Dear Editor,
Levels of phytosterols, including plant sterols and stanols, are increased in lipid storage disorders such as cerebrotendinous xanthomatosis (CTX) and sitosterolemia. Both are autosomal recessive diseases with heterogeneous clinical manifestations, such as tendon xanthoma, cataracts, and progressive neurologic abnormalities. Decreased bile acid synthesis in CTX leads to the accumulation of cholestanol [1], whereas increased intestinal absorption and decreased biliary excretion in sitosterolemia lead to increased sitosterol and campesterol levels [2]. If appropriately treated, the outcomes of both diseases can dramatically improve. However, it is difficult to measure phytosterol levels using standard diagnostic assays. For example, enzymatic colorimetry, based on the reaction with a C-5 double bond or the presence of a 3β-hydroxyl group, cannot differentiate between cholesterol and phytosterols because these groups are present in both sterol types [3,4]. Among the methods that can properly measure phytosterol levels, mass spectrometry (MS) is deemed superior because of its high analytical sensitivity and specificity. We developed and evaluated a triplex phytosterol assay utilizing gas chromatography (GC-MS). The study was approved by the Institutional Review Board of Seoul National University Hospital and Seoul National University Bundang Hospital (E-1901-001-998).
Ten microliters of serum and 60 µL of working internal standard (IS) (10 mg/L epicoprostanol in 90% ethanol) were mixed in glass tubes. One milliliter of 4% potassium hydroxide in 90% ethanol was added, and the mixture was incubated at 65℃ for 60 minutes. Liquid-liquid extraction was performed thrice: the sample was mixed with 1 mL distilled water and 2 mL hexane, centrifuged at 54×g for 7 minutes, and subjected to supernatant extraction. After drying with nitrogen gas, 100 µL of bis (trimethylsilyl) trifluoroacetamide and 10% trimethylchlorosilane (Regis Technologies, Inc., Morton Grove, IL, USA) were added, and the sample was incubated at 65℃ for 60 minutes. Then, the sample was introduced into the GC-MS system: an HP 6890N gas chromatograph coupled to an HP 5975 mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) with an HP-5MS capillary column (30 [L]×0.25 mm [i.d.], 0.25 µm [film thickness], Agilent Technologies). The column temperature gradient was programmed from 150℃ (hold for 2 minutes) to 270℃ at 30℃/minutes, to 290℃ at 10℃/minutes (hold for 7 minutes), to 300℃ at 10℃/minutes (hold for 2 minutes). Quantitative data were obtained by selected ion monitoring (m/z 306, 343, 357, and 370 for cholestanol, sitosterol, campesterol, and IS, respectively), and the three phytosterols and IS were clearly separated by GC-MS.
Various parameters of analytical performance, such as imprecision, linearity, lower limit of detection (LLOD) and quantification (LLOQ), ion suppression, recovery, and carryover effect, were evaluated for the assay. Within-run imprecision determined by five replicated analyses and between-run imprecision measured on five consecutive days were below 10.9% of CV for all compounds. According to the EP06-A CLSI guidelines [5], reproducible linearity was observed at five concentrations (0, 50, 100, 150, and 200 mg/L) for each phytosterol (r2>0.999). The LLOD and LLOQ were determined as the lowest concentration with a signal-to-noise ratio >3.0 and the lowest concentration with a precision <20% and accuracy <20%, respectively. The absolute matrix effect (ME) and recovery and process efficiencies were quantitatively analyzed according to the protocol by Matuszewski et al. [6], and were acceptable in all three phytosterols. The aforementioned results are depicted in Table 1. Furthermore, carryover was evaluated by injecting a reconstitution solvent blank immediately after the upper limit of quantification (ULOQ) of the standard curve. No significant carryover was observed; all concentrations of the solvent blank were <20% of the LLOQ.
Table 1. Analytical performance of the GC-MS assay for three phytosterols.
| Within-run imprecision (%) | Between-run imprecision (%) | ME (%) | Recovery (%) | PE (%) | LLOD (μmol/L) | LLOQ (μmol/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | Low | High | Low | High | |||
| Cholestanol | 2.13 | 0.82 | 8.64 | 4.92 | 150.8 | 118.7 | 90.0 | 80.2 | 135.8 | 95.2 | 0.3 | 6.8 |
| Sitosterol | 4.37 | 2.57 | 7.32 | 9.06 | 119.0 | 107.4 | 94.9 | 83.5 | 112.9 | 89.7 | 0.2 | 12.1 |
| Campesterol | 1.64 | 1.16 | 10.94 | 10.23 | 114.8 | 103.3 | 95.5 | 82.2 | 109.6 | 84.9 | 0.3 | 7.5 |
Abbreviations: ME, matrix effect; PE, process efficiency; LLOD, lower limit of detection; LLOQ, lower limit of quantification; GC-MS, gas chromatography-mass spectrometry.
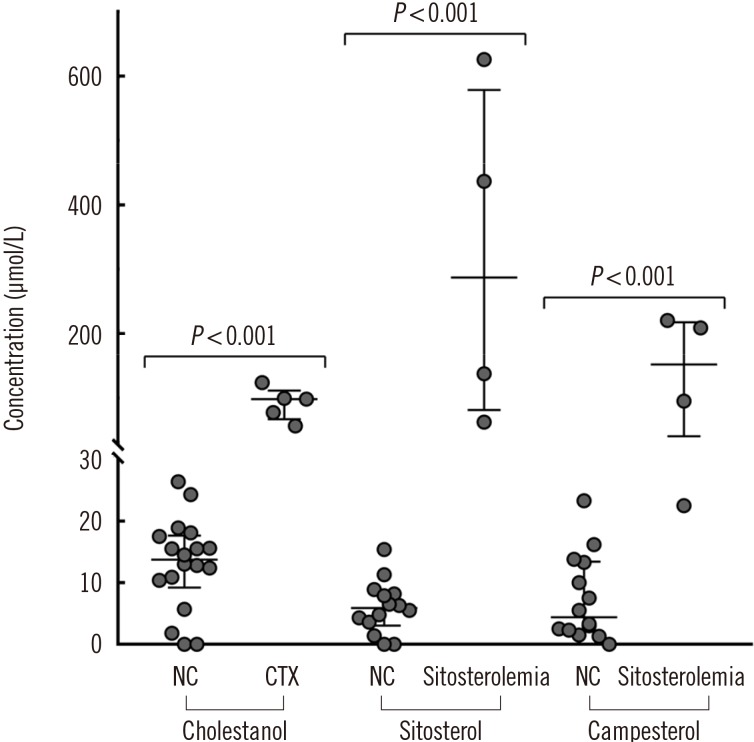
Phytosterol levels in serum samples of patients with CTX (N=5) and sitosterolemia (N=4) were compared with that in serum samples from normal controls (N=18; Fig. 1). Cholestanol level was higher in CTX patients (median [lower-upper quartile], 98.4 [77.7–99.5] µmol/L) than in normal controls (13.7 [10.5–17.0] µmol/L). Likewise, sitosterol and campesterol levels were higher in sitosterolemia patients (287.3 [119.1–483.9] µmol/L for sitosterol and 152.1 [77.1–211.9] µmol/L for campesterol) than in normal controls (5.9 [3.8–8.1] µmol/L for sitosterol and 4.4 [2.3–2.4] µmol/L for campesterol).
Fig. 1. Comparison of phytosterol levels between NCs (N=18) and patients with CTX (N=5) and sitosterolemia (N=4). The outer horizontal lines at both ends depict the upper and lower quartile values, and the middle horizontal line depicts the median. P value was calculated using the Mann–Whitney U test.
Abbreviations: NC, normal control; CTX, cerebrotendinous xanthomatosis.
No consensus has been reached on the true prevalence of CTX and sitosterolemia, with the latest reports estimating the prevalence as 5/100,000 [7] and <1/1,000,000 [8], respectively. Because of the low frequency of pediatric lipid testing and the difficulty in measuring phytosterol levels, these disorders are prone to underdetection [9,10]. Though not pathognomonic, cholestanol and sitosterol are biomarkers for CTX and sitosterolemia, respectively.
One limitation of our study is the small sample size; the rarity of CTX and sitosterolemia makes it difficult to obtain the desirable sample numbers. However, our triplex phytosterol assay by GC-MS showed excellent analytical performance and can be utilized in the clinical laboratory for diagnosing inherited lipid storage disorders. In conjunction with earlier screening, this would ultimately lead to better disease outcomes.
Acknowledgment
This study was supported by a grant from the Korea Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A120030).
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Kuriyama M, Fujiyama J, Kasama T, Osame M. High levels of plant sterols and cholesterol precursors in cerebrotendinous xanthomatosis. J Lipid Res. 1991;32:223–229. [PubMed] [Google Scholar]
- 2.Yoo EG. Sitosterolemia: a review and update of pathophysiology, clinical spectrum, diagnosis, and management. Ann Pediatr Endocrinol Metab. 2016;21:7–14. doi: 10.6065/apem.2016.21.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidambi S, Patel SB. Sitosterolaemia: pathophysiology, clinical presentation and laboratory diagnosis. J Clin Pathol. 2008;61:588–594. doi: 10.1136/jcp.2007.049775. [DOI] [PubMed] [Google Scholar]
- 4.Escola-Gil JC, Quesada H, Julve J, Martin-Campos JM, Cedo L, Blanco-Vaca F. Sitosterolemia: diagnosis, investigation, and management. Curr Atheroscler Rep. 2014;16:424. doi: 10.1007/s11883-014-0424-2. [DOI] [PubMed] [Google Scholar]
- 5.CSLI. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. Wayne, PA: Clinical and Laboratory Standards Institute; 2003. CLSI document EP06-A. [Google Scholar]
- 6.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 7.Lorincz MT, Rainier S, Thomas D, Fink JK. Cerebrotendinous xanthomatosis: possible higher prevalence than previously recognized. Arch Neurol. 2005;62:1459–1463. doi: 10.1001/archneur.62.9.1459. [DOI] [PubMed] [Google Scholar]
- 8.Patel SB. Sitosterolemia and other rare sterol disorders. In: Garg A, editor. Dyslipidemias: pathophysiology, evaluation and management. 1st ed. New York: Humana Press; 2015. pp. 235–250. [Google Scholar]
- 9.Lee MH, Lu K, Patel SB. Genetic basis of sitosterolemia. Curr Opin Lipidol. 2001;12:141–149. doi: 10.1097/00041433-200104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JH, Chung IH, Kim DH, Choi MH, Garg A, Yoo EG. Sitosterolemia presenting with severe hypercholesterolemia and intertriginous xanthomas in a breastfed infant: case report and brief review. J Clin Endocrinol Metab. 2014;99:1512–1518. doi: 10.1210/jc.2013-3274. [DOI] [PubMed] [Google Scholar]