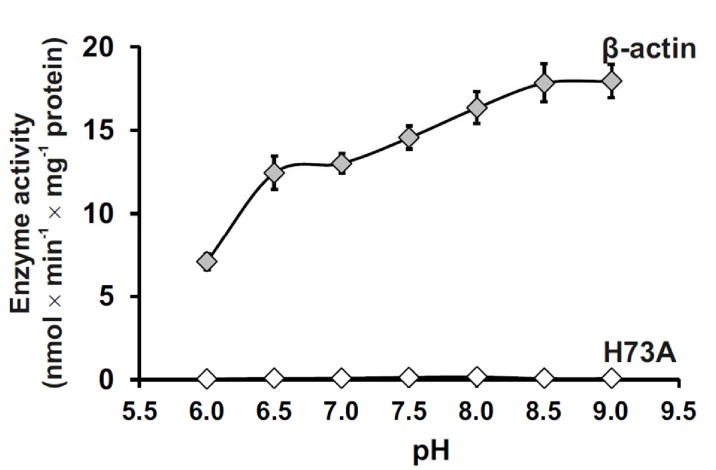
Figure 3. Effect of pH on SETD3 enzyme activity.
The pH-dependence of human SETD3 was determined with the use of a purified recombinant N-terminal His6-tagged SETD3 protein (aa 2–502). The enzyme preparation (0.045 µg protein) was incubated at 37°C for 10 min in the presence of 0.8 μM [1H+3H] AdoMet (80 pmol, ≈350 × 103 cpm) and either 5 µM (500 pmol, 22.34 µg) purified recombinant human β-actin or its mutated form (H73A), with the latter serving as a negative control. In all experiments, the reaction mixture contained the homogenous recombinant AdoHcy nucleosidase (1.6 mg protein, 600 nM, E. coli) and adenine deaminase (3.9 mg protein, 600 nM, B. subtilis) to prevent AdoHcy accumulation. The reaction was stopped and the proteins present in the assay mixture were precipitated by adding 10% trichloroacetic acid. This allowed for the separation and specific measurement of the radioactivity incorporated in the protein pellet (representing the extent of actin methylation) from the total radioactivity present in the assay mixture. Values are the means ± S.E. (error bars) of three separate experiments. If no error bar is visible, it is shorter than the height of the symbol.