Abstract
Cinnamomum camphora trees have a vast range of distribution in southern China and the seed oil has unique fatty acid (FA) properties and various bio-activities. In this work, Cinnamomum camphora seed oil (CCSO) was utilized to synthesize value-added cocoa butter substitute (CBS) by enzymatic interesterification. The synthesis was conducted in a solvent-free system by blending CCSO with fully hydrogenated palm oil under the catalysis of Lipozyme RM IM. The reacted products were assessed with physicochemical properties, i.e. FA composition, slip melting point (SMP), triacylglycerol (TAG), crystal polymorphism, microstructure, melting and crystallization properties and solid fat content (SFC). It showed that MCFAs (capric acid plus lauric acid) was the main fatty acid in products, accounting for over 45%. Comparing to physical blends, some novel TAG species such as LaLaLa and LaMLa/LaLaM were observed after enzymatic interesterification whereas SSS TAGs were reduced. IP presented a ball-like, well-distributed and nearly round crystal microstructure and a smaller crystal size. Moreover, it should be mentioned that SFC of IP ranging from 31.85 to 38.47% at 25 °C with most β′ crystal forms, was beneficial to improve the spreadability in term of confectionery products and baked goods. The SMP of the interesterified products was 35.75–36.15 °C which closed to the commercial CBS. Hence, the products synthesized can be used to as CBS, and the results in this study also showed CCSO have value-added applications.
Keywords: Cocoa butter substitute, Cinnamomum camphora seed oil, Enzymatic interesterification, Lipozyme RM IM
Introduction
Cinnamomum camphora tree is a large evergreen tree and widely distributed in subtropical areas, like southeastern China and northeastern Australia (Jiang et al. 2016). The oil from Cinnamomum camphora is a latest reported oil, recognized as a source of essential oil and utilized in medicine and perfume because of its bioactivities, like antioxidant, antibacterial, antifungal and insecticidal properties. According to the previous work, it turned out that Cinnamomum camphora seed oil (CCSO) can be used as edible oil as well (Liu et al. 2006; Xu et al. 2018). Medium-chain fatty acids (MCFAs) with chain lengths from C8 to C12 are abundant in CCSO, accounting for almost 95% (Hu et al. 2011). To our knowledge, the behavior of medium-chain triacylglycerol (MCT) oil are different from long-chain triacylglycerol (LCT) in terms of digestion, absorption, and metabolism in vivo. MCFAs can be easily hydrolyzed in the gastrointestinal tract, then directly transported and rapidly oxidized in the liver to provide quick energy via the portal system (Papamandjaris et al. 1998). It seemed that CCSO has the potential of alternative as economic oil, which is not completely exploited and utilized.
The cocoa butter (CB) is widely used in confectionery, baked goods and chocolate mainly. Despite of the huge amount of CB production consumed, the requirement is far outpacing supply and keeps growing year by year (Mohamed 2015). Meanwhile low content of CB in cocoa beans (Zaidul et al. 2007) and the climate conditions in western Africa, central America and southeast Asia, which are the main place of origin, constrain the supply of CB. Furthermore, crop failures and its chain problem result in higher price, with the increase of the gap between supply and requirement. High cost, low yields and extreme imbalances between supply and demand have spurred scholars to produce cocoa butter substitute (CBS) by restructuring economic vegetable oils as widespread researches (Mohamed 2015). To date, the enzymatic-catalyzed interesterification has drown much attention and is mostly utilized to produce CBS by restructuring economic oils, because of its great superiority on milder reaction conditions, less by-products and fewer pollutants generation. And fully hydrogenation can be utilized to prepare zero trans fatty acids and enhance lipid crystal structuration as commercial fats via interesterification (Petrauskaite et al. 1998; Ribeiro et al. 2009). Furthermore, fully hydrogenated palm oil (FHPO) has high amount of palmitic acids, tends to form β′-crystal fats during the catalysis and leads to favorable melting and crystallization properties of interesterified products (IP) (Aini and Miskandar 2007; de Oliveira et al. 2015). In the previous study, palm mid fraction (PMF), palm kernel stearin (PKS) and medium chain triacylglycerols (MCTs) were catalyzed by Lipozyme RM IM to gain low calorie CBS (Borhan et al. 2011). Yamoneka et al. (2018) found that the interesterified blend of 90% Irvingia gabonensis seeds fat and 10% Dacryodes edulis pulp oil with Lipozyme TL IM catalyzed had similar melting profile and polymorphic behavior compared to CB, was a good candidate as specialty fat like CBS.
Bahari and Akoh (2018) synthesized a cocoa butter equivalent (CBE) structured lipid from illipe butter and PMF using enzymatic interesterification and IP were shown to be no significant difference in terms of melting and crystallization properties with CB and therefore may potentially be utilized as a CBE. However, the utilization of CCSO in CBS is rarely studied.
In this work, the main objective is to gain a low-cost edible alternative for CB by modifying CCSO with FHPO and catalyzed by Lipozyme RM IM. The properties of product, such as slip melting point (SMP), FA profile, polymorphic form, microstructure, and solid fat content (SFC) were characterized by gas chromatography (GC), differential interference contrast microscope (DIC), X-ray diffractometry (XRD), and differential scanning calorimetry (DSC), respectively. Subsequently, scale-up experiments were conducted to determine the feasibility of enzymatic interesterification of CCSO and FHPO for producing CBS.
Materials and methods
Materials
FHPO was purchased from JiaTa Chemical Technology Co., Ltd (Shijiazhuang, Hebei, China). Cinnamomum camphora seeds were gained from local agriculture market. To avoid from the destroy of components in CCSO during the extract, the CO2 supercritical fluid extraction (SFE-CO2) was conduct here according to the method (Hu et al. 2011) with a minor modification. Lipozyme RM IM, a commercial immobilized 1, 3-specific lipase from R. miehei which stored at − 18 °C until usage, was purchased from Novozymes A/S (Bagsvaerd, Denmark). The specific activity of Lipozyme RM IM was 150 IU/g, having 0.35–0.45 g/mL bulk density, and 0.3–0.6 mm particle diameter. The GLC-463 standard fatty acid methyl esters (FAMEs) containing methyl esters C8–C20 saturated fatty acid, were purchased from Nu-Chek Prep Inc. (Elysian, MN, USA). All other reagents used were of either analytical or chromatographic grade.
Methods
Enzymatic interesterification
CCSO was blended with FHPO at different substrate mass ratios and Lipozyme RM IM of 10% total weight (w/w total reactants) was added to the blends in a 250 mL screw-capped Erlenmeyer flask, respectively. The experiment was performed as described in the report previously (Adhikari et al. 2010a) with slightly differences. The enzymatic interesterification reaction was performed at 65 °C for 8 h under the shaking water bath and mixing speed was set at 200 rpm. After the enzymatic interesterification, Lipozyme RM IM was removed from the mixtures by 0.45 μm polytetrafluoroethylene (PTFE) syringe membrane filters. The same volume of hexane (about twice volume of the sample) and 5 drops of phenolphthalein solution were added to the IP in order to remove free fatty acids and partial monoacylglycerols and diacylglycerols (Farmani et al. 2007). The reaction mixture was titrated with 0.5 mol/L KOH solution in 95% ethanol until a pink color appeared. Then each of the composite sample was washed with warm water until the pink color faded and disappeared. The upper layer was seeped through an anhydrous sodium sulfate column to drain off remaining moisture, and the solvents (hexane and some ethanol) were completely volatilized under nitrogen to obtain crude CBS.
Fatty acid composition analysis
The fatty acid compositions of physical blends (PB) and IP were determined. All of PB and IP samples were methylated according to the method described as the report by Zhu et al. (2012) to prepare FAMEs. Then the FAMEs were analyzed using a fused-silica capillary column (CP-Sil 88, 100 m × 0.25 mm × 0.2 μm i.d.) through gas chromatograph (model 6890N, Agilent Technologies, Santa Clara, CA, USA) equipped with an automatic sampler and a flame ionization detector (FID). The column was heated to 45 °C and then kept this temperature for 3 min. Then increased the temperature to 175 °C and kept this temperature for 27 min at a rate of 13 °C/min. The temperature was finally raised to 215 °C and kept it for 35 min at a rate of 4 °C/min. The injector temperature and detector temperature were maintained at 250 °C and 260 °C, respectively. As a carrier gas, nitrogen was used at a flow rate of 52 mL/min in split mode (50:1). The loading volume was 0.3 μL. Fatty acid compositions were confirmed by comparison with the retention times of four hundred and sixty-three standard fatty acid (GLC-463) methyl esters. Each sample was performed in triplicate.
Microstructure analysis
The samples were placed in a water bath and held at 65 °C for 30 min until they were totally melted. Microstructure of PB and IP was observed by a differential interference contrast microscope equipped with a Nikon ECLIPSE Ti-U port. 10 μL of melted samples were placed on a preheated microscope slide, maintained at 25 °C for 24 h (Adhikari et al. 2010b). The microstructure was observed and photographed under the microscope at 400 × magnification.
Determination of SMP
Melting point of samples was determined by the capillary tube method according to AOCS official method Ccl-25 (Tang et al. 2012). Each sample was measured for three times.
Texture analysis
Samples were set at 24 °C for 8 h in an oven and hardness analysis was performed with Texture Analyser (TA-XT Plus, Stable Microsystems Ltd., Surrey, UK) equipped with 5 kg load cell and probe (P/2) moving at pre-test speed of 1.0 mm/s, test speed of 2.0 mm/s and post-test speed of 10.0 mm/s, and the probe was penetrated at 5 mm depth along with trigger type of auto-0.1 g.
Polymorphic forms analysis by XRD
Samples were heated in a water bath of 65 °C until to be melted completely. Holding at 25 °C for 24 h in a particular container, the polymorphic forms of PB and IP crystals were obtained by X-ray diffraction (XRD, PAN alytical B.V., Almelo, Netherlands) using a Bruker D8-Focus diffractometer equipped with Cu K α radiation (λ = 1.54184 Å, 40 kV tube voltage, 35 mA current, scan diffraction angle were in the range of 18°–32° with 2°/min scanning rate) (Zhang et al. 2014).
Differential scanning calorimeter (DSC) and solid fat content analysis
Melting and crystallization characteristics of samples were determined with a differential scanning calorimeter DSC (Mettler-Toledo Co., Switzerland). Samples were placed into an aluminum pan with 10 ± 0.5 mg weight for DSC analysis, the result was compared with control group which use an empty control group. Heating to 80 °C and maintained at this temperature for some time until the fat samples melted to the liquid state absolutely. The temperature was subsequently decreased to − 60 °C at a rate of 10 °C/min and maintained for 10 min, the initial data was first recorded. Heating to 80 °C at a rate of 5 °C/min and held at 80 °C for 90 min, recorded this data. Then the samples were maintained at each measuring temperature (0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60 °C) for 30 min respectively before recording the data. After calculation, make a SFC-T curve. Each sample was measured twice.
Triacylglycerol composition (TAG)
The TAGs composition of raw materials (CCSO and FHPO), PB and IP were analyzed by a reversed-phase high-performance liquid chromatograph (HPLC) equipped with quaternionic pump and evaporative light-scattering detector (ELSD, All-tech 2000ES, USA). The method was carried out with some changes according to previous report (Zhang et al. 2014). The Agilent 1260 HPLC system was conducted at 55 °C and the eluent flow rate was controlled at 0.8 mL/min, besides, the nitrogen pressure was operated at 1.7 bar. Samples were dissolved in dichloromethane and separated by the Novo-Pak C18 column (150 × 3.9 mm, waters, Milford, MA, USA). 10 μL of sample was filtered by 0.22 μm PTFE syringe membrane filter before injecting into the system. Samples were eluted by acetonitrile (solvent B) and isopropanol/hexane (1:1, v/v, solvent D), and they were determined with the following profile: 0–10 min, 60% B; 20–50 min, 56% B; 50–60 min, 60% B; 60–70 min, 60% B. Each sample was examined twice. The separated TAGs were defined by means of comparing the retention time and equivalent carbon number (ECN). ECN was calculated according to the formula below.
where CN is the total carbon number except three carbons of glycerol in TAGs, and DB is the total number of double bonds on the fatty acids.
Statistical analysis
All the results shown in this study, as mean ± standard deviation, were subjected to analysis of variance (ANOVA). Duncan’s multiple range test was used to determine the significance level of P value (P < 0.05). Analysis was done with the SPSS software (SPSS 20.0, SPSS Inc., Chicago, USA).
Results and discussion
Fatty acid composition
Table 1 shows the fatty acids composition of the raw materials and products before and after interesterification at different mass ratio. Capric acid (53.87 ± 1.65%) was the predominant FA in CCSO, and followed by lauric acid (39.67 ± 1.39%), oleic acid (3.13 ± 0.24%) and myristic acid (1.13 ± 0.06%). This is consistent with the finds of Tang et al. (2012). FHPO had a superior content of palmitic acid (60.72 ± 2.60%) and followed by stearic acid (37.13 ± 1.87%) as expected. The contents of MCFAs in CCSO and FHPO were 93.78% and 1.83%, respectively. As we mentioned above, MCFAs could play a role in providing quick energy during digestion in vivo (Takeuchi et al. 2008). It seemed that CCSO may have an advantage over other economic plant oil as an energy source for short and intense activities, i.e. athletics. The interesterification catalyzed by Lipozyme RM IM was a procedure that alter the natural distribution of sn-1,3-positional fatty acids between TAG molecules without changing the total fatty acid composition (Mayamol et al. 2009). The total fatty acid composition has a slightly difference in IP and PB depending on the substrates mass ratio (CCSO: FHPO). Meanwhile, no significant difference was observed in total fatty acid between IP and PB at same substrates mass (p > 0.05). It illustrated that interesterification only promoted the rearrangement of fatty acids in TAGs molecules. In addition, trans fatty acid was not detected in IP under the testing conditions performed in this study.
Table 1.
Total fatty acid composition (Area %) of raw material (CCSO and FHPO), and products at different mass ratio of CCSO: FHPO before and after enzymatic interesterification
| CCSO | FHPO | Physical blends (CCSO: FHPO) | Interesterified products (CCSO: FHPO) | |||||
|---|---|---|---|---|---|---|---|---|
| 12:8 | 12:10 | 12:11 | 12:8 | 12:10 | 12:11 | |||
| C8:0 | 0.24 ± 0.11 | ND | 0.15 ± 0.01a | 0.13 ± 0.01b | 0.12 ± 0.01b | ND | ND | ND |
| C9:0 | ND | 0.42 ± 0.47 | 0.17 ± 0.00b | 0.19 ± 0.02a | 0.20 ± 0.02a | ND | ND | ND |
| C10:0 | 53.87 ± 1.65a | 0.12 ± 0.07c | 32.33 ± 0.22a | 29.44 ± 0.53b | 28.16 ± 0.77c | 22.86 ± 0.39d | 21.81 ± 0.90d | 22.17 ± 0.56d |
| C11:0 | ND | 0.79 ± 0.54 | 0.31 ± 0.01c | 0.36 ± 0.04b | 0.38 ± 0.02a | 1.70 ± 0.06d | 1.93 ± 0.11d | 2.57 ± 0.25d |
| C12:0 | 39.67 ± 1.39c | 0.50 ± 0.37c | 24.30 ± 0.90ab | 21.87 ± 0.08b | 20.93 ± 0.91c | 24.63 ± 0.68a | 23.83 ± 1.62c | 20.40 ± 0.15c |
| C14:0 | 1.13 ± 0.06a | 1.32 ± 0.05a | 1.21 ± 0.03bc | 1.22 ± 0.12bc | 1.22 ± 0.02bc | 2.77 ± 0.05ab | 2.34 ± 0.03a | 2.15 ± 0.02c |
| C15:0 | ND | 0.07 ± 0.00 | 0.03 ± 0.00b | 0.03 ± 0.00a | 0.03 ± 0.00a | ND | ND | ND |
| C16:0 | 0.92 ± 0.01c | 60.72 ± 2.60a | 24.58 ± 1.04c | 28.13 ± 0.22b | 29.52 ± 0.44a | 26.03 ± 0.86b | 28.37 ± 0.58a | 28.43 ± 0.64a |
| 9c C16:1 | ND | 0.18 ± 0.17b | 0.07 ± 0.01d | 0.08 ± 0.01d | 0.09 ± 0.00d | 0.91 ± 0.05c | 1.49 ± 0.35b | 2.55 ± 0.21a |
| C17:0 | ND | 0.14 ± 0.00 | 0.06 ± 0.00b | 0.06 ± 0.00a | 0.07 ± 0.00a | ND | ND | ND |
| C18:0 | 0.28 ± 0.01c | 31.73 ± 1.87a | 12.86 ± 0.11d | 14.53 ± 0.32c | 15.33 ± 0.29b | 14.40 ± 0.35c | 15.13 ± 0.18b | 15.86 ± 0.06a |
| 9c C18:1 | 3.13 ± 0.24a | 2.43 ± 2.68a | 2.85 ± 0.05d | 2.82 ± 0.12d | 2.80 ± 0.07d | 4.44 ± 0.12a | 3.40 ± 0.16c | 3.68 ± 0.27b |
| 9c12c C18:2 | 0.76 ± 0.06a | 1.24 ± 1.45a | 0.95 ± 0.03a | 0.98 ± 0.03a | 0.99 ± 0.03a | 0.68 ± 0.10b | 0.62 ± 0.09b | 0.58 ± 0.14b |
| C20:0 | ND | 0.33 ± 0.01 | 0.13 ± 0.01ab | 0.15 ± 0.01ab | 0.16 ± 0.01a | 1.60 ± 0.01b | 1.11 ± 0.03ab | 1.61 ± 0.06ab |
| ΣMCFAs | 93.78 ± 0.30a | 1.83 ± 0.23c | 57.27 ± 1.04a | 51.99 ± 0.44b | 49.79 ± 0.21c | 50.18 ± 1.02c | 47.57 ± 0.74d | 45.14 ± 0.92e |
| ΣUFAs | 2.60 ± 1.53a | 3.85 ± 4.30a | 3.87 ± 0.08d | 3.89 ± 0.15d | 3.88 ± 0.06d | 6.03 ± 0.05b | 5.51 ± 0.31c | 6.81 ± 0.22a |
| ΣSFAs | 96.11 ± 1.67a | 96.15 ± 4.30a | 96.13 ± 0.08a | 96.12 ± 0.15a | 96.12 ± 0.06a | 93.97 ± 0.05c | 94.49 ± 0.31b | 93.19 ± 0.22d |
| TFA | ND | ND | ND | ND | ND | ND | ND | ND |
All values were expressed as the mean ± standard deviation (n = 3). Values marked with different superscript letters in a row were significantly different from one another (p < 0.05); ΣMCFAs, ΣUSFA, ΣSFA and TFA represent total medium-chain fatty acids, total unsaturated fatty acids, total saturated fatty acids and total trans fatty acids, respectively
ND not detected
TAG composition
The composition of TAG is a vital parameter for evaluating IP, since it shows strong correlation with physical characteristics and crystalline form of fat. It was summarized that the TAGs composition of raw material and products before and after interesterification at different mass ratio in Table 2. In FHPO, PPS is the predominant TAG species, accounting for nearly 45%, followed by PPP (29.80%), PSS (22.92%) and SSS (2.01%). CCSO is rich with MCT (LaCC/CLaC plus MCC/LaLaC), accounting for nearly 90%, and others were small part of CCC (10.73%). It was observed that differences of TAG composition in products depend on the different substrate mass ratio conduct. To PB, there were no differences in the species of TAGs. After interesterification, new types of TAGs appeared, such as LaLaLa and LaLaM. A substantial decrease of PPP (7.95–12.05 to 0.50–1.14%), PPS (11.87–18.56 to 1.08–1.49%), PSS (7.16–7.64 to 0.42–0.68%) and a significant increase of newly generated TAGs were observed in Table 2. In addition, SSS could not be detected after the enzymatic reaction. The generation of new species of TAGs could be chalked up to the alteration of fatty acid arrangement on the TAG molecules, resulting in the change physicochemical characteristics. Anyhow, the results indicated that the enzymatic interesterification had occurred.
Table 2.
Triacylglycerol (TAG) Composition (Area %) of raw material (CCSO and FHPO), and products at different mass ratio of CCSO: FHPO before and after enzymatic interesterification
| ECN** | TAG | CCSO | FHPO | Physical blends (CCSO: FHPO) | Interesterified products (CCSO: FHPO) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 12:8 | 12:10 | 12:11 | 12:8 | 12:10 | 12:11 | ||||
| 30 | CCC | 10.73 | ND | 5.55 | 5.39 | 5.21 | 8.60 | 10.09 | 12.47 |
| 32 | LaCC/CLaC | 64.78 | ND | 46.17 | 49.23 | 51.19 | 19.90 | 18.91 | 20.53 |
| 34 | MCC + LaCLa/LaLaC | 26.99 | ND | 13.30 | 11.11 | 9.99 | 8.83 | 7.74 | 5.93 |
| 36 | LaLaLa | ND | ND | ND | ND | ND | 15.73 | 13.26 | 12.37 |
| 38 | LaMLa | ND | ND | ND | ND | ND | 21.59 | 20.78 | 19.18 |
| 40 | LaLaO + LaMM + LaPLa | ND | ND | ND | ND | ND | 8.18 | 7.94 | 6.68 |
| 42 | LaMO + LaPM | ND | ND | ND | ND | ND | 5.43 | 7.11 | 8.51 |
| 44 | LaPO + MMO | ND | ND | ND | ND | ND | 6.49 | 7.57 | 7.75 |
| 46 | OOL + PPL + POL | ND | ND | 0.39 | 0.29 | ND | 2.36 | 3.49 | 4.13 |
| 48 | PPP | ND | 29.80 | 7.95 | 9.99 | 12.05 | 1.14 | 1.20 | 0.50 |
| 50 | PPS | ND | 45.28 | 18.56 | 15.21 | 11.87 | 1.08 | 1.49 | 1.34 |
| 52 | PSS | ND | 22.92 | 7.64 | 7.53 | 7.16 | 0.68 | 0.42 | 0.61 |
| 54 | SSS | ND | 2.01 | 0.44 | 1.25 | 2.53 | ND | ND | ND |
All values were expressed as the mean ± standard deviation (n = 3)
C capric acid, La lauric acid, M myristic acid, O oleic acid, P palmitic acid, L linoleic acid, S stearic acid
**Equivalent carbon number
Melting point determination and Solid fat content
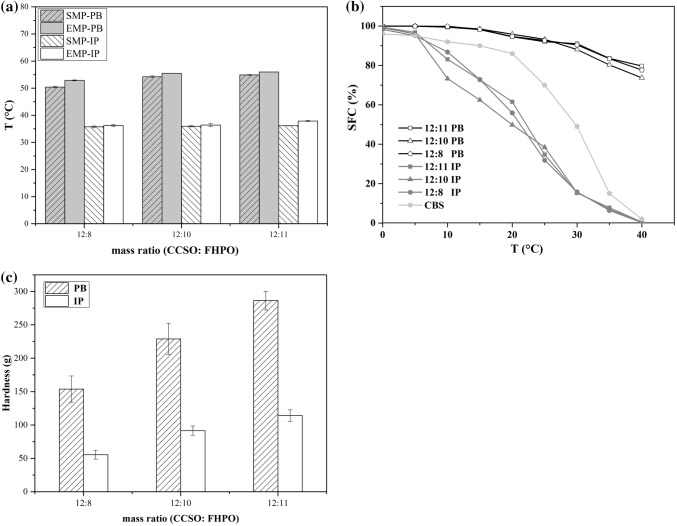
It is the prerequisite that CBS should maintain enough melting temperature because it is necessary to hold its peculiarity at room temperature. Melting point of the products after enzymatic interesterification were in the range of 32.6–37.7 °C (Fig. 1a), which were similar to the melting point of commercial CB (Mohamed 2015). Compared with PB (SMP: 50.40–54.90 °C; EMP: 52.90–56.00 °C), IP (SMP: 35.75–36.15 °C; EMP: 36.25–36.40 °C) indicated a narrower range of melting point. With the increase of FHPO content, the melting point tends to be higher. This could be attributed to the comparatively higher melting point TAGs in FHPO. In addition, it seemed that interesterification had the SMP and EMP of products decreased at all the mass ratio. This was consistent with previous report (Borhan et al. 2011). Karabulut et al. (2004) and Soares et al. (2009) suggested that the changes of melting point after interesterification might be affected by the length of fatty acids chain, the position and types of fatty acid in glycerol molecule, degree of unsaturation, trans fatty acid content and composition and species of TAGs during reactions. During the interesterification, fatty acids had rearranged in glycerol molecules at random and lead to the reduction of high melting point triacylglycerols, especially SSS (Table 2). Eventually, it presented a decreased manner in melting point of products after reaction.
Fig. 1.
Melting point of products at different mass ratio of CCSO and FHPO (a), solid fat content (SFC) of products with a commercial CBS as a contrast (b) and hardness of products before and after enzymatic interesterification at different mass ratios of CCSO and FHPO (c)
Solid fat content is a vital parameter which indicates solid residue of fat at different temperatures. According to the SFC curves of blends before and after interesterification shown in Fig. 1b, it was found that IP had lower percentage of solid fat than that of PB and commercial CBS at the same temperature. It revealed that IP became softer after enzymatic interesterification. The SFC value for the IP of different substrate mass ratio can be affected by the alteration of fatty acids composition and TAGs (Borhan et al. 2011). The PB may have a tendency to solidify at lower temperatures than either one of its substrates due to eutectic effect. Different SFC values at specific temperature range are relevant to some properties. It is a criterion to determine the percentage of solid fat particles in oils and fats at different measured temperature. The malleability of could be reflected by SFC determined at 10 °C (Gold et al. 2011). The SFC of IP at 10 °C varied from 73.26 to 83.81%, it was lower than PB which were in the range of 99.40–99.85%. Meanwhile, the SFC of commercial CBS was 92%. And It was reported that SFC at 25 °C indicated lipid plasticity and SFC represent the taste between 33 and 38 °C (Dian et al. 2007). IP may show a softer, tender and smooth sensation on the tongue, which demonstrating the application of IP in coatings, various baked products and as CBS. At 15–40 °C, the SFC value of IP was significantly different from that of PB, it decreased the risk of sandy and rough feelings. The sharpest decline of SFC values in IP were occurred at 20–30 °C, meanwhile commercial CBS were occurred at 30–35 °C. After interesterification, shifts to lower temperature ranges may be attributed to the exchange of fatty acids and the generation of new TAGs.
Textural analysis
The textural property of PB and IP samples were investigated using a penetration probing test and the maximum peak force (hardness) diagram was shown in Fig. 1c. Both PB and IP samples presented a harder texture depend on the increase of FHPO in mixtures. It suggested that hardness was influenced by the mixture proportion of samples. As far as we know, hardness was positively correlated with SFC values to some extent (Braipson-Danthine and Deroanne 2006). Thus, the changes of SFC in samples showed the same manner along with hardness tendency as expected here (Fig. 1b). Moreover, compared with PB, the hardness in IP samples were in lower values at the same mass ratio. It has declined from 286.32 g, 228.85 g and 153.73 g to 114.08 g, 91.51 g and 55.46 g when the mass ratio (CCSO: FHPO) was 12:11, 12:10 and 12:8, respectively. After interesterification, this may be attributed to the reduction of high melting point TAGs and generation of new shorter chain TAGs due to the rearrangement of fatty acids. Meanwhile, the hardness of IP sample at mass ratio of 12:11 reached to 114.08 g, and this was similar to that of CB-based chocolate according to the finding of Limbardo et al. (2017).
Microstructure
Crystal microstructures of samples were observed by a differential interference contrast microscope and summarized in Fig. 2. It figured out that the crystal microstructure changed after interesterification. When the mass ratio of CCSO: FHPO was at 12:11, long needle-like crystals were observed in PB, which showed a rough, branched, agminated shape. Whereas IP presented a well-distributed, smooth and nearly round pattern, and ball-like crystal microstructure were formed. The morphology of IP was found to be consistent with that observed by Biswas et al. (2017). Besides, after interesterification, crystal size was obviously decreased to less than 50 μm in diameter. This results could be attributed to the reduction of long chain trisaturated triacylglycerols, like SSS (Table 2) (Zhu et al. 2017). To our knowledge, this new microstructure could contribute to creamy texture and supple flavor in CBS. Thus, the IP with small and uniform crystal form had the tremendous potential to be ideal CBS applied in coating or bakery products.
Fig. 2.
The crystal microstructure of products before and after enzymatic interesterification at different mass ratios of CCSO and FHPO, with a 100 μm of bar as plotting scale, which were recorded by differential interference contrast microscope at 400 × magnification
Crystal polymorphism
Crystal polymorphism is an essential parameter to weigh crystalline phases that have different melting points (Mcgauley et al. 2002). α (hexagonal), β′ (orthorhombic), and β (triclinic) forms were the principal polymorphs of fat crystals. The most unstable crystalline phase was α crystal form, which short spacing was at 4.15 Å and melting point was the lowest (Solis-Fuentes et al. 2005). β crystal form was the most stable form while it often formed the plate shaped crystals. In application, β′ crystal form was more satisfactory than β due to its better surface, smooth texture, greater fluidity (Adhikari et al. 2012). β′ crystal form had 2 strong short spacings which at 3.80 and 4.20 Å, respectively. Besides, it also had 3 minor short spacings at 3.71, 3.97 and 4.27 Å (Dsouza et al. 1990). The polymorphic forms of PB and IP were exhibited in Fig. 3. The PB demonstrated 29.93–30.17, 28.27–30.05, 18.98–21.11 and 20.62–21.05% β′ crystal form at 4.35, 4.20, 4.03 and 3.80 Å in different substrate mass ratios, whereas the IP showed 25.53–25.92, 33.47–36.30, 16.78–18.16 and 21.23–22.45% β′ crystal form at 4.35, 4.20, 4.03 and 3.80 Å, respectively. Compared with PB, polymorphs of IP were mostly β′ form crystal, simple and single and they showed lower intensity of β′ crystal form at 4.35 and 4.03 Å and stronger intensity at 4.20 and 3.80 Å. This result may be caused by the rearrangement of fatty acids within and between TAGs so as to increase the species of TAGs. Similar crystal forms were found in IP samples and they were characterized with no α and β crystal forms but strong intensities at 3.80 and 4.20 Å which indicated β′ crystal forms. As known, β′ was regarded as the most desirable crystal form to produce CBS.
Fig. 3.
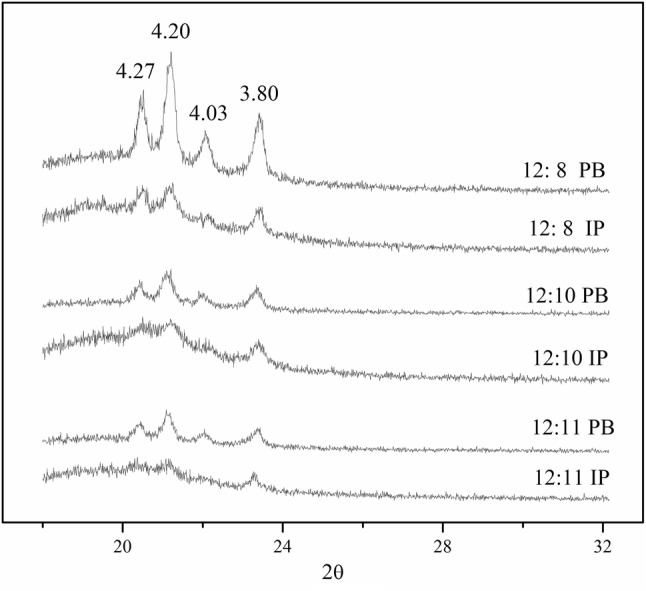
XRD spectra profiles of products before and after enzymatic interesterification at different mass ratio of CCSO and FHPO
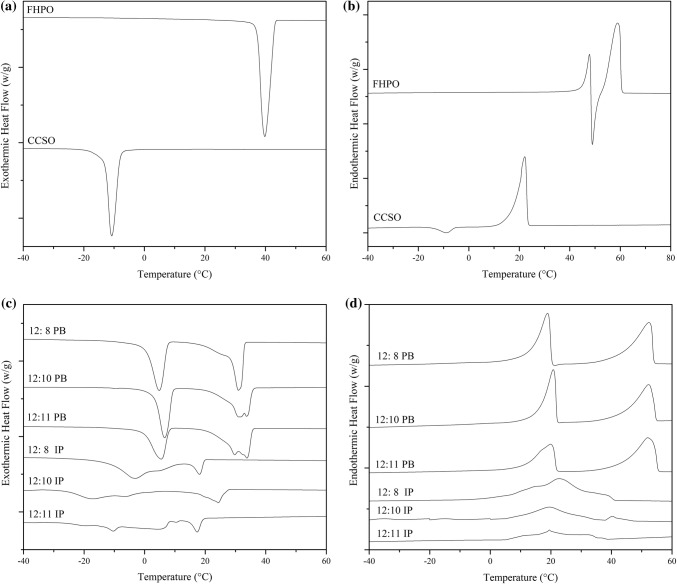
Thermal behaviors
The melting and crystallization thermograms profiles of CCSO, FHPO, PB and IP were tested by differential scanning calorimetry and shown in Fig. 4. The ultimate solid–liquid transitions temperatures were above 20 °C for CCSO and approximately 60 °C for FHPO. Sharp peaks were observed for FHPO and CCSO both in the melting and crystallization profiles. It seemed that almost all TAGs in raw materials melted in a narrow temperature range. Besides, an exothermic peak appeared in the FHPO melting curve (Fig. 4b), and recrystallization occurred. From the experimental results, melting happened mainly in lower temperature range for CCSO (10–22 °C) and higher temperature for FHPO (60 °C), and crystallization occurred mainly at about − 10 °C for CCSO and at 40 °C for FHPO. As for PB and IP, two temperature ranges of phase transitions could be easily distinguished wherever in the melting or crystallization profiles. Compared the melting and crystallization curves before and after interesterification, it was shown that IP had wider, smaller and weaker peaks instead of PB, indicating the formation of softer fats. This phenomenon was closely related to the reduction of high-melting TAGs especially SSS, this was consistent with the views of Alvarez and Akoh (2015). With the increase of FHPO, peaks were shifted towards higher temperature. After interesterification, the final solid–liquid transition temperature was reduced and below 40 °C, this might be attributed to the replacement of partly high-melting temperature TAGs by new TAGs with shorter chain or lower melting point fatty acid. Enzymatic reaction resulted in the rearrangement of fatty acids within and between TAGs so as to produce newly one and eventually affect the thermal behavior.
Fig. 4.
The DSC analysis of raw material (CCSO and FHPO) for crystallization thermograms (a) and melting thermograms (b). The comparison of crystallization thermograms (c) and melting thermograms (d) in products before and after enzymatic interesterification at different component ratio of CCSO and FHPO
Conclusion
With the development of CCSO as edible oil in daily life, it was used as substrate with the mixture of FHPO to synthesize CBS in this study. The reaction was carried out in a solvent-free system and catalyzed by Lipozyme RM IM. Meanwhile the physicochemical properties of products were evaluated. From the results presented in this study, enzymatic interesterification is a promising way to prepare structured lipids which can drastically improve fat quality. CCSO and FHPO appear to be potential raw materials to synthesize low-cost and low-calorie CBS, the products are attractive as fungible applications in formulating CBS and confectionery products.
Acknowledgements
This work was supported by the Natural Science Foundation of China (31460427, 31660470) and Young Scientists of Jiangxi Province (20171BCB23025).
References
- Adhikari P, Shin JA, Lee JH, Hu JN, Zhu XM, Akoh CC, Lee KT. Production of trans-free margarine stock by enzymatic interesterification of rice bran oil, palm stearin and coconut oil. J Sci Food Agric. 2010;90:703–711. doi: 10.1002/jsfa.3872. [DOI] [PubMed] [Google Scholar]
- Adhikari P, et al. Scaled-up production of zero-trans margarine fat using pine nut oil and palm stearin. Food Chem. 2010;119:1332–1338. doi: 10.1016/j.foodchem.2009.09.009. [DOI] [Google Scholar]
- Adhikari P, Shin JA, Lee JH, Kim HR, Kim IH, Hong ST, Lee KT. Crystallization, physicochemical properties, and oxidative stability of the interesterified hard fat from rice bran oil, fully hydrogenated soybean oil, and coconut oil through lipase-catalyzed reaction. Food Bioprocess Technol. 2012;5:2474–2487. doi: 10.1007/s11947-011-0544-4. [DOI] [Google Scholar]
- Aini IN, Miskandar MS. Utilization of palm oil and palm products in shortenings and margarines. Eur J Lipid Sci Technol. 2007;109:422–432. doi: 10.1002/ejlt.200600232. [DOI] [Google Scholar]
- Alvarez CA, Akoh CC. Enzymatic synthesis of infant formula fat analog enriched with capric acid. J Am Oil Chem Soc. 2015;92:1003–1014. doi: 10.1007/s11746-015-2662-z. [DOI] [Google Scholar]
- Bahari A, Akoh CC. Synthesis of a cocoa butter equivalent by enzymatic interesterification of illipe butter and palm midfraction. J Am Oil Chem Soc. 2018;95:547–555. doi: 10.1002/aocs.12083. [DOI] [Google Scholar]
- Biswas N, Cheow YL, Tan CP, Kanagaratnam S, Siow LF. Cocoa butter substitute (CBS) produced from palm mid-fraction/palm kernel oil/palm stearin for confectionery fillings. J Am Oil Chem Soc. 2017;94:235–245. doi: 10.1007/s11746-016-2940-4. [DOI] [Google Scholar]
- Borhan RH, Said M, Sahri MM. Enzymatic interesterification of palm products for producing low calorie cocoa butter substitutes. J Appl Sci. 2011;11:3750–3754. doi: 10.3923/jas.2011.3750.3754. [DOI] [Google Scholar]
- Braipson-Danthine S, Deroanne C. Determination of solid fat content (SFC) of binary fat blends and use of these data to predict SFC of selected ternary fat blends containing low-erucic rapeseed oil. J Am Oil Chem Soc. 2006;83:571–581. doi: 10.1007/s11746-006-1242-7. [DOI] [Google Scholar]
- de Oliveira GM, Ribeiro APB, dos Santos AO, Cardoso LP, Kieckbusch TG. Hard fats as additives in palm oil and its relationships to crystallization process and polymorphism. Lwt-Food Sci Technol. 2015;63:1163–1170. doi: 10.1016/j.lwt.2015.04.036. [DOI] [Google Scholar]
- Dian NLHM, Sundram K, Idris NA. Effect of chemical interesterification on triacylglycerol and solid fat contents of palm stearin, sunflower oil and palm kernel olein blends. Eur J Lipid Sci Tech. 2007;109:147–156. doi: 10.1002/ejlt.200600198. [DOI] [Google Scholar]
- Dsouza V, Deman JM, Deman L. Short spacings and polymorphic forms of natural and commercial solid fats—a review. J Am Oil Chem Soc. 1990;67:835–843. doi: 10.1007/BF02540502. [DOI] [Google Scholar]
- Farmani J, Hamedi M, Safari M, Madadlou A. Trans-free Iranian vanaspati through enzymatic and chemical transesterification of triple blends of fully hydrogenated soybean, rapeseed and sunflower oils. Food Chem. 2007;102:827–833. doi: 10.1016/j.foodchem.2006.06.015. [DOI] [Google Scholar]
- Gold IL, Ukhun ME, Akoh CC. Characteristics of eutectic compositions of restructured palm oil olein, palm kernel oil and their mixtures. J Am Oil Chem Soc. 2011;88:1659–1667. doi: 10.1007/s11746-011-1844-6. [DOI] [Google Scholar]
- Hu JN, et al. Characterization of medium-chain triacylglycerol (MCT)-enriched seed oil from Cinnamomum camphora (Lauraceae) and its oxidative stability. J Agric Food Chem. 2011;59:4771–4778. doi: 10.1021/jf200188r. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang J, Song L, Cao XS, Yao X, Tang F, Yue YD. GCxGC-TOFMS analysis of essential oils composition from leaves, twigs and seeds of Cinnamomum camphora L. Presl and their insecticidal and repellent activities. Molecules. 2016 doi: 10.3390/molecules21040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabulut I, Turan S, Ergin G. Effects of chemical interesterification on solid fat content and slip melting point of fat/oil blends. Eur Food Res Technol. 2004;218:224–229. doi: 10.1007/s00217-003-0847-4. [DOI] [Google Scholar]
- Limbardo RP, Santoso H, Witono JR (2017) The effect of coconut oil and palm oil as substituted oils to cocoa butter on chocolate bar texture and melting point. In: American Institute of Physics Conference Series, pp 7–17. 10.1063/1.4982281
- Liu CH, Mishra AK, Tan RX, Tang C, Yang H, Shen YF. Repellent and insecticidal activities of essential oils from Artemisia princeps and Cinnamomum camphora and their effect on seed germination of wheat and broad bean. Bioresour Technol. 2006;97:1969–1973. doi: 10.1016/j.biortech.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Mayamol PN, Balachandran C, Samuel T, Sundaresan A, Arumughan C. Zero trans shortening using rice bran oil, palm oil and palm stearin through interesterification at pilot scale. Int J Food Sci Technol. 2009;44:18–28. doi: 10.1111/j.1365-2621.2008.01627.x. [DOI] [Google Scholar]
- Mcgauley SE, Marangoni AG, Marangoni AG, Narine SS. Static crystallization behavior of cocoa butter and its relationship to network microstructure. Phys Prop Lipids. 2002;10:150. [Google Scholar]
- Mohamed IO. Enzymatic synthesis of cocoa butter equivalent from olive oil and palmitic-stearic fatty acid mixture. Appl Biochem Biotech. 2015;175:757–769. doi: 10.1007/s12010-014-1312-5. [DOI] [PubMed] [Google Scholar]
- Papamandjaris AA, MacDougall DE, Jones PJH. Medium chain fatty acid metabolism and energy expenditure: obesity treatment implications. Life Sci. 1998;62:1203–1215. doi: 10.1016/S0024-3205(97)01143-0. [DOI] [PubMed] [Google Scholar]
- Petrauskaite V, De Greyt W, Kellens M, Huyghebaert A. Physical and chemical properties of trans-free fats produced by chemical interesterification of vegetable oil blends. J Am Oil Chem Soc. 1998;75:489–493. doi: 10.1007/s11746-998-0252-z. [DOI] [Google Scholar]
- Ribeiro APB, Grimaldi R, Gioielli LA, Goncalves LAG. Zero trans fats from soybean oil and fully hydrogenated soybean oil: physico-chemical properties and food applications. Food Res Int. 2009;42:401–410. doi: 10.1016/j.foodres.2009.01.012. [DOI] [Google Scholar]
- Soares FASD, da Silva RC, da Silva KCG, Lourenco MB, Soares DF, Gioielli LA. Effects of chemical interesterification on physicochemical properties of blends of palm stearin and palm olein. Food Res Int. 2009;42:1287–1294. doi: 10.1016/j.foodres.2009.03.022. [DOI] [Google Scholar]
- Solis-Fuentes JA, Hernandez-Medel MD, Duran-de-Bazua MD. Determination of the predominant polymorphic form a of mango (Mangifera indica) almond fat by differential scanning calorimetry and X-ray diffraction. Eur J Lipid Sci Technol. 2005;107:395–401. doi: 10.1002/ejlt.200401072. [DOI] [Google Scholar]
- Takeuchi H, Sekine S, Kojima K, Aoyama T. The application of medium-chain fatty acids: edible oil with a suppressing effect on body fat accumulation. Asia Pac J Clin Nutr. 2008;17:320–323. [PubMed] [Google Scholar]
- Tang L, Hu JN, Zhu XM, Luo LP, Lei L, Deng ZY, Lee KT. Enzymatic interesterification of palm stearin with Cinnamomum camphora seed oil to produce zero-trans medium-chain triacylglycerols-enriched plastic fat. J Food Sci. 2012;77:C454–C460. doi: 10.1111/j.1750-3841.2012.02637.x. [DOI] [PubMed] [Google Scholar]
- Xu YX, Zhu XM, Ma XY, Xiong H, Zeng ZL, Peng HL, Hu JN. Enzymatic production of trans-free shortening from coix seed oil, fully hydrogenated palm oil and Cinnamomum camphora seed oil. Food Biosci. 2018;22:1–8. doi: 10.1016/j.fbio.2017.12.010. [DOI] [Google Scholar]
- Yamoneka J, Malumba P, Lognay G, Bera F, Blecker C, Danthine S. Enzymatic inter-esterification of binary blends containing Irvingia gabonensis seed fat to produce cocoa butter substitute. Eur J Lipid Sci Technol. 2018;120:10. doi: 10.1002/ejlt.201700423. [DOI] [Google Scholar]
- Zaidul ISM, Norulaini NAN, Omar AKM, Smith RL. Blending of supercritical carbon dioxide (SC-CO2) extracted palm kernel oil fractions and palm oil to obtain cocoa butter replacers. J Food Eng. 2007;78:1397–1409. doi: 10.1016/j.jfoodeng.2006.01.012. [DOI] [Google Scholar]
- Zhang X, Li L, Xie H, Liang ZL, Su JY, Liu GQ, Li B. Effect of temperature on the crystalline form and fat crystal network of two model palm oil-based shortenings during storage. Food Bioprocess Technol. 2014;7:887–900. doi: 10.1007/s11947-013-1078-8. [DOI] [Google Scholar]
- Zhu XM, et al. Physiochemical and oxidative stability of interesterified structured lipid for soft margarine fat containing Delta 5-UPIFAs. Food Chem. 2012;131:533–540. doi: 10.1016/j.foodchem.2011.09.018. [DOI] [Google Scholar]
- Zhu TW, Zhao YL, Zong MH, Li B, Zhang X, Wu H. Improvement of physical properties of palm stearin and soybean oil blends by enzymatic interesterification and their application in fast frozen food. RSC Adv. 2017;7:34435–34441. doi: 10.1039/C7RA02829F. [DOI] [Google Scholar]