Bonello and Peifer summarize recent advances in our understanding of how the scaffolding protein Scribble regulates polarity, adhesion, proliferation, and neuronal function.
Abstract
Key events ranging from cell polarity to proliferation regulation to neuronal signaling rely on the assembly of multiprotein adhesion or signaling complexes at particular subcellular sites. Multidomain scaffolding proteins nucleate assembly and direct localization of these complexes, and the protein Scribble and its relatives in the LAP protein family provide a paradigm for this. Scribble was originally identified because of its role in apical–basal polarity and epithelial integrity in Drosophila melanogaster. It is now clear that Scribble acts to assemble and position diverse multiprotein complexes in processes ranging from planar polarity to adhesion to oriented cell division to synaptogenesis. Here, we explore what we have learned about the mechanisms of action of Scribble in the context of its multiple known interacting partners and discuss how this knowledge opens new questions about the full range of Scribble protein partners and their structural and signaling roles.
Although realtors selling houses and cell biologists differ in many ways, both share an obsession with “location, location, location.” The animal body contains a vast array of cell types with an equally diverse set of functions. Key to the functioning of each cell type is the ability to put the correct cellular machinery in the correct subcellular location. Whether it is an embryonic epithelial cell segregating apical and basolateral proteins, a neuron building synaptic connections, a T cell progenitor undergoing asymmetric division, or a cochlear hair cell orienting actin-based stereocilia, assembling and positioning complex multicellular machines at the right place is critical. Evolution selected multidomain scaffolding proteins on which to assemble these diverse machines. Scribble and its family members provide a paradigm for this, assembling distinct adhesive, structural, or signaling protein complexes across a number of biological contexts, ranging from the establishment of apical–basal polarity to the assembly of a neuronal synapse to the regulation of proliferation in a tissue context.
The protein interaction domains of Scribble assemble diverse multiprotein machines
Scribble acts as an adaptor protein by facilitating key molecular interactions at distinct subcellular localizations. It does so by virtue of its domain structure and spatially restricted localization pattern. Scribble belongs to the LRR and postsynaptic density-95/Disc-large/ZO-1 (PDZ; LAP) family of proteins (Fig. 1; Bilder et al., 2000a; Santoni et al., 2002), characterized by 16 N-terminal leucine-rich repeats (LRR), two LAP-specific domains, and four PDZ domains. Drosophila melanogaster and Caenorhabditis elegans have only a single well-characterized LAP family member (Drosophila LAP-1 is essentially uncharacterized), whereas mammals have four LAP family proteins: Scribble, Erbin, Lano, and Densin-180 (Santoni et al., 2002).
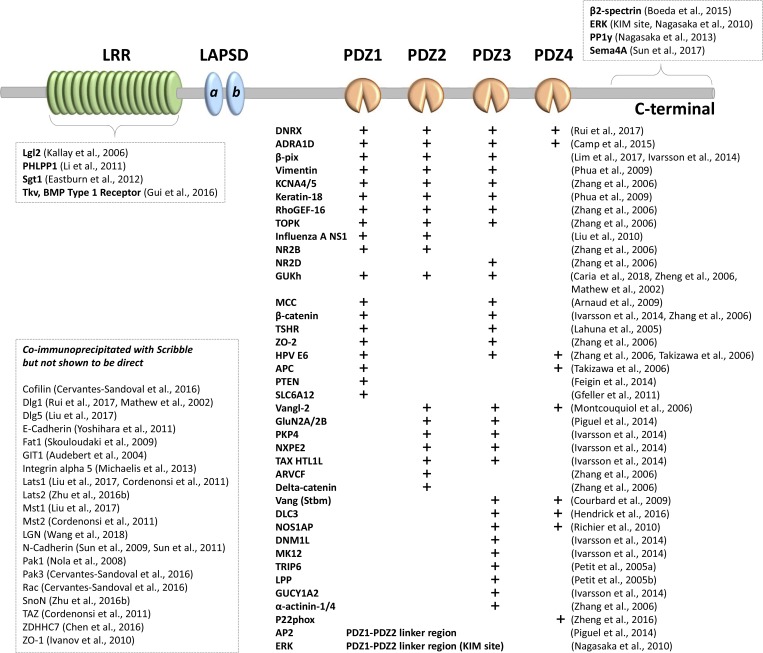
Figure 1.
Known Scribble-interacting proteins, mapped to the LRR, PDZ, or C-terminal region of Scribble. A plus sign (+) indicates a direct interaction with an individual PDZ domain, measured by peptide-phage display, yeast two-hybrid assay, binding assays using recombinantly expressed and purified GST-fusion proteins, or another biochemical strategy. No binding partners have been identified for the LAP-specific domains of Scribble. Proteins listed under coimmunoprecipitated with Scribble (inset box) have not been shown to directly interact with Scribble.
LRRs are a protein interaction domain found in diverse protein families with functions ranging from innate immunity to connecting neural circuitry. LRR repeats fold into an arc or horseshoe shape, providing both concave and convex surfaces for protein interaction (Enkhbayar et al., 2004). Key to Scribble function, LRRs are sufficient for cortical targeting of Scribble and its homologues in several biological contexts (Fig. 2), including localization to the plasma membrane of Drosophila neuroblasts (Albertson et al., 2004) or wing imaginal discs (Zeitler et al., 2004), the C. elegans embryonic epithelium (Legouis et al., 2003), or mammalian MDCK cells (Navarro et al., 2005). As is discussed below, in some biological contexts the LRR region is fully sufficient to rescue Scribble function. Surprisingly, however, relatively few known Scribble binding partners associate via the LRRs (Fig. 1)—the exceptions include Lgl, the Scribble partner in apical–basal polarity (Kallay et al., 2006).
Figure 2.
Contribution of Scribble domains to the subcellular localization and biological functions of Scribble. Gray bars indicate (1) regions sufficient for Scribble subcellular localization in different contexts (black text), (2) regions sufficient for particular biological functions (blue text), or (3) truncated versions that lack particular biological functions (red text). A broken line corresponds to a domain deletion. aC. elegans expresses LET-413, a member of the LAP protein family with only one PDZ domain. bIn addition to being restricted to the lateral membrane, Scribble colocalizes with β-catenin, indicating recruitment to adherens junctions. cA minimal Scribble construct containing the LRR domain and PDZ1 was required for effective membrane targeting. dData based on the circletail mouse mutant, a truncating mutation in Scribble that leads to the loss of PDZ3 and PDZ4.
PDZ domains are a distinct protein interaction domain, also found in diverse proteins, including the polarity partner for Scribble, Dlg. The mode of PDZ domain interaction with other proteins is well characterized, usually involving PDZ binding to the C terminus. Different PDZ domains have distinct preferences for the last four amino acids (Ernst et al., 2014). The ligand binding specificity of the individual Scribble PDZ domains has been characterized using peptides, providing clues as to possible partners (Zhang et al., 2006; Cai et al., 2014; Ivarsson et al., 2014). Each PDZ has its own binding preference, and binding may be regulated by motif phosphorylation (Sundell et al., 2018). Numerous partners bind Scribble PDZ domains (Fig. 1). The PDZ domains have different specificities; e.g., PDZ1, PDZ2, and PDZ3 show differential affinities for β-PIX (Lim et al., 2017), whereas Scribble PDZ1 is the major interactor with the C terminus of Guk-holder (GUKh; Caria et al., 2018). Nitric oxide synthase adaptor protein and NADPH oxidase, on the other hand, bind directly to the fourth Scribble PDZ domain (Richier et al., 2010; Zheng et al., 2016), a domain not required for binding either β-PIX or GUKh.
Scribble PDZ domains serve as key integration sites for molecular networks in both neurons and epithelial cells, playing a part in organizing multiprotein complexes. Perhaps the best characterized example is that at the synaptic terminal, where regulation of synaptic vesicle clustering and release depends on the coordinated activities of a number of proteins regulating F-actin organization (Fig. 3 A; Lin et al., 2016). Scribble is an integral component of synaptic protein complexes and can coimmunoprecipitate with β-catenin, the ADP-ribosylation factor-1 GTPase-activating protein (GAP) GIT1, the Rac/Cdc42 guanine nucleotide exchange factor β-PIX, and transmembrane receptor Neurexin (Audebert et al., 2004; Sun et al., 2009; Sun and Bamji, 2011; Rui et al., 2017). Scribble interacts with β-PIX and Neurexin via PDZ interactions. Together, this complex is thought to stimulate Rac1 activity, leading to localized actin polymerization at the presynaptic terminal (Rui et al., 2017). At the synapse, Scribble is part of another interactome involved in trafficking and recycling of NMDA receptors to the membrane. Scribble directly binds NMDA receptor subunits GluN2A and GluN2B through PDZ2 and PDZ3 interactions and can also bind the clathrin-mediated endocytosis regulator AP2, which regulates NMDA receptor internalization (Piguel et al., 2014).
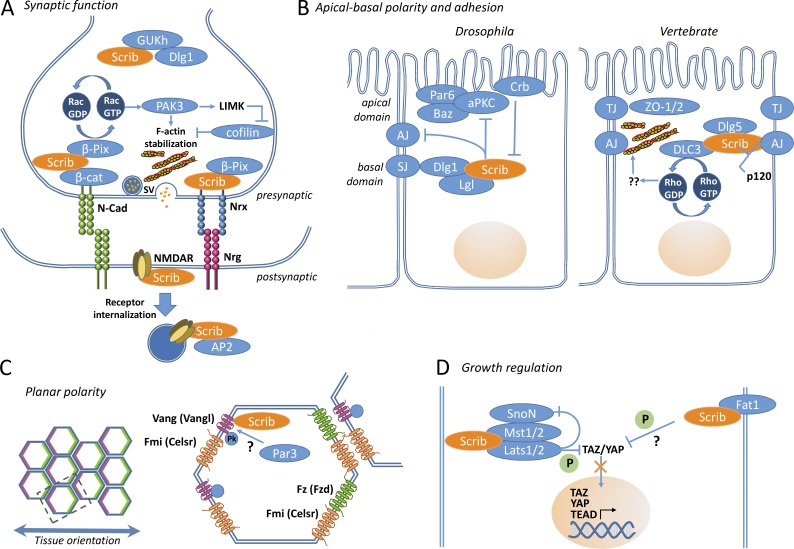
Figure 3.
Models for the roles of Scribble in organizing molecular interactomes involved in synaptogenesis, epithelial polarity, and adhesion, and growth regulation. (A) Cell adhesion molecules (Nrx/Nrg and N-Cad/β-Cat) are required for maintaining synaptic architecture and regulating neurotransmitter release. Both types of adhesion complexes interact with Scribble and β-PIX, facilitating localized Rac activity and F-actin polymerization. Polymerization of presynaptic actin is required for synaptic vesicle clustering and release from the active zone. Scribble also regulates trafficking of the NMDA receptor. (B) In the mature Drosophila ectoderm and imaginal disc epithelia Scribble localizes with Dlg and Lgl to the basolateral septate junctions. Scribble acts to antagonize aPKC and components of the adherens junction (AJ), excluding them from the basolateral domain. In turn, Scribble is antagonized by the apical polarity protein Crb. In cultured mammalian epithelial cells, Scribble regulates AJ and tight junction (TJ) organization, by stabilizing Ecad at the adherens junction via effects on p120 and via myosin stabilization mediated by DLC3. (C) Planar polarity, polarity across an epithelial sheet, is required for a number of processes, from sensory hair orientation to limb bud elongation. Core PCP proteins are asymmetrically localized and are conserved from Drosophila to mammals (vertebrate gene names in brackets). Vang and Fz localize to opposing sides of the cell, respectively, and form heterodimers between cells (Yang and Mlodzik, 2015). Scribble can physically interact with Vang as well as its vertebrate equivalent. Par3 is suggested to localize Vangl through an unknown mechanism. (D) Scribble interacts with several components of the Hippo signaling pathway, including the apical scaffold (Fat1) and the core kinase cassette (Mst1/2, Lats1/2). As a result, Scribble acts to restrain YAP and TAZ activity, transcriptional effectors of the Hippo cascade that control gene expression programs required for cell stemness, proliferation, survival, and EMT.
Scribble is also an important part of the junctional network that maintains epithelial apical–basal polarity and integrity. Many Scribble binding partners localizing to adherens or tight junctions have been identified, some by coimmunoprecipitation in a complex with the intact Scribble protein (e.g., ZO-1 or DLG5; Ivanov et al., 2010; Liu et al., 2017), and others for which the interaction is direct and maps to the PDZ domains (e.g., β-catenin, the Rho GAP DLC3; Ivarsson et al., 2014; Hendrick et al., 2016). However, it is not clear whether all these partners interact with Scribble simultaneously or even in the same cell type. Importantly, the differential contribution of the PDZ domains to engagement of Scribble with other proteins translates into significant functional consequences in vivo. For example, in Drosophila, scribble mutants lacking all four PDZ domains have severely disrupted septate junction formation, whereas mutants lacking only PDZ3 and PDZ4 domains display normal adherens and septate junctions (Zeitler et al., 2004; Fig. 2).
Scribble is a key player in the maintenance of apical–basal polarity
To appreciate the diverse roles of Scribble, we need to go back in time. The proper positioning of molecular machines underlies the global process of establishing cell polarity, epithelial apical–basal polarity being a cardinal example (reviewed in Campanale et al., 2017). Epithelia serve as barriers between body compartments, and thus must position different proteins on their apical and basolateral surfaces. In the 1990s, the molecular basis of apical–basal polarity remained largely an outline. We knew that cadherin-based adherens junctions were positioned at the apical end of the lateral cell interface, forming the boundary between apical and basolateral domains, and the roles for a set of apical determinants such as Par3/Bazooka and Crumbs were coming into focus. Proper apical–basal polarity is important for the diffusion barrier between the two surfaces of the epithelial sheet. This barrier is mediated by mammalian tight junctions, just apical to adherens junctions, or insect septate junctions, just basal to adherens junctions.
At this point, a bold but simple approach was used to identify molecular components essential for apical–basal polarity—isolate Drosophila mutants affecting epithelial integrity (Bilder and Perrimon, 2000). Fruit fly mothers endow their eggs with substantial stores of key cell biological players, often sufficient for embryonic development, allowing for a screen for maternal effect mutations disrupting epithelial morphogenesis, affecting cell adhesion, shape, and polarity. Using the simple approach pioneered by Nüsslein-Volhard and Wieschaus (1980), the cuticle secreted by the embryonic epidermis was assessed for defects in epithelial integrity. This revealed that “embryos that are maternally and zygotically mutant for scribble produce a corrugated cuticular surface that is riddled with holes.… [H]ence the name scribble.” The gene responsible encoded a probable scaffolding protein with an N-terminal LRR and a series of four PDZ domains. Consistent with a role in epithelial polarity, Scribble has a dynamic localization in embryos, evolving from an early apicolateral localization in the ectoderm to a position just basal to the cell–cell adherens junction, colocalizing with Coracle, a marker of fly septate junctions. Loss of Scribble leads to striking defects in ectodermal and epidermal apical–basal polarity, with apical and adherens junction proteins displaced basally (Bilder and Perrimon, 2000).
An examination of other fly epithelia revealed two other mutants sharing with scribble defects in polarity of epithelial follicle cells of ovaries—discs large (Dlg) and lethal giant larvae (Lgl; Bilder et al., 2000b). Dlg is also a multidomain scaffolding protein, with membrane-associated guanylate kinase (MAGUK) and PDZ domains, and Lgl contains a well-known protein interaction domain, WD40 repeats (Humbert et al., 2003). Loss of any of the three proteins disrupts epithelial architecture of follicle cells, and genetic interactions support similar roles in the embryonic epidermis. An even more intimate connection was suggested by the fact that correct localization of each protein to the lateral membrane requires function of the other proteins. Scribble, Dlg, and Lgl share another function: growth regulation. Dlg and Lgl were first identified because their loss results in dramatic overgrowth of imaginal discs (Gateff and Schneiderman, 1974; Woods et al., 1996), precursors of the adult epidermis. Together, this seminal work placed Scribble squarely in the middle of a protein module regulating apical–basal polarity in Drosophila, acting along the basolateral membrane to restrict localization of apical and junctional proteins.
Parallel work in the nematode C. elegans also revealed essential roles for Scribble in polarity maintenance (Legouis et al., 2000; Köppen et al., 2001; McMahon et al., 2001). Like Scribble, its nematode relative LET-413 localizes basolaterally. LET-413 mediates apical restriction of both the cadherin–catenin complex and Par3—in its absence, they spread all along the basolateral domain, and electron-dense adherens junctions likewise are not focused apically. However, consistent with a role in polarity maintenance rather than establishment, early localization of some apical proteins is normal in LET-413 mutants (Bossinger et al., 2004), and the morphogenesis defects of loss of LET-413 are not as severe as those of loss of worm E-cadherin (Ecad). In fact, some effects of LET-413 knockdown are alleviated by reducing cadherin function (Segbert et al., 2004). As in Drosophila, C. elegans Dlg-1 mutants share many of the same defects, and LET-413 is required for Dlg-1 localization (Bossinger et al., 2001; Köppen et al., 2001; McMahon et al., 2001; Mathew et al., 2002; Lockwood et al., 2008; Caria et al., 2018).
With regard to its role in apical–basal polarity, Scribble is often described as part of the Scribble-module, together with Dlg and Lgl. Despite the strong genetic interaction between these proteins in Drosophila, as well as a mutual dependence for localization to the septate junctions (Bilder et al., 2000b), there is no evidence for direct molecular interaction of Scribble with Dlg, whereas the evidence for direct interaction with Lgl is modest, and Scribble and Lgl often only partially overlap in localization (Bilder et al., 2000b; Kallay et al., 2006). In the case of Drosophila Dlg, the adapter protein GUKh is necessary to physically couple Scribble to Dlg (Mathew et al., 2002; Caria et al., 2018). One important task is to continue to sort out how these three proteins work together and which functions they carry out separately.
Placing Scribble in the polarity network and defining its mechanisms of action
How does the Scribble module restrict apical proteins and adherens junctions from ectopic basolateral sites? Proteins playing analogous roles in the apical domain, restricting localization of basolateral proteins, have been identified in flies (Fig. 3 B). These include the Crumbs/Stardust complex (Tepass et al., 1990; Tepass and Knust, 1993) and Bazooka (Par3), with its adherens junction and apical partners, atypical PKC (aPKC) and Par6 (Müller and Wieschaus, 1996). Intriguingly, both of these polarity modules also contain PDZ domain scaffolding proteins. The mature epidermis of Drosophila embryos and the imaginal disc epithelium served as important models for mapping regulatory interactions between the Scribble module and the Bazooka and Crumbs–containing apical polarity complexes, defining how these three protein modules cooperate and compete in maintaining apical–basal polarity. The basolateral Scribble module and the two apical complexes act in mutual antagonism, restricting protein localization of the other modules (Fig. 3 B). The Scribble module antagonizes the apical-polarizing activity of Bazooka, whereas the Crumbs complex antagonizes Scribble activity to maintain apical membrane identity (Bilder et al., 2003; Tanentzapf and Tepass, 2003). A more recently identified basolateral polarity module, the Yurt/Coracle group, also acts somewhat redundantly to maintain polarity (Laprise et al., 2006). This leads to a polarity system that is exceptionally robust: polarity can be partially reestablished in mutants that lose polarity early, owing to partial redundancy of the apical modules, whereas reducing function of the basolateral module partially compensates for loss of one of the apical modules. Together, these data provided important insights into the network of proteins maintaining mature epithelial polarity. However, our understanding of how these pathways are integrated at the molecular level remains incomplete, although phosphorylation and subsequent molecular events defining the reciprocal negative regulation between Lgl and aPKC are well documented (e.g., Betschinger et al., 2003, 2005; Hutterer et al., 2004).
One insight into the molecular mechanisms by which Scribble regulates polarity came from analysis of the functions of its different protein domains in Drosophila epithelia (Fig. 2; Zeitler et al., 2004). Strikingly, deleting the LRR or a very informative missense mutant in that domain eliminated Scribble function in both epithelial polarity and growth regulation, and also led to loss of membrane localization of the mutant protein. In contrast, deleting the PDZ domains was substantially less debilitating: in both embryos and imaginal discs, the mutant protein localized to the cortex and apical–basal polarity was largely unaffected, although ΔPDZ mutants did fail to assemble septate junctions (Fig. 2). The PDZ domains are required for full function in imaginal growth regulation and barrier function; in this role, only PDZ1 and PDZ2 are required, and they are also required to localize Scribble to septate junctions (Fig. 2). Strikingly similar results were seen with C. elegans LET-413, where the LRR domain is necessary for basolateral targeting, and sufficient, when the PDZ domains are deleted, for embryonic development (Fig. 2; Legouis et al., 2003). These data provide a foundation for future work identifying how LRR and PDZ protein partners contribute to these functions.
More recently an interesting premise has been explored: that Scribble mediates polarity via polarized trafficking of apical proteins, influencing their endocytic itineraries. This was prompted by the observation that null mutations in genes encoding key endocytic regulators, including clathrin heavy chain and dynamin, mimic the characteristic overgrown, disorganized, and multilayered eye disc phenotype of scribble mutants (Windler and Bilder, 2010). Although early endosomal internalization of cargo remains intact in Scribble module mutant cells, transport from endosomes to Golgi through the retromer pathway is disrupted (de Vreede et al., 2014). As a consequence, Crumbs, which is normally recycled to the plasma membrane via its interaction with the retromer complex, becomes trapped in subcortical compartments. This affects both polarity and growth regulation. Intriguingly, Bazooka, Par-6, and aPKC remain at the plasma membrane in Scribble module mutant cells (de Vreede et al., 2014). Work on LET-413 also implicated it in protein trafficking but suggested a somewhat different role, in which it acts as a Rab5 effector to regulate activation of Rab10 and promote endocytic recycling. Although the role of Rab10 in trafficking remains to be fully defined, like Rab10, LET-413 shows specificity for clathrin-independent cargo uptake from the basolateral plasma membrane (Liu et al., 2018). Further defining the role of Scribble together with or alongside the retromer complex will be informative.
The Drosophila renal tubules provide an alternative model for examining polarization during organogenesis (Denholm, 2013). Stratification of cell polarity and junctional proteins is similar to that in the epidermis. Interestingly, although Scribble and Bazooka are both essential for establishing cell polarity in this context, tubule cells mutant for Crumbs are unaffected at this stage. Instead, Crumbs becomes essential at subsequent stages for polarity stabilization during morphogenetic movements. The endocytic trafficking of Crumbs plays a role, as was observed in imaginal discs (Campbell et al., 2009). In a final Drosophila tissue, the adult midgut, although Scribble and Dlg localize to the septate junctions, they are not required for apical basal polarity or for septate junction maintenance (Chen et al., 2018).
Studies into the mechanisms by which Scribble establishes polarity have typically focused on direct actions at cell junctions or on regulating junctional protein trafficking; however, there may also be important downstream consequences at the level of transcription. Transcriptome-wide analysis of wing imaginal discs null for scribble or dlg shed light onto signaling and epigenetic regulators altered in these contexts (Bunker et al., 2015). These include activation of the JAK-STAT pathway and of the bZIP transcription factor Atf3. Parallel work revealed that Atf3 activation occurs downstream of ectopic aPKC activation. Strikingly, depleting Atf3 alleviates the abnormal distribution of polarity proteins and restores normal epithelial architecture in dlg mutants (Donohoe et al., 2018). It is intriguing to note that overexpressing Atf3 is associated with trafficking defects that parallel those observed in dlg mutants (Donohoe et al., 2018). Because this study identified several target genes of Atf3 associated with cytoskeletal organization and dynamics, tracing these molecular connections in the context of trafficking may provide interesting leads into understanding Scribble function.
Mammalian Scribble has similar but more limited roles in epithelial polarity and integrity
Parallel work in mammalian cell culture provided important insight into how Scribble might integrate junctional and apical–basal polarity cues. In cultured mammalian cells and in the intestinal and cochlear epithelia in vivo, Scribble localizes to the basolateral membrane and appears to overlap adherens junctions, although its localization relative to tight junctions remains less clear (Métais et al., 2005; Navarro et al., 2005; Montcouquiol et al., 2006; Ivanov et al., 2010; Yoshihara et al., 2011; Chen et al., 2016). Junctional localization can be mediated by either the LRRs or the PDZ plus C terminus (Fig. 2; Navarro et al., 2005) and requires N-terminal palmitoylation (Chen et al., 2016). Scribble recruitment to adherens junctions is Ecad dependent in MDCK cells (Navarro et al., 2005) and depends on DLG5 in MCF-10A cells (Liu et al., 2017). Junctional localization of Scribble may play a tumor suppressor role, because in several models altered Scribble localization is associated with tumor initiation or progression (Feigin et al., 2014; Wan et al., 2018).
Tests of Scribble function in cultured cells were also informative. MDCK and MCF-10A cells are prominent epithelial cell models. In MDCK cells, the consequences of Scribble knockdown depend on the level of protein reduction. Moderate reduction reduces Ecad-dependent cell adhesion and delays both the mesenchymal-to-epithelial transition and tight junction assembly. However, cells eventually polarize and become epithelial (Qin et al., 2005). A more complete Scribble depletion revealed a role for Scribble in Ecad retention at the cell cortex, suggesting it stabilizes p120catenin–Ecad coupling (Fig. 3 B; Lohia et al., 2012), thus preventing retromer from diverting Ecad to the Golgi. Potential interactions between Scribble and retromer-mediated trafficking are also implicated in Drosophila (de Vreede et al., 2014). Reduced cadherin-based adhesion may explain apical extrusion of Scribble knockdown cells plated in a wild-type MDCK epithelium (Norman et al., 2012). Others extended this work in 3D polarized epithelial cysts. Scribble is required for MCF7 cyst polarization, where it helps localize the RhoGAP DLC3, a regulator of RhoA-ROCK signaling, to cell–cell contacts (Fig. 3 B; Hendrick et al., 2016). DLC3 is also required for polarized 3D morphogenesis. Strikingly, targeting the DLC3 GAP domain to cell junctions by fusion to the Scribble LRR domain was sufficient to rescue Ecad organization. Thus, the role of Scribble in spatially regulating an active pool of DLC3 may implicate Scribble in other known DCL3 functions including endocytic trafficking (Braun et al., 2015).
Together, these data suggest that Scribble plays important roles in adhesion and polarity in mammalian epithelial cells, but the real test is in tissues in vivo. As will be discussed, mammalian Scribble has important roles in planar cell polarity (PCP) and asymmetric cell divisions, but studies of whole animal and conditional knockouts reveal that Scribble is not an essential regulator of epithelial cell polarity in most tissues. Many aspects of early to mid-embryonic mouse development proceed relatively normally in scribble mutants, from implantation through gastrulation and on to organogenesis. This contrasts dramatically with Ecad-deficient mice, which fail to implant (Larue et al., 1994). Scribble knockout mice die as neonates, and although they have severely impaired neural tube and abdominal wall closure; gonadal defects; and a disorganized, hyperplastic neuroepithelium in the cortex and other parts of the nervous system, other tissues are relatively normal (Murdoch et al., 2003; Zarbalis et al., 2004; Pearson et al., 2011).
Analysis of scribble mutants and conditional knockouts tested roles in other tissues. Scribble plays a clear role in the ectoderm-derived lens and corneal epithelium (Yamben et al., 2013). Homozygotes for the circletail allele of scribble have modest defects in lung branching morphogenesis and lumen formation, with epithelial cells within airways showing a disordered organization. Changes in the distribution of some tight junction proteins accompanied this defect. Lung explants treated with Scribble morpholinos also showed an obvious reduction in epithelial cohesion, suggesting that loss of junctional integrity may precede the effects on lumen morphology (Yates et al., 2013). Animals homozygous for the circletail allele also have subtle abnormalities in cardiomyocyte organization within the primary heart tube which manifest as gross abnormalities in heart formation at later stages of development (Phillips et al., 2007). Interestingly, the early defect in heart tube organization corresponds with the displacement of N-cadherin from the cardiomyocyte membrane. Characterization of both lung and cardiac defects in circletail mutants also highlighted a role for the PCP pathway in these tissues—in both cases, the distribution of planar polarity protein Vangl2 is disrupted (Phillips et al., 2007; Yates et al., 2013). These two models suggest that initial defects in cell–cell adhesion seen in circletail mutants may act as a catalyst for later morphological defects acting in concert with alterations to the planar polarity machinery.
In other tissues characterized, the contribution of Scribble to organization and function is not as pronounced. Conditional Scribble knockout in the prostate epithelium does not disrupt prostate development (Pearson et al., 2011), whereas conditional knockout in the developing skin reveals only a transient delay in formation of the permeability barrier during embryonic development, which later resolves (Pearson et al., 2015). Tight junction/adherens junction formation and apical–basal polarity are not impaired, but defects are seen in keratinocyte maturation. Finally, conditional knockout in kidneys had no effect (Hartleben et al., 2012). In several cases, conditional knockout or heterozygosity does accelerate tumorigenesis, e.g., leading to multifocal prostate hyperplasia (Pearson et al., 2011), and accelerated skin and lung tumorigenesis (Elsum et al., 2014; Pearson et al., 2015). Similarly, conditional mammary gland knockout did not disrupt initial gland architecture but at maturity led to ductal hyperplasia (Godde et al., 2014), a phenotype mimicked by an LRR point mutant (Feigin et al., 2014). Given essential roles for Scribble in epithelial development and polarity in Drosophila and C. elegans, what explains these more modest and tissue-specific defects in mammals? There may be partial functional overlap among the three epithelially expressed mammalian LAP family members. This is not likely the full explanation, however, as Drosophila scribble is certainly not fully redundant with the fly Erbin/Densin homologue (Lap1; Santoni et al., 2002). It will be exciting to determine the fate of double and triple mutant combinations of the epithelially expressed mouse family members, Scribble, Erbin, and Lano.
Scribble regulates polarity in another dimension—planar polarity: similar roles, different partners
Although apical–basal polarity is well known, many epithelial cells are also polarized along the perpendicular axis, parallel to the epithelial sheet. This tissue property is referred to as PCP (Devenport, 2014; Adler and Wallingford, 2017). First discovered in flies, PCP polarizes many animal tissues along a body or organ axis. Membrane and cytoskeletal proteins and macroscopic cellular structures all become polarized. In Drosophila, planar polarized tissues include the developing wing, where wing hairs, actin-rich protrusions, all point distally, or the eight photoreceptors within each of the eye’s ommatidia, which have stereotypical arrangements along the dorsal–ventral body axis. In mammals, hair cells of the cochlea all orient their actin-based stereocilia in parallel, a process critical for hearing. Many other mammalian tissues are planar polarized: for example, cell movements required for neural tube closure are also driven by correct PCP (Tissir and Goffinet, 2013).
Work in Drosophila identified a set of proteins required for planar polarity, many of which are polarized to one side of each cell along the relevant body axis (Fig. 3 C). Among these is Van Gogh (Vang), the mammalian homologue being Vangl. Strikingly, the classic mouse mutant Looptail affects Vangl. This mutant has severe defects in neural tube closure in homozygotes and subtle defects in body morphology in heterozygotes (Kibar et al., 2001; Murdoch et al., 2001). This led scientists to explore a second mutant with a similar phenotype, circletail, which they found disrupts scribble (Montcouquiol et al., 2003; Murdoch et al., 2003). Intriguingly, although animals heterozygous for either Looptail or circletail have only mild axial defects, animals simultaneously heterozygous for both have severe neural tube defects (Fig. 2), supporting the idea that they act in the same pathway. The role of Scribble in neural tube closure is clinically relevant, as human SCRIBBLE mutations are found in some patients with neural tube defects (Robinson et al., 2012; Kharfallah et al., 2017). Subsequently, collaborative roles for Scribble and Vangl in PCP were uncovered in diverse other mouse tissues, mediating axon guidance in the hindbrain (Walsh et al., 2011) and heart looping and subsequent cardiac development (Phillips et al., 2007). Functional connections also exist between the C. elegans Scribble relative LET-413 and the Vang homologue Vang-1 (Hoffmann et al., 2010).
Studies in the cochlea revealed effects on polarity at the single-cell level. Vangl loss disrupts stereocilia polarization in all hair cells. The Scribble circletail allele has a milder effect, altering polarization of only a subset of cochlear cells. This likely reflects the allele, which encodes a truncated protein retaining the LRRs and the first two PDZ domains (Fig. 2). Once again, double heterozygotes have strong phenotypes, suggesting that Vangl and Scribble work together. Consistent with this, the planar-polarized localization of Vangl is lost in circletail mutants, consistent with the requirement for Scribble PDZ3 and -4 domains for binding Vangl (Montcouquiol et al., 2006). However, Scribble itself is not planar polarized—instead, it localizes to the basolateral domain uniformly around the cell. Intriguingly, a very similar mechanism appears to link Drosophila Vang and Scribble in planar polarization of the wing hairs and photoreceptor cells (Courbard et al., 2009). As in the mouse, polarity is disrupted if the last two Scribble PDZ domains are removed (Fig. 2). These data are consistent with Scribble acting as an adapter linking Vangl to another protein, but the identity of that protein remains to be determined—one candidate is GUKh/NHS1, which also binds Dlg (Mathew et al., 2002; Walsh et al., 2011). The role of Scribble in PCP is independent of its role in apical–basal polarity, as in both mouse and fly PCP requires the last two PDZ domains (Fig. 2; Montcouquiol et al., 2006; Courbard et al., 2009), which are not essential for apical–basal polarity (Zeitler et al., 2004), and at least in the fly eye, other apical–basal polarity proteins do not seem to play similar roles in PCP (Courbard et al., 2009). Interestingly, in MDCK cells, knockdown of the well-characterized apical–basal polarity protein Par3 can mislocalize Vangl in a manner similar to Scribble knockdown, and overexpressing Par3 can rescue the Scribble-dependent localization defect (Fig. 3 C; Kharfallah et al., 2017). In this model, Par3 appears to be acting outside of its canonical function, since no other markers of apical–basal polarity were altered. Together, these data suggest that, as in its role in apical–basal polarity, Scribble mediates formation and stabilizes localization of a multiprotein complex, but one with largely or completely distinct partners. One possibility is that Scribble modulates vesicular trafficking of Vangl, as Vangl trafficking is important for PCP (Wansleeben et al., 2010; Giese et al., 2012). Future work testing this and other hypotheses and identifying other protein partners in the Scribble-mediated PCP protein complex will provide further insight.
Polarizing mitosis: Roles for Scribble in asymmetric cell division
Scribble also plays an important role in a third aspect of cell polarity: oriented and/or asymmetric cell divisions. In most tissues, mitotic spindles and subsequent division axes are oriented: for example, in epithelia, spindles orient parallel to the epithelial sheet, maintaining epithelial organization. In other cases, spindle orientation and the subsequent cytokinesis are used to produce daughter cells with different fates. To do so, fate determinants must align asymmetrically along the mitotic spindle, making spindle orientation critical.
Premier models for asymmetric cell division are Drosophila embryonic neuroblasts, neural stem/progenitor cells that divide to produce a larger daughter that maintains stem cell identity and a smaller one destined for neuronal differentiation, via inheritance of neuronal determinants (Homem and Knoblich, 2012). This division shares features with apical–basal polarity, since embryonic neuroblasts delaminate from the epithelial ectoderm. Apical polarity complex proteins such as Bazooka/Par3 define the apical domain, inherited by the stem cell daughter, and are required for spindle orientation and asymmetric localization of neural determinants. Scribble, Dlg, and Lgl are also enriched apically at prophase/metaphase, and then become uniformly cortical (Albertson and Doe, 2003). Scribble mutants have a reduced apical domain and defects in mitotic spindle asymmetry, leading to symmetric or even inverted divisions. Dissection of the role of the different protein interaction domains of Scribble revealed that the LRRs are both necessary and sufficient for Scribble cortical localization, although they cannot mediate apical enrichment (Fig. 2; Albertson et al., 2004). Although the LRRs alone can recruit the neural determinant Miranda to the cortex, both the LRRs and the PDZ domains are essential for correct division asymmetry (Fig. 2). Thus, as in epithelia, the two main Scribble protein interaction domains are both essential for its scaffolding function in defining polarized membrane domains. Mammalian Scribble has a strikingly similar role in the immune system. Asymmetric cell division and subsequent asymmetric distribution of fate determinants (some shared with fly neuroblasts) both require Scribble function, shaping the relative numbers of different T cell subsets (Pham et al., 2015). Conditional knockout suggests that Scribble plays a similar role in hematopoietic stem cell maintenance (Mohr et al., 2018). In contrast, it does not play a similar role in the erythrocyte lineage (Wölwer et al., 2017). One challenge for future work is to define proteins with which Scribble works in asymmetric division—the only identified partner in this process is GUKh (Albertson and Doe, 2003).
The role for Scribble in regulating epithelial organization may also have functional implications in symmetric cell divisions, a process characterized by parallel alignment of the mitotic spindle relative to the plane of the epithelium. In Drosophila, Scribble is essential for the planar orientation of mitotic spindle in cells of the wing disc epithelium (Nakajima et al., 2013). Although the molecular mechanisms governing this regulation are currently unknown, the close spatial proximity of the spindle poles relative to septate junctions in wild-type discs may hint at a scaffolding role for Scribble in this context. In line with this, Scribble was recently shown to exist as part of a ternary complex with Ecad and LGN, a known determinant for directing spindle orientation. Knockdown of either Ecad or Scribble was sufficient to attenuate their reciprocal interactions with LGN (Wang et al., 2018).
Vertebrate Scribble also defines spindle orientation in a third context, by a strikingly different mechanism. Like other vertebrates, the zebrafish central nervous system arises by invagination of the epithelial neural tube. Most cell divisions are parallel to the epithelial sheet, but during the neural keel/rod phase, this changes (Geldmacher-Voss et al., 2003; Žigman et al., 2011). Mitotic spindles set up parallel to the epithelial sheet, but then rotate 90° to be perpendicular to it. The tissue architecture means that the two daughter cells end up on opposite sides of the body midline. Spindle orientation becomes randomized in scribble mutants, and thus bilateral positioning of daughter cells is lost (Žigman et al., 2011). Surprisingly, this does not require PCP or apical PAR proteins, both partners in other Scribble-mediated events. Instead, Scribble is required for cortical localization of the cadherin–catenin complex, and N-cadherin knockdown phenocopies Scribble loss. Thus, in this tissue, the role of Scribble in spindle orientation appears more related to its roles in other epithelia; this tissue thus offers opportunities to define another macromolecular complex organized by Scribble to regulate polarized cell divisions.
Building synapses: Scribble as a scaffold regulating neural development and function
Neural function, behavior, and memory depend on assembly and turnover of a different subcellular organelle, the synapse. In many ways, it is analogous to a cell–cell junction, bringing two cells in close contact and acting as a signaling center (Fig. 3 A). In fact, classic cadherins and catenins play key roles in synapse architecture, as they do in cell junctions (Seong et al., 2015). In defining proteins key for synaptic architecture and function, one approach has been biochemical, seeking proteins enriched at pre- and postsynaptic membranes. One of the first identified, postsynaptic density protein-95 (PSD-95; also known as DLG4), was, together with the Scribble partner Dlg, a founding member of the MAGUK/PDZ domain protein family. Dlg and its relatives play roles in synaptic function in both flies and mammals, acting as protein scaffolds to assemble large multiprotein signaling complexes (Zhu et al., 2016a).
The close relationship between Dlg and Scribble in apical–basal polarity prompted researchers to explore potential roles for Scribble at the synapse. Drosophila Scribble localizes to synapses in a Dlg-dependent way, forming a complex with Dlg and the linking protein GUKh (Fig. 3 A). Fly scribble mutants have changes in synaptic vesicle number and synapse active zones, with effects on synaptic plasticity (Mathew et al., 2002). Mouse Scribble is also enriched at synapses of primary hippocampal neurons (Sun et al., 2009) and in their “spine” precursors (Moreau et al., 2010), where it colocalizes with the cadherin–catenin complex. Scribble coimmunoprecipitates with β-catenin, and Scribble synaptic localization is lost after β-catenin knockdown (Sun et al., 2009). As in Drosophila, Scribble knockdown alters synaptic vesicle clustering, although synapse number and localization of key synaptic proteins remain unchanged. Subsequent work reinforced connections between Scribble and the cadherin–catenin complex, suggesting that they work together to recruit the Rac/Cdc42 guanine nucleotide exchange factor β-PIX, stimulating local actin polymerization and synaptic vesicle recruitment (Fig. 3 A; Moreau et al., 2010; Sun and Bamji, 2011). Scribble loss alters synaptic maturation and pruning, with changes in learning, memory, and social behavior (Moreau et al., 2010). The effects on learning and memory are striking: Mice mutant for the truncated circletail allele have enhanced learning and memory (Moreau et al., 2010), and in Drosophila Scribble plays an important role in “active forgetting” (Cervantes-Sandoval et al., 2016). This latter work led to a model in which activating the dopamine receptor stimulates Scribble to induce formation of a “signalosome” including Rac1, Pak3, and Cofilin, which activates actin polymerization. Scribble may also affect signaling by regulating trafficking to and stability of neurotransmitter receptor complexes at the synapse, a role it has in regulating NMDA receptors (Fig. 3 A; Piguel et al., 2014). Postnatal Scribble knockout in hippocampal neurons revealed only subtle roles in learning and memory consolidation, suggesting that the effects are time and context dependent (Hilal et al., 2017). However, Scribble does promote axon myelination in postnatal oligodendrocytes (Jarjour et al., 2015). Thus, the synaptic roles of Scribble reinforce two broad themes: Scribble interacts with cell junction proteins to organize membrane domains and Scribble assembles and stabilizes multiprotein signaling complexes.
Scribble is an important regulator of cell proliferation
The other proteins of the Scribble module—Dlg and Lgl—were discovered for their striking role in regulating proliferation. Drosophila imaginal discs homozygous mutant for dlg, lgl, or scribble grow into large tumorous masses (Gateff and Schneiderman, 1974; Woods et al., 1996; Bilder et al., 2000b). These three genes were also independently identified in a Drosophila genetic screen for novel regulators of cell cycle progression, acting to negatively regulate entry into S phase (Brumby et al., 2004). Mammalian Scribble can also regulate proliferation, as indicated by the tumor suppressor role identified in mouse mutants and further emphasized by interactions between Scribble and oncogenic viral proteins in high-risk human papilloma virus and T-lymphotropic virus type, which promote ubiquitin-mediated degradation of Scribble or mislocalization, respectively (Javier and Rice, 2011).
Studies of Scribble and other fly tumor suppressors provided very interesting insights into interactions between cells in a tissue, how tissues control proliferation and repair damage, and how tumor suppressors regulate this behavior. The mechanisms by which Scribble loss drives proliferation are complex. Loss of scribble leads to global transcriptional changes involving several key signaling pathways (Bunker et al., 2015). Follow-up functional experiments revealed that in discs homozygous mutant for scribble, blocking JNK or JAK/STAT signaling is sufficient to block neoplastic overgrowth and restore tissue architecture (Bunker et al., 2015). However, activation of a single signaling pathway in isolation does not account for the full complement of neoplastic properties conferred by Scribble loss. Activating JNK together with blocking apoptosis, for example, results in tissue overgrowth but does not disrupt apical–basal polarity (Bunker et al., 2015). In a similar way, depleting the transcription factor Yorkie reduces wing disc overgrowth in scribble mutants but does not rescue the associated morphological defects (Doggett et al., 2011; Bunker et al., 2015). Thus, a future challenge is to define how these signals are integrated to activate a neoplastic gene expression program.
The neoplastic capacity of scribble mutant cells is dependent on their cellular environment. Mosaic analysis of imaginal discs revealed that clonal patches of scribble mutant cells do not hyperproliferate but instead are eliminated by competition with surrounding wild-type cells (Brumby and Richardson, 2003; Igaki et al., 2006, 2009; Yamamoto et al., 2017). Following this lead provided important insights. Activating the JNK pathway in scribble mutant cell clones is required for scribble cell elimination, acting via autonomous and nonautonomous mechanisms (Brumby and Richardson, 2003; Uhlirova et al., 2005; Igaki et al., 2006). A molecular mechanism was recently proposed for how JNK signaling acts in an autonomous manner within scribble mutant clones to facilitate their extrusion. This revealed the actin regulator Enabled/VASP (Ena) as essential for scribble mutant cell extrusion, acting downstream of Slit-Robo2 signaling. JNK signaling amplified Slit-Robo2-Ena signaling in scribble mutant cells but not in surrounding wild-type cells (Vaughen and Igaki, 2016). Although it is not known how the Slit-Robo2-Ena pathway is mechanically coupled to cell extrusion, disrupting Ecad-mediated adhesion is an important feature of the process (Vaughen and Igaki, 2016). In contrast, nonautonomous JNK signaling promotes wild-type neighbors to engulf scribble mutant cells (Ohsawa et al., 2011). Other neoplastic tumor suppressor genes can elicit a similar response, suggesting that a nonspecific mechanism for clearing oncogenic cells may be at play.
JNK signaling also acts to suppress hyperproliferation of scribble mutant clones (Brumby and Richardson, 2003; Igaki et al., 2009; Chen et al., 2012). Strikingly, ectopic proliferation induced by inhibiting JNK can be abrogated by impairing the Hippo signaling pathway (Doggett et al., 2011). Hippo signaling has emerged as an important regulator of organ size in both Drosophila and mammals; loss of Hippo regulation leads to unconstrained cell proliferation. Elevated expression of Hippo target genes occurs in homozygous scribble mutant discs and in mosaics where scribble mutant clones are neighbored by cells of compromised fitness (Chen et al., 2012; Verghese et al., 2012). At the molecular level, Scribble directly interacts with vertebrate Fat1 and Drosophila Fat (Skouloudaki et al., 2009), atypical cadherins that help link downstream Hippo pathway kinases to upstream events at the plasma membrane. In Drosophila, scribble acts downstream of Fat to regulate growth (Fig. 3 D; Verghese et al., 2012). Downstream of the apical regulatory scaffold, the Hippo pathway consists of a core kinase cassette that negatively regulates transcriptional machinery. During homeostasis, phosphorylation of transcriptional coactivators TAZ and YAP (mammalian Yorkie homologues) by LATS1/2 inhibits their activity by preventing translocation into the nucleus. Scribble can coimmunoprecipitate with MST1/2 and LATS1/2 (Fly Hpo and Wts, respectively; Fig. 3 D), as well as with TAZ (Cordenonsi et al., 2011), and may be essential for interactions among these proteins. Loss of Scribble attenuates TAZ/YAP phosphorylation, and there is an expanding body of evidence to indicate that the Scrib/YAP/TAZ complex is part of a much larger interactome (Mohseni et al., 2014; Clattenburg et al., 2015; Zhu et al., 2016b). For example, in mammary epithelial cells, the interaction of Scribble with LATS2 acts to limit the accumulation of SnoN and restrict its subcellular localization to the basolateral membrane (Fig. 3 D). Elevated levels of SnoN, and its translocation to the nucleus, lead to enhanced stability and transcriptional activity of TAZ, supporting the notion that Scribble acts to spatially organize signaling regimens upstream of proliferation (Zhu et al., 2016b).
The idea that proper tissue organization is a prerequisite for keeping proliferation in check is interesting, particularly in light of the role of adherens junctions and apical–basal polarity complexes in Hippo pathway regulation (reviewed in Genevet and Tapon, 2011; Richardson and Portela, 2017). Despite a well-characterized role for Scribble in maintaining adherens junction integrity in epithelia, it is notable that knocking down Ecad or α-catenin in the wing disc perturbs the Hippo pathway in ways that are distinct from loss of Scribble (Yang et al., 2015).
Studying oncogenic cooperation has also greatly aided our understanding of how Scribble interacts with key signaling pathways. One well-studied example of this synergy is seen in scribble mutant cells also overexpressing oncogenic Rasv12 (Brumby and Richardson, 2003; Pagliarini and Xu, 2003; Uhlirova et al., 2005; Chen et al., 2012). Clones mutant for scribble lose apical–basal polarity and die, but when RasV12 is coexpressed they develop into large tumors, which are much more aggressive than those expressing RasV12 alone. Interestingly, placing cells individually mutant for RasV12 and scribble next to one another is sufficient to promote tumor induction (Wu et al., 2010). In both situations, elevated JAK-STAT signaling is required to facilitate tumor growth. It was suggested that propagation of JNK signaling from scribble mutant to RasV12 cells facilitates JAK/STAT elevation in RasV12 cells (Wu et al., 2010). In support of this, RNA profiling of scribble mutant imaginal discs revealed an up-regulation of genes in the JAK-STAT signaling cascade (Bunker et al., 2015). Oncogenic cooperation also occurs in the Drosophila larval brain. As in epithelial tissue, scribble neuroblast clones are eliminated in a JNK-dependent manner, but clones mutant for both scribble and the junction-actin cross-linker and asymmetric cell division regulator canoe display tumor-like overgrowth (Rives-Quinto et al., 2017). In this situation, ectopic Ras expression activates the PI3K-Atk1 signaling pathway. Important insights into the molecular behavior of Scribble have also been gleaned from studies on oncogenic cooperation in mouse models. In a transgenic transplantation mouse model for mammary carcinoma, loss of Scribble in conjunction with oncogenic activation of c-myc significantly enhances the size of tumors compared with activating c-myc alone. The expression of c-myc induces both proliferation and apoptosis in mammary epithelial cells, the latter function requiring Scribble, in complex with β-PIX and GIT1, to activate the Rac–JNK–JUN pathway. Knockdown or mislocalization of Scribble was sufficient to override the proapoptotic signals from c-myc activation (Zhan et al., 2008).
Scribble also regulates cell migration and metastasis
The tumors induced by inactivating scribble in cells expressing activated Ras also exhibit metastatic properties (Pagliarini and Xu, 2003). Intriguingly, inactivation of other core polarity genes also imparts metastatic capacity on imaginal tissue when combined with RasV12 (Pagliarini and Xu, 2003). Consistent with Drosophila models, loss of mammalian scribble also cooperates with H-rRasv12 to promote invasion of mammary epithelial cells in organotypic cultures. In this context, Scribble normally restrains metastasis by suppressing MAPK-ERK signaling downstream of Ras (Dow et al., 2008). Interestingly, Scribble can directly interact with ERK, and the interaction maps to two well-conserved kinase interaction motif (KIM) docking sites. A scribble mutant lacking the C-terminal KIM docking site lost the ability to suppress invasion (Nagasaka et al., 2010). The restraint placed on MAPK-ERK signaling by Scribble is thought to have direct consequences for epithelial-to-mesenchymal transition (EMT), since forced expression of Scribble or knockdown of ERK1/2 leads to a down-regulation of ZEB1 and ZEB2, transcriptional regulators of the EMT (Elsum et al., 2013). It is important to note that the role of Scribble in mediating cell migration is likely to be context dependent, with Scribble acting in diverse cell types as an important integrator of signals required to promote cell migration. In many of these cases, Scribble regulates Rho GTPase gradients to drive front-to-back polarization required for directed cell migration. For example, in astrocytes, Scribble recruits β-PIX to the leading edge to facilitate localized Cdc42 activity (Osmani et al., 2006). Similarly, in response to directional cues, MCF-10A epithelial cells require Scribble to recruit Rac1 and Cdc42 to the leading edge to form stable lamellipodial protrusions (Dow et al., 2007). A promigratory function for Scribble has also been shown in dendritic cells and certain cancer cell lines, acting downstream of the interaction of Plexin-B1 with its transmembrane receptor Sema4A (Sun et al., 2017). Here, it is suggested that the interaction with Sema4A leads to reduced binding of Scribble to β-PIX, with consequences for polarized Cdc42 and Rac activity (Sun et al., 2017). Outside of its role in Rho GTPase regulation, Scribble has also been shown to promote directed migration of endothelial cells by regulating the turnover of integrin α5 at focal adhesions (Michaelis et al., 2013). As this summary illustrates, Scribble plays multiple potential roles in oncogenesis, and much remains to be learned in this regard.
Future directions
There are many exciting questions to address surrounding the biological functions of Scribble. For example, as a regulator of apical–basal polarity, genetic interactions between Scribble and other core polarity proteins are well mapped, but an understanding of the molecular nature of these interactions is lacking. There is also a substantial gap in our understanding of what links Scribble to key signaling events such as JNK signaling, a cascade that is essential for Scribble to impart proliferative constraint. To truly understand how Scribble integrates protein interactomes, we must acknowledge that its distinct biological functions are not mutually exclusive. For example, the apical–basal and planar-polarized cues feed directly into growth control mechanisms. It is enticing to theorize that Scribble may sometimes act at the interface of two or more biological functions. Exploring Scribble’s molecular mechanisms and interlocked interactomes will provide work for many of us for years to come.
Acknowledgments
Work in our lab is supported by National Institutes of Health (R35 GM118096).
The authors declare no competing financial interests.
References
- Adler P.N., and Wallingford J.B.. 2017. From Planar Cell Polarity to Ciliogenesis and Back: The Curious Tale of the PPE and CPLANE proteins. Trends Cell Biol. 27:379–390. 10.1016/j.tcb.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson R., and Doe C.Q.. 2003. Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nat. Cell Biol. 5:166–170. 10.1038/ncb922 [DOI] [PubMed] [Google Scholar]
- Albertson R., Chabu C., Sheehan A., and Doe C.Q.. 2004. Scribble protein domain mapping reveals a multistep localization mechanism and domains necessary for establishing cortical polarity. J. Cell Sci. 117:6061–6070. 10.1242/jcs.01525 [DOI] [PubMed] [Google Scholar]
- Arnaud C., Sebbagh M., Nola S., Audebert S., Bidaut G., Hermant A., Gayet O., Dusetti N.J., Ollendorff V., Santoni M.J., et al. 2009. MCC, a new interacting protein for Scrib, is required for cell migration in epithelial cells. FEBS Lett. 583:2326–2332. 10.1016/j.febslet.2009.06.034 [DOI] [PubMed] [Google Scholar]
- Audebert S., Navarro C., Nourry C., Chasserot-Golaz S., Lécine P., Bellaiche Y., Dupont J.L., Premont R.T., Sempéré C., Strub J.M., et al. 2004. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr. Biol. 14:987–995. 10.1016/j.cub.2004.05.051 [DOI] [PubMed] [Google Scholar]
- Betschinger J., Mechtler K., and Knoblich J.A.. 2003. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 422:326–330. 10.1038/nature01486 [DOI] [PubMed] [Google Scholar]
- Betschinger J., Eisenhaber F., and Knoblich J.A.. 2005. Phosphorylation-induced autoinhibition regulates the cytoskeletal protein Lethal (2) giant larvae. Curr. Biol. 15:276–282. 10.1016/j.cub.2005.01.012 [DOI] [PubMed] [Google Scholar]
- Bilder D., and Perrimon N.. 2000. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 403:676–680. 10.1038/35001108 [DOI] [PubMed] [Google Scholar]
- Bilder D., Birnbaum D., Borg J.P., Bryant P., Huigbretse J., Jansen E., Kennedy M.B., Labouesse M., Legouis R., Mechler B., et al. 2000a Collective nomenclature for LAP proteins. Nat. Cell Biol. 2:E114 10.1038/35017119 [DOI] [PubMed] [Google Scholar]
- Bilder D., Li M., and Perrimon N.. 2000b Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 289:113–116. 10.1126/science.289.5476.113 [DOI] [PubMed] [Google Scholar]
- Bilder D., Schober M., and Perrimon N.. 2003. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat. Cell Biol. 5:53–58. 10.1038/ncb897 [DOI] [PubMed] [Google Scholar]
- Boëda B., and Etienne-Manneville S.. 2015. Spectrin binding motifs regulate Scribble cortical dynamics and polarity function. eLife. 4:e04726 10.7554/eLife.04726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossinger O., Klebes A., Segbert C., Theres C., and Knust E.. 2001. Zonula adherens formation in Caenorhabditis elegans requires dlg-1, the homologue of the Drosophila gene discs large. Dev. Biol. 230:29–42. 10.1006/dbio.2000.0113 [DOI] [PubMed] [Google Scholar]
- Bossinger O., Fukushige T., Claeys M., Borgonie G., and McGhee J.D.. 2004. The apical disposition of the Caenorhabditis elegans intestinal terminal web is maintained by LET-413. Dev. Biol. 268:448–456. 10.1016/j.ydbio.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Braun A.C., Hendrick J., Eisler S.A., Schmid S., Hausser A., and Olayioye M.A.. 2015. The Rho-specific GAP protein DLC3 coordinates endocytic membrane trafficking. J. Cell Sci. 128:1386–1399. 10.1242/jcs.163857 [DOI] [PubMed] [Google Scholar]
- Brumby A.M., and Richardson H.E.. 2003. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 22:5769–5779. 10.1093/emboj/cdg548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumby A., Secombe J., Horsfield J., Coombe M., Amin N., Coates D., Saint R., and Richardson H.. 2004. A genetic screen for dominant modifiers of a cyclin E hypomorphic mutation identifies novel regulators of S-phase entry in Drosophila. Genetics. 168:227–251. 10.1534/genetics.104.026617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker B.D., Nellimoottil T.T., Boileau R.M., Classen A.K., and Bilder D.. 2015. The transcriptional response to tumorigenic polarity loss in Drosophila. eLife. 4:e03189 10.7554/eLife.03189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai P., Mu Y., Piao X., Hou N., Liu S., Gao Y., Wang H., and Chen Q.. 2014. Discovery and confirmation of ligand binding specificities of the Schistosoma japonicum polarity protein Scribble. PLoS Negl. Trop. Dis. 8:e2837 10.1371/journal.pntd.0002837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp N.D., Lee K.S., Wacker-Mhyre J.L., Kountz T.S., Park J.M., Harris D.A., Estrada M., Stewart A., Wolf-Yadlin A., and Hague C.. 2015. Individual protomers of a G protein-coupled receptor dimer integrate distinct functional modules. Cell Discov. 1:15011 10.1038/celldisc.2015.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanale J.P., Sun T.Y., and Montell D.J.. 2017. Development and dynamics of cell polarity at a glance. J. Cell Sci. 130:1201–1207. 10.1242/jcs.188599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K., Knust E., and Skaer H.. 2009. Crumbs stabilises epithelial polarity during tissue remodelling. J. Cell Sci. 122:2604–2612. 10.1242/jcs.047183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caria S., Magtoto C.M., Samiei T., Portela M., Lim K.Y.B., How J.Y., Stewart B.Z., Humbert P.O., Richardson H.E., and Kvansakul M.. 2018. Drosophila melanogaster Guk-holder interacts with the Scribbled PDZ1 domain and regulates epithelial development with Scribbled and Discs Large. J. Biol. Chem. 293:4519–4531. 10.1074/jbc.M117.817528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Sandoval I., Chakraborty M., MacMullen C., and Davis R.L.. 2016. Scribble Scaffolds a Signalosome for Active Forgetting. Neuron. 90:1230–1242. 10.1016/j.neuron.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Zheng B., DeRan M., Jarugumilli G.K., Fu J., Brooks Y.S., and Wu X.. 2016. ZDHHC7-mediated S-palmitoylation of Scribble regulates cell polarity. Nat. Chem. Biol. 12:686–693. 10.1038/nchembio.2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.L., Schroeder M.C., Kango-Singh M., Tao C., and Halder G.. 2012. Tumor suppression by cell competition through regulation of the Hippo pathway. Proc. Natl. Acad. Sci. USA. 109:484–489. 10.1073/pnas.1113882109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Sayadian A.C., Lowe N., Lovegrove H.E., and St Johnston D.. 2018. An alternative mode of epithelial polarity in the Drosophila midgut. PLoS Biol. 16:e3000041 10.1371/journal.pbio.3000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clattenburg L., Wigerius M., Qi J., Rainey J.K., Rourke J.L., Muruganandan S., Sinal C.J., and Fawcett J.P.. 2015. NOS1AP Functionally Associates with YAP To Regulate Hippo Signaling. Mol. Cell. Biol. 35:2265–2277. 10.1128/MCB.00062-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordenonsi M., Zanconato F., Azzolin L., Forcato M., Rosato A., Frasson C., Inui M., Montagner M., Parenti A.R., Poletti A., et al. 2011. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 147:759–772. 10.1016/j.cell.2011.09.048 [DOI] [PubMed] [Google Scholar]
- Courbard J.R., Djiane A., Wu J., and Mlodzik M.. 2009. The apical/basal-polarity determinant Scribble cooperates with the PCP core factor Stbm/Vang and functions as one of its effectors. Dev. Biol. 333:67–77. 10.1016/j.ydbio.2009.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denholm B. 2013. Shaping up for action: the path to physiological maturation in the renal tubules of Drosophila. Organogenesis. 9:40–54. 10.4161/org.24107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D. 2014. The cell biology of planar cell polarity. J. Cell Biol. 207:171–179. 10.1083/jcb.201408039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vreede G., Schoenfeld J.D., Windler S.L., Morrison H., Lu H., and Bilder D.. 2014. The Scribble module regulates retromer-dependent endocytic trafficking during epithelial polarization. Development. 141:2796–2802. 10.1242/dev.105403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett K., Grusche F.A., Richardson H.E., and Brumby A.M.. 2011. Loss of the Drosophila cell polarity regulator Scribbled promotes epithelial tissue overgrowth and cooperation with oncogenic Ras-Raf through impaired Hippo pathway signaling. BMC Dev. Biol. 11:57 10.1186/1471-213X-11-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe C.D., Csordás G., Correia A., Jindra M., Klein C., Habermann B., and Uhlirova M.. 2018. Atf3 links loss of epithelial polarity to defects in cell differentiation and cytoarchitecture. PLoS Genet. 14:e1007241 10.1371/journal.pgen.1007241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow L.E., Kauffman J.S., Caddy J., Zarbalis K., Peterson A.S., Jane S.M., Russell S.M., and Humbert P.O.. 2007. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene. 26:2272–2282. 10.1038/sj.onc.1210016 [DOI] [PubMed] [Google Scholar]
- Dow L.E., Elsum I.A., King C.L., Kinross K.M., Richardson H.E., and Humbert P.O.. 2008. Loss of human Scribble cooperates with H-Ras to promote cell invasion through deregulation of MAPK signalling. Oncogene. 27:5988–6001. 10.1038/onc.2008.219 [DOI] [PubMed] [Google Scholar]
- Eastburn D.J., Zegers M.M., and Mostov K.E.. 2012. Scrib regulates HGF-mediated epithelial morphogenesis and is stabilized by Sgt1-HSP90. J. Cell Sci. 125:4147–4157. 10.1242/jcs.108670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsum I.A., Martin C., and Humbert P.O.. 2013. Scribble regulates an EMT polarity pathway through modulation of MAPK-ERK signaling to mediate junction formation. J. Cell Sci. 126:3990–3999. 10.1242/jcs.129387 [DOI] [PubMed] [Google Scholar]
- Elsum I.A., Yates L.L., Pearson H.B., Phesse T.J., Long F., O’Donoghue R., Ernst M., Cullinane C., and Humbert P.O.. 2014. Scrib heterozygosity predisposes to lung cancer and cooperates with KRas hyperactivation to accelerate lung cancer progression in vivo. Oncogene. 33:5523–5533. 10.1038/onc.2013.498 [DOI] [PubMed] [Google Scholar]
- Enkhbayar P., Kamiya M., Osaki M., Matsumoto T., and Matsushima N.. 2004. Structural principles of leucine-rich repeat (LRR) proteins. Proteins. 54:394–403. 10.1002/prot.10605 [DOI] [PubMed] [Google Scholar]
- Ernst A., Appleton B.A., Ivarsson Y., Zhang Y., Gfeller D., Wiesmann C., and Sidhu S.S.. 2014. A structural portrait of the PDZ domain family. J. Mol. Biol. 426:3509–3519. 10.1016/j.jmb.2014.08.012 [DOI] [PubMed] [Google Scholar]
- Feigin M.E., Akshinthala S.D., Araki K., Rosenberg A.Z., Muthuswamy L.B., Martin B., Lehmann B.D., Berman H.K., Pietenpol J.A., Cardiff R.D., and Muthuswamy S.K.. 2014. Mislocalization of the cell polarity protein scribble promotes mammary tumorigenesis and is associated with basal breast cancer. Cancer Res. 74:3180–3194. 10.1158/0008-5472.CAN-13-3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gateff E., and Schneiderman H.A.. 1974. Developmental capacities of benign and malignant neoplasms of Drosophila. Wilhelm Roux Arch. Entwickl. Mech. Org. 176:23–65. 10.1007/BF00577830 [DOI] [PubMed] [Google Scholar]
- Geldmacher-Voss B., Reugels A.M., Pauls S., and Campos-Ortega J.A.. 2003. A 90-degree rotation of the mitotic spindle changes the orientation of mitoses of zebrafish neuroepithelial cells. Development. 130:3767–3780. 10.1242/dev.00603 [DOI] [PubMed] [Google Scholar]
- Genevet A., and Tapon N.. 2011. The Hippo pathway and apico-basal cell polarity. Biochem. J. 436:213–224. 10.1042/BJ20110217 [DOI] [PubMed] [Google Scholar]
- Gfeller D., Butty F., Wierzbicka M., Verschueren E., Vanhee P., Huang H., Ernst A., Dar N., Stagljar I., Serrano L., et al. 2011. The multiple-specificity landscape of modular peptide recognition domains. Mol. Syst. Biol. 7:484 10.1038/msb.2011.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese A.P., Ezan J., Wang L., Lasvaux L., Lembo F., Mazzocco C., Richard E., Reboul J., Borg J.P., Kelley M.W., et al. 2012. Gipc1 has a dual role in Vangl2 trafficking and hair bundle integrity in the inner ear. Development. 139:3775–3785. 10.1242/dev.074229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godde N.J., Sheridan J.M., Smith L.K., Pearson H.B., Britt K.L., Galea R.C., Yates L.L., Visvader J.E., and Humbert P.O.. 2014. Scribble modulates the MAPK/Fra1 pathway to disrupt luminal and ductal integrity and suppress tumour formation in the mammary gland. PLoS Genet. 10:e1004323 10.1371/journal.pgen.1004323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui J., Huang Y., and Shimmi O.. 2016. Scribbled optimizes BMP signaling through its receptor internalization to the Rab5 endosome and promote robust epithelial morphogenesis. PLoS Genet. 12:e1006424 10.1371/journal.pgen.1006424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartleben B., Widmeier E., Wanner N., Schmidts M., Kim S.T., Schneider L., Mayer B., Kerjaschki D., Miner J.H., Walz G., and Huber T.B.. 2012. Role of the polarity protein Scribble for podocyte differentiation and maintenance. PLoS One. 7:e36705 10.1371/journal.pone.0036705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick J., Franz-Wachtel M., Moeller Y., Schmid S., Macek B., and Olayioye M.A.. 2016. The polarity protein Scribble positions DLC3 at adherens junctions to regulate Rho signaling. J. Cell Sci. 129:3583–3596. 10.1242/jcs.190074 [DOI] [PubMed] [Google Scholar]
- Hilal M.L., Moreau M.M., Racca C., Pinheiro V.L., Piguel N.H., Santoni M.J., Dos Santos Carvalho S., Blanc J.M., Abada Y.K., Peyroutou R., et al. 2017. Activity-Dependent Neuroplasticity Induced by an Enriched Environment Reverses Cognitive Deficits in Scribble Deficient Mouse. Cereb. Cortex. 27:5635–5651. 10.1093/cercor/bhw333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Segbert C., Helbig G., and Bossinger O.. 2010. Intestinal tube formation in Caenorhabditis elegans requires vang-1 and egl-15 signaling. Dev. Biol. 339:268–279. 10.1016/j.ydbio.2009.12.002 [DOI] [PubMed] [Google Scholar]
- Homem C.C., and Knoblich J.A.. 2012. Drosophila neuroblasts: a model for stem cell biology. Development. 139:4297–4310. 10.1242/dev.080515 [DOI] [PubMed] [Google Scholar]
- Humbert P., Russell S., and Richardson H.. 2003. Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. BioEssays. 25:542–553. 10.1002/bies.10286 [DOI] [PubMed] [Google Scholar]
- Hutterer A., Betschinger J., Petronczki M., and Knoblich J.A.. 2004. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev. Cell. 6:845–854. 10.1016/j.devcel.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Igaki T., Pagliarini R.A., and Xu T.. 2006. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr. Biol. 16:1139–1146. 10.1016/j.cub.2006.04.042 [DOI] [PubMed] [Google Scholar]
- Igaki T., Pastor-Pareja J.C., Aonuma H., Miura M., and Xu T.. 2009. Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev. Cell. 16:458–465. 10.1016/j.devcel.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A.I., Young C., Den Beste K., Capaldo C.T., Humbert P.O., Brennwald P., Parkos C.A., and Nusrat A.. 2010. Tumor suppressor scribble regulates assembly of tight junctions in the intestinal epithelium. Am. J. Pathol. 176:134–145. 10.2353/ajpath.2010.090220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarsson Y., Arnold R., McLaughlin M., Nim S., Joshi R., Ray D., Liu B., Teyra J., Pawson T., Moffat J., et al. 2014. Large-scale interaction profiling of PDZ domains through proteomic peptide-phage display using human and viral phage peptidomes. Proc. Natl. Acad. Sci. USA. 111:2542–2547. 10.1073/pnas.1312296111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjour A.A., Boyd A., Dow L.E., Holloway R.K., Goebbels S., Humbert P.O., Williams A., and ffrench-Constant C.. 2015. The polarity protein Scribble regulates myelination and remyelination in the central nervous system. PLoS Biol. 13:e1002107 10.1371/journal.pbio.1002107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier R.T., and Rice A.P.. 2011. Emerging theme: cellular PDZ proteins as common targets of pathogenic viruses. J. Virol. 85:11544–11556. 10.1128/JVI.05410-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallay L.M., McNickle A., Brennwald P.J., Hubbard A.L., and Braiterman L.T.. 2006. Scribble associates with two polarity proteins, Lgl2 and Vangl2, via distinct molecular domains. J. Cell. Biochem. 99:647–664. 10.1002/jcb.20992 [DOI] [PubMed] [Google Scholar]
- Kharfallah F., Guyot M.C., El Hassan A.R., Allache R., Merello E., De Marco P., Di Cristo G., Capra V., and Kibar Z.. 2017. Scribble1 plays an important role in the pathogenesis of neural tube defects through its mediating effect of Par-3 and Vangl1/2 localization. Hum. Mol. Genet. 26:2307–2320. 10.1093/hmg/ddx122 [DOI] [PubMed] [Google Scholar]
- Kibar Z., Vogan K.J., Groulx N., Justice M.J., Underhill D.A., and Gros P.. 2001. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat. Genet. 28:251–255. 10.1038/90081 [DOI] [PubMed] [Google Scholar]
- Köppen M., Simske J.S., Sims P.A., Firestein B.L., Hall D.H., Radice A.D., Rongo C., and Hardin J.D.. 2001. Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat. Cell Biol. 3:983–991. 10.1038/ncb1101-983 [DOI] [PubMed] [Google Scholar]
- Lahuna O., Quellari M., Achard C., Nola S., Méduri G., Navarro C., Vitale N., Borg J.P., and Misrahi M.. 2005. Thyrotropin receptor trafficking relies on the hScrib-betaPIX-GIT1-ARF6 pathway. EMBO J. 24:1364–1374. 10.1038/sj.emboj.7600616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprise P., Beronja S., Silva-Gagliardi N.F., Pellikka M., Jensen A.M., McGlade C.J., and Tepass U.. 2006. The FERM protein Yurt is a negative regulatory component of the Crumbs complex that controls epithelial polarity and apical membrane size. Dev. Cell. 11:363–374. 10.1016/j.devcel.2006.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L., Ohsugi M., Hirchenhain J., and Kemler R.. 1994. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc. Natl. Acad. Sci. USA. 91:8263–8267. 10.1073/pnas.91.17.8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legouis R., Gansmuller A., Sookhareea S., Bosher J.M., Baillie D.L., and Labouesse M.. 2000. LET-413 is a basolateral protein required for the assembly of adherens junctions in Caenorhabditis elegans. Nat. Cell Biol. 2:415–422. 10.1038/35017046 [DOI] [PubMed] [Google Scholar]
- Legouis R., Jaulin-Bastard F., Schott S., Navarro C., Borg J.P., and Labouesse M.. 2003. Basolateral targeting by leucine-rich repeat domains in epithelial cells. EMBO Rep. 4:1096–1102. 10.1038/sj.embor.7400006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Yang H., Liu J., Schmidt M.D., and Gao T.. 2011. Scribble-mediated membrane targeting of PHLPP1 is required for its negative regulation of Akt. EMBO Rep. 12:818–824. 10.1038/embor.2011.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K.Y.B., Gödde N.J., Humbert P.O., and Kvansakul M.. 2017. Structural basis for the differential interaction of Scribble PDZ domains with the guanine nucleotide exchange factor β-PIX. J. Biol. Chem. 292:20425–20436. 10.1074/jbc.M117.799452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.C., Frei J.A., Kilander M.B., Shen W., and Blatt G.J.. 2016. A Subset of Autism-Associated Genes Regulate the Structural Stability of Neurons. Front. Cell. Neurosci. 10:263 10.3389/fncel.2016.00263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Golebiewski L., Dow E.C., Krug R.M., Javier R.T., and Rice A.P.. 2010. The ESEV PDZ-binding motif of the avian influenza A virus NS1 protein protects infected cells from apoptosis by directly targeting Scribble. J. Virol. 84:11164–11174. 10.1128/JVI.01278-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang S., Hang W., Gao J., Zhang W., Cheng Z., Yang C., He J., Zhou J., Chen J., and Shi A.. 2018. LET-413/Erbin acts as a RAB-5 effector to promote RAB-10 activation during endocytic recycling. J. Cell Biol. 217:299–314. 10.1083/jcb.201705136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li J., Li P., Wang Y., Liang Z., Jiang Y., Li J., Feng C., Wang R., Chen H., et al. 2017. Loss of DLG5 promotes breast cancer malignancy by inhibiting the Hippo signaling pathway. Sci. Rep. 7:42125 10.1038/srep42125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood C.A., Lynch A.M., and Hardin J.. 2008. Dynamic analysis identifies novel roles for DLG-1 subdomains in AJM-1 recruitment and LET-413-dependent apical focusing. J. Cell Sci. 121:1477–1487. 10.1242/jcs.017137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohia M., Qin Y., and Macara I.G.. 2012. The Scribble polarity protein stabilizes E-cadherin/p120-catenin binding and blocks retrieval of E-cadherin to the Golgi. PLoS One. 7:e51130 10.1371/journal.pone.0051130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D., Gramates L.S., Packard M., Thomas U., Bilder D., Perrimon N., Gorczyca M., and Budnik V.. 2002. Recruitment of scribble to the synaptic scaffolding complex requires GUK-holder, a novel DLG binding protein. Curr. Biol. 12:531–539. 10.1016/S0960-9822(02)00758-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon L., Legouis R., Vonesch J.L., and Labouesse M.. 2001. Assembly of C. elegans apical junctions involves positioning and compaction by LET-413 and protein aggregation by the MAGUK protein DLG-1. J. Cell Sci. 114:2265–2277. [DOI] [PubMed] [Google Scholar]
- Métais J.Y., Navarro C., Santoni M.J., Audebert S., and Borg J.P.. 2005. hScrib interacts with ZO-2 at the cell-cell junctions of epithelial cells. FEBS Lett. 579:3725–3730. 10.1016/j.febslet.2005.05.062 [DOI] [PubMed] [Google Scholar]
- Michaelis U.R., Chavakis E., Kruse C., Jungblut B., Kaluza D., Wandzioch K., Manavski Y., Heide H., Santoni M.J., Potente M., et al. 2013. The polarity protein Scrib is essential for directed endothelial cell migration. Circ. Res. 112:924–934. 10.1161/CIRCRESAHA.112.300592 [DOI] [PubMed] [Google Scholar]
- Mohr J., Dash B.P., Schnoeder T.M., Wolleschak D., Herzog C., Tubio Santamaria N., Weinert S., Godavarthy S., Zanetti C., Naumann M., et al. 2018. The cell fate determinant Scribble is required for maintenance of hematopoietic stem cell function. Leukemia. 32:1211–1221. 10.1038/s41375-018-0025-0 [DOI] [PubMed] [Google Scholar]
- Mohseni M., Sun J., Lau A., Curtis S., Goldsmith J., Fox V.L., Wei C., Frazier M., Samson O., Wong K.K., et al. 2014. A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nat. Cell Biol. 16:108–117. 10.1038/ncb2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M., Rachel R.A., Lanford P.J., Copeland N.G., Jenkins N.A., and Kelley M.W.. 2003. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 423:173–177. 10.1038/nature01618 [DOI] [PubMed] [Google Scholar]
- Montcouquiol M., Sans N., Huss D., Kach J., Dickman J.D., Forge A., Rachel R.A., Copeland N.G., Jenkins N.A., Bogani D., et al. 2006. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J. Neurosci. 26:5265–5275. 10.1523/JNEUROSCI.4680-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M.M., Piguel N., Papouin T., Koehl M., Durand C.M., Rubio M.E., Loll F., Richard E.M., Mazzocco C., Racca C., et al. 2010. The planar polarity protein Scribble1 is essential for neuronal plasticity and brain function. J. Neurosci. 30:9738–9752. 10.1523/JNEUROSCI.6007-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H.-A.J., and Wieschaus E.. 1996. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J. Cell Biol. 134:149–163. 10.1083/jcb.134.1.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch J.N., Doudney K., Paternotte C., Copp A.J., and Stanier P.. 2001. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum. Mol. Genet. 10:2593–2601. 10.1093/hmg/10.22.2593 [DOI] [PubMed] [Google Scholar]
- Murdoch J.N., Henderson D.J., Doudney K., Gaston-Massuet C., Phillips H.M., Paternotte C., Arkell R., Stanier P., and Copp A.J.. 2003. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum. Mol. Genet. 12:87–98. 10.1093/hmg/ddg014 [DOI] [PubMed] [Google Scholar]
- Nagasaka K., Nakagawa S., Yano T., Takizawa S., Matsumoto Y., Tsuruga T., Nakagawa K., Minaguchi T., Oda K., Hiraike-Wada O., et al. 2006. Human homolog of Drosophila tumor suppressor Scribble negatively regulates cell-cycle progression from G1 to S phase by localizing at the basolateral membrane in epithelial cells. Cancer Sci. 97:1217–1225. 10.1111/j.1349-7006.2006.00315.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaka K., Pim D., Massimi P., Thomas M., Tomaić V., Subbaiah V.K., Kranjec C., Nakagawa S., Yano T., Taketani Y., et al. 2010. The cell polarity regulator hScrib controls ERK activation through a KIM site-dependent interaction. Oncogene. 29:5311–5321. 10.1038/onc.2010.265 [DOI] [PubMed] [Google Scholar]
- Nagasaka K., Seiki T., Yamashita A., Massimi P., Subbaiah V.K., Thomas M., Kranjec C., Kawana K., Nakagawa S., Yano T., et al. 2013. A novel interaction between hScrib and PP1γ downregulates ERK signaling and suppresses oncogene-induced cell transformation. PLoS One. 8:e53752 10.1371/journal.pone.0053752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y., Meyer E.J., Kroesen A., McKinney S.A., and Gibson M.C.. 2013. Epithelial junctions maintain tissue architecture by directing planar spindle orientation. Nature. 500:359–362. 10.1038/nature12335 [DOI] [PubMed] [Google Scholar]
- Navarro C., Nola S., Audebert S., Santoni M.J., Arsanto J.P., Ginestier C., Marchetto S., Jacquemier J., Isnardon D., Le Bivic A., et al. 2005. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene. 24:4330–4339. 10.1038/sj.onc.1208632 [DOI] [PubMed] [Google Scholar]
- Nola S., Sebbagh M., Marchetto S., Osmani N., Nourry C., Audebert S., Navarro C., Rachel R., Montcouquiol M., Sans N., et al. 2008. Scrib regulates PAK activity during the cell migration process. Hum. Mol. Genet. 17:3552–3565. 10.1093/hmg/ddn248 [DOI] [PubMed] [Google Scholar]
- Norman M., Wisniewska K.A., Lawrenson K., Garcia-Miranda P., Tada M., Kajita M., Mano H., Ishikawa S., Ikegawa M., Shimada T., and Fujita Y.. 2012. Loss of Scribble causes cell competition in mammalian cells. J. Cell Sci. 125:59–66. 10.1242/jcs.085803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., and Wieschaus E.. 1980. Mutations affecting segment number and polarity in Drosophila. Nature. 287:795–801. 10.1038/287795a0 [DOI] [PubMed] [Google Scholar]
- Ohsawa S., Sugimura K., Takino K., Xu T., Miyawaki A., and Igaki T.. 2011. Elimination of oncogenic neighbors by JNK-mediated engulfment in Drosophila. Dev. Cell. 20:315–328. 10.1016/j.devcel.2011.02.007 [DOI] [PubMed] [Google Scholar]
- Osmani N., Vitale N., Borg J.P., and Etienne-Manneville S.. 2006. Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr. Biol. 16:2395–2405. 10.1016/j.cub.2006.10.026 [DOI] [PubMed] [Google Scholar]
- Pagliarini R.A., and Xu T.. 2003. A genetic screen in Drosophila for metastatic behavior. Science. 302:1227–1231. 10.1126/science.1088474 [DOI] [PubMed] [Google Scholar]
- Pearson H.B., Perez-Mancera P.A., Dow L.E., Ryan A., Tennstedt P., Bogani D., Elsum I., Greenfield A., Tuveson D.A., Simon R., and Humbert P.O.. 2011. SCRIB expression is deregulated in human prostate cancer, and its deficiency in mice promotes prostate neoplasia. J. Clin. Invest. 121:4257–4267. 10.1172/JCI58509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson H.B., McGlinn E., Phesse T.J., Schlüter H., Srikumar A., Gödde N.J., Woelwer C.B., Ryan A., Phillips W.A., Ernst M., et al. 2015. The polarity protein Scrib mediates epidermal development and exerts a tumor suppressive function during skin carcinogenesis. Mol. Cancer. 14:169 10.1186/s12943-015-0440-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit M.M., Crombez K.R., Vervenne H.B., Weyns N., and Van de Ven W.J.. 2005a The tumor suppressor Scrib selectively interacts with specific members of the zyxin family of proteins. FEBS Lett. 579:5061–5068. 10.1016/j.febslet.2005.08.012 [DOI] [PubMed] [Google Scholar]
- Petit M.M., Meulemans S.M., Alen P., Ayoubi T.A., Jansen E., and Van de Ven W.J.. 2005b The tumor suppressor Scrib interacts with the zyxin-related protein LPP, which shuttles between cell adhesion sites and the nucleus. BMC Cell Biol. 6:1 10.1186/1471-2121-6-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K., Shimoni R., Charnley M., Ludford-Menting M.J., Hawkins E.D., Ramsbottom K., Oliaro J., Izon D., Ting S.B., Reynolds J., et al. 2015. Asymmetric cell division during T cell development controls downstream fate. J. Cell Biol. 210:933–950. 10.1083/jcb.201502053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips H.M., Rhee H.J., Murdoch J.N., Hildreth V., Peat J.D., Anderson R.H., Copp A.J., Chaudhry B., and Henderson D.J.. 2007. Disruption of planar cell polarity signaling results in congenital heart defects and cardiomyopathy attributable to early cardiomyocyte disorganization. Circ. Res. 101:137–145. 10.1161/CIRCRESAHA.106.142406 [DOI] [PubMed] [Google Scholar]
- Phua D.C., Humbert P.O., and Hunziker W.. 2009. Vimentin regulates scribble activity by protecting it from proteasomal degradation. Mol. Biol. Cell. 20:2841–2855. 10.1091/mbc.e08-02-0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguel N.H., Fievre S., Blanc J.M., Carta M., Moreau M.M., Moutin E., Pinheiro V.L., Medina C., Ezan J., Lasvaux L., et al. 2014. Scribble1/AP2 complex coordinates NMDA receptor endocytic recycling. Cell Reports. 9:712–727. 10.1016/j.celrep.2014.09.017 [DOI] [PubMed] [Google Scholar]
- Qin Y., Capaldo C., Gumbiner B.M., and Macara I.G.. 2005. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J. Cell Biol. 171:1061–1071. 10.1083/jcb.200506094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H.E., and Portela M.. 2017. Tissue growth and tumorigenesis in Drosophila: cell polarity and the Hippo pathway. Curr. Opin. Cell Biol. 48:1–9. 10.1016/j.ceb.2017.03.006 [DOI] [PubMed] [Google Scholar]
- Richier L., Williton K., Clattenburg L., Colwill K., O’Brien M., Tsang C., Kolar A., Zinck N., Metalnikov P., Trimble W.S., et al. 2010. NOS1AP associates with Scribble and regulates dendritic spine development. J. Neurosci. 30:4796–4805. 10.1523/JNEUROSCI.3726-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rives-Quinto N., Franco M., de Torres-Jurado A., and Carmena A.. 2017. Synergism between canoe and scribble mutations causes tumor-like overgrowth via Ras activation in neural stem cells and epithelia. Development. 144:2570–2583. 10.1242/dev.148171 [DOI] [PubMed] [Google Scholar]
- Robinson A., Escuin S., Doudney K., Vekemans M., Stevenson R.E., Greene N.D., Copp A.J., and Stanier P.. 2012. Mutations in the planar cell polarity genes CELSR1 and SCRIB are associated with the severe neural tube defect craniorachischisis. Hum. Mutat. 33:440–447. 10.1002/humu.21662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui M., Qian J., Liu L., Cai Y., Lv H., Han J., Jia Z., and Xie W.. 2017. The neuronal protein Neurexin directly interacts with the Scribble-Pix complex to stimulate F-actin assembly for synaptic vesicle clustering. J. Biol. Chem. 292:14334–14348. 10.1074/jbc.M117.794040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni M.J., Pontarotti P., Birnbaum D., and Borg J.P.. 2002. The LAP family: a phylogenetic point of view. Trends Genet. 18:494–497. 10.1016/S0168-9525(02)02738-5 [DOI] [PubMed] [Google Scholar]
- Segbert C., Johnson K., Theres C., van Fürden D., and Bossinger O.. 2004. Molecular and functional analysis of apical junction formation in the gut epithelium of Caenorhabditis elegans. Dev. Biol. 266:17–26. 10.1016/j.ydbio.2003.10.019 [DOI] [PubMed] [Google Scholar]
- Seong E., Yuan L., and Arikkath J.. 2015. Cadherins and catenins in dendrite and synapse morphogenesis. Cell Adhes. Migr. 9:202–213. 10.4161/19336918.2014.994919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouloudaki K., Puetz M., Simons M., Courbard J.R., Boehlke C., Hartleben B., Engel C., Moeller M.J., Englert C., Bollig F., et al. 2009. Scribble participates in Hippo signaling and is required for normal zebrafish pronephros development. Proc. Natl. Acad. Sci. USA. 106:8579–8584. 10.1073/pnas.0811691106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., and Bamji S.X.. 2011. β-Pix modulates actin-mediated recruitment of synaptic vesicles to synapses. J. Neurosci. 31:17123–17133. 10.1523/JNEUROSCI.2359-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T., Yang L., Kaur H., Pestel J., Looso M., Nolte H., Krasel C., Heil D., Krishnan R.K., Santoni M.J., et al. 2017. A reverse signaling pathway downstream of Sema4A controls cell migration via Scrib. J. Cell Biol. 216:199–215. 10.1083/jcb.201602002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Aiga M., Yoshida E., Humbert P.O., and Bamji S.X.. 2009. Scribble interacts with beta-catenin to localize synaptic vesicles to synapses. Mol. Biol. Cell. 20:3390–3400. 10.1091/mbc.e08-12-1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundell G.N., Arnold R., Ali M., Naksukpaiboon P., Orts J., Güntert P., Chi C.N., and Ivarsson Y.. 2018. Proteome-wide analysis of phospho-regulated PDZ domain interactions. Mol. Syst. Biol. 14:e8129 10.15252/msb.20178129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa S., Nagasaka K., Nakagawa S., Yano T., Nakagawa K., Yasugi T., Takeuchi T., Kanda T., Huibregtse J.M., Akiyama T., and Taketani Y.. 2006. Human scribble, a novel tumor suppressor identified as a target of high-risk HPV E6 for ubiquitin-mediated degradation, interacts with adenomatous polyposis coli. Genes Cells. 11:453–464. 10.1111/j.1365-2443.2006.00954.x [DOI] [PubMed] [Google Scholar]
- Tanentzapf G., and Tepass U.. 2003. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat. Cell Biol. 5:46–52. 10.1038/ncb896 [DOI] [PubMed] [Google Scholar]
- Tepass U., and Knust E.. 1993. Crumbs and stardust act in a genetic pathway that controls the organization of epithelia in Drosophila melanogaster. Dev. Biol. 159:311–326. 10.1006/dbio.1993.1243 [DOI] [PubMed] [Google Scholar]
- Tepass U., Theres C., and Knust E.. 1990. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 61:787–799. 10.1016/0092-8674(90)90189-L [DOI] [PubMed] [Google Scholar]
- Tissir F., and Goffinet A.M.. 2013. Shaping the nervous system: role of the core planar cell polarity genes. Nat. Rev. Neurosci. 14:525–535. 10.1038/nrn3525 [DOI] [PubMed] [Google Scholar]
- Uhlirova M., Jasper H., and Bohmann D.. 2005. Non-cell-autonomous induction of tissue overgrowth by JNK/Ras cooperation in a Drosophila tumor model. Proc. Natl. Acad. Sci. USA. 102:13123–13128. 10.1073/pnas.0504170102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughen J., and Igaki T.. 2016. Slit-Robo Repulsive Signaling Extrudes Tumorigenic Cells from Epithelia. Dev. Cell. 39:683–695. 10.1016/j.devcel.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese S., Waghmare I., Kwon H., Hanes K., and Kango-Singh M.. 2012. Scribble acts in the Drosophila fat-hippo pathway to regulate warts activity. PLoS One. 7:e47173 10.1371/journal.pone.0047173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh G.S., Grant P.K., Morgan J.A., and Moens C.B.. 2011. Planar polarity pathway and Nance-Horan syndrome-like 1b have essential cell-autonomous functions in neuronal migration. Development. 138:3033–3042. 10.1242/dev.063842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S., Meyer A.S., Weiler S.M.E., Rupp C., Tóth M., Sticht C., Singer S., Thomann S., Roessler S., Schorpp-Kistner M., et al. 2018. Cytoplasmic localization of the cell polarity factor scribble supports liver tumor formation and tumor cell invasiveness. Hepatology. 67:1842–1856. 10.1002/hep.29669 [DOI] [PubMed] [Google Scholar]
- Wang X., Dong B., Zhang K., Ji Z., Cheng C., Zhao H., Sheng Y., Li X., Fan L., Xue W., et al. 2018. E-cadherin bridges cell polarity and spindle orientation to ensure prostate epithelial integrity and prevent carcinogenesis in vivo. PLoS Genet. 14:e1007609 10.1371/journal.pgen.1007609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansleeben C., Feitsma H., Montcouquiol M., Kroon C., Cuppen E., and Meijlink F.. 2010. Planar cell polarity defects and defective Vangl2 trafficking in mutants for the COPII gene Sec24b. Development. 137:1067–1073. 10.1242/dev.041434 [DOI] [PubMed] [Google Scholar]
- Windler S.L., and Bilder D.. 2010. Endocytic internalization routes required for delta/notch signaling. Curr. Biol. 20:538–543. 10.1016/j.cub.2010.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölwer C.B., Gödde N., Pase L.B., Elsum I.A., Lim K.Y., Sacirbegovic F., Walkley C.R., Ellis S., Ohno S., Matsuzaki F., et al. 2017. The Asymmetric Cell Division Regulators Par3, Scribble and Pins/Gpsm2 Are Not Essential for Erythroid Development or Enucleation. PLoS One. 12:e0170295 10.1371/journal.pone.0170295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D.F., Hough C., Peel D., Callaini G., and Bryant P.J.. 1996. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J. Cell Biol. 134:1469–1482. 10.1083/jcb.134.6.1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Pastor-Pareja J.C., and Xu T.. 2010. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature. 463:545–548. 10.1038/nature08702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Ohsawa S., Kunimasa K., and Igaki T.. 2017. The ligand Sas and its receptor PTP10D drive tumour-suppressive cell competition. Nature. 542:246–250. 10.1038/nature21033 [DOI] [PubMed] [Google Scholar]
- Yamben I.F., Rachel R.A., Shatadal S., Copeland N.G., Jenkins N.A., Warming S., and Griep A.E.. 2013. Scrib is required for epithelial cell identity and prevents epithelial to mesenchymal transition in the mouse. Dev. Biol. 384:41–52. 10.1016/j.ydbio.2013.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., and Mlodzik M.. 2015. Wnt-Frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt). Annu. Rev. Cell Dev. Biol. 31:623–646. 10.1146/annurev-cellbio-100814-125315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.C., Graves H.K., Moya I.M., Tao C., Hamaratoglu F., Gladden A.B., and Halder G.. 2015. Differential regulation of the Hippo pathway by adherens junctions and apical-basal cell polarity modules. Proc. Natl. Acad. Sci. USA. 112:1785–1790. 10.1073/pnas.1420850112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates L.L., Schnatwinkel C., Hazelwood L., Chessum L., Paudyal A., Hilton H., Romero M.R., Wilde J., Bogani D., Sanderson J., et al. 2013. Scribble is required for normal epithelial cell-cell contacts and lumen morphogenesis in the mammalian lung. Dev. Biol. 373:267–280. 10.1016/j.ydbio.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara K., Ikenouchi J., Izumi Y., Akashi M., Tsukita S., and Furuse M.. 2011. Phosphorylation state regulates the localization of Scribble at adherens junctions and its association with E-cadherin-catenin complexes. Exp. Cell Res. 317:413–422. 10.1016/j.yexcr.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Zarbalis K., May S.R., Shen Y., Ekker M., Rubenstein J.L., and Peterson A.S.. 2004. A focused and efficient genetic screening strategy in the mouse: identification of mutations that disrupt cortical development. PLoS Biol. 2:E219 10.1371/journal.pbio.0020219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitler J., Hsu C.P., Dionne H., and Bilder D.. 2004. Domains controlling cell polarity and proliferation in the Drosophila tumor suppressor Scribble. J. Cell Biol. 167:1137–1146. 10.1083/jcb.200407158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L., Rosenberg A., Bergami K.C., Yu M., Xuan Z., Jaffe A.B., Allred C., and Muthuswamy S.K.. 2008. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 135:865–878. 10.1016/j.cell.2008.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yeh S., Appleton B.A., Held H.A., Kausalya P.J., Phua D.C., Wong W.L., Lasky L.A., Wiesmann C., Hunziker W., and Sidhu S.S.. 2006. Convergent and divergent ligand specificity among PDZ domains of the LAP and zonula occludens (ZO) families. J. Biol. Chem. 281:22299–22311. 10.1074/jbc.M602902200 [DOI] [PubMed] [Google Scholar]
- Zheng W., Umitsu M., Jagan I., Tran C.W., Ishiyama N., BeGora M., Araki K., Ohashi P.S., Ikura M., and Muthuswamy S.K.. 2016. An interaction between Scribble and the NADPH oxidase complex controls M1 macrophage polarization and function. Nat. Cell Biol. 18:1244–1252. 10.1038/ncb3413 [DOI] [PubMed] [Google Scholar]
- Zhu J., Shang Y., and Zhang M.. 2016a Mechanistic basis of MAGUK-organized complexes in synaptic development and signalling. Nat. Rev. Neurosci. 17:209–223. 10.1038/nrn.2016.18 [DOI] [PubMed] [Google Scholar]
- Zhu Q., Le Scolan E., Jahchan N., Ji X., Xu A., and Luo K.. 2016b SnoN Antagonizes the Hippo Kinase Complex to Promote TAZ Signaling during Breast Carcinogenesis. Dev. Cell. 37:399–412. 10.1016/j.devcel.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žigman M., Trinh A., Fraser S.E., and Moens C.B.. 2011. Zebrafish neural tube morphogenesis requires Scribble-dependent oriented cell divisions. Curr. Biol. 21:79–86. 10.1016/j.cub.2010.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]