Abstract
Background:
Corrosion has been documented in modular knee implants, but it has not been related to negative patient outcomes. We performed an observational retrieval investigation of 13 Stryker Triathlon TS modular knee implants, 3 of which were revised because of osteolysis and adverse local tissue reactions secondary to fretting corrosion at the modular junctions.
Methods:
Modular surfaces were examined for the presence and severity of corrosion, and factors that may influence the development of corrosion were investigated. Scanning electron microscopy and energy-dispersive x-ray spectroscopy were performed to evaluate implants with severe corrosion, and tissue samples were sent for histopathological analysis.
Results:
Mild to severe corrosion was present in association with 62% of modular tibial components and 75% of modular femoral components. Although tibial corrosion was less prevalent than femoral corrosion, it occurred earlier and with greater severity. Scanning electron microscopy and energy-dispersive x-ray spectroscopy demonstrated the appearances of fretting and corrosion of the modular junctions. Histopathological analysis of specimens from the 3 patients with adverse local tissue reactions demonstrated severe reactions to metal debris, including 1 reaction that was consistent with an aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL).
Conclusions:
To our knowledge, ALVAL and pseudotumors have not previously been reported secondary to corrosion of modular knee replacements. The threaded taper design and the release of cobalt-chromium ions and/or debris are implicated in the occurrence of the adverse local tissue reactions, osteolysis, and soft-tissue damage that we observed in our investigation. Clinicians should be aware of this possible complication associated with modular knee implants.
Clinical Relevance:
This article should raise clinician awareness of adverse local tissue reactions secondary to corrosion, potentially resulting in earlier recognition of this complication.
Corrosion, wear debris, and associated adverse local tissue reactions have been reported extensively in the literature in relation to metal-on-metal and modular hip replacements but not modular knee implants1-10.
Modular junctions in hip implants are susceptible to crevice and fretting corrosion. As a consequence, these junctions have shown a propensity for increased osteolysis, loosening, and adverse local tissue reactions resulting in the requirement for revision1-11. These reactions include the type-IV hypersensitivity response characteristic of aseptic lymphocyte-dominated vasculitis-associated lesions (ALVAL), which have been associated with poor patient outcomes2,5-11.
Modular knee replacements are reserved for patients with severe instability or those undergoing revision, as they provide greater intraoperative flexibility in cases involving minimal bone stock and altered biomechanics. As the number of primary knee replacements continues to rise, so too does the number requiring revision12-14. The increased difficulty of these procedures and the inherent risk of failure with increased implant complexity are demonstrated by greater re-revision rates as compared with primary knee arthroplasty13,15.
Literature regarding the corrosion of modular knee implants is relatively scant, with 3 recently published articles identifying corrosion following modular total knee arthroplasty that was similar to that observed following total hip arthroplasty16-18. Arnholt et al., in a large retrieval study, found corrosion to be almost uniformly present in tibial and femoral components and identified multiple factors that contributed to the severity of the corrosion, including anatomical location (femoral), the type of modular junction (conical or threaded), and patient weight16.
However, there is even less literature regarding adverse local tissue reactions and negative patient outcomes secondary to corrosion. In the studies cited, no revisions were performed because of an adverse reaction to metal debris16,18. The authors of those studies hypothesized that fewer metal ions are released following total knee arthroplasty as compared with total hip arthroplasty, decreasing the likelihood of a local or systemic response and the clinical impact of the corrosion16,18.
We are aware of only 1 reported case of an adverse local tissue reaction secondary to corrosion at a modular interface following total knee arthroplasty; in that case, the patient had severe detritic synovitis that required extensive debridement19.
In 2016, 3 Stryker Triathlon TS revision knee systems were received in our laboratory after adverse local tissue reactions adjacent to the tibial baseplate had been noted in the operating room and severe corrosion on the tibial threaded junctions had been identified on retrieval analysis. This common modular knee device features a threaded taper that is similar in design to that of other implants from the same manufacturer, with comparable rates of success13. We are aware of 2 cases of failure at the modular junction due to loosening and fracture20,21, but we are not aware of any reports of adverse local tissue reactions with this system or of any severe reactions of this type in association with any modular knee replacement system. On the basis of the 3 cases mentioned above, we launched an investigation to review the Stryker Triathlon TS revision knee devices in our retrieval collection (n = 10,400 retrievals). The purpose of this investigation was to analyze the presence of corrosion, factors influencing the presence and severity of corrosion, corrosion as a contributor to implant failure, and clinical consequences associated with the use of this modular knee replacement. We aimed to increase clinician awareness of the possibility of this severe complication associated with modular knee implants as well as the possible implant and patient-related factors contributing to clinical outcomes.
Materials and Methods
Implant and Clinical Information
After having obtained approval from our institutional ethics committee, we identified all Stryker Triathlon TS revision knee system implants from our retrieval collection. There were a total of 13 Stryker Triathlon TS knee systems received in our laboratory between 2011 to 2017, from 11 different surgeons. All 13 tibial components were modular employing an intramedullary stem, whereas only 8 femoral components were modular. The demographic characteristics of the patients are summarized in Table I. The reasons for revision and clinical findings at the time of surgery as reported by the treating surgeons are presented in Table II and Table III, respectively.
TABLE I.
Patient Demographics, Implantation Time, and Reason for Removal
| Case | Sex | Age (yr) | Duration of Implantation (yr) | Reason for Implant Removal |
| 1 | M | 73 | 1.3 | Infection |
| 2 | F | 59 | 5.8 | Infection, loosening, osteolysis |
| 3 | F | 78 | 6.5 | Pain, loosening |
| 4 | M | 58 | 1.1 | Loosening, pain |
| 5 | F | 71 | 0.9 | Infection |
| 6 | F | 82 | 6.2 | Loosening, osteolysis |
| 7 | M | 50 | 7.9 | Infection |
| 8 | M | 52 | 2.4 | Infection |
| 9 | M | 68 | 7 | Infection |
| 10 | M | 69 | 3 | Infection |
| 11 | F | 83 | 4.4 | Pain, loosening, infection, osteolysis |
| 12 | F | 58 | 5 | Loosening, osteolysis |
| 13 | M | 63 | 4 | Osteolysis, pseudotumor |
| Total | 7M/6F | |||
| Average | 66.5 | 4.3 |
TABLE II.
Reasons for Implant Removal
| Reasons for Implant Removal | No. of Patients |
| Infection | 8 (62%) |
| Loosening | 6 (46%) |
| Osteolysis | 5 (38%) |
| Pain | 3 (23%) |
| Pseudotumor | 1 (8%) |
TABLE III.
Intraoperative Findings
| Intraoperative Findings | No. of Patients |
| Scar tissue and granulation | 6 (46%) |
| Component loosening | 6 (46%) |
| Infection | 6 (46%) |
| Metal debris/tissue staining/metallosis | 6 (46%) |
| Osteolysis | 4 (31%) |
| Adverse local tissue reaction (pseudotumor) | 3 (23%) |
Retrieval Analysis
The TS knee components were separated into their individual parts for further analysis. Analysis was performed with use of a stereomicroscope (Leica MZ10) at a maximum of 40× magnification to determine any degradation of the retrieved components at the bearing surfaces and the taper junction interfaces of the stems. Fretting and corrosion at the taper interface was scored independently with use of the modified Goldberg fretting corrosion score3 by 2 of the authors. Corrosion was graded at each of the interfaces into 5 groups of severity (ranging from none to severe). Corrosion was described as fretting due to a combination of wear secondary to micromovement at the interface of 2 surfaces under load, crevice corrosion at or immediately adjacent to a gap or crevice in 2 adjacent surfaces, and pitting (localized cavities) or etching marks (discoloration) on the surface finish. Scores from both examiners were averaged, with 0 indicating no corrosion and 4 indicating severe corrosion (Table IV). Implantation time, implant location, the presence of an augment or offset adaptor, and stem width were all investigated as possibly influencing the presence and severity of corrosion. Statistical analysis was performed for each possible factor. At the time of revision, the treating surgeons reported subjectively on possible patient-related contributing factors, including comorbidities, activity level, and patient weight.
TABLE IV.
Corrosion Scores*
| Corrosion Score |
|||||
| Tibia |
Femur |
||||
| Case | Duration of Implantation (yr) | Taper | Threaded Junction | Taper | Threaded Junction |
| 1 | 1.3 | 0 | 0 | 0 | 0 |
| 2 | 5.8 | 0 | 0 | NA | NA |
| 3 | 6.5 | 0 | 0 | 0 | 0 |
| 4 | 1.1 | 0.5 | 4 | NA | NA |
| 5 | 0.9 | 3 | 4 | NA | NA |
| 6 | 6.2 | 0 | 0 | 1.5 | 2 |
| 7 | 7.9 | 1.5 | 2 | 2 | 3 |
| 8 | 2.4 | 0 | 0.5 | 0 | 0.5 |
| 9 | 7 | 0 | 0 | 1 | 4 |
| 10 | 3 | 3 | 3 | NA | NA |
| 11 | 4.4 | 2.5 | 2.5 | 1 | 1 |
| 12 | 5 | 4 | 4 | NA | NA |
| 13 | 4 | 4 | 4 | 2.5 | 1.5 |
| Average | 4.3 | 1.42 | 1.85 | 1 | 1.5 |
0 = none, 1 = very mild, 2 = mild, 3 = moderate, 4 = severe. NA = not applicable.
Retrieved implants with severe corrosion were analyzed with use of a JCM 6000 scanning electron microscope (JEOL), which had the capacity to conduct energy-dispersive x-ray spectroscopy. The modular surfaces were analyzed to determine the extent of the corrosion, and energy-dispersive x-ray spectroscopy allowed for the correct identification of the corrosion deposits and their relative proportion in the material.
In the cases of the 3 patients who were found to have an adverse local tissue reaction adjacent to the tibial implant, tissue was excised and sent for histopathological analysis. The histological scoring system proposed by Campbell et al. was used to determine the likelihood of a type-IV hypersensitivity response characteristic of ALVAL10. All tissue samples were analyzed by the same pathologist, and each was given an ALVAL score based on the histological analysis3.
Results
The Stryker Triathlon TS knee system is specifically designed for revision total knee arthroplasty and other complex procedures. The modularity of the implant offers options for femoral and/or tibial stems and offset adaptors and/or extenders with threaded taper junctions requiring manual tightening intraoperatively22. In the present study, 11 implants employed CoCr (cobalt-chromium) alloy for the tibial and femoral components, 1 employed a mixed-metal combination (with a TiAlV [titanium-aluminum-vanadium]-alloy tibial stem and CoCr-alloy tibial baseplate), and 1 employed TiAlV only. All 13 implants had a modular interface on the tibial implant, and 4 had an additional tibial extender and stem. Eight implants had a modular femoral junction, and 5 of the 8 employed an offset adaptor. Several implants also employed a tibial or femoral augment. All implants employed the same X3 polyethylene insert.
The study group included 7 men and 6 women with a mean age of 66.5 years (range, 50 to 83 years) and a mean implantation time of 4.3 years (range, 0.9 to 7.9 years) (Table I). Infection was the most common reason for revision (Table II). Adverse local tissue reactions were noted in 3 patients (Table III). The initial reason for primary implantation in all cases was osteoarthritis.
Corrosion damage was identified in 14 of the 21 components, including 8 of the 13 tibial implants and 6 of the 8 femoral implants. Individual details and corrosion scores for the male threaded taper and female threaded junction modular components at each location are presented in Table IV.
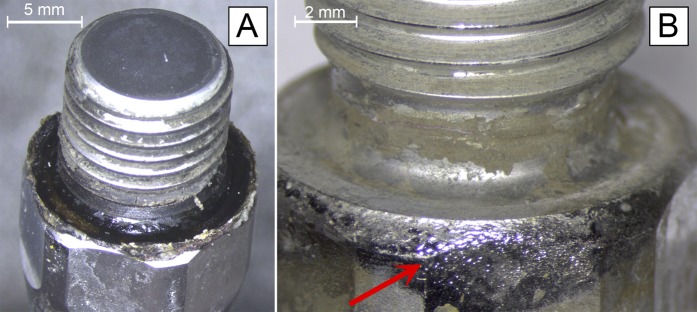
With regard to anatomical location, corrosion was more uniformly present in the femoral implants than in the tibial implants (75% [6 of 8] compared with 61.5% [8 of 13]). However, when corrosion occurred, it seemed to do so earlier and with greater severity in the tibial implant. Six tibial implants had moderate to severe corrosion (grade 2.5 or greater) on 1 or both surfaces after an average implantation time of 3.1 years, whereas 3 femoral implants had such corrosion after an average implantation time of 6.3 years (Table V, Fig. 1). Figure 1 demonstrates the average implantation time and average corrosion scores for tibial and femoral implants with corrosion (with implants with a score of 0 on both the taper and the threaded junction excluded). Given the small sample size, however, no significant conclusions could be drawn regarding the anatomical location or severity of corrosion. Figure 2 depicts severe corrosion, accompanied by black discoloration and corrosion deposits, on 2 of the tibial stems.
Fig. 1.
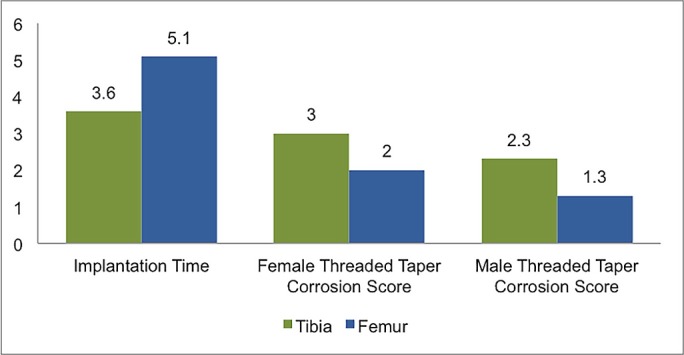
Graph demonstrating a comparison between tibial and femoral components with corrosion present (implants with corrosion scores of 0 are excluded). The first group of bars shows the implantation time (in years), and the second and third groups show the corrosion scores for female and male modular surfaces.
Fig. 2.
Photographs of 2 tibial stems with severe corrosion accompanied by extensive deposition of corrosion products (arrow) on the base of the threaded surface (Fig. 2-A) and the body of the stem (Fig. 2-B).
TABLE V.
Distribution of Corrosion Scores According to Anatomical Location and Average Implantation Time for Each Corrosion Score
| Tibia |
Femur |
|||
| Corrosion Score | No. of Implants | Duration of Implantation (yr) | No. of Implants | Duration of Implantation (yr) |
| 0 (no corrosion) | 5 (38%) | 5.4 | 2 (25%) | 3.9 |
| 1 (corrosion present) | 1 (8%) | 2.4 | 2 (25%) | 3.4 |
| 2 (mild) | 1 (8%) | 7.9 | 1 (13%) | 6.2 |
| 3 (moderate) | 2 (15%) | 3.7 | 2 (25%) | 6 |
| 4 (severe) | 4 (31%) | 2.8 | 1 (13%) | 7 |
| Total | 13 (100%) | 4.2 | 8 (100%) | 5 |
The width and length of the stem and the presence of an offset adaptor or augment had no identifiable or statistical relationship with the corrosion score. The present study included only 1 implant that employed a mixed-metal alloy and only 1 implant that employed TiAlV alone. Consequently, we cannot hypothesize about the differences in corrosion behavior of different materials.
There was no correlation between age or sex and the severity of corrosion. The weight was not known for all patients, but 2 of the patients who had implants with severe corrosion at the tibial junctions had a weight of 110 kg. The activity level also was not known for all patients, but, according to the operating surgeons, 4 of the 5 patients who had implants with severe corrosion (including the 2 patients who weighed 110 kg) were noted to have had a high activity level preoperatively. No patient with a corrosion score of <3 was reported as being overweight or having had a high activity level.
Scanning Electron Microscopy and Energy-Dispersive X-Ray Spectroscopy
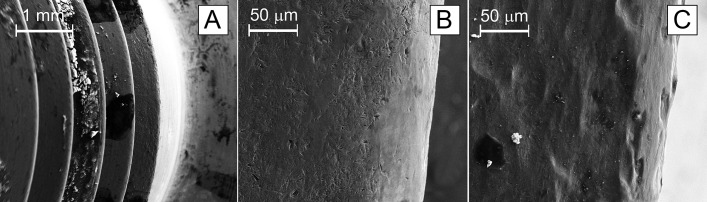
Scanning electron microscopy was performed on 4 of the implants that were deemed to have severe corrosion. Scanning electron microscopy demonstrated severe fretting corrosion, with black corrosion deposits, through most of the threaded junctions, with the findings seeming to worsen in the distal threads (Fig. 3). Energy-dispersive x-ray spectroscopy demonstrated greater peaks of chromium than cobalt. Cobalt has higher solubility in tissues and is therefore taken up faster with these findings of fretting corrosion23.
Fig. 3.
Scanning electron microscopy of distal thread edges, including an overview of the distal threads (Fig 3-A) and non-corroded (Fig. 3-B) and corroded (Fig. 3-C) surfaces.
Adverse Local Tissue Reactions and Histopathological Findings
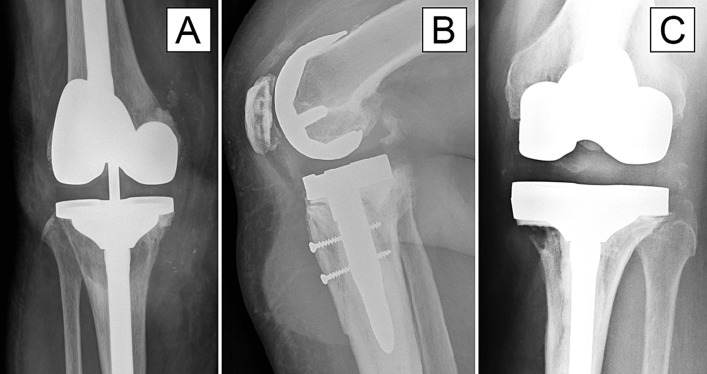
The 3 patients who had adverse local tissue reactions had several similarities. Only 1 of these patients had revision because of a pseudotumor, but all had implantation times of between 4 and 5 years, pre-revision pain, imaging studies that demonstrated progressive osteolysis and a collection of fluid or pseudotumor, and moderate to severe corrosion on the tibial threaded junction and stem. Pre-revision radiographs for all 3 of these patients are shown in Figure 4.
Fig. 4.
Radiographs showing adverse local tissue reactions in 3 patients (Cases 11, 12, and 13), including osteolysis around the tibial stem (Fig. 4-A), an obvious soft-tissue mass anterior to the tibial baseplate (Fig. 4-B), and severe medial osteolysis adjacent to the tibial baseplate (Fig. 4-C).
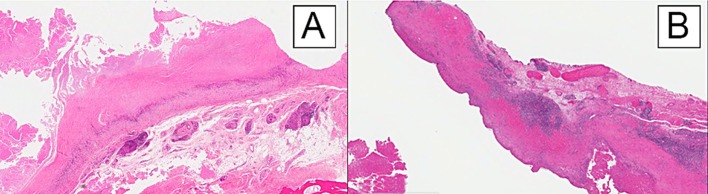
One patient (Case 11) had necrosis and an ALVAL score of 4 (low) but had an inflammatory infiltrate dominated by detritic laden macrophages, consistent with severe detritic synovitis (Fig. 5-A). Two patients (Cases 12 and 13) had necrosis and ALVAL scores of 9 (high) and 7 (moderate), respectively. One of those patients (Case 12) had a lymphocyte-dominated inflammatory infiltrate in perivascular aggregates typical of ALVAL (Fig. 5-B), whereas the other patient (Case 13) had a diffuse and unorganized lymphocytic response. However, given the extensive tissue necrosis of the synovium and the lymphocyte-dominated response, it was the pathologist’s opinion that there was a component of a hypersensitivity response such as ALVAL that was contributing to the clinical picture in the case of the latter patient (Case 13).
Fig. 5.
Photomicrographs of samples from patients with a low ALVAL score (Case 11) (Fig. 5-A) and a high ALVAL score (Case 12) (Fig. 5-B) (hematoxylin and eosin, ×10). Both images show a long band of severe hyaline necrosis. Figure 5-A demonstrates minimal scattered lymphocytes, whereas Figure 5-B shows large perivascular aggregates of lymphocytes, a finding characteristic of ALVAL.
Discussion
Although corrosion was more uniformly present in the femoral components in the present study, it occurred earlier and with greater severity in the tibial components. Patient weight was not known in all cases, and the reported activity level was a subjective judgment from the surgeon. However, 4 of the 5 patients with severe corrosion on the tibial or femoral interface were reported as being overweight and/or having a high activity level. These findings are in agreement with those reported by Arnholt et al., who found that patient weight was positively correlated with corrosion in the threaded taper junctions16.
We are unsure why the tibial junction corroded so severely in our cohort; however, the threaded junction design does add complexity to the construct. Specifically, the correct amount of torque prior to insertion is critical to properly engage the thread; the threaded taper design provides more surface area for corrosion to occur as compared with the more commonly used conical taper; and the threaded junction design has a roughened mating surface on the female boss, predisposing the interface to crevice corrosion. Therefore, increased patient weight, activity, and forces through the more susceptible threaded taper junctions may leave patients more susceptible to micromotion and corrosion. Clinicians should be aware of this possibility when considering the choice of implant and the type of modular junction in a society in which 58% of revision knee procedures are in overweight patients13.
On the basis of research on the hip, we know that metal wear debris and ions that are released secondary to corrosion induce pathological changes in the surrounding tissue4-6,24. Cobalt and chromium ions have been associated with this process, with cobalt ions being particularly toxic as their increased solubility enables them to be taken up by tissues faster23-25. Once taken up, cobalt ions enhance the secretion of several chemokines that function to attract inflammatory mediators involved in osteolysis4,24,25. Cobalt ions also have been shown to directly affect and inhibit osteoblasts and to promote bone resorption25. Osteolysis and increased bone defects have been shown to increase the amount of stress on tibial and femoral modular stems15,26-28. With increased stresses at the modular junctions, these implants are more susceptible to mechanical failure, opening at the interface, micromotion, and fretting5,15,28.
All 3 patients who were found to have an adverse local tissue reaction in the present study were shown to have moderate to severe corrosion on the tibial stem and threaded junction, with energy-dispersive x-ray spectroscopy indicating cobalt dispersion into surrounding tissues. In all 3 cases, osteolysis was one of the primary reasons for revision. It is likely that the release of cobalt ions into the surrounding tissues secondary to fretting and corrosion contributed to the osteolytic process. This process would have put greater stresses through the implant, increased micromotion and fretting, and exacerbated the clinical picture in patients with already depleted bone stock.
We are aware of only 1 reported case in which an adverse local tissue reaction in a modular knee was associated with histological findings consistent with severe detritic synovitis19. Histologically, all 3 of our patients in whom pseudotumors were noted in theater had severe adverse local tissue reactions to metallic debris, ranging from detritic synovitis to ALVAL, resulting in substantial soft-tissue necrosis.
The local tissue response associated with ALVAL may be a toxic reaction to excessive metal particles or ions or a hypersensitive response to a normal amount of debris8-10. Two of our patients had a hypersensitivity response, and, given the extensive corrosion on the threaded junctions and the clinical picture of progressing osteolysis, it is thought that these hypersensitive tissue responses were a direct consequence of the ions released secondary to the corrosion of the tibial taper and threaded junction.
The current study had several limitations. The sample size was small (n = 13), and therefore we were unable to draw any significant conclusions regarding factors influencing the occurrence or severity of corrosion in this modular knee implant. We also relied on intraoperative findings as reported by the surgeons and presumed no adverse local tissue reaction in the other cases. Secondary to the nature of the study design, the clinical records were incomplete and the patient activity level reports were subjective. We consequently relied on the inclusion of these details by the operating surgeon at the time of revision. Given that corrosion is not recognized as a possible cause for failure in modular knees, systemic blood cobalt and chromium blood levels were not evaluated and consequently cannot be discussed.
In agreement with the literature, we identified the presence of corrosion in modular knee implants. On the basis of this investigation, we can conclude that the threaded taper design is implicated and that patient-related factors may contribute to the severity of corrosion in these cases. Contrary to the previous literature on modular knees, we observed 3 adverse local tissue reactions secondary to corrosion, including ALVAL, that resulted in early and complex revisions of this type of device. With progressive osteolysis and soft-tissue destruction, these reactions are catastrophic for patients undergoing procedures with already depleted bone stock and complex surgical histories.
In summary, corrosion can occur in modular knee devices, and the release of metal ions can result in osteolysis and adverse local tissue reactions in patients with this type of implant (and, likely, other devices). We believe that both the threaded junction and patient factors contributed to the level of corrosion and adverse responses in this investigation. We hope that our findings will raise clinician awareness of adverse local tissue reactions secondary to corrosion and will stimulate further research in this important area.
Footnotes
Investigation performed at Centre for Implant Technology and Retrieval Analysis (CITRA), Perth, Australia.
Disclosure: No external funding was received in support of the current study. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (http://links.lww.com/JBJSOA/A78).
References
- 1.Kop AM, Swarts E. Corrosion of a hip stem with a modular neck taper junction: a retrieval study of 16 cases. J Arthroplasty. 2009. October;24(7):1019-23. Epub 2008 Oct 5. [DOI] [PubMed] [Google Scholar]
- 2.Kop AM, Keogh C, Swarts E. Proximal component modularity in THA—at what cost? An implant retrieval study. Clin Orthop Relat Res. 2012. July;470(7):1885-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg JR, Gilbert JL, Jacobs JJ, Bauer TW, Paprosky W, Leurgans S. A multicenter retrieval study of the taper interfaces of modular hip prostheses. Clin Orthop Relat Res. 2002. August;401:149-61. [DOI] [PubMed] [Google Scholar]
- 4.Dattani R. Femoral osteolysis following total hip replacement. Postgrad Med J. 2007. May;83(979):312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill IP, Webb J, Sloan K, Beaver RJ. Corrosion at the neck-stem junction as a cause of metal ion release and pseudotumour formation. J Bone Joint Surg Br. 2012. July;94(7):895-900. [DOI] [PubMed] [Google Scholar]
- 6.Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013. May 15;95(10):865-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Martino I, Assini JB, Elpers ME, Wright TM, Westrich GH. Corrosion and fretting of a modular hip system: a retrieval analysis of 60 rejuvenate stems. J Arthroplasty. 2015. August;30(8):1470-5. Epub 2015 Mar 14. [DOI] [PubMed] [Google Scholar]
- 8.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008. July;90(7):847-51. [DOI] [PubMed] [Google Scholar]
- 9.Watters TS, Cardona DM, Menon KS, Vinson EN, Bolognesi MP, Dodd LG. Aseptic lymphocyte-dominated vasculitis-associated lesion: a clinicopathologic review of an underrecognized cause of prosthetic failure. Am J Clin Pathol. 2010. December;134(6):886-93. [DOI] [PubMed] [Google Scholar]
- 10.Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010. September;468(9):2321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willert H., Buchhorn GH, Fayyazi A, Flury R, Windler M, Köster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005. January;87(1):28-36. [DOI] [PubMed] [Google Scholar]
- 12.National Joint Registry. 13th Annual report, 2016. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 2016. http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/13th%20Annual%20Report/07950%20NJR%20Annual%20Report%202016%20ONLINE%20REPORT.pdf. Accessed 2018 Jul 12.
- 13.Australian Orthopaedic Association. National Joint Replacement Registry- hip, knee and shoulder arthroplasty. 2016 Annual report. Adelaide: Australian Orthopaedic Association; 2016. [Google Scholar]
- 14.Kurtz SM, Ong KL, Lau E, Widmer M, Maravic M, Gómez-Barrena E, de Pina MdeF, Manno V, Torre M, Walter WL, de Steiger R, Geesink RG, Peltola M, Röder C. International survey of primary and revision total knee replacement. Int Orthop. 2011. December;35(12):1783-9. Epub 2011 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conlisk N, Howie CR, Pankaj P. The role of complex clinical scenarios in the failure of modular components following revision total knee arthroplasty: a finite element study. J Orthop Res. 2015. August;33(8):1134-41. Epub 2015 May 13. [DOI] [PubMed] [Google Scholar]
- 16.Arnholt CM, MacDonald DW, Tohfafarosh M, Gilbert JL, Rimnac CM, Kurtz SM, Klein G, Mont MA, Parvizi J, Cates HE, Lee GC, Malkani A, Kraay M; Implant Research Center Writing Committee. Mechanically assisted taper corrosion in modular TKA. J Arthroplasty. 2014. September;29(9)(Suppl):205-8. Epub 2014 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnholt CM, MacDonald DW, Malkani AL, Klein GR, Rimnac CM, Kurtz SM, Kocagoz SB, Gilbert JL; Implant Research Center Writing Committee. Corrosion damage and wear mechanisms in long-term retrieved CoCr femoral components for total knee arthroplasty. J Arthroplasty. 2016. December;31(12):2900-6. Epub 2016 May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin AJ, Seagers KA, Van Citters DW. Assessment of corrosion, fretting and material loss of retrieved modular total knee arthroplasties. J Arthroplasty. 2017. July;32(7):2279-84. Epub 2017 Feb 28. [DOI] [PubMed] [Google Scholar]
- 19.McMaster W. Adverse local tissue response of the knee with Morse-taper corrosion. J Arthroplasty. 2013. November;28(2):375-8. [DOI] [PubMed] [Google Scholar]
- 20.Boe CC, Fehring KA, Trousdale RT. Failure of the stem-condyle junction of a modular femoral stem in revision total knee arthroplasty. Am J Orthop (Belle Mead NJ). 2015. October;44(10):E401-3. [PubMed] [Google Scholar]
- 21.Lee SC, Nam CH, Jung KA, Lee JH, Ahn HS, Park HY. Disassembly of threaded junction between stem extension and femoral component in a total stabilizer revision total knee arthroplasty. Knee. 2014. March;21(2):628-30. Epub 2014 Jan 24. [DOI] [PubMed] [Google Scholar]
- 22.Stryker. Triathlon TS knee system surgical protocol. 2016. https://www.strykermeded.com/media/2195/triathlon-ts-including-cone-augments-technique.pdf/. Accessed 2018 Jul 12.
- 23.Shahgaldi BF, Heatley FW, Dewar A, Corrin B. In vivo corrosion of cobalt-chromium and titanium wear particles. J Bone Joint Surg Br. 1995. November;77(6):962-6. [PubMed] [Google Scholar]
- 24.Thomas P, Summer B, Sander CA, Przybilla B, Thomas M, Naumann T. Intolerance of osteosynthesis material: evidence of dichromate contact allergy with concomitant oligoclonal T-cell infiltrate and TH1-type cytokine expression in the peri-implantar tissue. Allergy. 2000. October;55(10):969-72. [DOI] [PubMed] [Google Scholar]
- 25.Queally JM, Devitt BM, Butler JS, Malizia AP, Murray D, Doran PP, O’Byrne JM. Cobalt ions induce chemokine secretion in primary human osteoblasts. J Orthop Res. 2009. July;27(7):855-64. [DOI] [PubMed] [Google Scholar]
- 26.Completo A, Simões JA, Fonseca F. Revision total knee arthroplasty: the influence of femoral stems in load sharing and stability. Knee. 2009. August;16(4):275-9. Epub 2009 Mar 18. [DOI] [PubMed] [Google Scholar]
- 27.Completo A, Simões JA, Fonseca F, Oliveira M. The influence of different tibial stem designs in load sharing and stability at the cement-bone interface in revision TKA. Knee. 2008. June;15(3):227-32. [DOI] [PubMed] [Google Scholar]
- 28.Woodgate IG, Rooney J, Mulford JS, Gillies RM. Mechanical failure of revision knee prosthesis at both femoral and tibial modular metaphyseal stem junctions. J Orthop Case Rep. 2016. Jan-Mar;6(1):40-3. [DOI] [PMC free article] [PubMed] [Google Scholar]