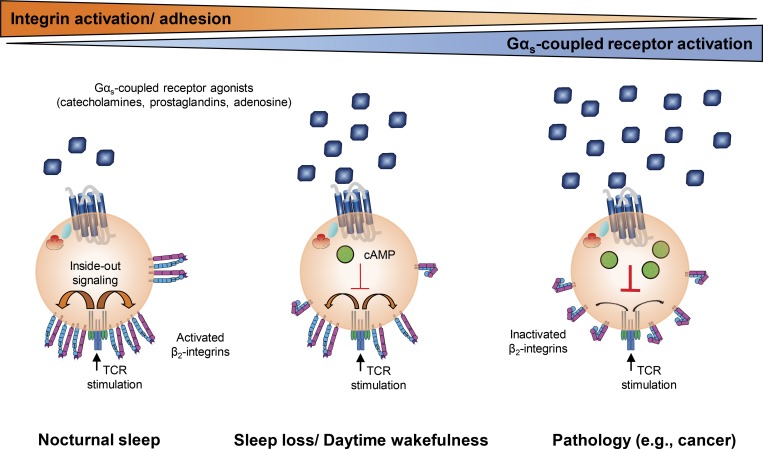
This study demonstrates a regulatory role of Gαs-coupled receptor agonists (catecholamines, prostaglandins, and adenosine) and sleep on integrin activation on T cells in humans. The findings point to a mechanism by which T cell responses are altered in several conditions characterized by aberrant levels of these substances.
Abstract
Efficient T cell responses require the firm adhesion of T cells to their targets, e.g., virus-infected cells, which depends on T cell receptor (TCR)–mediated activation of β2-integrins. Gαs-coupled receptor agonists are known to have immunosuppressive effects, but their impact on TCR-mediated integrin activation is unknown. Using multimers of peptide major histocompatibility complex molecules (pMHC) and of ICAM-1—the ligand of β2-integrins—we show that the Gαs-coupled receptor agonists isoproterenol, epinephrine, norepinephrine, prostaglandin (PG) E2, PGD2, and adenosine strongly inhibit integrin activation on human CMV- and EBV-specific CD8+ T cells in a dose-dependent manner. In contrast, sleep, a natural condition of low levels of Gαs-coupled receptor agonists, up-regulates integrin activation compared with nocturnal wakefulness, a mechanism possibly underlying some of the immune-supportive effects of sleep. The findings are also relevant for several pathologies associated with increased levels of Gαs-coupled receptor agonists (e.g., tumor growth, malaria, hypoxia, stress, and sleep disturbances).
Graphical Abstract
Introduction
The initiation and execution of efficient T cell responses require the recruitment of T cells to lymphoid and nonlymphoid tissues (Ley et al., 2007), as well as the formation of immunological synapses with antigen-presenting cells (APCs) or target cells (such as virus-infected or cancer cells; Scholer et al., 2008; Dustin and Long, 2010; Fooksman et al., 2010). The modulation of T cell adhesiveness by regulation of integrin activation is crucial to these steps. Recirculating T lymphocytes express high levels of membrane-bound β2-integrins (Dimitrov et al., 2009, 2010), which are maintained in a nonadhesive (inactive) state (Evans et al., 2009). Immediate activation (i.e., increase in affinity and avidity) of β2-integrins induced by chemokines allows the arrest of T cells on the endothelium and their subsequent extravasation into tissues (Ley et al., 2007). A similar activation of β2-integrins in response to TCR engagement by cognate peptides presented by MHC molecules (pMHC) on APCs or target cells is also required for the formation of stable immunological synapses (Dustin and Springer, 1989; Dustin and Long, 2010; Fooksman et al., 2010; Long, 2011).
Research on the regulation of integrin-mediated adhesion has focused over the past 35 yr exclusively on pro-adhesive signals, such as chemokines and pMHC. Only recently, the existence of anti-adhesive factors, such as Gαs (a heterotrimeric G protein subunit that activates the cAMP-dependent pathway)-coupled receptor agonists, nitric oxide, and carbon monoxide has become evident (Chigaev et al., 2008, 2011a,b, 2014). Specifically, it has been shown in monocytes that the chemokine-induced integrin affinity is down-regulated by anti-adhesive signaling derived from Gαs-coupled receptor agonists like amthamine (H2-histaminergic receptor agonist) and isoproterenol (β1/2-adrenergic receptor [AR] agonist; Chigaev et al., 2008, 2011b). The recruitment of cytotoxic leukocytes to the blood during daytime and acute physical or psychological stress has been suggested to be mediated by epinephrine (a β1/2-AR agonist), which induces a down-regulation of integrins, resulting in the de-adhesion of the cells from the endothelium of the marginal pool (Dimitrov et al., 2009, 2010). However, nothing is known about the effect of epinephrine or other Gαs-coupled receptor agonists on TCR-mediated integrin activation and formation of immunological synapses.
Several signaling molecules, including catecholamines (Wahle et al., 2005), prostaglandins (PGs; Scher and Pillinger, 2009; Kalinski, 2012), adenosine (Hoskin et al., 2008), dopamine (Yan et al., 2015), histamine, and serotonin (Kim et al., 2013) exert anti-inflammatory effects via their cognate Gαs-coupled receptors. Given the common intracellular mediator cAMP, here we asked whether these substances also share anti-adhesive properties. Sleep is known as a condition characterized by low levels of endogenous Gαs-coupled receptor agonists such as catecholamines (Dimitrov et al., 2015), PGs (Haack et al., 2009), and serotonin (Davies et al., 2014). We therefore additionally used sleep as an in vivo readout to assess effects of low levels of Gαs-coupled receptor agonists on adhesive properties of antigen-specific T cells in a physiological condition. In addition, because of the strong circadian rhythm in the levels of catecholamines (Dimitrov et al., 2015), PGs (Kamperis et al., 2004), serotonin (Davies et al., 2014), and adenosine (Chagoya de Sánchez et al., 1983), with a nadir during the rest phase, adhesion was measured across an entire day to detect a possible circadian rhythm of this parameter. For these purposes, we recruited healthy humans seropositive for CMV, because this chronic latent infection is characterized by a high number of antigen-specific T cells, allowing for the analysis of different T cell subsets. Adhesive properties of the cells were assessed by a new flow cytometry–based assay using soluble pMHC multimers for staining and activation of the antigen-specific T cells, and fluorescent intercellular adhesion molecule (ICAM)–1 multimers (mICAM-1) for visualization of activated β2-integrins (Dimitrov et al., 2018).
We show that catecholamines, PGE2, PGD2, and adenosine potently inhibit TCR-mediated integrin activation on human antigen-specific CD8+ T cells, even at low (physiological) concentrations, whereas nocturnal sleep up-regulates integrin activation by reducing Gαs-coupled receptor signaling. Our findings support the view that conditions of low levels of Gαs-coupled receptor agonists, as generated during sleep, favor the formation of immunological synapses and thereby enhance T cell responses.
Results and discussion
Epinephrine potently inhibits pMHC multimer-induced activation of β2-integrins on antigen-specific CD8+ T cells
We have recently developed a whole blood flow cytometry–based assay to monitor β2-integrin activation on antigen-specific CD8+ T cells by staining the cells with mICAM-1 upon antigen-specific stimulation (Dimitrov et al., 2018). Up-regulation of integrin activation on T cells occurs within a few minutes after TCR engagement and is, so far, the earliest measurable correlate of T cell functionality (as measured by cytokine production and expression of cytotoxic markers) following stimulation (Dimitrov et al., 2018). Thus, this assay is optimal for detecting acute effects of endogenous, fast-acting substances like catecholamines on T cell function.
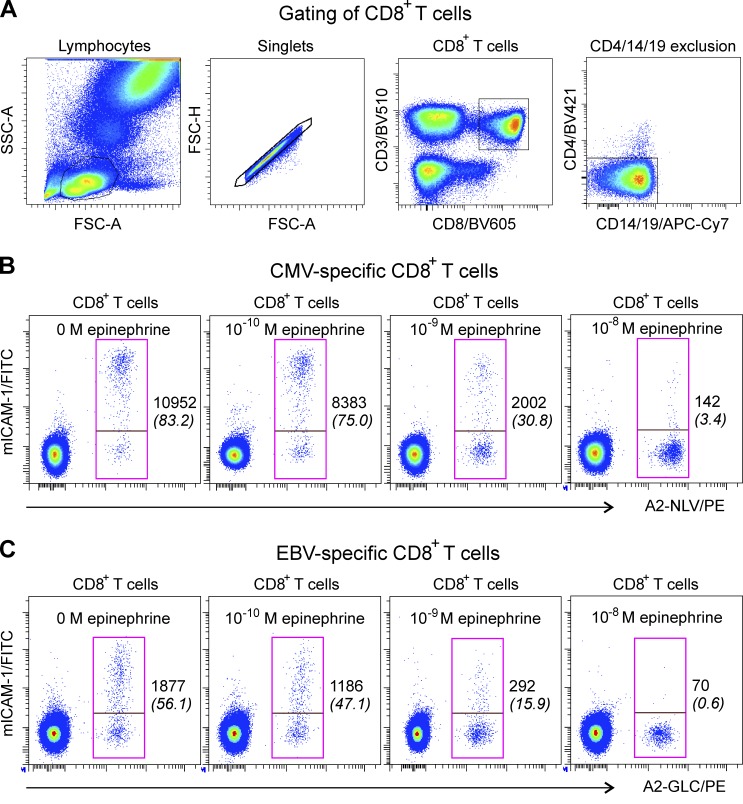
We first tested the effect of epinephrine at very low (10−10 M), stress/exercise-like (10−9 M), and high (10−8 M; pathophysiological, such as during hypoglycemia) concentrations on integrin activation. Whole blood from five HLA-A2+ and CMV-seropositive donors was coincubated with A2/NLV (CMV) or A2/GLC (EBV) pMHC multimers and mICAM-1 for 5 min. The gating strategy to identify CD8+ T cells is shown in Fig. 1 A. As previously shown (Dimitrov et al., 2018), more than two-thirds of A2/NLV+ cells and approximately 30% (with a large inter-individual variability) of A2/GLC+ cells were mICAM-1+ (left plots on Fig. 1, B and C, respectively, shown for one representative CMV- and EBV-seropositive donor; and Table 1). Preincubation of the blood cells for 5 min with epinephrine reduced β2-integrin activation by one-fourth to one-third at the lowest concentration and by 80% at stress/exercise-like levels, and almost completely inhibited it at high concentrations (Fig. 1, B and C; and Fig. 2, A and B). Previous assays assessing the effect of Gαs-coupled receptor agonists on integrin activation in monocytes relied on cell lines or processed blood, thus not allowing analysis of fast and fine-tuning effects relevant for in vivo situations (Chigaev et al., 2008, 2011b). In contrast, costaining of whole blood cells with pMHC and mICAM-1 proved to be a simple and sensitive method for detecting fast changes in β2-integrin activation on antigen-specific T cells induced by even very low physiological levels of epinephrine.
Figure 1.
Epinephrine potently inhibits pMHC multimer-induced β2-integrin activation on antigen-specific CD8+ T cells. Human whole blood cells were incubated for 5 min at 37°C in the presence of pMHC multimers (A2-NLV/PE or A2-GLC/PE) and mICAM-1. (A) Representative density plots showing the gating strategy used for defining pMHC+ CD8+ T cells. From left to right, the lymphocyte gate (on the forward scatter area [FSC-A]/side scatter area [SSC-A] plot), the FSC-A/FSC height (FSC-H) duplet exclusion, the gating of CD3+CD8+ cells, and the exclusion of CD4+, CD14+, and CD19+ events are shown. (B and C) CMV-specific (B) and EBV-specific (C) CD8+ T cells were stained with A2-NLV/PE or A2-GLC/PE multimers, respectively. Multimer binding to the TCR leads to activation of β2-integrins (left), which was detected by staining with mICAM-1. Preincubation of the cells for 5 min with 10−10 M (18 pg/ml), 10−9 M (180 pg/ml), or 10−8 M (1,800 pg/ml) epinephrine partially or completely blocked the activation of the β2-integrins. Numbers indicate the MFI of mICAM-1 binding on pMHC+ CD8+ T cells, and italic numbers in brackets indicate the frequency of mICAM-1+ cells among pMHC+ CD8+ T cells.
Table 1. Characteristics of CMV- and EBV-specific cells and EC50 values of Gαs-coupled receptor agonists.
| Total CMV-specific cells | Early differentiated CMV-specific cells | Intermediate differentiated CMV-specific cells | Late differentiated CMV-specific cells | Total EBV-specific cells | |
|---|---|---|---|---|---|
| % of A2-NLV+ or A2-GLC+ ± SEM | 100 | 32.8 ± 4.0 | 32.9 ± 5.1 | 30.6 ± 5.8 | 100 |
| % mICAM-1+ ± SEM | 83.0 ± 3.7 | 77.6 ± 4.6 | 83.5 ± 3.6 | 85.9 ± 5.0 | 30.3 ± 8.8 |
| MFI of mICAM-1+ ± SEM | 12,730 ± 1,904 | 8,459 ± 1,081 | 13,596 ± 1,796* | 15,423 ± 3,198* | 2,325 ± 988 |
| EC50 of isoproterenol, M | 1.4 × 10−10 | 1.27 × 10−10 | 1.47 × 10−10 | 1.58 × 10−10 | 9.9 × 10−11 |
| EC50 of epinephrine, M | 3.9 × 10−10 | 3.20 × 10−10 | 4.15 × 10−10* | 3.94 × 10−10* | 2.5 × 10−10 |
| EC50 of norepinephrine, M | 3.2 × 10−8 | 3.22 × 10−8 | 3.04 × 10−8 | 3.62 × 10−8 | 1.2 × 10−8 |
| EC50 of PGE2, pg/ml | 931 | 504 | 1,135* | 1,073* | 303 |
| EC50 of PGD2, pg/ml | 797 | 606 | 737 | 810 | 952 |
*, P ≤ 0.05 for comparisons between early versus intermediate or late differentiated CMV-specific T cells.
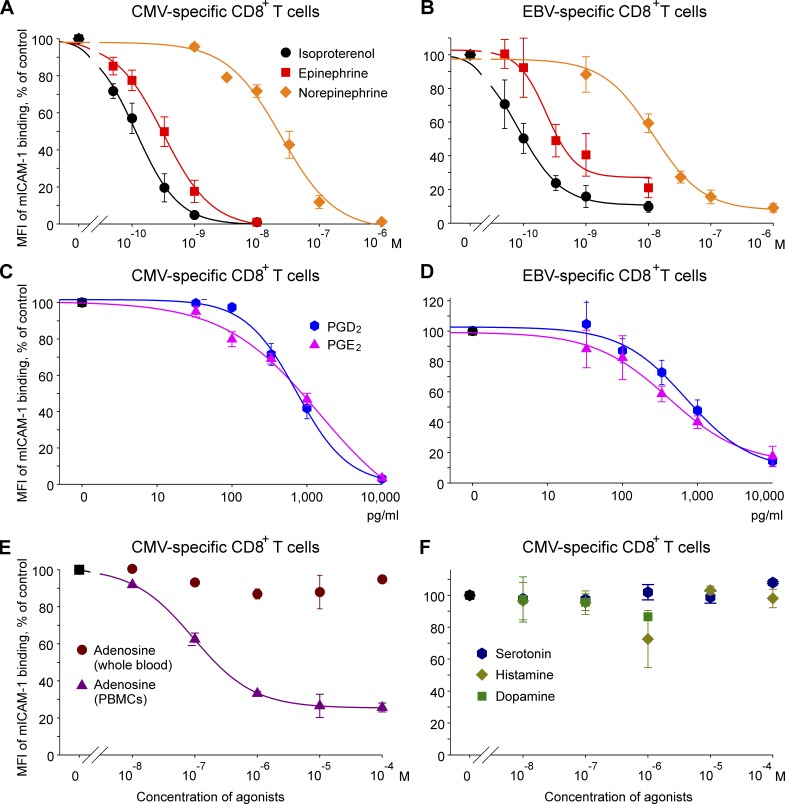
Figure 2.
Effect of Gαs-coupled receptor agonists on pMHC-induced β2-integrin activation on antigen-specific CD8+ T cells. (A–F) Whole blood cells and (for adenosine) PBMCs were preincubated in the presence or absence of the indicated Gαs-coupled receptor agonists at the indicated concentrations for 5 min followed by staining with CMV A2-NLV/PE (A, C, E, and F) or EBV A2-GLC/PE (B and D) multimers and mICAM-1 for 5 min at 37°C. Means ± SEM of the MFI of mICAM-1 binding are shown for the different agonists as percentage of the control (i.e., the sample without Gαs-coupled receptor agonists, which was set to 100%). The fitted standard curves were calculated by nonlinear regression; n = 5 (n = 3 for adenosine, serotonin, histamine, and dopamine).
Gαs-coupled receptor agonists down-regulate TCR-induced integrin activation on CMV- and EBV-specific T cells
Next, we determined the effect and relative potency of different Gαs-coupled receptor agonists on the regulation of β2-integrin activation. Isoproterenol, epinephrine, norepinephrine, PGE2, PGD2, and adenosine down-regulated β2-integrin activation in a dose-dependent and agonist-specific manner, whereas dopamine, histamine, and serotonin had no effect (Fig. 2).
The effective concentration generating 50% of the effect (EC50) for CMV-specific cells was ∼2× higher for epinephrine and 200× higher for norepinephrine compared with isoproterenol (Fig. 2 A and Table 1). This suggests that the effect is mediated by β2-ARs (rather than by β1- or β3-ARs), as the relative degree of binding affinity to the β2-AR and the impact on cAMP production following receptor activation follows the same order (isoproterenol ≈ 2× epinephrine ≫ norepinephrine; Hoffmann et al., 2004). Indeed, the inhibitory effect of epinephrine and norepinephrine was entirely abolished by preincubation with an antagonist of the Gαs-coupled β2-AR (Fig. S1). Reduction of integrin activation first became significant at a concentration of 5 × 10−11 M for isoproterenol (P = 0.031), at 10−10 M for epinephrine (P = 0.047), and at 10−8 M for norepinephrine (P = 0.016). Almost complete inhibition was achieved at isoproterenol and epinephrine concentrations of 10−8 M and at a norepinephrine concentration of 10−6 M.
PGE2 and PGD2 inhibited β2-integrin activation with similar EC50 on CMV-specific cells (Fig. 2 C and Table 1). Both PGs achieved a maximal effect at 10,000 pg/ml. However, PGE2 was more effective at lower concentrations: reduction of integrin activation first reached significance at a concentration of 100 pg/ml for PGE2 (P = 0.046) and 333 pg/ml for PGD2 (P = 0.044). The inhibitory effect of PGD2 was completely blocked after preincubation with an antagonist of the Gαs-coupled PGD2 receptor DP1 (Fig. S1). The effect of PGE2 was reversed by blocking the high-affinity Gαs-coupled PGE2 receptor EP4, but not by blocking the low-affinity receptor EP2 (presumably because we did not use very high levels of PGE2 [1,000 pg/ml]).
Adenosine inhibited integrin activation when peripheral blood mononuclear cells (PBMCs) were used (EC50: 9.6 × 10−8 M; Fig. 2 E), but was ineffective when using whole blood (probably due to the fast reuptake of adenosine by erythrocytes and the consequently extremely short half-life [<1 s] of this substance in whole blood; Ramakers et al., 2008). The reduction of integrin activation became significant at the lowest dose used (10−8 M; P = 0.045) and achieved a maximal effect at 10−5 M. No effect was observed for the other Gαs-coupled receptor agonists tested (Fig. 2 F). Although there is some evidence for an immunomodulatory effect of serotonin, dopamine, and histamine, this finding can probably be explained by the lack or relatively low expression of intact Gαs-coupled receptors for these neurotransmitters on T cells (Saha et al., 2001a,b; León-Ponte et al., 2007; Kmiecik et al., 2012).
The inhibitory effect of Gαs-coupled receptor agonists does not seem to be limited to particular antigen-specific T cells, as we found similar results for EBV-specific CD8+ T cells (Fig. 2, B and D; and Table 1). Here, reduction of integrin activation first became significant at a concentration of 10−10 M for isoproterenol (P = 0.032), 3.3 × 10−9 M for epinephrine (P = 0.032), 10−8 M for norepinephrine (P = 0.037), and 333 pg/ml for PGE2 and PGD2 (P ≤ 0.042). Maximum inhibition was achieved at isoproterenol and epinephrine concentrations of 10−8 M, at a norepinephrine concentration of 10−6 M, and for both PGs at concentrations of 10,000 pg/ml.
Catecholamines, PGs, and adenosine are known to directly suppress the production of cytokines and T cell proliferation (Wahle et al., 2005; Hoskin et al., 2008; Kalinski, 2012; Leone et al., 2015a). Our findings showing that these Gαs-coupled receptor ligands impair integrin activation reveal a new mechanism by which these molecules can exert immunosuppressive effects. Because the levels of Gαs-coupled receptor agonists needed to inhibit integrin activation ex vivo are observed in many physiological and pathological conditions, such as tumor growth, certain infections, hypoxia, and stress, these substances can be well expected to contribute to immunosuppressive effects in vivo (Lokshin et al., 2006; Brudvik and Taskén, 2012; Kalinski, 2012; Leone et al., 2015a,b).
Gαs-coupled receptor agonists down-regulate TCR-induced integrin activation depending on the differentiation status of CMV-specific T cells
We then tested whether mICAM-1 binding and the effects of Gαs-coupled receptor agonists depend on the differentiation status of antigen-specific T cells. As shown previously (Appay et al., 2002, 2008), CMV- and EBV-specific CD8+ T cells can be separated into three differentiation subsets: early (CD27+CD28+), intermediate (CD27+CD28−) and late (CD27−CD28−) differentiated cells, based on their CD27 and CD28 expression. For CMV-specific cells, percentages of the three subpopulations were similar (Table 1 and Fig. S2 A), whereas EBV-specific cells were highly enriched in the early differentiated subset (70.3% ± 7.1%).
The mean fluorescence intensity (MFI) of mICAM-1 binding was higher on intermediate and late differentiated compared with early differentiated cells (Table 1 and Fig. S2 B). The inhibition of integrin activation by epinephrine and PGE2 was strongest for the early differentiated cells, but there were no significant differences between subsets for the EC50 of isoproterenol, norepinephrine, and PGD2 (Table 1 and Fig. S2, C and D). The finding that epinephrine was more effective in reducing integrin activation in early compared with intermediate and late differentiated cells might be surprising, given that this subset expresses the lowest number of β2-adrenoreceptors (Dimitrov et al., 2009, 2010). However, it is not uncommon that receptor expression levels do not correlate with the cell’s responsiveness to a substance (Dimitrov et al., 2009), pointing to the presence of other factors, such as the molecules involved in the intracellular signaling cascade, that contribute to the magnitude of the substance effect as well.
Sleep increases β2-integrin activation on CMV- and EBV-specific CD8+ T cells
Given that nocturnal sleep is characterized by low levels of Gαs-coupled agonists, such as catecholamines and PGs, we compared effects of sleep versus nocturnal wakefulness on β2-integrin activation of CMV- and EBV-specific T cells. In a within-subject design, healthy CMV-positive participants spent one night in the sleep laboratory with sleep allowed between 11 pm and 7 am (sleep condition) and another night in which participants had to stay awake in bed during the same period (wake condition). Whole blood for the determination of integrin activation was collected repeatedly across an entire day, additionally enabling the detection of circadian rhythms.
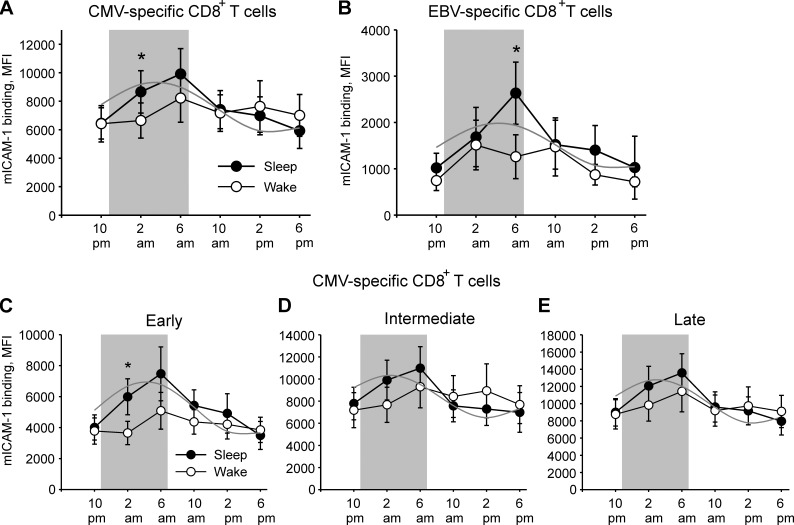
Compared with the wake condition, sleep significantly increased the MFI of mICAM-1 binding on pMHC+ CD8+ T cells (F(1,9) = 4.1; P = 0.05 and F(1,8) = 6.0; P = 0.05 for sleep/wake × period effect for CMV- and EBV-specific CD8+ T cells, respectively; see Fig. 3, A and B, for comparisons at single time points). For CMV-specific T cells, the effect was evident already at 2 am (i.e., 3 h after the beginning of the sleep opportunity). Cosinor analysis confirmed a significant circadian rhythm in mICAM-1 binding on CMV- and EBV-specific cells during the regular sleep–wake cycle (P ≤ 0.023; Fig. 3, A and B), which was abolished in the wake condition (P = 0.07 and 0.10 for CMV- and EBV-specific cells, respectively). The fitted cosine curve for the sleep–wake cycle showed an acrophase (peak time) at 3:33 am ± 32 min and 4:15 am ± 50 min for CMV- and EBV-specific cells, respectively.
Figure 3.
Sleep increases β2-integrin activation on antigen-specific CD8+ T cells. (A–E) Means ± SEM of the MFI of mICAM-1 binding on total CMV-specific (A) and EBV-specific (B) CD8+ T cells, as well as on early (CD27+CD28+; C), intermediate (CD27+CD28−; D) and late (CD27−CD28−; E) differentiated CMV-specific CD8+ T cell subpopulations after staining with pMHC multimers and mICAM-1 are shown for a regular sleep–wake cycle (sleep, filled circles) and continuous wakefulness (wake, open circles). Gray lines illustrate adapted cosine curves for confirmed circadian rhythms in the sleep condition; the shaded area depicts bedtime. Significance is indicated for pairwise comparisons between conditions using paired t tests following significant ANOVA results. n = 10 for CMV; n = 5 for EBV; *, P < 0.05.
Our results demonstrate that a couple of hours of sleep loss suffice to reduce the adhesion capacity of antigen-specific T cells. This finding shows that sleep has the potential to enhance the efficiency of effector T cell responses, which is especially relevant in light of the high prevalence of sleep disorders and conditions characterized by impaired sleep, such as depression, chronic stress, aging, and shift work (Akerstedt, 2003; Roth, 2007; Medina et al., 2014; Feinsilver and Hernandez, 2017).
Sleep selectively increases β2-integrin activation on early and intermediate differentiated CMV-specific CD8+ T cell subsets
mICAM-1 binding at baseline (10 pm) across the sleep and wake conditions was lowest in early differentiated cells (3,889 ± 570), higher in the intermediate differentiated subset (7,479 ± 1,055), and highest in late differentiated cells (8,868 ± 1,135; P ≤ 0.003 for early vs. intermediate, early vs. late, and intermediate vs late differentiated cells; Fig. 3, C–E). Compared with the wake condition, sleep significantly increased mICAM-1 binding on pMHC+ CD8+ T cells for the early and intermediate differentiated cells (F(1,9) = 7.7; P = 0.02 and F = 4.6; P = 0.005 for sleep/wake × period effect, respectively; see Fig. 3 for comparisons at single time points). Cosinor analysis confirmed a significant rhythm in mICAM-1 binding for all three subsets during the regular sleep–wake cycle (P ≤ 0.033), which descriptively was most pronounced for the early differentiated cells: with reference to peak values, mICAM-1 binding during nadir times was reduced by 33%, 30%, and 26%, respectively, in the three subpopulations. The fitted cosine curve showed an acrophase at 4:13 am ± 84 min for the early differentiated subset, at 2:19 am ± 76 min for the intermediate differentiated subset, and at 3:01 am ± 68 min for the late differentiated subset. Peak values of mICAM-1 binding (as derived from cosinor analyses) were significantly higher in the sleep condition compared with the wake condition (P ≤ 0.01), with a similar percentage change in all three subpopulations (57%, 56%, and 56%, respectively).
We could not measure integrin activation on undifferentiated (i.e., naive) T cells due to the inherent properties of the applied assay. However, because the early differentiated phenotype was the most responsive one, it is likely that adhesive properties of naive T cells are also affected by Gαs-coupled receptors. Previous experiments have shown that sleep is essential for forming adaptive immune responses, which has been most impressively demonstrated by several studies using vaccination (Lange et al., 2003, 2011; Benedict et al., 2012). The mechanisms of this boosting effect of sleep on antigen-specific responses are not well understood. If our findings are indeed transferrable to naive T cells, an increased adhesion of naive T cells to APCs might at least partly explain the adjuvant-like effect of sleep on vaccination responses, thus revealing a novel mechanism underlying this effect.
Sleep regulates β2-integrin activation by suppression of Gαs-coupled receptor signaling
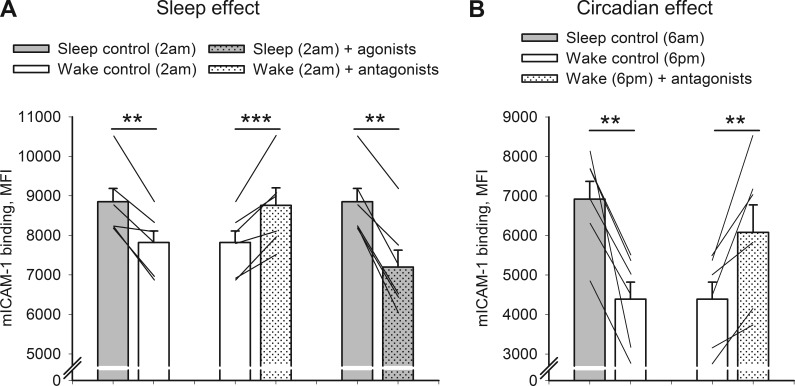
Next, we tested whether the effect of sleep versus wakefulness on integrin activation is mediated by soluble factors via a Gαs-coupled receptor–dependent way. We collected heparin plasma from healthy participants on one night while they were asleep and on another night while they stayed awake in bed. We then incubated cells from a sleeping CMV-positive donor with the plasma collected during the two conditions (sleep vs. wake) in the presence or absence of Gαs-coupled receptor agonists or antagonists to compare integrin activation. These plasma experiments confirmed our findings on a sleep-induced increase in integrin activation: Incubation of CMV-specific cells with plasma collected at 2 am (i.e., the time point of significant differences between sleep and wakefulness in the foregoing sleep experiment) from sleeping participants significantly increased integrin activation on CMV+ CD8+ T cells compared with plasma collected at 2 am during nocturnal wakefulness (Fig. 4 A, left). We could also replicate our finding on a circadian rhythm observed in integrin activation by comparing effects of plasma collected at 6 am from sleeping participants versus plasma collected at 6 pm (during regular daytime wakefulness; Fig. 4 B, left).
Figure 4.
Regulation of integrin activation by plasma collected during sleep versus wakefulness is mediated by Gαs-coupled receptor signaling. (A and B) Means ± SEM of the MFI of mICAM-1 binding are shown on CMV-specific CD8+ T cells incubated with plasma collected at 2 am during sleep versus wakefulness (A) and at 6 am (during nocturnal sleep) versus 6 pm (during daytime wakefulness; B). CMV-specific T cells were incubated with plasma collected at the indicated time points and conditions (sleep vs. wake) in the presence or absence of an antagonist composite (including β2-AR, EP4, and DP1 antagonists) or in the presence or absence of an agonist composite (including epinephrine, norepinephrine, PGE2, and PGD2). All experiments were performed in duplicates. Significance is indicated for pairwise comparisons between conditions or treatments using paired t tests. n = 6; **, P ≤ 0.01; ***, P < 0.001.
Blocking catecholamines and PGs in the plasma derived from wake participants using a composite of β2-AR, DP1, and EP4 receptor antagonists (which were the antagonists shown to block the effect of the respective receptor agonists; Fig. S1) enhanced integrin activation to levels comparable to the sleep condition (P < 0.01; Fig. 4). Adding a composite of catecholamines and PGs (epinephrine, norepinephrine, PGE2, and PGD2) at very low physiological concentrations mimicking sleep–wake differences (Haack et al., 2009; Dimitrov et al., 2015) to the plasma derived from sleeping participants significantly inhibited integrin activation (P < 0.01; Fig. 4 A, right). These results corroborate and add to our foregoing sleep experiment by proving that the effect of sleep deprivation is mediated by soluble factors present in the plasma and that these factors act via Gαs-coupled receptors. Even very low concentrations of Gαs-coupled receptors agonists that correspond to changes induced by a couple of hours without sleep significantly suppressed integrin activation, again demonstrating the great potency of endogenous Gαs-coupled receptor ligands to suppress T cell function.
General conclusions
We show here that several Gαs-coupled receptor ligands potently inhibit TCR-mediated integrin activation on antigen-specific CD8+ T cells, whereas sleep up-regulates integrin activation by suppressing Gαs-coupled receptor signaling. Given the importance of integrin activation for the formation of immunological synapses (Dustin and Springer, 1989; Abram and Lowell, 2009; Dustin and Long, 2010; Fooksman et al., 2010; Long, 2011), our data suggest a critical role of conditions like sleep that are characterized by low levels of Gαs-coupled receptor ligands, in boosting T cell responses (Irwin et al., 1999; Lange et al., 2011). These findings provide a potential mechanism for some immune-enhancing effects of sleep on a new, so far unrecognized, level, namely the formation of immunological synapses by T cell adhesion to APCs or target cells. The findings are also important for understanding immunosuppression present in a variety of pathologies characterized by increased levels of adenosine (e.g., hypoxia, sleep apnea, and tumor growth), catecholamines (e.g., chronic stress and sleep disturbances), or PGs (e.g., tumor growth and malaria) (Kilunga Kubata et al., 1998; Hoskin et al., 2008; Sloan et al., 2010; Brudvik and Taskén, 2012). Furthermore, the results suggest that Gαs-coupled receptors of catecholamines, PGs, and adenosine might constitute immune checkpoint inhibitors, which could be manipulated for therapeutic purposes in various diseases, such as cancer (Leone et al., 2015b; Ohta, 2016; Ricon et al., 2019).
Materials and methods
Participants and study procedure
Ten physically and mentally healthy participants (five men and five women) were included in the study (mean age, 24.7 yr; range, 19–34 yr; mean body mass index, 22.4 kg/m2; range, 20–25 kg/m2). Participants were selectively recruited for being HLA-A2+ and CMV seropositive. Five of these individuals also had detectable levels of EBV-specific CD8+ T cells, allowing us to quantify a second type of antigen-specific cells in this subsample. All participants were nonsmokers, did not suffer from sleep disturbances, and were not taking any medications (except for oral contraceptives in women) at the time of the experiments. None had a medical history of any relevant chronic disease or mental disorder. Acute illness was excluded by physical examination and routine laboratory investigation, including a chemistry panel, C-reactive protein concentration (<6 mg/liter), and a white blood cell count (<9,000/µl).
For investigation of the effect of sleep and the circadian system on adhesion properties of antigen-specific T cells, subjects participated in two experimental conditions, each starting at 8 pm and ending at 6 pm the following day, according to a within-subject crossover design. One condition (sleep) included a regular sleep–wake cycle, whereas in the other condition (wake), participants remained awake throughout the entire experimental period. In the sleep condition, sleep was allowed between 11 pm (lights off) and 7 am, whereas in the wake condition, participants stayed awake in bed in a half-supine position between 11 pm and 7 am. During this time they were listening to music and talking to the experimenter at normal room light. Both experimental sessions were separated by ≥2 wk for each participant. To control for a possible influence of the hormonal cycle in women, both experimental sessions took place within 3 to 6 d after the beginning of their menstrual cycle or the pill-free period.
On both conditions, blood was sampled at 10 pm, and then at 2 am, 6 am, 10 am, 2 pm, and 6 pm the next day. Blood was sampled via an intravenous forearm catheter, which was connected to a long thin tube and enabled blood collection from an adjacent room without disturbing the participant’s sleep. To prevent clotting, ∼500 ml of saline solution were infused throughout the night. The total volume of blood sampled during a session was 120 ml. Blood samples were always processed immediately after sampling. All participants spent one adaptation night in the sleep laboratory before the experimental sessions in order to become accustomed to the experimental setting.
For incubation of whole blood with various Gαs-coupled receptor agonists, blood was collected at 6 am from five sleeping participants. We used blood from sleeping participants for these in vitro experiments, as endogenous Gαs-coupled receptor agonists are lower during sleep than during wakefulness.
For the plasma experiments testing the Gαs-coupled receptor dependence of the sleep/wake effect, we collected heparin plasma from six healthy participants at 2 am on one night while they were asleep and on another night while they stayed awake. Plasma was also sampled at 6 am during regular sleep and at 6 pm during regular daytime wakefulness for investigation of circadian rhythm effects. Indomethacin (a PG synthetase inhibitor; Sigma-Aldrich) was added to the plasma at a concentration of 10 µg/ml before storage of the samples at −80°C until further use. These six participants were not tested for CMV seropositivity, as only their plasma (but not the antigen-specific T cells) was used for further experiments (described in Gαs-coupled receptor dependence of the effects of sleep…). Otherwise, the experimental procedures were comparable to the main sleep experiment. The study was approved by the Ethics Committee of the University of Tübingen, and all participants gave written informed consent.
pMHC and ICAM-1 multimers
We produced biotinylated pHLA-A*0201 monomers (CMV A2/NLVPMVATV [A2/NLV] and EBV A2/GLCTLVAML [A2/GLC]) in-house by conventional pMHC refolding, as previously described (Hadrup et al., 2015). We generated fluorescent pHLA-A*0201 multimers by coincubating monomers with streptavidin-PE or -APC (Thermo Fisher Scientific) at a 1:4 (streptavidin:pHLA-A2 monomer) molar ratio. Multimers were aliquoted and stored at −80°C in Tris-buffered saline buffer containing 16% glycerol (Hadrup et al., 2015). The final concentration of azide was 0.035%.
We generated fluorescent ICAM-1-Fc/anti-Fc multimeric complexes by coincubating 200 µg/ml of recombinant human ICAM-1-Fc (produced as previously described; Bengtsson et al., 2013) with polyclonal anti-human Fc-FITC F(ab′)2 fragments (Jackson ImmunoResearch) at a 1:4 (ICAM-1-Fc:IgG part of anti-Fc-FITC fragments) molar ratio at 4°C for 3 h. We stored them in aliquots at −20°C for ≤1 wk until use.
Cell stimulation and mICAM-1 staining
We used a flow cytometry assay based on whole blood staining that we recently developed to monitor β2-integrin activation on antigen-specific CD8+ T cells (Dimitrov et al., 2018). This assay measures integrin activation by visualizing the specific cellular binding of mICAM-1, reflecting an increased affinity and/or avidity of β2-integrins following TCR-induced activation. We incubated fresh heparinized whole blood (380 µl/test) for 5 min at 37°C in a water bath in 5-ml Falcon tubes (BD Biosciences) with 0.6 µg/ml CMV A2-NLV/PE or EBV A2-GLC/PE multimers and 6.25 µg/ml mICAM-1/FITC. Immediately after incubation, we fixed the samples and lysed erythrocytes with FACS-Lysing solution (BD Biosciences) containing 1 mM Ca2+ and 2 mM Mg2+ for 5 min, followed by washing with PBS/0.5% albumin/0.1% sodium azide. After centrifugation, we stained the cells for surface markers in order to identify CD8+ T cells and their subpopulations (early [CD27+CD28+], intermediate [CD27+CD28−], and late [CD27−CD28−] differentiated cells). The monoclonal antibodies CD4/BV421, CD3/BV510, CD8/BV605, CD28/PerCP-Cy5.5, CD27/APC, CD14/APC-Cy7, and CD19/APC-Cy7 (all from BioLegend) were added to the cells for 15 min at room temperature. All antibodies were used at pretested optimal concentrations. The samples were acquired on a LSR Fortessa (BD Biosciences) and analyzed using FACS DiVa version 8.0. We collected at least 50,000 CD8+ events for the antigen-specific assays. Results are presented as percentage of cells within the parent populations (only Fig. 1 and Fig. S2) or MFI of mICAM-1 binding on total pMHC+ cells.
In vitro effect of Gαs-coupled receptor agonists and antagonists
To investigate the effects of Gαs-coupled receptor agonists on the TCR-induced β2-integrin activation, whole blood taken at 6 am from five participants while asleep was preincubated for 5 min at 37°C in the presence or absence of (–)-isoproterenol (5 × 10−11–10−8 M), (–)-epinephrine (5 × 10−11–10−8 M), (–)-norepinephrine (10−9–10−6 M), PGD2 (10–10,000 pg/ml), PGE2 (10–10,000 pg/ml), dopamine (10−8–10−6 M; all from Sigma-Aldrich), adenosine (10−8–10−4 M), serotonin (10−8–10−4 M), or histamine (10−8–10−4 M; all from Carl Roth) before staining with pMHC multimers and mICAM-1 as described above. Due to the known rapid uptake of adenosine by erythrocytes when using whole blood (Ramakers et al., 2008), the assay for adenosine was also performed on PBMCs isolated in mononuclear cell preparation tubes (CPT; BD Biosciences) from three CMV-seropositive participants. In contrast to whole blood, PBMCs had to be stimulated with peptides (in addition to the pMHC multimers) to achieve optimal cell activation. Alterations in the MFI of mICAM-1 binding on the different in vitro drug treatments are presented as percentage of the control value (without Gαs-coupled receptor agonists), thereby eliminating interindividual differences in MFI.
To investigate whether the effects of the various ligands can be blocked by antagonists of the cognate Gαs-coupled receptors, whole blood was sampled at 9 am from three to four CMV-seropositive participants. The whole blood cells were then preincubated with the following antagonists at the indicated concentration before incubation of the cells with the agonists as described in Fig. S1: β2-AR antagonist ICI-118,551 (10−7 M), PGE2 EP4 receptor antagonist ONO-AE3-208 (3 × 10−6 M; both from Sigma Aldrich), PGE2 EP2 receptor antagonist PF-04418948 (3 × 10−6 M), and PGD2 DP1 receptor antagonist MK-0524 (3 × 10−6 M; Santa Cruz Biotechnology). Following 5 min of incubation, the cells were stained with CMV A2-NLV/PE and mICAM-1 for visualization of integrin activation.
Gαs-coupled receptor dependence of the effects of sleep (vs. wakefulness) on β2-integrin activation
To demonstrate that the sleep/wake difference in integrin activation was mediated by changes of soluble factors present in plasma acting via Gαs-coupled receptors, CMV-specific T cells were incubated with plasma collected during sleep or wakefulness in the presence or absence of Gαs-coupled receptor antagonists or agonists. 80 µl of whole blood taken at 6 am from one HLA-A2+ and CMV-seropositive participant during sleep (to keep endogenous Gαs-coupled receptor ligands low) was immediately preincubated in the presence or absence of a composite of the following antagonists: the β2-AR antagonist ICI-118,551 (10−7 M; Sigma-Aldrich), the PGE2 EP4 receptor antagonist ONO-AE3-208 (3 × 10−6 M; Sigma-Aldrich), and the PGD2 DP1 receptor antagonist MK-0524 (3 × 10−6 M; Santa Cruz Biotechnology) for 5 min at 37°C. These were the antagonists shown to block the effect of the respective agonist on integrin activation (Fig. S1). Blood was then incubated for 5 min at 37°C with 320 µl of heparin plasma that had been collected previously from participants during sleep or wakefulness (as described in Participants and study procedure), before the staining with CMV A2-NLV/PE and mICAM-1 as described above. To test whether Gαs-coupled receptor agonists at low levels corresponding to sleep/wake differences can reverse the effect of sleep on integrin activation, a composite of agonists ((−)-epinephrine [10−10 M], (−)-norepinephrine [0.5 × 10−9 M], PGD2 [30 pg/ml], and PGE2 [30 pg/ml]) was added to the whole blood collected from the CMV seropositive donor and incubated together with plasma derived from sleeping participants for 5 min at 37°C before the staining with CMV A2-NLV/PE and mICAM-1. We used a composite of the Gαs-coupled receptor antagonists and agonists for these plasma experiments in order to achieve conditions most comparable to those naturally present in the blood during sleep or wakefulness (where several Gαs-coupled receptor ligands are concomitantly suppressed or increased, respectively). We did not include adenosine or adenosine receptor blockers in these experiments because of the extremely short half-life (<1 s) of (endogenous or exogenous) adenosine in whole blood (Ramakers et al., 2008).
Statistical analysis
Data are presented as means ± SEM. For in vitro data, fitted standard curves were calculated by nonlinear regression using SigmaPlot and analyzed using paired t tests. Statistical analyses for the in vivo sleep experiments were generally based on repeated-measures ANOVA with subsequent post hoc contrasts. ANOVA factors were sleep/wake, representing the two experimental conditions; period (night/day); and time, reflecting respective single time points of the night (10 pm to 6 am) and the day (10 am to 6 pm). The differences in baseline measures (at 10 pm) between the sleep and wake conditions were used as covariates to correct for day-to-day variations in immune parameters. Degrees of freedom were corrected using the Greenhouse–Geisser procedure. To identify significant rhythms, Cosinor analysis was performed for the wake and sleep condition with Chronolab using a period of 24 h (Mojón et al., 1992).
Online supplemental material
Fig. S1 demonstrates that the inhibitory effect of epinephrine, norepinephrine, PGE2, and PGD2 on integrin activation is abolished by Gαs-coupled receptor antagonists. Fig. S2 shows the effect of epinephrine, norepinephrine, PGE2, and PGD2 on integrin activation in CMV-specific CD8+ T cell subpopulations.
Supplementary Material
Acknowledgments
We thank S. Stevanović for providing synthetic peptides (Department of Immunology, Institute for Cell Biology, University of Tübingen, Tübingen, Germany), S. Heidu and E.-M. Schmidt for excellent technical assistance and advice, James O. Chinnis, Jr., for helpful comments on the manuscript, as well as all the participants of the current study.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 654 to T. Lange and H.-G. Rammensee; SFB 685 to C. Gouttefangeas and H.-G. Rammensee), by grants from the German Federal Ministry of Education and Research to the German Center for Diabetes Research (01GI0925 to S. Dimitrov and J. Born), and by the European Research Council AdG 339842, MUTAEDITING (to H.-G. Rammensee).
The authors declare no competing financial interests.
Author contributions: S. Dimitrov, T. Lange, H.-G. Rammensee, J. Born, and L. Besedovsky conceived the study and designed the experiments with contributions from all authors; S. Dimitrov, C. Gouttefangeas, M. Szczepanski, J. Lehnnolz, S. Soekadar, and L. Besedovsky conducted the experiments; S. Dimitrov performed the data analysis and prepared the figures; A.T.R. Jensen provided critical reagents; S. Dimitrov and L. Besedovsky wrote the manuscript with contributions from all authors.
References
- Abram C.L., and Lowell C.A.. 2009. The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 27:339–362. 10.1146/annurev.immunol.021908.132554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerstedt T. 2003. Shift work and disturbed sleep/wakefulness. Occup. Med. (Lond.). 53:89–94. 10.1093/occmed/kqg046 [DOI] [PubMed] [Google Scholar]
- Appay V., Dunbar P.R., Callan M., Klenerman P., Gillespie G.M., Papagno L., Ogg G.S., King A., Lechner F., Spina C.A., et al. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379–385. 10.1038/nm0402-379 [DOI] [PubMed] [Google Scholar]
- Appay V., van Lier R.A., Sallusto F., and Roederer M.. 2008. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 73:975–983. 10.1002/cyto.a.20643 [DOI] [PubMed] [Google Scholar]
- Benedict C., Brytting M., Markström A., Broman J.E., and Schiöth H.B.. 2012. Acute sleep deprivation has no lasting effects on the human antibody titer response following a novel influenza A H1N1 virus vaccination. BMC Immunol. 13:1 10.1186/1471-2172-13-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson A., Joergensen L., Barbati Z.R., Craig A., Hviid L., and Jensen A.T.. 2013. Transfected HEK293 cells expressing functional recombinant intercellular adhesion molecule 1 (ICAM-1)--a receptor associated with severe Plasmodium falciparum malaria. PLoS One. 8:e69999 10.1371/journal.pone.0069999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudvik K.W., and Taskén K.. 2012. Modulation of T cell immune functions by the prostaglandin E(2) - cAMP pathway in chronic inflammatory states. Br. J. Pharmacol. 166:411–419. 10.1111/j.1476-5381.2011.01800.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagoya de Sánchez V., Hernández-Muñoz R., Díaz-Muñoz M., Villalobos R., Glender W., Vidrio S., Suárez J., and Yañez L.. 1983. Circadian variations of adenosine level in blood and liver and its possible physiological significance. Life Sci. 33:1057–1064. 10.1016/0024-3205(83)90661-6 [DOI] [PubMed] [Google Scholar]
- Chigaev A., Waller A., Amit O., and Sklar L.A.. 2008. Galphas-coupled receptor signaling actively down-regulates alpha4beta1-integrin affinity: a possible mechanism for cell de-adhesion. BMC Immunol. 9:26 10.1186/1471-2172-9-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigaev A., Smagley Y., and Sklar L.A.. 2011a Nitric oxide/cGMP pathway signaling actively down-regulates α4β1-integrin affinity: an unexpected mechanism for inducing cell de-adhesion. BMC Immunol. 12:28 10.1186/1471-2172-12-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigaev A., Smagley Y., Zhang Y., Waller A., Haynes M.K., Amit O., Wang W., Larson R.S., and Sklar L.A.. 2011b Real-time analysis of the inside-out regulation of lymphocyte function-associated antigen-1 revealed similarities to and differences from very late antigen-4. J. Biol. Chem. 286:20375–20386. 10.1074/jbc.M110.206185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigaev A., Smagley Y., and Sklar L.A.. 2014. Carbon monoxide down-regulates α4β1 integrin-specific ligand binding and cell adhesion: a possible mechanism for cell mobilization. BMC Immunol. 15:52 10.1186/s12865-014-0052-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S.K., Ang J.E., Revell V.L., Holmes B., Mann A., Robertson F.P., Cui N., Middleton B., Ackermann K., Kayser M., et al. 2014. Effect of sleep deprivation on the human metabolome. Proc. Natl. Acad. Sci. USA. 111:10761–10766. 10.1073/pnas.1402663111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S., Benedict C., Heutling D., Westermann J., Born J., and Lange T.. 2009. Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood. 113:5134–5143. 10.1182/blood-2008-11-190769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S., Lange T., and Born J.. 2010. Selective mobilization of cytotoxic leukocytes by epinephrine. J. Immunol. 184:503–511. 10.4049/jimmunol.0902189 [DOI] [PubMed] [Google Scholar]
- Dimitrov S., Besedovsky L., Born J., and Lange T.. 2015. Differential acute effects of sleep on spontaneous and stimulated production of tumor necrosis factor in men. Brain Behav. Immun. 47:201–210. 10.1016/j.bbi.2014.11.017 [DOI] [PubMed] [Google Scholar]
- Dimitrov S., Gouttefangeas C., Besedovsky L., Jensen A.T.R., Chandran P.A., Rusch E., Businger R., Schindler M., Lange T., Born J., and Rammensee H.G.. 2018. Activated integrins identify functional antigen-specific CD8+ T cells within minutes after antigen stimulation. Proc. Natl. Acad. Sci. USA. 115:E5536–E5545. 10.1073/pnas.1720714115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M.L., and Long E.O.. 2010. Cytotoxic immunological synapses. Immunol. Rev. 235:24–34. 10.1111/j.0105-2896.2010.00904.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M.L., and Springer T.A.. 1989. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 341:619–624. 10.1038/341619a0 [DOI] [PubMed] [Google Scholar]
- Evans R., Patzak I., Svensson L., De Filippo K., Jones K., McDowall A., and Hogg N.. 2009. Integrins in immunity. J. Cell Sci. 122:215–225. 10.1242/jcs.019117 [DOI] [PubMed] [Google Scholar]
- Feinsilver S.H., and Hernandez A.B.. 2017. Sleep in the Elderly: Unanswered Questions. Clin. Geriatr. Med. 33:579–596. 10.1016/j.cger.2017.06.009 [DOI] [PubMed] [Google Scholar]
- Fooksman D.R., Vardhana S., Vasiliver-Shamis G., Liese J., Blair D.A., Waite J., Sacristán C., Victora G.D., Zanin-Zhorov A., and Dustin M.L.. 2010. Functional anatomy of T cell activation and synapse formation. Annu. Rev. Immunol. 28:79–105. 10.1146/annurev-immunol-030409-101308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack M., Lee E., Cohen D.A., and Mullington J.M.. 2009. Activation of the prostaglandin system in response to sleep loss in healthy humans: potential mediator of increased spontaneous pain. Pain. 145:136–141. 10.1016/j.pain.2009.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadrup S.R., Maurer D., Laske K., Frøsig T.M., Andersen S.R., Britten C.M., van der Burg S.H., Walter S., and Gouttefangeas C.. 2015. Cryopreservation of MHC multimers: Recommendations for quality assurance in detection of antigen specific T cells. Cytometry A. 87:37–48. 10.1002/cyto.a.22575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C., Leitz M.R., Oberdorf-Maass S., Lohse M.J., and Klotz K.N.. 2004. Comparative pharmacology of human beta-adrenergic receptor subtypes--characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch. Pharmacol. 369:151–159. 10.1007/s00210-003-0860-y [DOI] [PubMed] [Google Scholar]
- Hoskin D.W., Mader J.S., Furlong S.J., Conrad D.M., and Blay J.. 2008. Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells (Review). Int. J. Oncol. 32:527–535 (Review). [PubMed] [Google Scholar]
- Irwin M., Thompson J., Miller C., Gillin J.C., and Ziegler M.. 1999. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J. Clin. Endocrinol. Metab. 84:1979–1985. 10.1210/jcem.84.6.5788 [DOI] [PubMed] [Google Scholar]
- Kalinski P. 2012. Regulation of immune responses by prostaglandin E2. J. Immunol. 188:21–28. 10.4049/jimmunol.1101029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamperis K., Hansen M.N., Hagstroem S., Hvistendahl G., Djurhuus J.C., and Rittig S.. 2004. The circadian rhythm of urine production, and urinary vasopressin and prostaglandin E2 excretion in healthy children. J. Urol. 171:2571–2575. 10.1097/01.ju.0000110421.71910.c0 [DOI] [PubMed] [Google Scholar]
- Kilunga Kubata B., Eguchi N., Urade Y., Yamashita K., Mitamura T., Tai K., Hayaishi O., and Horii T.. 1998. Plasmodium falciparum produces prostaglandins that are pyrogenic, somnogenic, and immunosuppressive substances in humans. J. Exp. Med. 188:1197–1202. 10.1084/jem.188.6.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.J., Bridle B.W., Ghia J.E., Wang H., Syed S.N., Manocha M.M., Rengasamy P., Shajib M.S., Wan Y., Hedlund P.B., and Khan W.I.. 2013. Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J. Immunol. 190:4795–4804. 10.4049/jimmunol.1201887 [DOI] [PubMed] [Google Scholar]
- Kmiecik T., Otocka-Kmiecik A., Górska-Ciebiada M., and Ciebiada M.. 2012. T lymphocytes as a target of histamine action. Arch. Med. Sci. 8:154–161. 10.5114/aoms.2012.27295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange T., Perras B., Fehm H.L., and Born J.. 2003. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom. Med. 65:831–835. 10.1097/01.PSY.0000091382.61178.F1 [DOI] [PubMed] [Google Scholar]
- Lange T., Dimitrov S., Bollinger T., Diekelmann S., and Born J.. 2011. Sleep after vaccination boosts immunological memory. J. Immunol. 187:283–290. 10.4049/jimmunol.1100015 [DOI] [PubMed] [Google Scholar]
- Leone R.D., Horton M.R., and Powell J.D.. 2015a Something in the air: hyperoxic conditioning of the tumor microenvironment for enhanced immunotherapy. Cancer Cell. 27:435–436. 10.1016/j.ccell.2015.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone R.D., Lo Y.C., and Powell J.D.. 2015b A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Comput. Struct. Biotechnol. J. 13:265–272. 10.1016/j.csbj.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-Ponte M., Ahern G.P., and O’Connell P.J.. 2007. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood. 109:3139–3146. 10.1182/blood-2006-10-052787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K., Laudanna C., Cybulsky M.I., and Nourshargh S.. 2007. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7:678–689. 10.1038/nri2156 [DOI] [PubMed] [Google Scholar]
- Lokshin A., Raskovalova T., Huang X., Zacharia L.C., Jackson E.K., and Gorelik E.. 2006. Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells. Cancer Res. 66:7758–7765. 10.1158/0008-5472.CAN-06-0478 [DOI] [PubMed] [Google Scholar]
- Long E.O. 2011. ICAM-1: getting a grip on leukocyte adhesion. J. Immunol. 186:5021–5023. 10.4049/jimmunol.1100646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina A.B., Lechuga D.A., Escandón O.S., and Moctezuma J.V.. 2014. Update of sleep alterations in depression. Sleep Sci. 7:165–169. 10.1016/j.slsci.2014.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojón A., Fernández J.R., and Hermida R.C.. 1992. Chronolab: an interactive software package for chronobiologic time series analysis written for the Macintosh computer. Chronobiol. Int. 9:403–412. 10.3109/07420529209064552 [DOI] [PubMed] [Google Scholar]
- Ohta A. 2016. A Metabolic Immune Checkpoint: Adenosine in Tumor Microenvironment. Front. Immunol. 7:109 10.3389/fimmu.2016.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers B.P., Pickkers P., Deussen A., Rongen G.A., van den Broek P., van der Hoeven J.G., Smits P., and Riksen N.P.. 2008. Measurement of the endogenous adenosine concentration in humans in vivo: methodological considerations. Curr. Drug Metab. 9:679–685. 10.2174/138920008786049249 [DOI] [PubMed] [Google Scholar]
- Ricon I., Hanalis-Miller T., Haldar R., Jacoby R., and Ben-Eliyahu S.. 2019. Perioperative biobehavioral interventions to prevent cancer recurrence through combined inhibition of beta-adrenergic and cyclooxygenase 2 signaling. Cancer. 125:45–56. 10.1002/cncr.31594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T. 2007. Insomnia: definition, prevalence, etiology, and consequences. J. Clin. Sleep Med. 3(5, Suppl):S7–S10. [PMC free article] [PubMed] [Google Scholar]
- Saha B., Mondal A.C., Basu S., and Dasgupta P.S.. 2001a Circulating dopamine level, in lung carcinoma patients, inhibits proliferation and cytotoxicity of CD4+ and CD8+ T cells by D1 dopamine receptors: an in vitro analysis. Int. Immunopharmacol. 1:1363–1374. 10.1016/S1567-5769(01)00068-6 [DOI] [PubMed] [Google Scholar]
- Saha B., Mondal A.C., Majumder J., Basu S., and Dasgupta P.S.. 2001b Physiological concentrations of dopamine inhibit the proliferation and cytotoxicity of human CD4+ and CD8+ T cells in vitro: a receptor-mediated mechanism. Neuroimmunomodulation. 9:23–33. 10.1159/000049004 [DOI] [PubMed] [Google Scholar]
- Scher J.U., and Pillinger M.H.. 2009. The anti-inflammatory effects of prostaglandins. J. Investig. Med. 57:703–708. 10.2310/JIM.0b013e31819aaa76 [DOI] [PubMed] [Google Scholar]
- Scholer A., Hugues S., Boissonnas A., Fetler L., and Amigorena S.. 2008. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity. 28:258–270. 10.1016/j.immuni.2007.12.016 [DOI] [PubMed] [Google Scholar]
- Sloan E.K., Priceman S.J., Cox B.F., Yu S., Pimentel M.A., Tangkanangnukul V., Arevalo J.M., Morizono K., Karanikolas B.D., Wu L., et al. 2010. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 70:7042–7052. 10.1158/0008-5472.CAN-10-0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle M., Neumann R.P., Moritz F., Krause A., Buttgereit F., and Baerwald C.G.. 2005. Beta2-adrenergic receptors mediate the differential effects of catecholamines on cytokine production of PBMC. J. Interferon Cytokine Res. 25:384–394. 10.1089/jir.2005.25.384 [DOI] [PubMed] [Google Scholar]
- Yan Y., Jiang W., Liu L., Wang X., Ding C., Tian Z., and Zhou R.. 2015. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 160:62–73. 10.1016/j.cell.2014.11.047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.