Abstract
While poor performance during a maximal graded exercise test (GXT) predicts cardiovascular events and premature mortality, the potential clinical importance of non-participation in a GXT, either for medical or non-medical reasons, is currently unknown. Data are from 4086 and 3547 Coronary Artery Risk Development in Young Adults (CARDIA) participants who attended the Year 7 (ages 25–37 years) and/or 20 exams (ages 38–50 years), respectively, which included a GXT. Cox proportional hazard models were used to examine the effect of GXT disposition (at Year 7 and 20, separately) on risk of non-fatal and fatal cardiovascular events and all-cause mortality obtained through 28 years of follow-up. A GXT was not conducted or completed according to protocol in 12.9% and 19.1% of participants attending the Year 7 and 20 exams, respectively. After adjustment, participants who missed the Year 20 GXT for medical reasons had a higher risk of cardiovascular events [HR: 4.06 (95% CI: 1.43, 11.5)] and all-cause mortality [HR: 3.07 (95% CI: 1.11, 12.3)] compared to GXT completers; participants who missed at Year 20 for non-medical reasons also had higher risk of all-cause mortality [HR: 2.53 (95% CI: 1.61, 3.99)]. Findings suggest that non-participation in a GXT, regardless of medical or non-medical reason, to be an important predictor of excess risk of adverse health outcomes and premature mortality. Additional patient follow-up, including identification of potential targets for intervention (e.g., weight management and smoking cessation programs), should be conducted at the point of a missed GXT.
Keywords: Cardiorespiratory fitness, Risk prediction, Adverse events, Cohort studies
1. Introduction
Cardiorespiratory fitness and cardiovascular response data collected as part of a maximal graded exercise test (GXT) protocol have consistently been shown to be predictors of non-fatal and fatal cardiovascular events and all-cause mortality (Mozaffarian et al., 2016; Ross et al., 2016; U.S. Department of Health and Human Services, 2008). Further, evidence suggests that cardiorespiratory fitness may be a more powerful predictor of premature mortality than traditional risk factors, including obesity (Ross et al., 2016). While information gleaned from a GXT has important clinical and public health relevance, one important requirement is that individuals participate in the protocol. There are several reasons why an individual may not participate in a maximal GXT, ranging from exclusion for medical reasons to voluntarily opting out (Armstrong et al., 2006). The underlying reasons for non-participation may provide additional useful clinical information about overall health status that extends beyond cardiorespiratory fitness and/or cardiovascular response to maximal exercise.
The Coronary Artery Risk Development in Young Adults (CARDIA) Study is unique in that a maximal GXT was successfully completed in 4659 participants (91.1%) at Year 0 (aged 18–30). Of the remaining 456 participants, 6.7% met medical exclusions, and 2.2% did not participate in the GXT for non-medical reasons; 1.2% and 0.8% of these 456 participants also did not take part in the GXT at the Year 7 (aged 25–37) and 20 (aged 38–50) exams, respectively. This comprehensive participation at the baseline exam is unusual among epidemiologic studies and allows for further identification of factors that can be used to predict subsequent risk for morbidity and mortality in a clinical setting.
In a previous CARDIA investigation (Zhu et al., 2010), several factors present at Year 0 predicted participation in one or both of the subsequent GXTs, including white race, non-smoker, lower body mass index (BMI), and higher reported physical activity. These findings serve as the basis for the current study, which was designed to examine if not participating in a GXT (overall, and further stratified by medical or non-medical reason) when the opportunity is presented is indicative of higher risk of non-fatal and fatal cardiovascular events and all-cause mortality over 28 years of follow-up. To optimize clinical relevance, a subsequent analysis was conducted to examine differences in potential targets for intervention and reported medical history that were obtained at the time of the exam, by GXT disposition.
2. Materials and methods
2.1. Design overview and study participants
CARDIA includes 5115 adults aged 18 to 30 years at the Year 0 examination (1985–86). Participants were recruited from four geographical locations (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA). Community-based sampling was performed in Birmingham, Chicago, and Minneapolis, while, in Oakland, participants were sampled from the membership of a large prepaid health care program. Recruitment efforts were successful in achieving a study population that was approximately balanced by age, race, sex, and education. Following the Year 0 examination, participants were re-examined 2, 5, 7, 10, 15, 20, 25, and 30 years later. Retention among survivors at Years 7 and 20 (when the GXT was offered to participants) was 81% and 72%, respectively (Coronary Artery Risk Development in Young Adults (CARDIA) Study, n.d.). At Years 0 and 7, the GXT was incorporated as part of the core exam, at Year 20 it was part of the CARDIA Fitness Study. All CARDIA participants provided informed consent and the institutional review boards at each participating center approve the study annually.
2.2. Data collection
2.2.1. Participant characteristics
Standardized questionnaires were used at Year 0 and at each follow-up visit to assess participant characteristics including, age, sociodemographic factors, health behaviors (including physical activity (Jacobs, 1989; Gabriel et al., 2014), diet (Lloyd-Jones et al., 2010), and smoking status), and reported medical history. All data collection measures are publicly available through the CARDIA website (http://www.cardia.dopm.uab.edu/) (Coronary Artery Risk Development in Young Adults (CARDIA) Study, n.d.).
2.2.2. Anthropometric measures
Height (stadiometer) and weight (balance-beam scale) were measured with the participant lightly clothed and without shoes (Cutter et al., 1991). BMI was calculated as weight (kg) divided by height squared (m2). Waist girth was measured to the closest 0.5 cm using anthropometric tape at the natural waist, midway between the iliac crest and the lowest lateral portion of the rib cage and anteriorly midway between the xiphoid process of the sternum and the umbilicus, at end-expiration.
2.2.3. Cardiovascular risk factors
Systolic and diastolic blood pressure was measured on the right arm, three times following 5-min of seated rest; the average of the last two measures was used. Blood samples were drawn from the antecubital vein for determination of insulin, glucose, and lipid concentrations. Insulin (Year 0) was measured by immunoassay (Herbert et al., 1965), and glucose was measured using the hexokinase-UV method (Manolio et al., 1990). Total cholesterol and triglyceride levels were enzymatically determined (Warnick, 1986). High density lipoprotein cholesterol (HDL-c) was measured after dextran sulfate-magnesium precipitation (Warnick et al., 1982), and low density lipoprotein cholesterol (LDL-c) was estimated using the Friedewald equation (Friedewald et al., 1972).
2.2.4. Exposure: GXT disposition
The CARDIA GXT was designed to assess maximal, symptom-limited cardiorespiratory fitness and utilized a modified Balke protocol (Sidney et al., 1992). This protocol included the following components: screening for medical eligibility; participant preparation for electrocardiogram (ECG); resting (supine) 12-lead ECG; pre-exercise (standing) 3-lead ECG and blood pressure; exercise on the treadmill (up to nine, 2-minute stages of increasing difficulty); recovery following exercise; and participant discharge.
At each visit, GXT disposition was determined using information obtained prior to (i.e., when ascertaining medical eligibility) and during the GXT protocol. Based on this information, participants were classified into one of three groups, including: (1) normal participation, defined as those with symptom limited maximal treadmill duration data from the GXT, (2) medical exclusion, operationalized as those meeting standard medical exclusions prior to (e.g., history of cardiac conditions) or during (e.g., abnormal blood pressure response) the GXT, or (3) other, no GXT defined as those who were (a) sick or injured on the day of the exam visit, and not rescheduled, (b) equipment malfunction on the day of the exam visit, and not rescheduled, or (c) participant refusal. For preliminary analysis, the medical exclusion, and other, no GXT categories were collapsed and labeled as missed GXT (versus normal participation).
2.2.5. Outcomes: cardiovascular morbidity and mortality and all-cause mortality
The CARDIA Endpoints Surveillance and Adjudication Subcommittee (ESAS), consisting of clinicians from all participating CARDIA field centers, uses pre-established criteria to classify morbidity endpoint events and underlying cause of death: http://www.cardia.dopm.uab.edu/images/more/recent/CARDIA_Endpoint_Events_MOO_v03_20_2015.pdf. Non-fatal and fatal cardiovascular events and deaths obtained through 28 years of annual event ascertainment through August 31, 2014 were used in analysis.
Every 6 months the participants were contacted by his/her field center to ascertain vital status and update contact information. For participants that could not be contacted, vital status searches were done annually, using various resources (e.g., social security death index, obituary search engines, contacting participant next-of-kin and/or friends). National Death Index searches were completed every 5 years. Medical records were requested for all suspected cardiovascular events; death certificates and autopsy reports (if performed) were requested for all deaths. When more information was needed, a member of the study team interviewed the next-of kin or an informed witness. Two members of the ESAS reviewed each record, applying standard outcome definitions to classify events; (Luepker et al., 2003; Rosamond et al., 2012; Madden et al., 1995; Adams et al., 1993; Easton et al., 2009) disagreements were resolved by the full CARDIA ESAS Committee (Armstrong et al., 2014).
2.3. Statistical analysis
Participant Year 0 characteristics were reported, stratified by Year 7 (and then, Year 20) GXT disposition categories (3-level variable) as mean with standard deviations (SD) reported for continuous variables and frequencies and proportions for categorical variables. Either Chi Square (χ2) tests or analysis of variance (ANOVA) was used to examine differences in Year 0 characteristics by GXT disposition.
Cox proportional hazard models were used to examine the effect of GXT disposition status (at Year 7 and 20, separately) on risk of non-fatal and fatal cardiovascular events and all-cause mortality through 28 years of follow-up. Minimally-adjusted models included: age (Year 7 or 20), race, sex, center, smoking status (never, current, former at Year 0 and Year 7 or 20), total self-reported physical activity (Year 0 and Year 7 or 20), BMI (Year 7 or 20), total cholesterol (Year 7 or 20), systolic blood pressure (Year 7 or 20), and cumulative incidence of type 2 diabetes mellitus (Year 7 or 20). Fully adjusted models also included Year 0 GXT duration. All covariates were selected a priori based on established associations with the exposure and/or outcome variables. Initial models included the two level exposure variable [missed GXT vs. normal participation (reference group)]. Then, all models were re-run using the three level exposure variable [other, no GXT or medical exclusion vs. normal participation (reference group)]. The unadjusted probability of an event during follow-up was plotted using the Kaplan-Meier method (Kaplan and Meier, 1958).
Differences in potentially modifiable risk factors and reported medical history, obtained at the time of the exam, by GXT disposition were determined using χ2 tests. In women, 6.3% (n = 103) and 1.4% (n = 24) reported either being pregnant or suspected pregnancy at the Year 7 and 20 exams, respectively. While not an overt contraindication, maximal exercise testing is not recommended in pregnant women (American College of Sports Medicine, 2015). Therefore, a sensitivity analysis was conducted excluding these women to determine if risk estimates differed. All statistical significance tests were two-sided with a type I error level set at p < 0.05. All analyses were generated with SAS/STAT software, version 9.4 (Cary, NC).
3. Results
As shown on Table 1, 3560 participants [87.1% of Year 7 attendees (n = 4086)] took part in the GXT protocol, 4.7% and 8.1% did not participate in the GXT due to medical or non-medical reasons, respectively. At Year 20, 2870 participants [80.9% of Year 20 attendees (n = 3547)] took part in the GXT protocol, 1.6% and 17.5% did not participate in the GXT due to medical or non-medical reasons. Across both exam years, individuals classified as other, no GXT had the lowest mean values of reported vigorous intensity physical activity (both p < 0.05) and GXT duration (both p < 0.001), and highest mean BMI (also highest proportion classified as obese), waist circumference, and triglycerides at Year 0 (all p < 0.001). Those with medical exclusions had the highest proportion of participants with fair/poor self-rated health (both p < 0.001) and highest mean values of insulin (both p < 0.001) at Year 0.
Table 1.
Year 0 (1985–86) characteristics by graded exercise test (GXT) disposition at the CARDIA Year 7 and 20 exam visits.
| Year 7 (1992–93; cohort ages 25–37 years) | Year 20 (2005–06; cohort ages 38–50 years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| n | Other, no GXT* | Medical exclusions† | Normal participation‡ | p value | n | Other, no GXT* | Medical exclusions† | Normal participation‡ | p value | |
|
| ||||||||||
| n = 332 | n = 194 | n = 3560 | n = 623 | n = 56 | n = 2870 | |||||
| Age, years | 4086 | 26.0 ± 3.7 | 25.0 ± 3.5 | 25.0 ± 3.6 | 0.073 | 3549 | 26.0 ± 3.8 | 27.0 ± 3.8 | 25.0 ± 3.6 | 0.14 |
| Sex, % | 4086 | < 0.001 | 3549 | 1.0 | ||||||
| Male | 38.0 | 29.4 | 46.5 | 43.2 | 42.9 | 43.3 | ||||
| Female | 62.0 | 70.6 | 53.5 | 56.8 | 57.1 | 56.7 | ||||
| Race, % | 4086 | < 0.001 | 3549 | < 0.001 | ||||||
| Black | 62.0 | 58.2 | 46.5 | 55.2 | 42.9 | 44.7 | ||||
| White | 38.0 | 41.8 | 53.5 | 44.8 | 57.1 | 55.3 | ||||
| Education, % | 4019 | 0.12 | 3491 | 0.002 | ||||||
| ≤High school degree | 70.1 | 71.9 | 66.0 | 68.9 | 72.7 | 63.3 | ||||
| Associate degree | 7.0 | 4.1 | 5.9 | 7.6 | 5.5 | 5.7 | ||||
| ≥Bachelor’s degree | 22.9 | 24.0 | 28.1 | 23.4 | 21.8 | 31.0 | ||||
| Marital status, % | 4082 | 0.94 | 3546 | 0.57 | ||||||
| Married or living with a significant other | 23.3 | 24.2 | 23.1 | 25.0 | 26.8 | 23.3 | ||||
| Other | 76.7 | 75.8 | 76.9 | 75.0 | 73.2 | 76.7 | ||||
| Full-time occupation, % | 4081 | 0.031 | 3546 | 0.095 | ||||||
| Yes | 54.8 | 51.0 | 59.2 | 57.2 | 46.4 | 59.5 | ||||
| No | 45.2 | 49.0 | 40.8 | 42.8 | 53.6 | 40.5 | ||||
| Difficulty for paying for basic needs, % | 4078 | < 0.001 | 3.544 | 0.03 | ||||||
| Not very hard | 93.7 | 92.8 | 96.7 | 94.7 | 92.9 | 96.7 | ||||
| Other | 6.3 | 7.2 | 3.3 | 5.3 | 7.1 | 3.4 | ||||
| Self-rated health, % | 4061 | < 0.001 | 3528 | < 0.001 | ||||||
| Fair/poor | 16.1 | 17.1 | 8.9 | 13.2 | 17.9 | 8.5 | ||||
| Good | 54.2 | 52.9 | 54.1 | 54.5 | 57.1 | 52.6 | ||||
| Excellent | 29.7 | 30.0 | 37.0 | 32.4 | 25.0 | 38.9 | ||||
| Current smoker, % | 4060 | 0.09 | 3529 | < 0.001 | ||||||
| Never | 54.4 | 50.8 | 59.0 | 54.4 | 48.2 | 61.4 | ||||
| Former | 13.3 | 16.6 | 13.1 | 13.1 | 25.0 | 13.8 | ||||
| Current | 32.3 | 32.6 | 27.9 | 32.5 | 26.8 | 24.8 | ||||
| Body mass index, % | 4086 | < 0.001 | 3549 | < 0.001 | ||||||
| Normal weight | 59.9 | 58.3 | 66.4 | 55.7 | 64.3 | 68.2 | ||||
| Overweight | 17.5 | 21.1 | 23.6 | 24.1 | 21.4 | 22.6 | ||||
| Obese | 22.6 | 20.6 | 10.0 | 20.2 | 14.3 | 9.2 | ||||
| Self-reported physical activity, exercise units | 4085 | 3548 | ||||||||
| Moderate intensity | 114.0 ± 113.9 | 110.0 ± 99.0 | 128.0 ± 107.6 | 0.057 | 116.0 ± 108.4 | 125.5 ± 133.5 | 127.5 ± 106.7 | 0.32 | ||
| Vigorous intensity | 184.0 ± 225.1 | 185.5 ± 202.1 | 237.0 ± 233.7 | < 0.001 | 213.0 ± 223.1 | 236.5 ± 197.1 | 233.0 ± 232.4 | 0.03 | ||
| Total | 305.5 ± 304.4 | 332.0 ± 254.9 | 373.0 ± 300.1 | < 0.001 | 324.5 ± 292.1 | 315.0 ± 307.3 | 368.0 ± 298.1 | 0.045 | ||
| Graded exercise test duration, seconds | 3978 | 480.0 ± 184.4 | 553.0 ± 170.2 | 600.0 ± 169.5 | < 0.001 | 3465 | 540.0 ± 176.5 | 569.0 ± 180.4 | 600.0 ± 168.0 | < 0.001 |
| Body mass index, kg/m2 | 4073 | 23.9 ± 7.3 | 23.5 ± 7.5 | 23.4 ± 4.5 | < 0.001 | 3536 | 24.3 ± 6.0 | 23.9 ± 5.5 | 23.3 ± 4.5 | < 0.001 |
| Waist circumference, cm | 4070 | 77.0 ± 15.7 | 75.8 ± 15.5 | 76.0 ± 10.6 | < 0.001 | 3533 | 78.5 ± 13.6 | 77.9 ± 11.6 | 75.5 ± 10.6 | < 0.001 |
| Systolic blood pressure, mm Hg | 4086 | 112.0 ± 13.3 | 112.0 ± 13.5 | 109.5 ± 10.5 | < 0.001 | 3549 | 111.0 ± 11.2 | 110.5 ± 10.9 | 109.0 ± 10.7 | < 0.001 |
| Diastolic blood pressure, mm Hg | 4086 | 71.0 ± 11.4 | 71.0 ± 12.0 | 68.0 ± 9.2 | < 0.001 | 3549 | 70.0 ± 9.4 | 68.0 ± 10.9 | 68.0 ± 9.3 | 0.002 |
| Total cholesterol, mg/dL | 4052 | 176.0 ± 34.0 | 175.0 ± 32.3 | 174.0 ± 33.0 | 0.76 | 3519 | 176.0 ± 34.6 | 167.0 ± 31.6 | 174.0 ± 32.8 | 0.16 |
| Low density lipoprotein cholesterol, mg/dL | 4038 | 107.0 ± 31.6 | 108.5 ± 31.9 | 106.0 ± 30.8 | 0.94 | 3509 | 108.0 ± 32.2 | 102.0 ± 32.4 | 107.0 ± 30.8 | 0.064 |
| High density lipoprotein cholesterol, mg/dL | 4052 | 52.0 ± 13.7 | 52.0 ± 13.7 | 52.0 ± 12.9 | 0.66 | 3519 | 51.0 ± 12.4 | 51.0 ± 15.1 | 53.0 ± 12.9 | 0.01 |
| Triglycerides, mg/dL | 4051 | 66.0 ± 77.4 | 65.0 ± 41.5 | 61.0 ± 45.4 | < 0.001 | 3516 | 65.0 ± 60.0 | 61.0 ± 74.2 | 60.0 ± 42.3 | < 0.001 |
| Insulin, uU/mL | 4030 | 6.7 ± 7.5 | 7.4 ± 6.5 | 6.1 ± 5.1 | < 0.001 | 3504 | 6.9 ± 6.5 | 7.0 ± 7.6 | 5.9 ± 5.2 | < 0.001 |
| Glucose, mg/dL | 4023 | 82.0 ± 28.5 | 82.0 ± 34.5 | 81.0 ± 10.5 | < 0.001 | 3496 | 82.0 ± 16.1 | 80.0 ± 9.0 | 81.0 ± 10.6 | 0.01 |
Individuals were categorized as: (*) other, no GXT defined as attending the exam visit, sick or injured at the time of the exam visit, but not rescheduled, equipment malfunction, and/or participant refusal, (†) medical exclusion defined as meeting medical exclusion(s) prior to or during the GXT, or (‡) normal participation defined as those with available GXT data.
The risk of non-fatal and fatal cardiovascular events and all-cause mortality of those that missed the GXT (for any reason), compared to those who participated in the GXT protocol, at Year 7 and 20 are shown in Table 2. In the minimally adjusted models, participants that missed the GXT at Year 7 had a higher risk of non-fatal and fatal cardiovascular events and all-cause mortality through 28 years of follow-up, compared to the normal participation group [HR: 1.58 (95% CI: 1.04, 2.41) and HR: 2.04 (95% CI: 1.42, 2.91), respectively]. The risk estimates were attenuated after additional adjustment for GXT duration at Year 0, but remained statistically significant for all-cause mortality [HR: 1.58 (95% CI: 1.05, 2.36)]. Based on the minimally adjusted models, participants that missed the GXT at Year 20 had a higher risk of non-fatal and fatal cardiovascular events and all-cause mortality than those that participated in the GXT protocol. Findings were similar in the fully adjusted models [HR: 1.66 (95% CI: 1.03, 2.70) and HR: 2.60 (95% CI: 1.76, 4.05), respectively].
Table 2.
Hazards ratios (HR) for associations of graded exercise test (GXT) disposition (missed GXT for any reason versus normal participation) at the CARDIA Year 7 (1992–93; ages 25–37 years) and 20 (2005–06; ages 38–50 years) exam visits with cardiovascular morbidity and mortalitya and all-cause mortalityb through 28 years of follow-up.
| Minimally-adjusted modele | Fully-adjusted modelf | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Events/N at risk | Events, % | Hazards ratio | 95% CI | Events/N at risk | Events, % | Hazards ratio | 95% CI | |||
| Cardiovascular morbidity and mortality | ||||||||||
| Year 7 | ||||||||||
| Missed GXTc | 41/383 | 10.7 | 1.58 | 1.04 | 2.41 | 35/346 | 10.1 | 1.46 | 0.94 | 2.26 |
| Normal participationd | 127/3456 | 3.7 | 1.0 | 126/3399 | 3.7 | 1.0 | ||||
| Total | 168/3839 | 4.4 | 161/3745 | 4.3 | ||||||
| Year 20 | ||||||||||
| Missed GXTc | 28/592 | 4.7 | 1.65 | 1.02 | 2.67 | 27/576 | 4.7 | 1.66 | 1.03 | 2.70 |
| Normal participationd | 58/2780 | 2.1 | 1.0 | 57/2720 | 2.1 | 1.0 | ||||
| Total | 86/3372 | 2.6 | 84/3296 | 2.5 | ||||||
| All-cause mortality | ||||||||||
| Year 7 | ||||||||||
| Missed GXTc | 50/388 | 12.9 | 2.04 | 1.42 | 2.91 | 36/350 | 10.3 | 1.58 | 1.05 | 2.36 |
| Normal participationd | 162/3456 | 4.7 | 1.0 | 158/3399 | 4.6 | 1.0 | ||||
| Total | 212/3844 | 5.5 | 194/3749 | 5.2 | ||||||
| Year 20 | ||||||||||
| Missed GXTc | 37/620 | 6.0 | 2.64 | 1.71 | 4.08 | 36/601 | 6.0 | 2.60 | 1.67 | 4.05 |
| Normal participationd | 55/2803 | 2.0 | 1.0 | 53/2743 | 1.9 | 1.0 | ||||
| Total | 92/3423 | 2.7 | 89/3344 | 2.7 | ||||||
Cardiovascular events includes fatal and non-fatal: acute myocardial infarct, heart failure, angina, peripheral artery disease, transient ischemic attack and stroke.
Death from any cause.
Missed GXT defined as no available GXT data, when the opportunity to perform a GXT was offered.
Normal participation defined as those with available GXT data.
Minimally-adjusted multivariable models adjusted for age (Year 7 or 20), race, sex, center, smoking status (current and former; Years 0 & 7 or 20), total self-reported physical activity (Years 0 & 7 or 20), body mass index (Year 7 or 20), total cholesterol (Year 7 or 20), systolic blood pressure (Year 7 or 20), and cumulative incidence of type 2 diabetes mellitus, determined using fasting glucose and medication use (Year 7 or 20) and via 2-hour oral glucose tolerance test and hemoglobin A1c (Year 20, only).
Fully-adjusted multivariate models adjusted for all variables included in the minimally-adjusted multivariable model plus Year 0 graded exercise test (GXT) duration.
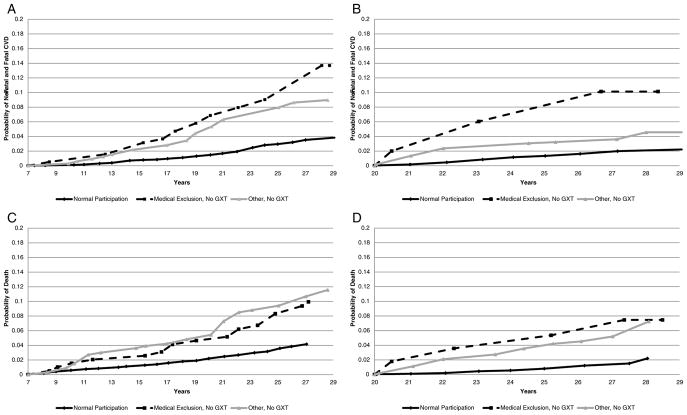
Table 3 presents the risk of non-fatal and fatal cardiovascular events and all-cause mortality through 28 years of follow-up using the 3-level GXT disposition exposure variable. At Year 7, results from minimally adjusted models suggest that those with medical exclusions had a statistically significant increased risk of non-fatal and fatal cardiovascular events and all-cause mortality, respectively, compared to those that participated in the GXT protocol. Also, participants classified as other, no GXT had a statistically significant increased risk of all-cause mortality. After full adjustment, the higher risk of non-fatal and fatal cardiovascular events among those with medical exclusions (versus normal participation) was borderline statistically significant [HR: 1.59 (95% CI: 0.99, 2.56)]. At Year 20, compared to those with normal participation, results from the minimally adjusted models showed participants with medical exclusions had a significantly higher risk of non-fatal and fatal cardiovascular events and participants classified as other, no GXT had a significantly higher risk of non-fatal and fatal cardiovascular events and all-cause mortality. After full adjustment, compared to those with normal participation, individuals with medical exclusions had a significantly higher risk of non-fatal and fatal cardiovascular events [HR: 4.06 (95% CI: 1.43, 11.5)] and all-cause mortality [HR: 3.70 (95% CI: 1.11, 12.3)]; participants classified as other, no GXT had a higher risk of all-cause mortality [HR: 2.53 (95% CI: 1.61, 3.99)]. The Kaplan-Meier curves showing the probability of non-fatal and fatal cardiovascular events and all-cause mortality from the time of the GXT through the follow-up period are shown in Fig. 1 (Panels A–D). When women reporting or suspecting pregnancy were excluded from the main analysis, findings were generally similar (data not shown). The one exception was that the risk estimate of all-cause mortality among participants excluded from the Year 7 GXT for medical reasons (versus normal participation) reached statistical significance [HR: 1.64 (95% CI: 1.02, 2.64)].
Table 3.
Hazards ratios (HR) for associations of graded exercise test (GXT) disposition (no GXT due to medical exclusion versus no GXT, but attended visit versus normal participation) at the CARDIA Year 7 (1992–93; ages 25–37 years) and 20 (2005–06; ages 38–50 years) exam visits with cardiovascular morbidity and mortalitya and all-cause mortalityb through 28 years of follow-up.
| Minimally-adjusted modelf | Fully-adjusted modelg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Events/N at risk | Events, % | Hazards ratio | 95% CI | Events/N at risk | Events, % | Hazards ratio | 95% CI | |||
| Cardiovascular morbidity and mortality | ||||||||||
| Year 7 | ||||||||||
| Other, no GXTc | 26/242 | 10.7 | 1.43 | 0.87 | 2.33 | 23/220 | 10.5 | 1.36 | 0.82 | 2.27 |
| Medical exclusiond | 15/141 | 10.6 | 1.90 | 1.06 | 3.40 | 12/126 | 9.5 | 1.66 | 0.88 | 3.15 |
| Normal participatione | 127/3456 | 3.7 | 1.0 | 126/3399 | 3.7 | 1.0 | ||||
| Total | 168/3839 | 4.4 | 161/3745 | 4.3 | ||||||
| Year 20 | ||||||||||
| Other, no GXTc | 23/545 | 4.2 | 1.52 | 0.92 | 2.51 | 23/534 | 4.3 | 1.51 | 0.91 | 2.51 |
| Medical exclusiond | 5/47 | 10.6 | 3.39 | 1.26 | 9.12 | 4/42 | 9.5 | 4.06 | 1.43 | 11.5 |
| Normal participatione | 58/2780 | 2.1 | 1.0 | 57/2720 | 2.1 | 1.0 | ||||
| Total | 86/3372 | 2.5 | 84/3296 | 2.5 | ||||||
| All-cause mortality | ||||||||||
| Year 7 | ||||||||||
| Other, no GXTc | 32/244 | 13.1 | 2.08 | 1.37 | 3.15 | 23/222 | 10.4 | 1.55 | 0.85 | 2.82 |
| Medical exclusiond | 18/144 | 12.5 | 1.97 | 1.18 | 3.30 | 13/128 | 10.2 | 1.59 | 0.99 | 2.56 |
| Normal participatione | 162/3456 | 4.7 | 1.0 | 158/3399 | 4.6 | 1.0 | ||||
| Total | 212/3844 | 5.5 | 194/3749 | 5.2 | ||||||
| Year 20 | ||||||||||
| Other, no GXTc | 34/568 | 6.0 | 2.61 | 1.67 | 4.07 | 33/555 | 5.9 | 2.53 | 1.61 | 3.99 |
| Medical exclusiond | 3/52 | 5.8 | 3.11 | 0.93 | 10.4 | 3/46 | 6.5 | 3.70 | 1.11 | 12.3 |
| Normal participatione | 55/2803 | 2.0 | 1.0 | 53/2743 | 1.9 | 1.0 | ||||
| Total | 92/3423 | 2.7 | 89/3344 | 2.7 | ||||||
Cardiovascular events includes fatal and non-fatal: Acute myocardial infarct, heart failure, angina, peripheral artery disease, transient ischemic attack and stroke.
Death from any cause.
Other, no GXT defined as attending the exam visit, sick or injured at the time of the exam visit, but not rescheduled, equipment malfunction, and/or participant refusal.
Medical exclusion defined as meeting medical exclusion(s) prior to or during the GXT.
Normal participation defined as those with available GXT data.
Minimally-adjusted multivariable models adjusted for age (Year 7 or 20), race, sex, center, smoking status (current and former; Years 0 & 7 or 20), total self-reported physical activity (Years 0 & 7 or 20), body mass index (Year 7 or 20), total cholesterol (Year 7 or 20), systolic blood pressure (Year 7 or 20), and cumulative incidence of type 2 diabetes mellitus, determined using fasting glucose and medication use (Year 7 or 20) and via 2-hour oral glucose tolerance test and hemoglobin A1c (Year 20, only).
Fully-adjusted multivariate models adjusted for all variables included in the minimally-adjusted multivariable model plus Year 0 graded exercise test (GXT) duration.
Fig. 1.
(Panels A–D). Kaplan-Meier Curve of non-fatal and fatal cardiovascular events and all-cause mortality during follow-up after the Year 7 (1992–93) or Year 20 CARDIA Exams (2005–06).
To optimize clinical relevance, Table 4 shows differences in potentially modifiable risk factors measured at the time of the Year 7 or Year 20 exam by GXT disposition status. Participants meeting medical exclusions had the highest proportion of prevalent hypertension (Year 20) and hypercholesterolemia (Year 7) and had the lowest proportion meeting physical activity guidelines (Years 7 and 20). Participants classified as other, no GXT had the highest proportion of prevalent hypertension (Year 7), type 2 diabetes (Year 7), unfavorable diet composition (Year 7 and 20), overweight or obesity (Year 7 and 20), and current smoker (Year 7 or 20). Differences in reported medical conditions collected at the Year 7 and 20 exams by GXT disposition status are also shown in Supplemental Table 1. Of note, the percentage of participants reporting a medical problem that limits exercise, was highest in those meeting medical exclusions at Year 7 and 20 (30.9% versus 37.5%). However, participants classified as other, no GXT had a higher prevalence than those with normal participation (Year 7: 27.0% versus 16.4%) and Year 20: 26.5% versus 15.5%, respectively).
Table 4.
Potential targets for clinical intervention at the time of the CARDIA exam visit by Year 7 (1992–93; ages 25–37 years) and 20 (2005–06; ages 38–50 years) graded exercise test (GXT) disposition status.
| Year 7 (1992–93; cohort ages 25–37 years) | Year 20 (2005–06; cohort ages 38–50 years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| n | Other, no GXT† | Medical exclusion‡ | Normal participation§ | p value | n | Other, no GXT† | Medical exclusion‡ | Normal participation§ | p value | |
|
| ||||||||||
| n = 332 | n = 194 | n = 3560 | n = 623 | n = 56 | n = 2870 | |||||
| Potential targets for clinical intervention | ||||||||||
| High blood pressure (measured)*, % | 4086 | < 0.001 | 3549 | < 0.001 | ||||||
| No | 71.7 | 79.9 | 97.5 | 68.1 | 67.9 | 80.1 | ||||
| Yes | 28.3 | 20.1 | 2.5 | 31.9 | 32.1 | 19.9 | ||||
| High blood cholesterol (measured)*, % | 4008 | < 0.001 | 3506 | |||||||
| No | 92.6 | 89.5 | 95.6 | 83.6 | 85.2 | 85.5 | 0.485 | |||
| Yes | 7.4 | 10.5 | 4.4 | 16.5 | 14.8 | 14.5 | ||||
| Type 2 diabetes mellitus (measured)*, % | 4086 | < 0.001 | 3527 | < 0.001 | ||||||
| No | 96.1 | 97.9 | 98.9 | 61.0 | 64.3 | 52.7 | ||||
| Yes | 3.9 | 2.1 | 1.1 | 39.0 | 35.7 | 47.3 | ||||
| Meets physical activity guidelines (measured)*, % | 4014 | 0.004 | 3527 | < 0.001 | ||||||
| No | 59.7 | 64.9 | 54.2 | 61.0 | 64.3 | 52.7 | ||||
| Yes | 40.3 | 35.1 | 45.8 | 39.0 | 35.7 | 47.3 | ||||
| AHA dietary criteria (measured)*, % | 3910 | 0.029 | 3094 | 0.493 | ||||||
| 0–2 components | 88.7 | 79.1 | 84.3 | 78.1 | 71.2 | 78.0 | ||||
| 3–5 components | 11.3 | 20.9 | 15.7 | 21.9 | 28.9 | 22.0 | ||||
| Body mass index (measured)*, % | 4086 | < 0.001 | 3549 | < 0.001 | ||||||
| Normal | 45.5 | 51.0 | 47.7 | 23.8 | 32.1 | 30.1 | ||||
| Overweight | 22.6 | 17.5 | 31.0 | 28.1 | 19.6 | 34.3 | ||||
| Obese | 31.9 | 31.4 | 21.3 | 48.1 | 48.2 | 35.5 | ||||
| Current smoker (measured)*, % | 4069 | 0.004 | < 0.001 | |||||||
| No | 66.8 | 67.4 | 73.9 | 72.9 | 74.1 | 82.3 | ||||
| Yes | 33.2 | 32.6 | 26.1 | 27.1 | 25.9 | 17.7 | ||||
High blood pressure defined as ≥140/90 mm Hg OR medication use for hypertension; high blood cholesterol defined as ≥240 mg/dL (fasting) OR medication use for hypercholesterolemia; type 2 diabetes mellitus defined as fasting glucose ≥100 mg/dL OR medication use for hypoglycemia OR 2-hour oral glucose tolerance test ≥200 mg/dL (Year 20, only), OR hemoglobin A1c ≥6.5% (Year 20, only); meets physical activity guidelines defined as reporting ≥300 exercise units of total (moderate to vigorous intensity) physical activity (Gabriel et al., 2014); AHA dietary criteria defined using the sum of meeting recommendations for the following criteria: Fruits and vegetable servings per day, sodium, fish, whole grains, and sugar-sweetened beverages (Lloyd-Jones et al., 2010); body mass index defined as normal (< 25 kg/m2), overweight (≥25 to < 30 kg/m2), or obese (≥30 kg/m2) based on measured height (m) and weight (kg); current smoker defined as cigarette, pipe, or cigar use at the time of the visit. Participants categorized as: (†) other, no GXT defined as attending the exam visit, sick or injured at the time of the exam visit, but not rescheduled, equipment malfunction, and/or participant refusal, (‡) medical exclusion defined as meeting medical exclusion(s) prior to or during the GXT, or (§) normal participation defined as those with available GXT data.
4. Discussion
The principal finding of the current study supports the premise that non-participation in a GXT may be clinically meaningful, rather than something to disregard or ignore. Specifically, study findings suggest a statistically significantly higher risk of non-fatal and fatal cardiovascular events in CARDIA participants who did not participate in the GXT (for any reason) at Year 20, as well as a statistically significantly higher risk of all-cause mortality in CARDIA participants who did not participate in the GXT (for any reason) at Year 7 and/or 20 through 28 years of follow-up. Upon further stratification, compared to those with normal participation, individuals that met medical exclusions at Year 7 and 20 had a statistically significant higher risk of all-cause mortality; a finding that was remarkably also shown in those who missed the Year 20 GXT for non-medical reasons, including refusal. However, these findings should be interpreted with caution given that non-participation in a GXT protocol that is part of a research study may be different than non-participation in a clinical setting.
In a recent Scientific Statement by the American Heart Association (AHA), Ross et al. (Ross et al., 2016) argues that the addition of cardiorespiratory fitness to more traditional cardiovascular risk factors would improve overall risk prediction for adverse events. Further, unlike current clinical practice, Ross and colleagues advocate for the implementation of routine cardiorespiratory fitness testing, ideally using a maximal GXT protocol, in all adults. Authors (Ross et al., 2016) argue this more widespread implementation would provide physicians with new opportunities to intervene, including encouraging physical activity participation to optimize cardiovascular health (Kokkinos et al., 2010; Kodama et al., 2009; Laukkanen et al., 2010). While Ross et al. (Ross et al., 2016) make a compelling case, the underlying assumption is that the patient is either sufficiently healthy (does not meet medical exclusions) and/or willing to participate in the GXT protocol. In CARDIA, GXT data were not available for 12.9% and 19.1% of participants attending the Year 7 and 20 exams, respectively. This represents a substantial proportion of individuals who would, theoretically, not benefit from patient counseling and intervention that poor performance on a GXT would elicit. Our study can inform future recommendations related to the importance of cardiorespiratory fitness as a clinical vital sign because findings suggest that missing a GXT may also be considered an indicator of underlying risk.
In terms of clinical action, rather than disregarding or ignoring a missed GXT, our findings suggest that physicians should be aware of this higher mortality risk and conduct additional follow-up to better understand the underlying reasons for non-participation and intervene, as necessary. While subsequent intervention is routine among patients that meet medical exclusions for the GXT, our findings suggest that additional patient follow-up may also be warranted for patients that cancel or do not show up for their scheduled GXT. Based on the Kaplan-Meier curves, the probability of an adverse event gradually increased overtime; risk was not immediate. This suggests the availability of several years for physicians to potentially counsel patients and implement treatment plans to reduce risk and optimize cardiovascular health (Lloyd-Jones et al., 2010). Further, individuals that did not participate in the GXT for non-medical reasons, had underlying – yet, modifiable –conditions and/or risk behaviors at the time of the exam that may explain non-participation. For example, across both exams, participants classified as other, no GXT had the highest proportion of overweight/ obesity and current smokers; therefore, focused behavioral intervention (including diet modification) for weight reduction or smoking cessation may have reduced risk of premature mortality. These findings illustrate the potential utility of conducting additional follow-up with the patient immediately following a missed GXT. However, additional studies are needed that utilize a clinical population to confirm these findings.
Current study findings also have implications for exercise training and epidemiologic studies. Clearly, maximal testing provides important information on underlying physiology at the outer limits of functional capacity. However, maximal testing can lead to biased samples, in that they only include those who are physically able and willing to undergo such testing. In turn, this greatly limits our understanding of the functional capacity (and related health consequences) of this at-risk population sub-group and supports the importance of missing data. Future studies should consider using all available information, including whether the individual participated in the protocol or not, when evaluating health risk.
Observational studies like CARDIA which included a GXT in a “take all comers” sample with no restriction on presumed exercise capacity may provide a real-life representation of the determinants and health consequences of being excluded and/or opting out of a maximal GXT protocol; findings that cannot be replicated with an experimental study design. Further, the results of the current study are directly applicable to the AHA Scientific Statement by Ross et al. (Ross et al., 2016), which encourages conduct routine cardiorespiratory fitness assessment in all adults to improve risk stratification and reduce the substantial public health burden attributable by cardiovascular diseases in the U.S. (Benjamin et al., 2017) However, as previously mentioned, studies using a clinical sample are still needed to confirm our findings. Other strengths include the use of adjudicated cardiovascular events and death and repeated measures of cardiorespiratory fitness that reflect important stages of adulthood. There are also limitations to consider including the relatively low number of cardiovascular and mortality events in CARDIA, which is largely a reflection of the still relatively young cohort. Further, results were limited to CARDIA participants that attended the Year 7 and 20 exams, which may influence external study validity. Also, the low sample sizes in the medical exclusion group at Year 20 (n = 56) resulted in large confidence intervals around the risk estimate, which reduces precision. Finally, in CARDIA the GXT was offered at no-cost to participants; therefore, we were unable to evaluate the health risks associated with not completing the test for monetary reasons, including lack of health insurance.
5. Conclusions
To our knowledge, this is the first study that examined the potential clinical importance of not participating in a GXT when the opportunity was provided, in relation to risk of non-fatal and fatal cardiovascular events and all-cause mortality. The research and clinical community should recognize the inherent bias when evaluating health risks only among individuals that are able and willing to perform a GXT. Also, physicians should conduct additional follow-up with patients unwilling to perform a GXT to identify possible targets for subsequent intervention to reduce risk for adverse events and optimize overall cardiovascular health.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the CARDIA participants.
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005). Additional support for this work was provided by the CARDIA Fitness Study (R01 HL078972 to BS & SS) and CARDIA Activity Study (R56 HL125423 to KPG, BS/SS).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2017.10.025.
Transparency document
The Transparency document associated with this article can be found, in online version.
Conflict of interest
None.
Disclosures
None.
References
- Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. ACSM’s Resources for the Exercise Physiologist: A Practical Guide for the Health Fitness Professional. Lippincott, Williams & Wilkins; 2015. [Google Scholar]
- Armstrong L, Balady GJ, Berry MJ, et al. ACSM’s Guidelines for Exercise Testing and Prescription. 7. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. [Google Scholar]
- Armstrong AC, Jacobs DR, Jr, Gidding SS, et al. Framingham score and LV mass predict events in young adults: CARDIA study. Int J Cardiol. 2014;172(2):350–355. doi: 10.1016/j.ijcard.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Accessed January 8, 2013];Coronary Artery Risk Development in Young Adults (CARDIA) Study Website. n.d http://www.cardia.dopm.uab.edu/
- Cutter GR, Burke GL, Dyer AR, et al. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control. Clin. Trials. 1991;12(1 Suppl):1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40(6):2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- Gabriel KP, Sidney S, Jacobs DR, Jr, et al. Convergent validity of a brief self-reported physical activity questionnaire. Med Sci Sports Exerc. 2014;46(8):1570–1577. doi: 10.1249/MSS.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Jacobs DR. Validity and reliability of short physical activity history: Cardia and the Minnesota Heart Health Program. J Cardpulm Rehabil. 1989;9:448–459. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- Kokkinos P, Myers J, Faselis C, et al. Exercise capacity and mortality in older men: a 20-year follow-up study. Circulation. 2010;122(8):790–797. doi: 10.1161/CIRCULATIONAHA.110.938852. [DOI] [PubMed] [Google Scholar]
- Laukkanen JA, Makikallio TH, Rauramaa R, Kiviniemi V, Ronkainen K, Kurl S. Cardiorespiratory fitness is related to the risk of sudden cardiac death: a population-based follow-up study. J Am Coll Cardiol. 2010;56(18):1476–1483. doi: 10.1016/j.jacc.2010.05.043. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- Madden KP, Karanjia PN, Adams HP, Jr, Clarke WR. Accuracy of initial stroke subtype diagnosis in the TOAST study. Trial of ORG 10172 in Acute Stroke Treatment. Neurology. 1995;45(11):1975–1979. doi: 10.1212/wnl.45.11.1975. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Savage PJ, Burke GL, et al. Association of fasting insulin with blood pressure and lipids in young adults. The CARDIA study. Arteriosclerosis. 1990;10(3):430–436. doi: 10.1161/01.atv.10.3.430. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: a report from the American Heart Association. Circulation. 2016;133(4):e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5(2):152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134(24):e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- Sidney S, Haskell WL, Crow R, et al. Symptom-limited graded treadmill exercise testing in young adults in the CARDIA study. Med Sci Sports Exerc. 1992;24(2):177–183. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. [Accessed date: 10 October 2008];Physical Activity Guidelines for Americans. 2008 www.health.gov/paguidelines.
- Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol. 1986;129:101–123. doi: 10.1016/0076-6879(86)29064-3. [DOI] [PubMed] [Google Scholar]
- Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28(6):1379–1388. [PubMed] [Google Scholar]
- Zhu N, Suarez-Lopez JR, Sidney S, et al. Longitudinal examination of age-predicted symptom-limited exercise maximum HR. Med Sci Sports Exerc. 2010;42(8):1519–1527. doi: 10.1249/MSS.0b013e3181cf8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.