Abstract
Hui details the cell biology underlying the action of immune checkpoint inhibitors.
Immunotherapy, harnessing the immune system to combat cancers, has yielded promising results in patient care. Monoclonal antibodies that block the “immune checkpoint” through PD-1, PD-L1, or CTLA-4 have demonstrated impressive clinical activities against a variety of tumors (1). However, durable benefit is only observed in a small fraction of patients. Identifying patients that will likely respond and extending the therapies to a larger population both require a better understanding of PD-1 and CTLA-4 function at the cell biological level. Here, the current knowledge of the cell biological mechanisms of PD-1, PD-L1, and CTLA-4 and their blocking antibodies is summarized.
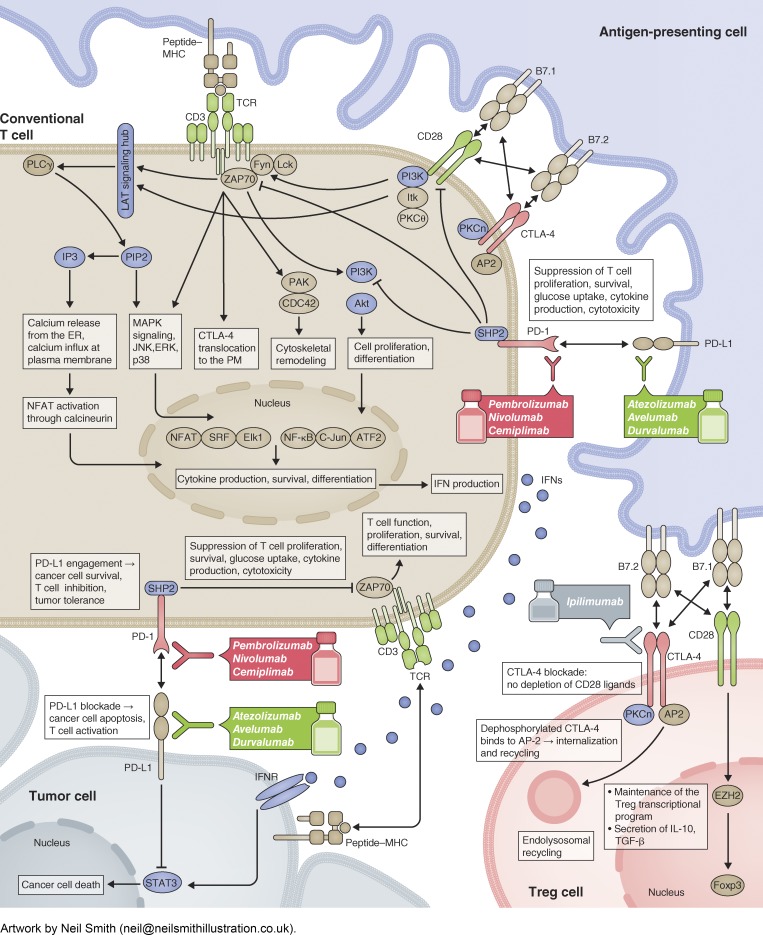
The ability of conventional T (Tcon) cells to target tumor cells depends on two types of signals. The first is an antigen-specific signal through the T cell receptor (TCR). TCR recognizes a peptide antigen presented by major histocompatibility complex (MHC) molecules on tumor cells or tumor-infiltrating antigen-presenting cells (APCs), and converts the extracellular binding event to highly coordinated intracellular signaling cascades that lead to T cell proliferation, cytokine production, and cytolytic activities. Initially, TCR-associated CD3 subunits become phosphorylated and recruit and activate the kinase ZAP70. ZAP70 phosphorylates the membrane adaptor LAT, leading to multivalent interactions between LAT, adaptors, and enzymes to form a signaling hub at the membrane. The LAT “signalosome” triggers Ca2+ signaling, cytoskeleton remodeling, and MAPK signaling to activate the T cell transcriptional program (2). The second type of signal is antigen-unspecific, mediated by cosignaling receptors—costimulatory, increasing the T cell response, or coinhibitory, attenuating T cell activity (3)—triggered by ligands on tumor cells or tumor-infiltrating APCs. CD28 is a prominent costimulatory receptor, whereas PD-1 and CTLA-4 are coinhibitory receptors. Upon binding to its ligand B7-1 or B7-2, displayed by APCs but not tumor cells, CD28 is phosphorylated and recruits kinases PKCθ, ITK, and PI3K to facilitate TCR signaling.
PD-1 on T cells is activated by its ligand PD-L1, expressed by diverse cell types, and present on exosomes. PD-L1/PD-1 binding triggers PD-1 phosphorylation and recruitment of the SHP2 phosphatase. PD-1–associated SHP2 dephosphorylates CD28 and TCR signaling components to inhibit the T cell response. Mechanisms other than SHP2 may exist, as suggested by a SHP2 knockout study (4). While operating as a brake to restrict over-reactive T cells and autoimmunity, PD-1 can be hijacked by tumors to evade immune surveillance. Normally, PD-1 expression on T cells is induced by TCR signaling and decreases to basal levels upon antigen clearance. Persistent antigen stimulation in the tumor microenvironment can lead to constitutively high PD-1 expression. Moreover, a variety of mechanisms can up-regulate PD-L1 in tumor tissues. Besides operating as the PD-1 ligand, PD-L1 was recently shown to inhibit interferon/STAT3-mediated apoptosis of tumor cells (5). The FDA has approved several PD-L1/PD-1 blocking antibodies for cancer immunotherapy, including anti-PD-1 (Pembrolizumab, Nivolumab, and Cemiplimab) and anti-PD-L1 (Atezolizumab, Avelumab, and Durvalumab).
CTLA-4 shares ligands with CD28: B7-1 and B7-2. Due to its higher affinity to B7 molecules, CTLA-4 can outcompete CD28 for these ligands. In Tcon cells, CTLA-4 is largely localized to intracellular vesicles and delivered to the cell surface upon TCR stimulation (6). CTLA-4 surface levels critically determine the response to self-antigens or immunotherapy. Importantly, mounting evidence establishes a pivotal role of CTLA-4 on regulatory T (Treg) cells—T cells with suppressive activity. Treg-intrinsic CTLA-4 is able to deplete B7 molecules from APCs via trans-endocytosis (7), which occurs in a PKCη-promoted manner (8). Many believe that CTLA-4 blockade antibodies (e.g., Ipilimumab) work primarily by blocking or depleting Tregs. Interestingly, Tregs in the tumor microenvironment express higher surface levels of CTLA-4 than Tregs at other sites (9). To what extent blocking Tcon-intrinsic CTLA-4 contributes to the therapeutic response is unknown.
Cosignaling receptors other than CTLA-4 are less well understood in Tregs. CD28 appears to support Treg function. Treg-intrinsic CD28 induces Ezh2 (10), a chromatin-modifying enzyme that up-regulates the Treg-maintaining transcription factor Foxp3. The function of PD-1 in Tregs is controversial and how PD-1/PD-L1 blockade affects Tregs needs further investigation.
Lastly, while tumor immunity is mostly studied in the context of T cells, more work is needed to understand the contributions of other immune cells, such as macrophages, dendritic cells, neutrophils, and natural killer cells (11). The quickly developing field of immune checkpoint blockade fuels renewed interest in the fundamental cell biological mechanisms of T cell function and regulation in the tumor microenvironment.
Acknowledgments
E. Hui is a Searle Scholar and a Pew Scholar in Biomedical Sciences.
The author declares no competing financial interests.
References
- 1.Ribas A., and Wolchok J.D.. 2018. Science. 359:1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huse M. 2009. J. Cell Sci. 122:1269–1273. [DOI] [PubMed] [Google Scholar]
- 3.Chen L., and Flies D.B.. 2013. Nat. Rev. Immunol. 13:227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rota G., et al. 2018. Cell Reports. 23:39–49. [DOI] [PubMed] [Google Scholar]
- 5.Gato-Canas M., et al. 2017. Cell Reports. 20:1818–1829. [DOI] [PubMed] [Google Scholar]
- 6.Walker L.S., and Sansom D.M.. 2015. Trends Immunol. 36:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou T.Z., et al. 2015. J. Immunol. 194:2148–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong K.F., et al. 2014. Nat. Immunol. 15:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson T.R., et al. 2013. J. Exp. Med. 210:1695–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DuPage M., et al. 2015. Immunity. 42:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassidy M.R., et al. 2017. EBioMedicine. 18:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]