Abstract
Daily consumption of nuts is recommended as a part of a healthy diet as they contain protein and are rich in beneficial fatty acids and essential nutrients. The nutritional qualities of nuts are affected by their fatty acid composition and other factors such as maturity. Oil oxidative stability is important to determine nut nutritional quality in terms of fatty acid composition over storage. Therefore, this study aimed to (a) assess the nutritional quality (photooxidative stability and nutrient composition) of almond, cashew, pistachio and canarium (a newly commercialised indigenous nut); and (b) explore differences in nutrient concentrations between immature and mature canarium nuts. A decrease in polyunsaturated fats after photooxidation in almond and pistachio was observed. Canarium oil did not change following photooxidation suggesting canarium may display a long shelf life when stored appropriately. Our study indicated that almond provided over 50% of the recommended daily intake for manganese whereas canarium intake provided 50% of the recommended daily intake for iron (for males). Pistachio was richer in potassium compared with other nuts and canarium was richer in boron, iron and zinc than other nut species. Mature canarium kernels were richer in boron, iron and zinc but contained less potassium than immature canarium. Therefore, the current study recommended to store kernels in dark to decrease oil photooxidation, and maturity of canarium kernels at the harvest time was important affecting nutrient concentrations of kernels.
Keywords: Agroforestry, Oil stability, Crude protein, Micro- and macro-nutrients, Recommended daily intake, Non-timber forest products
Introduction
World hunger is increasing and current estimates are that over 800 million of the human population are undernourished (FAO 2017). Moreover, over two billion people suffer from hidden hunger and do not receive recommended daily essential nutrients including iron and vitamin A (Hawkes and Fanzo 2017). However, food insecurity can be addressed promoting highly nutritious and indigenous food sources particularly in developing countries (Leakey 2018). Approximately fifty indigenous plant species are being domesticated (Leakey et al. 2012) and some of which are highly nutritious (Powell et al. 2015). Tree nuts, in particular, are increasingly being recognized as rich source of mineral nutrient and beneficial fatty acids (Couladis et al. 2003; Özcan 2004; Matthaus and Özcan 2006; Ros 2010) that improve metabolic processes (e.g. cardiovascular performances and vascular reactivity) (Ros 2010). More than 90% of world trade in tree nuts is based on just five main nut species including almonds, cashews, hazelnuts, pistachios and walnuts. However, many indigenous species of edible nuts exist that have great potential to be commercialised and address food insecurity in developing countries. The indigenous tree species including Canarium spp, produces edible nuts and has been commercialised (Walton et al. 2017; Bai et al. 2017a). The pili nuts (C. ovatum) and canarium nuts (C. indicum) are nutritionally rich especially in iron, phosphorus and zinc (Bai et al. 2017a, 2018a, b; Millena and Sagum 2018a, b). There are limited information with regard to nutritional composition of pili nuts (Millena and Sagum 2018a, b) but not canarium nuts. To the best knowledge of authors, the nutritional composition of canarium species has not been compared with nutritional values of widely used nut species.
The oil of tree nuts is rich in unsaturated fatty acids (Miraliakbari and Shahidi 2008; Özcan et al. 2009; Matthaus et al. 2015). The unsaturated fatty acids in tree nuts are unstable and shelf life of nut oils can be greatly reduced when the oil is not stored properly (Miraliakbari and Shahidi 2008; Gama et al. 2018b). Autooxidation occurs when oils are exposed to heat causing both polyunsaturated and monounsaturated fatty acids to be oxidised (Ali et al. 2017). Photooxidation occurs when oils are exposed to light (Khan and Shahidi 2002; Amaral et al. 2006). Photooxidation is associated with the amount of natural pigments and unsaturated fatty acids in oils (Khan and Shahidi 2002). Photooxidation is one of the major issues in edible oils leading to produce off flavour oils (Albuquerque et al. 2003). The autooxidative stability of nuts under different conditions have been widely investigated (Khan and Shahidi 2002; Amaral et al. 2006; Miraliakbari and Shahidi 2008). The autooxidative and photooxidative stability of oils may be similar (Miraliakbari and Shahidi 2008). However, the oil oxidative stability may not always be necessarily an indication of oil photooxidative stability (Özkan et al. 2016). Few studies have recently examined photooxidation in nut oils (Miraliakbari and Shahidi 2008; Özkan et al. 2016) (Albuquerque et al. 2003). Therefore, exploring photosensitivity of tree nut oils still remains a major gap of knowledge.
Many factors influence the nutrient concentrations and crude protein of nuts (Kodad and Socias i Company 2008; Gama et al. 2018a, b). For example, soil nutrient availability, plant varieties and location of sample collection are among the main factors that determine nutrient accumulation in plants (Kodad and Socias i Company 2008; Vanhanen and Savage 2013; Bai et al. 2017b, 2018a, b; Simsek et al. 2016). Nuts of unknown origin and variety display wide variations in their nutrient concentrations when sourced from retail outlets (Ni et al. 2016; Gama et al. 2018a). For example, variation in nutrient concentrations of almond, cashew and pistachio have been reported previously (Küçüköner and Yurt 2003; Kodad and Socias i Company 2008; Gama et al. 2018a). It is unclear which nut species would be best to address widespread nutrient deficiencies (e.g. iron).
The maturity of nuts may further affect their nutritional quality including sugar content, fatty acid composition and nutrient concentration (Nanos et al. 2002; Kazantzis et al. 2003; Matthaus et al. 2016). For example, early harvested almonds contained lower sugar and oil compared with mature almonds harvested later in the season (Nanos et al. 2002). Lower sugar concentrations in early harvested nuts has been associated with lack of completion in sugar transformation cycle (Kazantzis et al. 2003). The relationships between maturity stage and nut quality in almond and pistachio have been established (Matthaus et al. 2016). However, little attention has been paid to nutritional values of newly commercialised nuts at different maturity stages. This study aimed to compare nutritional quality of nuts including almond, cashew, pistachio and canarium. In particular, we (a) investigated photooxidative stability of almond, cashew, pistachio and canarium (a newly commercialised nut); (b) explored variation in their nutrient concentrations; and (c) assessed the effect of canarium maturity level on their mineral nutrient concentrations.
Materials and methods
Sample collection and preparation
Almond, cashew and pistachio samples were sourced from health food section of a retail outlet. No further preparation was undertaken for these samples prior to oil extraction and chemical analyses. Purple (mature) and green (immature) fruits of canarium were sourced from a plantation located at Kerevat, East New Britain, Papua New Guinea (PNG) in March 2016. The fruits were soaked in warm water for 5 min to remove the pulp followed by cracking of the shells (Walton et al. 2017). The kernels were placed in a laboratory oven to be dried at 50 °C until their moisture content reached approximately 3.0%. The kernels were sealed in zip-lock aluminium bags, and stored at 4 °C before further analysis.
Oil extraction and accelerated photooxidation
Each species of nut was divided into 12 replicates (4 nut species × 12 replicates) and each replicate had 10 g kernels. Kernels of each replicate were crushed and added to pentane. The mixture was stirred for 20 min and pentane was then removed from the oil using an air-tight vacuum rotator for 15 min. The 12 replicates of oil of each nut species was divided into two sub-sample of six replicates and stored in glass vials. One set of six replicates for each nut species (4 nut species × 6 replicates) was used to measure fatty acid composition at day 0. The second sub-sample of the oils (4 nut species × 6 replicates) was placed in a growth chamber for accelerated photooxidation at 27 °C with constant light for 14 days. The lids of the vials were removed and vials were shaken in the air for 20 s every 48 h throughout the incubation to ensure oxygen was not limited. At day 14, all oil samples were collected from the growth chamber and stored at 4 °C before determining their fatty acid compositions within a week of the end of the experiment.
Fatty acid compositions of the oils
Approximately 1 μl of oil of each replicate was added to a mixture of 0.7 ml dry methanol solution which contained butylated hydroxytoluene (BHT) and 25 μl of HCl (32%). The mixture was incubated overnight at 65 °C. After incubation, 0.5 ml of hexane and 0.5 ml of MilliQ water were added to mixture. The whole solution was agitated gently for 30 s. The functionalised oil was then collected from the mixture and the fatty acid compositions was measured using a Gas Chromatography–Mass Spectrometry (GCMS). In brief, FAME analysis was performed using an Elite-5MS (30 m × 0.25 mm × 0.25 µm) column in a PerkinElmer Clarus 580 Gas Chromatograph coupled to a PerkinElmer Clarus SQ8S Mass Spectrometer. The helium carrier gas had a constant flow of 1 ml/min. The injection port was at 280 °C with a split ratio of 30:1. The temperature program was operated at 50 °C for 0.5 min, ramping at 10 °C/min until 300 °C and holding for 1.0 min. The mass spectrometer analysed a mass range from 40 to 400 (m/z), from 3.1 to 26.5 min with ionization be 70 eV electron impact. Compounds were identified by comparison of mass spectra against the National Institute of Standards and Technology (NIST) (08) MS library. Proportions of each fatty acid were recorded for each oil sample.
Protein and minerals concentrations of the kernels
Kernels of each nut species (almond, cashew, pistachio and mature canarium kernels) were randomly assigned into five replicates (4 nut species × 5 replicates). Each replicate contained five kernels. The kernels of mature and immature canarium were also assigned into five replicates (2 nut maturity stages × 5 replicates). Each replicate also consisted of five kernels. Kernels of each replicate were crushed and homogenised. One sub-sample of each replicate was used to measure total nitrogen (N) using a LECO TruSpec analyser. A conversion factor of 6.25 was used to convert total N of the nuts to crude protein (Bai et al. 2017a). The second sub-sample of each replicate was used to assess the concentration of aluminium (Al), boron (B), calcium (Ca), copper (Cu), iron (Fe), potassium (K), magnesium (Mg), manganese (Mn), sodium (Na), phosphorus (P), sulphur (S) and zinc (Zn) using a Varian Vista Pro ICPOES instrument. Nut sample of each replicate (0.25 g) was weighed out and digested by heating to the temperature of 200 °C with 15 ml of a 5:1 mixture of nitric and perchloric acids. The digested samples were made up to 25 ml volume and analysed using a Varian (brand) Vista Pro (model) ICPOES instrument. The analytical wavelengths used included 396.152 (Al), 249.772 (B), 422.673 (Ca), 327.395 (Cu), 238.204 (Fe), 766.491 (K), 279.553 (Mg), 257.610 (Mn), 588.995 (Na), 213.618 (P), 181.972 (S), and 213.857 (Zn). Concentration results in mg/L were converted to mg/kg by blank subtraction, multiplying by the digest volume and dividing by the sample weight (Martini and Schilt 1976).
Statistical analysis
A t test was used to compare oils before and after photooxidation for each nut species. A one-way analysis of variance (ANOVA) was conducted to examine differences in nutrient concentrations among nut species followed by Tukey’s post hoc test when differences were significant at p < 0.05. Differences between mature and immature canarium were also assessed using a t test. SPSS Version 24 software was used to perform all statistical analyses in this study.
Results
Effects of photooxidation on fatty acid compositions of different nuts
After photooxidation, C16:1 significantly increased compared with that of day 0 only in almond and pistachio (Table 1). The C18:1 Cis significantly increased in cashew and pistachio after photooxidation. The C18:2 significantly decreased in both almond and pistachio after photooxidation (Table 1). Fatty acid compositions of canarium remained unchanged after photooxidation (Table 1).
Table 1.
Fatty acid compositions of different nut species at day 0 and day 14 following photo-oxidation
| Almond | Cashew | Canarium | Pistachio | |
|---|---|---|---|---|
| C16:0 | ||||
| Day 0 | 5.16 (0.02)b | 9.12 (0.13) | 29.9 (0.43) | 9.62 (0.15)b |
| Day 14 | 5.36 (0.05)a | 9.18 (0.10) | 28.7 (0.10) | 12.9 (0.83)a |
| C18:0 | ||||
| Day 0 | 1.13 (0.02) | 12.1 (0.58)a | 14.6 (0.63) | 1.10 (0.12) |
| Day 14 | 1.08 (0.73) | 8.75 (0.15)b | 17.8 (0.53) | 1.12 (0.04) |
| C18:1 Cis | ||||
| Day 0 | 72.7 (0.17) | 63.3 (0.53)b | 50.0 (0.45) | 59.8 (0.47)b |
| Day 14 | 73.4 (0.10) | 65.3 (0.52)a | 46.4 (1.21) | 63.1 (0.42)a |
| C18:1 Trans | ||||
| Day 0 | 0.25 (0.07)b | ND | 0.78 (0.45) | 1.82 (0.26)b |
| Day 14 | 1.10 (0.34)a | 0.01 (0.004) | 0.74 (0.002) | 3.00 (0.19)a |
| C18:2 | ||||
| Day 0 | 20.6 (0.11)a | 15.4 (0.28)b | 5.46 (0.32) | 27.5 (0.24)a |
| Day 14 | 18.9 (0.10)b | 16.6 (0.11)a | 6.20 (0.61) | 19.8 (1.30)b |
Different lower case letters between days 1 and 14 indicate significant differences in fatty acid compositions of the nuts at p < 0.05 before and after photooxidation
ND not detected
Chemical compositions of nuts
Almond was rich in protein, Al, Ca, Fe, Mn and Zn (Fig. 1; Table 2), cashew was rich in protein, Cu, Mg, Mn, Fe, and Zn (Fig. 1; Table 2). Canarium was rich in protein, B, Cu, Fe, Mn, P, and Zn (Fig. 1; Table 2). Pistachio was rich in protein, Cu, Fe, K, P and S (Fig. 1; Table 2). In terms of comparing nutrient concentrations of different nuts, protein concentration of almond and pistachio was significantly higher than that of cashew and canarium (Table 2). Canarium showed the highest B concentrations followed by pistachio, almond and cashew (Table 2). Almond had the highest Ca concentration compared with other nuts and the greatest Cu and Na was observed in cashew (Table 2). Canarium was richer in P concentrations than pistachio and provided over 60% of P daily recommended intake based on 50 g kernels day−1 (Table 3). The Zn concentration of the canarium was significantly higher than that of other nuts (Table 2). Whilst pistachio had the highest K concentrations, the S concentration of pistachio and mature canarium did not differ (Table 2). Mature and immature canarium differed only in their B, Fe, K, Mn and Zn concentrations (Table 4).
Fig. 1.
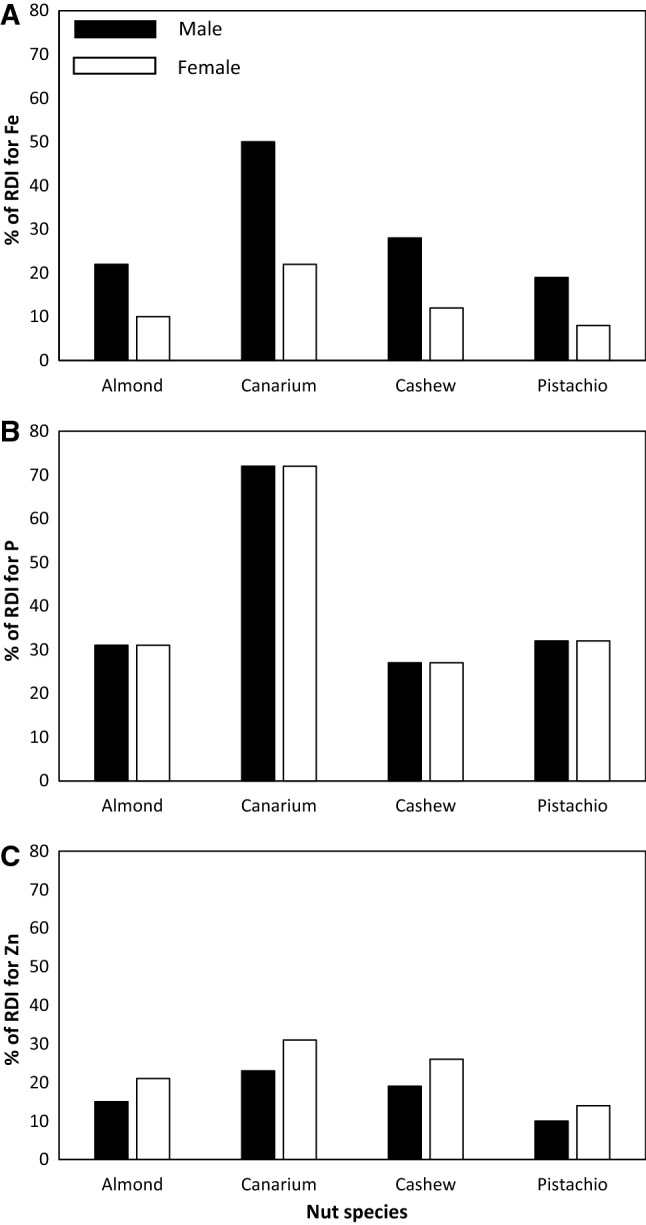
Recommended daily intake (RDI) of a iron (Fe), b phosphorus (P) and (C) zinc (Zn) for male (black columns) and female (white columns) in almond, canarium, cashew and pistachio
Table 2.
Chemical properties of almond, cashew, canarium and pistachio
| Almond | Cashew | Canarium (mature) | Pistachio | |
|---|---|---|---|---|
| Moisture content (%) | 2.21 (0.10)a | 2.27 (0.3)a | 3.01 (0.3)a | 3.27 (0.3)a |
| N (%) | 4.07 (0.04)a | 3.24 (0.13)b | 2.25 (0.04)c | 4.14 (0.09)a |
| Crude protein (%) | 25.4 (0.2)a | 20.2 (0.8)b | 14.0 (0.3)c | 25.8 (0.6)a |
| Al (mg/kg) | 15.1 (3)a | 2.44 (0.8)b | 3.46 (0.7)b | 4.07 (0.9)b |
| B (mg/kg) | 11.9 (1.6)b | 10.8 (4)b | 36.0 (4)a | 16.6 (4)b |
| Ca (%) | 0.22 (0.01)a | 0.025 (0.003)c | 0.08 (0.009)b | 0.10 (0.004)b |
| Cu (mg/kg) | 8.03 (0.4)c | 15.0 (0.4)a | 9.46 (0.5)bc | 11.0 (0.5)b |
| Fe (mg/kg) | 36.4 (2)bc | 46.0 (1)b | 81.0 (5)a | 31.4 (1)c |
| K (%) | 0.65 (0.02)b | 0.57 (0.02)b | 0.36 (0.02)c | 1.01 (0.02)a |
| Mg (%) | 0.25 (0.004)b | 0.20 (0.005)c | 0.46 (0.007)a | 0.10 (0.003)d |
| Mn (mg/kg) | 26.5 (0.9)a | 19.3 (1)b | 26.7 (12)a | 10.1 (0.6)c |
| Na (mg/kg) | 20.5 (5)bc | 79.6 (13)a | 8.87 (2)c | 41.8 (8)b |
| P (%) | 0.43 (0.01)b | 0.38 (0.01)b | 1.02 (0.04)a | 0.45 (0.01)b |
| S (%) | 0.15 (0.007)b | 0.17 (0.001)b | 0.21 (0.01)a | 0.22 (0.006)a |
| Zn (mg/kg) | 34.1 (1)c | 42.1 (0.4)b | 50.3 (2)a | 23.8 (0.9)d |
Different lower case letters at each row indicate significant differences among different nuts at p < 0.05. Mean standard errors have presented in parentheses
N nitrogen, Al aluminium, B boron, Ca calcium, Cu copper, Fe iron, K: potassium, Mg magnesium, Mn manganese, Na sodium, P phosphorus, S sulphur and Zn zinc. All data presented based on fresh weight
Table 3.
Recommended daily intake (RDI) of elements for age group between 31 and 50 years old and their percentages based on 50 g day−1 nut consumption
| DRI (mg day−1) | % of DRI (50 g nuts day−1) | |||||
|---|---|---|---|---|---|---|
| Cashew | Almond | Canarium (immature) | Canarium (mature) | Pistachio | ||
| Al | 70a | 1 | 0.17 | 0.39 | 0.24 | 0.29 |
| B | 20b,d | 3 | 3 | 6 | 9 | 4 |
| Ca | 1000b | 11 | 1 | 2.5 | 4 | 5 |
| Cu | 0.9b | 44 | 83 | 61 | 52 | 61 |
| K | 4700c | 7 | 6 | 6.5 | 4 | 11 |
| Mg | 420M–320Fb | 29–39 | 23–31 | 53–70 | 54–71 | 12–15 |
| Mn | 2.3M–1.8Fb | 57–73 | 42–53 | ND | 58–74 | 22–28 |
M Male, F Female, ND not determined
aEFSA (2008), total weekly intake (TWI) of 1 mg/kg bw/week calculated for a person of 70 kg
bTrumbo et al. 2001
cCampbell (2004)
dBased on UL which represents maximum daily intake that is not likely to pose any health risk, used when RDI is not determined due to the lack of sufficient data
Table 4.
Chemical properties of canarium, immature versus mature
| Canarium (immature) | Canarium (mature) | |
|---|---|---|
| Moisture content (%) | 3.11 (0.4) | 3.01 (0.3) |
| N (%) | 2.22 (0.04) | 2.25 (0.04) |
| Crude protein (%) | 13.8 (0.2) | 14.0 (0.3) |
| Al (mg/kg) | 5.53 (1.3) | 3.46 (0.7) |
| B (mg/kg) | 23.2 (3)b | 36.0 (4)a |
| Ca (%) | 0.05 (0.006) | 0.08 (0.009) |
| Cu (mg/kg) | 11.1 (0.7) | 9.46 (0.5) |
| Fe (mg/kg) | 57.5 (5)b | 81.0 (5)a |
| K (%) | 0.61 (0.03)a | 0.36 (0.02)b |
| Mg (%) | 0.45 (0.01) | 0.46 (0.007) |
| P (%) | 0.90 (0.03) | 1.02 (0.04) |
| S (%) | 0.17 (0.007) | 0.21 (0.01) |
| Zn (mg/kg) | 37.5 (3)b | 50.3 (2)a |
Different lower case letters at each row indicate significant differences between immature and mature canarium at p < 0.05. Mean standard errors have presented in parentheses
Discussion
Oil photooxidative stability of different nuts
Our data indicated that almond and pistachio were more sensitive to photooxidation compared to cashew and canarium. The presence of natural pigments and high unsaturated fatty acids increase oil instability under light (Khan and Shahidi 2002; Miraliakbari and Shahidi 2008; Özkan et al. 2016). Pistachio contains chlorophyll (Bolling et al. 2011). Therefore, photooxidative instability of pistachio could be explained by both the presence of natural pigments and high unsaturated fatty acid. It has been well documented that unsaturated fats are prone to heat, light and processing treatments (Khan and Shahidi 2002; Amaral et al. 2006; Miraliakbari and Shahidi 2008) due to the presence of carbons with double bonds in their molecular structures (Amaral et al. 2006). A higher susceptibility of almond and pistachio oil compared with cashew and canarium oil to photooxidation could be explained by their high unsaturated fat concentrations which decreased significantly after photooxidation. Therefore, the oils of almond and pistachio have shorter shelf-lives than cashew and canarium when exposed to light and hence should be stored in dark conditions.
Chemical properties of the different nuts
Our data indicated that almond, canarium, cashew and pistachio were a rich source of protein, and some essential nutrients (e.g. B, Ca, Fe, P, and Zn). The essential elements are crucial to maintain human health (Ros 2010). For example, dietary B prevents or reduces the severity of arthritis, promotes bone growth, improves brain function, and decreases the risk of some cancers (Nielsen and Meacham 2011). The B concentration of almond in our study was approximately 20% lower than that reported in some other studies (Naghii et al. 1996). Nut nutrient concentrations are associated with the origin and variety of the nuts (Evaristo et al. 2010; Gama et al. 2018a) which could help to explain the reported differences in B concentration in almond. In general, avocado, almond, cashew, peanut, pistachio and walnuts are considered as highly B-enriched food sources (Naghii et al. 1996; Hunt and Meacham 2001) and canarium can be also categorised as a B-enriched food source.
Almond, canarium, cashew and pistachio were rich in Fe, Mg, Mn and P. Daily intake of food needs to be nutrient balanced and some essential nutrients (e.g. Mg and Mn) are critical for human health due to their contribution in heart, brain and muscle function (Chiuve et al. 2013; De Baaij et al. 2015; Horning et al. 2015). Approximately 20% of maternal deaths have been associated with maternal iron-deficiency anaemia in Africa and Asia and over 2 billion people suffer from iron and vitamin A deficiencies (Horton and Ross 2003; Hawkes and Fanzo 2017). Pistachio was K-rich whereas canarium was P-rich (Tables 2, 3). Both K and P are important for bone health (Heaney, 2015). A daily intake of 4700 mg day−1 and 700 mg day−1 has been recommended for K and P, respectively (Trumbo et al. 2001; Campbell 2004). Therefore, a combination of mixed nut species may address dietary nutrient deficiencies.
Effects of nut maturity on nutrient concentrations
B, Fe and Zn content significantly increased in mature canarium nuts compared with immature nuts whereas K content significantly decreased. It is generally expected that nutrient concentrations in fruit increases while maturing (Clark and Smith 1988). However, only some nutrients change in kernels with maturity. For example, the harvesting time altered Cu, Fe, Na and Zn content in almond kernel but did not effect on Ca, Mg, Mn and K content (Piscopo et al. 2010). The observed decrease in K content of mature canarium kernels compared with immature kernels could be due to K limitation in the study site due to the fact that foliar K concentrations were under ideal concentrations (Bai et al. 2017b). K deficiency limits photosynthetic capacity of plants leading to a decreased K sink in plant parts (Pettigrew, 2008). Our study suggested that mature canarium nuts generally provide a better nutritional intake of some but not all nutrients.
Conclusion
This study aimed to investigate oil photooxidative stability and nutritional value of almond, canarium (newly commercialised indigenous nut), cashew, and pistachio. Cashew and canarium had higher oil photooxidative stability owing to their greater saturated fatty acid compositions compared with almond and pistachio. Thus, it is important to investigate photooxidative stability of oils to prevent degradation of beneficial fatty acids when stored. Our study also indicated a daily intake of 50 g day−1 of different nuts would provide different nutritional values for the consumer. For example, almond was a rich source of Ca and Mn, while cashew in Cu, Mn and Zn, canarium in B, Fe, Mg, Mn and P and pistachio in K. Therefore, kernels are recommended to be stored in dark to prevent oil photooxidation. This study highlighted the value of indigenous food sources (e.g. canarium nuts) to be used as valuable source of nutrients to decrease world hidden hunger.
Acknowledgements
We thank the University of the Sunshine Coast and the Papua New Guinea Government for support to undertake this study. Financial support was provided by the Australian Centre for International Agricultural Research (Project FST/2014/099).
Compliance with ethical standards
Conflict of interest
The authors declared that they have no conflict of interest.
References
- Albuquerque TAS, Pedreira PRB, Medina AN, Pereira JRD, Bento AC, Baesso ML. Time resolved thermal lens in edible oils. Rev Sci Instrum. 2003;74:694–696. doi: 10.1063/1.1512776. [DOI] [Google Scholar]
- Ali MA, Islam MA, Othman NH, Noor AM. Effect of heating on oxidation stability and fatty acid composition of microwave roasted groundnut seed oil. J Food Sci Technol. 2017;54:4335–4343. doi: 10.1007/s13197-017-2534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral JS, Casal S, Seabra RM, Oliveira BP. Effects of roasting on hazelnut lipids. J Agric Food Chem. 2006;54:1315–1321. doi: 10.1021/jf052287v. [DOI] [PubMed] [Google Scholar]
- Bai SH, Darby I, Nevenimo T, Hannet G, Hannet D, Poienou M, Grant E, Brooks P, Walton D, Randall B. Effects of roasting on kernel peroxide value, free fatty acid, fatty acid composition and crude protein content. PLoS ONE. 2017;12:e0184279. doi: 10.1371/journal.pone.0184279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai SH, Trueman SJ, Nevenimo T, Hannet G, Bapiwai P, Poienou M, Wallace HM. Effects of shade-tree species and spacing on soil and leaf nutrient concentrations in cocoa plantations at 8 years after establishment. Agric Ecosyst Environ. 2017;246:134–143. doi: 10.1016/j.agee.2017.06.003. [DOI] [Google Scholar]
- Bai SH, Tahmasbian I, Zhou J, Nevenimo T, Hanet G, Walton D, Randall B, Gama T, Wallace HM. A non-destructive determination of peroxide values, total nitrogen and mineral nutrients in an edible tree nut using hyperspectral imaging. Comput Electron Agric. 2018;151:492–500. doi: 10.1016/j.compag.2018.06.029. [DOI] [Google Scholar]
- Bai SH, Trueman SJ, Gama T, Jones K, Walton DA, Randall B, Wallace HM (2018b) Shelf life of macadamia kernels of different origin. Acta Hortic (in press)
- Bolling BW, Chen C-YO, McKay DL, Blumberg JB. Tree nut phytochemicals: composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr Res Rev. 2011;24:244–275. doi: 10.1017/S095442241100014X. [DOI] [PubMed] [Google Scholar]
- Campbell S. Dietary reference intakes: water, potassium, sodium, chloride, and sulfate. Clin Nutr Insight. 2004;30:1–4. [Google Scholar]
- Chiuve SE, Sun Q, Curhan GC, Taylor EN, Spiegelman D, Willett WC, Manson JE, Rexrode KM, Albert CM. Dietary and plasma magnesium and risk of coronary heart disease among women. J Am Heart Assoc. 2013;2:e000114. doi: 10.1161/JAHA.113.000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C, Smith G. Seasonal accumulation of mineral nutrients by kiwifruit 2. Fruit. New Phytol. 1988;108:399–409. doi: 10.1111/j.1469-8137.1988.tb04180.x. [DOI] [Google Scholar]
- Couladis M, Özcan M, Tzakou O, Akgül A. Comparative essential oil composition of various parts of the turpentine tree (Pistacia terebinthus L) growing wild in Turkey. J Sci Food Agric. 2003;83:136–138. doi: 10.1002/jsfa.1295. [DOI] [Google Scholar]
- De Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- EFSA, European Food Safety Authority Safety of aluminium from dietary intake-scientific opinion of the panel on food additives, flavourings, processing aids and food contact materials (AFC) EFSA J. 2008;6:754. doi: 10.2903/j.efsa.2008.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evaristo I, Batista D, Correia I, Correia P, Costa R. Chemical profiling of Portuguese Pinus pinea L. nuts. J Sci Food Agric. 2010;90:1041–1049. doi: 10.1002/jsfa.3914. [DOI] [PubMed] [Google Scholar]
- FAO . The state of food security and nutrition in the world 2017. Building resilience for peace and food security. Rome: FAO; 2017. [Google Scholar]
- Gama T, Wallace HM, Bai SH, Trueman SJ (2018a) Variability in crude protein and mineral nutrient content of almonds. Acta Hortic (in press)
- Gama T, Wallace HM, Trueman SJ, Hosseini-Bai S. Quality and shelf life of tree nuts: a review. Sci Hortic. 2018;242:116–126. doi: 10.1016/j.scienta.2018.07.036. [DOI] [Google Scholar]
- Hawkes C, Fanzo J. Nourishing the SDGs: global nutrition report 2017. London: Development Initiatives Bristol; 2017. [Google Scholar]
- Heaney RP. Sodium, potassium, phosphorus, and magnesium. Nutrition and bone health. Berlin: Springer; 2015. pp. 379–393. [Google Scholar]
- Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M. Manganese is essential for neuronal health. Ann Rev Nutr. 2015;35:71–108. doi: 10.1146/annurev-nutr-071714-034419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton S, Ross J. The economics of iron deficiency. Food Policy. 2003;28:51–75. doi: 10.1016/S0306-9192(02)00070-2. [DOI] [Google Scholar]
- Hunt C, Meacham SL. Aluminum, boron, calcium, cooper, iron, magnesium, manganese, molybdenum, phosphorus, potassium, sodium, and zinc: concentrations in common western foods and estimated daily. J Acad Nutr Diet. 2001;101:1058. doi: 10.1016/S0002-8223(01)00260-7. [DOI] [PubMed] [Google Scholar]
- Kazantzis I, Nanos GD, Stavroulakis GG. Effect of harvest time and storage conditions on almond kernel oil and sugar composition. J Sci Food Agric. 2003;83:354–359. doi: 10.1002/jsfa.1312. [DOI] [Google Scholar]
- Khan MA, Shahidi F. Photooxidative stability of stripped and non-stripped borage and evening primrose oils and their emulsions in water. Food Chem. 2002;79:47–53. doi: 10.1016/S0308-8146(02)00176-0. [DOI] [Google Scholar]
- Kodad O, Socias i Company R Variability of oil content and of major fatty acid composition in almond (Prunus amygdalus Batsch) and its relationship with kernel quality. J Agric Food Chem. 2008;56:4096–4101. doi: 10.1021/jf8001679. [DOI] [PubMed] [Google Scholar]
- Küçüköner E, Yurt B. Some chemical characteristics of Pistacia vera varieties produced in Turkey. Eur Food Res Technol. 2003;217:308–310. doi: 10.1007/s00217-003-0763-7. [DOI] [Google Scholar]
- Leakey RR. Converting ‘trade-offs’ to ‘trade-ons’ for greatly enhanced food security in Africa: multiple environmental, economic and social benefits from ‘socially modified crops. Food Secur. 2018;10:505–524. doi: 10.1007/s12571-018-0796-1. [DOI] [Google Scholar]
- Leakey RRB, Weber JC, Page T, Cornelius JP, Akinnifesi FK, Roshetko JM, Tchoundjeu Z, Jamnadass R. Tree domestication in agroforestry: progress in the second decade. In: Nair PK, Garrity D, editors. Agroforestry-the future of global land use. New York: Springer; 2012. pp. 145–173. [Google Scholar]
- Martini GD, Schilt AA. Investigation of the wet oxidation efficiencies of perchloric acid mixtures for various organic substances and the identities of residual matter. Anal Chem. 1976;48:70–74. doi: 10.1021/ac60365a032. [DOI] [Google Scholar]
- Matthaus B, Özcan MM. Quantification of fatty acids, sterols and tocopherols turpentine (Pistacia terebinthus Chia) wild growing in Turkey. J Agric Food Chem. 2006;54:7667–7671. doi: 10.1021/jf060990t. [DOI] [PubMed] [Google Scholar]
- Matthaus B, Ozcan MM, Al Juhaimi F. Oil content, fatty acid composition and tocopherol contents of turpentine (Pistachia terebinthus L.) and stonepine (Pinus pinea L.) nut oils. Z Arznei-Gewurzpfla. 2015;20:136–140. [Google Scholar]
- Matthaus B, Özcan MM, Al Juhaimi F. Fatty acid composition and tocopherol content of the kernel oil from apricot varieties (Hasanbey, Hacihaliloglu, Kabaasi and Soganci) collected at different harvest times. Eur Food Res Technol. 2016;242:221–226. doi: 10.1007/s00217-015-2533-8. [DOI] [Google Scholar]
- Millena CG, Sagum RS. Philippine Pili (Canarium ovatum, Engl.) varieties as source of essential minerals and trace elements in human nutrition. J Food Comp Anal. 2018;69:53–61. doi: 10.1016/j.jfca.2018.02.008. [DOI] [Google Scholar]
- Millena CG, Sagum RS. Physicochemical characterization and fatty acid profiling of different Philippine pili nut (Canarium ovatum, Engl.) varieties. J Am Oil Chem Soc. 2018;95:325–336. doi: 10.1002/aocs.12028. [DOI] [Google Scholar]
- Miraliakbari H, Shahidi F. Oxidative stability of tree nut oils. J Agric Food Chem. 2008;56:4751–4759. doi: 10.1021/jf8000982. [DOI] [PubMed] [Google Scholar]
- Naghii M, Wall P, Samman S. The boron content of selected foods and the estimation of its daily intake among free-living subjects. J Am Coll Nutr. 1996;15:614–619. doi: 10.1080/07315724.1996.10718638. [DOI] [PubMed] [Google Scholar]
- Nanos GD, Kazantzis I, Kefalas P, Petrakis C, Stavroulakis GG. Irrigation and harvest time affect almond kernel quality and composition. Sci Hortic. 2002;96:249–256. doi: 10.1016/S0304-4238(02)00078-X. [DOI] [Google Scholar]
- Ni Z, Tang F, Yu Q, Liu Y. Toxic and essential elements in five tree nuts from Hangzhou market, China. Food Addit Contam B. 2016;9:246–250. doi: 10.1080/19393210.2016.1186118. [DOI] [PubMed] [Google Scholar]
- Nielsen FH, Meacham SL. Growing evidence for human health benefits of boron. J Evid Based Complement Altern Med. 2011;16:169–180. doi: 10.1177/2156587211407638. [DOI] [Google Scholar]
- Özcan M. Characteristics of fruit and oil of terebinth (Pistacia terebinthus L) growing wild in Turkey. J Sci Food Agric. 2004;84:517–520. doi: 10.1002/jsfa.1632. [DOI] [Google Scholar]
- Özcan MM, Tzakou O, Couladis M. Essential oil composition of the turpentine tree (Pistacia terebinthus L.) fruits growing wild in Turkey. Food Chem. 2009;114:282–285. doi: 10.1016/j.foodchem.2008.08.094. [DOI] [Google Scholar]
- Özkan G, Kiralan M, Karacabey E, Çalik G, Özdemir N, Tat T, Bayrak A, Ramadan MF. Effect of hazelnut roasting on the oil properties and stability under thermal and photooxidation. Eur Food Res Technol. 2016;242:2011–2019. doi: 10.1007/s00217-016-2699-8. [DOI] [Google Scholar]
- Pettigrew WT. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol Plant. 2008;133:670–681. doi: 10.1111/j.1399-3054.2008.01073.x. [DOI] [PubMed] [Google Scholar]
- Piscopo A, Romeo FV, Petrovicova B, Poiana M. Effect of the harvest time on kernel quality of several almond varieties (Prunus dulcis (Mill.) D.A. Webb) Sci Hortic. 2010;125:41–46. doi: 10.1016/j.scienta.2010.02.015. [DOI] [Google Scholar]
- Powell B, Thilsted SH, Ickowitz A, Termote C, Sunderland T, Herforth A. Improving diets with wild and cultivated biodiversity from across the landscape. Food Secur. 2015;7:535–554. doi: 10.1007/s12571-015-0466-5. [DOI] [Google Scholar]
- Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652–682. doi: 10.3390/nu2070652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek S, Ozcan MM, Al Juhaimi F. Characteristics of turpentine (Pistacia terebinthus L.) fruit and fruit oil collected from two different locations. Z Arznei-Gewurzpfla. 2016;21:102–106. [Google Scholar]
- Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- Vanhanen LP, Savage GP. Mineral analysis of pine nuts (Pinus spp.) grown in New Zealand. Foods. 2013;2:143–150. doi: 10.3390/foods2020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton DA, Randall BW, Poienou M, Nevenimo T, Moxon J, Wallace HM. Shelf life of tropical Canarium nut stored under ambient conditions. Horticulturae. 2017;3:24. doi: 10.3390/horticulturae3010024. [DOI] [Google Scholar]


