Abstract
Navarro et al discuss new work using the gating-modifier toxin GxTx to investigate the molecular mechanism of Kv2.1 channel gating.
The Boa constrictor uses its powerful muscles to suffocate and kill its prey. Unlike the mighty Goliath, smart little David—spiders, scorpions, and other venomous creatures—uses an arsenal of toxins to inflict pain, paralyze, and kill. In the ion channel arena, the fearless investigator also uses toxins to attack difficult problems, as Tilley et al. do in this issue of the Journal of General Physiology, in a comprehensive study aimed at deciphering the gating mechanism of Kv2.1, a member of the voltage-gated potassium (Kv) channel family.
Gating-modifier toxins
Toxins that target voltage-gated ion channels work by two mechanisms: they either block the pore to prevent ion conduction (Garcia et al., 2001), or bind to the voltage-sensing domain (VSD) to alter the gating of the channel in response to changes in membrane voltage (Swartz, 2007). Many of the gating-modifier toxins are promiscuous; they target several types of channels, sometimes with opposite effects on gating. For example, Hanatoxin (HaTx), a peptide isolated from tarantula venom, inhibits Kv2.1 (Swartz and MacKinnon, 1997) but activates Kv1.2 (Milescu et al., 2013). By interacting with VSDs, gating-modifier toxins modify the kinetics of conformational transitions that are not directly associated with changes in conductance and thus are more difficult to investigate with electrophysiological approaches. Highlighting these silent transitions makes gating-modifier toxins valuable tools for dissecting the molecular mechanisms of voltage-gated ion channels.
Kv2.1 gating mechanism and the effects of GxTx
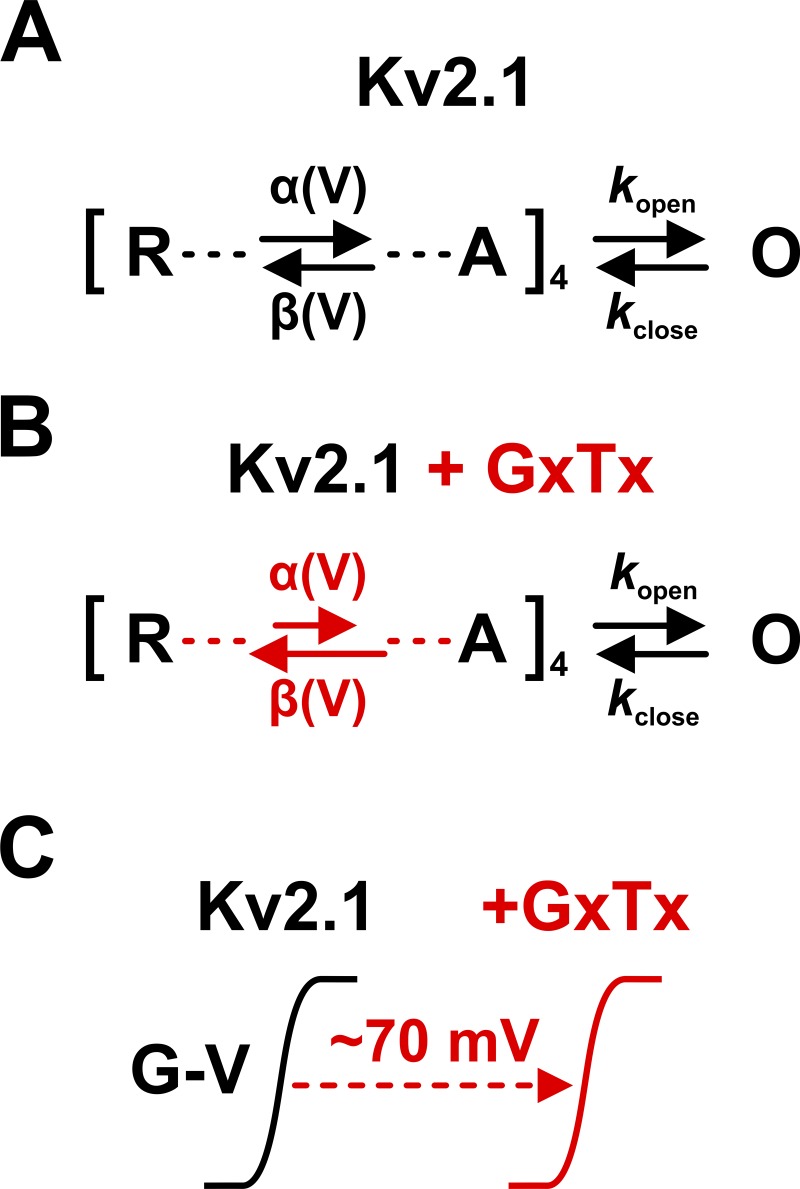
In this issue of JGP, Tilley et al. (2018) use a gating-modifier tarantula toxin, guangxitoxin-1E (GxTx), to investigate the gating mechanism of Kv2.1 by recording whole-cell, single-channel, and gating currents from Kv2.1 channels expressed in Chinese hamster ovary cells. We summarize their findings in Fig. 1. By cleverly interpreting the differences between data obtained in the presence and absence of toxin, and with the aid of kinetic modeling, the authors arrive at the gating mechanism represented in Fig. 1 A. In this mechanism, four VSDs independently undergo voltage-dependent transitions (∼3 e0 per VSD) from a resting state (R) occupied at more negative voltages to an activated state (A) favored by more positive voltages, potentially via intermediate states. When all four VSDs are activated, the channel can undergo a weakly voltage-sensitive (∼0.5 e0) final transition into the open state (O). While not necessarily true in every detail, this conceptual model is in agreement with previous studies (Schoppa et al., 1992; Hoshi et al., 1994; Horrigan and Aldrich, 1999; Islas and Sigworth, 1999). It also explains the new data obtained by Tilley et al., including the conductance-voltage (G-V) curve (Fig. 1 in Tilley et al., 2018), which could be fitted well with a product of two Boltzmann equations: one raised to the fourth power to capture the independent activation of the four VSDs, and the other to capture pore opening as a separate transition with weak voltage dependence.
Figure 1.
A gating model of Kv2.1 and the effects of GxTx. (A) Four identical VSDs transition independently from a resting state (R) to an activated state (A) with voltage-dependent activation and deactivation rates (α and β). When all VSDs are activated, the pore can open (O) with weakly voltage-dependent rates (kopen and kclose). (B) GxTx modifies the VSD activation and deactivation rates (α is reduced, β is increased) but not the pore opening. (C) Saturating GxTx (>100 nM) detains the voltage sensors in their resting conformation (R), making it harder for the channel to open and shifting the G-V activation curve. The channel can still open with toxin bound without change in unitary conductance.
GxTx interacts with a conserved helix-turn-helix motif within the Kv2.1 VSD (Milescu et al., 2009) where, according to Tilley et al., it has two key effects on the Kv2.1 gating mechanism (Fig. 1 B): decreasing the activation rate (α) and increasing the deactivation rate (β) of the VSD, without modifying the rates of the final pore opening transition (kopen and kclose). This means that GxTx binds to each VSD independently and shifts the activation of the bound VSD to more positive voltages. The resulting shift in the macroscopic G-V curve (Fig. 1 C) is toxin concentration dependent, and overall channel activation is limited by the bound sensors. Although GxTx shifts the G-V curve by as much as +70 mV at saturating concentrations (>100 nM), the channel is still able to open with toxin bound, without any change in unitary conductance, implying that it would reach the same maximum open probability if enough depolarization could be applied. Nevertheless, this G-V shift renders Kv2.1 channels silent within the physiological voltage range, which explains the toxicity of GxTx. The binding of the toxin to the channel is voltage dependent, as shown by previous studies (Milescu et al., 2009; Gupta et al., 2015), including Tilley et al.’s own work, in which they observed fluorescently labeled GxTx binding to cells expressing Kv2.1 but detaching upon depolarization (Tilley et al., 2014). As we describe below, the proposed gating mechanism for Kv2.1 and the interaction with GxTx are supported by a satisfying collection of experiments that leave no stone unturned and produce a cohesive set of results.
Macroscopic currents
Macroscopic currents are not only relatively easy to obtain but can also provide a wealth of information in properly designed experiments, as Tilley et al. (2018) demonstrate. They first subject Kv2.1 channels to depolarizing steps and find that a saturating concentration of GxTx increases the apparent time constant of activation (τa), shifting its voltage dependence by approximately +80 mV. Interestingly, when the voltage is stepped to +60 mV or higher, τa shows little dependence on toxin concentration (10 nM to 1 µM). This means that channels with one, two, three, or four toxin-bound VSDs have similar activation rates, but these rates are approximately three times slower than the activation rate of a toxin-free channel (Fig. 2 in Tilley et al., 2018). This result suggests that the toxin-bound VSDs become rate limiting at these extremely positive voltages.
In contrast to the increase in τa, the time constant of deactivation (τd) is decreased by GxTx. This is apparent in experiments in which the channel is first activated by a voltage step to +80 mV and then deactivated by hyperpolarizing steps between +40 and −100 mV. Below −50 mV, where τd is strongly voltage dependent, the same voltage-sensitive conformational transition limits the rate of deactivation with or without toxin, but this transition is accelerated three to four times by GxTx (Fig. 4 in Tilley et al., 2018). Above +10 mV, τd has little voltage dependence and has the same value in the presence of saturating GxTx as it has in the absence of toxin. Thus, deactivation at more negative potentials corresponds to the voltage-sensitive, toxin-dependent transition of the VSDs into a resting state, whereas deactivation at more positive voltages corresponds to the voltage-insensitive, toxin-independent closing of the pore.
In addition to increasing τa and decreasing τd, GxTx extends the time delay between the onset of a voltage step and the beginning of ionic current flow in a concentration dependent manner (Fig. 3 in Tilley et al., 2018). This delay, quantified by the degree of “sigmoidicity” (σ), can be used to infer the minimum number of conformational steps that precede pore opening (Zagotta et al., 1994). Without toxin, σ takes values from 3 at lower voltages to 1 at higher voltages, where the final (weakly voltage-dependent) pore opening transition becomes the rate-limiting step. At a lower concentration of GxTx (10 nM), σ is between 1 and 2 at most voltages but increases to between 2 and 3 at saturating GxTx, where the slow activation of multiple toxin-bound VSDs results in a longer sojourn along the channel activation pathway. Interestingly, the shifts in the voltage dependence of τa, τd, and σ that are caused by saturating GxTx can all be similarly compensated by approximately +80 mV, in agreement with a model where GxTx affects the voltage-dependent transitions (VSD activation) but has no effect on the voltage-insensitive ones (pore opening).
Single-channel currents
Although the above results are already very informative, Tilley et al. (2018) engaged next in a suite of single-channel experiments and report for the first time the effects of voltage sensor toxins on unitary Kv channel currents. Compared with macroscopic currents, single-channel data can disambiguate the relationship between kinetic mechanism and unitary conductance, and can also provide more direct kinetic information. For these experiments to work, a voltage stimulation protocol was designed to determine whether the single-channel events in a trace were generated by a toxin-free or by a toxin-bound channel. Thus, traces were flanked by voltage steps to 0 mV, where toxin-free channels can open, but toxin-bound channels cannot, as we already know from the whole-cell recordings. Very importantly, we unambiguously learn from these experiments that the channel can open with the toxin bound, but the toxin does not alter the Kv2.1 unitary conductance (Fig. 5 in Tilley et al., 2018), a possibility that would have invalidated the analysis of the G-V curve and its shift by GxTx.
Analysis of the open state dwell times reveals a single-exponential distribution in control (no toxin added), toxin-free (toxin added but not bound), and toxin-bound traces, with a time constant of ∼10 ms (slightly smaller in toxin-bound traces), similar to the τd extracted from macroscopic currents at the most positive voltages tested. In comparison, analysis of the closed dwell time distribution reveals two components in control and toxin-free traces, with time constants in the 1–10 ms range (Fig. 6 in Tilley et al., 2018). These two components are preserved in toxin-bound traces, but a third component emerges with a significantly greater time constant (20–40 ms), similar to τa obtained from macroscopic currents in the presence of GxTx (10–40 ms). Together, these results indicate that, under these conditions, the gating mechanism is dominated by a single open state, virtually unaffected by the presence of toxin, and contains multiple closed states, some of which become longer-lived in the presence of toxin. By detaining Kv2.1 channels in these long-lived closed states that correspond to VSDs being in their resting state, GxTx lowers the open probability of the toxin-bound channel at a given voltage.
Gating currents
The last piece of evidence comes from gating currents, which can highlight those state transitions associated with charge translocation during gating. For the proposed model (Fig. 1 A), these transitions would correspond to the activation and deactivation of each VSD, respectively characterized by the α and β rates, rather than to the opening and closing of the pore, which from the macroscopic and single-channel data appear to move little charge. Thus, the significant effect of GxTx on α (reduction) and β (increase) is unambiguous (Fig. 7 in Tilley et al., 2018). The “on” gating current, evoked by depolarizing voltage steps from −100 mV to different voltages (−80 to +120 mV), exhibits voltage dependence and becomes slower in the presence of toxin. In contrast, the “off” gating current, evoked by stepping from the activation voltage (between −80 and +120 mV) down to −140 mV, becomes faster. When stepping from −100 mV up to 0 mV and then stepping down to −140 mV, both on and off currents are entirely inhibited by toxin, indicating that the VSDs are completely at rest in this voltage range but start to activate at more positive voltages, in agreement with the macroscopic current activation.
To obtain the gating charge–voltage (Q-V) curve, the authors calculate the deactivation charge from the integral of the off current, obtained by stepping from different voltages down to −140 mV. From these data, the maximum gating charge translocated by each VSD was estimated at 3.125 e0, which is comparable with the 2.6 e0 estimate from the G-V curve and similar to previous measurements in Kv2.1 (Islas and Sigworth, 1999). Interestingly, the maximum charge is ∼1.35 times greater when the toxin is bound, which could mean, among other things, that the toxin-bound VSDs are slightly deeper into their resting conformation at extremely negative voltages. At saturating concentrations (>100 nM), GxTx shifts the Q-V curve by more than +70 mV (Fig. 7 in Tilley et al., 2018), similar to the shift observed in the G-V curve (Fig. 1 in Tilley et al., 2018). According to this shift, GxTx stabilizes the resting conformation of the VSD by 9.0 kBT (or 8,100 fold) and thus the overall closed state of the channel by 36 kBT: a significant effect.
Importantly, most of the gating charge reported by the on current, evoked by steps to positive voltages, settles fast, before the ionic current starts to flow (Figs. 1, 2, 7, and 8 in Tilley et al., 2018). This indicates that most of the charge translocated under these conditions corresponds to VSD activation and not to pore opening, further supporting the idea that kopen and kclose are weakly voltage sensitive. This fast-moving fraction of the activation charge is shifted +86 mV by GxTx, more than the shift of +73 mV in the total deactivation charge (Fig. 7 in Tilley et al., 2018), emphasizing the greater effects exerted by toxin on the earlier steps in the channel activation pathway. To more directly measure these effects, the on and off gating currents were fitted with exponential functions, and the time constants corresponding to the decay phase (τon and τoff), which is less contaminated by artifacts, were compared between control and saturating GxTx (Fig. 8 in Tilley et al., 2018). Clearly, GxTx decreases τoff in the −140 to +20 mV range and increases τon above +60 mV, although it is less clear what happens to the two time constants outside their tested voltage ranges.
Perspectives
Voltage-gated ion channels have gating mechanisms that are complex enough to resist casual decoding attempts, and all the more so when gating modifier toxins are added into the equation. The comprehensive study by Tilley et al. (2018) goes a long way toward understanding the intrinsic gating mechanism of Kv2.1 and the effects of GxTx on the channel. While this work has generated a stream of interesting data, several findings seem particularly important to us. First, the toxin does not modify the single channel conductance, which means that future experiments with Kv2.1 and GxTx can be designed with the assurance that all changes observed will be due to kinetic effects. Similar results were reported for other channel/toxin pairs, such as Cav3.1/ProTx-II (Edgerton et al., 2010) and Nav1.3/TsIV-5 (Kirsch et al., 1989). Indeed, we are not aware of any gating-modifier toxin that changes unitary conductance.
Second, GxTx has a profound effect on the Kv2.1 VSDs by detaining them in their early resting state, but without changing pore opening kinetics. Additionally, with or without toxin bound, the opening of the pore has minimal voltage sensitivity (0.5 e0) relative to the activation of the VSD (∼3 e0). Together, these results point to a weak coupling between VSD and pore in Kv2.1. This is definitely not the case in Kv1.2 (Shaker), a well-studied member of the Kv family (Hoshi et al., 1994; Zagotta et al., 1994; Islas and Sigworth, 1999). HaTx (a GxTx-related toxin) stabilizes the Kv1.2 VSDs in conformations intermediate between resting and fully activated but also modifies the kinetics of pore opening (Milescu et al., 2013), which exhibits significant voltage dependence (1.8 e0) and involves a conformational change of the VSD (Smith-Maxwell et al., 1998). Interestingly, HaTx also acts on Kv2.1 by inhibiting the later transitions of the VSDs.
Overall, these results stress the usefulness of gating-modifier toxins as a tool to explore the gating mechanisms of Kv2.1 and other voltage-gated ion channels. Kv2.1 has an important physiological role in muscle and neurons, and phosphorylation of its C terminus serves as a mechanism of neuronal plasticity (Misonou et al., 2004). To fully understand how voltage-gated ion channels work, one must continue to probe their function with new experiments, bearing in mind that membrane composition (Xu et al., 2008; Milescu et al., 2009), auxiliary proteins (Peltola et al., 2011), and phosphorylation state (Murakoshi et al., 1997) also affect the voltage sensing mechanisms of these molecular machines.
Acknowledgments
We acknowledge the many groundbreaking studies that we were not able to credit due to space requirements, as well as Autoosa Salari for insightful comments.
This work was supported in part by a National Institutes of Health Initiative for Maximizing Student Development fellowship (R25GM056901) and a University of Missouri Gus Ridgel Fellowship to M.A. Navarro.
The authors declare no competing financial interests.
José D. Faraldo-Gómez served as editor.
References
- Edgerton G.B., Blumenthal K.M., and Hanck D.A.. 2010. Inhibition of the activation pathway of the T-type calcium channel Ca(V)3.1 by ProTxII. Toxicon. 56:624–636. 10.1016/j.toxicon.2010.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M., Gao Y.-D., McManus O., and Kaczorowski G.. 2001. Potassium channels: from scorpion venoms to high-resolution structure. Toxicon. 39:739–748. 10.1016/S0041-0101(00)00214-2 [DOI] [PubMed] [Google Scholar]
- Gupta K., Zamanian M., Bae C., Milescu M., Krepkiy D., Tilley D.C., Sack J.T., Yarov-Yarovoy V., Kim J.I., and Swartz K.J.. 2015. Tarantula toxins use common surfaces for interacting with Kv and ASIC ion channels. eLife. 4:e06774 10.7554/eLife.06774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan F.T., and Aldrich R.W.. 1999. Allosteric voltage gating of potassium channels II. Mslo channel gating charge movement in the absence of Ca(2+). J. Gen. Physiol. 114:305–336. 10.1085/jgp.114.2.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W.N., and Aldrich R.W.. 1994. Shaker potassium channel gating. I: Transitions near the open state. J. Gen. Physiol. 103:249–278. 10.1085/jgp.103.2.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islas L.D., and Sigworth F.J.. 1999. Voltage sensitivity and gating charge in Shaker and Shab family potassium channels. J. Gen. Physiol. 114:723–742. 10.1085/jgp.114.5.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G.E., Skattebøl A., Possani L.D., and Brown A.M.. 1989. Modification of Na channel gating by an alpha scorpion toxin from Tityus serrulatus. J. Gen. Physiol. 93:67–83. 10.1085/jgp.93.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milescu M., Bosmans F., Lee S., Alabi A.A., Kim J.I., and Swartz K.J.. 2009. Interactions between lipids and voltage sensor paddles detected with tarantula toxins. Nat. Struct. Mol. Biol. 16:1080–1085. 10.1038/nsmb.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milescu M., Lee H.C., Bae C.H., Kim J.I., and Swartz K.J.. 2013. Opening the shaker K+ channel with hanatoxin. J. Gen. Physiol. 141:203–216. 10.1085/jgp.201210914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H., Mohapatra D.P., Park E.W., Leung V., Zhen D., Misonou K., Anderson A.E., and Trimmer J.S.. 2004. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat. Neurosci. 7:711–718. 10.1038/nn1260 [DOI] [PubMed] [Google Scholar]
- Murakoshi H., Shi G., Scannevin R.H., and Trimmer J.S.. 1997. Phosphorylation of the Kv2.1 K+ channel alters voltage-dependent activation. Mol. Pharmacol. 52:821–828. 10.1124/mol.52.5.821 [DOI] [PubMed] [Google Scholar]
- Peltola M.A., Kuja-Panula J., Lauri S.E., Taira T., and Rauvala H.. 2011. AMIGO is an auxiliary subunit of the Kv2.1 potassium channel. EMBO Rep. 12:1293–1299. 10.1038/embor.2011.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa N.E., McCormack K., Tanouye M.A., and Sigworth F.J.. 1992. The size of gating charge in wild-type and mutant Shaker potassium channels. Science. 255:1712–1715. 10.1126/science.1553560 [DOI] [PubMed] [Google Scholar]
- Smith-Maxwell C.J., Ledwell J.L., and Aldrich R.W.. 1998. Uncharged S4 residues and cooperativity in voltage-dependent potassium channel activation. J. Gen. Physiol. 111:421–439. 10.1085/jgp.111.3.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz K.J. 2007. Tarantula toxins interacting with voltage sensors in potassium channels. Toxicon. 49:213–230. 10.1016/j.toxicon.2006.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz K.J., and MacKinnon R.. 1997. Mapping the receptor site for hanatoxin, a gating modifier of voltage-dependent K+ channels. Neuron. 18:675–682. 10.1016/S0896-6273(00)80307-4 [DOI] [PubMed] [Google Scholar]
- Tilley D.C., Eum K.S., Fletcher-Taylor S., Austin D.C., Dupré C., Patrón L.A., Garcia R.L., Lam K., Yarov-Yarovoy V., Cohen B.E., and Sack J.T.. 2014. Chemoselective tarantula toxins report voltage activation of wild-type ion channels in live cells. Proc. Natl. Acad. Sci. USA. 111:E4789–E4796. 10.1073/pnas.1406876111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley D.C., Angueyra J.M., Eum K.S., Kim H., Chao L.H., Peng A.W., and Sack J.T.. 2018. The tarantula toxin GxTx detains K+ channel gating charges in their resting conformation. J. Gen. Physiol.:jgp.201812213 10.1085/jgp.201812213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Ramu Y., and Lu Z.. 2008. Removal of phospho-head groups of membrane lipids immobilizes voltage sensors of K+ channels. Nature. 451:826–829. 10.1038/nature06618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W.N., Hoshi T., Dittman J., and Aldrich R.W.. 1994. Shaker potassium channel gating. II: Transitions in the activation pathway. J. Gen. Physiol. 103:279–319. 10.1085/jgp.103.2.279 [DOI] [PMC free article] [PubMed] [Google Scholar]