Abstract
Hypothalamic Agrp neurons are critical regulators of food intake in adult mice. In addition to food intake, these neurons have been involved in other cognitive processes, such as the manifestation of stereotyped behaviors. Here, we evaluated the extent to which Agrp neurons modulate mouse behavior in spatial memory-related tasks. We found that activation of Agrp neurons did not affect spatial learning but altered behavioral flexibility using a modified version of the Barnes Maze task. Furthermore, using the Y-maze test to probe working memory, we found that chemogenetic activation of Agrp neurons reduced spontaneous alternation behavior mediated by the neuropeptide Y receptor-5 signaling. These findings suggest novel functional properties of Agrp neurons in memory-related cognitive processes.
Keywords: Memory, cognition, animal model, hypothalamus
Graphical abstract
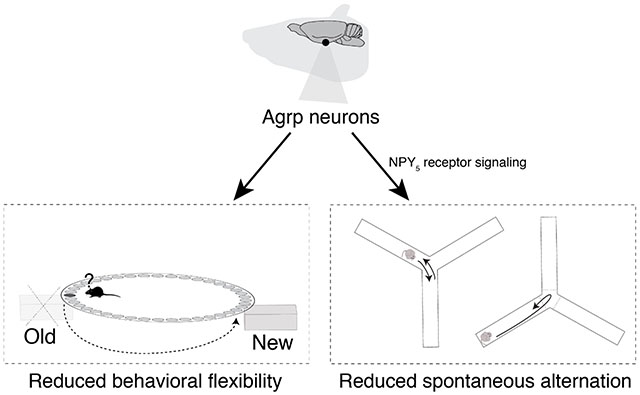
Introduction
Animals need to adapt different behavioral strategies in times of caloric needs to ensure survival. For instance, animals rely on spatial memory and working memory to exploit nutritional supplies and decision-making [1]. In fact, it is the strong influence of hunger on the behavior of the animal that has ignited the study of animal behavior since Pavlov [2, 3]. Agouti-related protein (Agrp)-producing neurons, located in the arcuate nucleus of the hypothalamus, are critical regulators of food intake in mice [4–7]. In addition to food intake, Agrp neurons are involved in behaviors that are not proximally involved in food ingestion [8–10]. For example, chemogenetic activation of Agrp neurons elicits repetitive and compulsive behaviors when mice are tested in the absence of food [9]. Despite these previous findings, we still know little about the influence of Agrp neurons in memory-related cognitive functions.
Here, we performed behavioral assays to assess memory-related cognitive processes in mice under conditions of Agrp neuron activation. We found that chemogenetic activation of Agrp neurons modulate the performance of mice in behavior tasks involving spatial learning and working memory.
Methods
Animals
All mice used in the experiments were 4–8 months old from both genders. AgrpTrpv1 mice and control animals are AgrpCreTm/+ :: Trpv1−/− ::R26-LSL-Trpv1Gt and Trpv1−/− :R26-LSL-Trpv1Gt/+, respectively [9, 11]. Similarly, AgrpTrpv1:VgatKO and control animals are AgrpCreTm/+:: Trpv1−/−::R26-LSL-Trpv1Gt/+::VgatFlox.Flox and Trpv1−/−::R26-LSL-Trpv1Gt/+::VgatFlox.Flox. AgrpCre and R26LSL-Trpv1, respectively. VgatFlox.Flox mice were backcrossed to Trpv1KO mice. All mice were kept in temperature- and humidity-controlled rooms, in a 12/12 hr light/dark cycle, with lights on from 7:00 AM–7:00 PM. Food and water were provided ad libitum unless otherwise stated. All procedures were approved by IACUC (Yale University).
Drugs
Drugs used were: capsaicin (3.33% Tween-80 in PBS; from Sigma) and CGP71683 hydrochloride (hereinafter referred as neuropeptide Y receptor 5 (NPY5R) antagonist) (in 5% DMSO, 5% Tween-80 in water; from Tocris). All drugs were injected in a volume of 10 ml/kg of body weight intraperitoneally (i.p.). Route of administration and dose of drugs were established according to previous study published by our group [9].
Modified Barnes Maze
A schematic representation of the Barnes Maze is given in Figure 1A. The Barnes Maze consists of a white elevated circular platform (91 × 122 cm) with 40 equidistant holes located around the edges. A dark Plexiglas escape chamber was placed under one of the holes (target hole) in which the animals could hide. Four reference cues were presented in the walls surrounding the maze. Animals were tested under a 300-watt light to create an aversive environment in the surface of the apparatus due to the bright illumination. Before each trial, mice were placed in a dark enclosed start box positioned in the center of the maze. Ten seconds later, the box was lifted, and animals were allowed to explore the maze. The protocol consisted of four days of training (learning or acquisition phase) in which animals were allowed to explore the apparatus for 180 seconds during each trial. During the intervals, animals returned to the home cage. At day 5, we tested the effect of activation of Agrp neurons in the recall of memory. Mice were not tested at days 6 and 7. At day 8, we re-tested mice in the maze to evaluate any long-lasting effects of Agrp neuron activation at day 5. At day 9, we performed the probe trial in which the escape chamber was removed, and animals were allowed to explore the maze for 180 seconds to evaluate exploratory behavior and behavior search strategy to find an alternative escape route upon activation of Agrp neurons. We performed four trials a day for each animal with an inter-trial interval of 15 minutes. During the inter-trial interval, mice were placed in the home cage and no food was provided. At day 13, we performed a reversal learning trial of 180 seconds after injection of capsaicin to all mice. Latency to reach the escape chamber and total distance traveled were recorded and measured automatically by a video tracking system (ANY-maze software, Stoelting Co, Wood Dale, IL).
Figure 1: Activation of Agrp neurons does not impair spatial learning.
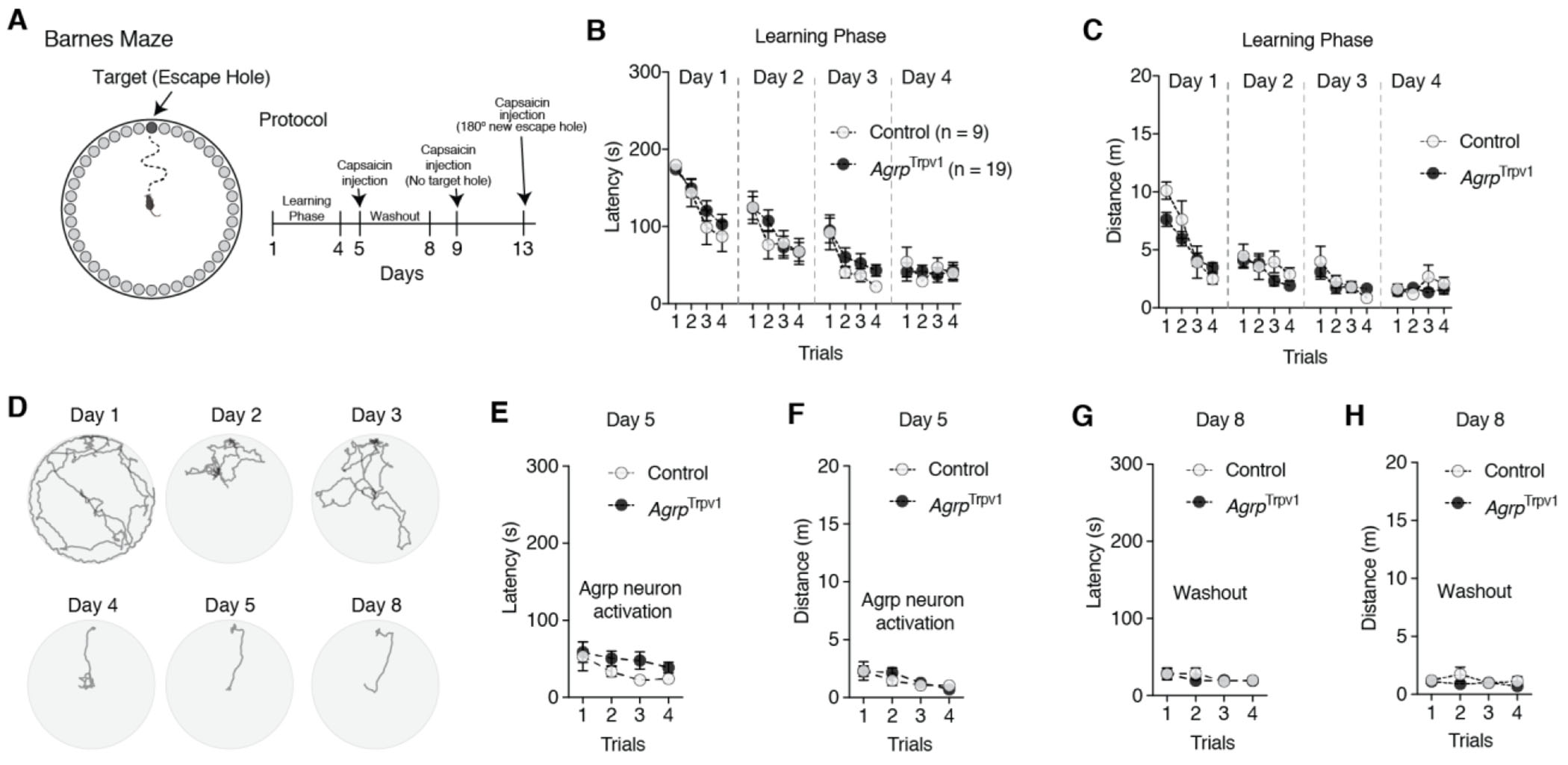
(A) Illustrative representation of Barnes Maze and experimental protocol. On days 5, 9, and 13 they received an injection of capsaicin (10 mg/kg, i.p.) before trials. Control (n = 9) and AgrpTrpv1 mice (n = 19) were tested. (B-C) Latency and distance traveled to reach the escape hole in the learning phase during trials across days. (D) Tracking data from a control animal representing one trial per day during the learning phase. (E-F) Latency and distance traveled to reach the escape hole upon Agrp neuron activation. (G-H) Latency and distance traveled to reach the escape hole three days after the injection of capsaicin, to probe the long-lasting effects of Agrp neuron activation. In B, C, E, F, G, and H symbols represent mean ± SEM. Statistically significant P values are provided in the panels.
Spontaneous alternation behavior (Y-Maze)
The Y-maze consisted of a black Plexiglas apparatus with three symmetrical enclosed arms (30 cm long, 8 cm wide and 15 cm high) at 120° angle from each other. The arms converged on an equilateral triangular center platform (5 × 5 × 5 cm). Animals were recorded under infrared illumination during the dark cycle. Prior to testing, animals received an injection of capsaicin (10 mg/kg, i.p.). Testing began when animals were placed in one arm of the Y-maze and allowed to explore the environment for 10 minutes. For AgrpTrpv1:VgatKO mice were placed in one arm of the Y-maze and allowed to explore the environment for 15 minutes. An animal was considered to enter an arm when 85% of the body surface was within the arm. The number of arm entries and the sequence of entries were recorded using a video tracking software (Any-Maze software, Stoelting Co., Wood Dale, IL). In the indicated experiments, NPY5R antagonist (30 mg/kg, i.p.) or vehicle were administered 30 minutes prior injection of capsaicin. Spontaneous alternation behavior was calculated as the ratio of correct alternations (A) to possible alternations (number of arm entries – 2) (Figure 3A). A correct alternation is considered when animals visit the three different arms of the Y-maze consecutively (e.g., ABC, ACB, CBA). The number of correct alternations is a conserved measure of working memory processes.
Figure 3: Activation of Agrp neurons impairs spontaneous alternation behavior in mice.
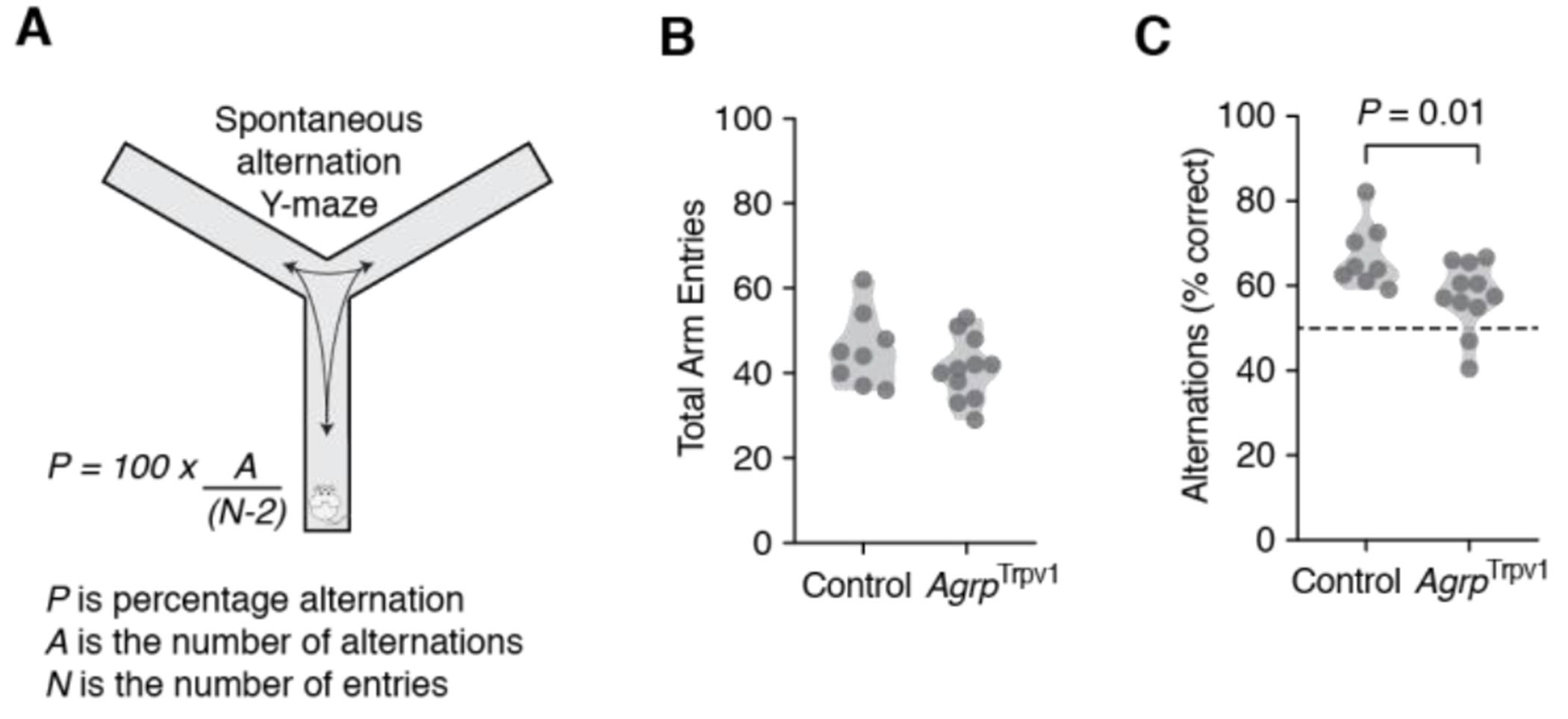
(A) Diagram illustrating the use of the Y-maze test to measure spontaneous alternation behavior in mice. Control (n = 8) and AgrpTrpv1 mice (n = 11) were tested immediately after receiving an injection of capsaicin (10 mg/kg, i.p.). (B) Violin plots represent the distribution in the total number of arm entries between groups. (C) Violin plots represent the distribution in the proportion of correct alternations between groups. Symbols represent individual values. Statistically significant P values are provided in the panels.
Statistical analysis and data plotting
Prism 8.0 was used to analyze data and plot figures. All figures were edited in Adobe Illustrator CS6/CC. Data were first subjected to a normality test using the D’Agostino & Pearson normality test or the Shapiro-Wilk normality test. When homogeneity was assumed, a parametric analysis of variance test was used. Two-way ANOVA with repeated measures was used to compare multiple groups and days/trials of testing. When necessary, Greenhouse-Geisser estimates of sphericity were used to correct for degrees of freedom. Holm-Sidak’s multiple comparisons test was used to find post-hoc differences among groups. The student’s t-test (paired and unpaired) and the Mann-Whitney U test were used to determine significance between two groups. Chi-square test was used to analyze the differences in the use of search strategies in the Barnes Maze. Statistical data are provided in the figures. P < 0.05 was considered statistically significant.
Results
To investigate to what extent Agrp neurons influence cognitive performance in memory-related tests that do not involve food cues, we tested the effect of the chemogenetic activation of these neurons in a modified version of the Barnes Maze test (Figure 1A). To activate Agrp neurons, we used AgrpTrpv1 mice [9, 11], AgrpTrpv1 mice expressed the Trpv1 receptor exclusively in the Agrp neurons. Trpv1 is a calcium receptor activated by capsaicin, its exogenous ligand. Thus, this animal model allows the chemogenetic activation of Agrp neurons by peripheral injection of capsaicin.
Unsurprisingly, during the learning phase both control and AgrpTrpv1 mice decreased the latency to reach the escape chamber [Figure 1B and 1D; day 1 (effect of trial, F3,78 = 22.31, P < 0.001; effect of genotype, F1,26 = 0.40, P = 0.53; interaction, F3,78 = 0.57, P = 0.64); day 2 (effect of trial, F3,78 = 7.65, P = 0.002; effect of genotype, F1,26 = 0.12, P = 0.73; interaction, F3,78 = 0.86, P = 0.47); day 3 (effect of trial, F3,78 = 11.02, P < 0.001; effect of genotype, F1,26 = 1.06, P = 0.31; interaction, F3,78 = 0.30, P = 0.83); day 4 (effect of trial, F3,78 = 0.74, P = 0.53; effect of genotype, F1,26 = 0.01, P = 0.91; interaction, F3,78 = 1.06, P = 0.37)] and the distance traveled [Figure 1C; day 1 (effect of trial, F3,78 = 36.30, P < 0.001; effect of genotype, F1,26 = 0.76, P = 0.39; interaction, F3,78 = 3.32, P = 0.024); day 2 (effect of trial, F3,78 = 3.24, P = 0.027; effect of genotype, F1,26 = 1.25, P = 0.2743; interaction, F3,78 = 0.86, P = 0.46); day 3 (effect of trial, F3,78 = 6.72, P = 0.0004; effect of genotype, F1,26 = 0.05, P = 0.82; interaction, F3,78 = 0.92, P = 0.43); day 4 (effect of trial, F3,78 = 0.99, P = 0.40; effect of genotype, F1,26 = 0.49, P = 0.49; interaction, F3,78 = 2.17, P = 0.09)] to a similar degree.
At day 5, chemogenetic activation of Agrp neurons by injection of capsaicin in AgrpTrpv1 mice did not alter the performance of mice in the Barnes Maze as demonstrated by the latency to enter the escape chamber (Figure 1E; effect of trial, F3,78 = 3.46, P = 0.02; effect of genotype, F1,26 = 1.26, P = 0.27; interaction, F3,78 = 0.50, P = 0.68) and the distance traveled in the apparatus (Figure 1F; effect of trial, F3,78 = 5.62, P = 0.0015; effect of genotype, F1,26 = 0.16, P = 0.69; interaction, F3,78 = 0.63, P = 0.60). We then evaluated whether testing animals upon chemogenetic activation of Agrp neurons had any long-lasting effects on behavior performance. At day 8, we tested the animals once again for four trials without injection of capsaicin. We did not observe any statistical differences in the latency to reach the escape chamber (Figure 1G; effect of trial, F3,78 = 1.91, P = 0.13; effect of genotype, F1,26 = 0.15, P = 0.71; interaction, F3,78 = 0.65, P = 0.58) or in the distance traveled in the apparatus (Figure 1H; effect of trial, F3,78 = 1.02, P = 0.39; effect of genotype, F1,26 = 1.22, P = 0.28; interaction, F3,78 = 1.22, P = 0.31). Thus, in mice that learnt the contingency of a spatial memory task that does not involve food rewards, chemogenetic activation of Agrp neurons does not disrupt the retrieval of the memory, the motivation to perform the test, and the long-term retrieval of the memory.
Next, we performed a probe trial with no escape hole (Figure 2A). Typically, the probe trial consists of a single short trial [12, 13]. However, we opted to run four trials to investigate the dynamic changes in behavior upon chemogenetic activation of Agrp neurons. During the probe trials, mice searched around the target area for the escape chamber (Figure 2B-F and Movie S1), corroborating activation of Agrp neurons did not alter memory recall (similar to Figure 1). Compared to control mice, chemogenetic activation of Agrp neurons led to a relative increase in the exploration of the target quadrant of the apparatus where the escape hole was previously located (Figure 2G-H; P = 0.002 using Holm-Sidak’s multiple comparisons test after two-way ANOVA: effect of quadrant, F2,52 = 184.6, P < 0.0001; effect of genotype, F1,26 = 2.52, P = 0.12; interaction, F2,52 = 6.41, P = 0.003 – all trials combined) and a decrease in the time exploring adjacent quadrants (Figure 2G-H; P = 0.02).
Figure 2: Activation of Agrp neurons alters spatial navigation in probe trials.
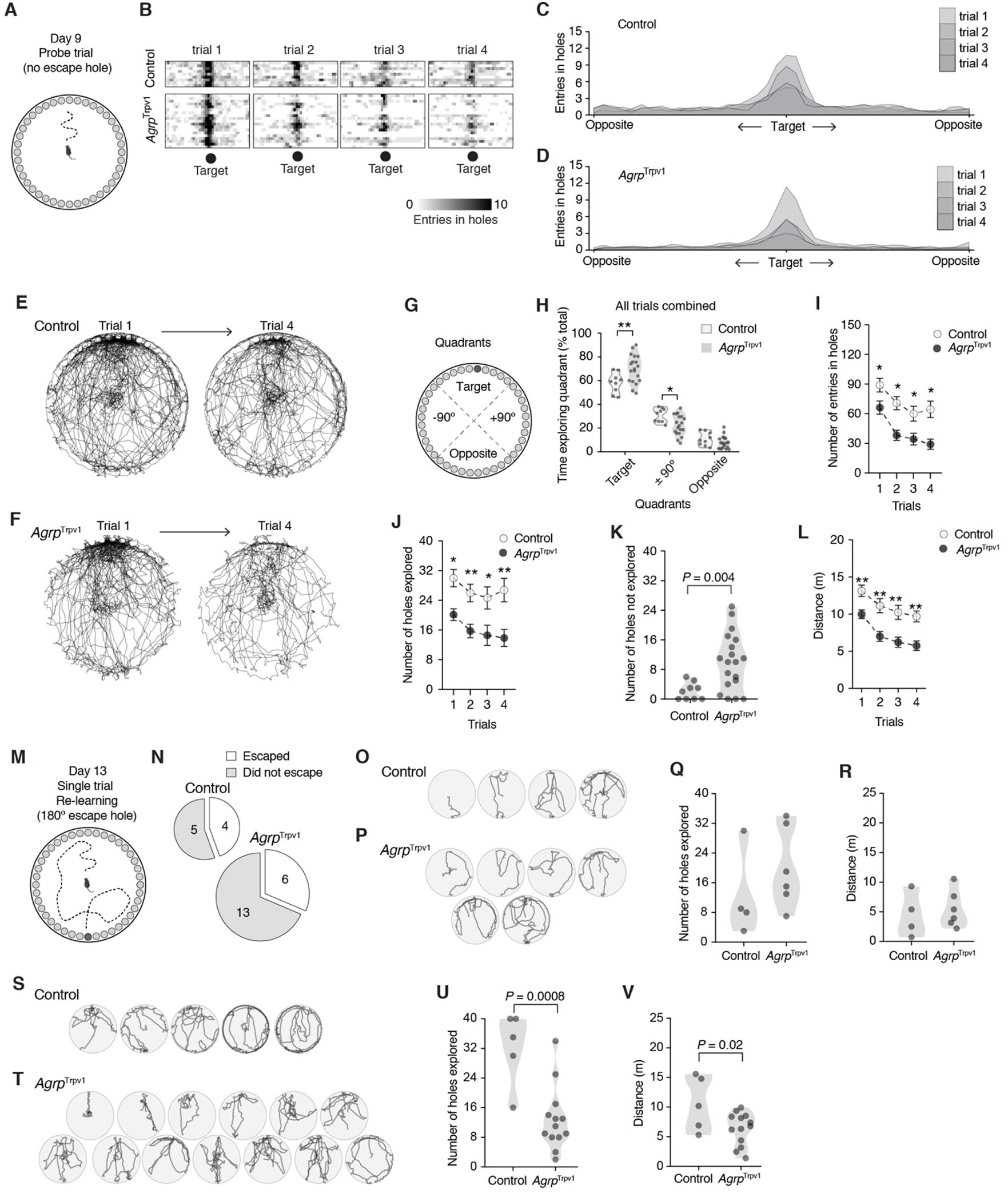
(A) Illustration of the Barnes Maze in the probe trials with the removal of the escape hole. (B) Heat plot showing the number of entries in each hole (X axis) across the four trials at day 9 (each box is one trial; each row is one mouse). (C-D) Quantification of data from B in the form of histogram distribution. (E-F) Cumulative superimposed tracking plots of control (n = 9) and AgrpTrpv1 mice (n = 9 randomly displayed to equal the number of control mice) in the first and the fourth trials of the probe test. (G) Illustration of the quadrants in the Barnes Maze in the probe trial. (H) Proportion of time mice spent exploring the quadrants in all probe trials combined. (I) Total number of entries in holes across the probe trials. (J) Total number of holes explored (out of 40 total) across the probe trials. (K) Total number of holes not explored during the probe test (4 trials combined; out of 40 holes). (L) Total distance traveled across the probe trials. (M) Illustration of the reversal learning trial with a new escape chamber placed at 180° of the original target hole. (N) Proportion of mice that escaped the Barnes Maze by entering the new escape chamber. (O-P) Tracking plots of all mice that escaped the trial by entering the new escape chamber. (Q) Total number of holes explored (out of 40 total) in the trial by the animals that entered the new escape hole. (R) Similar to Q, but the total distance traveled in the trial. (S-T) Similar to O-P, tracking plots of all mice that did not escape the apparatus during the reversal learning trial. (U) Similar to Q, but for mice that did not escape. (V) Similar to R, but for mice that did not escape. In I, J, and L symbols represent mean ± SEM. In H, K, Q, R, U, and V symbols represent individual values. Statistically significant P values are provided in the panels. For multiple comparisons tests, * denotes P < 0.05 and ** denotes P < 0.01.
We also measured other indexes of exploratory behavior during the probe trials. The total number of hole entries (Figure 2I; effect of trial, F2.57,67.05 = 5.39, P = 0.003; effect of genotype, F1,26 = 11.73, P = 0.002; interaction, F3,78 = 0.46, P = 0.70) and the total number of holes explored (Figure 2J; effect of trial, F2.46,63.99 = 23.50, P < 10−8; effect of genotype, F1,26 = 12.24, P = 0.001; interaction, F3,78 = 0.96, P = 0.41) were reduced upon chemogenetic activation of Agrp neurons. Concomitantly, the number of holes not explored across all four trials was increased in AgrpTrpv1 mice (Figure 2K; t23.66 = 4.15, P = 0.004, unpaired t test with Welch’s correction). In line with the decreased investigation of alternative holes to exit the apparatus, chemogenetic activation of Agrp neurons significantly decreased the distance traveled compared to control mice (Figure 2L; effect of trial, F3,78 = 23.17, P < 0.0001; effect of genotype, F1,26 = 17.10, P = 0.0003; interaction, F3,78 = 0.39, P = 0.76). Thus, in a probe trial with no escape chamber, chemogenetic activation of Agrp neurons seem to impair exploratory behavior and searching behavior for a new escape alternative, skewing mouse behavior towards previously acquired navigation strategies.
To further understand the consequences of these changes in search behavior, we ran another trial on day 13, in which we placed a new escape chamber at 180° from the original target hole (Figure 2M). Four out of 9 control mice and 6 out of 19 AgrpTrpv1 mice found the new escape chamber (Figure 2N; x21 = 0.44, P1-tail = 0.25, chi-squared test; and Figure 2O-P). We did not find a significant difference in the number of holes explored (Figure 2Q; t8 = 1.03, P = 0.33, unpaired t test) or the distance traveled in the apparatus between control and AgrpTrpv1 mice that were able to enter the new escape chamber (Figure 2R; t8 = 0.45, P = 0.66, unpaired t test). We next analyzed the behavior of mice that did not enter the new escape chamber during the trial (Figure 2S-T). In this subgroup of mice, chemogenetic activation of Agrp neurons decreased the total number of holes explored (Figure 2U; t16 = 4.102, P = 0.0008, unpaired t test) and the distance traveled in the apparatus (Figure 2V; t16 = 2.43, P = 0.02, unpaired t test) compared to control mice. Thus, the results from the modified Barnes Maze test implies that chemogenetic activation of Agrp neurons in mice suppresses the searching behavior to find an escape alternative, suggesting that in more natural conditions the overactivation of these neurons can impair behavior flexibility.
One possibility for the impaired behavior flexibility upon chemogenetic activation of Agrp neurons in the Barnes Maze probe trials is altered working memory. To more directly test for working memory, we measured the spontaneous alternation behavior of mice (Figure 3A). Spontaneous alternation is conserved across mammals [14, 15] and comprises the tendency of animals to alternate arms when exploring a maze [16–18]. The Y-maze test used here evaluates the spontaneous alternation behavior as a measurement of spatial working memory [19–21], which is independent of previous training or the use of food rewards. Chemogenetic activation of Agrp neurons did not alter the number of arm entries (Figure 3B; control: 45.75 ± 3.12, n = 8; AgrpTrpv1: 41.00 ± 2.26, n = 11; t17 = 1.26, P = 0.22, unpaired t test) but led to a reduced proportion of correct spontaneous alternations towards random choices (Figure 3C; control: 67.01 ± 2.69 %; AgrpTrpv1: 57.49 ± 2.42 %; t17 = 2.60, P = 0.01, unpaired t test). This result suggest chemogenetic activation of Agrp neurons reduces the performance in a test of working memory in mice.
Next, we investigated whether GABA release by Agrp neurons was involved in the impaired spontaneous alternation behavior upon chemogenetic Agrp neuronal activation. We generated AgrpTrpv1 mice with impaired GABA release selectively from Agrp neurons (Figure 4A) [22]. Using this mouse model, chemogenetic activation of Agrp neurons did not significantly change the number of arm entries (Figure 4B; control: 59.75 ± 4.92, n = 12; AgrpTrpv1:VgatKO: 57.00 ± 5.91, n = 9; t19 = 0.35, P = 0.72, unpaired t test) but decreased the number of spontaneous alternations (Figure 4C; control: 69.87 ± 2.01 %; AgrpTrpv1:VgatKO: 62.14 ± 2.53 %; t19 = 2.42, P = 0.02, unpaired t test) similar to our previous results (Figure 3). Thus, GABA release by Agrp neurons does not seem to mediate the reduced spontaneous alternation behavior upon chemogenetic activation of Agrp neurons.
Figure 4: NPY signaling, but not GABA release by Agrp neurons, blunts the effect of Agrp neuron activation on spontaneous alternation behavior.
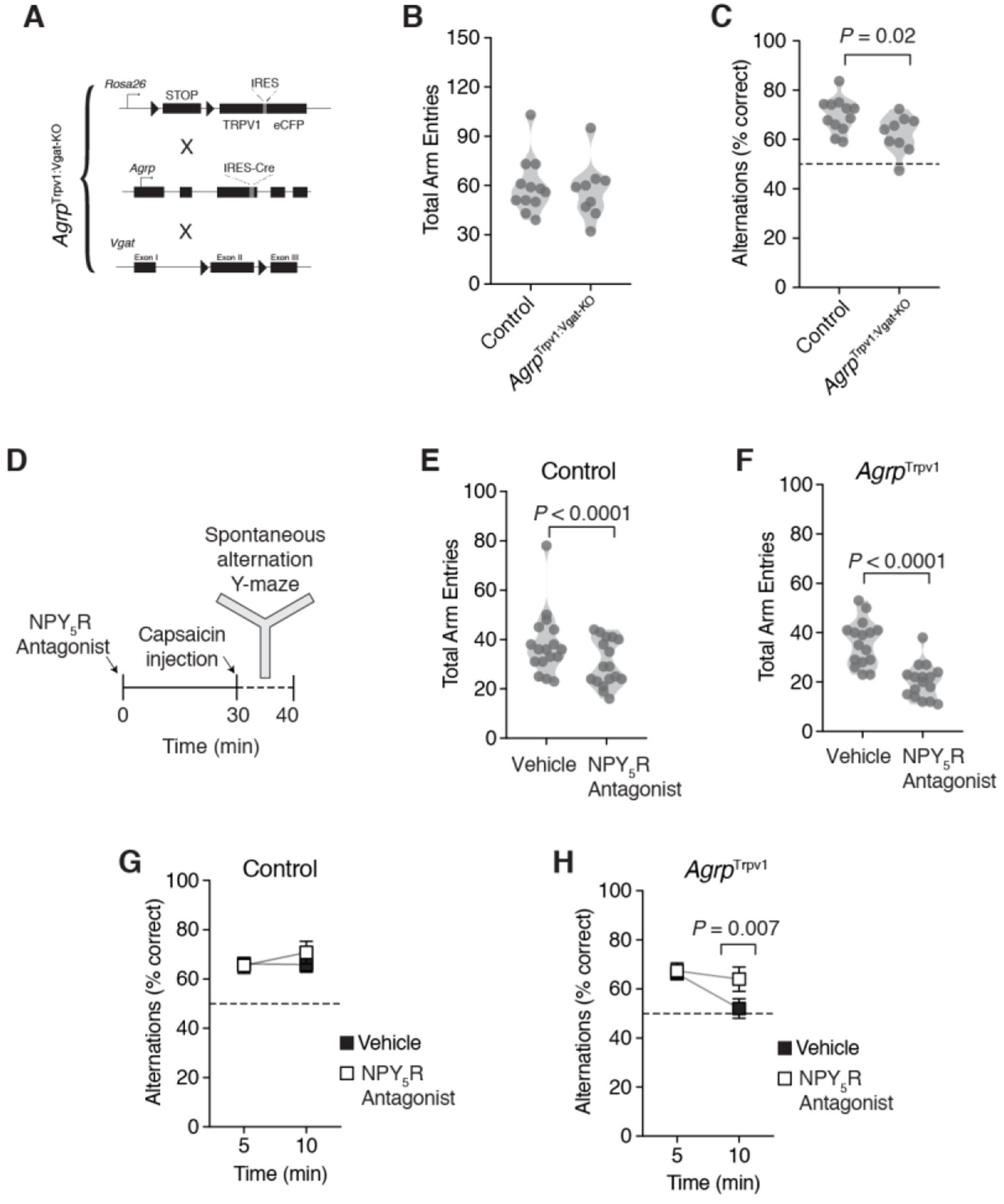
(A) Schematic strategy to generate the AgrpTrpv1:VgatKO mice. Mice are the result of a triple breeding cross; all lines were backcrossed to a Trpv1 knockout background. Control (n = 12) and AgrpTrpv1:VgatKO mice (n = 9) were tested immediately after receiving an injection of capsaicin (10 mg/kg, i.p.). (B) Violin plots represent the distribution in the total number of arm entries between groups. (C) Violin plots represent the distribution in the proportion of correct alternations between groups. (D) Study design showing pre-treatment of mice with the NPY5 receptor antagonist (CGP71683, 30 mg/kg, i.p.) or vehicle thirty minutes before the injection of capsaicin (10 mg/kg, i.p.). Control (n = 17) and AgrpTrpv1 mice (n = 16) were tested in the Y-maze immediately after the injection of capsaicin. The same animals were tested twice, in response to vehicle or CGP71683. Mice were randomly assigned to the different groups using a crossover study design. (E-F) Violin plots represent the distribution in the total number of arm entries in control and AgrpTrpv1 mice, respectively. (G-H) Proportion of correct alternations in five-minute bins in control and AgrpTrpv1 mice, respectively. In B, C, E and F symbols represent individual values. In G and H, symbols represent mean ± SEM. Statistically significant P values are provided in the panels.
Agrp neurons also release neuropeptide Y (NPY), which signal mainly through downstream NPY1 and NPY5 receptors [23–26]. In a previous study, we demonstrated that repetitive behaviors driven by the chemogenetic activation of Agrp neurons were mediated by NPY5 receptor signaling [9]. Hence, we sought to test the role of NPY5 receptor signaling in spontaneous alternation behavior upon chemogenetic Agrp neuron activation (Figure 4D). The NPY5 receptor antagonist CGP71683 (30 mg/kg, i.p.) reduced the total number of arm entries in both control (Figure 4E; vehicle: 38.18 ± 3.13, n = 17; NPY5R antagonist: 30.35 ± 2.24, n = 17; t16 = 3.17, P = 0.005, paired t test) and AgrpTrpv1 mice (Figure 4F; vehicle: 35.88 ± 2.28, n = 16; NPY5R antagonist: 20.25 ± 1.76, n = 16; t15 = 5.91, P < 0.0001, paired t test) compared to vehicle injected animals. In control animals, treatment with the NPY5R antagonist did not significantly change spontaneous alternation behavior (Figure 4G; effect of time, F1,16 = 1.23, P = 0.28; effect of drug treatment, F1,16 = 0.31, P = 0.58; interaction, F1,16 = 0.41, P = 0.52, two-way ANOVA with time as a repeated-measure). On the other hand, treatment of AgrpTrpv1 mice with the NPY5R antagonist blunted the reduction in spontaneous alternation behavior observed upon chemogenetic activation of Agrp neurons (Figure 4H; effect of time, F1,15 = 7.29, P = 0.01; effect of drug treatment, F1,15 = 2,15, P = 0.16; interaction, F1,15 = 4.70, P = 0.04, two-way ANOVA with time as a repeated-measure). Together, these findings suggest that Agrp neurons are capable of altering cognitive performance in mice at least partially via NPY5 receptor signaling in behavior tests that probe working memory and do not involve food related cues.
Discussion
Agrp neurons are classically involved in the promotion of hunger [5, 6]. This study provides support for the capacity of hypothalamic Agrp neurons to alter cognitive processes in behavior tasks that do not involve food cues or food rewards. More specifically, our findings suggest that chemogenetic activation of Agrp neurons can acutely shift the behavior repertoire of mice in memory-related tests, impairing the use of working memory and suppressing exploratory searching behavior towards a non-food related escape goal.
Animals use spatial memory to navigate and to recall their past locations [27]. Spatial learning and memory are assessed in rodents using a variety of approaches, such as the Morris Water Maze, the Barnes Maze, and the Radial Arm Maze [27–33]. Here, we used the Barnes Maze test as the least stressful option compared to the Morris Water Maze as it does not require mice to swim in cold water. Additionally, the Barnes Maze does not require food rewards as often used in the Radial Arm Maze [13, 34]. Using the Barnes Maze, we found that chemogenetic activation of Agrp neurons was capable of suppressing searching behavior for a new escape chamber when the previously memorized escape location was either removed or altered. This behavior could result from drive competition, in which activation of Agrp neurons would generate a motivational drive presumably for food that suppresses the drive to explore the aversive environment and find a new escape route [35]. However, (1) chemogenetic activation of Agrp neurons did not alter behavior performance during trials in which the escape chamber was at a previously memorized location; and (2) in the probe trials, mice in which the Agrp neurons were activated showed skewed exploration of the previously memorized escape hole compared to alternative holes. Thus, the motivation to find the escape chamber seem to be preserved upon chemogenetic activation of Agrp neurons, which would argue against a competition between motivational drives. An alternative explanation to these findings would be that activation of Agrp neurons alters behavioral flexibility [36–38] and working memory, favoring previously established memories.
The Y-maze test used to assess spontaneous alternation behavior [14–18] measures spatial working memory [19–21]. In this test, a score of 50% indicates random choice between two arms [39]. Performance below 50% indicates repetitive/perseverative choices [40, 41], while a performance above 50% indicates use of working memory in making behavior choices [42]. We found that chemogenetic activation of Agrp neurons impairs the performance in the task towards 50% performance, suggesting random choices and impaired use of working memory. The impaired performance in the spontaneous alternation test was mediated, at least partially, by NPY5 receptor signaling. These results are similar to a previous report from our laboratory demonstrating that chemogenetic activation of Agrp neurons led to repetitive, stereotypic behaviors via NPY5 receptor signaling [9]. In our studies we used peripheral injection of a pharmacological antagonist of NPY5 receptor signaling. Intriguingly, the same dose and route of administration of this pharmacological antagonist did not suppress Agrp neuron activation driven food intake in mice [9]. Together, these results suggest that the repetitive behaviors and reduced performance in the Y-maze test upon chemogenetic activation of Agrp neurons are likely not the product of the same cognitive processes involved in food intake. Alternatively, because NPY5 receptors are expressed in several downstream targets of Agrp neurons [26, 43] as well as in cortex and hippocampus [43–45], our studies cannot precisely identify which NPY5 receptor expressing neurons are important to blunt the effects of Agrp neurons on spontaneous alternation behavior. It could be that upon activation of Agrp neurons in testing conditions with no food available, neurons that express the NPY5 receptor - and are not direct targets of Agrp neurons - control the expression of these altered cognitive processes. Future studies should address this question using more specific tools to perform discrete manipulations of NPY signaling whilst conducting similar behavioral assessments of working memory.
Agrp neurons are considered bona fide motivational drivers of food intake. Our behavior results may suggest a broader view of the drive generated by the activity of these neurons, i.e. hunger. Agrp neuron activity seems to signal an ‘energy storage’ drive, rather than a drive specific to food appetite. In this case, the drive would be transmitted to other neuronal networks that would take the most adaptive solution to obtain energy, to decrease energy expenditure, and to store excess energy in body supplies (e.g., fat). In situations of increased food supply, food intake is the most adaptive solution to increase energy storage. Conversely, in conditions when food sources are scarcely available, decreasing the expenditure of energy in seemingly superfluous activities would be the most adaptive solution. Thus, in the case of the Barnes Maze test, when the escape chamber is removed or difficult to find (as in animals that did not enter the chamber in the reversal learning trial), Agrp neuron activity suppresses the search for the new solution, as this would presumably be the most cost-effective solution energetically. In support of this model, we and others have investigated the effects of Agrp neuron activity in a variety of behavior tests and have never found a strong suppression in the exploratory drive [9, 35, 46] as reported in the probe trials of the Barnes Maze. In fact, activation of Agrp neurons is typically related to increased activity levels that are interpreted as foraging activity in anticipation of food intake [9, 35, 46]. Comparisons between the physiological and behavioral processes controlled by Agrp neurons as well as other neurons involved in motivational states would help to clarify the extent to which this framework refers to what we denominate ‘hunger’ or refers to a more distinct motivational state. Were this model to hold, it would be intriguing to see the performance of mice in tests that involve food rewards, life-threatening conditions, and more naturalistic settings when the environment is less deterministic than that in the laboratory.
Our results highlight the involvement of evolutionarily conserved Agrp neurons in the mammalian hypothalamus in the coordination of complex cognitive functions in conditions that do not necessarily - or proximally in an ethological perspective - involve metabolic or appetite control. They provide experimental evidence to inquire about the functional organization of the mammalian brain and how neurons that generate motivational states communicate with neuronal networks involved in memory recall and executive functions. Moreover, our findings can raise novel insights in the understanding of several human conditions characterized by metabolic and cognitive dysfunctions, including obesity, anorexia nervosa, and Prader-Willi Syndrome. Since neuropeptide Y receptor-5 antagonists have been tested in clinical studies in humans, these compounds could be promptly tested in these human conditions.
Supplementary Material
Acknowledgements
We thank lab members and Dr. Ifat Levy for their comments on this manuscript. M.O.D. was supported by a NARSAD Young Investigator Grant ID 22709 from the Brain & Behavior Research Foundation, by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health (R01DK107916), by a pilot grant from the Yale Diabetes Research Center (P30 DK045735), by the Yale Center for Clinical Investigation Scholar Award, by the Whitehall Foundation, by the Charles H. Hood Foundation, Inc. (Boston, MA) and by a pilot grant from the Modern Diet and Physiology Research Center (The John B. Pierce Laboratory). M.O.D. also received support from the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq) and Coordenadoria de Aperfeicoamento de Pessoal de Nivel Superior (CAPES), Brazil. M.R.Z. and A.S. were partially supported by scholarships from CNPq and CAPES. The authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Hulgard K, Ratcliffe JM Niche-specific cognitive strategies: object memory interferes with spatial memory in the predatory bat Myotis nattereri. J. Exp. Biol, 217 (18) (2014), pp. 3293–3300. [DOI] [PubMed] [Google Scholar]
- 2.Pavlov IP and Anrep GV, Conditioned Reflexes. 2003: Dover Publications. [Google Scholar]
- 3.Dietrich MO and Horvath TL, Limitations in anti-obesity drug development: the critical role of hunger-promoting neurons. Nature Reviews Drug Discovery, 2012. 11(9): p. 675–691. [DOI] [PubMed] [Google Scholar]
- 4.Shutter JR, et al. , Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev, 1997. 11(5): p. 593–602. [DOI] [PubMed] [Google Scholar]
- 5.Hahn TM, et al. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci, 1998. 1(4): p. 271–2. [DOI] [PubMed] [Google Scholar]
- 6.Rossi M, et al. , A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology, 1998. 139(10): p. 4428–31. [DOI] [PubMed] [Google Scholar]
- 7.Gropp E, et al. , Agouti-related peptide-expressing neurons are mandatory for feeding. Nature Neuroscience, 2005. 8(10): p. 1289–1291. [DOI] [PubMed] [Google Scholar]
- 8.Burnett CJ, et al. , Hunger-Driven Motivational State Competition. Neuron, 2016. 92(1): p. 187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietrich MO, et al. , Hypothalamic Agrp Neurons Drive Stereotypic Behaviors beyond Feeding. Cell, 2015. 160(6): p. 1222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padilla SL, et al. , Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat Neurosci, 2016. 19(5): p. 734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruan HB, et al. , O-GlcNAc Transferase Enables AgRP Neurons to Suppress Browning of White Fat. Cell, 2014. 159(2): p. 306–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sunyer B, et al. , Barnes maze, a useful task to assess spatial reference memory in the mice. 2007.
- 13.Barnes CA, Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol, 1979. 93(1): p. 74–104. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen A, et al. , Spontaneous alternation behavior in humans, in Proceedings of the 23rd ACM Symposium on Virtual Reality Software and Technology 2017, ACM: Gothenburg, Sweden, p. 1–4. [Google Scholar]
- 15.Richman CL, et al. , Spontaneous alternation behavior in animals: A review. 1986. 5(4): p. 358–391. [Google Scholar]
- 16.Tolman EC, Purpose and cognition: the determiners of animal learning. Psychological Review, 1925. 32(4): p. 285–297. [Google Scholar]
- 17.Lalonde R, The neurobiological basis of spontaneous alternation. Neuroscience & Biobehavioral Reviews, 2002. 26(1): p. 91–104. [DOI] [PubMed] [Google Scholar]
- 18.Dember WN and Fowler H, Spontaneous alternation behavior. Psychol Bull, 1958. 55(6): p. 412–28. [DOI] [PubMed] [Google Scholar]
- 19.Drew WG, Miller LL , and Baugh EL, Effects of delta9-THC, LSD-25 and scopolamine on continuous, spontaneous alternation in the Y-maze. Psychopharmacologia, 1973. 32(2): p. 171–82. [DOI] [PubMed] [Google Scholar]
- 20.Sarter M, Bodewitz G , and Stephens DN, Attenuation of scopolamine-induced impairment of spontaneous alteration behaviour by antagonist but not inverse agonist and agonist beta-carbolines. Psychopharmacology (Berl), 1988. 94(4): p. 491–5. [DOI] [PubMed] [Google Scholar]
- 21.Hiramatsu M, Hyodo T , and Kameyama T, U-50488H, a selective kappa-opioid receptor agonist, improves carbon monoxide-induced delayed amnesia in mice. Eur J Pharmacol, 1996. 315(2): p. 119–25. [DOI] [PubMed] [Google Scholar]
- 22.Tong Q, et al. , Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nature neuroscience, 2008. 11(9): p. 998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerald C, et al. , A receptor subtype involved in neuropeptide-Y-induced food intake. Nature, 1996. 382(6587): p. 168–71. [DOI] [PubMed] [Google Scholar]
- 24.Pedrazzini T, et al. , Cardiovascular response, feeding behavior and locomotor activity in mice lacking the NPY Y1 receptor. Nat Med, 1998. 4(6): p. 722–6. [DOI] [PubMed] [Google Scholar]
- 25.Kanatani A, et al. , Role of the Y1 receptor in the regulation of neuropeptide Y-mediated feeding: comparison of wild-type, Y1 receptor-deficient, and Y5 receptor-deficient mice. Endocrinology, 2000. 141(3): p. 1011–6. [DOI] [PubMed] [Google Scholar]
- 26.Wolak ML, et al. , Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol, 2003. 464(3): p. 285–311. [DOI] [PubMed] [Google Scholar]
- 27.Healy SD and Jozet-Alves C, Spatial Memory, in Encyclopedia of Animal Behavior, Breed MD and Moore J, Editors. 2010, Academic Press: Oxford, p. 304–307. [Google Scholar]
- 28.Cole CJ and Josselyn SA, 4.27 - Transcription Regulation of Memory: CREB, CaMKIV, Fos/Jun, CBP, and SRF, in Learning and Memory: A Comprehensive Reference, Byrne JH, Editor. 2008, Academic Press: Oxford: p. 547–566. [Google Scholar]
- 29.Sweatt JD, Chapter 4 - Rodent Behavioral Learning and Memory Models, in Mechanisms of Memory (Second Edition), Sweatt JD Editor. 2010, Academic Press: London, p. 76–103. [Google Scholar]
- 30.Carter M and Shieh J, Chapter 2 - Animal Behavior, in Guide to Research Techniques in Neuroscience (Second Edition), Carter M and Shieh J, Editors. 2015, Academic Press: San Diego, p. 39–71. [Google Scholar]
- 31.Inman-Wood SL, et al. , Effects of prenatal cocaine on Morris and Barnes maze tests of spatial learning and memory in the offspring of C57BL/6J mice. Neurotoxicology and Teratology, 2000. 22(4): p. 547–557. [DOI] [PubMed] [Google Scholar]
- 32.Holmes A, et al. , Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. 2002. 1(1): p. 55–69. [DOI] [PubMed] [Google Scholar]
- 33.D’Hooge R and De Deyn PP, Applications of the Morris water maze in the study of learning and memory. Brain Research Reviews, 2001. 36(1): p. 60–90. [DOI] [PubMed] [Google Scholar]
- 34.Bach ME, et al. , Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the theta frequency. Cell, 1995. 81(6): p. 905–15. [DOI] [PubMed] [Google Scholar]
- 35.Burnett CJ, et al. , Hunger-Driven Motivational State Competition. Neuron, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laredo SA, et al. , Effects of defeat stress on behavioral flexibility in males and females: modulation by the mu-opioid receptor. Eur J Neurosci, 2015. 41(4): p. 434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiserer RS, et al. , Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer’s disease. Genes Brain Behav, 2007. 6(1): p. 54–65. [DOI] [PubMed] [Google Scholar]
- 38.Yasumura M, et al. , IL1RAPL1 knockout mice show spine density decrease, learning deficiency, hyperactivity and reduced anxiety-like behaviours. Sci Rep, 2014. 4: p. 6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kokkinidis L and Anisman H, Interaction between cholinergic and catecholaminergic agents in a spontaneous alternation task. Psychopharmacology (Berl), 1976. 48(3): p. 261–70. [DOI] [PubMed] [Google Scholar]
- 40.Kokkinidis L and Anisman H, Perseveration and rotational behavior elicited by d-amphetamine in a Y-maze exploratory task: differential effects of intraperitoneal and unilateral intraventricular administration. Psychopharmacology (Berl), 1977. 52(2): p. 123–8. [DOI] [PubMed] [Google Scholar]
- 41.Kokkinidis L and Anisman H, Abatement of stimulus perseveration following repeated d-amphetamine treatment: absence of behaviorally augmented tolerance. Pharmacol Biochem Behav, 1978. 8(5): p. 557–63. [DOI] [PubMed] [Google Scholar]
- 42.Anisman H and Kokkinidis LJP, Effects of scopolamine, d-amphetamine and other drugs affecting catecholamines on spontaneous alternation and locomotor activity in mice. 1975. 45(1): p. 55–63. [Google Scholar]
- 43.Kask A, et al. , The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neuroscience & Biobehavioral Reviews, 2002. 26(3): p. 259–283. [DOI] [PubMed] [Google Scholar]
- 44.Grove KL, et al. , Neuropeptide Y Y5 receptor protein in the cortical/limbic system and brainstem of the rat: expression on γ-aminobutyric acid and corticotropin-releasing hormone neurons. Neuroscience, 2000. 100(4): p. 731–740. [DOI] [PubMed] [Google Scholar]
- 45.AOKI C and PICKEL VM, Neuropeptide Y in Cortex and Striatum. 1990. 611(1): p. 186–205. [DOI] [PubMed] [Google Scholar]
- 46.Krashes MJ, et al. , Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. The Journal of Clinical Investigation, 2011. 121(4): p. 1424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


