Abstract
Argentina is a leading country in biodiesel production from soy. Extruded soy is a low-cost byproduct of the soybean oil industry, from which animal feeds are prepared as well as flour for human consumption. Soy proteins can be isolated from flours and digested with enzymes in order to obtain bioactive fractions. In this work, a commercial soy isolate (PRO-FAM 974) was characterized. Maximal solubility was achieved at a concentration of 90 mg/mL. Protein profiles obtained by SDS-PAGE showed that the isolate was constituted mostly by globulins. Conformational and thermal analyses (differential scanning calorimetry) showed that proteins were almost completely denatured. The isolate was hydrolyzed with a commercially available enzyme (COROLASE 7089). The peptide profile (MALDI-TOF) showed peptides ranging from 800 to 10,000 Daltons. We conclude that the product obtained has the potential to be used as functional ingredient for the development of functional foodstuffs, giving the opportunity to add value to the byproducts of the soybean oil industry.
Keywords: Protein isolates, Soy peptides, Hydrolysates, Functional foods, Enzymes
Introduction
In Argentina, soybean production is economically important. In 2015, Argentina was the third largest soybean producer in the world, with a production of 60.8 million tons. Furthermore, Argentina is the largest producer of biodiesel from soybean oil, reaching 2,580,000 tons/year and it is also the largest exporter of soybean flour, with 27.8 million tons sold in 2015 (Secretaría de Agricultura, Ganadería, Pesca y Alimentación 2016). Extruded soy is a low-cost byproduct of the soybean oil industry, from which animal feeds are prepared as well as flour for human consumption. Soy flour is a low-cost byproduct that contains between 40 and 45% (w/w) of protein.
The protein present in soy flour can be further concentrated by alkaline extraction and precipitation based on its isoelectric point, with an expected yield of 70% (w/w) and a purity of 91% (w/w). With the goal of increasing its functionality, soy protein can be hydrolyzed by means of acids, temperature, microbial fermentation or enzymatic action. Microbial fermentation and enzymatic action are safer and healthier, as in acid or thermal hydrolysates random ruptures may occur, which may lead to the liberation of toxic fractions (Vioque et al. 2001). Enzymatic hydrolysis increases protein digestibility and reduces its potential allergenicity (Peñas et al. 2006). Concomitantly, it is possible to obtain specific peptides with potential biological activity, which could be used in the formulation of new food products with high added value (Madureira et al. 2007; Pihlanto 2001).
Functional foods are those which confer a benefit beyond nutrition, improving health or reducing the risk of disease. In order to achieve these beneficial effects, functional foods must be consumed periodically as a part of a balanced diet (Madureira et al. 2007; Nagpal et al. 2012).
The concern of the consumers for their health has increased the demand for functional foods around the world. Conditions such as obesity, certain types of cancer, hypertension, high cholesterol, cardiac issues, stress or intestinal inflammation are related to dietary habits (Etzel 2004). Bioactive peptides are sequences of aminoacids capable of causing specific biological effects. These molecules are inactive within the intact protein and can be liberated by means of digestive and fermentative processes or by the action of specific proteolytic enzymes (Korhonen and Pihlanto 2003; Vinderola et al. 2008). A large number of peptides with different functional properties can be derived from protein sources such as eggs, milk, whey, fish and soybean, among others.
The different peptides obtained have been classified according to their bioactivity in opioid peptides, antihypertensives, antithrombotics, mineral transporters, antimicrobials, antioxidants, antitumorals, and immunomodulators (Fitzgerald and Meisel 2003). Our interest in obtaining functional peptides is based on their potential impact on the immune system, particularly the stimulating effects on innate immunity, as observed in previous studies in our laboratory with a soy protein hydrolysate (unpublished data). The aim of this work was to obtain and characterize a peptidic fraction derived from soy flour which could be incorporated into different alimentary matrices in order to add value to the extruded soy and soy flour produced in Argentina.
Materials and methods
Extraction of protein from soy flour
The procedure of Petruccelli and Añon (1995) and Paredes and Ordorica (1986) was used. Briefly, soy flour (Sireth, Molinos Chabas, Santa Fe, Argentina) was resuspended (10% w/v) in distilled water and the pH was adjusted to 9 with 0.25 M NaOH. The solution was agitated (30 min, 200 rpm, 25 °C), using an automatic agitator (SI 300, Jeio Tech, Japan) and centrifuged (3500×g, 10 °C, 30 min).
The supernatant was separated and pH was adjusted to 4.5 with 1 N HCl. The solution was further centrifuged (4000×g, 30 min, 4 °C) (Sorvall, model RC-3, USA), the supernatant was discarded and the pellet was resuspended in distilled water, adjusting the pH to 7 with 1.25 N NaOH. The solution was agitated for 5 additional min (180 × g, 25 °C), centrifuged (4000×g, 10 min,4 °C) and the supernatant was removed and lyophilized (100 millitorrs, − 85 °C) (Thermo Vac, USA). Total nitrogen was quantified by Kjeldhal, utilizing the semi-micro Kjedhal method (Digester K435 Buchi, Switzerland, distiller K350 Buchi, Switzerland and titler DL15 Mettler Toledo, Switzerland). Due to operational issues and for practical reasons, the remaining assays were performed with a commercially available soy protein isolate (PROFAM-974, ADM, Chicago, USA).
Characterization of PROFAM-974
Physico-chemical analysis
Nitrogen was quantified by Kjedhal to calculate the percentage of dried protein. Moisture was determined by gravimetry (101 ± 2 °C) until constant weight. Fat was determined by the Soxhlet method.
Protein profile
SDS-PAGE was performed to determine the protein profile of the soy flour isolate and the commercial isolate PROFAM-974. Electrophoresis was performed based on the Laemmli (1970) method. A 15% (w/v) acrylamide-bis acrylamide gel was prepared. A commercially available molecular weight marker was used (Fermentas, Thermo Scientific, USA).
The sample was treated with 2 × Laemmli buffer (2.5 mL 0.5 M Tris–HCl pH 6.8, 4 mL 10% SDS, 2 mL glycerol, 1 mL B-mercaptoethanol, bromophenol blue buffer, final volume 10 mL) and then heated (90 °C, 5 min). Samples were run at 10 mA through the gel concentrator and then at 20 mA until the sample front reached the end of the gel. The gel was stained (0.1% Coomasie Blue in 25% methanol and 10% acetic acid) for 30 min and rinsed (25% methanol and 10% acetic acid) until excess stain was removed. Electrophoresis was performed in a vertical mini-gel instrument (Bio Rad, Hercules, CA, USA). The power source was a POWER PAC HC (Bio Rad, Hercules, CA, USA).
Solubility of PROFAM-974
Different solutions (20, 40, 60, 90, 120, 160 and 180 mg/mL) of the commercial soy isolate PROFAM-978 were prepared in 0.1 M pH 7.2 phosphate buffer, with vigorous vortexing. Samples were centrifuged (9000×g, 10 min, 20 °C) and protein quantification in the supernatants was carried out spectrophotometrically (LKB Biochrom, Ultrospec II spectrophotometer, A280 nm). A calibration curve (BSA, Sigma) was used to determine the concentration of solubilized protein.
Thermal and conformational analysis of PROFAM-974
This analysis was performed to determine whether the commercial isolate would require a thermal treatment for protein denaturation and for the proper functioning of the enzyme (Corolase 7089) before hydrolysis. Differential scanning calorimetry (DSC) was applied, samples were heated (10 °C, 2 min) and the analysis was performed at a speed of 10 °C/min, from 10 to 130 °C (Mettler Toledo, DSC821e, Schwerzenbach, Switzerland).
Enzymatic hydrolysis of PROFAM-974
The digestion of the commercial isolate was performed keeping a constant protein concentration of 9% (w/v) while using different concentrations [0.1; 0.2; 0.3 and 0.5% (v/v)] of the commercial enzyme Corolase 7089 (ABF Ingredients Company, United Kingdom). Digestion was performed at 55 °C for 80 min at pH 7.2 in 0.1 M phosphate buffer. Samples were taken at 0; 1; 3; 5; 10; 20; 30; 60; 70 and 80 min during digestion. Digested samples were treated with 10% (w/v) trichloroacetic acid (TCA) and centrifuged (9000×g, 10 min, 20 °C). The degree of hydrolysis was determined by absorbance of the supernatant at 280 nm after precipitation with 10% trichloroacetic acid. The supernatant was obtained by centrifugation (9000×g, 10 min, 20 °C).
Preparation of hydrolysate
PROFAM-974 isolate was diluted (9% w/v) in 0.1 M phosphate buffer. Neutral endoprotease Corolase 7089 (ABF Ingredients Company, United Kingdom) was added at 0.2% (v/v). The hydrolysis was carried out in a shaker, in an Erlenmeyer flask (55° C, pH 7.3; 60 min, with agitation at 60 rpm) (SI-300, Jeio Tech, Korea). The digestion was stopped by heating (90 °C, 10 min). The digested sample was lyophilized (100 milliliters, − 85° C, 24 h) (Thermo Vac, USA).
Hydrolysate characterization
After digestion, a 0.5 ml aliquot was taken and treated with 0.5 mL of 10% TCA (w/v). The supernatant was obtained by centrifugation (9000×g, 10 min, 20 °C). The degree of hydrolysis was determined spectrophotometrically (A280 nm), as the ratio of soluble protein in TCA/Total Protein × 100.
Physico-chemical analysis of lyophilized hydrolysate
Moisture, sodium and phosphorus contents were quantified. Sodium was quantified by atomic absorption spectrophotometry (Horwitz 2000), using a GBC 906 atomic absorption spectrophotometer (GBC 906 AEE, GBC, Australia). Phosphorus content was determined by molecular absorption spectrophotometry (Ultrospec III, Pharmacia LKB, Sweden). The principle of the method relies in the reaction of inorganic phosphorus with molybdate in an acid medium to produce a phosphorus-molybdate complex that can be quantified spectrophotometrically at 340 nm (Henry et al. 1974).
Peptidic profile
The peptide profile was obtained using MALDI-TOF (Ultraflex II, Brunker, Daltonics, Germany) according to Coligan (1995), in a low molecular weight peptide matrix (up to 2700 Dalton, α-Cyano-4-hydroxycinnamic acid) in reflectron mode and in a matrix for large peptides (up to 20,000 Dalton, sinapinic acid) in lineal mode.
Peptide identification
The hydrolysate was treated with a resin (Zip-Tip C18, Emd Millipore, Ireland) to extract salts and to concentrate peptides, especially those smaller than 4 kDa. The purified sample was dehydrated (SpeedVac SVC 100H Centrifugal Evaporator, Savant, Germany) and resuspended in 10 μl of 0.1% (w/v) formic acid. The sample was analyzed by nano-HPLC (Easy-nLC 1000, Thermo Scientific, Germany) coupled to a mass spectrometer (Q Exactive, Thermo Scientific, Germany). The ionization of the samples was done by electrospray (ES081—Easy spray source kit, Thermo Scientific, Germany).
The analysis of the data was performed by a Proteome Discoverer 1.4, USA program with the following searching criteria: original data base: Glycinemax; enzyme: no enzyme; miscleavage: mass tolerance for precursor: 10 ppm, mass tolerance for fragments: 0.05 Da; dynamic modifications: oxidation (M); static modifications: carbamidemethylation; level of confidence of peptides: high. Protein complexes were separated (Easy-nLC 1000 apt, Thermo Scientific chromatographer) with a high degree of resolution using a reverse-phase column (Easy-Spray Accucore (P/N ES801), C18, 2.6 μm, 150α, 75 μm × 150 mm, Thermo Scientific, Germany) run at 45 °C. The chromatographic run (2 μl of sample/run) was performed with a gradient of solution A (water with 0.1% w/w formic acid) and solution B (acetonitrile with 0.1% w/w formic acid). All solvents and reagents used were of LC–MS quality. The ionizer that was used was Electro Spray brand Thermo Scientific, model EASY-SPRAY. Spray Voltage: 3.5 kV. The spectrometer used was a Thermo Scientific, model Q-Exact. The team has a High Collision Dissociation (HCD) cell and an Orbitrap analyzer. This equipment allows for identification of the peptides at the same time as they are separated by chromatography, obtaining complete MS and MSMS. We used a method that performs the greatest number of cycle measurements per unit time.
Statistical analysis
Concentrations of enzyme used over time were statistically analyzed. In the statistical analysis model the effect of concentration, time and the interaction of concentration with time were included, considering the repetitive measurement in each tube with a covariance autoregressive structure of first order. The analysis was performed with the procedure PROC MIXED of SAS V9.3 (SAS, Institute Inc., Cary, NC, USA).
Results
Protein profile of the isolate obtained from soy flour
A yellowish powder with 90 ± 0.145% (w/w) total nitrogen was obtained. The protein profile of the protein isolate obtained from soy flour is shown in Fig. 1. In lane B, the upper bands correspond to the 7 S β-conglycinin of approximately 150 KDa, constituted by three subunits, α, α´ y β. The remaining bands correspond to the 11S glycinin of approximately 350 kDa, formed by six subunits, acidic fractions A and basic fractions B, joined by a disulfide bridge.
Fig. 1.
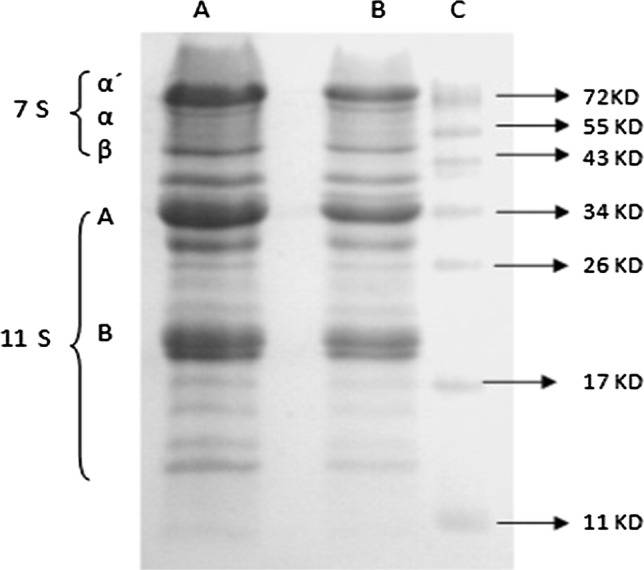
Denaturing polyacrylamide gel: 6 μg protein isolate Profam-974 (a), 6 μg protein isolate obtained from soy flour (b) stained with Coomasie blue, compared to the MW standard (c) (KD)
Characteristics PROFAM-974
The protein isolate contained 94 ± 0.144% (w/w) protein, 0.4 ± 0.02% (w/w) fat and 3.5 ± 0.18% moisture. In lane A (Fig. 1), the upper bands correspond to the 7 S β-conglycinin of approximately 150 KDa, constituted by three subunits, named α, α´ and β. The remaining bands correspond to the 11S glycinin of approximately 350 KDa, made of 6 subunits, acidic fractions A and basic fractions B, joined by a disulfide bridge.
Solubility and conformational analysis of isolate PROFAM-974
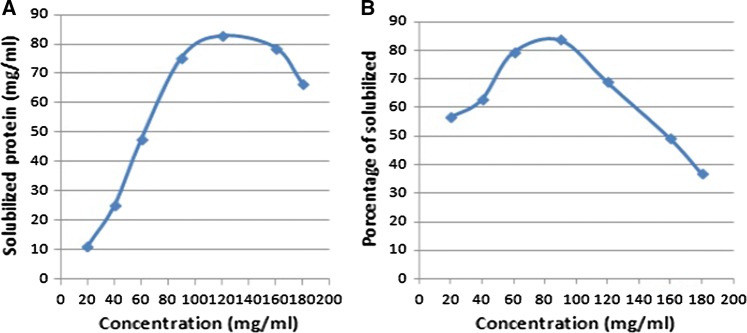
Maximum solubility was achieved at a concentration of 90 mg/mL (Fig. 2a). 83.75% (75.38 ± 0.5 mg/mL), remained in solution. Beyond this value, solubility decreased due to solution saturation (Fig. 2b). Thermal and conformational analyses of isolated proteins from PROFAM-974 showed that the isolate was highly denatured. Two small peaks were detected, which corresponded to 7S β -conglycinin at 77.53 °C and 11S glycinin at 98.67 °C, belonging to the native protein (data not shown).
Fig. 2.
a Solubility of Profam-974 (mg/ml) at different concentrations in 0.1 M, pH 7.2 phosphate buffer. b Percentage of solubilized protein at different concentrations of Profam-974 prepared in 0.1 M, pH 7.2 phosphate buffer
Hydrolysis of PROFAM-974 with Corolase 7089
The interaction between concentration and time of hydrolysis was statistically significant (p = 0.0049). Thus, the concentrations at each time-point were evaluated. Enzyme concentrations of 0.2% and 0.3% did not show significant differences at the time-points evaluated (p > 0.05). When the enzyme was used at 0.1% (w/w) and 0.2% (w/w), a significant difference including the time-point 10 min and beyond (p < 0.05) was observed. The concentration of enzyme at 0.3% (w/w) and 0.5% (w/w) showed significant differences only after 70 min of hydrolysis (p = 0.0212). For further studies, the enzyme was used at a concentration of 0.2% (w/w). Soluble protein in 10% (w/w) TCA after the digestion of PROFAM-974 with different enzyme concentrations and for different digestion times is shown in (Fig. 3).
Fig. 3.
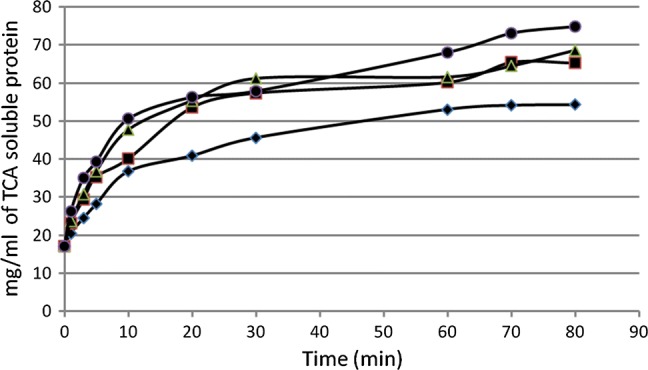
Concentration in mg/ml of soluble protein in 10% TCA after digestion of PRO-FAM 974 with 0.1% (filled diamond), 0.2% (filled square), 0.3% (filled triangle) or 0.5% (filled circle) of enzyme in different sampling times
Degree of hydrolysis for the different concentrations of the enzyme and obtention and characterization of the hydrolysate
The degree of hydrolysis of different enzyme concentrations at different sampling times is shown in (Fig. 4). A hydrolysate of 47.91 ± 0.005% degree of hydrolysis (DH %) was obtained at 60 min (0.2% v/v Corolase 7089) of hydrolysis under the previously described conditions. The hydrolysate showed a dry mass content of 98.01 ± 0.144%, 3.2 ± 0.346% sodium and 3.35 ± 0.569% phosphorus.
Fig. 4.
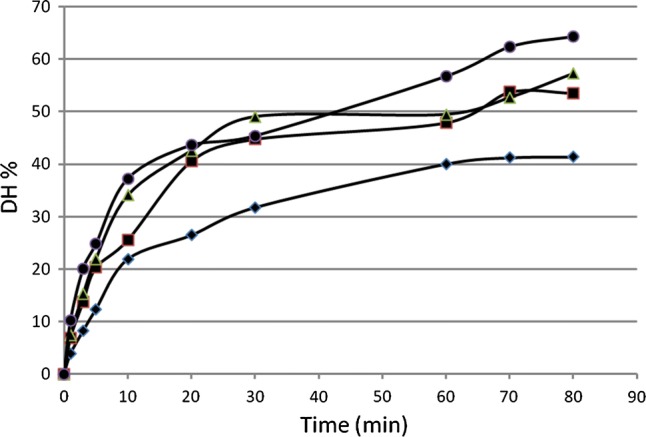
Degree of hydrolysis (DH) after digestion of PRO-FAM 974 with 0.1% (filled diamond), 0.2% (filled square), 0.3% (filled triangle) or 0.5% (filled circle) of enzyme at different sampling times
Peptide profile of the hydrolysate
The peptide profile prepared on a matrix for small peptides of up to 2700 Dalton (α-Cyano-4-hydroxycinnamic acid) in refrectron mode is shown in Fig. 5. The upper part of the image shows the spectrum obtained from a 1:10 dilution of the lyophilized sample, whereas the lower part is the analysis of a 1:100 dilution of the same sample. The peptide profile prepared on a matrix for large peptides up to 18,000 Dalton (sinapinic acid) with the instrument set in linear mode is shows in (Fig. 6). The upper part of the image shows the spectrum obtained from a 1:10 dilution of the lyophilized sample, the other two are the spectrums obtained from the analysis of a 1:100 dilution of the same sample. Analysis was done for spectra over 18,000 Dalton with negative results. The hydrolysate showed peptides with molecular masses between 800 and 10,000 Dalton.
Fig. 5.
Peptide profile performed in a matrix for small peptides up to 2700 Dalton (α-Cyano-4-hydroxycinnamic acid) in reflectron mode
Fig. 6.
Comparison of peptide profile performed in a large-peptide matrix (up to 18,000 Dalton, sinapinic acid) in lineal mode
It was possible to identify between 60 and 75% of all peptides which comprise the major proteins. Only those peptides with highest concentrations are shown. The number after the sequence represents the number of times the sequence was detected by the instrument.
The resulting peptides from the β-conglycinin, alpha’ chain were: RAELSEQDIFVIPA (43); LAGSKDNVISQIPSQVQE (13); KYRAELSEQDIFVIPA (14); VVDMNEGALFLPH (11); LAIPVNKPGRFESF (7). The peptides derived from the β-conglycinin alpha-subunit were: RAELSEQDIFVIPA (43); LQESVIVEISKEQ (20); LQESVIVEISKEQIRA (14); LRSRDPIYSNK (11); YDTKFEEINKVLFSR (7). The peptides derived from the β-conglycinin beta subunit (Fragment) were: LAFPGSAQDVERL (9); RAELSEDDVFVIPA (8); FHSEFEEINRVL (6). The peptides derived from the Glycinin G1 were: IIDTNSLENQLDQMPRR (57); IIDTNSLENQLDQMPRR (25) with oxidation in methionine; LQGENEGEDKGAIVT (22); LAGNQEQEFLKYQ (14); ALPEEVIQHTFN (9); LVPPQESQKRA (9). The peptides derived from the Glycinin G4 were: FNEGDVLVIPPGVPY (24); FNEGDVLVIPPGVPYW (17); LHLPSYSPYPRM (12); FNTNEDIAEKL (10); GLHLPSYSPYPRM (8) and the peptides derived from the Glycinin A3B4 subunit were: VVAEQGGEQGLEYVVF (49); FNEGDVLVIPPGVPY (24); FNEGDVLVIPPGVPYW (17).
Discussion
Isolating the protein from soy flour resulted in an easy and economically viable procedure. The product obtained had a satisfactory content of proteins comparable to that reported for isolates from soybean, kidney beans and field pea (Shevkani et al. 2014). The process was performed on an experimental scale. However, it can be applied to an industrial scale for a more rational use of soy by-products and to increase the value of soy flour.
The profiles of the soy protein isolate obtained from soy flour and from the commercial isolate PROFAM-974 were similar (Fig. 1), coinciding with what was reported for soy globulins (Barac et al. 2015; Chen et al. 2016). The commercially available PROFAM-974 soy protein showed to be an isolate of high purity, mostly constituted by globulins and with low lipid content. Globulins of legumes showed better in vitro digestibility than albumins (Ghumman et al. 2016). Its highest solubility at pH 7.2 was found when the protein concentration did not exceed 9% (w/v), the point at which the highest concentration was observed and where 83% of the protein remained soluble (Fig. 2a, b). Similar solubilities were reported at pH 7 for soy protein and from other legumes isolates (Barac et al. 2015; Shevkani et al. 2014).
The thermal and conformational analyses by DSC of PROFAM-974 showed that the proteins were mostly denatured, in coincidence with another study in which the isolate PROFAM-974 showed a 100% degree of denaturation (Lee et al. 2003). This can be attributed to the alkaline isolation and drying process, which permits hydrolysis without a previous thermal denaturation process to allow the enzyme (Corolase 7089, from Bacillus subtilis) to reach active sites.
The commercial enzyme used for the hydrolysis showed to be very efficient compared to the enzymes used by Kong et al. (2008) with only 18.36 DH % in 180 min. Ahmadifard et al. (2016) used the commercial enzyme Alcalase to hydrolyze soy protein, obtaining 12.50 DH % after 60 min, whereas Corolase 7089 reached 47.91 DH % at 60 min. A reasonably low concentration of enzyme was needed to obtain hydrolysates. The percentage of enzyme chosen (0.2% v/v) was the lowest concentration which allowed for the shortest time to obtain the hydrolysis products, thus reducing processing costs. The enzymatic hydrolysis process can be controlled in order to obtain a final product with the desired characteristics. Within 10 min, the enzyme reached 25% hydrolysis and a value of 40% by min 20 of treatment. Later on, the reaction slowed down considerably and no significant changes were observed between min 70 and 80. Kong et al. (2008) attributed the reduction in hydrolysis rate to the competition between unhydrolysed protein and the peptides being constantly formed during hydrolysis.
Generating hydrolysates from soy protein adds value and permits the use of these products in the food industry for the production of food, drinks and dietary supplements, increasing the content of nitrogen and achieving a greater assimilation by the organism. The products can also be used in the pharmaceutical and cosmetic industries, for example for facial creams (Singh et al. 2014).
In different studies the peptides derived from soy protein have demonstrated to have important immunomodulator and hypocholesterolemic properties (Cho et al. 2007). A peptide derived from the tryptic digestion of soy protein, made of thirteen aminoacids, called Soymetide-13 (MITLAIPVNKPGR), with the ability to stimulate phagocytosis by polymorphonuclear leucocytes in humans in vitro, has been reported (Takahiro et al. 2003). Furthermore, it was also demonstrated that the effect increased considerably when the peptide was reduced to a tetrapeptide (MITL) (Takahiro et al. 2005). Moreover, certain peptides derived from soy protein were reported to display anticancer (Kim et al. 2000), antihypertensive (Kitts and Weiler 2003) and antioxidant (Korhonen and Pihlanto 2003) properties.
A large number of identified bioactive peptides are the result of tryptic digestions (Chiang et al. 1999). Nevertheless, there are different microbial enzymes which have demonstrated high proteolytic capacity as is the case of the serin proteases produced by ground sporulated bacilli (Doi 1991) or Bacillus subtilis (Blinkovsky et al. 1994; Smith and Bradley 1987). In particular, these bacilli have been used by people from eastern countries for many years to ferment different foodstuffs, among them, soybean. One example is the product Nattô, a traditional japanese food based on soybean. After its fermentation with Bacillus subtilis, Nattô increases its digestibility thus favoring the absorption of nutrients up to 90% compared to 65% of absorption of boiled soybean (Sanz Pérez 2005). Bacillus subtilis Nattô has the ability to hydrolyze soy (Kuo et al. 2006).
Peptides with biofunctional properties derived from the hydrolysis of proteins from different sources have been reported (Fitzgerald and Meisel 2003). Most bioactive peptides come from tryptic digestions. Therefore, the study of hydrolysis with Corolase 7089 and endoprotease from Bacillus subtilis of PRO-FAM 974 could contribute new peptides with bioactive properties. Bacillus subtilis has shown the capacity to produce peptides in fermentative processes using soy proteins. However, enzymatic hydrolysis like the one performed in this study, is essential for a better control of the process and for obtaining specific sequences.
The SDS-PAGE allowed us the identification of proteins of the PRO-FAM 974 isolate. However, the technique lacks the necessary resolution to identify peptides. MALDI-TOF (Coligan et al. 1995) was chosen for this analysis. Peptides larger than 10,000 Dalton were not found, which indicates that the hydrolysate obtained at 60 min with 47.91% degree of hydrolysis could be an interesting candidate to incorporate to different alimentary matrices, potentially reducing the allergenicity of soy proteins and increasing its assimilation. Moreover, the peptide profile showed that the hydrolysate was rich in peptides with molecular masses between 800 and 1500 Dalton, which is in line with the size of peptides reported to display immunostimulating properties (Singh et al. 2014).
The modification of the identified identical peptides of Glycinin G1: IIDTNSLENQLDQMPRR (57); IIDTNSLENQLDQMPRR (25) with oxidation in methionine could be due to the processing of the sample since methionine is susceptible to be oxidized by oxygen. The identified identical peptides of Glycinin G4: FNEGDVLVIPPGVPY (24); FNEGDVLVIPPGVPYW (17) and of the subunit Glycinin A3B4: FNEGDVLVIPPGVPY (24); FNEGDVLVIPPGVPYW (17) relies in the fact that they share these sequences. The identified identical peptides of β-conglycinin, alpha’ chain: RAELSEQDIFVIPA (43) and of the β-conglycinin alpha-subunit: RAELSEQDIFVIPA (43) is due to the fact that they share these sequences.
Conclusion
The high availability of extruded soy as a byproduct of the oil production generates a great opportunity to obtain proteins and peptides of added value which can also help to improve and design new foodstuffs with health benefits. The methodologies used in this study allowed to characterize the protein isolate and to obtain deeper knowledge on the aminoacid sequence of the different peptides that were generated after the enzymatic treatment. This treatment can be controlled to produce peptides of different sizes to search for different biofunctional properties. According to the molecular weights of the peptides obtained by the hydrolysis of the commercial soy protein isolate, it is evident that they may display functional potential in relation to the immunomodulation of the gut mucosa, a topic that will be addressed in future work.
Acknowledgements
The authors thank Dr. María Cristina Añón, Centro de Investigación y Desarrollo en Criotecnología de Alimentos (CIDCA, UNLP-CONICET) for collaborating with the thermal and conformational studies of the protein isolate, Dr. Silvia Moreno, Centro de Estudios Químicos y Biológicos por Espectrometría de Masa (CEQUIBIEM) for collaborating with the mass spectrometry studies. Dr. Sandra Elizabeth Perez and Daniel Starck, Centro de Investigación Veterinaria de Tandil (CIVETAN) for collaborating with the language review.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest in this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmadifard N, Murueta JHC, Abedian-Kenari A. Comparison the effect of three commercial enzymes for enzymatic hydrolysis of two substrates (rice bran protein concentrate and soy-been protein) with SDS-PAGE. J Food Sci Technol. 2016;53:1279. doi: 10.1007/s13197-015-2087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barac MB, Pesic MB, Stanojevic SP. Comparative study of the functional properties of three legume seed isolates: adzuki, pea and soy vean. J Food Sci Technol. 2015;52:2779. doi: 10.1007/s13197-014-1298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinkovsky AM, Khmelnitsky YL, Dordick JS. Organosoluble enzyme-polymer complexes: a novel type of biocatalyst for nonaqueous media. Biotechnol Technol. 1994;8:33. doi: 10.1007/BF00207630. [DOI] [Google Scholar]
- Chen N, Zhao M, Chassenieux C, Nicolai T. Data on the characterization of native soy globulin by SDS-Page, light scattering and titration. Data Brief. 2016;9:749–752. doi: 10.1016/j.dib.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang WD, Shih CJ, Chu YH. Functional properties of soy protein hydrolysate produced from a continuous membrane reactor system. Food Chem. 1999;65:189–194. doi: 10.1016/S0308-8146(98)00193-9. [DOI] [Google Scholar]
- Cho SJ, Marcel AJ, Lee HC. Cholesterol lowering mechanism of soybean protein hydrolysate. J Agric Food Chem. 2007;55(26):10599–10604. doi: 10.1021/jf071903f. [DOI] [PubMed] [Google Scholar]
- Coligan JE, Dunn BM, Ploegh HL (1995) Matrix-assisted laser desorption/ionization time-of-flight mass analysis of peptides. In: Speicher DW, Wingfield PT (eds) (Contributed by William J. Henzel and John T. Stults), current protocols in protein science, Volume 1. Wiley, New York, Unit 16.2
- Doi HR. Proteolytic activities in bacillus. Curr Opin Biotechnol. 1991;2:682–684. doi: 10.1016/0958-1669(91)90034-3. [DOI] [PubMed] [Google Scholar]
- Etzel MR. Manufacture and use of dairy protein fractions. J Nutrit. 2004;134(4):996S–1002S. doi: 10.1093/jn/134.4.996S. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RJ, Meisel H (2003) Milk protein hydrolysates and bioactive peptides. In: Fox PF, McSweeney PLH (eds) Advanced dairy chemistry, Volume 1, 3rd Edition, Part B, pp 675–698. Kluwer/Plenum, New York/Dordrecht, pp 675–698
- Ghumman A, Kaur A, Singh N. Functionality and digestibility of albumins and globulins from lentil and horse gram and their effect on starch rheology. Food Hydrocoll. 2016 [Google Scholar]
- Henry RJ, Cannon DC, Winkelma JW. Clinical chemistry, principles and techniques. 2. Hagerstown: Harper and Row; 1974. pp. 1354–1369. [Google Scholar]
- Horwitz W. Official methods of analysis of AOAC International, vols 1 and 2. 17. Gaithersburg: AOAC International; 2000. [Google Scholar]
- Kim SE, Kim HH, Kim JY, Kang YI, Wo HJ, Lee HJ. Anticancer activity of hydrophobic peptides from soy proteins. BioFactors. 2000;12(1–4):151–155. doi: 10.1002/biof.5520120124. [DOI] [PubMed] [Google Scholar]
- Kitts DD, Weiler K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr Pharm Des. 2003;9(16):1309–1323. doi: 10.2174/1381612033454883. [DOI] [PubMed] [Google Scholar]
- Kong XZ, Guo MM, Hua Y, Dong C, Zhang C. Enzymatic preparation of 610 immunomodulating hydrolysates from soy proteins. Bioresour Technol. 2008;611(99):8873–8879. doi: 10.1016/j.biortech.2008.04.056. [DOI] [PubMed] [Google Scholar]
- Korhonen H, Pihlanto A. Bioactive peptides: new challenges and opportunities for the dairy andustry. Int Dairy J. 2003;16(2006):945–960. [Google Scholar]
- Kuo LC, Cheng WY, Wu RY, Huang CJ, Lee KT. Hydrolysis of black soybean isoflavone glycoside by Bacillus subtilis natto. Appl Microbiol Biotechnol. 2006;73(2):314–320. doi: 10.1007/s00253-006-0474-7. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleave of structural proteins during the assembly of the head of bacteria pHage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee KH, Ryu HS, Rhee KC. Protein Solubility Characteristics of Commercial Soy Protein Products. J Amer Oil Chem Soc. 2003;80:85. doi: 10.1007/s11746-003-0656-6. [DOI] [Google Scholar]
- Madureira AC, Pereira A, Gómes M, Pintado MalcataF. Bovine whey proteins—overview on their main biological properties. Food Res Int. 2007;40:1197–1211. doi: 10.1016/j.foodres.2007.07.005. [DOI] [Google Scholar]
- Nagpal R, Kumar A, Kumar M, Behare PV, Jain S, Yadav H. Probiotics, their health benefits and applications for developing healthier foods: a review. FEMS Microbiol Lett. 2012;334:1–15. doi: 10.1111/j.1574-6968.2012.02593.x. [DOI] [PubMed] [Google Scholar]
- Paredes LO, Ordorica FC. Production of safflower protein isolates: composition, yield and protein quality. J Sci Food Agric. 1986;37:1097–1103. doi: 10.1002/jsfa.2740371107. [DOI] [Google Scholar]
- Peñas E, Prestarno G, Baeza M, Martinez M, Gomez R. Effect of combined high pressure and enzimatic treatments on the hidrolysis and inmunoreactivity of dairy whey prot. Int Dairy J. 2006;16:831–839. doi: 10.1016/j.idairyj.2005.08.009. [DOI] [Google Scholar]
- Petruccelli S, Añon MC. Thermal aggregation of soy protein isolates. J Agric Food Chem. 1995;43:3035–3041. doi: 10.1021/jf00060a009. [DOI] [Google Scholar]
- Pihlanto A. Bioactive peptides derived from bovine whey proteins: opioid and ACE-inhibitorypeptides. Trends Food Sci Technol. 2001;11:347–536. doi: 10.1016/S0924-2244(01)00003-6. [DOI] [Google Scholar]
- Sanz Pérez B (2005) El variado mundo de los alimentos funcionales nutracéuticos y suplementos dietéticos. Cascales Angosto M, Espinos Pérez D, García Barreno P (eds) En Bioquímica y fisiopatología de la nutrición. Instituto de España. Madrid, pp 133–208
- Secretaria de Agricultura, Ganadería, Pesca y alimentación (2016). www.sagpya.mecon.gob.ar
- Shevkani K, Singh N, Kaur A, Rana JC. Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocoll. 2014;43(2015):679–689. [Google Scholar]
- Singh BP, Vij S, Hati S. Functional significance of bioactive peptides derived from soybean. Peptides. 2014 doi: 10.1016/j.peptides.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Smith KE, Bradley RL. Activity of four anzyme-based cleaners for ultrafiltrations systems against proteins in skim milk and whey. J Dairy Sac. 1987;70:243. doi: 10.3168/jds.S0022-0302(87)80003-6. [DOI] [Google Scholar]
- Takahiro T, Katsuki K, Masakazu T, Makoto T, Taiji M, Masaaki Y. Soymetide, an immunostimulating peptide derived from soybean β-conglycinin, is an fMLP agonist. FEBS Lett. 2003;540(1–3):206–210. doi: 10.1016/s0014-5793(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Takahiro T, Kyoya T, Masaaki Y. Anti-alopecia mechanisms of soymetide-4, an immunostimulating peptide derived from soy β-conglycinin. Peptides. 2005;26(5):707–711. doi: 10.1016/j.peptides.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Vinderola CG, de Moreno de LeBlanc A, Perdigón G, Matar C (2008) Biologically active peptides released in fermented milk: role and functions. In: Farnworth ER (ed) Handbook of fermented functional foods 2nd edition, Chapter 7. CRC Press, Taylor & Francis Group, Boca Raton, pp 209–242. ISBN: 9781420053265
- Vioque J, Clemente A, Pedroche J, Yust MM, Millán F (2001) Obtention and uses of protein hydrolysates. Grasas y Aceites, ISSN 1988-4214. 10.3989/gya.2001.v52.i2.385 [DOI]