Abstract
Influence of two apple cultivars (cvs. Cripps Pink and Idared) and two commercial strains of Saccharomyces bayanus (Lalvin EC1118, Fermol Blanc) on the chemical composition and sensory characteristics of apple wines was tested. Chemical parameters (alcohol, sugar-free extract, reducing sugars, titratable and volatile acidity) of the analyzed wines were strongly affected by apple variety. Ash and sugar-free extracts in Cripps Pink wines were significantly higher than Idared wines. Polyphenols and main organic acids were determined in apple juice and wines. Chlorogenic acid was the most abundant polyphenolic compound with the significantly higher concentrations detected for Idared wines. Total phenolic acids, as well as total flavan-3-ols content, were also higher for wines made from Idared variety where fermentation was conducted with Fermol blanc yeast. Among organic acids significantly higher succinic acid content was determined in wines where Fermol Blanc yeast was used while Lalvine EC1118, irrespective of apple variety, significantly influenced the concentration of lactic acid. Sensory evaluation showed the pronounced influence of variety but also the yeast used, singling out Idared cultivar and Fermol Blanc yeast achieving the best overall quality results.
Keywords: Apple wine, Cripps Pink, Idared, Polyphenols, Organic acids
Introduction
Apple (Malus domestica), a member of the Rosaceae family, is an important and most widely grown temperate fruit crop in the world. Besides fresh consumption, a significant amount of apple is also processed into juice, juice concentrate, apple wine and canned products (Rana and Bhushan 2016). In recent years there is also a significant interest in apple wine production in Croatia. During fermentation, yeasts synthesize a vast number of flavor compounds and different types of yeasts show some significant metabolic differences. Apart from the choice of yeast, many of the fermentation factors like temperature, the composition of apple juice, nitrogen and other nutrients, the presence of other microorganisms and ratio of inoculum to the substrate can affect fermentation process and the eventual sensory characteristics of apple wine. Thus, the quality of apple wine is strongly affected by organic acid composition and phenolic compounds profile (Peng et al. 2015). Vinson et al. (2001) have found that apples had the highest content of free phenolics among 10 other commonly consumed fruits, but the distribution of phenolic compounds and antioxidant capacity in apple is depending on the variety, type of tissues and the ripening stage (Alberti et al. 2017). It is widely known that polyphenolic compounds have antioxidant activity, free-radical scavenging capacity, coronary heart disease prevention, and anticarcinogenic properties (Peng et al. 2005; Satora et al. 2008; Parmar et al. 2017). In addition, the content and profile of phenolics is an important quality indicator since their concentration can strongly contribute to the bitterness, astringency, color stability and overall mouthfeel of the wine (Nogueira et al. 2008; Satora et al. 2008). Winemaking processes like fermentation, maceration, fining and aging can modulate the composition of polyphenolic compounds in apple wines (Guyot et al. 2003; Symoneaux et al. 2014). There are five major groups of polyphenols found in apple wines: flavan-3-ols (catechin and procyanidins), hydroxycinnamic acids (5-caffeoylquinic or chlorogenic acid and 4-p-coumaroylquinic acid), dihydrochalcones (phloretin glucoside and xylol glucoside), flavonols and anthocyanins (Nogueira et al. 2008). In general, typical culinary and dessert apples tend to have lower total phenolic content than apples usually used for wine production (McKay et al. 2011). The quality of apple wines, in general, is also connected with concentrations of main organic acids (Kunicka-Styczynska and Pogorzelski 2009; McKay et al. 2011). The major acid in apples is (S)-(−)-malic acid representing more than 90% of the total acidity. In addition to malic acid, the presence of pyruvic, tartaric, citric, lactic and succinic acid was previously reported (Kunicka-Styczynska and Pogorzelski 2009; Valles et al. 2005).
It is generally accepted that apple varieties classified as “cider” apples are the most suitable for the production of apple wine. The most part belongs to the old varieties survived thanks to the use in the apple wine production. Considering that in Croatia there are no apple cultivars used for wine production as well as no research on the subject, cultivars Idared and Cripps Pink were used. Moreover, chosen cultivars are among the most popular cultivars of dessert apples in Croatia.
The aim of this study was to determine an influence of two apple cultivars (cvs. Cripps Pink and Idared) and two commercial strains of Saccharomyces bayanus (Lalvin EC1118, Fermol Blanc) on the chemical composition and sensory characteristics of apple wines.
Materials and methods
Apple juice, yeasts, and fermentation
Apple juice was obtained from Cripps Pink and Idared variety apples. The fruit (150 kg for each variety) was harvested ripe and was stored in a cold room before crushing and pressing. Selected and cleaned fruit was fragmented and press by the courtesy of Grgar producer, Novo Čiće, Croatia. The apple juice yields ranged from 50 to 60%. After pressing fruit, juice was treated with potassium metabisulphite and ascorbic acid (Aromax, AEB group, Italy) to obtain 50 mg L−1 of SO2. Pectic substances were removed by treatment of juice with pectinolytic enzyme Lallzyme HC (2 g per 100 L; Lallemand, Canada). After 24 h of sedimentation clear juice of each variety was separated from sediments and divided into 10 L glass bottles, totally 6 glass bottles per variety.
Two active wine yeast strains Saccharomyces bayanus Fermol Blanc (AEB group, Italy) and EC 1118 (Lalvin, Canada) were used for the inoculation of the juice of each variety (106 cells/mL). Inoculation rate was 30 g dry weight of yeast per 100 L. All treatments (two varieties inoculated with two active yeast strains) were carried out in triplicate. Alcoholic fermentation was conducted for 21 days at 15 °C. After the fermentation, the young wines were separated from the lees and sulphurized. Free SO2 in young wines was adjusted to 50 mg L−1. Clarified young wines were subjected to the analysis.
Biochemical analysis
Chemicals
Epigallocatechin, procyanidin B1, and procyanidin B2 were purchased from Extrasynthese (Geney, France). Caffeic acid, caftaric acid, chlorogenic acid, p-coumaric acid, ferulic acid, gallic acid, (−)-epicatechin, (+)-catechin, epicatechin-gallate, quercetin-3-O-glucoside, quercetin, isoleucine, and N-acetyl-l-cysteine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Malic, succinic, lactic and citric acid were purchased from Riedel–de–Haen (Seelze, Germany). All chromatographic solvents were HPLC grade purchased from J. T. Baker (Deventer, Netherlands). Formic acid and 85%-orthophosphoric acid were obtained from Fluka (Buchs, Switzerland). Boric acid, sodium hydroxide, and ethanol were obtained from Kemika (Zagreb, Hrvatska).
Enological parameters of must and wine
Basic chemical analyses of must and wine were done using methods proposed by O.I.V. (2007).
Organic acids analysis (HPLC)
Mass concentrations of malic, citric, succinic and lactic acid were determinate by HPLC. Wines were filtered by Phenex-PTFE 0.45 μm syringe filters (Phenomenex, Torrance, USA) prior analysis of organic acids.
Agilent 1050 System (Agilent, Germany), equipped with an autosampler, column thermostat, and UV–Vis detector were used. The separation of organic acids was performed on Aminex HPX-87H (Biorad, CA, USA) column (300 × 7.8 mm, 9 μm) at 65 °C. The detection wavelength was 210 nm. Injected sample volume was 10 μL. The mobile phase was 0.065% H3PO4 with isocratic elution at flow rate of 0.6 mL min−1 for determination of malic, citric, succinic and lactic acid.
Free amino acids in apple must (FAN)
Free amino acids (FAN) were determined using an o-phthaldialdehyde/N-acetyl-l-cysteine spectrophotometric assay (Dukes and Butzke 1998). The analyses were performed on Jena Specord 400 spectrophotometer. The assay was based on the derivatization of primary amino groups with an o-phthaldialdehyde/N-acetyl-l-cysteine (OPA/NAC) reagent. The resulting isoindole derivates formed rapidly and were stable at a wavelength of 335 nm. Isoleucine was used for constructing a calibration curve.
Polyphenols analysis (HPLC)
Individual phenolic compounds were determined by HPLC method according to Tomaz and Maslov 2016. Separation, identification, and quantification of 18 individual polyphenolic compounds in apple wines were performed on Agilent 1100 Series system (Agilent, Palo Alto, USA), equipped with DAD and FLD (Agilent 1200) coupled to Agilent ChemStation Software (version B.01.03). Apple wine samples were filtered through 0.45 μm PTFE membrane filters and then injected (20 μL) on a reversed-phase column Luna Phenyl-Hexyl (4.6 × 250 mm; 5 μm particle, Phenomenex, Torrance, USA) with a Phenyl guard column (4.0 × 3.0), thermostated at 50 °C. The solvents were water/phosphoric acid (99.5:0.5, v/v, solvent A) and acetonitrile/water/phosphoric acid; 50:49.5:0.5, v/v/v, solvent B), and the flow rate was 0.9 ml min−1. The HPLC elution program started with an isocratic elution of 100% A for 7 min, and continued with linear gradient of 0% B to 20% B in 35 min, to 40% B in 40 min, isocratic elution at 40% B for 5 min, to 80% B in 50 min, and to 100% A for further 5 min. The chromatograms were recorded at 280, 320, 360 nm by diode array detector and at excitation wavelength 225 nm and emission wavelength at 320 nm. UV–vis spectra were recorded in the range from 200 to 700 nm. Phenolic compounds were identified by matching the retention time of each chromatographic peak with external standards and DAD spectrum. Quantification of individual phenolic peaks was performed by the external standard method. In the case of procyanidin B3 and B4, the calibration of structurally related compound (procyanidin B2) was used. All the samples were analyzed in the triplicate.
LC–MS analysis
For peak assignment, phenolic compounds in apple wines were confirmed by HPLC–ESI–MS with Agilent 1200 series system (Agilent, Germany) coupled on-line to an Agilent model 6410 mass spectrometer fitted with ESI source. The column was the same as described in the previous section. The mass spectra of phenolic compounds were recorded in the negative mode. The electrospray ionization (ESI) were as follows; drying gas (N2) flow and temperature, 8 L min−1 and 300 °C, respectively; nebulizer pressure was 30 psi, capillary voltage was 4500 V. Fragmentation voltage was 135 V.
Sensory analysis
Sensory evaluation of apple wines samples was performed using descriptive sensory analysis tasting form. The sensory panel included 7 qualified judges, all of them highly experienced in the evaluation of wine quality. The sensory attributes used were 6 taste attributes (sweetness, acidity, bitterness, astringency, complexity, and overall quality) and 6 aroma descriptors (very pleasant, average, simple, mature, complex to unpleasant). All samples were presented to the judges at a temperature of 10 °C. During the analysis, the judges evaluated the wines and indicated the intensity of each attribute on a structured line scale ranging from 0 (the lowest intensity) to 10 (the highest intensity). This experiment was carried out in duplicate in independent sessions.
Statistical analysis
Factorial analysis of variance (ANOVA) and Tukey HSD comparison test were used to interpret statistical differences in means, if any, at the p < 0.05 confidence level. Principal component analysis (PCA) of the polyphenols data was performed. All statistical analysis was performed using the software SAS 9.3 (SAS Institute, Cary, NC, USA).
Results and discussion
Table 1 shows the mean value of basic parameters in Cripps Pink and Idared apple juice. The sugar concentration was slightly higher in Cripps Pink juice while other parameters were similar. The concentrations of malic acid were comparable to earlier studies showing that in apple wine it is in the greatest quantity, representing 90% of the total organic acid content (Suni et al. 2000). In both apple juices, total acidity was relatively low especially compared to data dealing with the use of malolactic fermentation where malic acid concentration was up to 8 g L−1, while the pH values were similar ranging from 3.8 to 3.9 g L−1 (Reuss et al. 2010). The ratio of sugar/acidity serves as an industrial indicator because the balance between sugars and organic acids influence the taste of the beverage. According to Alberti et al. (2016) the unripe fruit showed sugar to acid ratio less than 20 which emphasized industrial suitability while after maturation this ratio decreased due to the increase in sugar concentrations. Our results are in agreement with Alberti et al. (2016) with the ratio being 28 for Idared and 35 for Crips Pink juices. The nitrogen content of must is very important for yeast growth, fermentation rate and time to complete fermentation. According to Blateyron and Sablayrolles (2001) the minimum amount of nitrogen necessary for a correct fermentation ranged between 120 and 140 mg L−1 of must. FAN analysis of Cripps Pink and Idared juices had indicated that juice contained favorable concentrations of nitrogen compounds for yeast growth, especially in Idared juice (135 mg L−1). These results were comparable to the investigation of Alberti et al. (2016) where total nitrogen was measured in ripe apples of Gala, Lis Gala and Fuji Suprema varieties ranging from 79 mg L−1 in Lis Gala up to 283 mg L−1 in Gala apple juices.
Table 1.
The chemical composition of apple juice
| Apple cultivar | Reducing sugars (g/L) | Titratable aciditya (g/L) | Malic acid (g/L) | Citric acid (g/L) | pH | FAN (mg/L) |
|---|---|---|---|---|---|---|
| Idared | 113.00 | 3.90 | 3.56 | 0.11 | 3.85 | 135.00 |
| Cripps Pink | 141.00 | 4.00 | 3.82 | 0.13 | 3.80 | 116.00 |
aExpressed as g/L of malic acid
The chemical compositions of apple wines are shown in Table 2. The fermenting sugars of juices were used almost entirely by both yeasts used in the experiment. Hence, the obtained wines could be classified as “dry”. Apple variety had a strong influence on alcohol concentration in wines thus higher values (7.91 and 7.94 vol %) were observed for Cripps Pink wines. This is probably due to higher concentrations of fermentable sugars in Cripps Pink juices. Moreover, the significant difference (p < 0.01) were noticed for ash and sugar-free extract in wines, where Cripps Pink wines had higher amounts. There was no marked difference in the level of titratable acidity and pH value in all tested samples. On the contrary, Cripps Pink wines had slightly higher values of volatile acidity (0.53 and 0.48 g L−1) compared to Idared wine. The variation noted in volatile acidity can be explained either by the synthesis of acetic acid by yeast strains used, what was not the case in our study, or by the ripening stage of these apples and higher sugar level in Cripps Pink juices, which was in accordance with the results of Alberti et al. (2016). The composition of organic acids in apple wines was also analyzed (Table 2). Statistical differences between organic acids concentrations were noticed, especially for succinic and lactic acid. The yeast strain used had a major influence on observed differences. The higher concentrations of succinic acid were found in Idared and Cripps Pink wines fermented with Fermol Blanc, 0.35 and 0.31 g L−1 respectively. Valles et al. (2005) reported concentrations of 0.40 g L−1 produced by Saccharomyces bayanus yeasts in wines fermented at 18 °C. Succinic acid is quantitatively the third product of alcoholic fermentation and its concentrations depend on of the yeast strain. In grape wines, concentrations are between 0.6 and 1.2 g L−1 where this acid contributes to wine acidity. On the other hand, the significant increase in lactic acid concentrations can be noticed for EC1118 yeast strain (0.28 g L−1). Low concentrations of lactic acid can be produced by yeasts during fermentation, but mainly its origin is from malolactic bacteria activity in wine. Malic acid concentration decreased in Idared wines from 3.56 g L−1 in juice to 3.33 g L−1 (Fermol blanc) and 3.34 g L−1 (EC1118) in wines, while that change was a bit higher in Cripps Pink wines, from 3.82 g L−1 in juice to 3.79 g L−1 (Fermol blanc) and 3.76 g L−1 in wines using EC1118 yeast strain. Thus, yeast strains used in this experiment showed a low affinity towards malic acid degradation what is contrary to earlier findings where the majority of Saccharomyces cerevisiae strains can assimilate malic acid up to 3 g L−1 (Redzepovic et al. 2003; Satora et al. 2008).
Table 2.
The chemical composition of apple wines
| Idared | Cripps pink | |||
|---|---|---|---|---|
| Fermol blanc | EC1118 | Fermol blanc | EC1118 | |
| Alcohol (vol.%) | 6.51 ± 0.035b | 6.43 ± 0.036b | 7.91 ± 0.068a | 7.94 ± 0.036a |
| Reducing sugars (g/L) | 4.73 ± 0.058c | 5.33 ± 0.115b | 5.27 ± 0.153b | 5.63 ± 0.058a |
| Titratable aciditya (g/L) | 4.00 ± 0.100a | 4.13 ± 0.115a | 4.03 ± 0.058a | 4.10 ± 0,01a |
| Volatile acidity (g/L)b | 0.38 ± 0.020c | 0.43 ± 0.017bc | 0.53 ± 0.031a | 0.48 ± 0.026ab |
| pH | 3.81 ± 0.006b | 3.85 ± 0.006a | 3.79 ± 0.006b | 3.76 ± 0.010c |
| Ash (g/L) | 1.90 ± 0.015d | 2.03 ± 0.058c | 2.64 ± 0.026b | 2.84 ± 0.025a |
| Sugar-free extract (g/L) | 15.9 ± 0.058c | 15.5 ± 0.115d | 22.1 ± 0.058b | 23.1 ± 0.153a |
| Organic acids (g/L) | ||||
| Malic acid | 3.33 ± 0.049b | 3.34 ± 0.025b | 3.79 ± 0.020a | 3.76 ± 0.012a |
| Citric acid | 0.29 ± 0.006a | 0.28 ± 0.006a | 0.25 ± 0.012b | 0.27 ± 0.006ab |
| Succinic acid | 0.35 ± 0.015a | 0.16 ± 0.012c | 0.31 ± 0.021b | 0.11 ± 0.006d |
| Lactic Acid | 0.18 ± 0.006b | 0.28 ± 0.017a | 0.14 ± 0.006c | 0.28 ± 0.015a |
Values with different superscript letters in the same row are significantly different according to the Tukey test (p < 0.05)
aexpressed as g/L of malic acid
bg/L of acetic acid
Polyphenolic composition of apple juice and wines
The polyphenolic composition of apple juices and wines are shown in Table 3. Our results showed the marked difference between used varieties where Idared juice total polyphenols content was 240.15 mg L−1 while Cripps Pink had the lower amount of 80.64 mg L−1. Relatively lower concentration of polyphenols in analyzed apple juice can be explained with polyphenol oxidase activity or their adsorption into the cell-wall matrix in the apple pomace during pressing (Nogueira et al. 2008). Our results pointed out chlorogenic acid as the most abundant individual polyphenol compound in apple juice ranging from 38.53 to 140.66 mg L−1 what was in agreement with previous work by Budak et al. (2015). Among other polyphenols, according to our results, procyanidin B2 and epicatechin can be singled out, in general representing together with other detected flavan-3-ol compounds more than 50% of total polyphenols in apple juices. The content of phenolic compounds in analyzed Idared and Cripps Pink apple wines showed quantitative changes during fermentation. Reduction of the total phenol content during fermentation was also observed in earlier studies (Alberti et al. 2016; Nogueira et al. 2008). On the contrary, Budak et al. (2015) reported that phenolic substances were increased by fermentation which was also observed for Cripps Pink wines (Table 3). As previously reported, apple wines had relatively high amounts of various polyphenols (Satora et al. 2008) even five to six times higher than in white grape wines (Kunicka-Styczynska and Pogorzelski 2009). Same authors have found 527 to 706 mg L−1 of total polyphenols in apple wines, while Alberti et al. (2016) reported 203–374 mg L−1 in wines from ripe Gala, Lis Gala and Fuji Suprema varieties. In Latvian apple wines, total polyphenols as chlorogenic acid equivalents (CAE mg L−1) were between 1028 and 1526 mg L−1 (Riekstina-Dolge et al. 2014). Compared to them, our results were the lowest with concentrations of total polyphenols ranging from 110.72 to 145.42 mg L−1 in Cripps Pink wines, and 210.85–228.17 mg L−1 in Idared wines. It can be assumed that Idared wines should have greater antioxidant activity compared to Cripps Pink wines. Among the individual polyphenols analyzed, chlorogenic acid was the most abundant compound ranging from 33.82 mg L−1 (Cripps Pink) to 86.79 mg L−1 (Idared) in analysed apple wine samples which was in agreement with earlier published data by Satora et al. (2008) and Riekstina-Dolge et al. (2014). Chlorogenic acid with other polyphenols like epicatechin and its polymers, quercetin and its glycosides, and some vitamins show the highest antioxidant activity (Lu and Foo 2000). Podsedek et al. (2000) found that products obtained from apple varieties characterized by lower polyphenol oxidase activity usually contain a higher amount of chlorogenic acid what can be confirmed by our results where apple wines obtained from Idared variety had lower polyphenol oxidase activity and on the basis of this higher total polyphenols and chlorogenic acid concentrations. Yeasts used in this research showed no significant influence on chlorogenic acid concentrations (Table 3). A second most abundant group of polyphenols in apple wines were flavan-3-ols; epigallocatechin and epicatechin. Epigallocatechin prevailed in all analyzed wines from 34.96 to 46.53 mg L−1 in Cripps Pink and from 66.66 to 69.18 mg L−1 in Idared wines. Moreover, epicatechin was measured in lower concentrations, from 23.51 to 27.87 mg L−1 in Idared wines and 19.42–22.31 mg L−1 in Cripps Pink wines. Nevertheless, our findings showed notably higher amounts of epicatechin compared to the results by Satora et al. (2008). As regards apple juice, Podsedek et al. (2000) observed similar concentrations in Idared apples. The significant increase in flavan-3-ols (epigallocatechin, catechin) and caffeic acid concentrations during fermentation in both varieties was observed. This can be explained by yeast metabolism and hydrolysis of caftaric acid which can release free caffeic acid and tartaric acid (Fracassetti et al. 2011). The same observation was reported by Nogueira et al. (2008). Procyanidin B2 prevailed among other measured procyanidins (B1, B3, B4) in both apple varieties what was not the case in earlier studies (Satora et al. 2008). Similarly, Idared variety showed significantly higher concentrations of procyanidin B2 in wine (21.20 and 23.86 mg L−1) than Cripps Pink variety (11.41 and 16.43 mg L−1). Lower concentrations of flavonols (quercetin) in all wines were observed. According to Nogueira et al. (2008), low flavonols content may be an indicator of the poor extractability of the polyphenols from the peel during apple processing. Budak et al. (2015) detected gallic acid only in apple juices which was not in agreement with our results. On the contrary, gallic acid was present in all wines ranging from 0.75 to 0.80 mg L−1. Yeast strains used in the study did not show great difference regarding polyphenol content of wines. These small differences can be due to different yeast cell wall structure, feature and capacity to adsorb different apple wine molecules such as certain phenolics (Morata et al. 2003; Nogueira et al. 2008). Polyphenol content data was subjected to principal component analysis (Supplementary Fig. 1). First two principal components (component 1 and 2) explained 66 and 23% of data variation for wine samples. According to principal component analysis, the first two principal components clearly demonstrated that there was segregation of apple varieties. Irrespective of the yeasts strain, Idared wines were in positive correlation with first principal component. Most of the procyanidins and flavan-3-ols were strongly correlated with first principal component, especially procyanidin B1 (0.286), procyanidin B2 (0.267), catechin (0.283) and epigallocatechin (0.281). Similarly, most of phenolic acids were also in strong positive correlation with first component like ferulic acid (0.289), chlorogenic acid (0.283), syringic acid (0.286). Some of phenolic acids like gallic acid (-0.274) and caffeic acid (-0.259) shown negative correlation with first principal component. However, Cripps Pink wines were negatively related to first principal component and more separated due to yeast strain used.
Table 3.
Mass concentrations (mg/L) of phenolic compounds in apple juice and wine
| mg/L | Idared Apple juice | Cripps Pink Apple juice | Idared | Cripps Pink | ||
|---|---|---|---|---|---|---|
| EC1118 | Fermol blanc | EC1118 | Fermol blanc | |||
| Chlorogenic acid | 140.66 ± 3.21 | 38.53 ± 0.62 | 81.33 ± 1.17b | 86.79 ± 0.9a | 47.27 ± 1.21c | 33.82 ± 1.32d |
| Syringic acid | 3.62 ± 0.43 | 0.51 ± 0.09 | 1.66 ± 0.03a | 1.59 ± 0.07a | 0.91 ± 0.02b | 0.57 ± 0.01c |
| Ferulic acid | 0.39 ± 0.02 | 0.16 ± 0.01 | 0.44 ± 0.07a | 0.43 ± 0.05a | 0.24 ± 0.03b | 0.18 ± 0.01b |
| Caftaric acid | 0.60 ± 0.11 | 0.14 ± 0.03 | 0.14 ± 0.01b | 0.17 ± 0b | 0.25 ± 0.01a | 0.13 ± 0b |
| Caffeic acid | 0.78 ± 0.09 | 0.90 ± 0.10 | 2.86 ± 0.24b | 2.97 ± 0.13b | 4.93 ± 0.17a | 4.17 ± 0.12a |
| Coumaric acid | 0.31 ± 0–04 | n.d. | 0.11 ± 0.01a | 0.10 ± 0.01a | n.d. | n.d. |
| Gallic acid | n.d. | n.d. | 0.75 ± 0.04a | 0.74 ± 0.05a | 0.79 ± 0.02a | 0.80 ± 0.02a |
| Protocatehuic acid | 1.30 ± 0.07 | 0.89 ± 0.03 | 1.03 ± 0.01a | 0.99 ± 0.02a | 0.99 ± 0.07a | 1.04 ± 0.08a |
| Total phenolic acids | 146.36 ± 2.19 | 40.32 ± 0.14 | 86.87 ± 0.78b | 93.78 ± 0.46a | 53.19 ± 0.21c | 39.03 ± 0.17d |
| Quercetin-3-glucoside | 0.43 ± 0.03 | 0.47 ± 0.01 | n.d. | n.d. | 0.36 ± 0.04a | n.d. |
| Quercetin | n.d. | 1.02 ± 0.02 | 1.32 ± 0.01a | 1.44 ± 0.03a | 1.44 ± 0.01a | 1.38 ± 0a |
| Total flavonols | 0.43 ± 0.04 | 1.49 ± 0.02 | 1.32 ± 0.07b | 1.44 ± 0.06b | 1.80 ± 0.01a | 1.38 ± 0.03b |
| Procyanidin B1 | 5.80 ± 0.12 | 0.90 ± 0.04 | 4.05 ± 0.02a | 4.25 ± 0.04a | 0.85 ± 0.03b | 0.60 ± 0.02b |
| Procyanidin B2 | 25.00 ± 0.15 | 12.36 ± 0.16 | 21.20 ± 0.11a | 23.86 ± 0.24a | 16.43 ± 0.16b | 11.41 ± 0.21b |
| Procyanidin B3 | 6.24 ± 0.16 | 1.39 ± 0.13 | n.d. | n.d. | n.d. | n.d. |
| Procyanidin B4 | 1.78 ± 0.08 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Catechin | 3.25 ± 0.10 | 1.22 ± 0.03 | 4.63 ± 0.23b | 5.67 ± 0.09a | 1.88 ± 0.17c | 1.82 ± 0.09c |
| Epicatechin | 35.86 ± 1.14 | 17.34 ± 0.23 | 23.51 ± 0.46ab | 27.87 ± 0.34a | 22.31 ± 0.20bc | 19.42 ± 0.12c |
| Epicatechingallate | 1.19 ± 0.07 | 0.17 ± 0.10 | 0.23 ± 0.06a | 0.14 ± 0.03b | 0.23 ± 0.04a | 0.16 ± 0.04b |
| Epigallocatechin | 12.94 ± 0.12 | 4.80 ± 0.04 | 66.66 ± 1.27a | 69.18 ± 0.98a | 46.53 ± 0.42b | 34.96 ± 0.32c |
| Total flavan-3-ols | 92.06 ± 1.36 | 38.18 ± 0.08 | 120.28 ± 2.13b | 132.80 ± 1.88a | 88.23 ± 0.32c | 68.37 ± 0.22d |
| Total polyphenol content | 240.15 ± 4.21 | 80.64 ± 1.81 | 210.85 ± 3.62b | 228.17 ± 2.83a | 145.42 ± 1.19c | 110.72 ± 1.90d |
Values with different superscript letters in the same row are significantly different according to the Tukey test (p < 0.05)
n.d. Not detected
Sensory evaluation of apple wines
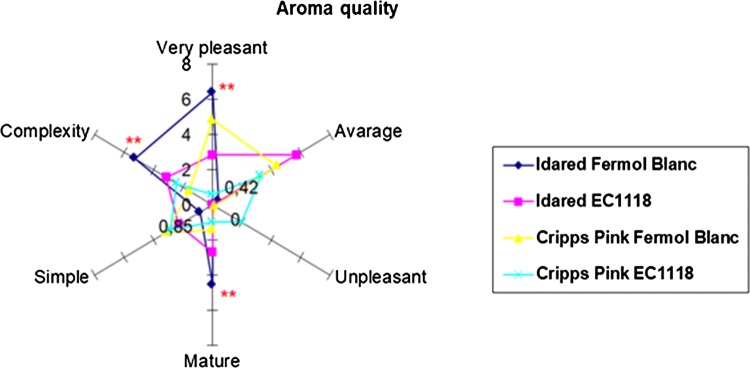
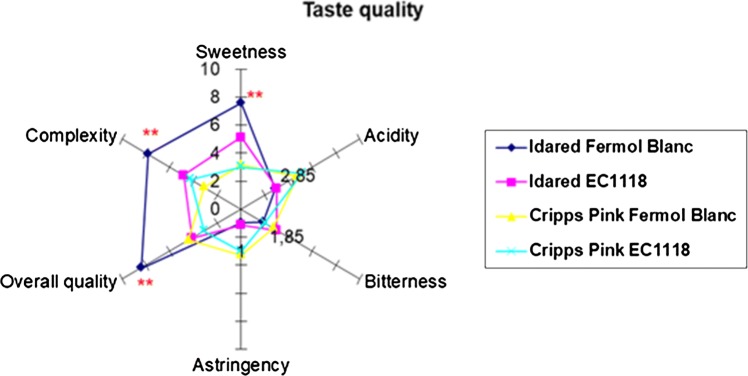
The results of the sensory evaluations of the apple wine samples, mean values of sensory attributes and level of significance by **P < 0.01 are shown in Figs. 1 and 2.
Fig. 1.
Aroma quality sensory scores of wines prepared from Idared and Cripps Pink variety apples fermented with different yeasts strains Fermol Blanc and EC 1118
Fig. 2.
Taste quality sensory scores of wines prepared from Idared and Cripps Pink variety apples fermented with different yeasts strains Fermol Blanc and EC 1118
The difference in the quality of aroma and taste was influenced by the variety of apple and yeast used. Wherein the variety Idared fermented with yeast strain Fermol Blanc was organoleptically best-scored apple wine singled to aroma quality described as very pleasant, complex and mature. In addition, the quality of taste of Idared wines was described by better overall quality, the complexity of taste and pronounced sweetness. According to Satora et al. (2008), pleasant and well-harmonized taste and aroma of Idared wines were strongly influenced by relatively high amounts of polyphenol compounds. The Idared wine fermented with Fermol Blanc strain had shown the highest overall concentration on polyphenols (228.17 mg L−1), the highest concentration of succinic acid (0.35 g L−1) and the lowest volatile acidity (0.38 g L−1). Better aroma impressions in Idared wines could be also connected with better nitrogen supply in corresponding juice since concentration and composition of amino acids in wines and musts can have an important effect on the aromatic complexity of wines. As it can be seen significantly better aroma and taste quality was noticed only in the Idared wines produced by Fermol Blanc yeast strain. On the contrary, Cripps Pink wines were described to be simpler and average on aroma, whereas the quality of taste was described as being astringent and highly acid with lower overall quality. Cripps Pink wines had a lower organoleptic quality which was a little bit unexpected according to chemical parameters (higher concentrations of alcohol, extract and reducing sugars). Astringency in taste was also pronounced in Cripps Pink wines what was not correlated with the polyphenolic composition of same samples. As seen, yeast strains used have shown the different impact on wine sensory quality. Between them, better overall quality in taste and very pleasant and mature aromas were noticed by Fermol Blanc strain used regardless of apple variety.
Conclusion
Apple wines produced using two different apple variety fermented with two yeast strains expressed significant variation in the composition of organic acids and some polyphenol compounds. The basic chemical parameters (alcohol, sugar-free extract, reducing sugars, titratable and volatile acidity) of the analyzed wines were strongly affected by apple variety. Whereas, yeast strains used in this study had a major influence on organic acids composition, especially succinic acid concentration. Yeast strain Fermol Blanc proved to be a better producer of succinic acid than an EC1118 strain. Idared variety has shown higher concentrations of polyphenols than Cripps Pink. Moreover, chlorogenic acid seems to be predominant phenol compound in apple juices and wines. From the results of this study, it can be concluded that the variety Idared showed more appropriate characteristic for apple wine production in respect of the variety Cripps Pink. In general, on the overall apple wine quality, the varietal effect was greater than yeast strain even though a significant effect of yeast strain on chemical composition was observed, wherein a Fermol Blanc commercial yeast strain presented better results.
References
- Alberti A, dos Santos TPM, Zielinski AAF, dos Santos CME, Braga CM, Demiate IM, Nogueira A. Impact on chemical profile in apple juice and cider made from unripe, ripe and senescent dessert varieties. Food Sci Technol-LEB. 2016;65:436–443. doi: 10.1016/j.lwt.2015.08.045. [DOI] [Google Scholar]
- Alberti A, Ferreira Zielinski AA, Couto M, Judacewski P, Igarashi Mafra L, Nogueria A. Distribution of phenolic compounds and antioxidant capacity in apples tissues during ripening. J Food Sci Technol. 2017;54(6):1511–1518. doi: 10.1007/s13197-017-2582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blateyron L, Sablayrolles JM. Stuck and slow fermentations in enology: statistical study of causes and effectiveness of combined additions of oxygen and diammonium phosphate. J Biosci Bioeng. 2001;91:184–189. doi: 10.1016/S1389-1723(01)80063-3. [DOI] [PubMed] [Google Scholar]
- Budak NH, Ozçelik F, Güzel-Seydim ZB. Antioxidant activity and phenolic content of apple cider. Turk JAF Sci Tech. 2015;3:356–360. doi: 10.24925/turjaf.v3i6.356-360.265. [DOI] [Google Scholar]
- Dukes BC, Butzke CE. Rapid determination of primary amino acids in grape juice using an o-phthaldialdehyde/N-acetyl-l-cysteine spectrophotometric assay. Am J Enol Viticult. 1998;49:125–134. [Google Scholar]
- Fracassetti D, Lawrence N, Tredoux AGJ, Tirelli A, Nieuwoudt HH, Du Toit WJ. Quantification of glutathione, catechin and caffeic acid in grape juice and wine by a novel ultra-performance liquid chromatography method. Food Chem. 2011;128:1136–1142. doi: 10.1016/j.foodchem.2011.04.001. [DOI] [Google Scholar]
- Guyot S, Marnet N, Sanoner P, Drilleau JF. Variability of the polyphenolic composition of cider apple (Malus domestica) fruits and juices. J Agr Food Chem. 2003;51:6240–6247. doi: 10.1021/jf0301798. [DOI] [PubMed] [Google Scholar]
- Kunicka-Styczynska A, Pogorzelski E. L-Malic acid effect on organic acid profiles and fermentation by-products in apple wines. Czech J Food Sci. 2009;27:S228–S231. doi: 10.17221/1063-CJFS. [DOI] [Google Scholar]
- Lu YR, Foo LY. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000;68: 81–85:-3. [Google Scholar]
- McKay SJ, Bradeen JM, Luby JJ. Prediction of genotypic values for apple fruit texture traits in a breeding population derived from ‘Honeycrisp’. J Am Soc Hort Sci. 2011;136:408–414. doi: 10.21273/JASHS.136.6.408. [DOI] [Google Scholar]
- Morata A, Gomez-Cordoves MC, Suberviola J, Bartolome B, Colomo B, Suarez JA. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J Agr Food Chem. 2003;51(14):4084–4088. doi: 10.1021/jf021134u. [DOI] [PubMed] [Google Scholar]
- Nogueira A, Guyot S, Marnet N, Lequere JM, Drilleau JF, Wosiacki G. Effect of alcoholic fermentation in the content of phenolic compounds in cider processing. Braz Arch Biol Techn. 2008;51:1025–1032. doi: 10.1590/S1516-89132008000500020. [DOI] [Google Scholar]
- O.I.V (International organization of vine and wine) (2007) Compendium of international methods of wine and must analysis. Paris
- Parmar N, Singh N, Kaur A, Thakur S. Comparison of color, anti-nutritional factors, minerals, phenolic profile and protein digestibility between hard-to-cook and easy-to-cook grains from different kidney bean (Phaseolus vulgaris) accessions. J Food Sci Technol. 2017;54(4):1023–1034. doi: 10.1007/s13197-017-2538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YY, Liu FH, Peng YY, Ye JN. Determination of polyphenols in apple juice and cider by capillary electrophoresis with electrochemical detection. Food Chem. 2005;92:169–175. doi: 10.1016/j.foodchem.2004.08.001. [DOI] [Google Scholar]
- Peng B, Lei Y, Zhao H, Cui L. Response surface methodology for optimization of fermentation process parameters for improving apple wine quality. J Food Sci Technol. 2015;52(11):7513–7518. doi: 10.1007/s13197-015-1872-6. [DOI] [Google Scholar]
- Podsedek A, Wilska-Jeszka J, Anders B, Markowski J. Compositional characterisation of some apple varieties. Eur Food Res Techol. 2000;210:268–272. doi: 10.1007/s002179900101. [DOI] [Google Scholar]
- Rana S, Bhushan S. Apple phenolics as nutraceuticals: assessment, analysis and application. J Food Sci Technol. 2016;53(4):1727–1738. doi: 10.1007/s13197-015-2093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redzepovic S, Orlic S, Majdak A, Kozina B, Volschenk H, Viljoen-Bloom M. Differential malic acid degradation by selected strains of Saccharomyces during alcoholic fermentation. Int J Food Microbiol. 2003;83:49–61. doi: 10.1016/S0168-1605(02)00320-3. [DOI] [PubMed] [Google Scholar]
- Reuss RM, Stratton JE, Smith DA, Read PE, Cuppett SL, Parkhurst AM. Malolactic fermentation as a technique for the deacidification of hard apple cider. J Food Sci. 2010;75:C74–C78. doi: 10.1111/j.1750-3841.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- Riekstina-Dolge R, Kruma Z, Cinkmanis I, Straumite E, Sabovics M, Tomsone L (2014) Influence of oenococcus oeni and oak chips on the chemical composition and sensory properties of cider. In: Straumite E (ed) 9th Baltic conference on food science and technology—Food for consumer well-being: Foodbalt 2014. FoodBalt. pp 178–183
- Satora P, Sroka P, Duda-Chodak A, Tarko T, Tuszynski T. The profile of volatile compounds and polyphenols in wines produced from dessert varieties of apples. Food Chem. 2008;111:513–519. doi: 10.1016/j.foodchem.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Suni M, Nyman M, Eriksson NA, Bjork L, Bjorck I. Carbohydrate composition and content of organic acids in fresh and stored apples. J Sci Food Agr. 2000;80:1538–1544. doi: 10.1002/1097-0010(200008)80:10<1538::AID-JSFA678>3.0.CO;2-A. [DOI] [Google Scholar]
- Symoneaux R, Baron A, Marnet N, Bauduin R, Chollet S. Impact of apple procyanidins on sensory perception in model cider (part 1): polymerisation degree and concentration. Food Sci Technol-LEB. 2014;57:22–27. doi: 10.1016/j.lwt.2013.11.016. [DOI] [Google Scholar]
- Tomaz I, Maslov L. Simultaneous determination of phenolic compounds in different matrices using phenyl-hexyl stationary phase. Food Anal Method. 2016;9:401–410. doi: 10.1007/s12161-015-0206-7. [DOI] [Google Scholar]
- Valles BS, Bedrinana RP, Tascon NF, Garcia AG, Madrera RR. Analytical differentiation of cider inoculated with yeast (Saccharomyces cerevisiae) isolated from Asturian (Spain) apple juice. Food Sci Technol-LEB. 2005;38:455–461. doi: 10.1016/j.lwt.2004.07.008. [DOI] [Google Scholar]
- Vinson JA, Su XH, Zubik L, Bose P. Phenol antioxidant quantity and quality in foods: fruits. J Agr Food Chem. 2001;49:5315–5321. doi: 10.1021/jf0009293. [DOI] [PubMed] [Google Scholar]