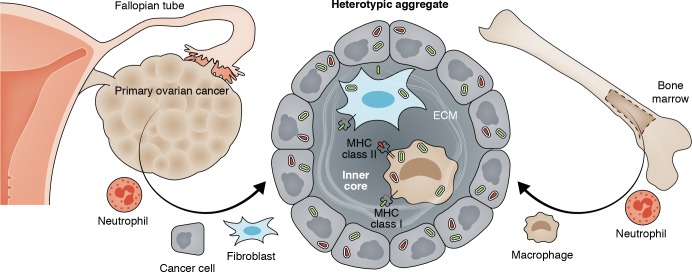
Fibroblasts form heterotypic aggregates with disseminating ovarian cancer cells, facilitating peritoneal metastasis.
Abstract
In this issue of JEM, Gao et al. (https://doi.org/10.1084/jem.20180765) demystify the exceptional metastatic success of ovarian cancer, the most lethal female malignancy: fibroblasts form heterotypic aggregates with disseminating cancer cells, thereby providing them with reciprocal signaling and matrix for adherence.
Metastatic growth is a pivotal, often lethal step in cancer progression in which cancer cells leave the primary tumor to travel to distal sites where they form new (i.e., secondary) tumors (Langley and Fidler, 2011). While the shedding of cancer cells from primary tumors is common, the formation of secondary tumors at distant sites is, fortuitously for the patient, usually highly inefficient. Only <0.01% of circulating cancer cells successfully establish secondary growth (Fidler, 1970). This lack of success is likely due to anoikis (literally meaning “without home”) that occurs when cancer cells leave the tissue microenvironment, also called tumor stroma (literally meaning “bed”), provided by the primary tumor. Loss of stroma means loss of attachment of the cancer cells to the extracellular matrix (ECM) in the stromal tumor microenvironment.

Insights from Hans Schreiber.
Loss of attachment deprives the cancer cells of essential growth and survival signals. Therefore, isolated cancer cells are often much less tumorigenic than those embedded in stroma, and immune destruction of cancer stroma substantially reduces the success of cancer cells to implant and cause tumors (Singh et al., 1992).
As a prominent exception to this rule, high-grade serous ovarian cancer (HGSOC) has found an efficient way to overcome this fundamental hurdle. For reasons that had remained mostly obscure, cancer cells leaving the primary ovarian tumor establish metastases highly successfully in the abdominal or pleural cavities. This property makes HGSOC the most lethal female malignancy. In this issue of JEM, Gao et al. report the reasons for the metastatic success of HGSOC cells: the formation of heterotypic spheroids of HGSOC cells with fibroblasts. This discovery was made possible by using intrapatient-paired tumor sample sets, three for each patient: primary tumor, ascites, and metastasis. These precious resources have been prominently missing from most previous studies (Bowtell et al., 2015), and their availability allowed Gao et al. (2019) to overcome the problems with interpatient variability. Thus, Gao et al. (2019) compared matched primary, ascites, and metastatic cancer samples from individual HGSOC patients with the three types of matched samples from patients with low-grade serous ovarian cancer (LGSOC), a cancer that also produces ascitic cancer cells but has a more favorable prognosis. The authors observed that HGSOC ascites contained large cellular aggregates while LGSOC ascitic cells were finely dispersed as single cells. The largest qualitative difference was the remarkably increased percentage of fibroblasts in HGSOC ascites. Histologically, the HGSOC aggregates showed a prominent inner core of fibroblasts that was surrounded by the cancer cells, and Gao et al. (2019) termed these heterotypic aggregates “metastatic units.” Metastatic units adhered more avidly to fibronectin and mesothelium-covered surfaces and were significantly more invasive in vitro. Also, ascitic tumor cells from a HGSOC patient established metastases in mice more efficiently than cancer cells derived from the primary tumor or a metastasis of the same HGSOC patient. No significant differences in these assays were found comparing cancer cells from the three types of matched samples from LGSOC patients.
Heterotypic aggregates are responsible for the unusual metastatic success of HGSOC, the most lethal female malignancy. When the tumor cells leave the primary ovarian cancer to enter the abdominal cavity, they do not travel alone. Instead, they rapidly form small aggregates by surrounding an inner tissue core of fibroblasts, macrophages, and ECM. This inner core provides the HGSOC cells with attachment and reciprocal signals to escape death by anoikis. The signals also help the aggregates attaching to mesothelium-covered surfaces and establish metastatic growth. Neutrophils are essential in the recruitment of progenitors of macrophages from the BM and in the recruitment of fibroblasts from local perivascular reservoirs. All HGSOCs harbor numerous patient-specific mutations that may be recognized by T cells as cross-presented antigens on fibroblasts and/or macrophages in tumor stroma. This would provide a truly cancer-specific, truly personalized approach to stromal targeting in cancer therapy.
HGSOC ascitic cancer cells specifically reduced expression of E-cadherin mRNA while up-regulating ITGA5 expression encoding integrin subunit α5. No integrin was dominantly up-regulated in LGSOC ascitic tumor cells. Deleting ITGA5 by CRISPR/Cas technology severely reduced adhesion and metastatic success of HGSOC cells. All evidence is consistent with the notion that integrin α5β3 provides ascitic HGSOC cells with the essential capability of attaching to an initial primitive fibronectin/collagen matrix for pro-survival signaling to escape death due to anoikis while traveling in the peritoneal cavity. Previous work already demonstrated that the loss of E-cadherin causes epithelial–mesenchymal transition (EMT) to allow the cancer cells to leave the primary tumor and metastasize by up-regulating integrin α5β1 (Sawada et al., 2008). This allowed the cancer cells to attach to the primitive fibronectin matrix to receive key mitogenic signals. Also, there is an inverse correlation between ITGA5 levels and ovarian cancer patient survival (Sawada et al., 2008).
Fibroblasts not only provide the primitive matrix for attachment of the HGSOC cells but are also an essential part of the bidirectional signaling loop: epidermal growth factor receptor (EGFR)–positive cancer cells release TGFβ1 that activates fibroblasts to release EGF and produce ECM components for the cancer cells to receive pro-survival signals, mobilize energy sources, and express ITGA5 needed for attachment (Curtis et al., 2018). However, the metastatic success of HGSOC probably also depends on the up-regulation of additional genes. Thus, >700 genes were overexpressed in HGSOC ascites cells when compared to primary as well as metastatic HGSOC cancer cells, and these genes were involved in multiple biological processes. By contrast, <20 genes were overexpressed in LGSOC ascites cells when compared to primary as well as metastatic LGSOC cancer cell samples.
The activation state of cancer-associated fibroblasts (CAFs) generally correlates with the aggressiveness of cancers (see references in Arina et al., 2016), and CAFs increase the proliferation, metastasis, and chemoresistance of ovarian cancer (Wang et al., 2016; Curtis et al., 2018). Interestingly, the other major component of cancer stroma, tumor-associated macrophages (TAMs), are also found in the center of the heterotypic spheroids in the ascites of HGSOC patients (Yin et al., 2016). TAMs also participate in the bidirectional EGF/EGFR signaling axis. Surprisingly, the sources of the major components of cancer stroma have only been conclusively identified rather recently through experiments using parabiotic and chimeric mice. TAMs come from the bone marrow (BM), whereas tumor endothelial cells and CAFs come from local sessile stem cell reservoirs (see references in Arina et al., 2016). These mesenchymal stem cell reservoirs are of perivascular origin and are found in every organ, even though fibroblasts obtained from different anatomical sites differ (see references in Arina et al., 2016). It remains unclear how fibroblasts exit the ovarian primary tumor as envisioned by Gao et al. (2019) and whether the fibroblasts also come from stem cell sources at other sites. The latter is consistent with the finding that intraperitoneal injection of spontaneous murine HGSOC cells induced such heterotypic spheroids.
Peyton Rous already found that the success of a tumor implant depends directly on whether it elicits a vascularizing stroma reaction (Rous, 1910). Thus, the spheroids must vascularize after adhering to mesothelial surfaces, and the initiating critical cell is likely the neutrophil. Neutrophils produce tissue inhibitor of metalloproteases (TIMP)–free matrix metalloproteinase-9 and neutrophil elastase to degrade SDF-1 that normally locks CXCR4-positive stromal mesenchymal, hematopoietic, and angiopoietic progenitors at their sites of origin (Petit et al., 2002). High neutrophil–lymphocyte ratios correlate with poorer survival in various, including ovarian, cancers. By contrast, elimination of neutrophils inhibits tumor growth (Pekarek et al., 1995) and macrophage recruitment into the peritoneal cavity (see references in Pekarek et al., 1995).
The realization that tumor-associated fibroblasts play a decisive role in the aggressiveness of ovarian cancer calls for a reevaluation of realistic possibilities to target the stroma of cancers specifically and effectively. The authors used the Abelson-kinase inhibitor imatinib to inhibit CAFs or liposome clodronate to destroy TAMs. However, both drugs are toxic and inhibited metastatic spread only transiently. Already two generations ago, outstanding immunologists showed that the most effective targets for cancer rejection were the so-called unique tumor-specific rejection antigens recognized by T cells. In 1995, these unique antigens were shown to be caused by nonsynonymous single nucleotide variants (nsSNVs) and shown to be effective targets for mutation-specific adoptive T cell transfer (Monach et al., 1995). These nsSNVs are now also simply referred to as mutant neoantigens, and it appears that these random, mutant, truly cancer-specific antigens represent the relevant targets of most successful human immunotherapies (see references in Deniger et al., 2018). HGSOC has an average of 46 nsSNVs; if only half of them were expressed at sufficient levels, the set of usually 12 different antigen-presenting HLA molecules could create >250 cancer-specific targets. At least some of these nsSNVs will be cross-presented by BM-derived as well as non–BM-derived stromal cells, both of which must be targeted to prevent cancer escape in experimental models (Zhang et al., 2007). HGSOC diagnosed with ascites is presently almost invariably lethal, but most of these patients can be effectively treated by chemotherapy, which results in a relapse-free interval often lasting >1 yr. Thus HGSOC is an ideal starting point for mutation-specific T cell therapy because this interval could be used to generate a set of autologous T cell receptors for specifically targeting the patient’s neoantigens. Indeed, recent results showed that autologous, truly cancer-specific T cells to mutant antigens could be induced in five of seven HGSOC patients and that responses are not limited by a relatively low mutational burden (Deniger et al., 2018). Such autologous TCRs transduced into autologous peripheral T cells and adoptively transferred into the patient during remission may well prevent relapse of HGSOC and would represent a truly personalized, truly cancer-specific therapy. The research by Gao et al. (2019) is an important guide to focus on those mutant neoantigens that are highly expressed to become effective cancer-specific targets, not only for the cancer cell but also for the tumor stroma.
Acknowledgments
H. Schreiber is supported by National Institutes of Health grants R01-CA22677 and R01-CA37156.
References
- Arina A., et al. 2016. Proc. Natl. Acad. Sci. USA. 113:7551–7556. 10.1073/pnas.1600363113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowtell D.D., et al. 2015. Nat. Rev. Cancer. 15:668–679. 10.1038/nrc4019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis M., et al. Cell Metab. 2018 doi: 10.1016/j.cmet.2018.08.007. [DOI] [Google Scholar]
- Deniger D.C., et al. 2018. Clin. Cancer Res. 24:5562–5573. 10.1158/1078-0432.CCR-18-0573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler I.J. 1970. J. Natl. Cancer Inst. 45:773–782. [PubMed] [Google Scholar]
- Gao Q., et al. J. Exp. Med. 2019 doi: 10.1084/jem.20180765. [DOI] [Google Scholar]
- Langley R.R., and Fidler I.J.. 2011. Int. J. Cancer. 128:2527–2535. 10.1002/ijc.26031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monach P.A., et al. 1995. Immunity. 2:45–59. 10.1016/1074-7613(95)90078-0 [DOI] [PubMed] [Google Scholar]
- Pekarek L.A., et al. 1995. J. Exp. Med. 181:435–440. 10.1084/jem.181.1.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit I., et al. 2002. Nat. Immunol. 3:687–694. 10.1038/ni813 [DOI] [PubMed] [Google Scholar]
- Rous P. 1910. J. Exp. Med. 12:344–366. 10.1084/jem.12.3.344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K., et al. 2008. Cancer Res. 68:2329–2339. 10.1158/0008-5472.CAN-07-5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., et al. 1992. J. Exp. Med. 175:139–146. 10.1084/jem.175.1.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., et al. 2016. Cell. 165:1092–1105. 10.1016/j.cell.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M., et al. 2016. J. Clin. Invest. 126:4157–4173. 10.1172/JCI87252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., et al. 2007. J. Exp. Med. 204:49–55. 10.1084/jem.20062056 [DOI] [PMC free article] [PubMed] [Google Scholar]