Alper and Zanic highlight new findings from Sallé et al. about the forces that can control asymmetric cell division.
Abstract
Asymmetric cell division relies on microtubule-based forces to asymmetrically position the mitotic apparatus. In this issue, Sallé et al. (2019. J. Cell Biol. https://doi.org/10.1083/jcb.201807102) use magnetic tweezers to induce asymmetric division in sea urchin zygotes, demonstrating that asymmetry could arise from a time-dependent weakening of centering forces.
Cell division is a highly orchestrated process, critical to the growth and development of multicellular organisms. In symmetrically dividing cells, the cell division plane is precisely centered, ensuring each of the daughter cells inherits half of the genetic and nongenetic material when separated from her sister cell. However, there are many cases in which a cell divides asymmetrically; for example, this is the norm for budding yeast. Complex organisms require certain cell divisions to be asymmetric, particularly during early development as an essential driver of cell specialization. Even when cells divide asymmetrically, the position of the division plane defines what each daughter inherits from the mother.
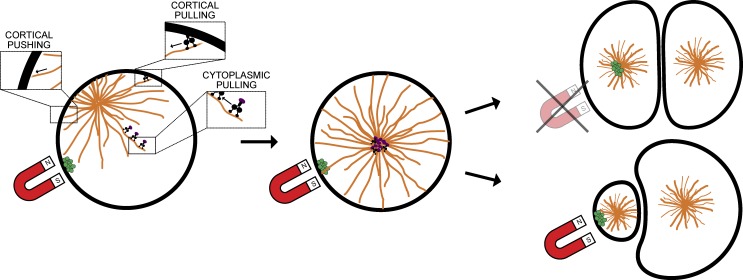
A critical question underlying these processes is: how does the cell control the location of the division plane? Much evidence suggests the ultimate location of the division plane relies on mechanical forces that position relevant intracellular components, including the nucleus, centrosomes, and spindle. Eukaryotic cells generally use stiff, dynamic microtubule polymers and their associated molecular motors to exert these forces. Three types of microtubule-based forces have been identified in the positioning process (Fig. 1). When growing microtubules encounter the cell perimeter, they can push against the cell cortex; such pushing forces acting all around the cell perimeter provide a robust centering mechanism (1). If microtubule ends become anchored at the cell cortex, the combination of microtubule depolymerization and the action of cortical dynein motors can exert pulling forces that reel in the microtubules, along with any intracellular components attached to them, toward the cell periphery. These cortical pulling forces typically act to decenter the cellular structures, although they may also drive centering, if precisely balanced (2, 3). In addition to forces exerted at the cortical interface, microtubule-based molecular motors can exert forces along the length of the microtubule track throughout the cytoplasm (4–6). These types of forces are known as the cytoplasmic pulling forces.
Figure 1.
Asymmetric division occurs through a reduction of microtubule centering forces in sea urchin zygotes. Magnetic forces localize motor proteins attached to magnetic beads (green) at the cell cortex (black), but nuclear microtubule aster (orange) centration, potentially driven by cortical pushing, cortical pulling, and cytoplasmic pulling mechanisms (insets), is unaffected by the presence of these asymmetric force generators (left). Once centration is complete (middle), the centering forces are attenuated and the beads bias the microtubule aster toward the cortex in the presence of magnetic forces, but migrate to the centered aster in the absence of magnetic forces. Ultimately, the position of the division plane, be it symmetric or asymmetric, is determined by the position of the aster (right).
Traditional approaches to investigate the positioning mechanisms of cell division machinery include genetic perturbations of key molecular players and laser severing of specific microtubule subpopulations. More recently, researchers have used magnetic tweezers to directly apply and measure the mechanical forces involved in spindle positioning and nuclear migration. In a pioneering study, Garzon-Coral et al. (1) injected magnetic beads into single-cell Caenorhabditis elegans embryos and measured the effective stiffness and damping of the spindle centering machinery. Building on this approach, Tanimoto et al. (7) recently measured the centering forces of sperm asters in large, symmetrically dividing sea urchin embryos.
In this issue, Sallé et al. used magnetic tweezers to create “cortical caps” of dynein motors locally concentrated on magnetic beads, effectively inducing an artificial asymmetry in the distribution of cortical force generators within sea urchin eggs (Fig. 1). They found that locally elevated concentrations of cortical motors did not affect sperm pronucleus centering, resulting in an initially centered zygote nucleus upon fertilization. Even when the sperm entered the egg near the cortical cap, the male pronucleus moved toward the center, unaffected by the cortical cap. Only after fusing with the female pronucleus did the zygote nucleus move toward the cortical cap, ultimately resulting in an asymmetric cell division of a cell that would normally divide symetrically.
Sallé et al. (8) measured the force required to maintain the cortical cap during centration and decentration, and found that this force remained constant during the entire process. However, when they allowed the magnetic beads to aggregate at the aster center by lowering the magnetic force during the initial centering, and subsequently applied the magnetic force, they found it was much easier to decenter the previously centered aster. Together, these results suggest that asymmetry arose through a significant reduction in aster centering forces after the initial centration was complete.
What molecular processes drive the proposed temporal evolution of the microtubule-based centering forces in sea urchin zygotes? Because centering forces were likely produced by dynein motors in the cytoplasm pulling on astral microtubules, regulation may involve temporal changes in microtubule network organization or dynein motor activity. However, Sallé et al. (8) did not observe substantial differences in the radial distribution of microtubules following the centration phase. Similarly, a global reduction in dynein activity after centration would have slowed centripetally moving beads upon their release from the magnetic trap, contrary to Sallé et al.’s (8) observations. In contrast, if cytoplasmic pulling forces were a consequence of the drag forces associated with centripetal vesicle transport (as suggested by Kimura et al. [9]), once the transported vesicles had all accumulated at the cell center, this centration mechanism would no longer be at the cell’s disposal. Thus, the accumulation of the cargo at the cell center would result in weakening of the cortical pulling forces, allowing the decentering phase to take over.
Sallé et al. (8) demonstrate a novel possible mechanism leading to asymmetric cell division and their findings raise many important questions. Specifically, this work highlights the necessity to identify the cargo and the dynein–cargo linkers involved in the cytoplasmic pulling process. Previous studies in C. elegans have implicated a role for endomembrane compartments in cytoplasmic pulling, it would be interesting to know whether similar cargos play this role in sea urchin embryos. Additionally, tracking individual cargos in real time during and after centration and perturbing the relative levels and spatiotemporal distributions of motors and cargo would provide critical insights into the underlying mechanisms of force regulation.
Asymmetric cell divisions are typically encoded by asymmetric localization of key molecular factors. In budding yeast, dynein asymmetrically localizes to spindle poles. Asymmetric divisions of C. elegans zygotes rely on asymmetric localization of the PAR polarity proteins. In addition to such spatial regulation, earlier studies of the temporal regulation of centering forces in C. elegans provided evidence that asymmetric cortical pulling forces act before the onset of asymmetric spindle positioning, but are balanced by tethering of astral microtubules to the cortex on the opposite side of the spindle (10). It is only upon release of these tethers that asymmetric positioning took place. Thus, temporal regulation of centering forces may represent a general mechanism used by cells in asymmetric divisions. The work by Sallé et al. (8) demonstrates that, even in cells that divide symmetrically, the forces that drive centering may become weaker over time. Therefore, both spatial and temporal regulation of centering and decentering mechanisms ultimately leads to either asymmetric or symmetric cell division.
Cell division is a universal process that takes place in cells of remarkably diverse sizes and shapes. Sallé et al. (8) provided us with a glimpse into the downstream mechanisms driving this process in sea urchin embryos. More broadly, geometric cues from a cell’s environment, including orientation of a cell’s long axis before division (Hertwig’s rule), cell–cell adhesions, mechanical stresses, and the topology of a cell’s neighbors, strongly influence the location of a cell’s division plane. Given the richness of upstream signaling mechanisms that drive the symmetry or asymmetry of cell division, future work is necessary to determine the complex integration of distinct forces driving cell fate in vastly diverse cellular contexts.
Acknowledgments
J. Alper would like to acknowledge financial support from Clemson University, a National Institutes of Health (NIH) National Institute of General Medical Sciences grant (P20GM109094) and an NIH National Institute of Allergy and Infectious Diseases (grant R15AI137979). M. Zanic acknowledges the support from the Human Frontier Science Program, the Searle Scholars Program, and the NIH grant R35GM119552.
The authors declare no competing financial interests.
References
- 1.Garzon-Coral C., et al. . 2016. Science. 352:1124–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grill S.W., et al. . 2001. Nature. 409:630–633. [DOI] [PubMed] [Google Scholar]
- 3.Laan L., et al. . 2012. Cell. 148:502–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamaguchi M.S., and Hiramoto Y.. 1986. Dev. Growth Differ. 28:143–156. [DOI] [PubMed] [Google Scholar]
- 5.Kimura A., and Onami S.. 2005. Dev. Cell. 8:765–775. [DOI] [PubMed] [Google Scholar]
- 6.Wühr M., et al. . 2010. Curr. Biol. 20:2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanimoto H., et al. . 2018. Nat. Phys. 14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sallé J., et al. J. Cell Biol. 2019 doi: 10.1083/jcb.201807102. [DOI] [Google Scholar]
- 9.Kimura K., and Kimura A.. 2011. Proc. Natl. Acad. Sci. USA. 108:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labbé J.-C., et al. . 2004. J. Cell Biol. 167:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]