Abstract
Background: Early gastric carcinoma (EGC) with pure signet ring cell carcinoma (pSRCC) has been reported to have favourable prognosis and low risk of lymph node metastasis (LNM). However, risk factors of LNM and clinicopathological features for early gastric mixed signet ring cell carcinoma (mSRCC) remain poorly investigated. The aim of this study was to identify risk factors of LNM and compare clinicopathological characteristics and prognosis of early gastric pSRCC with mSRCC.
Methods: This retrospective study was conducted at our center between 2005 and 2015 in 796 patients underwent radical gastrectomies combined with lymph node dissections, A total of 160 patients with early gastric SRCC underwent gastrectomies with lymph node dissections were reviewed, in which 79 cases were pSRCC and 81 cases were mSRCC. Risk factors of LNM and clinicopathologic features of these two groups were statistically compared, including age, gender, tumor location, gross pattern, size, invasion depth, lymphovascular invasion (LVI), helicobacter pylori (Hp) infection, atrophic gastritis, ulcer finding and LNM. Patients were follow-up for post-resection survival. The 5-year survival and disease-specific survival rate were estimated with the Kaplain-Meier method with a log-rank test and compared between the two groups.
Results: Tumor size (P<0.05), invision depth (P<0.05) and LVI (P < 0.0001) were risk factors of LNM, LVI (P < 0.0001) was independent risk factor of LNM in 160 patients. Univariate analysis reviewed LVI (P < 0.0001) as the risk factor in the pSRCC group, and the risk factors of LNM in the mSRCC included LVI (P < 0.0001) and tumor size (P<0.05). Multivariable analysis revealed two independent risk factors in the mSRCC group: 1) tumor size (P < 0.05), and 2) LVI (P < 0.0001). The significant characteristics in two groups included the male gender (P < 0.0001), gross pattern (P < 0.05), LVI (P < 0.01), and Hp infection (P < 0.01). The difference of LNM rate between expanded indication and out of indication in 160 patients was significant (P=0.03). The overall 5-year survival rate for early gastric SRCC was 96.3%. There was no significant difference in the overall survival and disease-specific survival between the two groups.
Conclusions: Although with similar post-resection survival, the independent risk factors of LNM in the early mSRCC group, compared to those in the early pSRCC group, included large tumor size and LVI. Early gastric mSRCC had more aggressive clinicopathological features than pSRCC.
Keywords: Early gastric carcinoma (EGC), Pure signet ring cell cancer (pSRCC), Mixed signet ring cell cancer (mSRCC), Lymph node metastasis(LNM)
Introduction
Gastric cancer remains one of leading cancer in incidence and mortality in the world, especially for SRCC that is usually fatal because of wide-spread LNM and distant metastasis 1. However, patients with EGC resection would have a much better prognosis with the 5-year survival rate of over 90%, as reported in China, Japan, and Western countries 2. The standard clinical practice guidelines for treatment of EGC include radical gastrectomy with nodal dissection 3. Recent studies reveal a very low risk of LNM in EGC, including early gastric SRCC 4, suggesting a significant role of endoscopic resection in EGC therapy with the endoscopic techniques such as endoscopic mucosa resection (EMR) and endoscopic submucosal dissection (ESD) compared with surgical resection, endoscopic therapy has the same long-term therapeutic effect, better quality of life 5, and excellent prognosis 6-8.
Clinical decision on endoscopic, vs, surgical resection of EGC relies upon an accurate assessment of risk of LNM. Overall, tumor size, histological type, LVI, and depth of invasion are independent risk factors for LNM in EGC 9, while risk factors of LNM for early pSRCC and mSRCC stay elusive. Accordig to the World Health Organization (WHO) diagnostic criteria 10, SRCC was classified as part of gastric adenocarcinoma with dismal prognosis. Recently, Zheng et al. 11 reported that mixed gastric cancer has more aggressive features than the pure Lauren intestinal-type or diffuse-type gastric cancer, because of deeper infiltration, greater dimension, and more frequent LNM. However, the published studies on clinicopathological features and prognosis of early mSRCC are scarce.
The purpose of the present study was to investigate risk factors of LNM, clinicopathological characteristics, and prognosis of early gastric pSRCC and mSRCC to guide a better precision therapeutic strategy.
Methods
Patient selection and groups
A total of consecutive 796 patients were identified with radical gastrectomy and lymph node dissection for EGC at the Division of Digestive Surgery, Department of Surgery, the Nanjing Drum Tower Hospital over the period between 2005 and 2015. Each pathology report was reviewed for pathologic diagnosis on the basis of the WHO diagnostic criteria 10. In the cohort of 796 EGCs, 79 (9.9%) cases were diagnosed as pSRCC and 81 (10.2%) as mSRCC. This study was approved by the Ethics Committee of the Affiliated Drum Tower Hospital Affiliated to Nanjing University.
Clinicopathological analysis
Electronic patient medical records of each selected patient were reviewed for demographic information, medical history, surgical note, pathological report, postoperative hospital course, and therapeutic outcomes. Resected specimens were routinely processed with a standard pathology gastric cancer resection protocol. After gross examination, the specimen was transversely sectioned at the interval of 4 mm in width, embedded in paraffin, and routinely stained with hematoxylin and eosin. Two experienced pathologists independently retrieved and investigated each case blindly without the knowledge of clinical and endoscopic findings, several specialists discussed and reached a consensus in difficult cases.
The WHO diagnostic critiera for EGC was followed. As such, pSRCC was defined as a predominant component (>50%) isolated carcinoma cells containing intracytoplasmic mucin, and mSRCC was defined as adenocarcinoma with a minor component (10 - 50%) of isolated carcinoma cells containing intracytoplasmic mucin 10. Tumor location, maximal size, gross pattern (on the basis of the Paris classification) 12, invasion depth, which was divided as M (intramucosal) and SM (submucosal invasion), and LVI were also recorded. The total numbers of lymph nodes retrieved and involved by carcinoma in each case were abstracted from the pathology report. Poor differentiation as indiscernible tubules and glands in less than 50% of tumor on a section, and moderate differentiation with discernible glands/tubules between 50% and 95%, based on the Vienna criteria 13. Ulceration was defined as rupture of the muscularis mucosae and fibrosis in the SM layer by histological findings. Hp infection status was determined by a rapid urease test or more of the tests of biopsy specimens and any positive result defined Hp infection. The definition of atrophic gastritis is based on the criteria of the updated Sydney System for the classification of Gastritis 14. The definitions of expanded indication and out of indication were based on the Japanese classification of gastric carcinoma [Japanese Gastric Cancer Association, 2011].
Post-resection survival
Post-resection outcomes were investigated through routine scheduled in-office visits, which were documented in the patient electronic medical record. Telephone interview were performed routinely at 6, 12, and 18 months, and then annually after the surgery to assess the general situation of each patient by the investigators. Patient post-resection survival was calculated from the day of surgery to the day of the last follow-up interview or the day of death of any causes.
Statistical Analysis
Statistical analyses were carried out with SPSS 22.0 (IBM, Armonk, New York, USA). Quantitative data were expressed as mean ± standard deviation (SD). Absolute and relative frequencies for categorical variables were analyzed with rate. The two-tailed Student's t and the Mann Whitney U tests were used for analysis of normally distributed and non-normally continuous variables, respectively. Univariate analysis of risk factors of LNM and the relationship with clinicopathological features was assessed by the Chi-square or Fisher exact test. Multivariate analysis by a logistic regression model was employed to investigate independent risk factors of LNM. Patient's overall survival was estimated by the Kaplan - Meier method with a log-rank test. A P value of < 0.05 was considered statistically significant.
Results
Demographic and clinicopathological characteristics
Baseline and clinicopathological characteristics of 160 patients were summarized in Table 1. The mean age was 53.1years, and the male-to-female ratio was 1.4:1. Gastric body and gastric antrum were 31.9% and 40.0%, respectively. Depressed type (0-III gross pattern) was found in 65 patients (40.0%) and 40 lesions (25.0%) were IIc gross pattern. The proportion of patients with tumor size over 2cm was 45%. Mucosal and submucosal lesions were 58.1% and 41.9%, respectively. Of these 160 patients, 16 (10.0%) patients had Lymphovascular invasion, 72 (45.0%) patients had Hp infection, 87 (54.4%) patients occured atrophic gastritis and 92 (57.5%) patients had Ulcer findings.
Table 1.
Demographic and clinical characteristics in 160 patients with early pure and mixed signet-ring cell gastric carcinoma.
| Age (year) | |
| median (range) | 52(20-86) |
| mean | 53.1 |
| Gender | |
| Male | 93(58.1%) |
| Female | 67(41.9%) |
| Location | |
| cardia | 11(6.9%) |
| body | 51(31.9%) |
| angularis | 19(11.9%) |
| antrum | 64(40.0%) |
| pylorus | 15(9.4%) |
| Gross pattern | |
| 0-I | 3(1.9%) |
| 0-2a | 19(11.9%) |
| 0-2b | 33(20.6%) |
| 0-2c | 40(25.0%) |
| 0-3 | 65(40.6%) |
| Tumor size(cm) | |
| ≤2.0 | 88(55.0%) |
| >2.0 | 72(45.0%) |
| Invasion depth | |
| M | 93(58.1%) |
| SM | 67(41.9%) |
| Lymphovascular invasion | |
| Absence | 144(90.0%) |
| Presence | 16(10.0%) |
| Hp infection | |
| Absence | 88(55.0%) |
| Presence | 72(45.0%) |
| Atrophic gastritis | |
| Absence | 73(45.6%) |
| Presence | 87(54.4%) |
| Histological type | |
| Pure | 79(49.4%) |
| Mixed | 81(50.6%) |
| Ulcer finding | |
| Absence | 68(42.5%) |
| Presence | 92(57.5%) |
M: intramucosal; SM: submucosal invasion; Hp: helicobacter pylori.
Risk factors of lymph node metastasis
Univarite analysis showed that tumor size(P < 0.05), invasion depth (P < 0.05) and LVI (P < 0.0001) were risk factors of LNM with the total 160 SRCCs in Table 2, multivariate analysis revealed LVI with the odds ratio of 25.6 was the significant independent risk factor of LNM (95% confidence interval: 6.7 - 98.7) (P < 0.0001). By univariate analysis (Table 3), significant risk factor of LNM in pSRCC was LVI (P < 0.0001), in which 2 cases of LNM all appeared LVI (100%). Multivariate analysis failed to find its independent risk factors.
Table 2.
Univariate and Multivariate analysis of risk factors for lymph node metastasis in 160 patients with early pure and mixed signet-ring cell gastric carcinoma.
| Univariate analysis of risk factors for LNM in 160 patients with early gastric SRCC | |||||
| Clinicopathologic Feature | Total Number | Lymph Node Metastasis | Percent | P Value | |
| Absence | Presence | ||||
| Age (year) | |||||
| ≤65 | 133 | 103 | 30 | 22.6 | NS |
| >65 | 27 | 24 | 3 | 11.1 | |
| Gender | |||||
| Male | 93 | 74 | 19 | 20.4 | NS |
| Female | 67 | 53 | 14 | 20.9 | |
| Location | |||||
| cardia | 11 | 9 | 2 | 18.2 | NS |
| body | 51 | 42 | 9 | 17.6 | |
| angularis | 19 | 17 | 2 | 10.5 | |
| antrum | 64 | 48 | 16 | 25.0 | |
| pylorus | 15 | 11 | 4 | 26.7 | |
| Gross pattern | |||||
| 0-I | 3 | 3 | 0 | 0.0 | NS |
| 0-2a | 19 | 15 | 4 | 21.1 | |
| 0-2b | 33 | 27 | 6 | 18.2 | |
| 0-2c | 40 | 31 | 9 | 22.5 | |
| 0-3 | 65 | 51 | 14 | 21.5 | |
| Tumor size(cm) | |||||
| ≤2.0 | 88 | 76 | 12 | 13.6 | <0.05 |
| > 2.0 | 72 | 51 | 21 | 29.2 | |
| Invasion depth | |||||
| M | 93 | 79 | 14 | 15.1 | <0.05 |
| SM | 67 | 48 | 19 | 28.4 | |
| Lymphovascular invasion | |||||
| Absence | 144 | 124 | 20 | 13.9 | <0.0001 |
| Presence | 16 | 3 | 13 | 81.3 | |
| Hp infection | |||||
| Absence | 88 | 69 | 19 | 27.5 | NS |
| Presence | 72 | 58 | 14 | 19.4 | |
| Atrophic gastritis | |||||
| Absence | 73 | 61 | 12 | 16.4 | NS |
| Presence | 87 | 66 | 21 | 24.1 | |
| Histological type | |||||
| Pure | 79 | 68 | 11 | 13.9 | NS |
| Mixed | 81 | 59 | 22 | 27.2 | |
| Ulcer finding | |||||
| Absence | 68 | 50 | 18 | 26.5 | NS |
| Presence | 92 | 77 | 15 | 16.3 | |
| Multivariate analysis of risk factors for LNM in 160 patients with early gastric SRCC | |||||
| P value | Odds ratio | 95% Confidence intervals | |||
| Lymphovascular invasion | <0.0001 | 25.6 | 6.7 - 98.7 | ||
| Tumor size | NS | 2.4 | 1.0 - 6.0 | ||
| Invasion depth | NS | 1.0 | 0.3 - 2.5 | ||
NS: not significant; SRCC: signet-ring cell gastric carcinoma; Hp: helicobacter pylori; LNM: lymph node metastasis; M: intramucosal; SM: submucosal invasion.
Table 3.
Univariate analysis of risk factors for lymph node metastasis in 79 patients with early pure signet-ring cell gastric carcinoma.
| Clinicopathologic Feature | Total Number | Lymph Node Metastasis | Percent | P Value | |
|---|---|---|---|---|---|
| Absence | Presence | ||||
| Age (year) | |||||
| ≤65 | 69 | 58 | 11 | 15.9 | NS |
| >65 | 10 | 10 | 0 | 0 | |
| Gender | |||||
| Male | 33 | 29 | 4 | 12.1 | NS |
| Female | 46 | 39 | 7 | 15.2 | |
| Location | |||||
| cardia | 4 | 3 | 1 | 25.0 | NS |
| body | 23 | 20 | 3 | 13.0 | |
| angularis | 10 | 9 | 1 | 10.0 | |
| antrum | 32 | 28 | 4 | 12.5 | |
| pylorus | 10 | 8 | 2 | 20.0 | |
| Gross pattern | |||||
| 0-I | 2 | 2 | 0 | 0.0 | NS |
| 0-2a | 9 | 8 | 1 | 11.1 | |
| 0-2b | 21 | 18 | 3 | 14.3 | |
| 0-2c | 24 | 20 | 4 | 16.7 | |
| 0-3 | 23 | 20 | 3 | 13.0 | |
| Tumor size(cm) | |||||
| ≤ 2.0 | 49 | 43 | 6 | 12.2 | NS |
| > 2.0 | 30 | 25 | 5 | 16.7 | |
| Invasion depth | |||||
| M | 51 | 45 | 6 | 11.8 | NS |
| SM | 28 | 23 | 5 | 17.9 | |
| Lymphovascular invasion | |||||
| Absence | 77 | 68 | 9 | 11.7 | <0.0001 |
| Presence | 2 | 0 | 2 | 100.0 | |
| Hp infection | |||||
| Absence | 53 | 45 | 8 | 15.1 | NS |
| Presence | 26 | 23 | 3 | 11.5 | |
| Atrophic gastritis | |||||
| Absence | 39 | 33 | 6 | 15.4 | NS |
| Presence | 40 | 35 | 5 | 12.5 | |
| Ulcer finding | |||||
| Absence | 35 | 30 | 5 | 14.3 | NS |
| Presence | 44 | 38 | 6 | 13.6 | |
NS: not significant; Hp: helicobacter pylori; M: intramucosal; SM: submucosal invasion.
In the mSRCC group, significant risk factors of LNM included LVI (P < 0.0001) and tumor size of > 1.0cm (P < 0.05); no LNM was discovered in tumors with the size of < 1.0 cm. None of other risk factors of LNM were statistically significant, as shown in Table 4. The multivariate analysis revealed two significant independent risk factors of LNM in mSRCC: 1) tumor size with the odds ratio of 2.1 (95% confidence interval: 1.0 - 4.1) (P < 0.05), and 2) LVI with the odds ratio of 22.2 (95% confidence interval: 4.8 - 103.1) (P < 0.0001) (Table 3).
Table 4.
Univariate and Multivariate analysis of risk factors for lymph node metastasis in 81 patients with early mixed signet-ring cell gastric carcinoma
| Univariate analysis of risk factors for LNM in 81patients with early gastric mSRCC | |||||
| Clinicopathologic Feature | Total Number | Lymph Node Metastasis | Percent (%) | P Value | |
| Absence | Presence | ||||
| Age (year) | |||||
| ≤65 | 64 | 45 | 19 | 29.7 | NS |
| >65 | 17 | 14 | 3 | 17.6 | |
| Gender | |||||
| Male | 60 | 45 | 15 | 25.0 | NS |
| Female | 21 | 14 | 7 | 33.3 | |
| Location | |||||
| cardia | 7 | 6 | 1 | 14.3 | NS |
| body | 28 | 22 | 6 | 21.4 | |
| angularis | 9 | 8 | 1 | 11.1 | |
| antrum | 32 | 20 | 12 | 37.5 | |
| pylorus | 5 | 3 | 2 | 40.0 | |
| Gross pattern | |||||
| 0-I | 1 | 1 | 0 | 0.0 | NS |
| 0-2a | 10 | 7 | 3 | 30.0 | |
| 0-2b | 12 | 9 | 3 | 25.0 | |
| 0-2c | 16 | 11 | 5 | 31.3 | |
| 0-3 | 42 | 31 | 11 | 26.2 | |
| Tumor size(cm) | |||||
| ≤ 2.0 | 39 | 33 | 6 | 15.4 | < 0.05 |
| > 2.0 | 42 | 26 | 16 | 38.1 | |
| Invasion depth | |||||
| M | 42 | 34 | 8 | 19.0 | NS |
| SM | 39 | 25 | 14 | 35.9 | |
| Lymphovascular invasion | |||||
| Absence | 67 | 56 | 11 | 16.4 | <0.0001 |
| Presence | 14 | 3 | 11 | 78.6 | |
| Hp infection | |||||
| Absence | 35 | 24 | 11 | 31.4 | NS |
| Presence | 46 | 35 | 11 | 23.9 | |
| Atrophic gastritis | |||||
| Absence | 34 | 28 | 6 | 17.6 | NS |
| Presence | 47 | 31 | 16 | 34.0 | |
| Ulcer finding | |||||
| Absence | 33 | 20 | 13 | 39.4 | NS |
| Presence | 48 | 39 | 9 | 18.8 | |
| Mixed pathological type | |||||
| p/D | 62 | 45 | 17 | 27.4 | NS |
| m/D | 19 | 14 | 5 | 26.3 | |
| Multivariate analysis of risk factors for LNM in 81patients with early gastric mSRCC | |||||
| P value | Odds ratio | 95% Confidence intervals | |||
| Tumor size | < 0.05 | 2.1 | 1.0 - 4.1 | ||
| Lymphovascular invasion | <0.0001 | 22.2 | 4.8 - 103.1 | ||
NS: not significant; p/D:poorly differentiated adenocarcinoma; m/D:middle differentiated adenocarcinoma; mSRCC: mixed signet-ring cell carcinoma; Hp: helicobacter pylori; LNM: lymph node metastasis; M: intramucosal; SM: submucosal invasion.
Comparison of clinicopathological features between pSRCC and mSRCC
As shown in Table 5, differences in clinicopathological characteristics between pSRCC and mSRCC groups were significant for the followings: 1) gender was much higher in mSRCC (74.1%) than in pSRCC (41.8%) (P < 0.0001); 2) tumor gross pattern that demonstrated a much higher percentage cases with ulcer (pattern 0-III) in mSRCC (51.9%) than in pSRCC (29.1%), while the frequencies in flat (pattern 0-IIb) and slightly depressed (pattern 0-IIc) gross patterns were lower in mSRCC than in pSRCC (P < 0.05); 3) LVI that showed a significantly higher proportion of cases in mSRCC (17.3%) than in pSRCC (2.5%) (P < 0.01); 4) Hp infection that was much more frequent in mSRCC (56.8%) than in pSRCC (32.9%) (P < 0.01). However, there was no significant difference in age, location, size, invasion depth, atrophic gastritis, and ulcerative findings between the mSRCC and pSRCC groups. More frequent LNM was observed in the mSRCC (27.2%) than in the pSRCC (13.9%) groups, although the difference was not statistically significant.
Table 5.
Comparison of clinicopathological characteristics between early pure and mixed signet-ring cell gastric carcinoma
| Clinicopathologic Feature | Mixed SRCC | Pure SRCC | P value |
|---|---|---|---|
| Age (year) | |||
| mean±SD | 54.8±12.0 | 51.3±13.0 | NS |
| median(range) | 54(20-69) | 51.5(27-69) | |
| Gender | |||
| Male | 60(74.1) | 33(41.8) | <0.0001 |
| Female | 21(25.9) | 46(58.2) | |
| Location | |||
| cardia | 7(8.6) | 4(5.1) | NS |
| body | 28(34.6) | 23(29.1) | |
| angularis | 9(11.1) | 10(12.7) | |
| antrum | 32(39.5) | 32(40.5) | |
| pylorus | 5(6.2) | 10(12.7) | |
| Gross pattern | |||
| 0-I | 1(1.2) | 2(2.5) | < 0.05 |
| O-2a | 10(12.3) | 9(11.4) | |
| O-2b | 12(14.8) | 21(26.6) | |
| O-2c | 16(19.8) | 24(30.4) | |
| O-3 | 42(51.9) | 23(29.1) | |
| Tumor size(cm) | |||
| mean±SD | 2.5±1.3 | 2.3±1.6 | NS |
| median(range) | 2.2(0.4-3.5) | 2(0.2-6.5) | |
| Invasion depth | |||
| M | 42(51.8) | 51(64.5) | NS |
| SM | 39(48.2) | 28(35.5) | |
| Lymphovascular invasion | |||
| Absence | 67(82.7) | 77(97.5) | < 0.01 |
| Presence | 14(17.3) | 2(2.5) | |
| Hp infection | |||
| Absence | 35(43.2) | 53(67.1) | < 0.01 |
| Presence | 46(56.8) | 26(32.9) | |
| Atrophic gastritis | |||
| Absence | 34(42.0) | 39(49.4) | NS |
| Presence | 47(58.0) | 40(50.6) | |
| Lymph node metastasis | |||
| Absence | 59(72.8) | 68(86.1) | NS |
| Presence | 22(27.2) | 11(13.9) | |
| Ulcer finding | |||
| Absence | 33(40.7) | 35(44.3) | NS |
| Presence | 48(59.3) | 44(55.7) |
NS: not significant; SD: standard deviation; Hp: helicobacter pylori; M: intramucosal; SM: submucosal invasion.
Comparison of LNM between expanded indication and out of indication
In the pSRCC group, LNM rate in patients with expanded indication was 8.7% (2/23), and out of indication was 16.1% (9/56). In the mSRCC group, LNM rate in patients with expanded indication was 0(0/8), and out of indication was 30.1% (22/73). There was no statistical difference between the above two groups (P=0.32 and P=0.06, respectively). In all 160 patients, LNM rate between the expanded indication (2/31, 6.5%) and out of indication (31/129, 24%) had statistically significant differences (P=0.03) (Table 6). The clinicopathological characteristics in 2 cases with LNM for expanded indication were showed in Table 7.
Table 6.
Comparison of lymph node metastasis rate between expanded indication and out of indication.
| Expanded indication | Out of indication | P Value | |||||
|---|---|---|---|---|---|---|---|
| Total | LNM | %LNM | Total | LNM | %LNM | ||
| Pure SRCC | 23 | 2 | 8.7 | 56 | 9 | 16.1 | 0.32 |
| Mixed SRCC | 8 | 0 | 0 | 73 | 22 | 30.1 | 0.06 |
| Pure and mixed SRCC | 31 | 2 | 6.5 | 129 | 31 | 24.0 | 0.03 |
SRCC: signet-ring cell carcinoma; LNM: lymph node metastasis.
Table 7.
Clinicopathological characteristics in 2 cases with lymph node metastasis for expanded indication.
| Clinicopathologic Feature | Case1 | Case2 |
|---|---|---|
| Age (year) | 41 | 56 |
| Gender | Female | Female |
| Location | Antrum | Antrum |
| Gross pattern | 2b | 2a |
| Tumor size (cm) | 1cm | 0.5cm |
| Invasion depth | m | m |
| Lymphovascular invasion | 0 | 0 |
| Hp infection | 0 | 0 |
| Atrophic gastritis | 0 | 0 |
| Ulcer finding | 0 | 0 |
Hp: helicobacter pylori.
Post-resection survival
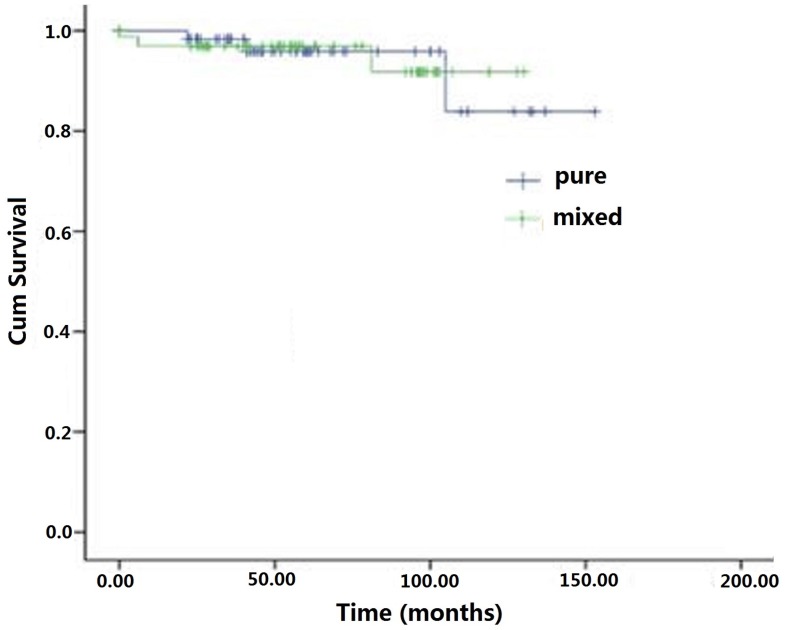
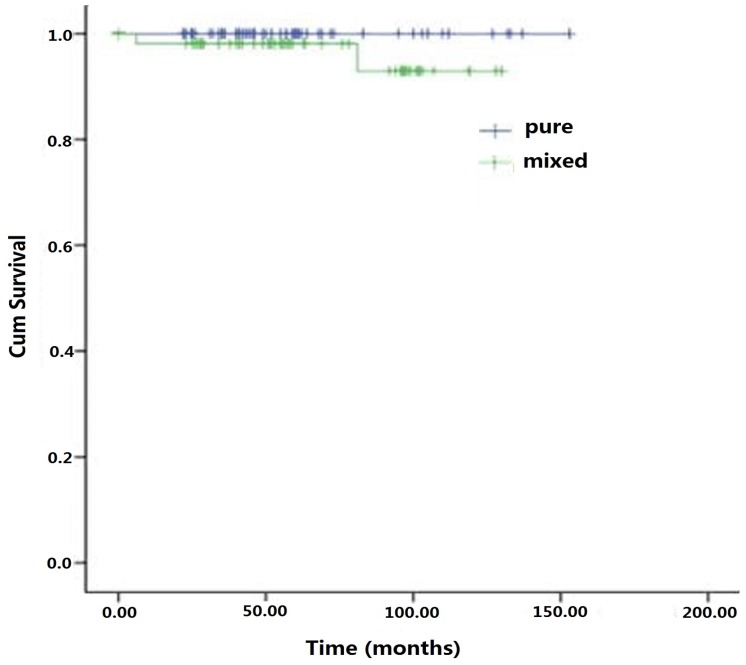
At the last follow-up interview of 160 patients in the cohort, 46 (28.8%) patients were lost and 114 (71.2%) patients completed the 5 year survival survey. The overall average survival time for the cohort was 62 months (range: 6-153). The 5-year survival rate was 96.3%. In the mSRCC group, 54 (66.7%, 54/81) completed the follow up and 3 patients died, two of whom died of the disease and one for other cause. In the pSRCC group, 60 (75.9%, 60/79) had the survival information with 3 deaths for unrelated diseases. There was no statistically significant difference in the average survival months between mSRCC (58.5 + 25.2 months) and pSRCC (66.7 + 26.9 months) groups (P> 0.05) (Figure 1). No significant difference in disease-specific survival was observed between the two groups (P > 0.05) (Figure 2).
Figure 1.
Overall survival curves of early signet ring cell gastric carcinomas patients underwent radical gastrectomies combined with lymph node dissections.
Figure 2.
Specific survival curves of early signet ring cell gastric carcinomas patients underwent radical gastrectomies combined with lymph node dissections.
Discussion
The characteristics of early gastric SRCC, including LNM, clinicopathological features and prognosis, are still in dispute 4,8,15. To achieve an agreement on the treatment options of early gastric SRCC, these aspects need to be further explored. Several studies had reported that risk factors for LNM in early gastric SRCC, tumor diameter is greater than 2cm, submucous infiltration and lymphatic vascular infiltration are risk factors and independent risk factors for LNM 15.
In our research, the risk factor for LNM in patients with pSRCC was LVI. In addition, we found that patients of pSRCC with high LNM rate were younger (≤65, 15.9%; >65, 0), had larger tumor size (2-2.9cm, 3/16, 18.8%; >3cm, 3/22, 13.6%), deeper invasion (SM, 5/28, 17.9%), although there was no difference in statistics. With respect to the risk factors for LNM in mSRCC, our finding demonstrated that patients with larger tumor size (>2cm) and more LVI are more likely to have LNM. At the same time, we observed that patients with mSRCC of LNM have earlier ages (≤65, 29.7%; >65, 17.6%), more atrophic gastritis (17/49, 34.7%) and deeper invasion depth (M, 8/42, 19.0%; SM, 14/39, 35.9%) despite unremarkble statistics differences. We think that the reason for this phenomenon is mSRCC have thin gastric mucosa because of atrophic gastritis, and larger tumor load because of larger tumor size, tumor cells easily penetrate the mucosa to the lymph and blood vessels of the lamina propria and grow LNM. On the other side, the deeper depth of invasion, the tumor cells are closer to the lymph and blood vessels of the lamina propria, and the LNM is more likely to occur. Patients with LNM are more frequent in younger patients whether pSRCC or mSRCC probably because of the rapid growth of the tumor cell. In our study, the mixed components of mSRCC included 19 moderate differentiated and 62 poorly differentiated histologic types. Although there is no significant difference in LNM, previous studies have suggested that the LNM of poorly differentiated gastric adenocarcinoma is higher and the prognosis is poorer 16, so surgical treatment is strongly recommended.
Our study showed that the lesion features such as male gender, a depressed gross type, Hp infection and LVI in the mSRCC group were more common than in the pSRCC group. The histology with mSRCC has been reported as one of the independent risk factors of LNM in EGC 17. Our results demonstrated the previous studies that more LNM occured in mSRCC than in pSRCC (27.2% vs 13.9%) and athough there was no significant difference between two groups. In consistent with the finding of previous reports 12, the factors that revealed more aggressive biologic characteristics in mSRCC, such as positive LVI, and positive LNM, were more prominently associated with the mSRCC group than pSRCC group in EGC. Zheng et al. 11 explained the reason for the aggressive features in mixed-type gastric cancer was that mSRCC have more aggressive behavior such as proliferation, apoptosis, angiogenesis, mucin secretion, and cell adhesion according to increased expression of proteins such as Ki-67, EMMPRIN (extracellular matrix metalloproteinase inducer), and VEGF (vascular endothelial growth factor), which are involved in the angiogenetic process and cell proliferation in mixed-type gastric cancer. Park et al. 18 showed that mixed-type gastric cancer frequently showed CpG island hypermethylation. So, the mixed-type gastric cancer seems to be more aggressive than the pure-type. In addition, our study found that more male gender patients in mSRCC probably because men have bad habits of smoking and drinking, which are high risk factors for the occurrence of gastric cancer. We also discovered more depressed gross patterns, Hp infection in mSRCC may be related to ulcerative lesion and aggressive features. We thought that the mixed type of poorly differentiated histology is closely connected with strong aggressive behaviors and poor prognosis of mSRCC.
In agreement with the results of other studies, this research indicated that SRCC in EGC have a good prognosis 19. Several studies have reported some factors contributing to the better prognosis of early gastric SRCC. Gronnier et al. 20 suggested that it is associated with younger in age. Kim et al. 19 reported that it is related to early discovery, the lesion is endoscopically concave, rich mucin protein in the cytoplasm and eccentric nucleus, the cancer cells are easily detected with pathological examination. In addition, the lower lymph node metastasis rate contributes to the better prognosis. Our research showed that early gastric SRCC either pure or mixed histology had no significant differences in the overall survival rate and the specific survival rate after the gastrectomy and lymph node dissection. However, the therapy options of mSRCC should be carefully considered based on characteristics of stronger invasiveness and higher LNM rate.
Recently, endoscopic therapeutic techniques, such as EMR or ESD, have been widely accepted as an alternate treatment to keep the quality of life for a subgroup of EGCs 21. Technically, endoscopic therapy is used to resect the mucosa or the submucosa layer, without regional lymph nodes dissected. Thus, recognizing patients with high risk of LNM is significantly crucial for the application of endoscopic therapy. Our study showed that early gastric pSRCC had low LNM rate and good prognosis, early gastric mSRCC had relative high LNM rate and more aggressive features. Taking all these data into account, we can predict the therapy program of early gastric SRCC. Early SRCC, whether pure or mixed, has lymphatic vascular invasion, larger tumor size and deeper invasion depth, which are not suitable for endoscopic resection. However, it is considered that early mSRCC has more aggressive behaviors and should be much thought carefully for endoscopic resection.
In the current study, patients with out of indication had higher LNM rate than expanded indication whether pSRCC or mSRCC. As the sample size expands, we can find that there is a significant statistical difference between the expanded indication and out of indication (P=0.03). We will speculate that the difference between them is more obvious when the number of samples is increasing. Therefore, patients who are not satisfied with the expanded indications of endoscopic resection have a higher LNM risk, gastrectomy combined with lymph node dissection is highly recommended. Of course, we should also carefully evaluate the indications of endoscopic resection, because our study found that 2 cases of pSRCC met the expanded indications had LNM and their size were not more 1cm, although the cases was fewer.
There are several main limitations in our study: 1) it was a retrospective study with a non-randomized design, selection bias was, inevitably, exist. But, we used a stringent study protocol with a consecutive patient selection procedure and a uniform exclusion method to minimize selection bias; 2) the variation in the number of lymph nodes retrieved was unavoidably present because of inconsistent surgical methods of lymphadenectomy among surgeons. However, the average number of lymph nodes studied in the cohort was high. We believe that any analysis on the risk of LNM in early gastric SRCC would have similar outcomes; 3) relatively short follow-up time. Therefore, a mass scale, multicenter, prospective and comparative cohort study is essential to verify the importance of the strategy.
Despite above problems, we think this study still has its own values: 1) a relatively large number of consecutive early gastric SRCC surgical resection cases (N=160) specifically for comparison of risk factors of LNM and clinicopathological features between mSRCC and pSRCC, which is rare; 2) in the analysis of risk factors for LNM and comparison of clinicopathological characteristic, the factors included in this study were more than previous studies, such as Hp infection, atrophic gastritis and ulcers; Comparison of LNM between expanded indication and out of indication was instructive to choices of treatment; 3) there are more factors leading to the formation of mSRCC and help us to explore the mechanism of its development; 4) unified implementation with the most rigorous investigation protocol by professional and skilled gastrointestinal pathologists guided with the WHO diagnostic code on early gastric SRCC. In conclusions, by analyzing risk factors of LNM, clinicopathological features and prognosis of pSRCC and mSRCC, it improves awareness of higher rate of LNM and more aggressive features in tumors with mixed histology of SRCC, helps to guide the endoscopic treatment of early gastric SRCC.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81572338 and 81672380, 81201909, 81602089), the Nanjing Medical Science and Technology Development Program (Nos. YKK12072, YKK15061 and YKK16078). This work was also part of a C-class sponsored research project of the Jiangsu Provincial Six Talent Peaks (WSN-078).
Ethical statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki and later versions. The exemption from the informed consent requirement is permitted by the Ethics Committee of the Affiliated Drum Tower Hospital Affiliated to Nanjing University.
References
- 1.Ishikawa S, Togashi A, Inoue M, Honda S, Nozawa F, Toyama E. et al. Indications for EMR/ESD in cases of early gastric cancer: relationship between histological type, depth of wall invasion, and lymph node metastasis. Gastric Cancer. 2007;10:35–38. doi: 10.1007/s10120-006-0407-2. [DOI] [PubMed] [Google Scholar]
- 2.Huang Q, Fang C, Shi J, Sun Q, Wu H, Gold JS. et al. Differences in Clinicopathology of Early Gastric Carcinoma between Proximal and Distal Location in 438 Chinese Patients. Sci Rep. 2015;5:13439. doi: 10.1038/srep13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo SS, Wu CW, Chen JH, Li AF, Hsieh MC, Shen KH. et al. Surgical results of early gastric cancer and proposing a treatment strategy. Ann Surg Oncol. 2007;14:340–347. doi: 10.1245/s10434-006-9077-x. [DOI] [PubMed] [Google Scholar]
- 4.Chiu CT, Kuo CJ, Yeh TS, Hsu JT, Liu KH, Yeh CN. et al. Early signet ring cell gastric cancer. Dig Dis Sci. 2011;56:1749–1756. doi: 10.1007/s10620-010-1487-8. [DOI] [PubMed] [Google Scholar]
- 5.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 6.Ha TK, An JY, Youn HK, Noh JH, Sohn TS, Kim S. Indication for endoscopic mucosal resection in early signet ring cell gastric cancer. Ann Surg Oncol. 2008;15:508–513. doi: 10.1245/s10434-007-9660-9. [DOI] [PubMed] [Google Scholar]
- 7.Abe N, Watanabe T, Sugiyama M, Yanagida O, Masaki T, Mori T. et al. Endoscopic treatment or surgery for undifferentiated early gastric cancer? Am J Surg. 2004;188:181–184. doi: 10.1016/j.amjsurg.2003.12.060. [DOI] [PubMed] [Google Scholar]
- 8.Kunisaki C, Shimada H, Nomura M, Matsuda G, Otsuka Y, Akiyama H. Therapeutic strategy for signet ring cell carcinoma of the stomach. Br J Surg. 2004;91:1319–1324. doi: 10.1002/bjs.4637. [DOI] [PubMed] [Google Scholar]
- 9.Fang C, Shi J, Sun Q, Gold JS, Xu GF, Liu WJ. et al. Risk factors of lymph node metastasis in early gastric carcinomas diagnosed by WHO criteria in 379 Chinese patients. J Dig Dis. 2016;17:526–537. doi: 10.1111/1751-2980.12385. [DOI] [PubMed] [Google Scholar]
- 10.Flejou JF. [WHO Classification of digestive tumors: the fourth edition] Ann Pathol. 2011;31:S27–31. doi: 10.1016/j.annpat.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Zheng HC, Li XH, Hara T, Masuda S, Yang XH, Guan YF. et al. Mixed-type gastric carcinomas exhibit more aggressive features and indicate the histogenesis of carcinomas. Virchows Arch. 2008;452:525–534. doi: 10.1007/s00428-007-0572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570–578. doi: 10.1055/s-2005-861352. [DOI] [PubMed] [Google Scholar]
- 13.Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130–131. doi: 10.1136/gut.51.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stolte M, Meining A. The updated Sydney system: classification and grading of gastritis as the basis of diagnosis and treatment. Can J Gastroenterol. 2001;15:591–598. doi: 10.1155/2001/367832. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Zhu G, Zhang H, Gao H, Xue Y. Clinicopathologic features of gastric carcinoma with signet ring cell histology. J Gastrointest Surg. 2010;14:601–606. doi: 10.1007/s11605-009-1127-9. [DOI] [PubMed] [Google Scholar]
- 16.Park JM, Jang YJ, Kim JH, Park SS, Park SH, Kim SJ. et al. Gastric cancer histology: clinicopathologic characteristics and prognostic value. J Surg Oncol. 2008;98:520–525. doi: 10.1002/jso.21150. [DOI] [PubMed] [Google Scholar]
- 17.Huh CW, Jung DH, Kim JH, Lee YC, Kim H, Kim H. et al. Signet ring cell mixed histology may show more aggressive behavior than other histologies in early gastric cancer. J Surg Oncol. 2013;107:124–129. doi: 10.1002/jso.23261. [DOI] [PubMed] [Google Scholar]
- 18.Park SY, Kook MC, Kim YW, Cho NY, Kim TY, Kang GH. Mixed-type gastric cancer and its association with high-frequency CpG island hypermethylation. Virchows Arch. 2010;456:625–633. doi: 10.1007/s00428-010-0916-6. [DOI] [PubMed] [Google Scholar]
- 19.Kim BS, Oh ST, Yook JH, Kim BS. Signet ring cell type and other histologic types: differing clinical course and prognosis in T1 gastric cancer. Surgery. 2014;155:1030–1035. doi: 10.1016/j.surg.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Gronnier C, Messager M, Robb WB, Thiebot T, Louis D, Luc G. et al. Is the negative prognostic impact of signet ring cell histology maintained in early gastric adenocarcinoma? Surgery. 2013;154:1093–1099. doi: 10.1016/j.surg.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Hyung WJ, Cheong JH, Kim J, Chen J, Choi SH, Noh SH. Application of minimally invasive treatment for early gastric cancer. J Surg Oncol. 2004;85:181–185. doi: 10.1002/jso.20018. discussion 186. [DOI] [PubMed] [Google Scholar]