Abstract
Humans have dopamine D5 receptors (hD5R) with single-nucleotide polymorphisms and a diminished function. We generated hD5F173L cDNA that has a decreased response to D5R agonist-mediated increase in cAMP production and increased production of reactive oxygen species, relative to wild-type hD5R (hD5WT) cDNA expressed in Chinese hamster ovary cells. To investigate the role of hD5F173L in the pathogenesis of salt-sensitive hypertension, we generated transgenic mice overexpressing hD5F173L or hD5WT and fed them normal (0.8% NaCl) or high (4% NaCl) salt diet. On normal salt diet, the blood pressure, and renal NADPH oxidase activity and angiotensin type 1 receptor (AT1R) expression were higher in hD5F173L than hD5WT transgenic mice. After 2 weeks on high salt diet, the blood pressure and renal NADPH oxidase activity, but not AT1R expression, were increased in hD5F173L but not in hD5WT transgenic mice. Candesartan, an AT1R antagonist, decreased the blood pressure and NADPH oxidase activity in hD5F173L but not in hD5WT transgenic mice. We suggest that the ability of the hD5R to negatively regulate the renal NADPH oxidase activity and AT1R function may have important implications in the pathogenesis of salt-sensitive blood pressure. However, the mechanisms involved in regulating the balance of renal D5R and AT1R function in the oxidative stress-mediated salt-sensitive blood pressure remain to be determined.
Keywords: D5R, AT1R, NADPH oxidase, salt-sensitive hypertension
INTRODUCTION
Hypertension is a multifactorial disorder resulting from the interactions between environmental and genetic factors.1,2 Epidemiological and interventional studies have demonstrated a clear relationship between salt intake and hypertension.3 Reactive oxygen species (ROS) are also known to be involved in the development of salt-sensitive hypertension and renal disease.4,5 A major source of ROS in the kidney is nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt (NAD(P)H) oxidase, which is an enzyme complex consisting of six subunits: membrane subunits p22phox and gp91phox, cytosolic components p40phox, p47phox and p67phox and a low-molecular weight G protein rac1 or rac2. Several homologues of gp91phox in mammalian non-phagocytic cells have been reported, including Nox 1, Nox 2, Nox 3, Nox 4 and Nox 5; Nox 5 is present in humans but not rodents. The rodent kidney mainly expresses Nox 1, Nox2 and Nox 4, that act as O2−-producing NAD(P)H oxidases.6,7
Abnormalities in dopamine production and receptor function have been described in human essential hypertension and rodent models of genetic hypertension.2,8–11 The effects of dopamine are exerted by two families of cell surface receptors that belong to the superfamily of G protein-coupled receptors; D1-like receptors (D1R and D5R) stimulate adenylyl cyclases, whereas D2-like receptors (D2R, D3R and D4R) inhibit adenylyl cyclases.2,8–11 Compared with D1R, the D5R has a higher affinity for dopamine, and exhibits constitutive activity.10,11 The locus of the human D5R (hD5R), 4p15.1–16.1, and its pseudogenes, 2p11.1-p11.2, 1q21.1, have been linked to human essential hypertension.12–14 Moreover, humans have D5R gene single-nucleotide polymorphisms with a diminished function.15
We have reported that the disruption of the D5R gene (Drd5) in mice increases blood pressure. The elevated blood pressure in Drd5−/− mice was suggested to be caused, in part, by an increased ROS production via an increased renal NADPH oxidase and phospholipase D activities.10,11 Dopamine, via its receptors, including the D5R, decreases renal tubular sodium transport. Decreasing the renal dopamine production or dopamine receptor expression or function results in sodium retention, contributing to the development of hypertension.2,8–11,16–21 However, the regulation of sodium transport by dopamine and its receptors also involves interaction with other G protein-coupled receptors.16 Indeed, the negative interaction between D5R and angiotensin type 1 receptor (AT1R) is involved in the regulation of renal sodium transport and pathogenesis of hypertension.16,22,23 We hypothesize that hD5R negatively regulates renal NADPH oxidase and AT1R function that may be important in the normal regulation of blood pressure. Moreover, impaired hD5R function may result in salt-sensitive hypertension.
To investigate the role and mechanism of D5R-mediated regulation of blood pressure and salt sensitivity, we generated mutant D5R with a single-nucleotide polymorphism, hD5F173L. We transfected the hD5F173L, and hD5 wild-type (hD5WT) cDNAs into Chinese hamster ovary (CHO) cells and tested their ability to regulate cyclic adenosine monophosphate (cAMP) and ROS production, in response to a D1R/D5R agonist, fenoldopam, which in the absence of D1R is a selective D5R agonist and vice versa. After establishing that hD5F173L, relative to hD5WT, has a decreased ability to increase cAMP production and negatively regulates ROS production, hD5F173L and hD5WT transgenic mice were generated that were studied for the effect of high salt intake on renal oxidative stress, AT1R expression and blood pressure.
METHODS
Materials
Cytochrome C, diphenyleneiodonium, fenoldopam, SCH23390, superoxide dismutase and candesartan were purchased from Sigma-Aldrich, St. Louis, MO, USA. NADPH was from ICN Biomedicals, Aliso Viejo, CA, USA. Lucigenin was from Molecular Probes (Grand Island, NY, USA), Invitrogen (Carlsbad, CA, USA), Eugene (Shanghai, China). Polyclonal AT1 antibody (N-10) and monoclonal anti-Nox 2 and Nox 4 antibodies were from Santa Cruz Biotechnology, Santa Cruz, CA, USA.
hD5WT and hD5F173L cell transfections
The full-length hD5WT and hD5F173L cDNAs, subcloned into a pcDNA6/V5-His vector between the EcoRI and Xbal sites, respectively, were transfected into CHO cells using LT1 transfection reagents.10 The successful transfection of hD5 cDNA was verified by immunoblotting for His/V5 expression. Empty vector-transfected CHO cells served as negative controls.
Cyclic AMP accumulation assay
The CHO cells, heterologously expressing hD5WT, hD5F173L or empty vector were grown to 70% confluence in six-well plates and pre-treated with 1 μM phorbol 12-myristate 13-acetate for 5 min in the presence of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (1 μM) before the D1R/D5R agonist fenoldopam (10 nM, 100 nM, 1 μM) treatment for 0 and 30 mins; in the absence of D1R, fendoldopam is a specific D5R agonist. Cell lysates were prepared to determine protein concentration by using the BCA protein assay kit (Thermo-Scientific, Rockford, IL, USA) and cAMP concentration by using the Direct Cyclic AMP Enzyme immunoassay kit (Arbor Assays, Ann Arbor, MI, USA), following the manufacturer’s procedures. All assays were performed in duplicates.
Detection of ROS
The oxidation of 2’,7’-dichlorodihydrofluorescein diacetate was used to measure ROS in the CHO cells heterologously expressing hD5WT, hD5F173L or empty vector. To test the effect of cellular NADPH oxidase inhibition on ROS production, cells were pre-treated with an inhibitor of NADPH oxidase (diphenyleneiodonium (DPI), 10 mM per 30 min) (Sigma), and then treated with H2O2 (50 μM per 2 h, ThermoScientific) or vehicle. The cells were incubated with fresh 2’,7’- dichlorodihydrofluorescein diacetate (10 mM per 30 mins) at 37 °C. 2’,7’-Dichlorodihydrofluorescein diacetate fluorescence was measured using a microplate reader in 96-well plates at an excitation wave length of 485nm and an emission wave length of 530nm. ROS production was expressed in arbitrary units corrected for the protein concentration (AU mg−1 protein). All assays were performed in duplicate.
Transgenic mice and blood pressure measurement
The full-length hD5WT and hD5F173L cDNAs were each subcloned into an expression plasmid under the CMV promoter. The transgenic mice were generated by the microinjection method in oocytes. The genotype of the transgenic mice was verified by polymerase chain reaction using the primers, 5′-GGACCGCTACTGGGCCATCT-3′ and 5′-GGGTCTTGAGAACCTTGGTC-3′, and the sequence of the amplified 488 bp fragment of hD5R was analyzed.
The mice (C57Bl/6) were fed a normal NaCl diet (0.8%) for 14 days that was subsequently changed to a high NaCl diet (4%) for 15 days, and then injected intraperitoneally with candesartan (AT1R antagonist, 1 mg kg−1 body weight per day) or vehicle (saline, control) daily for 10 days. Thereafter, blood pressures were measured from the aorta via the femoral artery under pentobarbital anesthesia.10,11 The kidneys were harvested, immunoblotted for AT1R, Nox2 and Nox4 and assayed for NADPH oxidase activity.
NADPH oxidase activity assay
NADPH oxidase activity (light units per g protein per minute) of membrane proteins from mouse kidney tissues was measured by NADPH-induced chemiluminescence with 5 μmol l−1 lucigenin and 100 μmol l−1 NADPH.11 The specificity of the NADPH-dependent O2− production was verified by treatment with the flavoprotein inhibitor diphenyleneiodonium.
Immunoblotting
Mouse kidney homogenates were prepared for immunoblotting as previously reported.11 The samples were immunoblotted with well characterized anti-Nox2, anti-Nox4 and anti-AT1R antibodies. Uniformity of protein loading and membrane transfer was determined by immunoblotting for glyceraldehyde-3-phosphate dehydrogenase (GADPH).
Statistical analysis
Data are expressed as mean±s.e.m. Comparison of more than two groups was made by one-way or two-way factorial analysis of variance, Holm–Sidak test and a comparison between two groups utilized the t-test. A value of P <0.05 was considered statistically significant.
RESULTS
cAMP and ROS production in CHO cells
Basal cAMP levels in plasmid-transfected CHO cells (0.54±0.02 pmol mg−1 protein per min) were similar to CHO cells expressing hD5F173L (0.52±0.03), but less than that noted in CHO cells expressing hD5WT (0.84±0.03) (P <0.05, one-way analysis of variance, Holm–Sidak test). The higher basal cAMP levels in the hD5WT indicate that the D5R receptor is constitutively active, in agreement with previous reports,10 Fenoldopam (10−8 −10−6 M, 30 min) significantly increased cAMP production in hD5WT and but not in D5F173L or vector-transfected cells (Figure 1).
Figure 1.
cAMP accumulation in CHO cells. cAMP accumulation was determined in the presence of 1 mM 3-isobutyl-1-methylxanthine in hD5F173L−, hD5WT− and empty vector-transfected CHO cells treated with a D1-like receptor agonist, fenoldopam (10−6 M, 30 min) (n=3/group, mean±s.e., in some, error bars are smaller than the symbols, *P<0.05, one-way analysis of variance, Holm–Sidak test). In the absence of D1R, fenoldopam is a D5R agonist.
We next determined the ability of hD5R to regulate ROS production. In the basal state, ROS production was similar in plasmid-transfected CHO cells (39300±200 arbitrary units/mg protein), CHO cells expressing hD5F173L (38500±120) and CHO cells expressing hD5WT (39300±200). The ability of 50 mM H2O2 to increase ROS production was greater in CHO cells transfected with plasmid (44900±200) or hD5F173L (44500±300) than CHO cells transfected with hD5WT (41200±80) (Figure 2).
Figure 2.
ROS production in CHO cells. The ROS production in hD5F173L−, hD5WT− and empty vector-transfected CHO cells treated with or without H2O2 (50 μM per 2 h) was measured by 2′,7′- dichlorodihydrofluorescein diacetate (n=3/group, mean±SE, *P<0.05, one-way analysis of variance, Holm–Sidak test).
Blood pressures in hD5R transgenic mice
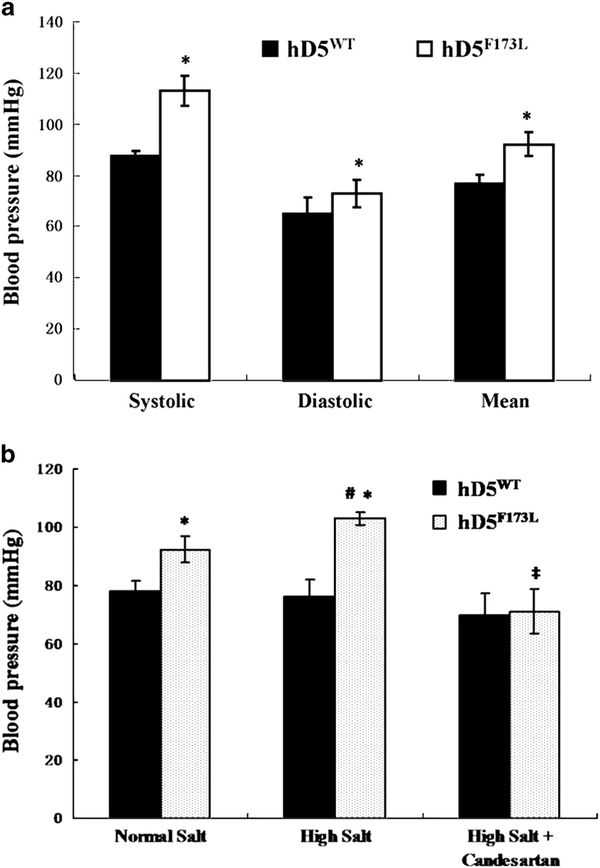
On normal salt diet (0.8% NaCl×14 days), the systolic, diastolic and mean arterial blood pressures, measured under pentobarbital anesthesia, were higher in 3-month-old hD5F173L than hD5WT transgenic mice (Figure 3a). A high salt diet (4% NaCl×15 days), increased further the blood pressures in hD5F173L but had no effect in hD5WT mice. The high blood pressures of hD5F173L transgenic mice on high salt diet were normalized after 10 days of candesartan, an AT1R antagonist, which had no effect in hD5WT transgenic mice (Figure 3b).
Figure 3.
Arterial blood pressure in hD5WT and hD5F173L transgenic mice. (a) Systolic, diastolic and mean arterial blood pressures (measured under pentobarbital anesthesia) in hD5WT and hD5F173L transgenic mice on normal salt diet (0.8% NaCl). (n=6, *P<0.05 vs. hD5WT, t-test). (b) Mean arterial blood pressure in hD5WT and hD5F173L mice fed normal salt (0.8% NaCl) or high salt diet (4% NaCl) for 14 and 15 days, respectively, and then treated with candesartan (1 mg kg−1 body weight per day × 10 days) (n=6, *P<0.05 vs. hD5WT, t-test; #P<0.05 vs. others, two-way analysis of variance; ‡P<0.05 vs. hD5F173L-normal salt diet and hD5F173L-high salt diet, two-way analysis of variance, Holm–Sidak test).
Expression of NADPH oxidase and AT1R proteins in hD5R transgenic mice
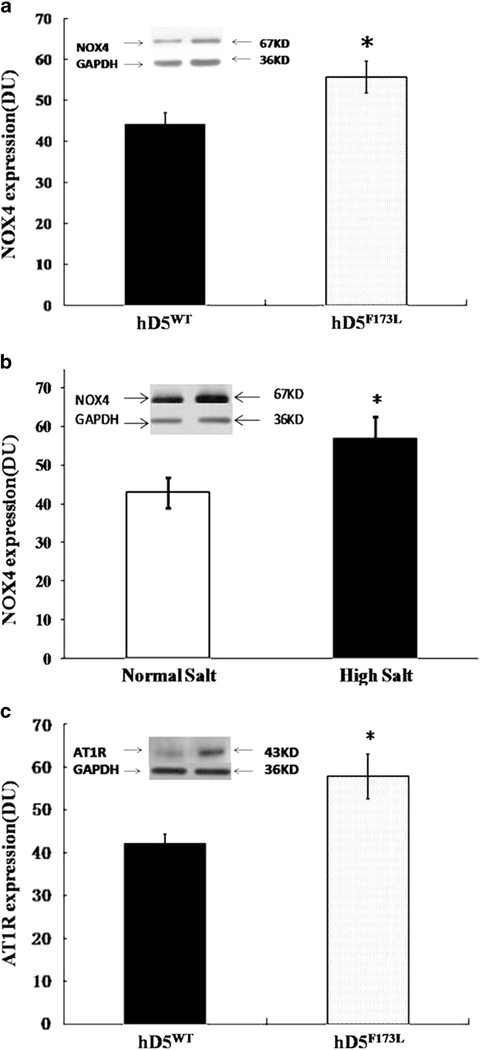
On normal salt diet, the renal expression of Nox4 but not Nox2 (data not shown) was higher in hD5F173L than D5WT transgenic mice (Figure 4a). On high salt diet the renal expression of Nox4 (but not Nox 2, data not shown) was also higher in hD5F173L (Figure 4b) than hD5WT transgenic mice (data not shown). In addition, renal AT1R expression was greater in hD5F173L than hD5WT transgenic mice on a normal salt diet (Figure 4c). However, AT1R protein expression was not affected by high salt diet in either strain (data not shown).
Figure 4.
Renal NOX4 and AT1R protein expression in hD5WT and hD5F173L transgenic mice. (a) Renal NOX4 protein expression (67 kDa) determined by immunoblotting in hD5WT and hD5F173L transgenic mice fed normal salt diet (n=6, *P<0.05 vs. hD5WT, t-test). (b) Renal NOX4 protein expression (67 kDa) determined by immunoblotting in hD5F173L transgenic mice on normal or high salt diet (n=6, *P<0.05 vs. normal salt, t-test). (c) AT1R expression (43 kDa) determined by immunoblotting in hD5WT and hD5F173L mice on normal salt diet (n=6, *P<0.05 vs. hD5WT, t-test). All immunoblotting results are expressed as relative DUs. Immunoblots of NOX4, AT1R, and GAPDH are depicted in the inset. DU, density unit.
NADPH oxidase activity in hD5R transgenic mice
Consistent with the increased renal Nox4 expression in hD5F173L mice (Figure 4a), renal NADPH oxidase activity was also increased in hD5F173L relative to hD5WT mice on a normal salt diet (Figure 5). High salt intake increased renal NADPH oxidase activity in both hD5F173L and hD5WT mice but it remained greater in hD5F173L than hD5WT transgenic mice (Figure 5). The dietary salt-induced increase in renal NADPH oxidase activity was decreased by candesartan treatment in hD5F173L transgenic mice but was not affected in hD5WT transgenic mice (Figure 5).
Figure 5.
Renal NADPH oxidase activity in hD5WT and hD5F173L transgenic mice. NADPH oxidase activity, measured by lucigenin chemiluminescence, in kidney membranes from hD5WT and hD5F173L mice on a normal NaCl (0.8% NaCl) and high NaCl (4% NaCl) diets for 14 and 15 days, respectively, and treated with candesartan (1 mg kg−1 body weight per day) for 10 days. Results are expressed as light units (U) per μg protein per min (n=6, *P<0.05 vs. normal salt; #P<0.05 vs. hD5WT normal salt; ‡P<0.05 vs. hD5F173L high salt, t-test).
DISCUSSION
Dopamine, via its receptors, including the D5R, has been shown as an important regulator of blood pressure.2,8–11,16,17,23,24 In this study, we found that the blood pressures were increased in transgenic mice with the hD5R mutant, hD5F173L, which had almost no ability to increase cAMP production in response to agonist stimulation. The hD5F173L mice shows markly increased Na/K ATPase levels and activities (Supplementary Data 1) and similar water and energy intake (Supplementary Data 2) compared with in hD5WT mice on normal salt diet. A high salt intake increased further the blood pressure in hD5F173L but had no effect in hD5WT transgenic mice, which demonstrated the disorders of sodium excreation and salt sensitivity in hD5F173L mice. We have reported that the blood pressures of transgenic mice expressing the D5R mutant hD5S390G, which had an increase in cAMP production in response to agonist stimulation, were similar to those measured in hD5WT transgenic mice (data not shown). The current study reconfirmed the importance of D5R in the regulation of blood pressure and salt sensitivity.10,11,23
It has been reported that oxidative stress is involved in the development of salt-sensitive hypertension.25–27 In vivo animal studies have demonstrated that increased ROS production in the renal cortex28 and medulla27 has an important role in the pathogenesis of salt-sensitive hypertension. In both human and experimental salt-sensitive hypertension, ROS production is increased and antioxidant capacity is decreased.26,27 ROS can inactivate the renal and vascular NO pathway, resulting in increased renal sodium transport28 and afferent arteriolar resistance,29 caused by impaired endothelium-dependent and -independent vasorelaxation.30 ROS have also been shown to directly upregulate ion channels, transporters and pumps.31 These effects could lead to a decrease in sodium excretion, resulting in volume expansion and subsequently, hypertension.
We had reported that the renal expression and activity of NADPH oxidase were increased in D5−/− mice relative to with D5WT mice, and that the D5R agonist (in the absence of D1R) fenoldopam inhibited the superoxide production in HEK-293 cells transfected with hD5WT.11 In this study, in CHO cells transfected with the mutant hD5F173L, superoxide production was increased and cAMP production was decreased relative to CHO cells transfected with hD5WT. Nox4 expression and NADPH oxidase activity were greater in hD5F173L mice than hD5WT transgenic mice on normal salt diet. High salt diet increased the Nox4 expression in hD5F173L but not in hD5WT transgenic mice. High salt diet also increased NADPH oxidase activity in both hD5F173L and hD5WT transgenic mice but blood pressure was increased further in hD5F173L but not in hD5WT transgenic mice. High salt intake has been reported to increase ROS generation accompanied by enhanced renal NADPH oxidase expression and activity32,33 and blood pressure in predisposed animals, for example, salt-sensitive rats.25–27 In the current study, the increased NADPH oxidase activity (which presumably increased ROS production) 11 induced by high salt intake was unable to increase blood pressure in hD5WT transgenic mice. This could be taken to indicate that the D5R may mitigate the production of ROS by increasing the activity of anti-oxidants, including heme-oxygenase 1,34 paraoxonase 235 and superoxide dismutase,36 among others. Moreover, the D5R can interact with other dopamine receptor subtypes to limit the production of ROS.36
The renin–angiotensin system is paramount in the regulation of blood pressure.37,38 In vitro and in vivo studies have demonstrated that angiotensin II, via AT1R, stimulated the activity and expression of NADPH oxidase to increase the production of ROS.38–41 Candesartan, an AT1R antagonist, decreased renal NADPH oxidase activity in hD5F173L mice fed a high salt diet but the effect that was not observed in hD5WT mice. Thus hD5F173L may interact with renin–angiotensin system to increase ROS production. Renal D5R and AT1R not only counter regulate each other’s effect on sodium excretion but also their expression.16,22,23 In the current study, the renal AT1R expression was higher in hD5F173L than hD5WT transgenic mice fed normal salt diet. Although renal AT1R expression was no longer different between hD5F173L than D5WT mice, AT1R blockade normalized blood pressure in hD5F173L mice on high salt diet, but had no effect on the normal blood pressure of hD5WT transgenic mice. This could be taken to indicate that on high salt diet AT1R function is increased even though renal AT1R expression was not increased in D5F173L mice. Dopamine and its receptors and angiotensin II, via AT1R, have been shown to counter regulate each other in the regulation of renal tubule signaling, sodium transport, blood pressure and renal injury kidney.22,23,42–45 Thus a proper balance between dopamine and the renin–angiotensin systems in the kidney is needed to maintain normal renal sodium excretion and their abnormal interaction leads to sodium retention and ultimately an increase in blood pressure.
In conclusion, we have shown that the hD5R mutant, hD5F173L increases ROS production and blood pressure that is, in part, related to an impaired ability to negatively regulate AT1R function. The expression of the hD5R variant, hD5F173L in mice results in salt-sensitive hypertension and increased renal expression of AT1R similar to the germline deletion of D5R in mice.23 However, the mechanisms involved in regulating the balance of renal D5R and AT1R in the oxidative stress-mediated salt-sensitive blood pressure remain to be determined.
Supplementary Material
ACKNOWLEDGEMENTS
These studies were supported, in part, by grants from National Natural Science Foundation (China) (30971186, 81370358), National Science and Technology Major Project (China) (2011ZX09307–302-03) and National Institutes of Health (USA) (P01HL068696, PAJ).
Footnotes
Supplementary Information accompanies the paper on Hypertension Research website (http://www.nature.com/hr)
References
- 1.Basson J, Simino J, Rao DC. Between candidate genes and whole genomes: time for alternative approaches in blood pressure genetics. Curr Hypertens Rep 2012; 14: 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asghar M, Tayebati SK, Lokhandwala MF, Hussain T. Potential dopamine-1 receptor stimulation in hypertension management. Curr Hypertens Rep 2011; 13: 294–302. [DOI] [PubMed] [Google Scholar]
- 3.Whelton PK, Appel LJ, Sacco RL, Anderson CA, Antman EM, Campbell N, Dunbar SB, Frohlich ED, Hall JE, Jessup M, Labarthe DR, MacGregor GA, Sacks FM, Stamler J, Vafiadis DK, Van Horn LV. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation 2012; 126: 2880–2889. [DOI] [PubMed] [Google Scholar]
- 4.Okamura DM, Himmelfarb J. Tipping the redox balance of oxidative stress in fibrogenic pathways in chronic kidney disease. Pediatr Nephrol 2009; 24: 2309–2319. [DOI] [PubMed] [Google Scholar]
- 5.Jaimes EA, Zhou MS, Pearse DD, Puzis L, Raij L. Upregulation of cortical COX-2 in salt-sensitive hypertension: role of angiotensin II and reactive oxygen species. Am J Physiol Renal Physiol 2008; 294: F385–F392. [DOI] [PubMed] [Google Scholar]
- 6.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA 2000; 97: 8010–8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montezano AC, Touyz RM. Oxidative stress, Noxs, and hypertension: experimental evidence and clinical controversies. Ann Med 2012; 44: S2–S16. [DOI] [PubMed] [Google Scholar]
- 8.Ueda A, Ozono R, Oshima T, Yano A, Kambe M, Teranishi Y, Katsuki M, Chayama K. Disruption of the type 2 dopamine receptor gene causes a sodium-dependent increase in blood pressure in mice. Am J Hypertens 2003; 16: 853–858. [DOI] [PubMed] [Google Scholar]
- 9.Johnson TL, Tulis DA, Keeler BE, Virag JA, Lust RM, Clemens S. The dopamine D3 receptor knockout mouse mimics aging-related changes in autonomic function and cardiac fibrosis. PLoS One 2013; 8: e74116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Z, Zheng S, Asico LD, Yu P, Bek M, Sibley DR, Jose PA. Increased PLD activity and elevated blood pressure in D5 receptor knockout mice. Am J Physiol Heart Circ Physiol 2005; 288: H55–H61. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS, Wang X, Quinn MT, Sibley DR, Romero GG, Felder RA, Jose PA. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol 2006; 290: R96–R104. [DOI] [PubMed] [Google Scholar]
- 12.Allayee H, de Bruin TW, Michelle Dominguez K, Cheng LS, Ipp E, Cantor RM, Krass KL et al. Genome scan for blood pressure in Dutch dyslipidemic families reveals linkage to a locus on chromosome 4p. Hypertension 2001; 38: 773–778. [DOI] [PubMed] [Google Scholar]
- 13.Grandy DK, Allen LJ, Zhang Y, Magenis RE, Civelli O. Chromosomal localization of three human D5 dopamine receptor genes. Genomics 1992; 13: 968–973. [DOI] [PubMed] [Google Scholar]
- 14.Cooper RS, Luke A, Zhu X, Kan D, Adeyemo A, Rotimi C, Bouzekri N, Ward R, Rorimi C. Genome scan among Nigerians linking blood pressure to chromosomes 2, 3, and 19. Hypertension 2002; 40: 629–633. [DOI] [PubMed] [Google Scholar]
- 15.Cravchik A, Gejman PV. Functional analysis of the human D5 dopamine receptor missense and nonsense variants: differences in dopamine binding affinities. Pharma-cogenetics 1999; 9: 199–206. [PubMed] [Google Scholar]
- 16.Jose PA, Soares-da-Silva P, Eisner GM, Felder RA. Dopamine and G protein-coupled receptor kinase 4 in the kidney: role in blood pressure regulation. Biochim Biophys Acta 2010; 1802: 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang MZ, Yao B, Wang S, Fan X, Wu G, Yang H, Yin H, Yang S, Harris RC. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest 2011; 121: 2845–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aperia A, Bertorello A, Seri I. Dopamine causes inhibition of Na_-K_-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol Renal Fluid Electrolyte Physiol 1987; 252: F39–F45. [DOI] [PubMed] [Google Scholar]
- 19.Bacic D, Kaissling B, McLeroy P, Zou L, Baum M, Moe OW. Dopamine acutely decreases apical membrane Na/H exchanger NHE3 protein in mouse renal proximal tubule. Kidney Int 2003; 64: 2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grider JS, Ott CE, Jackson BA. Dopamine D1 receptor-dependent inhibition of NaCl transport in the rat thick ascending limb: Mechanism of action. Eur J Pharmacol 2003; 473: 185–190. [DOI] [PubMed] [Google Scholar]
- 21.Pedrosa R, Jose PA, Soares-Da-Silva P. Defective D1-like receptor-mediated inhibition of Cl_/HCO3-exchanger in immortalized SHR proximal tubular epithelial cells. Am J Physiol Renal Physiol 2004; 286: F1120–F1126. [DOI] [PubMed] [Google Scholar]
- 22.Zeng C, Yang Z, Wang Z, Jones J, Wang X, Altea J, Mangrum AJ, Hopfer U, Sibley DR, Eisner GM, Felder RA, Jose PA. Interaction of angiotensin II type 1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension 2005; 45: 804–810. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, Pascua A, Lu Q, Wang X, Villar VA, Jones JE, Wang Z, Periasamy A, Lau YS, Soares-da-Silva P, Creswell K, Guillemette G, Sibley DR, Eisner G, Gildea JJ, Felder RA, Jose PA. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest 2008; 118: 2180–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinoshita S, Sidhu A, Felder FA. Defective dopamine-1 receptor adenylate cyclase coupling in the proximal convoluted tubule from the spontaneously hypertensive rat. J Clin Invest 1989; 84: 1849–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori T, Ogawa S, Cowely AW Jr, Ito S. Role of renal medullary oxidative and/or carbonyl stress in salt-sensitive hypertension and diabetes. Clin Exp Pharmacol Physiol 2012; 39: 125–131. [DOI] [PubMed] [Google Scholar]
- 26.Ando K, Fujita M. Reactive oxygen species and the central nervous system in salt-sensitive hypertension: possible relationship with obesity-induced hypertension. Clin Exp Pharmacol Physiol 2012; 39: 111–116. [DOI] [PubMed] [Google Scholar]
- 27.Manning RD Jr, Tian N, Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol 2005; 25: 311–317. [DOI] [PubMed] [Google Scholar]
- 28.Varela M, Herrera M, Garvin JL. Inhibition of Na-K-ATPase in thick ascending limbs by NO depends on O2- and is diminished by a high-salt diet. Am J Physiol Renal Physiol 2004; 287: F224–F230. [DOI] [PubMed] [Google Scholar]
- 29.Wilcox CS. Redox regulation of the afferent arteriole and tubuloglomerular feedback. Acta Physiol Scand 2003; 179: 217–223. [DOI] [PubMed] [Google Scholar]
- 30.Banday AA, Muhammad AB, Fazili FR, Lokhandwala M. Mechanisms of oxidative stress-induced increase in salt sensitivity and development of hypertension in Sprague-Dawley rats. Hypertension 2007; 49: 664–671. [DOI] [PubMed] [Google Scholar]
- 31.Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol 1998; 275: C1–24. [DOI] [PubMed] [Google Scholar]
- 32.Kim YH, Hwang JH, Noh JR, Gang GT, Tadi S, Yim YH, Jeoung NH, Kwak TH, Lee SH, Kweon GR, Kim JM, Shong M, Lee IK, Lee CH. Prevention of salt-induced renal injury by activation of NAD(P)H:quinone oxidoreductase 1, associated with NADPH oxidase. Free Radic Biol Med 2012; 52: 880–888. [DOI] [PubMed] [Google Scholar]
- 33.Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol 2003; 14: 2775–2782. [DOI] [PubMed] [Google Scholar]
- 34.Lu Q, Yang Y, Villar VA, Asico L, Jones JE, Yu P, Li H, Weinman EJ, Eisner GM, Jose PA. D5 dopamine receptor decreases NADPH oxidase, reactive oxygen species and blood pressure via heme oxygenase-1. Hypertens Res 2013; 36: 684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Zhang Y, Cuevas S, Villar VA, Escano C, Asico D, Yu L, Grandy P, Felder DK, Armando RA, Jose I, Paraoxonase PA. 2 decreases renal reactive oxygen species production, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of NADPH oxidase. Free Radic Biol Med 2012; 53: 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuevas S, Villar VA, Jose PA, Armando I. Renal dopamine receptors, oxidative stress, and hypertension. Int J Mol Sci 2013; 14: 17553–17572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crowley SD, Coffman TM. Recent advances involving the renin-angiotensin system. Exp Cell Res 2012; 318: 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navar LG, Prieto MC, Satou R, Kobori H. Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol 2011; 11: 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang GX, Lu XM, Kimura S, Nishiyama A. Role of mitochondria in angiotensin II-induced reactive oxygen species and mitogen-activated protein kinase activation. Cardiovasc Res 2007; 76: 204–212. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto E, Tamamaki N, Nakamura T, Kataoka K, Tokutomi Y, Dong YF, Fukuda M, Matsuba S, Ogawa H, Kim-Mitsuyama S. Excess salt causes cerebral neuronal apoptosis and inflammation in stroke-prone hypertensive rats through angiotensin II-induced NADPH oxidase activation. Stroke 2008; 39: 3049–3056. [DOI] [PubMed] [Google Scholar]
- 41.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 2004; 95: 937–944. [DOI] [PubMed] [Google Scholar]
- 42.Zeng C, Wang Z, Hopfer U, Asico LD, Eisner GM, Felder RA, Jose PA. Rat strain effects of AT1 receptor activation on D1 dopamine receptors in immortalized renal proximal tubule cells. Hypertension 2005; 46: 799–805. [DOI] [PubMed] [Google Scholar]
- 43.Chugh G, Lokhandwala MF, Asghar M. Altered functioning of both renal dopamine D1 and angiotensin II type 1 receptors causes hypertension in old rats. Hypertension 2012; 59: 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang S, Yao B, Zhou Y, Yin H, Zhang MZ, Harris RC. Intrarenal dopamine modulates progressive angiotensin II-mediated renal injury. Am J Physiol Renal Physiol 2012; 302: F742–F749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li D, Scott L, Crambert S, Zelenin S, Eklöf AC, Di Ciano L, Ibarra F, Aperia A. Binding of losartan to angiotensin AT1 receptors increases dopamine D1 receptor activation. J Am Soc Nephrol 2012; 23: 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.