Abstract
Iranian Ultrafiltered White cheese was produced by using different blends of coagulants (100:0, 75:25, 50:50, 25:75 and 0:100; Rhizomucor miehei and camel chymosin, respectively) and ripened for 90 days. The effect of different combinations of these coagulants on chemical composition, proteolysis and residual coagulant activity of the cheeses were studied. The results showed that pH, fat-in-dry matter, salt-in-dry matter and protein contents of the cheeses were significantly influenced by type and concentration of the coagulants. The difference between proteolytic activities of the two coagulants resulted in different levels of proteolysis in the cheeses. A direct relationship was determined between using higher concentrations of R. miehei and increasing the hydrolysis of αs1-casein in the cheeses, during ripening. The residual coagulant activity was influenced by the type and concentration of the coagulant as well. In conclusion, R. miehei provided a higher level of proteolysis and residual coagulant activity compared with camel chymosin.
Keywords: Ultrafiltered cheese, Rhizomucor miehei, Camel chymosin, Residual coagulant activity, Proteolysis
Introduction
In many cheese varieties, proteolytic enzymes are the main agents that play an important role during cheese manufacture and ripening. The activity of residual coagulant and indigenous proteinases (i.e. plasmin and cathepsin) are responsible for primary proteolysis and changes casein fractions including αs1- and β-caseins and derived peptides. On the other hand, secondary proteolysis is occurred by proteolytic and peptidolytic activity of starter and non-starter microorganisms (Fox et al. 1993; Hesari et al. 2006). Proteolytic activity of residual chymosin can change the rheological behavior of cheese, i.e., increase flowability and decrease the stretch ability. However, activity of proteolytic coagulant during ripening may lead to decrease of shelf-life of high moisture cheeses, e.g., soft or UF cheeses. So, some methods such as reducing the amount of coagulant used and storage temperature are implemented in order to limit the activity of residual chymosin during ripening (Sheehan et al. 2004; Moynihan et al. 2014).
Retention of milk clotting-enzymes in the curd is a significant factor for cheese quality and influences the proteolysis of cheese during ripening. While a small part of rennet remains in the curd after manufacturing of traditional cheeses, almost all the rennet is retained in the curd of UF cheeses because of addition of it to the retentate (Broome and Limsowtin 1998; Karami et al. 2009). On the other hand, due to whey removal occurred during the manufacturing, the concentrations of major constituents are increased in the conventional cheeses. However, prevention of whey removal during UF cheese production lead to presence of whey proteins at high concentration in the curd and may inhibit the activities of chymosin and microbial rennet. This results to slower proteolysis and production of amino acids and alters flavor development during ripening of UF cheeses comparing with the conventional ones (Benfeldt 2006; Karami et al. 2009).
Calf rennet is traditionally used as a proteolytic enzyme in conventional cheese-making; however, because of high cost of calf rennet, it was substituted by enzymes from other animals or microorganisms and use of these enzymes has increasingly been expanded (Hayaloglu et al. 2014). Camel chymosin (CC) is a camel origin coagulant and can be used as proteolytic enzyme in cheese manufacturing. Although the clotting activity of CC is strong, the overall proteolytic activity of this enzyme is reduced (Moynihan et al. 2014).
Iranian UF white cheese is produced from UF-treated and pasteurized bovine milk with mesophilic starter culture and commercial recombinant chymosin in dairy plants (Karami et al. 2009). Rhizomucor miehei as a microbial rennet (MR) is mainly used for production of this type of cheese. The MR has higher proteolytic activity than calf rennet and hydrolyses both αs1-casein and β-casein at a similar rate (Awad et al. 1999). Contribution of bovine rennet to proteolysis in Iranian UF white cheese has been previously studied (Hesari et al. 2006, 2007). Nevertheless, the effect of CC and MR on proteolysis in UF white cheese has not been investigated in detail. The present study was carried out to investigate the influence of CC and MR in five different combinations on chemical composition, proteolysis and residual coagulant activity of Iranian UF white cheese during 90 days of ripening.
Materials and methods
Materials
Raw cows’ milk and cheese production equipment were provided by Damaneh Sahand Co. (Tabriz, Iran). Mesophilic homofermentative starter culture with Lactococcus lactis spp. lactis and Lactococcus lactis spp. cremoris (DM-230) were obtained from Danisco Deutschland GmbH (Niebüll, Germany). Recombinant chymosin [Fromase® 2200 TL Granualte (≥ 2200 International Milk Clotting Unit (IMCU) g−1)] as microbial rennet from R. miehei was obtained from DSM Food Specialties (Seclin, Cedex, France). Camel Chymosine [CHY-MAX® M, 1000 International Milk Clotting Unit (IMCU) mL−1] was provided from Chr. Hansen (Hørsholm, Denmark).
Methods
Cheese manufacture
Manufacture of UF white cheese was carried out in two separate trials in consecutive weeks in Damaneh Sahand dairy plant (Tabriz, Iran) as introduced by Tetra-pack incorporation (Bylund 1995) and adapted by Hesari et al. (2006). The cheese making details are given in previous work (Soltani et al. 2016). The experimental design was implemented according to milk clotting activity of the enzymes, as follows:
C0: 100% of MR + 0% of CC (32 g of MR per 1000 kg of retentate)
C25: 75% of MR + 25% of CC (24 g of MR + 12 g of CC per 1000 kg of retentate)
C50: 50% of MR + 50% of CC (16 g of MR + 24 g of CC per 1000 kg of retentate)
C75: 25% of MR + 75% of CC (8 g of MR + 36 g of CC per 1000 kg of retentate)
C100: 0% of MR + 100% of CC (48 g of CC per 1000 kg of retentate).
After production of the cheeses as described by Soltani et al. (2016), sampling of cheeses produced for analysis was implemented in duplicate after 1, 30, 60 and 90 days of ripening.
Gross chemical analysis
A cheese of each trial was analyzed in duplicate for total solids by the oven drying method at 102 ± 1 °C (IDF 1982), fat by the Van Gulik method (Ardo and Polychroniadou 1999), and total nitrogen by the micro-Kjeldahl method (IDF 1993). The pH was monitored by mixing 10 g of grated cheese with 10 ml of distilled water and measuring the pH value of the resultant slurry using a digital pH meter (model SevenCompact S220K, Mettler-Toledo, Greifensee, Switzerland).
Proteolysis
Water–soluble nitrogen (WSN) and 12% trichloroacetic acid soluble nitrogen (TCA–SN) fractions of the cheeses were analyzed by the method described by Hayaloglu et al. (2005) and presented as % of total nitrogen. The levels of free amino acid (FAA) in the WSN fraction of the cheeses were determined by the methods described by Hayaloglu (2007).
Urea-PAGE of caseins and densitometry
After freeze-drying the water-insoluble fractions of the cheeses, a Protean II XI vertical slab gel unit (Bio-Rad Laboratories Ltd., Watford, UK) was used for urea polyacrylamide gel electrophoresis (urea-PAGE) of samples according to the method of Andrews (1983). The gels then were stained directly by the method of Blakesley and Boezi (1977) with Coomassie Brilliant Blue G-250. Next, the gels were destained by pure water and gel slabs were digitized using a scanner (HP ScanJet software, ScanJet G4010, Hewlett Packard, Palo Alto, CA). Scans of the electrophoretograms were used to quantify bands using densitometric software (Image Master TotalLab Phoretix 1D Pro software, Keel House, Newcastle upon Tyne, UK). The caseins were determined quantitatively by integration of peak volumes and areas using the densitometer.
RP-HPLCs of water-soluble fractions
The WSN fractions of the cheeses were freeze-dried and analyzed by reverse-phase high performance liquid chromatography (RP-HPLC) using a Shimadzu LC 20 AD Prominence HPLC system (Shimadzu Corporation, Kyoto, Japan) according to method described by Hayaloglu et al. (2011).
Residual coagulant activity (RCA)
The RCA was determined during ripening by RP-HPLC according to the method described by Hurley et al. (1999), which measures the rate of cleavage of a synthetic heptapeptide substrate (H-Pro-Thr-Clu-Phe-[p-nitro-Phe]-Arg-Leu-OH; code H-1002, Bachem, Bubendorf, Switzerland). As described in Hurley et al. (1999), prepared sample mixture was injected into a Shimadzu LC-20AD Prominence HPLC system (Shimadzu Corp., Kyoto, Japan) consisted of diode array detector model SPD-M20A equipped with a pump system with an auto sampler model SIL-20A HT, CTO-20A column heater and DGU-20A5 degasser units. A gradient solvent system consisting of 0.1% (v/v) trifuoroacetic acid (TFA) in HPLC-grade water (solvent A) and 0.1% TFA (v/v) in acetonitrile (Merck, Darmstadt, Germany) as solvent B was used for separation at 300 nm using a C8 RP column with size of 250 × 4.6 mm, 300 Å pore size, 5 μm particle (Inertsil, WP 300, GL Science, Tokyo, Japan). The column was equilibrated initially for 5 min with 15% B. A gradient was then generated by increasing the concentration of B as follows: 15–45% B over 20 min, 45–95% B over 3 min, maintaining at 95% B for 2 min, and finally returning to equilibration conditions over 3 min. The RCA of the cheeses were expressed as following formula.
Statistical analysis
The data obtained from two trials were analysed statistically using the analysis of variance (ANOVA) of SPSS program (SPSS package program, version 16.0, SPSS Inc., USA). Different groups were statistically defined by Duncan’s multiple range tests. Analysis was performed for 1, 30, 60 and 90 days of ripening. The obtained results were considered significant at α = 0.05.
Results and discussion
Chemical composition and pH
Changes in chemical composition and pH value of Iranian UF white cheeses during ripening are shown in Table 1. pH, fat-in-dry matter (FDM), salt-in-dry matter (SDM) and total protein content of the cheeses changed significantly (P < 0.05) with a change in both blends of enzymes used and ripening period. However, no significant differences were observed in total solids content of the cheeses (P > 0.05). A few technological changes during cheese production particularly in the salt and rennet addition steps may cause to differences in pH value and SDM contents of the cheeses (Moynihan et al. 2014). The pH of the cheeses ranged from 4.48 to 4.67 at day 1 of ripening. These values are in accordance with previous studies for Iranian UF White cheese (Hesari et al. 2006; Karami et al. 2009). On the other hand, cheese pH increased towards the end of ripening due to utilization of lactic acid, formation of non-acidic decomposition products and liberation of alkaline products (e.g., NH3) during hydrolysis of protein (McSweeney and Fox 1993; Awad 2006).
Table 1.
The chemical composition and pH value of Iranian UF White cheeses produced using different combinations of proteases from MR and CC [100% of MR + 0% of CC (C0), 75% of MR + 25% of CC (C25), 50% of MR + 50% of CC (C50), 25% of MR + 75% of CC (C75) and 0% of MR + 100% of CC (C100)] after 1, 30, 60 and 90 days of ripening
| Variables | Days | C0 | C25 | C50 | C75 | C100 | P treatment | P ripening |
|---|---|---|---|---|---|---|---|---|
| pH | 1 | 4.49 ± 0.01 | 4.41 ± 0.05 | 4.67 ± 0.01 | 4.49 ± 0.08 | 4.53 ± 0.14 | * | * |
| 30 | 4.76 ± 0.09 | 4.67 ± 0.14 | 4.79 ± 0.07 | 4.65 ± 0.02 | 4.72 ± 0.08 | * | ||
| 60 | 4.61 ± 0.01 | 4.60 ± 0.03 | 4.64 ± 0.04 | 4.53 ± 0.01 | 4.60 ± 0.01 | * | ||
| 90 | 4.80 ± 0.03 | 4.69 ± 0.07 | 4.81 ± 0.01 | 4.88 ± 0.02 | 4.78 ± 0.01 | * | ||
| Dry matter (%) | 1 | 38.52 ± 0.13 | 38.59 ± 1.06 | 38.50 ± 0.31 | 38.57 ± 0.20 | 38.15 ± 0.32 | n.s. | n.s. |
| 30 | 38.65 ± 0.33 | 38.79 ± 0.17 | 38.05 ± 0.34 | 39.02 ± 0.13 | 39.00 ± 0.30 | n.s. | ||
| 60 | 37.47 ± 0.28 | 37.76 ± 0.11 | 37.91 ± 1.22 | 37.97 ± 0.79 | 37.71 ± 0.24 | n.s. | ||
| 90 | 38.55 ± 0.38 | 38.86 ± 0.22 | 38.51 ± 0.39 | 38.69 ± 0.19 | 38.51 ± 0.38 | n.s. | ||
| Fat-in-dry matter (%) | 1 | 50.62 ± 0.18 | 50.38 ± 1.43 | 50.49 ± 0.35 | 51.48 ± 0.33 | 50.41 ± 0.45 | * | * |
| 30 | 51.29 ± 1.05 | 50.72 ± 1.26 | 50.86 ± 1.81 | 50.24 ± 1.10 | 50.74 ± 0.11 | * | ||
| 60 | 49.86 ± 1.22 | 49.43 ± 1.13 | 49.49 ± 0.76 | 50.20 ± 0.51 | 49.33 ± 0.09 | n.s. | ||
| 90 | 50.96 ± 0.80 | 51.25 ± 0.62 | 50.62 ± 0.13 | 51.28 ± 0.85 | 50.79 ± 0.14 | * | ||
| Salt-in-dry matter (%) | 1 | 5.88 ± 0.11 | 5.85 ± 0.05 | 5.92 ± 0.03 | 5.91 ± 0.02 | 5.89 ± 0.07 | * | ** |
| 30 | 5.59 ± 0.08 | 5.63 ± 0.09 | 5.59 ± 0.04 | 5.54 ± 0.16 | 5.60 ± 0.06 | * | ||
| 60 | 2.97 ± 0.07 | 2.93 ± 0.01 | 2.97 ± 0.03 | 2.94 ± 0.01 | 2.96 ± 0.03 | * | ||
| 90 | 4.89 ± 0.07 | 4.88 ± 0.05 | 4.87 ± 0.05 | 4.93 ± 0.05 | 4.90 ± 0.02 | * | ||
| Total protein (%) | 1 | 12.20 ± 0.39 | 11.97 ± 1.29 | 13.02 ± 0.30 | 11.81 ± 0.29 | 12.32 ± 0.22 | * | * |
| 30 | 14.07 ± 0.67 | 12.77 ± 0.18 | 13.73 ± 0.04 | 14.27 ± 0.89 | 14.82 ± 0.26 | * | ||
| 60 | 14.69 ± 0.39 | 12.80 ± 0.59 | 14.97 ± 0.51 | 14.40 ± 0.17 | 14.49 ± 0.29 | * | ||
| 90 | 13.02 ± 0.07 | 14.15 ± 0.01 | 15.62 ± 1.03 | 13.99 ± 0.41 | 13.86 ± 0.45 | * |
Presented values are the means of two replicate trials
n.s. non-significant
*P < 0.05; **P < 0.01
The type and concentration of coagulants did not influence significantly (P > 0.05) the total solids content of the cheeses. The values for total solid content of the cheeses were similar to the values reported by Hesari et al. (2006) and slightly higher than the values reported by Karami et al. (2009) for Iranian UF White cheese. Several researchers have reported that the type and concentration of coagulant did not affect the dry matter content in various types of cheeses (Kandarakis et al. 1999; Guven et al. 2008; Yasar and Guzeler 2011). Significant differences (P < 0.05) were determined in terms of FDM between the cheeses at 1, 30 and 90 d of ripening. Changes in FDM of the cheeses exhibited a parallel change for DM during ripening. Similar results have reported by Al-Otaibi and Wilbey (2004, 2005) for UF White cheese. Diffusion between salt and water molecules may cause DM changes (P > 0.05) and consequently led to changes in the FDM contents of cheeses analyzed (Guinee and Fox 2004). Significant (P < 0.05), but not substantial changes were determined in SDM contents of chesses analyzed during ripening that were in line with changes in total solid contents of the cheeses.
Total protein contents of the cheeses were significantly (P ≤ 0.05) affected by the coagulants. The type, concentration and blend rate of coagulants influence the gel strength (Hayaloglu et al. 2014). This situation may causes more or less differences in total protein contents between the cheeses. On the other hand, fluctuations were observed in total protein contents of the cheeses during ripening. These changes may affected by proteolysis, diffusion of water-soluble nitrogen into brine (Al-Otaibi and Wilbey 2004; Guven et al. 2006) and water holding characteristics of some compounds formed during ripening (Hayaloglu et al. 2002, 2005).
Proteolysis
Soluble nitrogen fractions
The effects of type or concentration of coagulant on the level of WSN in experimental cheeses were significant (P ≤ 0.05). The levels of WSN in the cheeses increased during ripening (Table 2). The cheeses produced using higher level of MR (C0 and C25) had higher levels of WSN values than the cheeses produced using higher level of CC (C75 and C100) during ripening period (P < 0.05) probably due to lower general proteolytic activity of CC compared with MR (Kappeler et al. 2006; Govindasamy-Lucey et al. 2004). A similar trend was observed in TCA–SN values of the cheeses during ripening (Table 2). However, because of the liberation of intermediate and lower molecular weight peptides, the differences in the level of TCA–SN in the cheeses were greater as ripening progressed (Hayaloglu et al. 2014). Hayaloglu et al. (2014) and Moynihan et al. (2014) reported that different coagulant enzymes caused significant differences in pH 4.6-SN, WSN or TCA–SN in Malatya and Mozzarella cheeses. Similar trends during ripening for pH 4.6-SN or TCA–SN have been reported by Kandarakis et al. (1999) for Feta cheeses produced using calf or fermentation produced rennet. Due to the proteolytic activity of starter bacteria, the levels of WSN and TCA–SN increased as ripening progressed for all experimental cheeses, as reported by other authors (Hayaloglu et al. 2005; Hayaloglu 2007).
Table 2.
Levels of soluble nitrogen fractions of Iranian UF White cheeses produced using different combinations of proteases from MR and CC [100% of MR + 0% of CC (C0), 75% of MR + 25% of CC (C25), 50% of MR + 50% of CC (C50), 25% of MR + 75% of CC (C75) and 0% of MR + 100% of CC (C100)] after 1, 30, 60 and 90 days of ripening
| Variablesa | Days | C0 | C25 | C50 | C75 | C100 | P treatment | P ripening |
|---|---|---|---|---|---|---|---|---|
| WSN, % of TN | 1 | 11.71 ± 0.21 | 11.49 ± 0.07 | 9.78 ± 0.25 | 9.56 ± 0.56 | 9.13 ± 0.53 | * | * |
| 30 | 11.90 ± 0.20 | 11.41 ± 0.31 | 11.07 ± 0.48 | 10.95 ± 0.51 | 9.71 ± 0.08 | * | ||
| 60 | 12.67 ± 0.76 | 12.22 ± 0.80 | 11.56 ± 0.61 | 11.25 ± 0.41 | 10.38 ± 0.17 | n.s. | ||
| 90 | 13.89 ± 0.63 | 12.42 ± 0.50 | 12.09 ± 0.20 | 11.95 ± 0.47 | 12.26 ± 0.62 | n.s. | ||
| TCA–SN, % of TN | 1 | 5.80 ± 0.43 | 6.49 ± 0.26 | 5.72 ± 0.14 | 6.17 ± 0.30 | 4.95 ± 0.45 | n.s. | ** |
| 30 | 8.14 ± 0.44 | 7.63 ± 0.11 | 5.82 ± 0.38 | 6.10 ± 0.38 | 5.36 ± 0.55 | * | ||
| 60 | 10.48 ± 1.30 | 10.15 ± 0.47 | 8.97 ± 0.67 | 8.76 ± 0.22 | 6.26 ± 0.80 | * | ||
| 90 | 13.12 ± 0.48 | 10.38 ± 0.11 | 9.05 ± 0.22 | 9.97 ± 0.93 | 8.99 ± 0.14 | ** |
Presented values are the means of two replicate trials
n.s. non-significant, WSN water-soluble nitrogen, TCA–SN 12% trichloroacetic acid-soluble nitrogen, TN total nitrogen
*P < 0.05; **P < 0.01
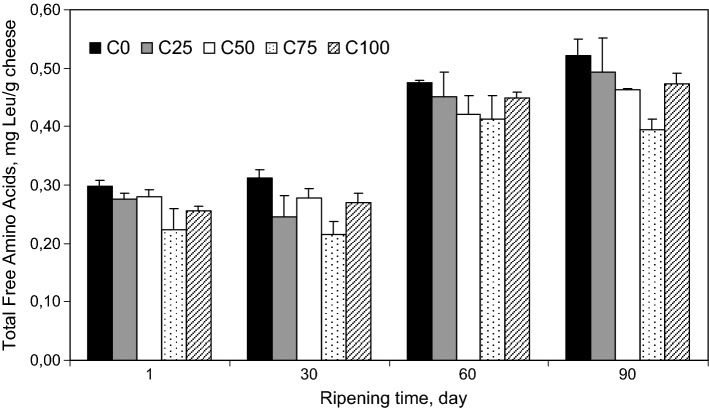
The type or concentration of coagulant enzymes significantly (P < 0.05) influenced the total FAAs of the cheeses. As shown in Fig. 1, the highest and the lowest values of FAA were observed during ripening (with exception of day 60) in the cheeses produced by using 100% of MR (C0) and 25% of MR (C75), respectively. The levels of total FAA declined steadily by increasing levels of CC. According to Fig. 1, the total FAA concentration of C100 was higher than C75 and close to C25 or C50. So, it is noticeable that not only the type but also the level of coagulant affects the formation of total FAA. Total FAAs are the final product of proteolysis and liberation of them from casein is the cause of their presence in the cheese at any stage of ripening (Sousa et al. 2001). So, the higher proteolytic activity of MR compared with CC caused a higher hydrolysis of casein and higher levels of FAA in the C0 compared to other cheeses (Hayaloglu et al. 2014; Moynihan et al. 2014).
Fig. 1.
Levels of free amino acid in Iranian UF White cheeses produced using different combinations of proteases from MR and CC [100% of MR + 0% of CC (C0), 75% of MR + 25% of CC (C25), 50% of MR + 50% of CC (C50), 25% of MR + 75% of CC (C75) and 0% of MR + 100% of CC (C100)] after 1, 30, 60 and 90 days of ripening
Urea-PAGE patterns of caseins
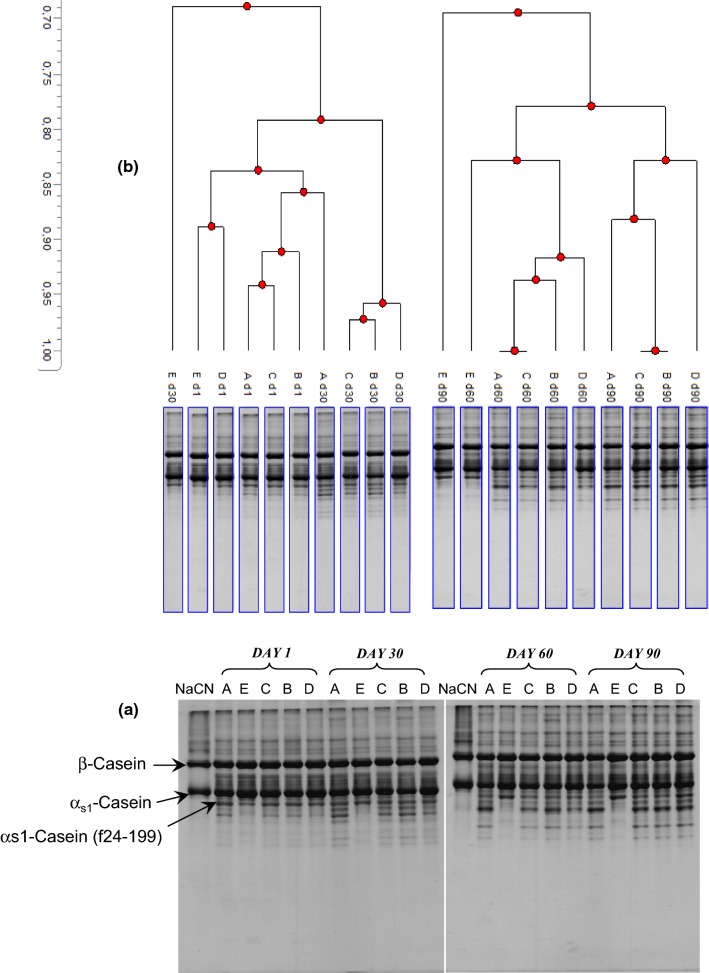
Urea-PAGE electrophoretogram and dendrogram of the water-insoluble fractions of the cheeses during ripening are presented in Fig. 2a and b, respectively. The degradation of αs1- and β-caseins were considerably slow until 30 d of ripening; however, the hydrolysis of αs1-CN and formation of its degradation products accelerated after 30 d and it was more intense after 60 d of ripening. The degradation patterns of αs1-CN in the cheeses were affected by the type or concentration of the coagulants during ripening. The level of degradation in αs1-CN was lower in cheeses produced using higher levels of CC (C75 and C100) compared to cheeses produced using higher levels of MR (C0 and C25). While the αs1-CN (f 24-199) was cleaved in cheeses produced using coagulant containing MR after 30 d of ripening, αs1-CN (f 24-199) was not hydrolysed further in cheese produced using CC (C100). The C100 was not grouped with other cheeses at any sampling time as shown Fig. 2b. Cheeses were distinguished by the levels of enzyme types on the dendrogram; however, the best separation was observed by ripening time. Bansal et al. (2009) reported that Cheddar cheese made using recombinant camel chymosin as coagulant presented lower level hydrolysis of αs1-CN compared to cheese made by calf chymosin. On the other hand, type or concentration of the coagulants did not affect the degradation of β-CN in the cheeses. Hayaloglu et al. (2014) reported that the concentration of coagulants did not influence the patterns of both αs1-CN and β-CN in this type of cheese, while using protease from calf rennet and MR resulted in differences in urea-PAGE patterns of Malatya cheese after 60 d of ripening. The same urea-PAGE patterns for αs1-CN and β-CN is reported by Yasar and Guzeler (2011) for Kasar cheese produced by using proteases from calf rennet and MR. In this context, Kubis et al. (2001) and Dave et al. (2003), reported that no relation was found between the concentration of rennet and the degradation of β-CN, while the direct relationship was observed between the rennet concentration and the degradation level of αs1-CN in Cheddar and Mozzarella cheeses, respectively.
Fig. 2.
Urea-PAGE electrophoretogram (a) and dendrogram (b) of the water-insoluble fractions of Iranian UF White cheeses produced using different combinations of proteases from MR and CC [100% of MR [(A=C0]) + 0% of CC, 75% of MR + 25% of CC [(B=C25)], 50% of MR + 50% of CC [(C=C50)], 25% of MR + 75% of CC [(D=C75)] and 0% of MR + 100% of CC [(E=C100)]] after 1, 30, 60 and 90 days of ripening
RP-HPLC peptide profiles of water-soluble fractions
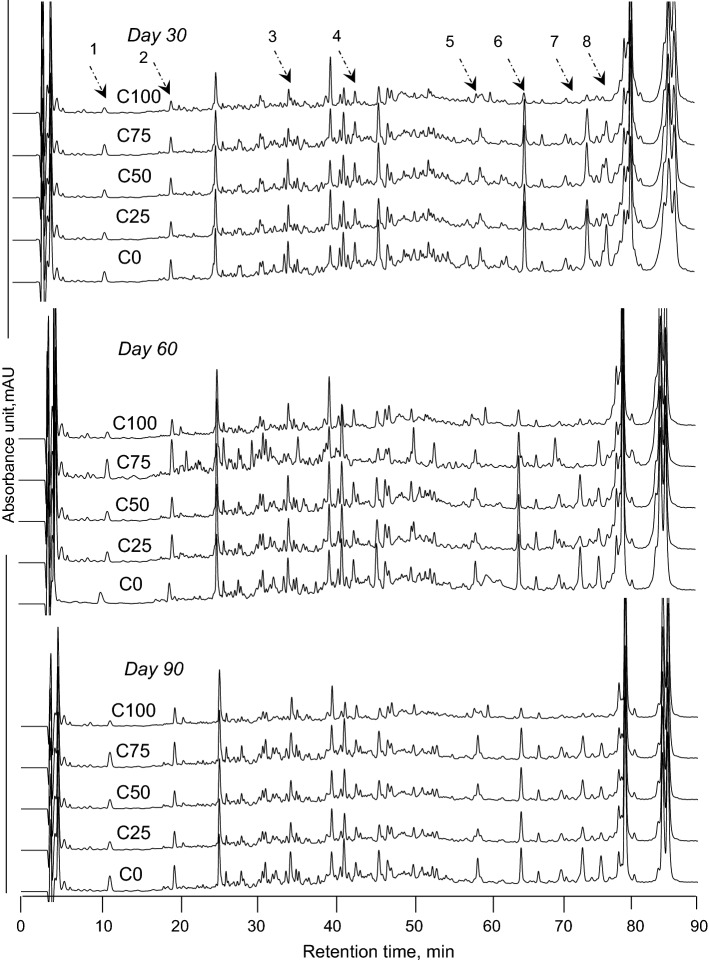
RP-HPLC peptide profiles of the cheeses after 30, 60 and 90 d of ripening are shown in Fig. 3. Major differences were observed between the chromatograms of the water-soluble fractions of the cheeses at early elution time (5–20 min) during ripening. Moreover, the quantitative differences between the peak concentrations in the cheeses were pronounced after 25 min and particularly between 40 and 80 min of elution time. With increasing CC concentration in the composition of coagulant, the peak concentrations decreased. So, C75 and C100 which produced using higher concentration of CC had lower peptide peak concentration than C0, C25 and C50. These differences reflected the lower general proteolytic activity of CC (Bansal et al. 2009; Moynihan et al. 2014). Hydrophobic and high molecular mass peptides along with whey proteins are eluted between 70 and 100 min of retention time (Hayaloglu et al. 2011). In our study, the peaks eluted at 79 and 85 min was belonged to α-lactalbumin and β-lactoglobulin, respectively that is similar to results reported by Hesari et al. (2006). These two proteins were identified and confirmed by injection of their analytical standards under the same chromatographic conditions.
Fig. 3.
Reversed phase-HPLC peptides profiles of Iranian UF White cheeses produced using different combinations of proteases from MR and CC [100% of MR + 0% of CC (C0), 75% of MR + 25% of CC (C25), 50% of MR + 50% of CC (C50), 25% of MR + 75% of CC (C75) and 0% of MR + 100% of CC (C100)] after 1, 30, 60 and 90 days of ripening
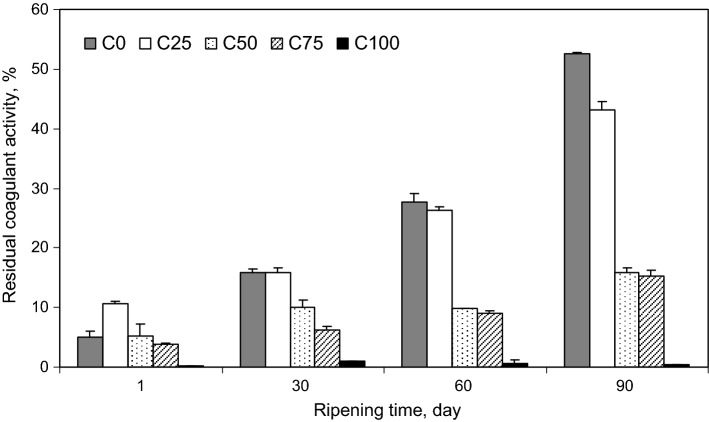
Residual coagulant activity
The amount of residual coagulant changes the biochemical and physical characteristics of cheeses. Activity of residual coagulant can also catalyze the primary hydrolysis of caseins into peptides and lead to secondary proteolysis by enzymes from lactic acid bacteria (Hesari et al. 2006; Hayaloglu et al. 2014). The levels of residual coagulant activity of the experimental cheeses during ripening are shown in Fig. 4. Significant differences were observed between the activity of MR and CC in the cheeses during ripening and the residual coagulant activity increased as ripening progressed. In this context, Maniou et al. (2013) reported that the level of residual chymosin after 60 d of ripening compared with the first day was increased and decreased in Feta cheese made with HP-treated starter and non-treated starter, respectively. The level of residual MR was significantly higher than the level of residual CC in the cheeses (P < 0.05). Therefore, C75 and C100 which were produced using higher levels of CC had the lowest level of residual coagulant among the cheeses analyzed. This was in agreement with the results of proteolysis and urea-PAGE (see Table 2, Fig. 2), which showed lower contents of WSN and TCA–SN and less hydrolysis of αs1-CN in the C75 and C100 due to lower proteolytic activity of CC compared with MR (Bansal et al. 2009; Moynihan et al. 2014).
Fig. 4.
Residual coagulant activity (as percentage of peak area ratio of product and substrate) of Iranian UF White cheeses produced using different combinations of proteases from MR and CC [100% of MR + 0% of CC (C0), 75% of MR + 25% of CC (C25), 50% of MR + 50% of CC (C50), 25% of MR + 75% of CC (C75) and 0% of MR + 100% of CC (C100)] after 1, 30, 60 and 90 days of ripening
Conclusion
The type and concentration of coagulants used for production of the cheeses significantly influenced the pH, FDM, SDM and protein contents of the experimental cheeses during ripening. While pH was established in the lowest level in C75 after 30, 60 and 90 d of ripening, FDM and protein contents were generally increased during ripening in cheeses produced using higher levels of CC (C75 and C100). SDM content of C0 was also higher than C100 during ripening with exception of the first day. Because of higher proteolytic activity of MR compared with CC, cheeses produced using higher levels of MR (C0 and C25) had higher proteolysis values than the other cheeses during ripening. While the degradation of αs1-CN was influenced by the type or concentration of the coagulants used, the β-CN degradation was not influenced by any of these factors. Using MR or CC as the coagulating agent resulted in higher and lower residual coagulant activity in the cheeses, respectively. To optimize the coagulating and proteolytic action of coagulant during ripening in UF white cheese, MR and CC can be blended at a ratio of 75:25 and 50:50, respectively. The results indicated that CC in combination with MR can be used in the production of Iranian UF White cheese.
Acknowledgements
The authors thank the Damaneh Sahand dairy plant (Khosroshah, Tabriz, Iran) for providing raw materials and production equipment for cheese making. The study was partially supported by Inonu University (Malatya, Turkey) Scientific and Research Projects Unit (Project No: 2013/30).
Abbreviations
- UF cheese
Ultrafiltered cheese
- CC
Camel chymosin
- MR
Microbial rennet
- C0
Cheese produced using 100% of microbial rennet + 0% of camel chymosin
- C25
Cheese produced using 75% of microbial rennet + 25% of camel chymosin
- C50
Cheese produced using 50% of microbial rennet + 50% of camel chymosin
- C75
Cheese produced using 25% of microbial rennet + 75% of camel chemosin
- C100
Cheese produced using 0% of microbial rennet + 100% of camel chmosin
- d
Day
Conflict of interest
The authors on this manuscript declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Al-Otaibi MM, Wilbey RA. Effect of temperature and salt on the maturation of white salted cheese. Int J Dairy Technol. 2004;57:57–63. doi: 10.1111/j.1471-0307.2004.00123.x. [DOI] [Google Scholar]
- Al-Otaibi MM, Wilbey RA. Effect of chymosin and salt reduction on the quality of ultrafiltrated white-salted cheese. J Dairy Res. 2005;72:234–242. doi: 10.1017/S0022029905000762. [DOI] [PubMed] [Google Scholar]
- Andrews AT. Proteinases in normal bovine milk and their action on caseins. J Dairy Res. 1983;50:45–55. doi: 10.1017/S0022029900032519. [DOI] [PubMed] [Google Scholar]
- Ardo Y, Polychroniadou Y. Laboratory manual for chemical analysis of cheese. Luxembourg: Office for Official Publications of the European Communities; 1999. [Google Scholar]
- Awad S. Texture and flavour development in Ras cheese made from raw and pasteurised milk. Food Chem. 2006;97:394–400. doi: 10.1016/j.foodchem.2005.05.012. [DOI] [Google Scholar]
- Awad S, Luthi-Peng QQ, Puhan Z. Proteolytic activities of Suparen and Rennilase on buffalo, cow, and goat whole casein and β-casein. J Agricul Food Chem. 1999;47:3632–3639. doi: 10.1021/jf981365u. [DOI] [PubMed] [Google Scholar]
- Bansal N, Drake MA, Piraino P, Broe ML, Harboe M, Fox PF, McSweeney PLH. Suitability of recombinant camel (Camelus dromedarius) chymosin as a coagulant for Cheddar cheese. Int Dairy J. 2009;19:510–517. doi: 10.1016/j.idairyj.2009.03.010. [DOI] [Google Scholar]
- Benfeldt C. Ultrafiltration of cheese milk: effect on plasmin activity and proteolysis during cheese ripening. Int Dairy J. 2006;16:600–608. doi: 10.1016/j.idairyj.2005.10.016. [DOI] [Google Scholar]
- Blakesley RW, Boezi JA. A new staining technique for proteins in polyacrylamide gels using coomassie brillant blue G250. Anal Biochem. 1977;82:580–582. doi: 10.1016/0003-2697(77)90197-X. [DOI] [PubMed] [Google Scholar]
- Broome MC, Limsowtin GKY. Milk coagulants. Austr J Dairy Technol. 1998;53:188–190. [Google Scholar]
- Bylund G. Dairy processing. Tetra pak processing systems AB. Lund: Lund University Publications; 1995. [Google Scholar]
- Dave RI, McMahon DJ, Oberg CJ, Broadbent JR. Influence of coagulant level on proteolysis and functionality of Mozzarella cheese made using direct acidification. J Dairy Sci. 2003;86:114–126. doi: 10.3168/jds.S0022-0302(03)73590-5. [DOI] [PubMed] [Google Scholar]
- Fox PF, Law J, McSweeney PLH, Wallace J. Biochemistry of cheese ripening. In: Fox PF, editor. Cheese: chemistry, physics and microbiology. 3. London: Chapman & Hall; 1993. pp. 379–438. [Google Scholar]
- Govindasamy-Lucey S, Jaeggi JJ, Bostley AL, Johnson ME, Lucey JA. Standardization of milk using cold ultrafiltration retentates for the manufacture of Parmesan cheese. J Dairy Sci. 2004;87:2789–2799. doi: 10.3168/jds.S0022-0302(04)73406-2. [DOI] [PubMed] [Google Scholar]
- Guinee TP, Fox PF. Salt in cheese: physical, chemical and biological aspects. In: Fox PF, Mc Sweeney PLH, Cogan TM, Guinee TP, editors. Cheese: chemistry, physics and microbiology. London: Elsevier Academic Press; 2004. pp. 207–259. [Google Scholar]
- Guven M, Yerlikaya S, Hayaloglu AA. Influence of salt concentration on the characteristics of Beyaz cheese, a Turkish white-brined cheese. Lait. 2006;86:73–81. doi: 10.1051/lait:2005043. [DOI] [Google Scholar]
- Guven M, Cadun C, Karaca OB, Hayaloglu AA. Influence of rennet concentration on ripening characteristics of Halloumi cheese. J Food Biochem. 2008;32:615–627. doi: 10.1111/j.1745-4514.2008.00187.x. [DOI] [Google Scholar]
- Hayaloglu AA. Comparisons of different single strain starter cultures for their effects on ripening and grading of Beyaz cheese. Int J Food Sci Technol. 2007;42:930–938. doi: 10.1111/j.1365-2621.2006.01312.x. [DOI] [Google Scholar]
- Hayaloglu AA, Guven M, Fox PF. Microbiological, biochemical and technological properties of Turkish White cheese ‘Beyaz Peynir’. Int Dairy J. 2002;12:635–648. doi: 10.1016/S0958-6946(02)00055-9. [DOI] [Google Scholar]
- Hayaloglu AA, Guven M, Fox PF, McSweeney PLH. Influence of starters on chemical, biochemical, and sensory changes in Turkish White-brined cheese during ripening. J Dairy Sci. 2005;88:3460–3474. doi: 10.3168/jds.S0022-0302(05)73030-7. [DOI] [PubMed] [Google Scholar]
- Hayaloglu AA, Topcu A, Koca N. Peynir analizleri [Cheese analysis] In: Hayaloglu AA, Ozer B, editors. Peynir Biliminin Temelleri [Principles of Cheese Science] Izmir: Sidas; 2011. pp. 489–562. [Google Scholar]
- Hayaloglu AA, Karatekin B, Gurkan H. Thermal stability of chymosin or microbial coagulant in the manufacture of Malatya, a Halloumi type cheese: proteolysis, microstructure and functional properties. Int Dairy J. 2014;38:136–144. doi: 10.1016/j.idairyj.2014.04.001. [DOI] [Google Scholar]
- Hesari J, Ehsani MR, Khosroshahi A, McSweeney PLH. Contribution of rennet and starter to proteolysis in Iranian UF White cheese. Le Lait. 2006;86:291–302. doi: 10.1051/lait:2006011. [DOI] [Google Scholar]
- Hesari J, Ehsani MR, Mosavi MAE, McSweeney PLH. Proteolysis in ultra-filtered and conventional Iranian white cheese during ripening. Int J Dairy Technol. 2007;60:211–220. doi: 10.1111/j.1471-0307.2007.00337.x. [DOI] [Google Scholar]
- Hurley MJ, O’Driscoll BM, Kelly AL, McSweeney PLH. Novel assay for the determination of residual coagulant activity in cheese. Int Dairy J. 1999;9:553–558. doi: 10.1016/S0958-6946(99)00118-1. [DOI] [Google Scholar]
- IDF (1982) Determination of the total solid content (cheese and processed cheese). IDF Standard 4A, International Dairy Federation, Brussels, Belgium
- IDF (1993) Determination of the nitrogen (Kjeldahl method) and calculation of the crude protein content. IDF Standard 2B, International Dairy Federation, Brussels, Belgium
- Kandarakis I, Moschopoulou E, Anifantakis E. Use of fermentation produced chymosin from E. coli in the manufacture of Feta cheese. Milchwissenschaft. 1999;54:24–26. [Google Scholar]
- Kappeler SR, van den Brink HM, Rahbek-Neilsen H, Farah Z, Puhan Z, Hansen EB, Johansen E. Characterization of recombinant camel chymosin reveals superior properties for the coagulation of bovine and camel milk. Biochem Biophys Res Commun. 2006;342:647–654. doi: 10.1016/j.bbrc.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Karami M, Ehsani MR, Mousavi SM, Rezaei K, Safari M. Changes in the rheological properties of Iranian UF-Feta cheese during ripening. Food Chem. 2009;112:539–544. doi: 10.1016/j.foodchem.2008.06.003. [DOI] [Google Scholar]
- Kubis I, Sousa MJ, Walsh-O’Grady D, Kelly AL, McSweeney PLH. Proteolysis in Cheddar-type cheese made from goat’s milk. Milchwissenschaft. 2001;56:557–560. [Google Scholar]
- Maniou D, Tsala A, Moschopoulou E, Giannoglou M, Taoukis P, Moatsou G. Effect of high-pressure-treated starter on ripening of Feta cheese. Dairy Sci Technol. 2013;93:11–20. doi: 10.1007/s13594-012-0060-y. [DOI] [Google Scholar]
- McSweeney PLH, Fox PF. Methods of chemical analysis. In: Fox PF, editor. Cheese, chemistry, physics and microbiology. New York: Chapman & Hall; 1993. pp. 389–438. [Google Scholar]
- Moynihan AC, Govindasamy-Lucey S, Jaeggi JJ, Johnson ME, Lucey JA, McSweeney PLH. Effect of camel chymosin on the texture, functionality, and sensory properties of low-moisture, part-skim Mozzarella cheese. J Dairy Sci. 2014;97:85–96. doi: 10.3168/jds.2013-7081. [DOI] [PubMed] [Google Scholar]
- Sheehan JJ, O’Sullivan K, Guinee TP. Effect of coagulant type and storage temperature on the functionality of reduced-fat Mozzarella cheese. Lait. 2004;84:551–566. doi: 10.1051/lait:2004031. [DOI] [Google Scholar]
- Soltani M, Boran OS, Hayaloglu AA. Effect of various blends of camel chymosin and microbial rennet (Rhizomucor miehei) on microstructure and rheological properties of Iranian UF White cheese. LWT-Food Sci Technol. 2016;68:724–728. doi: 10.1016/j.lwt.2016.01.028. [DOI] [Google Scholar]
- Sousa MJ, Ardo Y, McSweeney PLH. Advances in the study of proteolysis during cheese ripening. Int Dairy J. 2001;11:327–345. doi: 10.1016/S0958-6946(01)00062-0. [DOI] [Google Scholar]
- Yasar K, Guzeler N. Effects of coagulant type on the physicochemical and organoleptic properties of Kashar cheese. Int J Dairy Technol. 2011;64:372–379. doi: 10.1111/j.1471-0307.2011.00679.x. [DOI] [Google Scholar]