Preface
Neurons are like modern cities, dependent on robust transport mechanisms. Like the best mass transit systems, trafficking within neurons must be tailored to respond to local requirements. Neurons depend on both high-speed, long-distance transport and localized dynamics to correctly deliver cargos and tune synaptic responses. Here, we focus on the mechanisms that provide localized regulation of the transport machinery, including the cytoskeleton and molecular motors, to yield compartment-specific trafficking within the axon initial segment, axon terminal, dendrites, and spines. The synthesis of these mechanisms provides a sophisticated and responsive transit system for the cell.
Keywords: actin, axon initial segment, axon terminal, cytoplasmic dynein, cytoskeleton, dendrites, dendritic spines, kinesin, microtubules
Introduction
Neurons are large, polarized, and highly active cells that rely continuously on directed intracellular transport. In the soma, newly synthesized proteins bud from the Golgi in transport vesicles that must be trafficked out to sites of need at the cell periphery 1. In axons, the transport of newly synthesized proteins occurs over distances of up to a meter, taking from minutes to months 2. In dendrites, dynamic transport is required for the synaptic plasticity underlying learning and memory 3. While all of these processes involve a core set of cytoskeletal proteins and molecular motors, the specific needs and composition of each compartment are distinct.
Here, we focus on how common mechanisms of cytoskeletal-based transport are differentially executed to regulate trafficking within specific subcellular domains of the neuron, or adapted to complement local morphology and function. Neuronal compartments differ markedly in their cytoskeletal organization (Figure 1). For example, the functional distinction between axons and dendrites is mirrored by underlying differences in the microtubule organization between these regions 4. Further, neither the axon nor dendrites exhibit a uniform cytoskeletal organization. Within axons, microtubules are differentially organized within the axon initial segment (AIS), the mid-axon, and the axon terminal 5. Similarly, within dendrites, cytoskeletal organization can vary significantly in proximal versus distal processes, and is distinctly different within dendritic spines 6,7. These differences in cytoskeletal organization have profound effects on cytoskeletal-based transport mechanisms, and allow for exquisite local control of organelle trafficking within specific subcellular domains.
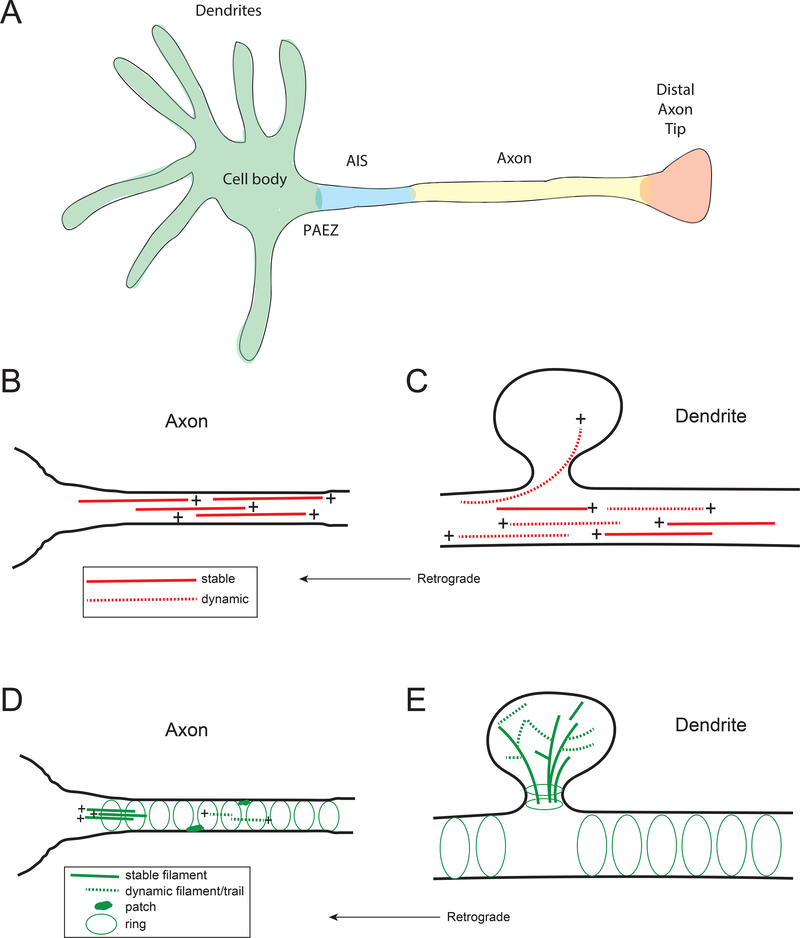
Figure 1: Overview of neuronal compartments and their cytoskeletal architecture.
(A) Functionally and/or structurally distinct compartments are found in many types of neurons. These compartments include the cell body, dendrites, pre-axonal exclusion zone (PAEZ), axon initial segment (AIS), axon shaft, and distal axon terminal. (B) Super-resolution microscopy has shown actin filaments in periodic rings organized by interactions with ankyrin G and βIV spectrin at the AIS. Actin rings are also found along the axon shaft, in association with ankyrin B and βII spectrin 53,54,56,158. Other actin structures identified in axons include stable actin patches and dynamic actin trails 56,57. (C) Dendritic spines are highly enriched in a dense network of both stable and dynamic F-actin. Actin rings are also present in dendrites 56. (D) Microtubules in the axon are almost uniformly plus-end out and tiled into overlapping arrays 4. In C. elegans 5, the number of microtubules in an axon decreases with increasing distance from the soma; a similar organization may occur in mammalian neurons. Microtubules in the proximal and mid-axon are characterized by post-translational modifications known to accumulate on stable microtubules. (E) Microtubules along dendritic shafts are organized with mixed polarity in mammalian neurons, while a more uniform plus end-out distribution is found in distal dendrites 4. Dynamic microtubules can invade dendritic spines 137,139, possibly depositing motor or cargo in the process.
New research is teasing apart the locally specific mechanisms that allow for the precise targeting of newly synthesized proteins and the effective long-distance transport of key signaling molecules. These studies are beginning to reveal common themes, including the overall role of cytoskeletal polarity, the modulation of cytoskeletal tracks by post-translational modifications (PTMs), the contribution of microtubule-associated proteins (MAPs), and the precise control of multiple motors bound to the same cargo. This review will explore how common cytoskeletal components that contribute to neuronal trafficking, including microtubules, actin, associated proteins that regulate filament organization and dynamics (Box 1), and the molecular motors that drive transport along the cytoskeleton (Box 2), are adapted locally to fulfill distinct functions. The basic mechanisms of axonal transport and neuronal polarity have been reviewed recently 1,8–11, allowing us to focus here on how these mechanisms are specifically tailored to provide a locally adapted, regionally responsive transport system for the neuron.
Box 1: Cytoskeletal organization and regulation.
Neurons can be divided into compartments (e.g. dendrite, spine, AIS, mid-axon, distal axon) with distinct trafficking needs (Fig. 1A). Throughout the neuron, the cytoskeleton is comprised of microtubules, actin filaments, and intermediate filaments, but only microtubules and actin filaments serve as tracks for intracellular transport.
Microtubules:
α and β tubulin form an obligate dimer that undergoes reversible head-to-tail assembly to form protofilaments; ~13 protofilaments associate laterally to form a microtubule 25 nm in diameter 146. In neurons, microtubules are organized radially in the soma, and in a unipolar, tiled array in axons with plus-ends directed outward 5. Microtubules in dendrites exhibit either reversed polarity with plus-ends oriented inward in invertebrate model systems 147, or mixed polarity, with up to 40% of microtubules in mammalian dendrites oriented with plus-ends toward the soma 4. Microtubule dynamics are tightly regulated, with dendritic microtubules generally more dynamic than axonal microtubules 12 (Fig. 1B,C).
MAPs:
Microtubule-associated proteins (MAPs) may bind along the length of microtubules, specifically recognize plus- or minus-ends, or sever microtubules 148. MAPs affect microtubule dynamics and may be specifically localized within neurons. MAPs such as tau and MAP2 promote stable microtubules 109, while others, such as doublecortin, regulate trafficking 110.
+TIPs:
Canonical +TIPs EB1 and EB3 bind specifically to tubulin subunits at growing, but not stable or shrinking, microtubule plus-ends. Other +TIPs are recruited to plus-ends via direct interactions with EBs, such as CLIP-170. +TIPs can affect microtubule dynamics as well as modulate specific interactions between the plus-end and either cargos or the cell cortex 149.
Microtubule nucleation:
Microtubules are nucleated by the γ-tubulin ring complex (γ-TURC), which binds to microtubule minus-ends and is enriched at the centrosome 150. In neurons, non-centrosomal nucleation plays a more significant role, mediated by γ-TURC at Golgi outposts 96 and along existing microtubules 97.
Post-translational tubulin modifications:
Neurons express many tubulin isoforms and these tubulins can be differentially modified by tyrosination/detyrosination, acetylation, and polyglutamylation 151. PTMs occur on either free tubulin dimers or the assembled polymer, thus reflecting the overall stability of the filament. PTMs can modify the binding of motors and MAPs, affecting the efficiency of trafficking 152.
Actin:
Actin forms diverse structures in axonal and dendritic processes, including rings, patches, and filaments, and is highly enriched in dendritic spines (Fig. 1D,E). A diverse array of actin-binding proteins modulates assembly, organization, and dynamics, including nucleators, crosslinkers, and severing proteins 153.
Box 2: Molecular motors drive trafficking in neurons.
Organelle transport in neurons is driven by kinesin, dynein, and myosin motors. Complex transport dynamics are precisely regulated by a suite of activators, adaptors, and scaffolding proteins.
Kinesins:
Kinesins are generally plus-end directed microtubule motors that drive long-distance movement in neurons. There is a broad superfamily of kinesins; within neurons, kinesin-1, kinesin-2, kinesin-3, and kinesin-4 motors drive organelle transport toward the plus-ends of microtubules in the soma, axons, and dendrites 11. In axons, kinesin motors drive anterograde transport from the soma to the distal tip 8, while in dendrites, kinesin motors may drive cargos both into and out of dendritic processes 103,110,154. Other kinesins contribute to microtubule organization in neurons 9.
Dynein:
Cytoplasmic dynein is the major minus end-directed microtubule motor for organelle and vesicle transport in eukaryotic cells. This includes the retrograde transport of cargos from the axon tip to soma 155, the anterograde transport of organelles from the soma into dendrites 100, and the retrograde transport of cargos such as signaling endosomes from dendrites to the soma 117.
Myosins:
Actin-based motors, including myosin I, myosin II, myosin V, and myosin VI, locally organize F-actin and drive local cargo dynamics including short-range motility and organelle tethering 15–17,59.
Motor activators, adaptors, and scaffolds:
Some factors, such as dynactin, are broadly required; dynactin binds directly to cytoplasmic dynein and is required to activate dynein-mediated organelle transport 156. Other factors, such as kinesin binding protein 22 and dynein adaptors BicD2 157, Hook1 31,65, and Lis1 are more specific in function. Multiple scaffolding proteins, including huntingtin, JIP1, JIP3, and TRAK1/2 regulate opposing dynein and kinesin motors bound to the same cargo 42.
The distinct morphologies and functions of each neuronal compartment (axon vs. dendrite, spine vs. shaft, AIS vs. axon terminal) predict that there will be pronounced differences in the regulation of trafficking in each compartment. Importantly, there is no single predominant mechanism; instead, compartment size and function, and the kinetics required to maintain this function, determine the ‘optimal’ regulatory scheme. For example, the AIS serves as a proximal filter for anterograde transport along the axon that also must permissively allow for robust retrograde transport. The axon terminal is often very far from the nucleus, yet must effectively send high-speed signals such as neurotrophins down the line to effect gene expression. Finally, the dendritic arbor is dynamic and activity-responsive, but can also be highly elaborated with a large internal volume, thus requiring very effective two-way trafficking. Below, we provide high-resolution snapshots of recent advances in our understanding of localized regulation and trafficking within subcellular regions of interest, including the mid-axon, the AIS, the axon terminal, dendrites, and spines. Together, these snapshots provide a more comprehensive picture of organelle trafficking within the neuron.
Trafficking along the mid-axon
The neuronal axon can extend more than a meter in length, and is generally permissive for sustained microtubule-based transport of organelles, vesicles, and proteins. Transport along the mid-axon is the best-studied aspect of neuronal trafficking to date 8, primarily due to the unipolar array of microtubules in this region and the higher stability of the axonal microtubule cytoskeleton, as compared to the higher levels of dynamic instability and cytoskeletal remodeling seen in the distal axonal terminal or in dendrites. Thus, axonal microtubules are preferentially enriched in PTMs that accumulate on stable microtubules, such as detyrosination 12,13.
Kinesin-1 and other motors of the kinesin superfamily, including members of the kinesin-2, kinesin-3, and kinesin-4 families drive anterograde axonal transport. In contrast, cytoplasmic dynein is the sole motor driving long-range retrograde axonal transport. Myosin motors, interacting with actin filaments also contribute to axonal transport, likely facilitating the initiation and termination of microtubule-motor-driven runs 14 as well as driving motility in regions with low microtubule density or high actin density 15–17.
Motor recruitment and activity on specific cargos of interest is still a very active topic of investigation, but generally appears to be regulated by organelle-associated GTPases such as Arl8, Rab3, Rab4 and Rab7. For example, Arl8b regulates the kinesin-1-dependent motility of lysosomes into axons 18, Rab3 regulates the kinesin-3-dependent motility of synaptic vesicle precursors 19, Rab4 regulates the anterograde transport of vesicles by kinesin-2 20, and the dynein-driven motility of late endosomes/lysosomes is regulated by Rab7 21. Additional levels of regulation are provided by motor effectors such as kinesin binding protein, a negative regulator of some kinesin motors 22. Cytoplasmic dynein is regulated by a growing list of effectors. Dynactin, the first to be identified 23,24, is a multi-subunit complex that binds directly to dynein and enhances initial engagement of the motor with its track25,26. BICD2, Hook1, and Hook3 are effectors that enhance the affinity of the dynein-dynactin interaction and thus the processive motility of the active motor complex 27–31, while Lis1 and NDEL1 enhance the attachment of dynein to the microtubule 32,33.
In the simplest model for regulation of motor-driven transport along the axon, anterograde cargos would be driven by a plus end-directed kinesin motor, while retrograde cargos would be driven by the minus-end directed motor cytoplasmic dynein. However, both biochemical and cellular studies indicate that opposing kinesin and dynein motors are simultaneously and stably bound to cargos undergoing transport along the axon. This has been shown experimentally for late endosomes/lysosomes 34, prion protein vesicles 35, and autophagosomes 36. Both genetic approaches and inhibitor studies demonstrate that the activities of cargo-attached motors are interdependent, as manipulations that target either kinesin or dynein motors often result in a bidirectional block in axonal transport (reviewed in 37).
If kinesin and dynein motors are both bound to a cargo, then how are the opposing activities of these motors regulated? Again, the simplest possible model posits that motors are engaged in a tug-of-war, resulting in stochastic switching between anterograde- and retrograde-directed runs. This model, at least to a first approximation, can account for the motility of some bidirectional cargo such as late endosomes/lysosomes, which undergo frequent switches in direction as they move along the axon 34. However, this model cannot account for cargos such as autophagosomes, which make long unidirectional runs along the axon 36. Instead, accumulating evidence indicates that the motility of many organelles moving along the axon may be tightly regulated by scaffolding proteins including JIP1 38, JIP3 39, and Milton 40, also known as TRAK1/2 41. Many of these scaffolding proteins can bind to both kinesin and dynein motors, and thus directly regulate their activities 42. For example, the binding of JIP1 to kinesin-1 can modulate the transition from an auto-inhibited state, in which the kinesin motor domain is docked to its tail domain, to an extended conformation in which the motor is fully active. Modulation of kinesin activity is dependent on the phosphorylation state of JIP1 38,43, allowing for the tuning of organelle motility within the neuron. Similarly, the motility of mitochondria along the axon is regulated by the scaffolding protein Milton, also known as TRAK1/2 40,41 in response to local changes in calcium concentration 44.
Further, scaffolding proteins may be a major factor in maintaining the highly processive, unidirectional motility of some cargos moving along the mid-axon, as these proteins may bind upstream regulator kinases and phosphatases in addition to interacting directly with motors. For example, autophagosomes are formed at the axon tip and initially move in a bidirectional manner driven by kinesins and dynein 36. The binding of JIP1 is correlated with a switch to unidirectional, dynein-driven motility toward the soma 43. Of note, JIP1 can bind to both a kinase (JNK) and a phosphatase (MKP1) 42,43, suggesting an integrated regulatory unit may lead to sustained transport of axonal cargos over long distances.
Perhaps the most interesting outstanding question in regard to transport along the mid-axon relates to regulation of transport initiation and termination. As cargos process along the axon from soma to terminal, they cannot run along a single microtubule track. Both serial EM reconstructions 45 and a recently developed light microscopy approach 5 indicate that microtubules are approximately 6–7 μm long in C. elegans and tiled in an overlapping manner along the length of the axon. In mammalian neurons, microtubule length may be more variable, with a mean length of ~100 μm determined by serial EM of sensory neurons 46, and a mean length of 4 μm and a range of 0.5 – 20 μm in developing hippocampal neurons 47. Live imaging indicates that organelles pause at microtubule ends 5. This pausing is likely due to a diffusive search following detachment from the initial track and prior to rebinding to another microtubule, as pause length is inversely correlated with local microtubule density 5. As microtubule length likely limits cargo run lengths in vivo, mechanisms that promote efficient interactions of motors with microtubules will affect the overall efficiency of long-distance vesicle transport.
The axon initial segment
The AIS is a 20–50 μm region initially characterized for its distinct ion channel composition and its role in action potential initiation 48. However, the AIS also functions as a gatekeeper to exclude somatodendritic proteins from the axon 10. The cytoskeletal structure of this region is densely packed and appears to be uniquely tuned to route motors and cargoes as needed (Figure 2). While cargo can pass freely through the AIS from the axon to the soma 49, cargo movement from the soma to axon is more restricted 50,51. In this section, we will discuss how both actin and microtubules are organized at the AIS, and how this organization may contribute to the differential regulation of trafficking into and out of the axonal compartment.
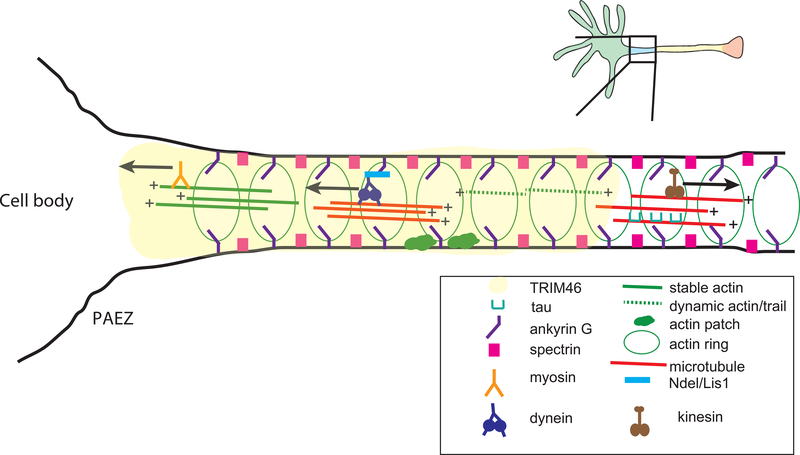
Figure 2: The cytoskeletal organization of the AIS.
A cytoskeletal hallmark of the AIS is the periodic arrangement of proteins including ankyrin G, spectrin, and actin. Bundled microtubules oriented plus-end outward are required for axodendritic cargo sorting at the AIS. While kinesin motors carry cargo into the axon, dynein and myosins assist with re-routing of somatodendritic cargos as needed. Axon-specific MAPs (e.g. TRIM46, tau) and motor adaptors (e.g. NDEL) contribute to cargo sorting at the AIS or pre-axonal exclusion zone (PAEZ).
Actin in the AIS
The structure of the AIS is resistant to perturbations of either F-actin or microtubules 52, suggesting that the cytoskeleton is highly stabilized in this region. The membrane-tethered scaffolding protein ankyrin G has emerged as the structural hallmark of the AIS, coordinating the clustering of other key AIS proteins including voltage-gated sodium channels (Nav2.1), neurofascin, βIV-spectrin, and actin 10. To gain further insight into the cytoskeletal structure of the AIS, Xu et al. (2013) utilized stochastic optical reconstruction microscopy (STORM) to reveal the presence of highly periodic adducin-capped F-actin rings in the AIS and along the length of the axon. Strikingly, the 190-nm spacing of these rings corresponds to the dimerized length of βIV-spectrin, which adopts a periodic structure that alternates with that of actin 53. The organizations of ankyrin G and neurofascin in the AIS are also periodic 54, suggesting that regular spacing of structural elements may be a defining feature of this region. Actin rings contribute to both the structure and function of the AIS, acting as a scaffold to maintain the organization of the underlying microtubule cytoskeleton 55 and as a barrier to limit the lateral diffusion of membrane proteins between rings 51,56.
Numerous other actin structures have been described in the axon and AIS. STED microscopy of SiR-Actin in living neurons revealed the existence of discrete actin patches at the plasma membrane that colocalize with presynaptic markers like Bassoon 56. Live imaging has also revealed dynamic actin structures known as actin trails along the length of the axon, including in the AIS, originating from “hotspots” of endosomal actin nucleation 57. The contribution of these patches and hotspots to long-range trafficking is unclear; they may be more important for the short-range transport of presynaptic components within the axon. Phalloidin-positive actin patches deep within the cytoplasm have also been described, including at the AIS 58. These observations have led to a model in which the interaction of myosin motors with these actin patches may contribute to cargo sorting 59. However, more recent data emphasize the importance of microtubules and microtubule motor proteins in the sorting of organelle and vesicular cargo at the AIS, reviewed below.
Microtubules in the AIS
Microtubules are critical for the formation and maintenance of the AIS. EM studies have described a network of highly fasciculated microtubules surrounded by a dense protein coat that becomes more globular over development 60,61. This coat is enriched in a number of AIS-associated proteins, including ankyrin G and short actin filaments, distinct from the more stable cortically associated actin rings. The C-terminal tail of ankyrin G, which projects 30–150 nm from the plasma membrane into the cytosolic space 52,61, can interact with microtubules in the AIS, which may provide stability to the overall cytoskeletal structure. Moreover, ankyrin G directly interacts with EB3, which tracks the growing plus tips of microtubules and is enriched in the AIS 62,63. The interaction of EBs and ankyrin G is mutually essential for the proper localization of these proteins to the AIS, and suggests a major organizational role for microtubules in this region 62,63.
MAPs and motors are key for the function of the AIS. Given the uniform plus-end outward orientation of axonal microtubules, kinesin is well-poised to carry cargos into the axon, while the activity of both dynein 64,65 and myosins (as discussed above) counteracts this motility and re-routes somatodendritic cargo out of the AIS. Recently, the MAP TRIM46 has been identified to play a crucial role in trafficking at the AIS by regulating the structure and function of this region. TRIM46 localizes to proximal axonal microtubules in the AIS, forming microtubule bundles of uniform plus-end outward polarity. The loss of TRIM46 leads to aberrant mixed microtubule polarity, resulting in an increase in bidirectional motility in the AIS and decreased axonal trafficking efficiency 66.
Several dynein effectors are critical for its ability to re-route somatodendritic cargo back towards the soma from the axon. Loss of function of the GTPase Rab5 significantly increases the penetration of somatodendritic cargoes into the axon. While Rab5 is ubiquitously expressed, its interaction with the Fused Toes (FTS)-Hook-FTS and Hook-interacting protein (FHIP) (FHF) complex in the proximal axon enhances dynein-dependent retrieval of somatodendritic cargos and processive motility towards the soma 65. In addition, the dynein effector NDEL1 localizes to the AIS through an interaction with ankyrin G, and promotes dynein-driven transport back to the soma together with its effector Lis1. Loss of NDEL or Lis1 results in kinesin-driven somatodendritic cargos passing freely into the axon 64. Thus, trafficking in the AIS is critically dependent on uniform microtubule polarity and the proper regulation of opposing motor pools.
The PAEZ
The region just proximal to the AIS at the transition point between the soma and axon has also emerged as an important subcellular compartment termed the pre-axonal exclusion zone (PAEZ) 67. In live imaging studies, Bonifacino and colleagues observed that most somatodendritic cargoes are deflected before they reach the AIS, and that this repulsion can be overcome by forced targeting of the axonally-directed kinesin-1 to these cargos 67. This suggests that some motors, like kinesin-1, are capable of effectively navigating the PAEZ, while others are not. For those somatodendritic vesicles that make it past the PAEZ and into the AIS, dynein in concert with its effectors works to retrieve and redirect these cargos towards the appropriate compartment, as described above 65. Further studies of the PAEZ will shed new light on this critical region, where cytoskeletal proteins and motors transition from somatic to axonal arrangements and functions.
The distal axon terminal
The axon terminal is the most distal subcellular region, innervating targets at distances up to 20,000x greater than the diameter of the soma 8. This extreme separation from the soma defines the needs and constraints of this region. Efficient anterograde and retrograde microtubule-based transport are required to ensure a continuous supply of new cellular resources and appropriate recycling or retrograde signaling. The cytoskeleton in this region is sparse and highly dynamic, with regulatory mechanisms adapted for efficient transport delivery and initiation (Figure 3A). We focus most of our discussion on mature, healthy neurons and constitutive transport processes in the axon terminal. However, the distal cytoskeleton and MAPs also have important roles in development and injury 68–70.
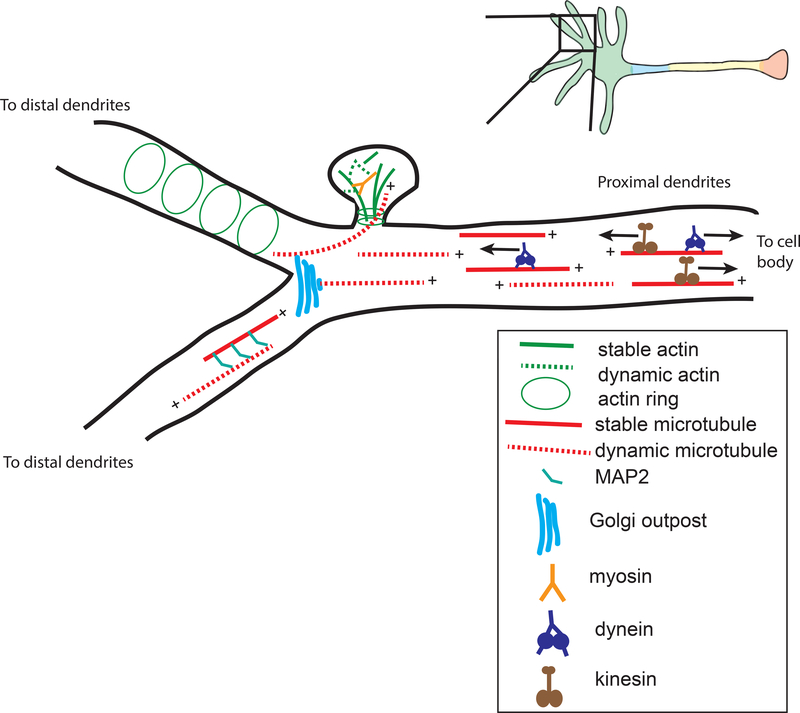
Figure 3. The cytoskeletal organization of dendrites.
The dendritic shaft is enriched in microtubules, while dendritic spines are actin-rich; both stable and dynamic microtubule and actin filaments are present. Actin rings are found in the shaft and spine neck, and may provide structural support. Due to the mixed polarity of dendritic microtubules, both dynein and kinesins regulate the transport of retrograde and anterograde cargo, while myosins mediate trafficking into and out of spines. Microtubules may be transported into dendrites by motors or nucleated at Golgi outposts, and are enriched in MAPs such as MAP2. In this compartment, long-range transport must be balanced against the dynamics of the underlying microtubule tracks and overall dendritic morphology.
Actin and myosin in the axon terminal
The axon terminal is rich in a dynamic population of actin that is most often studied in the context of the developing growth cone and the mature presynaptic terminal. Interestingly, a recent study found that this population of distal actin is resistant to actin depolymerization by cytochalasin D and latrunculin B, as all conditions showed dense F-actin patches in approximately half of hippocampal neurites 71. Actin is also concentrated in the presynaptic terminal of mature neurons, where it acts as a scaffold to dock vesicles 72 and coordinates neurotransmitter release and recycling 73. Myosin II has also been implicated in neurotransmitter release at presynaptic terminals, while myosin VI and GIPC1 may coordinate synaptic vesicle recycling and endocytosis 16. Additional studies are required to elucidate how actin and myosin regulate trafficking in the axon terminal as well as how this interfaces with microtubule-based transport. This transition is critical for retrograde transport, discussed below, as actin depolymerization is required for the retrograde flux of neurotrophic factor signaling endosomes 74.
Anterograde trafficking and the delivery of distal cargo
The bulk of new axonal proteins and organelles are synthesized in the soma and transported outward to the axon 8. As motors and adaptors move from the mid-axon to the terminal, they encounter a sparse and highly dynamic microtubule cytoskeleton. Recent work from C. elegans clearly shows that the dense, stable, overlapping microtubules of the axon shaft become sparse in the distal axon, with fewer overlapping microtubules and increased gaps 5. These microtubules are also highly dynamic, as evidenced by increased EB3 dynamics and enriched tyrosinated α-tubulin 26,75.
Materials transported to the distal axon are usually shared among multiple axonal branches and terminals, and the distance-dependent distribution of resources depends on the cargo. In vivo imaging of mitochondria in zebrafish sensory neurons found that mitochondrial density decreased in a distance-dependent manner along the axon 76. Further, flux density decreased with axon branching by a factor of two per axonal branch 76. Although some resources, like mitochondria, are low in the distal axon terminal, others are distally enriched. For example, constitutive macroautophagosome formation in cultured neurons is enriched in the distal axon terminal 77.
The mechanisms regulating cargo release in the axon terminal are not yet clear. Best studied has been the delivery of synaptic cargoes to the distal presynaptic terminal, a subset among the many en passant synapses along the axon shaft. The simplest model posits that cargo dissociates from the ends of the microtubules once they reach the axon terminal; however, this explanation is not sufficient to account for selective cargo delivery to one out of many terminals 78. Instead, it is likely that the regulated disengagement or inactivation of kinesin in the target terminal contributes to localized delivery. At least four models for presynaptic cargo delivery have been proposed, although further studies are required to establish the underlying mechanisms: 1) kinesin dissociation via Rab3 nucleotide exchange; 2) the phosphorylation-dependent release of motors from cargo; 3) phosphorylation-dependent inhibition of retrograde transport, promoting distal enrichment; and 4) distal delivery followed by continuous anterograde/retrograde cycling along synapses 78,79.
Retrograde transport initiation
Many cargos initiate retrograde transport in the axon terminal, including autophagosomes, neurotrophic-factor-associated signaling endosomes, endosomes, mitochondria, and lysosomes 8. Efficient retrograde transport is crucial for autophagosome maturation and function, preventing the distal accumulation of aging proteins and organelles 43. Rates of retrograde flux also affect the long-distance signaling of neurotrophic factors from axon terminal to soma 80. The key barrier to the efficiency of retrograde transport is the sparse and dynamic nature of microtubules at the axon terminal. EM studies have consistently shown a low and variable number of microtubules at terminals 81; more recent super-resolution studies demonstrate a lower density of more highly dynamic microtubules at the end of the axon 26. At least two major mechanisms have been shown to contribute to the robust attachment of retrograde cargos with microtubule tracks (Figure 3B).
First, both dynein and dynactin are distally enriched at the axon terminal. A direct interaction between kinesin-1 and cytoplasmic dynein maintains a distal pool of dynein, driven by slow axonal transport 82. Dynactin, as well as microtubule +TIPs including EB3, CLIP-170, and Lis1, are also enriched in the axon terminal, where they are required for retrograde transport initiation 69,75,83. Second, specific interactions among dynein, dynactin, and +TIPs are regulated by the local enrichment of tyrosinated tubulin to promote efficient retrograde transport initiation. The dynamic nature of distal microtubules ensures that the local microtubule lattice is rich in tyrosinated α-tubulin, which recruits +TIPs with CAP-Gly domains, such as CLIP-170 and p150Glued, the largest subunit of the dynactin complex 26,84–86. Constitutive retrograde transport initiation in neurons requires both of these proteins, as well as EB1/EB3 and Lis1 75,83,87. Specifically, the CAP-Gly domain of p150Glued interacts with EB3 and CLIP-170 83. EB3 binds to the growing end of dynamic microtubules and can recruit CLIP-170, and dynein/dynactin-bound cargo lands on microtubules via this EB3/CLIP-170 enrichment 26,75. This pathway is regulated by phosphorylation, as CLIP-170 assumes an autoinhibited, inactive conformation when phosphorylated in its second serine-rich domain 88. Thus, local dephosphorylation of CLIP-170 may allow temporal control of initiation. The dephosphorylated, active form of CLIP-170 forms an extended 135-nm linker that effectively increases the search radius for cargo capture, allowing for efficient initiation despite the sparse microtubule cytoskeleton in the distal axon 26.
However, if this were the sole mechanism of enhanced transport initiation, most retrograde transport initiation would be confined to the distal 1 – 2 μm of dynamic microtubules. An additional, non-exclusive pathway takes advantage of the spatial enrichment of tyrosinated α-tubulin in the distal axon. The C-terminal EEY motif of tyrosinated α-tubulin binds CAP-Gly-domain proteins such as p150Glued and CLIP-170 with high affinity 84–86. Thus, dynactin can bind directly and robustly to the tyrosinated microtubule lattice 26,89. CLIP-170 also binds preferentially to tyrosinated microtubules 26,86, further enhancing the robust association of retrograde cargo with the microtubule. Together, these mechanisms combine to provide robust and spatially specific regulation of transport initiation in the distal axon terminal.
Dendrites
The dendritic shaft
Microtubules are the primary cytoskeletal component of the dendritic shaft (Figure 4). The inverted or mixed microtubule polarity found along the dendritic shaft of mammalian neurons is a unique cytoskeletal property that distinguishes these processes from the axon 4. In distal dendrites, however, there is a more uniform plus end-out organization of microtubules, similar to that found in the distal axon 4. While recent advances in microscopy have revealed the presence of actin rings in dendrites as well 56,90, little is known about these structures compared to their axonal counterparts and microtubule-based trafficking has been studied in more detail. It is known that neuronal activity exerts a profound effect on dendritic microtubule dynamics, as well as the structure of the dendritic arbor itself, promoting the addition and retraction of branches and changing the stability of the microtubule tracks 91,92. Therefore, sustained long-range microtubule-based transport within the dendritic compartment presents a navigational challenge for the neuron.
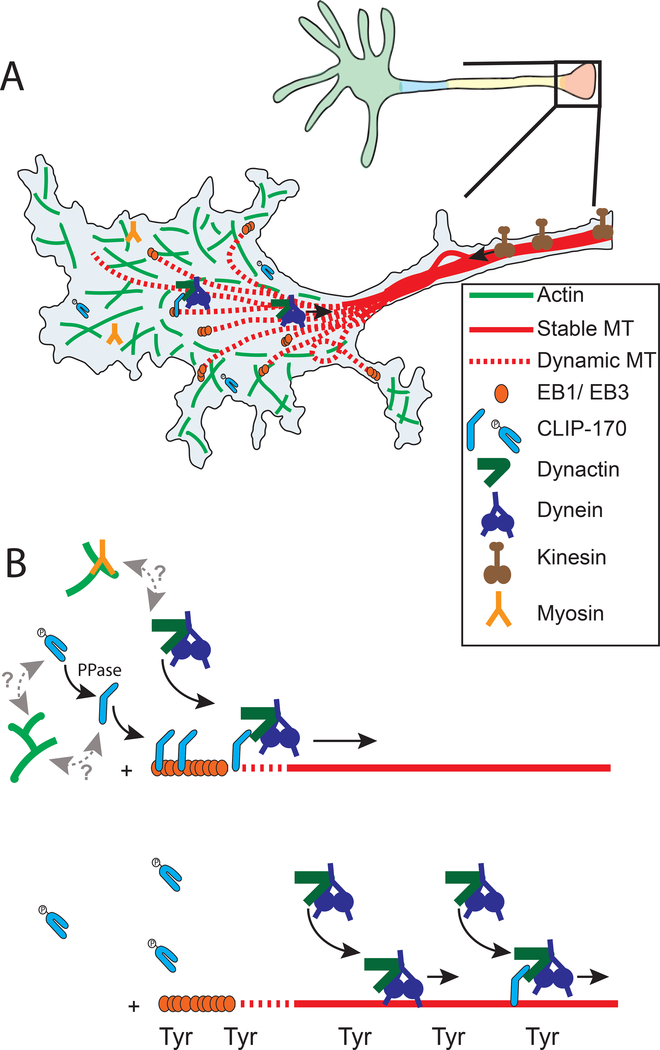
Figure 4. The cytoskeletal organization of the distal axon terminal.
(A) The cytoskeleton of the distal axon terminal is characterized by a dense actin network and sparse microtubule array. Although the morphology of a neuronal growth cone is depicted here, these features are also found in mature axon terminals. This contrasts with the dense, tiled array of more stable microtubules found in the mid-axon. Anterograde cargos are delivered by kinesin motors, primarily from the kinesin-1, −2, and −3 families. Actin and myosin coordinate the delivery, retention and release of pre-synaptic cargo. Retrograde cargo transport is driven by the dynein-dynactin complex. (B) Multiple mechanisms regulate the loading of retrograde cargo onto microtubule plus-ends. These regulatory proteins include +TIPs such as EB3, CLIP-170, DCTN1, and Lis1. Initiation is tuned by the phosphorylation state of CLIP-170 (top), which assumes an open conformation when dephosphorylated, and then binds to the plus-tips of microtubules, to enhance the recruitment of the dynein-dynactin complex. The local enrichment of tyrosinated microtubules (bottom), also enhances the recruitment of the dynein-dynactin complex to initiate retrograde transport.
Multiple mechanisms have been shown to contribute to the development of the mixed polarity of dendritic microtubules. Several microtubule motors are involved in the delivery of microtubules to dendrites, including kinesin-5s that restrict 93, and kinesin-6s and −12s that promote 94, the transport of minus-end outward microtubules into dendrites. In addition, dynein brings plus-end outward microtubules into both axons and dendrites 95. Moreover, there is increasing evidence that dendritic microtubules do not all originate at the centrosome, but can polymerize at Golgi outposts stationed at dendritic branch points 96 in a process that relies on γ-tubulin, CLASPs, and augmin 97–99.
The identity of microtubule motors that regulate dendritic transport has been elucidated primarily by monitoring the transport of fluorescently tagged motors or cargos from the cell body. Dynein and several kinesins have been experimentally localized to dendrites using induced-dimerization approaches or chimeric constructs 100–102. However, the majority of kinesins tested preferentially localized to axons but not dendrites, suggesting that dendritic kinesins may possess unique properties enabling them to navigate the more complex microtubule environment found in this cellular compartment 103. Accumulating data indicate that there are multiple levels of regulation controlling the sorting of cargos to dendrites, including motor/microtubule and motor/cargo interactions, as recently reviewed by Kapitein and Hoogenraad 104 and Bentley and Banker 1. Here we will focus primarily on the unique cytoskeletal features that regulate trafficking within dendrites.
Microtubule-based motors in dendrites are subject to regulation at a number of levels. For one, microtubules are differentially modified in dendrites compared to axons. These differential PTMs can influence the behavior of both MAPs and motors. Mid-axonal microtubules are enriched in acetylated and detyrosinated tubulins, which are considered hallmarks of microtubule stability, compared to dendritic microtubules 105. For example, kinesin-1 motors interact preferentially with stable, detyrosinated and acetylated microtubules 106–108, in contrast to kinesin-5 motors which preferentially interact with tyrosinated microtubules 93, potentially contributing to the differential localization of these proteins in the neuron (kinesin-1: axon, kinesin-5: dendrite). Dendritic and axonal microtubules are also distinguished by different populations of MAPs. For example, MAP2 is considered the canonical dendritic MAP, while tau is associated with axonal microtubules 109. Doublecortin-like protein kinase 1 (DCLK1), which also binds to microtubules, has recently been shown to regulate the transport of KIF1-mediated dendritic cargos 110, demonstrating an emerging role for MAPs in the regulation of motor activity in dendrites.
There are also data suggesting that motor phosphorylation and differential associations with adaptors contribute to the regulation of dendritic trafficking. For example, CDK5-dependent phosphorylation enhances the microtubule binding and trafficking of kinesin-5 in dendrites 93. On the other hand, CaMKII-dependent phosphorylation inhibits dendritic trafficking mediated by KIF17 by disrupting the interaction of the motor with its dendrite-specific adaptor Mint1 111. Additional well-characterized examples of dendrite-specific adaptors include TRAK2, which preferentially mediates the transport of mitochondria in dendrites 41 and GRIP1, which targets NR2B-bound kinesin-1 to dendrites 112.
Neuronal activity exerts a profound influence on microtubule-based transport in dendrites. An NMDA-dependent model of long-term depression (LTD) in cultured neurons alters the distribution of EB3 from its usual comet-like appearance, indicative of dynamic microtubules, to a more diffuse pattern 91. Strikingly, this effect was only apparent in dendrites, not axons. Similar effects on EB3 density and dynamics were observed following bicuculline treatment to enhance synaptic signaling 91,103, suggesting that activity may have a net negative effect on dendritic microtubule dynamics. Consistent with this stabilization of the underlying microtubule tracks, neuronal activity also increases the speed of KIF21B- and KIF1A-dependent transport in dendrites 103,113. The motility of some kinesins can also be enhanced by activity-dependent phosphorylation events, including the CaMKII-dependent phosphorylation of KIF3A and the light chain of kinesin-1 114,115. In these studies, kinesin phosphorylation enhanced the loading and/or synaptic delivery of N-cadherin and AMPA receptors, respectively, suggesting a feedback loop between dendritic trafficking and neuronal activity.
There is an emerging appreciation for the idea that multiple motor types contribute to the net trafficking of a given cargo. In axons, this is regulated by the coordinated activation/inactivation of opposing dynein and kinesins 42. However, multiple motors types can also actively work together to contribute to different aspects of transport, as has been described for kinesin-1 and kinesin-2 in the trafficking of APC in axons 116. The microtubule environment of dendrites is unique in that its mixed polarity allows for the possibility that dynein and kinesins could cooperate to mediate the trafficking of cargo in the same direction. As most microtubules are plus-end outward, dynein may preferentially regulate retrograde motility in dendrites, reflective of this underlying microtubule orientation. While mechanisms of kinesin-directed motility are less clear, it has been suggested that the population of plus-end inward microtubules is more stable than those that are plus-end outward 6. Thus, kinesins may prefer to traffic along more stable tracks. For example, dynein, KIF21B, and likely other kinesins all contribute to the retrograde trafficking of BDNF/TrkB signaling endosomes in dendrites 103,117. The cooperation of multiple motors in driving cargo movement may be particularly beneficial in dendrites, where sustained long-range transport must be balanced against the cytoskeletal dynamics that underlie plasticity.
Dendritic spines
A key feature of many neuronal dendrites is the presence of dendritic spines, small protrusions that are the primary sites of excitatory synapse formation. The primary purpose of the spine is to act as a compartment limiting local changes in plasticity to specific synapses 118. This requires the coordination of transport from the dendrite into spines, localized insertion/removal of proteins at the postsynaptic density, and morphological changes over a rapid time course.
The distinctive cytoskeletal composition of dendritic spines was first revealed by EM, which showed that spines are highly enriched in actin compared to the dendritic shaft, where microtubules predominate 7. This dense F-actin network is comprised of both branched and linear actin filaments 119 as well as both stable and dynamic populations of actin 120. The regulation of the dynamic actin content of spines is dependent on a number of molecular factors including the spine-specific activities of actin-binding proteins including Arp2/3 121, profilin 122, and cofilin 123, and small GTPases of the Rho family 124.
Neuronal activity is a critical regulator of the actin content of dendritic spines. Activation of NMDA or AMPA receptors induces spine outgrowth and enlargement, respectively, via actin-dependent mechanisms 125. In addition, the dynamics of actin branching and polymerization are enhanced upon neuronal depolarization 126,127. Importantly, the interaction between actin and glutamate receptors is mutual, as actin dynamics are crucial for the proper recycling of AMPA receptors in spines 128 and actin depolymerization causes the loss of synaptic NMDA and AMPA receptor accumulations, suggesting that actin regulates the insertion and/or maintenance of receptors at the synapse 129.
Given the density of actin in dendritic spines, it is no surprise that members of the myosin motor family are critically important in these structures. Inhibition of myosin II results in a less mature spine morphology 130, and non-muscle myosin IIb may transport actin itself into the spine head 131. The myosin II heavy chain MYH7H is also expressed in hippocampal dendritic spines and regulates morphology 132. Myosin motors are also implicated in activity-dependent trafficking into spines. Myosin Va, myosin Vb and myosin VI regulate the movement of AMPA receptors into spines and are critical for long-term synaptic plasticity 133–136.
The activity-dependent entry of microtubules into spines has been reported. Microtubules transiently sample a small percentage of dendritic spines at a given time, correlated with an enlargement in spine morphology 137–139. Importantly, depolarization or NMDA activation increases the frequency of these events, and may target microtubules to particular spines 139,140. In spines, the microtubule and actin cytoskeletons are linked, as microtubules can regulate actin dynamics through the interaction of EB3 with p140CAP/SNIP 138 and the actin-binding protein drebrin in spines 141. While the purpose of these transient entries into spines is unclear, they may allow for the rapid delivery of specific motor/cargo pairs to a given synapse, as has been shown for KIF1A/synaptotagmin-IV 142.
Conclusions and perspectives
The mature neuron is composed of multiple subdomains, each with a distinctive cytoskeletal organization. This local structure creates unique transport challenges and requirements within each region. The dynamic interplay between the local cytoskeleton, motors, and gradients of regulatory elements within each subdomain makes it clear that neuronal trafficking must be understood in a subcellular context. Although each subdomain faces different challenges and needs, local regulation is achieved using a core set of cytoskeletal and molecular tools. We are only beginning to discover how these tools are differentially employed and executed to allow for the precise navigation of the neuronal cytoskeletal architecture, and many critical questions lie ahead.
At the molecular level, the actin and microtubule tracks play important individual roles in each subdomain. However, they also interact via overlapping regulatory pathways and proteins. How are actin and microtubules coordinated within each domain? This may occur, in part, at the level of motor and adaptor proteins, which are sensitive to cytoskeletal PTMs or arrangement of the local cytoskeleton into parallel or antiparallel arrays. It will be important to assess how motors, such as a canonical dendritic kinesins, respond when placed in a subdomain with different cytoskeletal arrays or PTMs, taking advantage of recent advances in optogenetic and pharmacological motor targeting assays.
The development of axonal-dendritic polarity has been intensely studied, but the development of each subdomain is less well understood. When and how are these domains specified, and how is the local cytoskeleton maintained and remodeled over the lifetime of an organism? What degree of overlap exists among neighboring compartments? The axonal injury literature provides one example of a subdomain that is apparently competent to assume a different state when perturbed in the mature neuron: the formation of a growth cone-like structure at the site of axotomy in what used to be the mid-axon shaft 70.
Even in mature, healthy neurons, there are many remaining questions. Cajal and subsequent neuroscientists have described a diversity of neuronal morphologies, likely adapted for different functions. How does the unique morphology and function of a given neuronal subtype influence its trafficking constraints and requirements? For example, the highly branched axonal morphology of midbrain projection neurons 143 may act as a stressor on distal axon terminals. These neurons appear to be more susceptible to defects in retrograde transport initiation that do not significantly disrupt function in other neuronal subtypes. Patients with point mutations in the CAP-Gly domain of p150Glued that disrupt distal retrograde transport initiation, but not mid-axon transport, develop a form of Parkinsonism known as Perry syndrome, characterized by predominant midbrain degeneration 144. This example demonstrates the utility of framing subdomain challenges and needs within the context of a given neuronal subtype to identify stress points and generate new questions.
Going forward, studying trafficking in co-culture systems and in vivo will further shed light on specific aspects of neuronal transport 145. For example, the effects of neuronal activity and aging on transport dynamics need to be explored both in vitro and within a more physiological context. Further, intercellular interactions need to be considered, for example, the effects of myelination on axonal transport or the role of glia in the development and maintenance of cytoskeletal compartments in neurons. In the future, studies detailing the specific mechanisms locally regulating organelle motility within specific neuronal subdomains; coupled to an improved understanding of transport dynamics within the context of the living organism will be required to fully understand the sophisticated and responsive transit systems on which these cells rely.
Literature Cited
- 1.Bentley M & Banker G The cellular mechanisms that maintain neuronal polarity. Nat Rev Neurosci 17, 611–622, doi: 10.1038/nrn.2016.100 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Maday S & Holzbaur EL Autophagosome biogenesis in primary neurons follows an ordered and spatially regulated pathway. Dev Cell 30, 71–85, doi: 10.1016/j.devcel.2014.06.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehlers MD Dendritic trafficking for neuronal growth and plasticity. Biochemical Society transactions 41, 1365–1382, doi: 10.1042/BST20130081 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Baas PW, Deitch JS, Black MM & Banker GA Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci U S A 85, 8335–8339 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yogev S, Cooper R, Fetter R, Horowitz M & Shen K Microtubule Organization Determines Axonal Transport Dynamics. Neuron 92, 449–460, doi: 10.1016/j.neuron.2016.09.036 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yau KW et al. Dendrites In Vitro and In Vivo Contain Microtubules of Opposite Polarity and Axon Formation Correlates with Uniform Plus-End-Out Microtubule Orientation. J Neurosci 36, 1071–1085, doi: 10.1523/JNEUROSCI.2430-15.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matus A, Ackermann M, Pehling G, Byers HR & Fujiwara K High actin concentrations in brain dendritic spines and postsynaptic densities. Proc Natl Acad Sci U S A 79, 7590–7594 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maday S, Twelvetrees AE, Moughamian AJ & Holzbaur EL Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron 84, 292–309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matamoros AJ & Baas PW Microtubules in health and degenerative disease of the nervous system. Brain Res Bull 126, 217–225, doi: 10.1016/j.brainresbull.2016.06.016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leterrier C & Dargent B No Pasaran! Role of the axon initial segment in the regulation of protein transport and the maintenance of axonal identity. Semin Cell Dev Biol 27, 44–51, doi: 10.1016/j.semcdb.2013.11.001 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Hirokawa N & Tanaka Y Kinesin superfamily proteins (KIFs): Various functions and their relevance for important phenomena in life and diseases. Exp Cell Res 334, 16–25, doi: 10.1016/j.yexcr.2015.02.016 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Kollins KM, Bell RL, Butts M & Withers GS Dendrites differ from axons in patterns of microtubule stability and polymerization during development. Neural Dev 4, 26, doi: 10.1186/1749-8104-4-26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown A, Li Y, Slaughter T & Black MM Composite microtubules of the axon: quantitative analysis of tyrosinated and acetylated tubulin along individual axonal microtubules. J Cell Sci 104 (Pt 2), 339–352 (1993). [DOI] [PubMed] [Google Scholar]
- 14.McIntosh BB, Holzbaur EL & Ostap EM Control of the initiation and termination of kinesin-1-driven transport by myosin-Ic and nonmuscle tropomyosin. Curr Biol 25, 523–529, doi: 10.1016/j.cub.2014.12.008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saxton WM & Hollenbeck PJ The axonal transport of mitochondria. J Cell Sci 125, 2095–2104, doi: 10.1242/jcs.053850 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kneussel M & Wagner W Myosin motors at neuronal synapses: drivers of membrane transport and actin dynamics. Nat Rev Neurosci 14, 233–247, doi: 10.1038/nrn3445 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Gramlich MW & Klyachko VA Actin/Myosin-V- and Activity-Dependent Inter-synaptic Vesicle Exchange in Central Neurons. Cell reports 18, 2096–2104, doi: 10.1016/j.celrep.2017.02.010 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Farias GG, Guardia CM, De Pace R, Britt DJ & Bonifacino JS BORC/kinesin-1 ensemble drives polarized transport of lysosomes into the axon. Proc Natl Acad Sci U S A, doi: 10.1073/pnas.1616363114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niwa S, Tanaka Y & Hirokawa N KIF1Bbeta- and KIF1A-mediated axonal transport of presynaptic regulator Rab3 occurs in a GTP-dependent manner through DENN/MADD. Nat Cell Biol 10, 1269–1279, doi: 10.1038/ncb1785 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Dey S, Banker G & Ray K Anterograde Transport of Rab4-Associated Vesicles Regulates Synapse Organization in Drosophila. Cell reports 18, 2452–2463, doi: 10.1016/j.celrep.2017.02.034 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson M et al. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol 176, 459–471, doi: 10.1083/jcb.200606077 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kevenaar JT et al. Kinesin-Binding Protein Controls Microtubule Dynamics and Cargo Trafficking by Regulating Kinesin Motor Activity. Curr Biol 26, 849–861, doi: 10.1016/j.cub.2016.01.048 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Gill SR et al. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J Cell Biol 115, 1639–1650 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holzbaur EL et al. Homology of a 150K cytoplasmic dynein-associated polypeptide with the Drosophila gene Glued. Nature 351, 579–583, doi: 10.1038/351579a0 (1991). [DOI] [PubMed] [Google Scholar]
- 25.Ayloo S et al. Dynactin functions as both a dynamic tether and brake during dynein-driven motility. Nature Communications (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nirschl JJ, Magiera MM, Lazarus JE, Janke C & Holzbaur EL alpha-Tubulin Tyrosination and CLIP-170 Phosphorylation Regulate the Initiation of Dynein-Driven Transport in Neurons. Cell reports 14, 2637–2652, doi: 10.1016/j.celrep.2016.02.046 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Splinter D et al. BICD2, dynactin, and LIS1 cooperate in regulating dynein recruitment to cellular structures. Mol Biol Cell 23, 4226–4241, doi: 10.1091/mbc.E12-03-0210 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKenney RJ, Huynh W, Tanenbaum ME, Bhabha G & Vale RD Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes. Science 345, 337–341, doi: 10.1126/science.1254198 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urnavicius L et al. The structure of the dynactin complex and its interaction with dynein. Science 347, 1441–1446, doi: 10.1126/science.aaa4080 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroeder CM & Vale RD Assembly and activation of dynein-dynactin by the cargo adaptor protein Hook3. J Cell Biol 214, 309–318, doi: 10.1083/jcb.201604002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olenick MA, Tokito M, Boczkowska M, Dominguez R & Holzbaur EL Hook Adaptors Induce Unidirectional Processive Motility by Enhancing the Dynein-Dynactin Interaction. J Biol Chem 291, 18239–18251, doi: 10.1074/jbc.M116.738211 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toropova K et al. Lis1 regulates dynein by sterically blocking its mechanochemical cycle. eLife 3, doi: 10.7554/eLife.03372 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klinman E & Holzbaur EL Stress-Induced CDK5 Activation Disrupts Axonal Transport via Lis1/Ndel1/Dynein. Cell reports 12, 462–473, doi: 10.1016/j.celrep.2015.06.032 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hendricks AG et al. Motor coordination via a tug-of-war mechanism drives bidirectional vesicle transport. Curr Biol 20, 697–702, doi: 10.1016/j.cub.2010.02.058 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Encalada SE, Szpankowski L, Xia CH & Goldstein LS Stable kinesin and dynein assemblies drive the axonal transport of mammalian prion protein vesicles. Cell 144, 551–565, doi: 10.1016/j.cell.2011.01.021 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maday S, Wallace KE & Holzbaur EL Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol 196, 407–417, doi: 10.1083/jcb.201106120 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hancock WO Bidirectional cargo transport: moving beyond tug of war. Nat Rev Mol Cell Biol 15, 615–628, doi: 10.1038/nrm3853 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu MM & Holzbaur EL JIP1 regulates the directionality of APP axonal transport by coordinating kinesin and dynein motors. J Cell Biol 202, 495–508, doi: 10.1083/jcb.201302078 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abe N et al. Sunday driver interacts with two distinct classes of axonal organelles. J Biol Chem 284, 34628–34639, doi: 10.1074/jbc.M109.035022 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stowers RS, Megeath LJ, Gorska-Andrzejak J, Meinertzhagen IA & Schwarz TL Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron 36, 1063–1077 (2002). [DOI] [PubMed] [Google Scholar]
- 41.van Spronsen M et al. TRAK/Milton Motor-Adaptor Proteins Steer Mitochondrial Trafficking to Axons and Dendrites. Neuron 77, 485–502, doi: 10.1016/j.neuron.2012.11.027 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Fu MM & Holzbaur EL Integrated regulation of motor-driven organelle transport by scaffolding proteins. Trends Cell Biol, doi: 10.1016/j.tcb.2014.05.002 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu M-M, Nirschl JJ & Holzbaur ELF LC3 binding to the scaffolding protein JIP1 regulates processive dynein-driven transport of autophagosomes. Dev Cell 29, 577–590 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X & Schwarz TL The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell 136, 163–174, doi: 10.1016/j.cell.2008.11.046 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chalfie M & Thomson JN Organization of neuronal microtubules in the nematode Caenorhabditis elegans. J Cell Biol 82, 278–289 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bray D & Bunge MB Serial analysis of microtubules in cultured rat sensory axons. J Neurocytol 10, 589–605 (1981). [DOI] [PubMed] [Google Scholar]
- 47.Yu W & Baas PW Changes in microtubule number and length during axon differentiation. J Neurosci 14, 2818–2829 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bender KJ & Trussell LO The physiology of the axon initial segment. Annu Rev Neurosci 35, 249–265, doi: 10.1146/annurev-neuro-062111-150339 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Maday S & Holzbaur EL Compartment-Specific Regulation of Autophagy in Primary Neurons. J Neurosci 36, 5933–5945, doi: 10.1523/JNEUROSCI.4401-15.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song AH et al. A selective filter for cytoplasmic transport at the axon initial segment. Cell 136, 1148–1160, doi: 10.1016/j.cell.2009.01.016 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Albrecht D et al. Nanoscopic compartmentalization of membrane protein motion at the axon initial segment. J Cell Biol 215, 37–46, doi: 10.1083/jcb.201603108 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leterrier C et al. Nanoscale Architecture of the Axon Initial Segment Reveals an Organized and Robust Scaffold. Cell reports 13, 2781–2793, doi: 10.1016/j.celrep.2015.11.051 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Xu K, Zhong G & Zhuang X Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 339, 452–456, doi: 10.1126/science.1232251 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong G et al. Developmental mechanism of the periodic membrane skeleton in axons. eLife 3, doi: 10.7554/eLife.04581 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qu Y, Hahn I, Webb SE, Pearce SP & Prokop A Periodic actin structures in neuronal axons are required to maintain microtubules. Mol Biol Cell 28, 296–308, doi: 10.1091/mbc.E16-10-0727 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D’Este E, Kamin D, Gottfert F, El-Hady A & Hell SW STED nanoscopy reveals the ubiquity of subcortical cytoskeleton periodicity in living neurons. Cell reports 10, 1246–1251, doi: 10.1016/j.celrep.2015.02.007 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Ganguly A et al. A dynamic formin-dependent deep F-actin network in axons. J Cell Biol 210, 401–417, doi: 10.1083/jcb.201506110 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watanabe K et al. Networks of polarized actin filaments in the axon initial segment provide a mechanism for sorting axonal and dendritic proteins. Cell reports 2, 1546–1553, doi: 10.1016/j.celrep.2012.11.015 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arnold DB & Gallo G Structure meets function: actin filaments and myosin motors in the axon. Journal of neurochemistry 129, 213–220, doi: 10.1111/jnc.12503 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palay SL, Sotelo C, Peters A & Orkand PM The axon hillock and the initial segment. J Cell Biol 38, 193–201 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones SL, Korobova F & Svitkina T Axon initial segment cytoskeleton comprises a multiprotein submembranous coat containing sparse actin filaments. J Cell Biol 205, 67–81, doi: 10.1083/jcb.201401045 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leterrier C et al. End-binding proteins EB3 and EB1 link microtubules to ankyrin G in the axon initial segment. Proc Natl Acad Sci U S A 108, 8826–8831, doi: 10.1073/pnas.1018671108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freal A et al. Cooperative Interactions between 480 kDa Ankyrin-G and EB Proteins Assemble the Axon Initial Segment. J Neurosci 36, 4421–4433, doi: 10.1523/JNEUROSCI.3219-15.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuijpers M et al. Dynein Regulator NDEL1 Controls Polarized Cargo Transport at the Axon Initial Segment. Neuron 89, 461–471, doi: 10.1016/j.neuron.2016.01.022 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Guo X, Farias GG, Mattera R & Bonifacino JS Rab5 and its effector FHF contribute to neuronal polarity through dynein-dependent retrieval of somatodendritic proteins from the axon. Proc Natl Acad Sci U S A 113, E5318–5327, doi: 10.1073/pnas.1601844113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Beuningen SF et al. TRIM46 Controls Neuronal Polarity and Axon Specification by Driving the Formation of Parallel Microtubule Arrays. Neuron 88, 1208–1226, doi: 10.1016/j.neuron.2015.11.012 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Farias GG, Guardia CM, Britt DJ, Guo X & Bonifacino JS Sorting of Dendritic and Axonal Vesicles at the Pre-axonal Exclusion Zone. Cell reports 13, 1221–1232, doi: 10.1016/j.celrep.2015.09.074 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bearce EA, Erdogan B & Lowery LA TIPsy tour guides: how microtubule plus-end tracking proteins (+TIPs) facilitate axon guidance. Frontiers in cellular neuroscience 9, 241, doi: 10.3389/fncel.2015.00241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Voelzmann A, Hahn I, Pearce SP, Sanchez-Soriano N & Prokop A A conceptual view at microtubule plus end dynamics in neuronal axons. Brain Res Bull 126, 226–237, doi: 10.1016/j.brainresbull.2016.08.006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bradke F, Fawcett JW & Spira ME Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci 13, 183–193, doi: 10.1038/nrn3176 (2012). [DOI] [PubMed] [Google Scholar]
- 71.Chia JX, Efimova N & Svitkina TM Neurite outgrowth is driven by actin polymerization even in the presence of actin polymerization inhibitors. Mol Biol Cell, doi: 10.1091/mbc.E16-04-0253 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sankaranarayanan S, Atluri PP & Ryan TA Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat Neurosci 6, 127–135, doi: 10.1038/nn1002 (2003). [DOI] [PubMed] [Google Scholar]
- 73.Cingolani LA & Goda Y Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci 9, 344–356, doi: 10.1038/nrn2373 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Harrington AW et al. Recruitment of actin modifiers to TrkA endosomes governs retrograde NGF signaling and survival. Cell 146, 421–434, doi: 10.1016/j.cell.2011.07.008 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moughamian AJ, Osborn GE, Lazarus JE, Maday S & Holzbaur EL Ordered recruitment of dynactin to the microtubule plus-end is required for efficient initiation of retrograde axonal transport. J Neurosci 33, 13190–13203, doi: 10.1523/JNEUROSCI.0935-13.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Plucinska G et al. In vivo imaging of disease-related mitochondrial dynamics in a vertebrate model system. J Neurosci 32, 16203–16212, doi: 10.1523/JNEUROSCI.1327-12.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maday S & Holzbaur EL Autophagosome assembly and cargo capture in the distal axon. Autophagy 8, 858–860, doi: 10.4161/auto.20055 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rizzoli SO Synaptic vesicle recycling: steps and principles. EMBO J 33, 788–822, doi: 10.1002/embj.201386357 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gibbs KL, Greensmith L & Schiavo G Regulation of Axonal Transport by Protein Kinases. Trends Biochem Sci 40, 597–610, doi: 10.1016/j.tibs.2015.08.003 (2015). [DOI] [PubMed] [Google Scholar]
- 80.Wang T et al. Flux of signalling endosomes undergoing axonal retrograde transport is encoded by presynaptic activity and TrkB. Nat Commun 7, 12976, doi: 10.1038/ncomms12976 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bodaleo FJ & Gonzalez-Billault C The Presynaptic Microtubule Cytoskeleton in Physiological and Pathological Conditions: Lessons from Drosophila Fragile X Syndrome and Hereditary Spastic Paraplegias. Front Mol Neurosci 9, 60, doi: 10.3389/fnmol.2016.00060 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Twelvetrees AE et al. The Dynamic Localization of Cytoplasmic Dynein in Neurons Is Driven by Kinesin-1. Neuron 90, 1000–1015, doi: 10.1016/j.neuron.2016.04.046 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moughamian AJ & Holzbaur EL Dynactin is required for transport initiation from the distal axon. Neuron 74, 331–343, doi: 10.1016/j.neuron.2012.02.025 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peris L et al. Tubulin tyrosination is a major factor affecting the recruitment of CAP-Gly proteins at microtubule plus ends. J Cell Biol 174, 839–849, doi: 10.1083/jcb.200512058 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weisbrich A et al. Structure-function relationship of CAP-Gly domains. Nat Struct Mol Biol 14, 959–967, doi: 10.1038/nsmb1291 (2007). [DOI] [PubMed] [Google Scholar]
- 86.Bieling P et al. CLIP-170 tracks growing microtubule ends by dynamically recognizing composite EB1/tubulin-binding sites. J Cell Biol 183, 1223–1233, doi:jcb.200809190 [pii] 10.1083/jcb.200809190 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lloyd TE et al. The p150(Glued) CAP-Gly domain regulates initiation of retrograde transport at synaptic termini. Neuron 74, 344–360, doi: 10.1016/j.neuron.2012.02.026 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee HS et al. Phosphorylation controls autoinhibition of cytoplasmic linker protein-170. Mol Biol Cell 21, 2661–2673, doi: 10.1091/mbc.E09-12-1036 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McKenney RJ, Huynh W, Vale RD & Sirajuddin M Tyrosination of alpha-tubulin controls the initiation of processive dynein-dynactin motility. EMBO J 35, 1175–1185, doi: 10.15252/embj.201593071 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bar J, Kobler O, van Bommel B & Mikhaylova M Periodic F-actin structures shape the neck of dendritic spines. Scientific reports 6, 37136, doi: 10.1038/srep37136 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kapitein LC et al. NMDA receptor activation suppresses microtubule growth and spine entry. J Neurosci 31, 8194–8209, doi: 10.1523/JNEUROSCI.6215-10.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wong RO & Ghosh A Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci 3, 803–812, doi: 10.1038/nrn941nrn941 [pii] (2002). [DOI] [PubMed] [Google Scholar]
- 93.Kahn OI, Sharma V, Gonzalez-Billault C & Baas PW Effects of kinesin-5 inhibition on dendritic architecture and microtubule organization. Mol Biol Cell 26, 66–77, doi: 10.1091/mbc.E14-08-1313 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin S, Liu M, Mozgova OI, Yu W & Baas PW Mitotic motors coregulate microtubule patterns in axons and dendrites. J Neurosci 32, 14033–14049, doi: 10.1523/JNEUROSCI.3070-12.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zheng Y et al. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat Cell Biol 10, 1172–1180, doi: 10.1038/ncb1777 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ori-McKenney KM, Jan LY & Jan YN Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron 76, 921–930, doi: 10.1016/j.neuron.2012.10.008 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sanchez-Huertas C et al. Non-centrosomal nucleation mediated by augmin organizes microtubules in post-mitotic neurons and controls axonal microtubule polarity. Nat Commun 7, 12187, doi: 10.1038/ncomms12187 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nguyen MM et al. Gamma-tubulin controls neuronal microtubule polarity independently of Golgi outposts. Mol Biol Cell 25, 2039–2050, doi: 10.1091/mbc.E13-09-0515 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Efimov A et al. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev Cell 12, 917–930, doi: 10.1016/j.devcel.2007.04.002 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kapitein LC et al. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr Biol 20, 290–299, doi: 10.1016/j.cub.2009.12.052 (2010). [DOI] [PubMed] [Google Scholar]
- 101.Jenkins B, Decker H, Bentley M, Luisi J & Banker G A novel split kinesin assay identifies motor proteins that interact with distinct vesicle populations. J Cell Biol 198, 749–761, doi: 10.1083/jcb.201205070 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang CF & Banker G The translocation selectivity of the kinesins that mediate neuronal organelle transport. Traffic 13, 549–564, doi: 10.1111/j.1600-0854.2011.01325.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ghiretti AE et al. Activity-Dependent Regulation of Distinct Transport and Cytoskeletal Remodeling Functions of the Dendritic Kinesin KIF21B. Neuron 92, 857–872, doi: 10.1016/j.neuron.2016.10.003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kapitein LC & Hoogenraad CC Which way to go? Cytoskeletal organization and polarized transport in neurons. Mol Cell Neurosci 46, 9–20, doi: 10.1016/j.mcn.2010.08.015 (2011). [DOI] [PubMed] [Google Scholar]
- 105.Hammond JW et al. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol Biol Cell 21, 572–583, doi: 10.1091/mbc.E09-01-0044 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nakata T & Hirokawa N Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J Cell Biol 162, 1045–1055, doi: 10.1083/jcb.200302175 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Konishi Y & Setou M Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat Neurosci 12, 559–567, doi: 10.1038/nn.2314 (2009). [DOI] [PubMed] [Google Scholar]
- 108.Reed NA et al. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol 16, 2166–2172, doi:S0960–9822(06)02207-X [pii] 10.1016/j.cub.2006.09.014 (2006). [DOI] [PubMed] [Google Scholar]
- 109.Dehmelt L & Halpain S The MAP2/Tau family of microtubule-associated proteins. Genome biology 6, 204, doi: 10.1186/gb-2004-6-1-204 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lipka J, Kapitein LC, Jaworski J & Hoogenraad CC Microtubule-binding protein doublecortin-like kinase 1 (DCLK1) guides kinesin-3-mediated cargo transport to dendrites. EMBO J 35, 302–318, doi: 10.15252/embj.201592929 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guillaud L, Wong R & Hirokawa N Disruption of KIF17-Mint1 interaction by CaMKII-dependent phosphorylation: a molecular model of kinesin-cargo release. Nat Cell Biol 10, 19–29, doi: 10.1038/ncb1665 (2008). [DOI] [PubMed] [Google Scholar]
- 112.Hoogenraad CC, Milstein AD, Ethell IM, Henkemeyer M & Sheng M GRIP1 controls dendrite morphogenesis by regulating EphB receptor trafficking. Nat Neurosci 8, 906–915, doi: 10.1038/nn1487 (2005). [DOI] [PubMed] [Google Scholar]
- 113.Neupert C et al. Regulated Dynamic Trafficking of Neurexins Inside and Outside of Synaptic Terminals. J Neurosci 35, 13629–13647, doi: 10.1523/JNEUROSCI.4041-14.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hoerndli FJ et al. Neuronal Activity and CaMKII Regulate Kinesin-Mediated Transport of Synaptic AMPARs. Neuron 86, 457–474, doi: 10.1016/j.neuron.2015.03.011 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ichinose S, Ogawa T & Hirokawa N Mechanism of Activity-Dependent Cargo Loading via the Phosphorylation of KIF3A by PKA and CaMKIIa. Neuron 87, 1022–1035, doi: 10.1016/j.neuron.2015.08.008 (2015). [DOI] [PubMed] [Google Scholar]
- 116.Ruane PT et al. Tumour Suppressor Adenomatous Polyposis Coli (APC) localisation is regulated by both Kinesin-1 and Kinesin-2. Scientific reports 6, 27456, doi: 10.1038/srep27456 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liot G et al. Mutant Huntingtin alters retrograde transport of TrkB receptors in striatal dendrites. J Neurosci 33, 6298–6309, doi: 10.1523/JNEUROSCI.2033-12.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sabatini BL, Maravall M & Svoboda K Ca(2+) signaling in dendritic spines. Current opinion in neurobiology 11, 349–356 (2001). [DOI] [PubMed] [Google Scholar]
- 119.Korobova F & Svitkina T Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol Biol Cell 21, 165–176, doi: 10.1091/mbc.E09-07-0596 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Honkura N, Matsuzaki M, Noguchi J, Ellis-Davies GC & Kasai H The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron 57, 719–729, doi: 10.1016/j.neuron.2008.01.013 (2008). [DOI] [PubMed] [Google Scholar]
- 121.Racz B & Weinberg RJ Organization of the Arp2/3 complex in hippocampal spines. J Neurosci 28, 5654–5659, doi: 10.1523/JNEUROSCI.0756-08.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Neuhoff H et al. The actin-binding protein profilin I is localized at synaptic sites in an activity-regulated manner. Eur J Neurosci 21, 15–25, doi: 10.1111/j.1460-9568.2004.03814.x (2005). [DOI] [PubMed] [Google Scholar]
- 123.Hotulainen P et al. Defining mechanisms of actin polymerization and depolymerization during dendritic spine morphogenesis. J Cell Biol 185, 323–339, doi: 10.1083/jcb.200809046 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tashiro A, Minden A & Yuste R Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex 10, 927–938 (2000). [DOI] [PubMed] [Google Scholar]
- 125.Fischer M, Kaech S, Wagner U, Brinkhaus H & Matus A Glutamate receptors regulate actin-based plasticity in dendritic spines. Nat Neurosci 3, 887–894 (2000). [DOI] [PubMed] [Google Scholar]
- 126.Star EN, Kwiatkowski DJ & Murthy VN Rapid turnover of actin in dendritic spines and its regulation by activity. Nat Neurosci 5, 239–246, doi: 10.1038/nn811 (2002). [DOI] [PubMed] [Google Scholar]
- 127.Okamoto K, Nagai T, Miyawaki A & Hayashi Y Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci 7, 1104–1112, doi: 10.1038/nn1311 (2004). [DOI] [PubMed] [Google Scholar]
- 128.Gu J et al. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat Neurosci 13, 1208–1215, doi:nn.2634 [pii] 10.1038/nn.2634 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Allison DW, Gelfand VI, Spector I & Craig AM Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci 18, 2423–2436 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Koskinen M, Bertling E, Hotulainen R, Tanhuanpaa K & Hotulainen P Myosin IIb controls actin dynamics underlying the dendritic spine maturation. Mol Cell Neurosci 61, 56–64, doi: 10.1016/j.mcn.2014.05.008 (2014). [DOI] [PubMed] [Google Scholar]
- 131.Hodges JL, Newell-Litwa K, Asmussen H, Vicente-Manzanares M & Horwitz AR Myosin IIb activity and phosphorylation status determines dendritic spine and post-synaptic density morphology. PLoS One 6, e24149, doi: 10.1371/journal.pone.0024149 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rubio MD, Johnson R, Miller CA, Huganir RL & Rumbaugh G Regulation of synapse structure and function by distinct myosin II motors. J Neurosci 31, 1448–1460, doi: 10.1523/JNEUROSCI.3294-10.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Osterweil E, Wells DG & Mooseker MS A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis. J Cell Biol 168, 329–338, doi: 10.1083/jcb.200410091 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lise MF et al. Involvement of myosin Vb in glutamate receptor trafficking. J Biol Chem 281, 3669–3678, doi: 10.1074/jbc.M511725200 (2006). [DOI] [PubMed] [Google Scholar]
- 135.Wang Z et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 135, 535–548, doi: 10.1016/j.cell.2008.09.057 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Correia SS et al. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci 11, 457–466, doi: 10.1038/nn2063 (2008). [DOI] [PubMed] [Google Scholar]
- 137.Gu J, Firestein BL & Zheng JQ Microtubules in dendritic spine development. J Neurosci 28, 12120–12124, doi:28/46/12120 [pii] 10.1523/JNEUROSCI.2509-08.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jaworski J et al. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron 61, 85–100, doi:S0896–6273(08)00967–7 [pii] 10.1016/j.neuron.2008.11.013 (2009). [DOI] [PubMed] [Google Scholar]
- 139.Hu X, Viesselmann C, Nam S, Merriam E & Dent EW Activity-dependent dynamic microtubule invasion of dendritic spines. J Neurosci 28, 13094–13105, doi:28/49/13094 [pii] 10.1523/JNEUROSCI.3074-08.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Merriam EB et al. Dynamic microtubules promote synaptic NMDA receptor-dependent spine enlargement. PLoS One 6, e27688, doi: 10.1371/journal.pone.0027688 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Merriam EB et al. Synaptic regulation of microtubule dynamics in dendritic spines by calcium, F-actin, and drebrin. J Neurosci 33, 16471–16482, doi: 10.1523/JNEUROSCI.0661-13.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McVicker DP et al. Transport of a kinesin-cargo pair along microtubules into dendritic spines undergoing synaptic plasticity. Nat Commun 7, 12741, doi: 10.1038/ncomms12741 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Matsuda W et al. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci 29, 444–453, doi: 10.1523/JNEUROSCI.4029-08.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Farrer MJ et al. DCTN1 mutations in Perry syndrome. Nature genetics 41, 163–165, doi: 10.1038/ng.293 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sleigh JN, Vagnoni A, Twelvetrees AE & Schiavo G Methodological advances in imaging intravital axonal transport. F1000Res 6, 200, doi: 10.12688/f1000research.10433.1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nogales E Structural insight into microtubule function. Annu Rev Biophys Biomol Struct 30, 397–420, doi: 10.1146/annurev.biophys.30.1.397 (2001). [DOI] [PubMed] [Google Scholar]
- 147.Stone MC, Roegiers F & Rolls MM Microtubules have opposite orientation in axons and dendrites of Drosophila neurons. Mol Biol Cell 19, 4122–4129, doi: 10.1091/mbc.E07-10-1079 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mandelkow E & Mandelkow EM Microtubules and microtubule-associated proteins. Curr Opin Cell Biol 7, 72–81 (1995). [DOI] [PubMed] [Google Scholar]
- 149.van de Willige D, Hoogenraad CC & Akhmanova A Microtubule plus-end tracking proteins in neuronal development. Cell Mol Life Sci 73, 2053–2077, doi: 10.1007/s00018-016-2168-3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zheng Y, Wong ML, Alberts B & Mitchison T Nucleation of microtubule assembly by a gamma-tubulin-containing ring complex. Nature 378, 578–583, doi: 10.1038/378578a0 (1995). [DOI] [PubMed] [Google Scholar]
- 151.Janke C The tubulin code: molecular components, readout mechanisms, and functions. J Cell Biol 206, 461–472, doi: 10.1083/jcb.201406055 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chakraborti S, Natarajan K, Curiel J, Janke C & Liu J The emerging role of the tubulin code: From the tubulin molecule to neuronal function and disease. Cytoskeleton (Hoboken) 73, 521–550, doi: 10.1002/cm.21290 (2016). [DOI] [PubMed] [Google Scholar]
- 153.Pollard TD Actin and Actin-Binding Proteins. Cold Spring Harbor perspectives in biology 8, doi: 10.1101/cshperspect.a018226 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Franker MA et al. Three-Step Model for Polarized Sorting of KIF17 into Dendrites. Curr Biol 26, 1705–1712, doi: 10.1016/j.cub.2016.04.057 (2016). [DOI] [PubMed] [Google Scholar]
- 155.Hirokawa N, Sato-Yoshitake R, Yoshida T & Kawashima T Brain dynein (MAP1C) localizes on both anterogradely and retrogradely transported membranous organelles in vivo. J Cell Biol 111, 1027–1037 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Waterman-Storer CM, Karki S & Holzbaur EL The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1). Proc Natl Acad Sci U S A 92, 1634–1638 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hoogenraad CC & Akhmanova A Bicaudal D Family of Motor Adaptors: Linking Dynein Motility to Cargo Binding. Trends Cell Biol 26, 327–340, doi: 10.1016/j.tcb.2016.01.001 (2016). [DOI] [PubMed] [Google Scholar]
- 158.He J et al. Prevalent presence of periodic actin-spectrin-based membrane skeleton in a broad range of neuronal cell types and animal species. Proc Natl Acad Sci U S A 113, 6029–6034, doi: 10.1073/pnas.1605707113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]