorption by another substance in the system with the contaminant, such as CDOM in aquatic environments,316,320 which produces short-lived reactive transients that react with the contaminants.321–327 Recent developments in remote sensing techniques and surface measurements are providing new insights into the spatial and temporal distribution of the transients (e.g., hydrogen peroxide and superoxide),328 as well as additional information on the spatial and temporal distribution of CDOM in aquatic ecosystems.329–333 Enhanced terrestrial runoff due to more frequent heavy precipitation events could result in increased CDOM concentrations and reduced penetration of UV radiation into surface waters. Taking this climate-change effect together with potential super-recovery of stratospheric ozone,314 one can speculate that the overall effect might be a shift from direct to indirect UV-induced transformations of chemical and biological contaminants.
6. Interactive effects of solar UV radiation and climate change on tropospheric air quality and composition
6.1. Trifluoroacetic acid (TFA) is the main degradation product of HCFCs, HFCs, and HFOs in the atmosphere. A recent review confirms that amounts of TFA from these halocarbons are small relative to other sources and therefore currently unlikely to pose a risk to humans and the environment
Few new studies on TFA have been published since the review by Solomon et al.334 In a study conducted in Beijing, China, between 2013 and 2014, mean measured concentrations of TFA in the atmosphere were reported to be 1.5 ± 0.2 ng m−3, mainly in the gaseous phase (1.4 ± 0.2 ng m−3) with little in the particulate phase (0.06 ± 0.008 ng m−3).335 The mean concentration in air is four-million times less than an occupational standard for protective action of 0.6 mg m−3 from the US Department of Energy PAC Database,336 suggesting that atmospheric concentrations pose minimal risk to humans.
TFA is considered very stable in the environment; it is not degraded by the main atmospheric oxidants, the hydroxyl radicals (˙OH). However, it can be degraded under laboratory conditions. A study has shown that TFA can be photolysed in water in the presence of electrolysed sulfuric acid (S2O 2−), UV radiation and visible light (220–460 nm).337 This process may have some utility in an industrial setting. Electrolysed sulfuric acid does not occur in nature and the reaction is only efficient under very acidic conditions–also not normally seen in nature–so that this reaction is of little relevance to the fate of TFA in the environment.
6.2. A number of common compounds are currently being discussed as alternative refrigerants. The negative impacts of their uses on air quality are expected to be small, but do not appear to have been evaluated recently
These so-called “natural” refrigerants include ammonia, hydrocarbons, and carbon dioxide, and are being considered as replacements for HFCs. Ammonia has long been used as a commercial refrigerant. Exposure to high concentrations has detrimental impacts on human health. In the atmosphere, ammonia participates in the formation of aerosols, thus affecting air quality but also contributing to climate cooling through scattering of solar radiation.338 Some model estimates of concentrations of atmospheric ammonia are less than observed values by factors of 2–4, indicating that the sources and sinks of ammonia are not well known.339 Nevertheless, full replacement of halocarbons by ammonia would still constitute a negligible fraction (<1%) of current global ammonia emissions. Due to its toxicity, ammonia probably will not be used except in large-scale facilities340 where it currently accounts for ca. 15% of the total refrigerant market.341 Short chain hydrocarbons (e.g., propane and n-butane) are also being proposed as refrigerants, and could have some minor impacts on air quality,342 although this does not appear to have been recently re-assessed. Carbon dioxide has been used for many years as a refrigerant and has few consequences for air quality, but engineering challenges have limited its widespread use.
6.3. The recovery of stratospheric ozone is expected to reduce UV radiation in the troposphere outside of the tropics, decreasing ground-level ozone in cities but increasing it in rural areas
UV-B radiation is a significant driver in the generation of ozone at ground level, particularly in polluted environments, leading to a small but not insignificant dependence of air quality on stratospheric ozone.126 As a result, increases in stratospheric ozone will slow the production and destruction of ozone at ground level. Larger concentrations of ozone in rural areas will be detrimental to agriculture and natural ecosystems, while smaller concentrations in urban areas are expected to be less damaging to human health. Model calculations343 have suggested that UV-driven ozone destruction will be slower in rural areas, tending to increase its concentrations. In contrast, concentrations of urban ozone are expected to decrease due to slower production in a lower UV environment (see Fig. 1212). However, this expectation is based on older models that did not have sufficient spatial resolution for confident assessment. Many other factors are equally or more important in determining ambient concentrations of ozone. These include changes in emission of ozone precursors (nitrogen oxides and volatile organic compounds), and climate-driven changes in temperature, humidity, and stratospheric-tropospheric circulation.344,345 As a result, the net effect of UV reductions and these other factors on ground level ozone remains uncertain, and a challenge for management of air quality.
Fig. 12.
Schematic illustration of the evolution of ground-level ozone in urban air and its outflow, as a function of distance from emission sources. Dashed curve gives reference using current UV radiation, while the solid curve is for decreased UV radiation expected upon recovery of stratospheric ozone. The green hatched area shows the resultant de-crease in urban ozone, while the red hatched area shows the increase in regional (background) ozone.
These changes in ground-level ozone may be exacerbated in the future if stratospheric ozone amounts exceed pre-ozone depletion levels (“super-recovery”), as expected for middle and high latitudes under some representative emissions scenarios.346 For example, at Northern Hemisphere mid-latitudes, the super-recovery could double rural ozone increments relative to those estimated by Zhang et al.343 for the “normal” recovery to pre-ozone depletion levels. Over the tropics, small reductions in stratospheric ozone are expected, with only minor effects on ground level ozone.346 Under some proposed geo-engineering schemes, even larger stratospheric ozone values would be expected at mid-latitudes, and could lead to additional increases (up to 5%) in global ground-level ozone,347 with potentially significant impacts on air quality.
6.4. Evidence linking poor air quality to adverse health effects on humans and the environment continues to mount, but the effects of individual pollutants remain difficult to discern because they frequently occur together
Because changes in stratospheric ozone will have small but potentially significant impacts on air quality at ground level,126 this has implications for human and environmental health. A recent WHO Global Burden of Disease assessment for outdoor air pollution348 concludes that approximately 3 million premature deaths occurred globally in 2012 due to poor outdoor air quality. Other studies have arrived at similar estimates of mortality based on summing inferred effects of particulate matter (e.g., from industrial combustion processes, dust, fires) and ozone.349 However, there is difficulty in separating the effects of particulate matter and ozone because they generally co-occur.126,350,351
The combined impacts of air pollution and rising temperature are receiving increased attention. Epidemiologic studies of the 2003 European heat wave have ascribed a significant fraction of observed mortality to air pollution.352–354
A recent clinical trial has directly investigated this interaction and found that simultaneous experimental exposure to higher ozone and higher temperature was associated with a constellation of physiological responses indicative of slower dissolution of blood clots.355 Additional clinical trials are needed to further elucidate these mechanisms in anticipation of future changes in both climate and air quality.
7. Materials damage due to solar UV radiation and temperature
Exposure to solar UV radiation has adverse effects on materials. Outdoor service lifetimes of materials are influenced if not determined by the rates of degradation through weathering. Increased exposure to UV radiation at some geographical locations, coupled with rising ambient temperatures due to climate change, accelerate the degradation rates of materials. The consequent decrease in service life can be countered by stabilisation technologies in the case of plastics, and surface coating or treatment for wood products. Here we assess the recent advances in understanding of the mechanisms of UV radiation-induced degradation that would help the development of stabilisers or coatings in commonly used plastics, wood, and textile materials.
7.1. Surface yellowing of wood on exposure to UV radiation is correlated with the extent of chemical modification, allowing simpler monitoring of oxidation rates in wood using yellowing measurements
Yellowing of wood356–358 and signatures for oxidation products in the infra-red (IR) spectra359,360 are indicative of solar UV-induced oxidative degradation of wood. These changes are more pronounced during initial stages of exposure358–360 and are localised in a thin surface layer of wood360 and bamboo.361
The fractional crystallinity of cellulose in bamboo increases during photodegradation as the amorphous cellulose fraction is preferentially degraded.361 Monitoring the progress of the photooxidative reactions is critical in the development of protective coatings for wood.362 However, the spectroscopic methods used are tedious. The rate of yellowing was shown to be well correlated with changes due to oxidation as visualised by infra-red and novel hyperspectral imaging for photodegradation of chestnut wood under simulated solar UV radiation.358 A similar correlation was also reported between the residual lignin content and tensile strength during natural weathering of fir-wood.360 If generally applicable, such correlations allow easier, non-invasive measurements (particularly yellowness index) to be used to assess the progress of oxidative changes in the wood.
7.2. The effects of higher ambient temperatures and other factors on the service life of wood and plastics outdoors can now be better estimated
Outdoor service life of common plastics such as polyethylene363,364 and polypropylene365 is determined primarily by solar UV radiation dose and sample temperature. Relationships that estimate the increased weathering of these at higher ambient temperatures are commonly available.366,367 However, a relationship that also includes the intensity of solar UV irradiation, sample temperature, and the partial pressure of oxygen was recently reported and validated for polypropylene (PP).368 This approach allows for a more reliable estimation of service life of PP.
Similar effects hold for wood species such as wood of Norway spruce,369 as well as beech wood.370 The discoloration of beech wood at 20–60 h of exposure to solar UV radiation was greater at 100% humidity than at 0%.370 As weathered wood and wood-plastic composites are more susceptible to subsequent biodegradation by decaying fungi outdoors,371 weathering promotes further degradation in these materials. This has implications for protection against UV-induced weathering of wood at locations of high ambient humidity coupled with high temperatures.
7.3. Heat treatment of wood to control degradation by UV radiation shows mixed results with the majority of studies indicating no advantage in terms of UV stability
Industrial heat treatment of wood (at 180–240 °C) is claimed to stabilise the wood against solar UV radiation and weathering.372 Heat treatment of woods can yield a stable hydrophobic surface with improved resistance to decay.373 However, heat treatment of poplar, black locust374 and other varieties of wood375,376 showed no such improvement in UV stability. Even where light-induced discolouration was controlled by this treatment, spectroscopic data still showed marked degradation due to heat treatment.377 The presence of different types of extractives378,379 in wood might account for the variability in results; extractives are organic compounds in wood that can be extracted with solvents. Variables affecting the level of UV stability delivered by thermal treatment of wood has to be further studied before it can find widespread commercial use in outdoor applications.
7.4. Both bulk chemical modifications and surface coatings effectively control solar UV-induced degradation of wood
Bulk modification of rubber wood with isopropenyl acetate to enhance their durability and hydrophobicity also improve their solar UV stability.380 In laboratory exposures to simulated solar UV radiation, the wood showed photobleaching instead of yellowing after 250 h of accelerated weathering, while the untreated control yellowed deeply. Copper ethanolamine surface coating381 used to stabilise Japanese larch wood against degradation by solar UV radiation can also be effective as a UV-protective primer as found for Southern pine wood.382 However, copper compounds are well known to leach out during use383 with potential environmental impacts. Conventional UV stabilisers used in surface varnishes that are effective in softwood and bright hardwood products do not prevent bleaching of dark woods (rosewood, ebony, mahogany, or black walnut) or heat-treated wood exposed to UV radiation.378 Better stabilisers that can control photodamage of dark wood need to be developed.
7.5. Lignin used as a filler in polypropylene acts as a stabiliser against degradation by solar-simulated UV radiation
Lignin, a natural, complex organic polymer, making up a large part of woody plant tissue, is also a byproduct of the paper industry, and is being used to increase stabilisation of materials because of its antioxidant properties. For example, lignin filler is generally known to contribute antioxidant properties in polyethylene and polypropylene (PP).384,385 Compared to powdered wood, poplar-derived lignin is a superior UV stabiliser and antioxidant.386 The elastic modulus of composites filled with wood powder was reduced by ca. 30% when exposed to laboratory-accelerated weathering for 960 h, while no change (or even a slight increase) was obtained in lignin-filled PP under the same conditions. Use of wood-derived lignin as opposed to powdered wood in wood-plastic composites shows promise provided economic feasibility in large-scale manufacturing can be demonstrated.
7.6. Nanoparticles and nanofillers are increasingly used as effective solar UV stabilisers in materials
Nanofillers can act as UV stabilisers in plastics, wood387,388 and in textile fibres.389 In spruce wood, for instance, zinc oxide (ZnO) nanoplates generated in situ using a precursor absorbed into the wood, reduced total colour change by 75% compared to untreated wood after exposure to UV radiation for 102 h.390 A similar effect was found with nano-ZnO in poplar wood,391 composites of beech wood and polyethylene,392 and nanotitania in bamboo and polyethylene composite.392 These technologies are still at the research stage. Although they show promise as effective UV radiation stabilising technologies, further development is needed before their practical importance can be assessed.
Nanoparticles work similarly in protecting textile fibres. Recent laboratory studies have demonstrated the potential of surface treating textile fibres with nanoparticles for improving their solar UV radiation stability. Nanoparticles of zinc oxide used with cotton,389,393 nylon,394 and aramids395 show promise. Aramids are specialised fibres used in thermally-resistant industrial textiles. Nanoparticles of titania,396 graphene,397 and gold398 have also been explored for surface protection of fibres from UV radiation. For instance, cotton fibres functionalised with ZnO and carbon nanotube nanocomposites at a level of 22% by weight increased the UV protection factor (UPF) of cotton from 6 to 40.399 Treatments used in these studies have not been scaled up to allow assessment of their production in commercial processes. Also, their economic feasibility needs to be studied before they can be recommended for potential large-scale use.
7.7. Solar thermal collectors made of polycarbonate plastic have lower environmental impacts compared to conventional collectors made of glass and metal
However, they have shorter service lifetimes because of premature loss of optical and other properties on exposure to solar UV radiation. Solar thermal collectors made of plastic can be a practical and cost-effective technology for production of electricity.400 An all-polycarbonate collector in an aluminium frame weighs only a third of a conventional metal/glass collector but has been shown to be 8–15% lower in efficiency. However, it is lower in cost and the life cycle energy used in its manufacture is recovered in only 3.8 years of operation as opposed to 8.3 years for conventional collectors.401 A serious drawback of polycarbonate402 or polypropylene403 used in collectors is their loss of optical and other properties on weathering. The service life of plastic encapsulants in conventional photovoltaic modules is also determined by weathering-related changes.404 Advances in stabilisation that improve the lifespan of plastics used in solar energy applications will help advance this clean energy technology.
7.8. Generation of micro- and nanoplastics in the marine environment is accelerated by UV radiation as well as high temperatures
Combined effects of solar UV radiation and high temperatures cause plastic debris in the ocean environment to break-up into micro- and nanoscale particles.405 These microplastics are present in oceans406 as well as freshwater bodies269 and they concentrate persistent chemicals in sea water and, via ingestion, provide a credible pathway of these chemicals into the marine food chain (ref. 273 and section 4.12). Physiological effects of ingestion of these particles on a range of marine organisms have been reported.268,274 Since generation of microplastics is a UV radiation-initiated fragmentation process, better UV-stabilisation of plastic products, especially packaging products, will help slow down the rate of production of particles in the environment.
Acknowledgements
The Environmental Effects Assessment Panel gratefully acknowledges the following: Generous contributions by UNEP/Ozone Secretariat and the World Meteorological Organisation for the convened author meeting were much appreciated. Participation for the following authors is also acknowledged: Drs Pieter Aucamp and Krishna Pandey, were supported by UNEP. Dr Anthony Andrady, Prof. Paul Barnes, Dr Germar Bernhard, Dr Janice Longstreth, Dr Sasha Madronich, Dr Kevin Rose, Prof. Craig Williamson, and Dr Robert Worrest were supported by the U.S. Global Change Research Program (GB was also supported by Biospherical Instruments Inc.). Prof. Alkiviadis Bais, Greek General Secretarial for Research and Technology; Prof. Donat-P. Häder, Bundesministerium für Umwelt Naturschutz und Reaktorsicherheit; Dr Anu Heikkilä, World Meteorological Organization, Global Atmosphere Watch; Assoc. Prof. Samuel Hylander, Linnaeus University and the Centre for Ecology and Evolution in Microbial model Systems (EEMiS); Prof. Robyn Lucas, Australia National Health and Medical Research Council Career Development Fellowship, the Telethon Kids Institute and the Australian National University; Dr Richard McKenzie, New Zealand Government’s Ministry for the Environment through the Ministry of Business, Innovation and Employment’s research contract C01X1008; Dr Rachel Neale, National Health and Medical Research Council (Aust); Dr Patrick Neale, Smithsonian Institution; Prof. Nigel Paul, UK Department of Environment, Fisheries and Rural Affairs; Prof. Milla Rautio, Canada Research Chair Program; Prof. Stephen Wilson, the Centre for Atmospheric Chemistry, University of Wollongong (Aust.); Dr Richard Zepp, National Exposure Research Laboratory, Exposure Methods & Measurement Division, U.S. Environmental Protection Agency. The following are gratefully acknowledged for contribution of information and/or analysis of data used in this report: Dr Aparna Lal, Ms Erin Overholt, Dr Rachel Slatyer, Dr Erin Walsh. We thank Cor de Kock, of Haaften, NL, for the photograph of Prof. Jan van der Leun.
Footnotes
Dedication

This Progress Report is dedicated to the memory of Professor Jan van der Leun, founding co-chair in 1987, member of the UNEP Environmental Effects Assessment Panel (EEAP), and contributor to the section on human health. In 1971, through attending a dedicated meeting of the National Academy of Sciences, Jan became involved in assessing the impact of a looming threat of a thinning ozone layer. He quantified the effect on skin cancer, became a renowned expert on the subject and conducted important research on the dose-effect in UV carcinogenesis to improve his assessments. We will remember Jan for his untiring dedication also to other health impacts, focussing on the interactions with climate change, and in presenting the broad scientific basis for protecting the stratospheric ozone layer. For the EEAP his enduring legacy lies in the remarkable contribution he made in communicating the importance to policymakers, scientists and the public, of phasing out the substances that were depleting the ozone layer and consequently leading to increasing UV radiation. He indeed had a particular talent for emphasising and linking the scientific and political implications of protecting the ozone layer. He has left lasting and warm memories of his presence and leadership; we will keep these memories of a collegial diplomat and personal friend as we carry on his good work.
Jan Cornelis van der Leun, 14 June, 1928–6 July, 2016
He was a man amongst men, we shall not see his like again.
References
- 1.Solomon S, Ivy DJ, Kinnison D, Mills MJ, Neely RR and Schmidt A, Emergence of healing in the Antarctic ozone layer, Science, 2016, 353, 269–274 WMO, Antarctic Ozone Bulletin; 5/2015, ed. [DOI] [PubMed] [Google Scholar]
- 2.Braathen G, World Meteorological Organisation, http://www.wmo.int/pages/prog/arep/WMOAntarcticOzoneBulletins2015.html, 2015
- 3.Butler A, Daniel JS, Portmann RW, Ravishankara A, Young PJ, Fahey DW and Rosenlof KH, Diverse policy implications for future ozone and surface UV in a changing climate, Environ. Res. Lett, 2016, 11(6), 064017 [Google Scholar]
- 4.IPCC, Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change Report No, Cambridge, United Kingdom and New York, NY, USA, 2013, p. 1355 http://www.ipcc.ch/. [Google Scholar]
- 5.Bais AF, McKenzie RL, Bernhard G, Aucamp PJ, Ilyas M, Madronich S and Tourpali K, Ozone depletion and climate change: impacts on UV radiation, Photochem. Photobiol. Sci, 2015, 14, 19–52. [DOI] [PubMed] [Google Scholar]
- 6.Meul S, Dameris M, Langematz U, Abalichin J, Kerschbaumer A, Kubin A and Oberländer-Hayn S, Impact of rising greenhouse gas concentrations on future tropical ozone and UV exposure, Geophys. Res. Lett, 2016, 43, 2919–2927 [Google Scholar]
- 7.Li F, Vikhliaev YV, Newman PA, Pawson S, Perlwitz J, Waugh DW and Douglass AR, Impacts of interactive stratospheric chemistry on Antarctic and Southern Ocean climate change in the Goddard Earth Observing System, Version 5 (GEOS-5), in J. Climate, 2016, vol. 29, pp. 3199–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UNEP EEAP, Environmental effects of ozone depletion and its interactions with climate change: progress report, 2015, Photochem. Photobiol. Sci, 2016, 15, 141–174 [DOI] [PubMed] [Google Scholar]
- 9.Bai K, Chang N-B and Gao W, Quantification of relative contribution of Antarctic ozone depletion to increased austral extratropical precipitation during 1979–2013, J. Geophys. Res.: Atmos, 2016, 121, 1459–1474 [Google Scholar]
- 10.Tao L, Hu Y and Liu J, Anthropogenic forcing on the Hadley circulation in CMIP5 simulations, Clim. Dynam, 2016, 46, 3337–3350 [Google Scholar]
- 11.WMO, Scientific Assessment of Ozone Depletion: 2014, World Meteorological Organisation Report No. 55, Geneva, Switzerland, 2015. [Google Scholar]
- 12.Polvani LM, Camargo SJ and Garcia RR, The importance of the Montreal Protocol in mitigating the potential intensity of tropical cyclones, J. Clim, 2016, 29, 2275–2289 [Google Scholar]
- 13.Nordhaus WD, The economics of hurricanes and implications of global warming, Clim. Change Econom, 2010, 01, 1–20 [Google Scholar]
- 14.Fountoulakis I, Bais AF, Fragkos K, Meleti C, Tourpali K and Zempila MM, Short- and long-term variability of spectral solar UV irradiance at Thessaloniki, Greece: effects of changes in aerosols, total ozone and clouds, Atmos. Chem. Phys, 2016, 16, 2493–2505 [Google Scholar]
- 15.Zempila M-M, Koukouli M-E, Bais A, Fountoulakis I, Arola A, Kouremeti N and Balis D, OMI/Aura UV product validation using NILU-UV ground-based measurements in Thessaloniki, Greece, Atmos. Environ, 2016, 140, 283–297 [Google Scholar]
- 16.Brogniez C, Auriol F, Deroo C, Arola A, Kujanpää J, Sauvage B, Kalakoski N, Pitkänen MRA, Catalfamo M, Metzger JM, Tournois G and Da Conceicao P, Validation of satellite-based noontime UVI with NDACC ground-based instruments: influence of topography, environment and overpass time, Atmos. Chem. Phys. Disc, 2016, 16(23), 15049–15074 [Google Scholar]
- 17.Peltoniemi J, Gritsevich M, Hakala T, Dagsson-Waldhauserová P, Arnalds Ó, Anttila K, Hannula H-R, Kivekäs N, Lihavainen H and Meinander O, Soot on snow experiment: bidirectional reflectance factor measurements of contaminated snow, Cryosphere, 2015, 9, 2323–2337 [Google Scholar]
- 18.McKenzie RL, Aucamp PJ, Bais AF, Björn LO, Ilyas M and Madronich S, Ozone depletion and climate change: Impacts on UV radiation, Photochem. Photobiol. Sci, 2011, 10, 182–198 [DOI] [PubMed] [Google Scholar]
- 19.Norval M, Björn LO and de Gruijl FR, Is the action spectrum for the UV-induced production of previtamin D3 in human skin correct?, Photochem. Photobiol. Sci, 2009, 9, 11–17 [DOI] [PubMed] [Google Scholar]
- 20.Bouillon R, Eisman J, Garabedian M, Holick M, Kleinschmidt J, Suda T and Lucas K, Action spectrum for the production of previtamin D3 in human skin, CIE Report No, Vienna, 2006. [Google Scholar]
- 21.van Dijk A, den Outer P, van Kranen H and Slaper H, The action spectrum for vitamin D3: initial skin reaction and prolonged exposure, Photochem. Photobiol. Sci, 2016, 15, 896–909 [DOI] [PubMed] [Google Scholar]
- 22.Webb AR, Kline L and Holick MF, Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitaminD3 synthesis in human skin, J. Clin. Endocrinol. Metab, 1988, 67, 373–378 [DOI] [PubMed] [Google Scholar]
- 23.Xiang F, Harrison S, Nowak M, Kimlin M, Van der Mei I, Neale RE, Sinclair C, Lucas RM and Aus DSIG, Weekend personal ultraviolet radiation exposure in four cities in Australia: influence of temperature, humidity and ambient ultraviolet radiation, J. Photochem. Photobiol.,B, 2015, 143, 74–81 [DOI] [PubMed] [Google Scholar]
- 24.Murray HC, Maltby VE, Smith DW and Bowden NA, Nucleotide excision repair deficiency in melanoma in response to UVA, Exp. Hematol. Oncol, 2015, 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budden T, Davey RJ, Vilain RE, Ashton KA, Braye SG, Beveridge NJ and Bowden NA, Repair of UVB-induced DNA damage is reduced in melanoma due to low XPC and global genome repair, Oncotarget, 2016, 7(38), 60940–60953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karran P and Brem R, Protein oxidation, UVA and human DNA repair, DNA Repair, 2016, 44, 178–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamenisch Y, Baban TS, Schuller W, von Thaler AK, Sinnberg T, Metzler G, Bauer J, Schittek B, Garbe C, Rocken M and Berneburg M, UVA-Irradiation induces melanoma invasion via the enhanced Warburg effect, J. Invest. Dermatol, 2016, 136(9), 1866–1875 [DOI] [PubMed] [Google Scholar]
- 28.Eding CB, Domert J, Waster P, Jerhammar F, Rosdahl I and Ollinger K, Melanoma growth and progression after ultraviolet a irradiation: impact of lysosomal exocytosis and cathepsin proteases, Acta Derm.-Venereol, 2015, 95, 792–797 [DOI] [PubMed] [Google Scholar]
- 29.Damian DL, Matthews YJ, Phan TA and Halliday GM, An action spectrum for ultraviolet radiation-induced immunosuppression in humans, Br. J. Dermatol, 2011, 164, 657–659 [DOI] [PubMed] [Google Scholar]
- 30.Xiang F, Lucas R, Hales S and Neale R, Incidence of non-melanoma skin cancer in relation to ambient UV radiation in white populations, 1978–2012: empirical relationships, JAMA Dermatol, 2014, 150, 1063–107 [DOI] [PubMed] [Google Scholar]
- 31.Stefansson H, Tryggvadottir L, Olafsdottir EJ, Mooney E, Olafsson JH, Sigurgeirsson B andJonasson JG, Cutaneous melanoma in Iceland: changing Breslow’s tumour thickness, J. Eur.Acad. Dermatol. Venereol, 2015, 29, 346–352 [DOI] [PubMed] [Google Scholar]
- 32.Gordon D, Gillgren P, Eloranta S, Olsson H, Gordon M, Hansson J and Smedby KE, Time trends in incidence of cutaneous melanoma by detailed anatomical location and patterns of ultraviolet radiation exposure: a retrospective population-based study, Melanoma Res, 2015, 25, 348–356 [DOI] [PubMed] [Google Scholar]
- 33.Hoejberg L, Gad D, Gyldenkerne N, Bastholt L and R. Academy of Geriatric Cancer, Trends in melanoma in the elderly in Denmark, 1980–2012, Acta Oncol, 2016, 55(Suppl 1), 52–58 [DOI] [PubMed] [Google Scholar]
- 34.Helvind NM, Holmich LR, Smith S, Glud M, Andersen KK, Dalton SO and Drzewiecki KT, Incidence of in situ and invasive melanoma in Denmark from 1985 through 2012: a national database study of 24 059 melanoma cases, J. Am. Med. Assoc. Dermatol, 2015, 151, 1087–1095 [DOI] [PubMed] [Google Scholar]
- 35.Bay C, Kejs AM, Storm HH and Engholm G, Incidence and survival in patients with cutaneous melanoma by morphology, anatomical site and TNM stage: aDanish Population-based Register Study 1989–2011, Cancer Epidemiol, 2015, 39, 1–7 [DOI] [PubMed] [Google Scholar]
- 36.Greveling K, Wakkee M, Nijsten T, van den Bos RR and Hollestein LM, Epidemiology of Lentigo Maligna and Lentigo Maligna Melanoma in the Netherlands, 1989–2013, J. Invest. Dermatol, 2016, 136(10), 1955–1960 [DOI] [PubMed] [Google Scholar]
- 37.van der Leest RJ, Zoutendijk J, Nijsten T, Mooi WJ, van der Rhee JI, de Vries E and Hollestein LM, Increasing time trends of thin melanomas in The Netherlands: What are the explanations of recent accelerations?, Eur. J. Cancer, 2015, 51, 2833–2841 [DOI] [PubMed] [Google Scholar]
- 38.Ondrusova M, Psenkova M and Suchansky M, Long-term trends in descriptive epidemiology of malignant melanoma in the Slovak Republic, Value Health, 2015, 18, A431. [DOI] [PubMed] [Google Scholar]
- 39.Cossu A, Casula M, Cesaraccio R, Lissia A, Colombino M, Sini MC, Budroni M, Tanda F, Paliogiannis P and Palmieri G, Epidemiology and genetic susceptibility of malignant melanoma in North Sardinia, Italy, Eur. J. Cancer Prev, 2016. DOI: 10.1097/CEJ.0000000000000223 \ [DOI] [PubMed] [Google Scholar]
- 40.Ambrosini-Spaltro A, Dal Cappello T, Deluca J, Carriere C, Mazzoleni G and Eisendle K, Melanoma incidence and Breslow tumour thickness development in the central Alpine region of South Tyrol from 1998 to 2012: a population-based study, J. Eur. Acad. Dermatol. Venereol, 2015, 29, 243–248 [DOI] [PubMed] [Google Scholar]
- 41.Piscitelli P, Neglia C, Falco A, Rivezzi M, Agnello N, Argentiero A, Chitano G, Distante C, Della Rosa G, Vinci G, De Donno A, Distante A and Romanini A, Melanoma in the Italian population and regional environmental influences: a national retrospective survey on 2001– 2008 hospitalization records, Int. J. Environ. Res. Public Health, 2015, 12, 9102–9118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puig S, Marcoval J, Paradelo C, Azon A, Bartralot R, Bel S, Bigata X, Boada A, Campoy A, Carrera C, Curco N, Dalmau J, Ferrandiz C, Ferreres JR, Formigon M, Gallardo F, Gonzalez A, Just M, Llistosella E, Marti RM, Nogues ME, Pedragosa R, Pujol JA, Roldan-Marin R, Sabat M, Salleras M, Smandia JA, Zaballos P, Plana E and Malvehy J, Melanoma incidence increases in the elderly of Catalonia but not in the younger population: effect of prevention or consequence of immigration?, Acta Derm.-Venereol, 2015, 95, 422–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaikh WR, Dusza SW, Weinstock MA, Oliveria SA, Geller AC and Halpern AC, Melanoma thickness and survival trends in the United States, 1989 to 2009, J. Natl. Cancer Inst, 2016, 108(1) DOI: 10.1093/jnci/djv294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell LB, Kreicher KL, Gittleman HR, Strodtbeck K, Barnholtz-Sloan J and Bordeaux JS, Melanoma incidence in children and adolescents: decreasing trends in the United States, J. Pediatr, 2015, 166, 1505–1513 [DOI] [PubMed] [Google Scholar]
- 45.Whiteman DC, Green AC and Olsen CM, The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031, J. Invest. Dermatol, 2016, 136, 1161–1171 [DOI] [PubMed] [Google Scholar]
- 46.Sella T, Goren I, Shalev V, Shapira H, Zandbank J, Rosenblum J, Kimlin MG and Chodick G, Incidence trends of keratinocytic skin cancers and melanoma in Israel 2006–11, Br. J. Dermatol, 2015, 172, 202–207 [DOI] [PubMed] [Google Scholar]
- 47.Razi S, Rafiemanesh H, Ghoncheh M, Khani Y and Salehiniya H, Changing trends of types of skin cancer in Iran, Asian Pac. J. Cancer Prev, 2015, 16, 4955–4958 [DOI] [PubMed] [Google Scholar]
- 48.Lucas RM, Norval M and Wright CY, Solar ultraviolet radiation in Africa: a systematic review and critical evaluation of the health risks and use of photoprotection, Photochem. Photobiol. Sci, 2016, 15, 10–23 [DOI] [PubMed] [Google Scholar]
- 49.Abbas M and Kalia S, Trends in non-melanoma skin cancer (basal cell carcinoma and squamous cell carcinoma) in Canada: a descriptive analysis of available data, J. Cutan. Med. Surg, 2016, 20, 166–175 [DOI] [PubMed] [Google Scholar]
- 50.Asgari MM, Moffet HH, Ray GT and Quesenberry CP, Trends in basal cell carcinoma incidence and identification of high-risk subgroups, 1998–2012, J. Am. Med. Assoc. Dermatol, 2015, 151, 976–981 [DOI] [PubMed] [Google Scholar]
- 51.Callens J, Van Eycken L, Henau K and Garmyn M, Epidemiology of basal and squamous cell carcinoma in Belgium: the need for a uniform and compulsory registration, J. Eur. Acad. Dermatol. Venereol, 2016, 30, 1912–1918 [DOI] [PubMed] [Google Scholar]
- 52.Rogers HW, Weinstock MA, Feldman SR and Coldiron BM, Incidence estimate of non-melanoma skin cancer (keratinocyte carcinomas) in the U.S. Population, 2012, J. Am. Med. Assoc. Dermatol, 2015, 151, 1081–1086 [DOI] [PubMed] [Google Scholar]
- 53.Rudolph C, Schnoor M, Eisemann N and Katalinic A, Incidence trends of nonmelanoma skin cancer in Germany from 1998 to 2010, J. Dtsch. Dermatol. Ges, 2015, 13, 788–797 [DOI] [PubMed] [Google Scholar]
- 54.Doran CM, Ling R, Byrnes J, Crane M, Searles A, Perez D and Shakeshaft A, Estimating the economic costs of skin cancer in New South Wales, Australia, BMC Public Health, 2015, 15,952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gordon LG, Elliott TM, Wright CY, Deghaye N and Visser W, Modelling the healthcare costs of skin cancer in South Africa, BMC Health Serv. Res, 2016, 16, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guy GP Jr., Machlin SR, Ekwueme DU and Yabroff KR, Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011, Am. J. Prev. Med, 2015, 48, 183–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doran CM, Ling R, Byrnes J, Crane M, Shakeshaft AP, Searles A and Perez D, Benefit-cost analysis of three skin cancer public education mass-media campaigns implemented in New South Wales, Australia, PLoS One, 2016, 11, e0147665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fortes C, Mastroeni S, Segatto MM, Hohmann C, Miligi L, Bakos L and Bonamigo R, Occupational exposure to pesticides with occupational sun exposure increases the risk for cutaneous melanoma, J. Occup. Environ. Med, 2016, 58, 370–375 [DOI] [PubMed] [Google Scholar]
- 59.Trakatelli M, Barkitzi K, Apap C, Majewski S, De Vries E and Epiderm group, Skin cancer risk in outdoor workers: a European multicenter case-control study, J. Eur. Acad. Dermatol. Venereol, 2016, 30(Suppl 3), 5–11 [DOI] [PubMed] [Google Scholar]
- 60.John SM, Trakatelli M, Gehring R, Finlay K, Fionda C, Wittlich M, Augustin M, Hilpert G, Barroso Dias JM, Ulrich C and Pellacani G, CONSENSUS REPORT: Recognizing non-melanoma skin cancer, including actinic keratosis, as an occupational disease - A Call to Action, J. Eur. Acad. Dermatol. Venereol, 2016, 30(Suppl 3), 38–45 [DOI] [PubMed] [Google Scholar]
- 61.Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Vakirlis E, Trakatelli M, Kyrgidis A and Ioannides D, Farmers develop more aggressive histologic subtypes of basal cell carcinoma. Experience from a tertiary hospital in Northern Greece, J. Eur. Acad. Dermatol. Venereol, 2016, 30(Suppl 3), 17–20 [DOI] [PubMed] [Google Scholar]
- 62.Sanlorenzo M, Wehner MR, Linos E, Kornak J, Kainz W, Posch C, Vujic I, Johnston K, Gho D, Monico G, McGrath JT, Osella-Abate S, Quaglino P, Cleaver JE and Ortiz-Urda S, The risk of melanoma in airline pilots and cabin crew: a meta-analysis, J. Am. Med. Assoc. Dermatol, 2015, 151, 51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seite S, Fourtanier A, Moyal D and Young AR, Photodamage to human skin by suberythemal exposure to solar ultraviolet radiation can be attenuated by sunscreens: a review, Br. J. Dermatol, 2010, 163, 903–91 [DOI] [PubMed] [Google Scholar]
- 64.Moreno S, Soria X, Martinez M, Marti RM and Casanova JM, Epidemiology of melanocytic naevi in children from Lleida, Catalonia, Spain: protective role of sunscreen in the developmentof acquired moles, Acta Derm.-Venereol, 2016, 96, 479–484 [DOI] [PubMed] [Google Scholar]
- 65.Sinikumpu SP, Huilaja L, Jokelainen J, Auvinen J, Timonen M and Tasanen K, Association of multiple melanocytic naevi with education, sex and skin type: A northern Finland birth cohort 1966 study with 46 years’ follow-up, Acta Derm.-Venereol, 2017, 97 DOI: 10.2340/00015555-2509 [DOI] [PubMed] [Google Scholar]
- 66.Ghiasvand R, Weiderpass E, Green AC, Lund E and Veierod MB, Sunscreen use and subsequent melanoma risk: A population-based cohort study, J. Clin. Oncol, 2016, 33, 3976–3983 [DOI] [PubMed] [Google Scholar]
- 67.Green A, Williams G, Neale R, Hart V, Leslie D, Parsons P, Marks GC, Gaffney P, Battistutta D, Frost C, Lang C and Russell A, Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial, Lancet, 1999, 354, 723–729 [DOI] [PubMed] [Google Scholar]
- 68.Green AC, Williams GM, Logan V and Strutton GM, Reduced melanoma after regular sunscreen use: randomized trial follow-up, J. Clin. Oncol, 2011, 29, 57–63 [DOI] [PubMed] [Google Scholar]
- 69.Olsen CM, Wilson LF, Green AC, Bain CJ, Fritschi L, Neale RE and Whiteman DC, Cancers in Australia attributable to exposure to solar ultraviolet radiation and prevented by regular sunscreen use, Aust. NZ J. Public Health, 2015, 39, 471–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanchez G, Nova J, Rodriguez-Hernandez AE, Medina RD, Solorzano-Restrepo C, Gonzalez J, Olmos M, Godfrey K and Arevalo-Rodriguez I, Sun protection for preventing basal cell and squamous cell skin cancers, Cochrane Database Syst. Rev, 2016, 7, CD011161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eastabrook S, Chang P and Taylor MF, Melanoma risk: adolescent females’ perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage, Global Health Promot, 2016. DOI: 10.1177/1757975916639871. [DOI] [PubMed] [Google Scholar]
- 72.Gellen E, Janka E, Tamas I, Adam B, Horkay I, Emri G and Remenyik E, Pigmented naevi and sun protection behaviour among primary and secondary school students in an Eastern Hungarian city, Photodermatol., Photoimmunol. Photomed, 2016, 32, 98–106 [DOI] [PubMed] [Google Scholar]
- 73.Flannery C, Burke LA, Grainger L, Williams P and Gage H, Risky sun tanning behaviours amongst Irish University students: a quantitative analysis, Ir. J. Med. Sci, 2016, 185(4), 887–893 [DOI] [PubMed] [Google Scholar]
- 74.Mortier L, Lepesant P, Saiag P, Robert C, Sassolas B, Grange F, Lhomel C and Lebbe C, Comparison of sun protection modalities in parents and children, J. Eur. Acad. Dermatol. Venereol, 2015, 29(Suppl 2), 16–19 [DOI] [PubMed] [Google Scholar]
- 75.Nahar VK, Allison Ford M, Brodell RT, Boyas JF, Jacks SK, Biviji-Sharma R, Haskins MA and Bass MA, Skin cancer prevention practices among malignant melanoma survivors: A systematic review, J. Cancer Res. Clin. Oncol, 2016, 142, 1273–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tripp MK, Peterson SK, Prokhorov AV, Shete SS, Lee JE, Gershenwald JE and Gritz ER, Correlates of sun protection and sunburn in children of melanoma survivors, Am. J. Prev.Med, 2016, 51(3), e77–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Secnikova Z, Gopfertova D, Hoskova L, Hercogova J, Dzambova M, Jirakova A, Rajska L, Rob F and Smerhovsky Z, Significantly higher incidence of skin cancer than other malignancies in patients after heart transplantation. A retrospective cohort study in the Czech Republic, Biomed. Pap. Med. Fac. Univ Palacky Olomouc, Czechoslovakia, 2015, 159, 648–651. [DOI] [PubMed] [Google Scholar]
- 78.Vajdic CM, Mayson E, Dodds AJ, O’Brien T, Wilcox L, Nivison-Smith I, Le Marsney R, Daniels B, Ashton LJ and C. s. investigators, Second cancer risk and late mortality in adult Australians receiving allogeneic hematopoietic stem cell transplantation: a population-based cohort study, Biol. Blood Marrow Transplant, 2016, 22, 949–956 [DOI] [PubMed] [Google Scholar]
- 79.Robbins HA, Clarke CA, Arron ST, Tatalovich Z, Kahn AR, Hernandez BY, Paddock L, Yanik EL, Lynch CF, Kasiske BL, Snyder J and Engels EA, Melanoma risk and survival among organ transplant recipients, J. Invest. Dermatol, 2015, 135, 2657–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goncalves CP, Trope BM and Ramos ESM, Non-melanoma skin cancer in renal transplant recipients: a study in a Brazilian reference center, Clin. Cosmet. Invest. Dermatol, 2015, 8, 339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuschal C, Thoms KM, Schubert S, Schafer A, Boeckmann L, Schon MP and Emmert S, Skin cancer in organ transplant recipients: effects of immunosuppressive medications on DNA repair, Exp. Dermatol, 2012, 21, 2–6. [DOI] [PubMed] [Google Scholar]
- 82.Korostil IA and Regan DG, Varicella-zoster virus in Perth, Western Australia: seasonality and reactivation, PLoS One, 2016, 11, e0151319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zak-Prelich M, Borkowski JL, Alexander F and Norval M, The role of solar ultraviolet irradiation in zoster, Epidemiol. Infect, 2002, 129, 593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jung HS, Kang JK and Yoo SH, Epidemiological study on the incidence of herpes zoster in nearby Cheonan, Korean J. Pain, 2015, 28, 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu PY, Wu HD, Chou TC and Sung FC, Varicella vaccination alters the chronological trends of herpes zoster and varicella, PLoS One, 2013, 8, e77709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cahoon E, Engels E, Freedman M, Norval M and Pfeiffer R, Ultraviolet radiation and Kaposi sarcoma in a nationwide US cohort of HIV-infected men, J. Natl. Cancer Inst, 2017, 109(5) DOI: 10.1093/jnci/djw267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rivolta C, Royer-Bertrand B, Rimoldi D, Schalenbourg A, Zografos L, Leyvraz S and Moulin A, UV light signature in conjunctival melanoma; not only skin should be protected from solar radiation, J. Hum. Genet, 2016, 61, 361–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harbour JW, Uveal and conjunctival melanoma: close together-but only distantly related, Oncology, 2016, 30(1), 44. [PubMed] [Google Scholar]
- 89.Mundy KM, Nichols E and Lindsey J, Socioeconomic disparities in cataract prevalence, characteristics, and management, Semin. Ophthalmol, 2016, 31, 358–363. [DOI] [PubMed] [Google Scholar]
- 90.Yu JM, Yang DQ, Wang H, Xu J, Gao Q, Hu LW, Wang F, Wang Y, Yan QC, Zhang JS and Liu Y, Prevalence and risk factors of lens opacities in rural populations living at two different altitudes in China, Int. J. Ophthalmol, 2016, 9, 610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu M, Yu J, Gao Q, Wang Y, Hu L, Zheng Y, Wang F and Liu Y, The relationship between disability-adjusted life years of cataracts and ambient erythemal ultraviolet radiation in China, J. Epidemiol, 2015, 25, 57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dolgin E, The myopia boom, Nature, 2015, 519, 276–278. [DOI] [PubMed] [Google Scholar]
- 93.Jin JX, Hua WJ, Jiang X, Wu XY, Yang JW, Gao GP, Fang Y, Pei CL, Wang S, Zhang JZ, Tao LM and Tao FB, Effect of outdoor activity on myopia onset and progression in school-aged children in northeast China: the Sujiatun Eye Care Study, BMC Ophthalmol, 2015, 15, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He M, Xiang F, Zeng Y, Mai J, Chen Q, Zhang J, Smith W, Rose K and Morgan IG, Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial, J. Am. Med. Assoc, 2015, 314, 1142–1148. [DOI] [PubMed] [Google Scholar]
- 95.Yazar S, Hewitt AW, Black LJ, McKnight CM, Mountain JA, Sherwin JC, Oddy WH, Coroneo MT, Lucas RM and Mackey DA, Myopia is associated with lower vitamin D status in young adults, Invest. Ophthalmol. Visual Sci, 2014, 55, 4552–4559. [DOI] [PubMed] [Google Scholar]
- 96.Tideman JW, Polling JR, Voortman T, Jaddoe VW, Uitterlinden AG, Hofman A, Vingerling JR, Franco OH and Klaver CC, Low serum vitamin D is associated with axial length and risk of myopia in young children, Eur. J. Epidemiol, 2016, 31, 491–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Read SA, Collins MJ and Vincent SJ, Light exposure and eye growth in childhood, Invest. Ophthalmol. Visual Sci, 2015, 56, 6779–6787. [DOI] [PubMed] [Google Scholar]
- 98.Institute of Medicine, Dietary Reference Intakes for Calcium and Vitamin D, Institute of Medicine of the National Academies Report No, 2010. http://www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D.aspx.
- 99.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM and Endocrine S, Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline, J. Clin. Endocrinol. Metab, 2011, 96, 1911–1930. [DOI] [PubMed] [Google Scholar]
- 100.Wu F, Wills K, Laslett LL, Oldenburg B, Seibel MJ, Jones G and Winzenberg T, Cut-points for associations between vitamin D status and multiple musculoskeletal outcomes in middle-aged women, Osteopor. Int, 2016. DOI: 10.1007/s00198-016-3754-9. [DOI] [PubMed] [Google Scholar]
- 101.Kimlin MG, Lucas RM, Harrison SL, van der Mei I, Armstrong BK, Whiteman DC, Kricker A, Nowak M, Brodie AM and Sun J, The contributions of solar ultraviolet radiation exposure and other determinants to serum 25-hydroxyvitamin D concentrations in Australian adults: the AusD Study, Am. J. Epidemiol, 2014, 179, 864–874. [DOI] [PubMed] [Google Scholar]
- 102.Rice SA, Carpenter M, Fityan A, Vearncombe LM, Ardern-Jones M, Jackson AA, Cooper C, Baird J and Healy E, Limited exposure to ambient ultraviolet radiation and 25- hydroxyvitamin D levels: a systematic review, Br. J. Dermatol, 2015, 172, 652–661. [DOI] [PubMed] [Google Scholar]
- 103.Feng H, Xun P, Pike K, Wills AK, Chawes BL, Bisgaard H, Cai W, Wan Y and He K, In utero exposure to 25(OH) D and risk of childhood asthma, wheeze and respiratory tract infections: A meta-analysis of birth cohort studies, J. Allergy Clin. Immunol, 2016. DOI: 10.1016/j.jaci.2016.06.065. [DOI] [PubMed] [Google Scholar]
- 104.Hollams E, Teob S, Kusel M, Holt B, Holt K, Inouye M, de Klerk N, Zhang G, Sly P, Hart P and Holt P, Vitamin D over the first decade and susceptibility to childhood allergy and asthma, J. Allergy. Clin. Immunol, 2016. DOI: 10.1016/j.jaci.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 105.Martineau AR, Cates CJ, Urashima M, Jensen M, Griffiths AP, Nurmatov U, Sheikh A and Griffiths CJ, Vitamin D for the management of asthma, Cochrane Database Syst. Rev, 2016, 9, CD011511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lucas RM, Byrne SN, Correale J, Ilschner S and Hart PH, Ultraviolet radiation, vitamin D and multiple sclerosis, Neurodegener. Dis. Manag, 2015, 5, 413–424. [DOI] [PubMed] [Google Scholar]
- 107.Wright F and Weller RB, Risks and benefits of UV radiation in older people: More of a friend than a foe?, Maturitas, 2015, 81, 425–431. [DOI] [PubMed] [Google Scholar]
- 108.Lindqvist PG, Epstein E, Nielsen K, Landin-Olsson M, Ingvar C and Olsson H, Avoidance of sun exposure as a risk factor for major causes of death: A competing risk analysis of the Melanoma in Southern Sweden cohort, J. Intern. Med, 2016, 280(4), 375–387. [DOI] [PubMed] [Google Scholar]
- 109.Cashman KD, Dowling KG, Skrabakova Z, Gonzalez-Gross M, Valtuena J, De Henauw S, Moreno L, Damsgaard CT, Michaelsen KF, Molgaard C, Jorde R, Grimnes G, Moschonis G, Mavrogianni C, Manios Y, Thamm M, Mensink GB, Rabenberg M, Busch MA, Cox L, Meadows S, Goldberg G, Prentice A, Dekker JM, Nijpels G, Pilz S, Swart KM, van Schoor NM, Lips P, Eiriksdottir G, Gudnason V, Cotch MF, Koskinen S, Lamberg-Allardt C, Durazo-Arvizu RA, Sempos CT and Kiely M, Vitamin D deficiency in Europe: pandemic?, Am. J. Clin. Nutr, 2016, 103, 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schleicher RL, Sternberg MR, Lacher DA, Sempos CT, Looker AC, Durazo-Arvizu RA, Yetley EA, Chaudhary-Webb M, Maw KL, Pfeiffer CM and Johnson CL, The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25- hydroxyvitamin D shows recent modest increases, Am. J. Clin. Nutr, 2016, 104, 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Australian Bureau of Statistics, Australian Health Survey: Biomedical Results for Nutrients, 2011– 12, Australian Bureau of Statistics Report No. 4364.0.55.006, 6 November 2014 2014. http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4364.0.55.006Chapter2002011-12.
- 112.Palacios C and Gonzalez L, Is vitamin D deficiency a major global public health problem?, J. Steroid Biochem. Mol. Biol, 2014, 144(Pt A), 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martin CA, Gowda U and Renzaho AM, The prevalence of vitamin D deficiency among dark-skinned populations according to their stage of migration and region of birth: A meta-analysis, Nutrition, 2016, 32, 21–32 [DOI] [PubMed] [Google Scholar]
- 114.Scragg RK, Stewart AW, McKenzie RL, Reeder AI, Liley JB and Allen MW, Sun exposure and 25-hydroxyvitamin D3 levels in a community sample: quantifying the association with electronic dosimeters, J. Expo. Sci. Environ. Epidemiol, 2016, 175, 1320–1328 [DOI] [PubMed] [Google Scholar]
- 115.Datta P, Philipsen PA, Olsen P, Petersen B, Johansen P, Morling N and Wulf HC, Major inter-personal variation in the increase and maximal level of 25-hydroxy vitamin D induced by UVB, Photochem. Photobiol. Sci, 2016, 15, 536–545. [DOI] [PubMed] [Google Scholar]
- 116.Felton SJ, Cooke MS, Kift R, Berry JL, Webb AR, Lam PM, de Gruijl FR, Vail A and Rhodes LE, Concurrent beneficial (vitamin D production) and hazardous (cutaneous DNA damage) impact of repeated low-level summer sunlight exposures, Br. J. Dermatol, 2016, 175(6), 1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, Wedge DC, Fullam A, Alexandrov LB, Tubio JM, Stebbings L, Menzies A, Widaa S, Stratton MR, Jones PH and Campbell PJ, Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin, Science, 2015, 348, 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bogh MK, Schmedes AV, Philipsen PA, Thieden E and Wulf HC, Interdependence between body surface area and ultraviolet B dose in vitamin D production: a randomized controlled trial, Br. J. Dermatol, 2011, 164, 163–169. [DOI] [PubMed] [Google Scholar]
- 119.Kockott D, Herzog B, Reichrath J, Keane K and Holick MF, New approach to develop optimized sunscreens that enable cutaneous vitamin D formation with minimal erythema risk, PLoS One, 2016, 11, e0145509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Robinson SA and Erickson DJ, Not just about sunburn - the ozone hole’s profound effect on climate has significant implications for Southern Hemisphere ecosystems, Glob. Chang. Biol, 2015, 21, 515–527. [DOI] [PubMed] [Google Scholar]
- 121.Bornman JF, Barnes PW, Robinson SA, Ballaré CL, Flint SD and Caldwell MM, Solar ultraviolet radiation and ozone depletion-driven climate change: Effects on terrestrial ecosystems, Photochem. Photobiol. Sci, 2015, 14, 88–107. [DOI] [PubMed] [Google Scholar]
- 122.Royles J, Amesbury MJ, Roland TP, Jones GD, Convey P, Griffiths H, Hodgson DA and Charman DJ, Moss stable isotopes (carbon-13, oxygen-18) and testate amoebae reflect environmental inputs and microclimate along a latitudinal gradient on the Antarctic Peninsula, Oecologia, 2016, 181, 931–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hideg E, Jansen MAK and Strid A, UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates?, Trends Plant Sci, 2013, 18, 107–115. [DOI] [PubMed] [Google Scholar]
- 124.Czegeny G, Wu M, Der A, Eriksson LA, Strid A and Hideg E, Hydrogen peroxide contributes to the ultraviolet-B (280–315 nm) induced oxidative stress of plant leaves through multiple pathways, FEBS Lett, 2014, 588, 2255–2261. [DOI] [PubMed] [Google Scholar]
- 125.Muller-Xing R, Xing Q and Goodrich J, Footprints of the sun: Memory of UV and light stress in plants, Front. Plant Sci, 2014, 5, 474–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Madronich S, Shao M, Wilson SR, Solomon KR, Longstreth JD and Tang XY, Changes in air quality and tropospheric composition due to depletion of stratospheric ozone and interactions with changing climate: implications for human and environmental health, Photochem. Photobiol. Sci, 2015, 14, 149–169. [DOI] [PubMed] [Google Scholar]
- 127.Choudhary KK and Agrawal SB, Cultivar specificity of tropical mung bean (Vigna radiata L.) to elevated ultraviolet-B: Changes in antioxidative defense system, nitrogen metabolism and accumulation of jasmonic and salicylic acids, Environ. Exp. Bot, 2014, 99, 122–132 [Google Scholar]
- 128.Koukounaras A, Siomos AS, Gerasopoulos D and Karamanoli K, Genotype, ultravioletirradiation, and harvesting time interaction effects on secondary metabolites of whole lettuce and browning of fresh-cut product, J. Hortic. Sci. Biotechnol, 2016, 91, 491–496. [Google Scholar]
- 129.Biever JJ and Gardner G, The relationship between multiple UV-B perception mechanisms and DNA repair pathways in plants, Environ. Exp. Bot, 2016, 124, 89–99. [Google Scholar]
- 130.Mazza CA and Ballaré CL, Photoreceptors UVR8 and phytochrome B cooperate to optimize plant growth and defense in patchy canopies, New Phytol, 2015, 207, 4–9. [DOI] [PubMed] [Google Scholar]
- 131.Yin RH, Skvortsova MY, Loubery S and Ulm R, COP1 is required for UV-B-induced nuclear accumulation of the UVR8 photoreceptor, Proc. Natl. Acad. Sci. U. S. A, 2016, 113, E4415–E4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Binkert M, Crocco CD, Ekundayo B, Lau K, Raffelberg S, Tilbrook K, Yin R, Chappuis R, Schalch T and Ulm R, Revisiting chromatin binding of the Arabidopsis UV-B photoreceptor UVR8, BMC Plant Biol, 2016, 16, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao C, Mao K, You CX, Zhao XY, Wang SH, Li YY and Hao YJ, Molecular cloning and functional analysis of a UV-B photoreceptor gene, MdUVR8 (UV Resistance Locus 8), from apple, Plant Sci, 2016, 247, 115–126. [DOI] [PubMed] [Google Scholar]
- 134.Ulm R and Jenkins GI, Q&A: How do plants sense and respond to UV-B radiation?, BMC Biol, 2015, 13, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Demkura PV and Ballaré CL, UVR8 mediates UV-B-induced Arabidopsis defense responses against Botrytis cinerea by controlling sinapate accumulation, Mol. Plant, 2012, 5, 642–652. [DOI] [PubMed] [Google Scholar]
- 136.Ballaré CL, Light regulation of plant defense, Annu. Rev. Plant Biol, 2014, 65, 335–363. [DOI] [PubMed] [Google Scholar]
- 137.Chen MH, Huang YY, Liu G, Qin F, Yang S and Xu X, Effects of enhanced UV-B radiation on morphology, physiology, biomass, leaf anatomy and ultrastructure in male and female mulberry (Morus alba) saplings, Environ. Exp. Bot, 2016, 129, 85–93. [Google Scholar]
- 138.Randriamanana TR, Nissinen K, Moilanen J, Nybakken L and Julkunen-Tiitio R, Long-term UV-B and temperature enhancements suggest that females of Salix myrsinifolia plants are more tolerant to UV-B than males, Environ. Exp. Bot, 2015, 109, 296–305. [Google Scholar]
- 139.Maja MM, Kasurinen A, Holopainen T, Julkunen-Tiitto R and Holopainen JK, The effect of warming and enhanced ultraviolet radiation on gender-specific emissions of volatile organic compounds from European aspen, Sci. Total Environ, 2016, 547, 39–47. [DOI] [PubMed] [Google Scholar]
- 140.Boeckler GA, Gershenzon J and Unsicker SB, Phenolic glycosides of the Salicaceae and their role as anti-herbivore defenses, Phytochemistry, 2011, 72, 1497–1509. [DOI] [PubMed] [Google Scholar]
- 141.Randriamanana TR, Lavola A and Julkunen-Tiitto R, Interactive effects of supplemental UV-B and temperature in European aspen seedlings: Implications for growth, leaf traits, phenolic defense and associated organisms, Plant Physiol. Biochem, 2015, 93, 84–93. [DOI] [PubMed] [Google Scholar]
- 142.Xu XA, Zhao HX, Zhang XL, Hanninen H, Korpelainen H and Li CY, Different growth sensitivity to enhanced UV-B radiation between male and female Populus cathayana, Tree Physiol, 2010, 30, 1489–1498. [DOI] [PubMed] [Google Scholar]
- 143.IPPC, Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, ed. Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR and White LL, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2014, p. 1132. [Google Scholar]
- 144.Wolf A, Zimmerman NB, Anderegg WRL, Busby PE and Christensen J, Altitudinal shifts of the native and introduced flora of California in the context of 20th-century warming, Glob. Ecol. Biogeogr, 2016, 25, 418–429. [Google Scholar]
- 145.Wang H, Ma XC, Zhang L, Siemann E and Zou JW, UV-B has larger negative impacts on invasive populations of Triadica sebifera but ozone impacts do not vary, J. Plant Ecol, 2016, 9, 61–68. [Google Scholar]
- 146.Ruhland CT, Dyslin MJ and Krenz JD, Wyoming big sagebrush screens ultraviolet radiation more effectively at higher elevations, J. Arid Environ, 2013, 96, 19–22. [Google Scholar]
- 147.Wang Q-W, Nagano S, Ozaki H, Morinaga S-I, Hidema J and Hikosaka K, Functional differentiation in UV-B-induced DNA damage and growth inhibition between highland and lowland ecotypes of two Arabidopsis species, Environ. Exp. Bot, 2016, 131, 110–119. [Google Scholar]
- 148.Stark S, Vaisanen M, Ylanne H, Julkunen-Tiitto R and Martz F, Decreased phenolic defence in dwarf birch (Betula nana) after warming in subarctic tundra, Polar Biol, 2015, 38, 1993–2005. [Google Scholar]
- 149.Feister U, Cabrol N and Hader D, UV irradiance enhancements by scattering of solar radiation from clouds, Atmosphere, 2015, 6, 1211–1228. [Google Scholar]
- 150.Heisler GM, Grant RH and Gao W, Individual- and scattered-tree influences on ultraviolet irradiance, Agric. Forest Meteorol, 2003, 120, 113–126. [Google Scholar]
- 151.Björn LO, On the history of phyto-photo UV science (not to be left in skoto toto and silence), Plant Physiol. Biochem, 2015, 93, 3–8. [DOI] [PubMed] [Google Scholar]
- 152.Nenadis N, Llorens L, Koufogianni A, Diaz L, Font J, Abel Gonzalez J and Verdaguer D, Interactive effects of UV radiation and reduced precipitation on the seasonal leaf phenolic content/composition and the antioxidant activity of naturally growing Arbutus unedo plants, J. Photochem. Photobiol., B, 2015, 153, 435–444. [DOI] [PubMed] [Google Scholar]
- 153.Sullivan JH, Gitz DC, Liu-Gitz L, Xu CP, Gao W and Slusser J, Coupling short-term changes in ambient UV-B levels with induction of UV-screening compounds, Photochem. Photobiol, 2007, 83, 863–870. [DOI] [PubMed] [Google Scholar]
- 154.Barnes PW, Flint SD, Rye RJ, Tobler MA, Barkley AE and Wargent JJ, Rediscovering leaf optical properties: New insights into plant acclimation to solar UV radiation, Plant Physiol. Biochem, 2015, 93, 94–100. [DOI] [PubMed] [Google Scholar]
- 155.Barnes PW, Tobler MA, Keefover-Ring K, Flint SD, Barkley AE, Ryel RJ and Lindroth RL, Rapid modulation of ultraviolet shielding in plants is influenced by solar ultraviolet radiation and linked to alterations in flavonoids, Plant Cell Environ, 2016, 39, 222–230. [DOI] [PubMed] [Google Scholar]
- 156.Barnes PW, Flint SD, Tobler MA and Ryel RJ, Diurnal adjustment in UV-sunscreen protection is widespread among higher plants, Oecologia, 2016, 181, 55–63. [DOI] [PubMed] [Google Scholar]
- 157.Wargent JJ, Nelson BCW, McGhie TK and Barnes PW, Acclimation to UV-B radiation and visible light in Lactuca sativa involves up-regulation of photosynthetic performance and orchestration of metabolome-wide responses, Plant Cell Environ, 2015, 38, 929–940. [DOI] [PubMed] [Google Scholar]
- 158.Kim S-G, Yon F, Gaquerel E, Gulati J and Baldwin IT, Tissue specific diurnal rhythms of metabolites and their regulation during herbivore attack in a native tobacco, Nicotiana attenuata, PLoS One, 2011, 6, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Takeuchi T, Newton L, Burkhardt A, Mason S and Farre EM, Light and the circadian clock mediate time-specific changes in sensitivity to UV-B stress under light/dark cycles, J. Exp. Bot, 2014, 65, 6003–6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Horak E and Farre EM, The regulation of UV-B responses by the circadian clock, Plant Signal. Behav, 2015, 10, e1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Goodspeed D, Chehab EW, Min-Venditti A, Braam J and Covington MF, Arabidopsis synchronizes jasmonate-mediated defense with insect circadian behavior, Proc. Natl. Acad. Sci. U. S. A, 2012, 109, 4674–4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Julkunen-Tiitto R, Nenadis N, Neugart S, Robson M, Agati G, Vepsäläinen J, Zipoli G, Nybakken L, Winkler B and Jansen MAK, Assessing the response of plant flavonoids to UV radiation: an overview of appropriate techniques, Phytochem. Rev, 2015, 14, 273–297. [Google Scholar]
- 163.Schreiner M, Mewis I, Huyskens-Keil S, Jansen MAK, Zrenner R, Winkler JB, O’Brien N and Krumbein A, UV-B-induced secondary plant metabolites - potential benefits for plant and human health, Crit. Rev. Plant Sci, 2012, 31, 229–240. [Google Scholar]
- 164.Wargent JJ, UV LEDs in horticulture: From biology to application, Acta Hortic, 2016, 1134(4), 25–32. [Google Scholar]
- 165.Martinez-Luscher J, Sanchez-Diaz M, Delrot S, Aguirreolea J, Pascual I and Gomes E, Ultraviolet-B alleviates the uncoupling effect of elevated CO2 and increased temperature on grape berry (Vitis vinifera cv. Tempranillo) anthocyanin and sugar accumulation, Aust. J. Grape Wine Res, 2016, 22, 87–95. [Google Scholar]
- 166.Rinaldo AR, Cavallini E, Jia Y, Moss SMA, McDavid DAJ, Hooper LC, Robinson SP, Tornielli GB, Zenoni S, Ford CM, Boss PK and Walker AR, A grapevine anthocyanin acyltransferase, transcriptionally regulated by VvMYBA, can produce most acylated anthocyanins present in grape skins, Plant Physiol, 2015, 169, 1897–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Singh SK, Reddy KR, Reddy VR and Gao W, Maize growth and developmental responses to temperature and ultraviolet-B radiation interaction, Photosynthetica, 2014, 52, 262–271. [Google Scholar]
- 168.Suchar VA and Robberecht R, Integration and scaling of UV-B radiation effects on plants: from DNA to leaf, Ecol. Evolut, 2015, 5, 2544–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Di Ferdinando M, Brunetti C, Agati G and Tattini M, Multiple functions of polyphenols in plants inhabiting unfavorable Mediterranean areas, Environ. Exp. Bot, 2014, 103, 107–116 [Google Scholar]
- 170.Swann AL, Hoffman FM, Koven CD and Randerson JT, Plant responses to increasing 2 CO reduce estimates of climate impacts on drought severity, Proc. Natl. Acad. Sci. U. S. A, 2016, 113, 10019 R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Negash L and Björn LO, Stomatal closure by ultraviolet radiation, Physiol. Plant, 1986, 66, 360–364. [Google Scholar]
- 172.He JM, Zhang Z, Wang RB and Chen YP, UV-B-induced stomatal closure occurs via ethylene-dependent NO generation in Vicia faba, Funct. Plant Biol, 2011, 38, 293–302 [DOI] [PubMed] [Google Scholar]
- 173.Tossi V, Lamattina L, Jenkins GI and Cassia RO, Ultraviolet-B-induced stomatal closure in Arabidopsis is regulated by the UV RESISTANCE LOCUS8 photoreceptor in a nitric oxide-dependent mechanism, Plant Physiol, 2014, 164, 2220–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Eisinger W, Swartz TE, Bogomolni RA and Taiz L, The ultraviolet action spectrum for stomatal opening in broad bean, Plant Physiol, 2000, 122, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jansen MAK and vandenNoort RE, Ultraviolet-B radiation induces complex alterations in stomatal behaviour, Physiol. Plant, 2000, 110, 189–194. [Google Scholar]
- 176.Doupis G, Bosabalidis AM and Patakas A, Comparative effects of water deficit and enhanced UV-B radiation on photosynthetic capacity and leaf anatomy traits of two grapevine (Vitis vinifera L.) cultivars, Theoret. Exp. Plant Physiol, 2016, 28, 131–141. [Google Scholar]
- 177.Wijewardana C, Henry WB, Gao W and Reddy KR, Interactive effects on CO2, drought, and ultraviolet-B radiation on maize growth and development, J. Photochem. Photobiol., B, 2016, 160, 198–209. [DOI] [PubMed] [Google Scholar]
- 178.Martinez-Luscher J, Morales F, Sanchez-Diaz M, Delrot S, Aguirreolea J, Gomes E and Pascual I, Climate change conditions (elevated CO2 and temperature) and UV-B radiation affect grapevine (Vitis vinifera cv. Tempranillo) leaf carbon assimilation, altering fruit ripening rates, Plant Sci, 2015, 236, 168–176. [DOI] [PubMed] [Google Scholar]
- 179.Williamson CE, Overholt EP, Brentrup JA, Pilla RM, Leach TH, Schladow SG, Warren JD, Urmy SS, Sadro S, Chandra S and Neale PJ, Sentinel responses to droughts, wildfires, and floods: effects of UV radiation on lakes and their ecosystem services, Front. Ecol. Environ, 2016, 14, 109–109. [Google Scholar]
- 180.Monteith DT, Stoddard JL, Evans CD, de Wit H, Forsius M, Høgåsen T, Wilander A, Skjelkvåle BL, Jeffries DS, Vuorenmaa J, Keller B, Kopácek J and Vesely J, Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry, Nat. Biotechnol, 2007, 450, 537–541. [DOI] [PubMed] [Google Scholar]
- 181.Williamson CE, Overholt EP, Pilla RM, Leach TH, Brentrup JA, Knoll LB, Mette EM and Moeller RE, Ecological consequences of long-term browning in lakes, Sci. Rep, 2015, 5, 18666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Finstad AG, Helland IP, Ugedal O, Hesthagen T and Hessen DO, Unimodal response of fish yield to dissolved organic carbon, Ecol. Lett, 2014, 17, 36–43. [DOI] [PubMed] [Google Scholar]
- 183.Benoit PO, Beisner BE and Solomon CT, Growth rate and abundance of common fishes is negatively related to dissolved organic carbon concentration in lakes, Can. J. Fish. Aquat. Sci, 2016, 73, 1230–1236. [Google Scholar]
- 184.Robidoux M, del Giorgio P and Derry A, Effects of humic stress on the zooplankton from clear and DOC-rich lakes, Freshwater Biol, 2015, 60, 1263–1278. [Google Scholar]
- 185.Kelly PT, Craig N, Solomon CT, Weidel BC, Zwart JA and Jones SE, Experimental whole-lake increase of dissolved organic carbon concentration produces unexpected increase in crustacean zooplankton density, Glob. Chang. Biol, 2016, 22, 2766–2775. [DOI] [PubMed] [Google Scholar]
- 186.Fichot CG, Kaiser K, Hooker SB, Amon RMW, Babin M, Bélanger S, Walker SA and Benner R, Pan-Arctic distributions of continental runoff in the Arctic Ocean, Sci. Rep, 2013, 3, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vonk JE, Tank SE, Bowden WB, Laurion I, Vincent WF, Alekseychik P, Amyot M, Billet MF, Canário J, Cory RM, Deshpande BN, Helbig M, Jammet M, Karlsson J, Larouche J, MacMillan G, Rautio M, Walter Anthony KM and Wickland KP, Reviews and syntheses: effects of permafrost thaw on Arctic aquatic ecosystems, Biogeosciences, 2015, 12, 7129–7167. [Google Scholar]
- 188.Fichot CG, Lohrenz SE and Benner R, Pulsed, cross-shelf export of terrigenous dissolved organic carbon to the Gulf of Mexico, J. Geophys. Res. Oceans, 2014, 119, 1176–1194. [Google Scholar]
- 189.Baum A, Rixen T and Samiaji J, Relevance of peat draining rivers in central Sumatra for the riverine input of dissolved organic carbon into the ocean, Estuar. Coastal Shelf Sci, 2007, 73, 563–570. [Google Scholar]
- 190.Moore S, Gauci V, Evans CD and Page SE, Fluvial organic carbon losses from a Bornean blackwater river, Biogeosciences, 2011, 8, 901–909. [Google Scholar]
- 191.DeVilbiss SE, Zhou ZZ, Klump JV and Guo LD, Spatiotemporal variations in the abundance and composition of bulk and chromophoric dissolved organic matter in seasonally hypoxia-influenced Green Bay, Lake Michigan, USA, Sci. Total Environ, 2016, 565, 742–757. [DOI] [PubMed] [Google Scholar]
- 192.Gueguen C, Mokhtar M, Perroud A, McCullough G and Papakyriakou T, Mixing and photoreactivity of dissolved organic matter in the Nelson/Hayes estuarine system (Hudson Bay, Canada), J. Mar. Syst, 2016, 161, 42–48. [Google Scholar]
- 193.Huovinen P, Ramirez J and Gomez I, Underwater optics in sub-Antarctic and Antarctic coastal ecosystems, PLoS One, 2016, 11, e0154887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Perry M and Troccoli A, Impact of a fire burn on solar irradiance and PV power, Sol. Energy, 2015, 114, 167–173. [Google Scholar]
- 195.Urmy S, Williamson CE, Leach TH, Schladow SG, Overholt E and Warren JD, Vertical redistribution of zooplankton in an oligotrophic lake associated with reduction in ultraviolet radiation by wildfire smoke, Geophys. Res. Lett, 2016, 43(8), 3746–3753. [Google Scholar]
- 196.Havens K, Paerl H, Phlips E, Zhu M, Beaver J and Srifa A, Extreme weather events and climate variability provide a lens to how shallow lakes may respond to climate change, Water, 2016, 8, 229. [Google Scholar]
- 197.Havens K, Fulton III RS, Beaver JR, Samples EE and Colee J, Effects of climate variability on cladoceran zooplankton and cyanobacteria in a shallow subtropical lake, J. Plankt. Res, 2016, 38, 418–430. [Google Scholar]
- 198.Strock KE, Saros JE, Nelson SJ, Birkel SD, Kahl JS and McDowell WH, Extreme weather years drive episodic changes in lake chemistry: implications for recovery from sulfate deposition and long-term trends in dissolved organic carbon, Biogeochemistry, 2016, 127, 353–365. [Google Scholar]
- 199.Magnuson JJ, Robertson DM, Benson BJ, Whynne RH, Livinstone DM, Arai T, Assel RA, Barry RG, Card V, Kuusisto E, Granin NG, Prowse KM, Steward KM and Vuglinski VS, Historical trends in lake and river ice cover in the Northern Hemisphere., Science, 2000, 289, 1743–1746. [DOI] [PubMed] [Google Scholar]
- 200.Hylander S, Kiørboe T, Snoeijs P, Sommaruga R and Nielsen TG, Concentrations of sunscreens and antioxidant pigments in Arctic Calanus spp. in relation to ice cover, ultraviolet radiation, and the phytoplankton spring bloom, Limnol. Oceanogr, 2015, 60, 2197–2206. [Google Scholar]
- 201.Rose KC, Hamilton DP, Williamson CE, McBride CG, Fischer JM, Olson MH, Saros JE, Allan MG and Cabrol N, Light attenuation characteristics of glacially-fed lakes, J. Geophys. Res. Biogeosci, 2014, 119, 1446–1457. [Google Scholar]
- 202.Sommaruga R, When glaciers and ice sheets melt: consequences for planktonic organisms, J. Plankt. Res, 2015, 37, 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Boyd PW and Doney SC, Modelling regional responses by marine pelagic ecosystems to global climate change, Geophys. Res. Lett, 2002, 29, 53. [Google Scholar]
- 204.Cetina-Heredia P, Roughan M, van Sebille E, Feng M and Coleman MA, Strengthened currents override the effect of warming on lobster larval dispersal and survival, Glob. Chang. Biol, 2015, 21, 4377–4386. [DOI] [PubMed] [Google Scholar]
- 205.Johnson CR, Banks SC, Barrett NS, Cazassus F, Dunstan PK, Edgar GJ, Frusher SD, Gardner C, Haddon M, Helidoniotis F, Hill KL, Holbrook NJ, Hosie GW, Last PR, Ling SD, Melbourne-Thomas J, Miller K, Pecl GT, Richardson AJ, Ridgway KR, Rintoul SR, Ritz DA, Ross DJ, Sanderson JC, Shepherd SA, Slotvvinski A, Swadling KM and Taw N, Climate change cascades: Shifts in oceanography, species’ ranges and subtidal marine community dynamics ineastern Tasmania, J. Exp. Mar. Biol. Ecol, 2011, 400, 17–32. [Google Scholar]
- 206.Evangelista H, Wainer I, Sifeddine A, Correge T, Cordeiro RC, Lamounier S, Godiva D, Shen CC, Le Cornec F, Turcq B, Lazareth CE and Hu CY, Ideas and perspectives: Southwestern tropical Atlantic coral growth response to atmospheric circulation changes induced by ozone depletion in Antarctica, Biogeosciences, 2016, 13, 2379–2386. [Google Scholar]
- 207.Häder D-P, Williamson CE, Wängberg S-Å, Rautio M, Rose KC, Gao K, Helbling EW, Sinha RP and Worrest R, Effects of UV radiation on aquatic ecosystems and interactions with other environmental factors, Photochem. Photobiol. Sci, 2015, 14, 108–126. [DOI] [PubMed] [Google Scholar]
- 208.Wong C-Y, Teoh M-L, Phang S-M, Lim P-E and Beardall J, Interactive effects of temperature and UV radiation on photosynthesis of Chlorella, Strains from polar, temperate and tropical environments: differential impacts on damage and repair, PLoS One, 2015, 10, e0139469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Villafane VE, Guendulain-Garcia SD, Valadez F, Rosiles-Gonzalez G, Walter Helbling E and Banaszak AT, Antagonistic and synergistic responses to solar ultraviolet radiation and increased temperature of phytoplankton from cenotes (sink holes) of the Yucatan Peninsula, Mexico, Freshwater Sci, 2015, 34, 1282–1292. [Google Scholar]
- 210.Rautenberger R, Huovinen P and Gomez I, Effects of increased seawater temperature on UV tolerance of Antarctic marine macroalgae, Mar. Biol, 2015, 162, 1087–1097. [Google Scholar]
- 211.Rose KC, Winslow LA, Read JS and Hansen GJA, Climate-induced warming of lakes can be either amplified or suppressed by trends in water clarity, Limnol. Oceanog. Lett, 2016, 1, 44–53. [Google Scholar]
- 212.Beardall J, Stojkovic S and Gao K, Interactive effects of nutrient supply and other environmental factors on the sensitivity of marine primary producers to ultraviolet radiation: implications for the impacts of global change, Aquat. Biol, 2014, 22, 5–23. [Google Scholar]
- 213.Chen H, Guan W, Zeng G, Li P and Chen S, Alleviation of solar ultraviolet radiation (UVR)- induced photoinhibition in diatom Chaetoceros curvisetus by ocean acidification, J. Mar. Biol. Assoc, 2015, 95, 661–667. [Google Scholar]
- 214.Li W, Gao K and Beardall J, Nitrate limitation and ocean acidification interact with UV-B to reduce photosynthetic performance in the diatom Phaeodactylum tricornutum, Biogeosciences, 2015, 12, 2383–2393. [Google Scholar]
- 215.Carrillo P, Medina-Sanchez JM, Herrera G, Duran C, Segovia M, Cortes D, Salles S, Korbee N, Figueroa FL and Mercado JM, Interactive effect of UVR and phosphorus on the coastal phytoplankton community of the western Mediterranean Sea: unravelling eco-physiological mechanisms, PLoS One, 2015, 10, e0142987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Villafane VE, Valinas MS, Cabrerizo MJ and Helbling EW, Physio-ecological responses of Patagonian coastal marine phytoplankton in a scenario of global change: role of acidification, nutrients and solar UVR, Mar. Chem, 2015, 177, 411–420. [Google Scholar]
- 217.Duran C, Medina-Sanchez JM, Herrera G and Carrillo P, Changes in the phytoplankton-bacteria coupling triggered by joint action of UVR, nutrients, and warming in Mediterranean high-mountain lakes, Limnol. Oceanogr, 2016, 61, 413–429. [Google Scholar]
- 218.Gordillo FJL, Aguilera J, Wiencke C and Jimenez C, Ocean acidification modulates the response of two Arctic kelps to ultraviolet radiation, J. Plant. Physiol, 2015, 173, 41–50. [DOI] [PubMed] [Google Scholar]
- 219.Al-Aidaroos AM, El-Sherbiny MMO, Satheesh S, Mantha G, Agusti S, Carreja B and Duarte CM, Strong sensitivity of Red Sea zooplankton to UV-B radiation, Estuaries Coasts, 2015, 38, 846–853. [Google Scholar]
- 220.Won E-J, Han J, Lee Y, Kumar KS, Shin K-H, Lee S-J, Park HG and Lee J-S, In vivo effects of UV radiation on multiple endpoints and expression profiles of DNA repair and heat shock protein (Hsp) genes in the cycloid copepod Paracyclopina nana, Aquat. Toxicol, 2015, 165, 1–8. [DOI] [PubMed] [Google Scholar]
- 221.Schneider T, Grosbois G, Vincent WF and Rautio M, Carotenoid accumulation in copepods is related to lipid metabolism and reproduction rather than to UV-protection, Limnol. Oceanogr, 2016, 61, 1201–1213. [Google Scholar]
- 222.Nevalainen L, Rantala MV, Luoto TP, Ojala AEK and Rautio M, Long-term changes in pigmentation of arctic Daphnia provide potential for reconstructing aquatic UV exposure, Quatern. Sci. Rev, 2016, 144, 44–50. [Google Scholar]
- 223.Nevalainen L, Luoto TP, Rantala MV, Galkin A and Rautio M, Role of terrestrial carbon in aquatic UV exposure and photoprotective pigmentation of meiofauna in subarctic lakes, Freshwater Biol, 2015, 60, 2435–2444. [Google Scholar]
- 224.Connelly SJ, Walling K, Wilbert SA, Catlin DM, Monaghan CE, Hlynchuk S, Meehl PG, Resch LN, Carrera JV, Bowles SM, Clark MD, Tan LT and Cody JA, UV-stressed Daphnia pulex increase fitness through uptake of Vitamin D3, PLoS One, 2015, 10, e0131847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ekvall MT, Hylander S, Walles T, Yang X and Hansson L-A, Diel vertical migration, size distribution and photoprotection in zooplankton as response to UV-A radiation, Limnol. Oceanogr, 2015, 60, 2048–2058. [Google Scholar]
- 226.Hansson LA, Bianco G, Ekvall MT, Heuschele J, Hylander S and Yang X, Instantaneous threat escape and differentiated refuge demand among zooplankton taxa, Ecology, 2016, 97, 279–285. [DOI] [PubMed] [Google Scholar]
- 227.Valiñas MS and Helbling EW, Metabolic and behavioral responses of the reef fish Patagonotothen cornucola to ultraviolet radiation: influence of the diet, J. Exp. Mar. Biol. Ecol, 2016, 474, 180–184. [Google Scholar]
- 228.Levis NA and Johnson JR, Level of UV-B radiation influences the effects of glyphosate-based herbicide on the spotted salamander, Ecotoxicology, 2015, 24, 1073–1086. [DOI] [PubMed] [Google Scholar]
- 229.Debecker S, Sommaruga R, Maes T and Stoks R, Larval UV exposure impairs adult immune function through a trade-off with larval investment in cuticular melanin, Funct. Ecol, 2015, 29, 1292–1299. [Google Scholar]
- 230.Brüsin M, Svensson PA and Hylander S, Individual changes in zooplankton pigmentation in relation to ultraviolet radiation and predator cues, Limnol. Oceanogr, 2016, 61, 1337–1344. [Google Scholar]
- 231.Lindholm M, Wolf R, Finstad A and Hessen DO, Water browning mediates predatory decimation of the Arctic fairy shrimp Branchinecta paludosa, Freshwater Biol, 2016, 61, 340–347. [Google Scholar]
- 232.Valiñas MS, Bermejo P, Galbán L, Laborda L, Häder D-PV, Villafañe VE and Helbling EW, Combined impact of ultraviolet radiation and increased nutrients supply: a test of the potential anthropogenic impacts on the benthic amphipod Amphitoe valida from Patagonian waters (Argentina), Front. Environ. Sci, 2014. DOI: 10.3389/fenvs.2014.00032. [DOI] [Google Scholar]
- 233.Valiñas MS and Helbling WE, Sex-dependent effects of ultraviolet radiation on the marine amphipod Ampithoe valida (Ampithoidae), J. Photochem. Photobiol., B, 2015, 147, 75–82. [DOI] [PubMed] [Google Scholar]
- 234.Williamson CE and Rose KC, When UV meets freshwater, Science, 2010, 329, 637–639. [DOI] [PubMed] [Google Scholar]
- 235.Leach TH, Williamson CE, Theodore N, Fischer JM and Olson MH, The role of ultraviolet radiation in the diel vertical migration of zooplankton: an experimental test of the transparency-regulator hypothesis, J. Plankt. Res, 2015, 37, 886–896. [Google Scholar]
- 236.Fischer JM, Olson MH, Theodore N, Williamson CE, Rose KC and Hwang J, Diel vertical migration of copepods in mountain lakes: the changing role of ultraviolet radiation across a transparency gradient, Limnol. Oceanogr, 2015, 60, 252–262. [Google Scholar]
- 237.Overholt EP, Rose KC, Williamson CE, Fischer J and Cabrol N, Behavioral responses of freshwater calanoid copepods to the presence of ultraviolet radiation: avoidance and attraction, J. Plankt. Res, 2016, 38, 16–26. [Google Scholar]
- 238.Bok MJ, Porter ML and Cronin TW, Ultraviolet filters in stomatopod crustaceans: diversity, ecology and evolution, J. Exp. Biol, 2015, 218, 2055–2066. [DOI] [PubMed] [Google Scholar]
- 239.Williamson CE, Fischer JM, Bollens SM, Overholt EP and Breckenridge JK, Toward a more comprehensive theory of zooplankton diel vertical migration: Integrating ultraviolet radiation and water transparency into the biotic paradigm, Limnol. Oceanogr, 2011, 56, 1603–1623. [Google Scholar]
- 240.Neale PJ and Kieber DJ, Assessing biological and chemical effects of UV in the marine environment: Spectral weighting functions, in Causes and Environmental Implications of Increased UV-B Radiation, ed. Hester RE and Harrison RM, Royal Society of Chemistry, 2000, pp. 61–83. [Google Scholar]
- 241.Harrison JW and Smith REH, Effects of ultraviolet radiation on the productivity and composition of freshwater phytoplankton communities, Photochem. Photobiol. Sci, 2009, 8, 1218–1232. [DOI] [PubMed] [Google Scholar]
- 242.Neale PJ and Thomas BC, Inhibition by ultraviolet and photosynthetically available radiation lowers model estimates of depth-integrated picophytoplankton photosynthesis: global predictions for Prochlorococcus, and Synechococcus, Glob. Chang. Biol, 2016, 23, 293–306. [DOI] [PubMed] [Google Scholar]
- 243.Neale PJ, Pritchard AL and Ihnacik R, UV effects on the primary productivity of picophytoplankton: biological weighting functions and exposure response curves of Synechococcus, Biogeosciences, 2014, 11, 2883–2895. [Google Scholar]
- 244.Neale PJ and Thomas BC, Solar irradiance changes and phytoplankton productivity in Earth’s ocean following astrophysical ionizing radiation events, Astrobiology, 2016, 16, 245–258. [DOI] [PubMed] [Google Scholar]
- 245.Li T, Bai Y, Li G, He X, Chen C-TA, Gao K and Liu D, Effects of ultraviolet radiation on marine primary production with reference to satellite remote sensing, Front. Earth Sci, 2015, 9, 237–247. [Google Scholar]
- 246.Einarsson E, Svard SG and Troell K, UV irradiation responses in Giardia intestinalis, Exp. Parasitol, 2015, 154, 25–32. [DOI] [PubMed] [Google Scholar]
- 247.Baker-Austin C, Trinanes JA, Taylor NGH, Hartnell R, Siitonen A and Martinez-Urtaza J, Emerging Vibrio risk at high latitudes in response to ocean warming, Nat. Climate Chang, 2013, 3, 73–77. [Google Scholar]
- 248.O’Reilly CM, Sharma S, Gray DK, Hampton SE, Read JS, Rowley RJ, Schneider P, Lenters JD, McIntyre PB, Kraemer BM, Weyhenmeyer GA, Straile D, Dong B, Adrian R, Allan MG, Anneville O, Arvola L, Austin J, Bailey JL, Baron JS, Brookes JD, de Eyto E, Dokulil MT, Hamilton DP, Havens K, Hetherington AL, Higgins SN, Hook S, Izmest’eva LR, Joehnk KD, Kangur K, Kasprzak P, Kumagai M, Kuusisto E, Leshkevich G, Livingstone DM, MacIntyre S, May L, Melack JM, Mueller-Navarra DC, Naumenko M, Noges P, Noges T, North RP, Plisnier P-D, Rigosi A, Rimmer A, Rogora M, Rudstam LG, Rusak JA, Salmaso N, Samal NR, Schindler DE, Schladow SG, Schmid M, Schmidt SR, Silow E, Soylu ME, Teubner K, Verburg P, Voutilainen A, Watkinson A, Williamson CE and Zhang G, Rapid and highly variable warming of lake surface waters around the globe, Geophys. Res. Lett, 2015, 42, 10773–10781. [Google Scholar]
- 249.Williamson CE, Zepp RG, Lucas RM, Madronich S, Austin AT, Ballaré CL, Norval M, Sulzberger B, Bais A, McKenzie RL, Robinson SA, Häder D-P, Paul ND and Bornman JF, Solar ultraviolet radiation in a changing climate, Nat. Climate Chang, 2014, 4, 434–441. [Google Scholar]
- 250.Le Grand M and Cliquet S, Impact of culture age on conidial germination, desiccation and UV tolerance of entomopathogenic fungi, Biocontrol Sci. Technol, 2013, 23, 847–859. [Google Scholar]
- 251.Rueda Paramo ME, Lopez Lastra CC, Garcia JJ, Fernandes EKK, Marreto RN and Luz C, Effect of ultraviolet-A radiation on the production of Leptolegnia chapmanii (Saprolegniales: Saprolegniaceae) zoospores on dead Aedes aegypti, (Diptera: Culicidae) larvae and their larvicidal activity, J. Invertebr. Pathol, 2015, 130, 133–135. [DOI] [PubMed] [Google Scholar]
- 252.Kim S and Choi K, Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: A mini-review, Environ. Int, 2014, 70, 143–157. [DOI] [PubMed] [Google Scholar]
- 253.Bratkovics S, Wirth E, Sapozhnikova Y, Pennington P and Sanger D, Baseline monitoring of organic sunscreen compounds along South Carolina’s coastal marine environment, Mar. Pollut. Bull, 2015, 101, 370–377. [DOI] [PubMed] [Google Scholar]
- 254.Arpin-Pont L, Bueno MJM, Gomez E and Fenet H, Occurrence of PPCPs in the marine environment: a review, Environ. Sci. Pollut. Res, 2016, 23, 4978–4991. [DOI] [PubMed] [Google Scholar]
- 255.Tsui MMP, Leung HW, Wai TC, Yamashita N, Taniyasu S, Liu WH, Lam PKS and Murphy MB, Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface waters from different countries, Water Res, 2014, 67, 55–65. [DOI] [PubMed] [Google Scholar]
- 256.Gao CJ, Liu LY, Ma WL, Zhu NZ, Jiang L, Li YF and Kannan K, Benzonphenone-type UV filters in urine of Chinese young adults: Concentration, source and exposure, Environ. Pollut, 2015, 203, 1–6. [DOI] [PubMed] [Google Scholar]
- 257.Tsui MMP, Leung HW, Lam PKS and Murphy MB, Seasonal occurrence, removal efficiencies and preliminary risk assessment of multiple classes of organic UV filters in wastewater treatment plants, Water Res, 2014, 53, 58–67. [DOI] [PubMed] [Google Scholar]
- 258.Lindholm-Lehto PC, Ahkola HSJ, Knuutinen JS and Herve SH, Occurrence of pharmaceuticals in municipal wastewater, in the recipient water, and sedimented particles of northern Lake Paijanne, Environ. Sci. Pollut. Res, 2015, 22, 17209–17223. [DOI] [PubMed] [Google Scholar]
- 259.Kotnik K, Kosjek T, Zegura B, Filipic M and Heath E, Photolytic fate and genotoxicity of benzophenone-derived compounds and their photodegradation mixtures in the aqueous environment, Chemosphere, 2016, 147, 114–123. [DOI] [PubMed] [Google Scholar]
- 260.Sang ZY and Leung KSY, Environmental occurrence and ecological risk assessment of organic UV filters in marine organisms from Hong Kong coastal waters, Sci. Total Environ, 2016, 566, 489–498. [DOI] [PubMed] [Google Scholar]
- 261.Alonso MB, Luisa Feo M, Corcellas C, Gago-Ferrero P, Bertozzi CP, Marigo J, Flach L, Meirelles ACO, Carvalho VL, Azevedo AF, Torres JPM, Lailson-Brito J, Malm O, Silvia Diaz-Cruz M, Eljarrat E and Barcelo D, Toxic heritage: Maternal transfer of pyrethroid insecticides and sunscreen agents in dolphins from Brazil, Environ. Pollut, 2015, 207, 391–402. [DOI] [PubMed] [Google Scholar]
- 262.Langford KH, Reid MJ, Fjeld E, Oxnevad S and Thomas KV, Environmental occurrence and risk of organic UV filters and stabilizers in multiple matrices in Norway, Environ. Int, 2015, 80, 1–7. [DOI] [PubMed] [Google Scholar]
- 263.Sanchez-Quiles D and Tovar-Sanchez A, Are sunscreens a new environmental risk associated with coastal tourism?, Environ. Int, 2015, 83, 158–170. [DOI] [PubMed] [Google Scholar]
- 264.Ozaez I, Aquilino M, Morcillo G and Martinez-Guitarte JL, UV filters induce transcriptional changes of different hormonal receptors in Chironomus riparius embryos and larvae, Environ. Pollut, 2016, 214, 239–247. [DOI] [PubMed] [Google Scholar]
- 265.Kinnberg KL, Petersen GI, Albrektsen M, Minghlani M, Awad SM, Holbech BF, Green JW, Bjerregaard P and Holbech H, Endocrine-disrupting effect of the ultraviolet filter benzophenone-3 in zebrafish, Danio rerio, Environ. Toxicol. Chem, 2015, 34, 2833–2840. [DOI] [PubMed] [Google Scholar]
- 266.Galletti A, Seo S, Joo SH, Su C and Blackwelder P, Effects of titanium dioxide nanoparticles derived from consumer products on the marine diatom Thalassiosira pseudonana, Environ. Sci. Pollut. Res, 2016, 23, 21113–21122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Thompson RC, Microplastics in the marine environment: sources, consequences and solutions, in Marine Anthropogenic Litter, ed. Bergmann M, Gutow L and Klages M, Springer International Publishing, 2015, pp. 185–200. [Google Scholar]
- 268.Duis K and Coors A, Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects, Environ. Sci. Eur, 2016, 28, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Klein S, Worch E and Knepper TP, Occurrence and spatial distribution of microplastics in river shore sediments of the Rhine-Main area in Germany, Environ. Sci. Technol, 2015, 49, 6070–6076. [DOI] [PubMed] [Google Scholar]
- 270.Lusher A, Microplastics in the marine environment: distribution, interactions and effects, in Marine Anthropogenic Litter, ed. Bergmann M, Gutow L and Klages M, Springer International Publishing, 2016, pp. 245–307. [Google Scholar]
- 271.Suhrhoff TJ and Scholz-Bottcher BM, Qualitative impact of salinity, UV radiation and turbulence on leaching of organic plastic additives from four common plastics - A lab experiment, Mar. Pollut. Bull, 2016, 102, 84–94. [DOI] [PubMed] [Google Scholar]
- 272.Browne MA, Underwood AJ, Chapman MG, Williams R, Thompson RC and van Franeker JA, Linking effects of anthropogenic debris to ecological impacts, Proc. R. Soc. B. Biol. Sci, 2015, 282, 20142929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Van Cauwenberghe L, Devriese L, Galgani F, Robbens J and Janssen CR, Microplastics in sediments: A review of techniques, occurrence and effects, Mar. Environ. Res, 2015, 111, 5–17. [DOI] [PubMed] [Google Scholar]
- 274.Wesch C, Bredimus K, Paulus M and Klein R, Towards the suitable monitoring of ingestion of microplastics by marine biota: A review, Environ. Pollut, 2016, 218, 1200–1208. [DOI] [PubMed] [Google Scholar]
- 275.Avio CG, Gorbi S and Regoli F, Plastics and microplastics in the oceans: From emerging pollutants to emerged threat, Mar. Environ. Res, 2016. DOI: 10.1016/j.marenvres.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 276.Richardson SD, Plewa MJ, Wagner ED, Schoeny R and DeMarini DM, Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research, Mutat. Res, 2007, 636, 178–242. [DOI] [PubMed] [Google Scholar]
- 277.Wu Q-Y, Li C, Du Y, Wang W-L, Huang H and Hu H-Y, Elimination of disinfection byproduct formation potential in reclaimed water during solar light irradiation, Water Res, 2016, 95, 260–267. [DOI] [PubMed] [Google Scholar]
- 278.Peleato NM and Andrews RC, Comparison of three-dimensional fluorescence analysis methods for predicting formation of trihalomethanes and haloacetic acids, J. Environ. Sci, 2015, 27, 159–167. [DOI] [PubMed] [Google Scholar]
- 279.Awad J, van Leeuwen J, Chow C, Drikas M, Smernik RJ, Chittleborough DJ and Bestland E, Characterization of dissolved organic matter for prediction of trihalomethane formation potential in surface and sub-surface waters, J. Hazard Mater, 2016, 308, 430–439. [DOI] [PubMed] [Google Scholar]
- 280.Li WT, Jin J, Li Q, Wu CF, Lu H, Zhou Q and Li AM, Developing LED UV fluorescence sensors for online monitoring DOM and predicting DBPs formation potential during water treatment, Water Res, 2016, 93, 1–9. [DOI] [PubMed] [Google Scholar]
- 281.Hu HY, Du Y, Wu QY, Zhao X, Tang X and Chen Z, Differences in dissolved organic matter between reclaimed water source and drinking water source, Sci. Total Environ, 2016, 551, 133–142. [DOI] [PubMed] [Google Scholar]
- 282.Quintão FJO, Freitas JRL, de Fátima Machado C, Aquino SF, de Queiroz Silva S and de RJ Cássia Franco Afonso, Characterization of metformin by-products under photolysis, photocatalysis, ozonation and chlorination by high-performance liquid chromatography coupled to high-resolution mass spectrometry, Rapid Commun. Mass Spec, 2016, 30, 2360–2368. [DOI] [PubMed] [Google Scholar]
- 283.Armbruster D, Happel O, Scheurer M, Harms K, Schmidt TC and Brauch HJ, Emerging nitrogenous disinfection byproducts: transformation of the antidiabetic drug metformin during chlorine disinfection of water, Water Res, 2015, 79, 104–118. [DOI] [PubMed] [Google Scholar]
- 284.Bradley PM, Journey CA, Button DT, Carlisle DM, Clark JM, Mahler BJ, Nakagaki N, Qi SL, Waite IR and VanMetre PC, Metformin and other pharmaceuticals widespread in wadeable streams of the southeastern United States, Environ. Sci. Technol. Lett, 2016, 3, 243–249. [Google Scholar]
- 285.Austin AT and Vivanco L, Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation, Nature, 2006, 442, 555–558. [DOI] [PubMed] [Google Scholar]
- 286.Rey A, Mind the gap: non-biological processes contributing to soil CO2 efflux, Glob. Chang. Biol, 2015, 21, 1752–1761. [DOI] [PubMed] [Google Scholar]
- 287.Austin AT, Mendez MS and Ballare CL, Photodegradation alleviates the lignin bottleneck for carbon turnover in terrestrial ecosystems, Proc. Natl. Acad. Sci. U. S. A, 2016, 113, 4392–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Day TA, Guenon R and Ruhland CT, Photodegradation of plant litter in the Sonoran Desert varies by litter type and age, Soil Biol. Biochem, 2015, 89, 109–122. [Google Scholar]
- 289.Gaxiola A and Armesto JJ, Understanding litter decomposition in semiarid ecosystems: linking leaf traits, UV exposure and rainfall variability, Front. Plant Sci, 2015, 6 DOI: 10.3389/fpls201500140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Baker NR and Allison SD, Ultraviolet photodegradation facilitates microbial litter decomposition in a Mediterranean climate, Ecology, 2015, 96, 1994–2003. [DOI] [PubMed] [Google Scholar]
- 291.Lin Y, Scarlett RD and King JY, Effects of UV photodegradation on subsequent microbial decomposition of Bromus diandrus, litter, Plant Soil, 2015, 395, 263–271. [Google Scholar]
- 292.Gliksman D, Rey A, Seligmann R, Dumbur R, Sperling O, Navon Y, Haenel S, De Angelis P, Arnone JA and Grünzweig JM, Biotic degradation at night, abioric degradation at day: positive feedbacks on litter decomposition in drylands, Glob. Chang. Biol, 2016. DOI: 10.1111/gcb.13465. [DOI] [PubMed] [Google Scholar]
- 293.Yanni SF, Suddick EC and Six J, Photodegradation effects on CO2 emissions from litter and SOM and photo-facilitation of microbial decomposition in a California grassland, Soil Biol. Biochem, 2015, 91, 40–49. [Google Scholar]
- 294.Araujo PI and Austin AT, A shady business: Pine afforestation alters the primary controls on litter decomposition along a precipitation gradient in Patagonia, Argentina, J. Ecol, 2015, 103, 1408–1420. [Google Scholar]
- 295.Hewins DB and Throop HL, Leaf litter decomposition is rapidly enhanced by the co-occurrence of monsoon rainfall and soil-litter mixing across a gradient of coppice dune development in the Chihuahuan Desert, J. Arid Environ, 2016, 129, 111–118. [Google Scholar]
- 296.Austin AT and Ballare CL, Dual role of lignin in plant litter decomposition in terrestrial ecosystems, Proc. Natl. Acad.Sci. U. S. A, 2010, 107, 4618–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Heffernan O, The mystery of the expanding tropics, Nature, 2016, 530, 21–23. [DOI] [PubMed] [Google Scholar]
- 298.Erickson DJ, Sulzberger B, Zepp RG and Austin AT, Effects of stratospheric ozone depletion, solar UV radiation, and climate change on biogeochemical cycling: interactions and feedbacks, Photochem. Photobiol. Sci, 2015, 14, 127–148. [DOI] [PubMed] [Google Scholar]
- 299.Santin C, Doerr SH, Merino A, Bryant R and Loader NJ, Forest floor chemical transformations in a boreal forest fire and their correlations with temperature and heating duration, Geoderma, 2016, 264, 71–80. [Google Scholar]
- 300.Ward CP, Sleighter RL, Hatcher PG and Cory RM, Insights into the complete and partial photooxidation of black carbon in surface waters, Environ. Sci.: Processes Impacts, 2014, 16, 721–731. [DOI] [PubMed] [Google Scholar]
- 301.Brown DRN, Jorgenson MT, Douglas TA, Romanovsky VE, Kielland K, Hiemstra C, Euskirchen ES and Ruess RW, Interactive effects of wildfire and climate on permafrost degradation in Alaskan lowland forests, J. Geophys. Res. Biogeosci, 2015, 120, 1619–1637. [Google Scholar]
- 302.Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, Jones C, Le Quéré C, Myneni RB, Piao S and Thornton P, Carbon and Other Biogeochemical Cycles, in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, ed. Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V and Midgley PM, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2013, pp. 465–570. [Google Scholar]
- 303.Cory RM, Ward CP, Crump BC and Kling GW, Sunlight controls water column processing of carbon in arctic fresh waters, Science, 2014, 345, 925–928. [DOI] [PubMed] [Google Scholar]
- 304.Grosse G, Goetz S, McGuire AD, Romanovsky VE and Schuur EAG, Changing permafrost in a warming world and feedbacks to the Earth system, Environ. Res. Lett, 2016, 11 DOI: 10.1088/1748- [DOI] [Google Scholar]
- 305.Sulzberger B and Arey JS, Impacts of polar changes on the UV-induced mineralization of terrigenous dissolved organic matter, Environ. Sci. Technol, 2016, 50, 6621–6631. [DOI] [PubMed] [Google Scholar]
- 306.Ward CP and Cory RM, Complete and partial photo-oxidation of dissolved organic matter draining permafrost soils, Environ. Sci. Technol, 2016, 50, 3545–3553. [DOI] [PubMed] [Google Scholar]
- 307.Cory RM, Harrold KH, Neilson BT and Kling GW, Controls of dissolved organic matter (DOM) degradation in a headwater stream: the influence of photochemical and hydrological conditions in determining light-limitation or substrate-limitation of photo-degradation, Biogeosci. Discuss, 2015, 12, 9793–9838. [Google Scholar]
- 308.Cory RM, Crump BC, Dobkowski JA and Kling GW, Surface exposure to sunlight stimulates CO2 release from permafrost soil carbon in the Arctic, Proc. Natl. Acad. Sci. U. S. A, 2013, 110, 3429–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Schuur EAG, McGuire AD, Schaedel C, Grosse G, Harden JW, Hayes DJ, Hugelius G, Koven CD, Kuhry P, Lawrence DM, Natali SM, Olefeldt D, Romanovsky VE, Schaefer K, Turetsky MR, Treat CC and Vonk JE, Climate change and the permafrost carbon feedback, Nature, 2015, 520, 171–179. [DOI] [PubMed] [Google Scholar]
- 310.Wang JJ, Jiao Y, Rhew RC and Chow AT, Haloform formation in coastal wetlands along a salinity gradient at South Carolina, United States, Environ. Chem, 2016, 13, 745–756. [Google Scholar]
- 311.Liu YN, Thornton DCO, Bianchi TS, Arnold WA, Shields MR, Chen J and Yvon-Lewis SA, Dissolved organic matter composition drives the marine production of brominated very short-lived substances, Environ. Sci. Technol, 2015, 49, 3366–3374. [DOI] [PubMed] [Google Scholar]
- 312.Stemmler I, Hense I and Quack B, Marine sources of bromoform in the global open ocean - global patterns and emissions, Biogeosciences, 2015, 12, 1967–1981. [Google Scholar]
- 313.Tegtmeier S, Ziska F, Pisso I, Quack B, Velders GJM, Yang X and Kruger K, Oceanic bromoform emissions weighted by their ozone depletion potential, Atmos. Chem. Phys, 2015, 15, 13647–13663. [Google Scholar]
- 314.Butler AH, Daniel JS, Portmann RW, Ravishankara AR, Young PJ, Fahey DW and Rosenlof KH, Diverse policy implications for future ozone and surface UV in a changing climate, Environ. Res. Lett, 2016, 11(6), 064017. [Google Scholar]
- 315.Liu QY and Shank GC, Solar radiation-enhanced dissolution (photodissolution) of particulate organic matter in Texas estuaries, Estuaries Coasts, 2015, 38, 2172–2184. [Google Scholar]
- 316.Mopper K, Kieber DJ and Stubbins A, Chapter 8 - Marine Photochemistry of Organic Matter: Processes and Impacts, in Biogeochemistry of Marine Dissolved Organic Matter, ed. Carlson DAHA, Academic Press, Boston, 2nd edn, 2015, pp. 389–450. [Google Scholar]
- 317.Schiebel HN, Wang XC, Chen RF and Peri F, Photochemical release of dissolved organic matter from resuspended salt marsh sediments, Estuaries Coasts, 2015, 38, 1692–1705. [Google Scholar]
- 318.Song GS, Richardson JD, Werner JP, Xie HX and Kieber DJ, Carbon monoxide photoproduction from particles and solutes in the Delaware Estuary under contrasting hydrological conditions, Environ. Sci. Technol, 2015, 49, 14048–14056. [DOI] [PubMed] [Google Scholar]
- 319.McNeill K and Canonica S, Triplet state dissolved organic matter in aquatic photochemistry: reaction mechanisms, substrate cope, and photophysical properties, Environ. Sci.: Processes Impacts, 2016, 11, 1381–1399. [DOI] [PubMed] [Google Scholar]
- 320.Batista APS, Teixeira A, Cooper WJ and Cottrell BA, Correlating the chemical and spectroscopic characteristics of natural organic matter with the photodegradation of sulfamerazine, Water Res, 2016, 93, 20–29. [DOI] [PubMed] [Google Scholar]
- 321.Chu CH, Erickson PR, Lundeen RA, Stamatelatos D, Alaimo PJ, Latch DE and McNeill K, Photochemical and nonphotochemical transformations of cysteine with dissolved organic matter, Environ. Sci. Technol, 2016, 50, 6363–6373. [DOI] [PubMed] [Google Scholar]
- 322.Kohn T, Mattle MJ, Minella M and Vione D, A modeling approach to estimate the solar disinfection of viral indicator organisms in waste stabilization ponds and surface waters, Water Res, 2016, 88, 912–922. [DOI] [PubMed] [Google Scholar]
- 323.Li R, Zhao C, Yao B, Li D, Yan SW, O’Shea KE and Song WH, Photochemical transformation of aminoglycoside antibiotics in simulated natural waters, Environ. Sci. Technol, 2016, 50, 2921–2930. [DOI] [PubMed] [Google Scholar]
- 324.Lundeen RA, Chu CH, Sander M and McNeill K, Photooxidation of the antimicrobial, nonribosomal peptide Bacitracin A by singlet oxygen under environmentally relevant conditions, Environ. Sci. Technol, 2016, 50, 8586–8595. [DOI] [PubMed] [Google Scholar]
- 325.Lyu XJ, Li WW, Lam PKS and Yu HQ, Insights into perfluorooctane sulfonate photodegradation in a catalyst-free aqueous solution, Sci. Rep, 2015, 5 DOI: 10.1038/srep09353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Minella M, Maurino V, Minero C and Vione D, A model assessment of the ability of lake water in Terra Nova Bay, Antarctica, to induce the photochemical degradation of emerging contaminants, Chemosphere, 2016, 162, 91–98. [DOI] [PubMed] [Google Scholar]
- 327.Wammer KH, Anderson KC, Erickson PR, Kliegman S, Moffatt ME, Berg SM, Heitzman JA, Pflug NC, McNeill K, Martinovic-Weigelt D, Abagyan R, Cwiertny DM and Kolodziej EP, Environmental photochemistry of Altrenogest: Photoisomerization to a bioactive product with increased environmental persistence via reversible photohydration, Environ. Sci. Technol, 2016, 50, 7480–7488. [DOI] [PubMed] [Google Scholar]
- 328.Powers LC and Miller WL, Photochemical production of CO and CO2 in the Northern Gulf of Mexico: Estimates and challenges for quantifying the impact of photochemistry on carbon cycles, Mar. Chem, 2015, 171, 21–35. [Google Scholar]
- 329.Kieber DJ, Keene WC, Frossard AA, Long MS, Maben JR, Russell LM, Kinsey JD, Tyssebotn IMB, Quinn PK and Bates TS, Coupled ocean-atmosphere loss of marine refractory dissolved organic carbon, Geophys. Res. Lett, 2016, 43, 2765–2772. [Google Scholar]
- 330.Lu C-J, Benner R, Fichot CG, Fukuda H, Yamashita Y and Ogawa H, Sources and transformations of dissolved lignin phenols and chromophoric dissolved organic matter in Otsuchi Bay, Japan, Front. Mar. Sci, 2016, 3 DOI: 10.3389/fmars.2016.00085. [DOI] [Google Scholar]
- 331.Olmanson LG, Brezonik PL, Finlea JC and Bauer ME, Comparison of Landsat 8 and Sandsat 7 for regional measurements of CDOM and water clarity in lakes, Remote Sens. Environ, 2016, 185, 119–128. [Google Scholar]
- 332.Slonecker ET, Jones DK and Pellerin BA, The new Landsat 8 potential for remote sensing of colored dissolved organic matter (CDOM), Mar. Pollut. Bull, 2016, 107, 518–527. [DOI] [PubMed] [Google Scholar]
- 333.Zheng Z, Ren J, Li Y, Huang C, Liu G, Du C and Lyu H, Remote sensing of diffuse attenuation coefficient patterns from Landsat 8 OLI imagery of turbid inland waters: A case study of Dongting Lake, Sci. Total Environ, 2016, 573, 39–54. [DOI] [PubMed] [Google Scholar]
- 334.Solomon KR, Velders GJ, Wilson SR, Madronich S, Longstreth J, Aucamp PJ and Bornman JF, Sources, fates, toxicity, and risks of trifluoroacetic acid and its salts: Relevance to substances regulated under the Montreal and Kyoto Protocols, J. Toxicol. Environ. Health, Part B, 2016, 19, 289–304. [DOI] [PubMed] [Google Scholar]
- 335.Guo Y, Zeng H, Zheng R, Li S, Barnett AG, Zhang S, Zou X, Huxley R, Chen W and Williams G, The association between lung cancer incidence and ambient air pollution in China: A spatiotemporal analysis, Environ. Res, 2016, 144, 60–65. [DOI] [PubMed] [Google Scholar]
- 336.US DOE, Protective Action Criteria (PAC), U.S. Department of Energy, https://sp.eota.energy.gov/pac/, accessed September, 2016.
- 337.Hori H, Manita R, Yamamoto K, Kutsuna S and Kato M, Efficient photochemical decomposition of trifluoroacetic acid and its analogues with electrolyzed sulfuric acid, J. Photochem. Photobiol., A, 2017, 332, 167–173. [Google Scholar]
- 338.Shindell DT, The social cost of atmospheric release, Clim. Change, 2015, 130, 313–326. [Google Scholar]
- 339.Battye WH, Bray CD, Aneja VP, Tong D, Lee P and Tang YH, Evaluating ammonia (NH3) predictions in the NOAA National Air Quality Forecast Capability (NAQFC) using in situ aircraft, ground-level, and satellite measurements from the DISCOVER-AQ Colorado campaign, Atmos. Environ, 2016, 140, 342–351. [Google Scholar]
- 340.Reisch MS, Why interest in ammonia refrigerants is surging, Chem. Eng. News, 2016, 94, 27–28. [Google Scholar]
- 341.GTZ Proklima, Natural Refrigerants, Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH, https://www.giz.de/expertise/html/3372.html, accessed September, 2016.
- 342.Velders GJM, Madronich S, Clerbaux C, Derwent R, Grutter M, Hauglustaine D, Incecik S, Ko M, Libre JM, Nielsen OJ, Stordal F and Zhu T, Chemical and Radiative Effects of Halocarbons and their ReplacementCompounds, in IPCC Special Report on “Safeguarding the Ozone Layer and the Global Climate System”, 2005, pp. 133–180. [Google Scholar]
- 343.Zhang H, Wu S, Huang Y and Wang Y, Effects of stratospheric ozone recovery on photochemistry and ozone air quality in the troposphere, Atmos. Chem. Phys, 2014, 14, 4079–4086. [Google Scholar]
- 344.Banerjee A, Maycock AC, Archibald AT, Abraham NL, Telford P, Braesicke P and Pyle JA, Drivers of changes in stratospheric and tropospheric ozone between year 2000 and 2100, Atmos. Chem. Phys, 2016, 16, 2727–2746. [Google Scholar]
- 345.Markakis K, Valari M, Engardt M, Lacressonnière G, Vautard R and Andersson C, Mid-21st century air quality at the urban scale under the influence of changed climate and emissions – case studies for Paris and Stockholm, Atmos. Chem. Phys, 2016, 16, 1877–1894. [Google Scholar]
- 346.Butler AH, Daniel JS, Portmann RW, Ravishankara AR, Young PJ, Fahey DW and Rosenlof KH, Diverse policy implications for future ozone and surface UV in a changing climate, Environ. Res. Lett, 2016, 11(6), 064017. [Google Scholar]
- 347.Nowack PJ, Abraham NL, Braesicke P and Pyle JA, Stratospheric ozone changes under solar geoengineering: implications for UV exposure and air quality, Atmos. Chem. Phys, 2016, 16, 4191–4203. [Google Scholar]
- 348.World Health Organization, Ambient air pollution: A global assessment of exposure and burden of disease, 2016, p. 121. [Google Scholar]
- 349.Lelieveld J, Evans JS, Fnais M, Giannadaki D and Pozzer A, The contribution of outdoor air pollution sources to premature mortality on a global scale, Nature, 2015, 525, 367–371. [DOI] [PubMed] [Google Scholar]
- 350.Cakmak S, Hebbern C, Vanos J, Crouse DL and Burnett R, Ozone exposure and cardiovascular-related mortality in the Canadian Census Health and Environment Cohort (CANCHEC) by spatial synoptic classification zone, Environ. Pollut, 2016, 214, 589–599. [DOI] [PubMed] [Google Scholar]
- 351.Atkinson RW, Mills IC, Walton HA and Anderson HR, Fine particle components and health-a systematic review and meta-analysis of epidemiological time series studies of daily mortality and hospital admissions, J. Expos. Sci. Environ. Epidemiol, 2015, 25, 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Green HK, Andrews N, Armstrong B, Bickler G and Pebody R, Mortality during the 2013 heatwave in England - How did it compare to previous heatwaves? A retrospective observational study, Environ. Res, 2016, 147, 343–349. [DOI] [PubMed] [Google Scholar]
- 353.Filleul L, Cassadou S, Medina S, Fabres P, Lefranc A, Eilstein D, Le Tertre A, Pascal L, Chardon B, Blanchard M, Declercq C, Jusot JF, Prouvost H and Ledrans M, The relation between temperature, ozone, and mortality in nine french cities during the heat wave of 2003, Environ. Health Perspect, 2006, 114, 1344–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Stedman JR, The predicted number of air pollution related deaths in the UK during the August 2003 heatwave, Atmos. Environ, 2004, 38, 1087–1090. [Google Scholar]
- 355.Kahle JJ, Neas LM, Devlin RB, Case MW, Schmitt MT, Madden MC and Diaz-Sanchez D, Interaction effects of temperature and ozone on lung function and markers of systemic inflammation, coagulation, and fibrinolysis: A crossover study of healthy young volunteers, Environ. Health Perspect, 2015, 123, 310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Cogulet A, Blanchet P and Landry V, Wood degradation under UV irradiation: A lignin characterization, J. Photochem. Photobiol., B, 2016, 158, 184–191. [DOI] [PubMed] [Google Scholar]
- 357.Salas C, Moya R and Vargas-Fonseca L, Optical performance of finished and unfinished tropical timbers exposed to ultraviolet light in the field in Costa Rica, Wood Mater. Sci. Eng, 2016, 11, 62–78. [Google Scholar]
- 358.Bonifazi G, Calienno L, Capobianco G, Monaco AL, Pelosi C, Picchio R and Serranti S, A new approach for the modelling of chestnut wood photo-degradation monitored by different spectroscopic techniques, Environ. Sci. Pollut. Res, 2016, 1–11. [DOI] [PubMed] [Google Scholar]
- 359.Timar MC, Varodi AM and Gurău L, Comparative study of photodegradation of six wood species after short-time UV exposure, Wood Sci. Technol, 2016, 50, 135–163. [Google Scholar]
- 360.Živković V, Arnold M, Pandey KK, Richter K and Turkulin H, Spectral sensitivity in the photodegradation of fir wood (Abies alba Mill.) surfaces: Correspondence of physical and chemical changes in natural weathering, Wood Sci. Technol, 2016, 50, 989–1002. [Google Scholar]
- 361.Kim YS, Lee KH and Kim JS, Weathering characteristics of bamboo (Phyllostachys puberscence) exposed to outdoors for one year, J. Wood. Sci, 2016, 62, 332–338. [Google Scholar]
- 362.Capobianco G, Calienno L, Pelosi C, Scacchi M, Bonifazi G, Agresti G, Picchio R, Santamaria U, Serranti S and Monaco AL, Protective behaviour monitoring on wood photo-degradation by spectroscopic techniques coupled with chemometrics, Spectrochim. Acta, Part A, 2016, 172, 34–42. [DOI] [PubMed] [Google Scholar]
- 363.Ali SS, Qazi IA, Arshad M, Khan Z, Voice TC and Mehmood CT, Photocatalytic degradation of low density polyethylene (LDPE) films using titania nanotubes, Environ. Nanotechnol. Monit. Manage, 2016, 5, 44–53. [Google Scholar]
- 364.Yagoubi W, Abdelhafidi A, Sebaa M and Chabira SF, Identification of carbonyl species of weathered LDPE films by curve fitting and derivative analysis of IR spectra, Polym. Test, 2015, 44, 37–48. [Google Scholar]
- 365.Massardier V and Louizi M, Photodegradation of a polypropylene filled with lanthanide complexes, Polímeros, 2015, 25, 515–522. [Google Scholar]
- 366.Baker W, McCauley K and Tsang J-S, Sustaining the unsustainabile: Mitigation and monitoring for modern materials, AIC News, 2015, 40(5), 1, 3–6. [Google Scholar]
- 367.Raccurt O, Delord C, Bouquet C and Couturier R, Correlation between Solar Mirror Degradation and Colorimetric Measurement of Protective Back Layer, Energy Procedia, 2014, 49, 1700–1707. [Google Scholar]
- 368.Lv Y, Huang Y, Yang J, Kong M, Yang H, Zhao J and Li G, Outdoor and accelerated laboratory weathering of polypropylene: A comparison and correlation study, Polym. Degrad. Stab, 2015, 112, 145–159. [Google Scholar]
- 369.Gupta BS, Jelle BP, Hovde PJ and Gao T, Wood coating failures against natural and accelerated climates, Proc. Instit. Civil Eng. Construct. Mater, 2015, 168, 3–15. [Google Scholar]
- 370.Tolvaj L, Popescu C-M, Molnar Z and Preklet E, Effects of air relative humidity and temperature on photodegradation processes in beech and spruce wood, BioResources, 2016, 11, 296–305. [Google Scholar]
- 371.Catto AL, Montagna LS, Almeida SH, Silveira RMB and Santana RMC, Wood plastic composites weathering: Effects of compatibilization on biodegradation in soil and fungal decay, Int. Biodeter. Biodegrad, 2016, 109, 11–22. [Google Scholar]
- 372.Altgen M and Militz H, Photodegradation of thermally-modified Scots pine and Norway spruce investigated on thin micro-veneers, Eur. J. Wood Prod, 2016, 74, 185–190. [Google Scholar]
- 373.Candelier K, Thevenon M-F, Petrissans A, Dumarcay S, Gerardin P and Petrissans M, Control of wood thermal treatment and its effects on decay resistance: A review, Ann. Forest Sci, 2016, 73, 571–583. [Google Scholar]
- 374.Nemeth R, Tolvaj L, Bak M and Alpar T, Colour stability of oil-heat treated black locust and poplar wood during short-term UV radiation, J. Photochem. Photobiol., A, 2016, 329, 287–292. [Google Scholar]
- 375.Kubovský I, Kačík F and Reinprecht L, The impact of UV radiation on the change of colour and composition of the surface of lime wood treated with a CO2 laser, J. Photochem. Photobiol., A, 2016, 322–323, 60–66. [Google Scholar]
- 376.Xing D, Wang S and Li J, Effect of artificial weathering on the properties of industrial-scale thermally modified wood, BioResources, 2015, 10, 8238–8252. [Google Scholar]
- 377.Cirule D, Meija-Feldmane A, Kuka E, Andersons B, Kurnosova N, Antons A and Tuherm H, Spectral sensitivity of thermally modified and unmodified wood, BioResources, 2016, 11, 324–335. [Google Scholar]
- 378.Passauer L, Prieto J, Müller M, Rössler M, Schubert J and Beyer M, Novel color stabilization concepts for decorative surfaces of native dark wood and thermally modified timber, Prog. Org. Coat, 2015, 89, 314–322. [Google Scholar]
- 379.Shen H, Cao J, Sun W and Peng Y, Influence of post-extraction on photostability of thermally modified scots pine wood during artificial weathering, BioResources, 2016, 11, 4512–4525 [Google Scholar]
- 380.Nagarajappa GB and Pandey KK, UV resistance and dimensional stability of wood modified with isopropenyl acetate, J. Photochem. Photobiol., B, 2016, 155, 20–27. [DOI] [PubMed] [Google Scholar]
- 381.Isaji S and Kojima Y, Application of copper monoethanolamine solutions as primers for semitransparent exterior wood stains, Eur. J. Wood Prod, 2016, 1–10, DOI: 10.1007/s00107-016-1078-2. [DOI] [Google Scholar]
- 382.Zhang J, Kamdem DP and Temiz A, Weathering of copper–amine treated wood, Appl. Surf. Sci, 2009, 256, 842–846. [Google Scholar]
- 383.Humar M, Pohleven F and Žlindra D, Influence of water, properties on leaching of copper-based preservatives from treated wood, Wood Res, 2006, 51, 69–76. [Google Scholar]
- 384.Gadioli R, Waldman WR and De Paoli MA, Lignin as a green primary antioxidant for polypropylene, J. Appl. Polym. Sci, 2016, 133, 43558. [Google Scholar]
- 385.Gregorová A, Cibulková Z, Košíková B and Šimon P, Stabilization effect of lignin in polypropylene and recycled polypropylene, Polym. Degrad. Stab, 2005, 89, 553–558 [Google Scholar]
- 386.Peng Y, Liu R and Cao J, Characterization of surface chemistry and crystallization behavior of polypropylene composites reinforced with wood flour, cellulose, and lignin during accelerated weathering, Appl. Surf. Sci, 2015, 332, 253–259. [Google Scholar]
- 387.Hazarika A and Maji TK, Ultraviolet resistance and other physical properties of softwood polymer nanocomposites reinforced with ZnO nanoparticles and nanoclay, Wood Mater. Sci. Eng, 2017, 12, 24–39. [Google Scholar]
- 388.Nikolic M, Lawther JM and Sanadi AR, Use of nanofillers in wood coatings: a scientific review, J. Coat. Technol. Res, 2015, 12, 445–461. [Google Scholar]
- 389.Akpolat LB, Çakır BA, Topel Ö and Hoda N, Synthesis of TiO2 nanoparticles by self-assembling reverse micelle cores of PS-b-PAA for functional textile applications, Mater. Res. Bull, 2015, 64, 117–122. [Google Scholar]
- 390.Guo H, Fuchs P, Cabane E, Michen B, Hagendorfer H, Romanyuk YE and Burgert I, UV-protection of wood surfaces by controlled morphology fine-tuning of ZnO nanostructures, in Holzforschung, 2016, vol. 70, pp. 699–708. [Google Scholar]
- 391.Dong Y, Yan Y, Ma H, Zhang S, Li J, Xia C, Shi SQ and Cai L, In situ chemosynthesis of ZnO nanoparticles to endow wood with antibacterial and UV-resistance properties, J. Mater. Sci. Technol, 2016. DOI: 10.1016/j.jmst.2016.03.018. [DOI] [Google Scholar]
- 392.Rasouli D, Dintcheva NT, Faezipour M, La Mantia FP, Mastri Farahani MR and Tajvidi M, Effect of nano zinc oxide as UV stabilizer on the weathering performance of wood-polyethylene composite, Polym. Degrad. Stab, 2016, 133, 85–91. [Google Scholar]
- 393.Kramer RB, Kramer R, Marshall N, Rosenberg J and Gupta RB, Fabric having ultraviolet radiation protection, enhanced resistance to degradation, and enhanced resistance to fire, U.S. Patent9234310, 2016.
- 394.Rezaei F, Maleknia L, Valipour P and Chizari Fard G, Improvement properties of nylon fabric by corona pre-treatment and nano coating, J. Textile Inst, 2016, 107, 1223–1231. [Google Scholar]
- 395.Wang B, Duan Y and Zhang J, Titanium dioxide nanoparticles-coated aramid fiber showing enhanced interfacial strength and UV resistance properties, Mater. Des, 2016, 103, 330–338. [Google Scholar]
- 396.Karimi L, Yazdanshenas ME, Khajavi R, Rashidi A and Mirjalili M, Functional finishing of cotton fabrics using graphene oxide nanosheets decorated with titanium dioxide nanoparticles, J. Textile Inst, 2016, 107, 1122–1134. [Google Scholar]
- 397.Berendjchi A, Khajavi R, Yousefi AA and Yazdanshenas ME, Improved continuity of reduced graphene oxide on polyester fabric by use of polypyrrole to achieve a highly electro-conductive and flexible substrate, Appl. Surf. Sci, 2016, 363, 264–272. [Google Scholar]
- 398.Yao Y, Tang B, Chen W, Sun L and Wang X, Sunlight-induced coloration of silk, Nanoscale Res. Lett, 2016, 11, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Bharathi Yazhini K and Gurumallesh Prabu H, Study on flame-retardant and UV-protection properties of cotton fabric functionalized with ppy-ZnO-CNT nanocomposite, RSC Adv, 2015, 5, 49062–49069 [Google Scholar]
- 400.Colangelo G, Favale E, Miglietta P and de Risi A, Innovation in flat solar thermal collectors: A review of the last ten years experimental results, Renewable Sustainable Energy Rev, 2016, 57, 1141–1159 [Google Scholar]
- 401.Chen G, Doroshenko A, Koltun P and Shestopalov K, Comparative field experimental investigations of different flat plate solar collectors, Sol. Energy, 2015, 115, 577–588 [Google Scholar]
- 402.Allan J, Dehouche Z, Stankovic S and Mauricette L, Performance testing of thermal and photovoltaic thermal solar collectors, Energy Sci. Eng, 2015, 3, 310–326. [Google Scholar]
- 403.Wallner GM, Povacz M, Hausner R and Lang RW, Lifetime modeling of polypropylene absorber materials for overheating protected hot water collectors, Sol. Energy, 2016, 125, 324–331 [Google Scholar]
- 404.Jones-Albertus R, Feldman D, Fu R, Horowitz K and Woodhouse M, Technology advances needed for photovoltaics to achieve widespread grid price parity, Prog. Photovolt: Res. Appl, 2016, 24, 1272–1283 [Google Scholar]
- 405.Lambert S and Wagner M, Characterisation of nanoplastics during the degradation of polystyrene, Chemosphere, 2016, 145, 265–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Qiu Q, Peng J, Yu X, Chen F, Wang J and Dong F, Occurrence of microplastics in the coastal marine environment: First observation on sediment of China, Mar. Pollut. Bull, 2015, 98, 274–280 [DOI] [PubMed] [Google Scholar]


