Abstract
This study aimed to establish the optimal conditions of temperature (31–43 °C) and coconut pulp concentration in water 1:3–1:9 (w/v) for the growth of Lactobacillus reuteri LR 92 or DSM 17938 in coconut milk beverage, using a central composite face centered design. The optimized conditions were used for analysis of the viability during the fermentation process, pH, production of sugars and organic acids by High Performance Liquid Chromatography (HPLC) and reuterin production. Coconut milk provided adequate substrate for L. reuteri growth without supplementation. The optimal parameters for L. reuteri viability were: concentration 1:3 (w/v) and 37 °C for LR 92 and concentration 1:3 (w/v) and 34 °C for DSM 17938. Chemical analysis showed that the naturally occurring sucrose in the matrix (ca. 4.4 g/L) was used for cell multiplication and the strains differed in the production and content of organic acids. After fermentation until pH 4.5 ± 0.1, the samples were stored at 4 °C for 30 days and the final cell viability in coconut milk was 7.55 ± 0.07 log CFU/mL for L. reuteri LR 92 and 8.57 ± 0.09 log CFU/mL for DSM 17938. It was detected 0.15 ± 0.03 mM and 0.14 ± 0.04 mM of reuterin produced by DSM 17938 and LR 92, respectively. L. reuteri DSM 17938 presented a great decrease of pH and post acidification after storage. The LR 92 strain showed low post acidification. These results showed that coconut milk provides adequate matrix for the development of new fermented functional beverages.
Keywords: Plant-based milk, Probiotic, Response surface, Sugars profile, Organic acids profile, Reuterin
Introduction
Non-dairy beverages consumption has increased in the last years due to several factors such as: food allergies and restrictions, desire to limit animal-based foods due to health concerns, and vegetarian and vegan diets (McCarthy et al. 2017). In this context, sales of non-dairy milk have grown steadily over the last 5 years, growing 61% since 2012 (IFT 2018).
Plant-based milks are suspensions of dissolved and disintegrated plant material in water, resembling cow’s milk in appearance (Mäkinen et al. 2015). Coconut milk consists of a liquid extracted from the solid endosperm of mature coconut (Cocos nucifera L.) and can be mixed with water for consumption as a beverage. This product contains lauric acid as a functional component, which can promote the development of brain functions, stimulate immune system defenses and maintain elasticity of blood vessels (Seow and Gwee 1997; Sethi et al. 2016).
The development of products containing plant-based milks is relatively new and therefore, studies are conducted to enhance the nutritional benefits of these foods (Jeske et al. 2018). Several authors have demonstrated that vegetable milks are suitable matrices for the growth of beneficial microorganisms, producing beverages with great market potential due to the possibilities of creating new functional products using different processes and ingredients (Santos et al. 2014; Bernat et al. 2015a; Zannini et al. 2018). In addition, fermentation may be essential for flavor development of the beverages (Jeske et al. 2018).
Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host (FAO/WHO 2001). The species Lactobacillus reuteri showed probiotic properties, as it can decrease colic in newborns, attenuate acute diarrhea in children, and reduce symptoms of gingivitis in the elderly (Dinleyici et al. 2015; Kraft-Bodi et al. 2015; Fatheree et al. 2017). This species has already shown growth in oat milk and almond milk, reaching approximate counts of 9 log CFU/mL and 8 log CFU/mL at the end of the fermentation, respectively (Bernat et al. 2015a, b). L. reuteri synthesizes reuterin, an antimicrobial compound, in media where there is excess of glycerol under anaerobic conditions. The in situ production of this compound in fermented milk was achieved, contributing to the microbiological stability of the product during product storage (Langa et al. 2013).
The objective of this work was to optimize the best conditions for the growth of Lactobacillus reuteri strains LR 92 and DSM 17938 in coconut milk with addition of glycerol, using a central factorial centered design (α = ± 1). Under the optimized conditions, to monitor the fermentation process, detect the production of reuterin in fermented products and evaluate stability during refrigerated storage (4 °C for 30 days).
Materials and methods
Coconut milk
The mature coconut pulp was purchased from the local market in Londrina–PR, Brazil. The pulp was sanitized with water, grated and homogenized. The coconut pulp was mixed with distilled water at 70 °C previously heated in glass flasks (proportions of the experimental design) in a food processor for household use (Philips Walita, Brazil) for 2 min. The suspension was filtered through a synthetic fabric strainer and the solid wastes were discarded. After obtaining the coconut milk, the sample was added to glass flasks and pasteurized at 95 °C for 5 min (Siriphanich et al. 2011) in digital water bath (Novatecnica, Brazil).
Bacterial strains
For the fermentation of coconut milk, a commercial lyophilized culture of Lactobacillus reuteri LR 92 (DSM 26866, Sacco–Italy) and chewable tablets of Provance (Aché – Biogaia) containing Lactobacillus reuteri DSM 17938 were used. L. reuteri LR 92 were activated (0.1% w/v) in pasteurized coconut milk containing 20% (v/v) glycerol (Synth). The DSM 17938 strain contained in one Provance tablet was reactivated in 9 mL sterile MRS broth (Himedia), incubated at 37 °C for 24 h, centrifuged at 12,000×g for 20 min at 4 °C (Eppendorf, 5804/5804 R) and washed twice with saline solution 0.9% (w/v). The total biomass obtained was added to 100 mL of pasteurized coconut milk with 20% (v/v) of sterile glycerol. The strains were divided into portions of 10 mL, which were kept frozen at − 20 °C. The pre-inoculum were obtained through two activations in coconut milk at 37 °C for 24 h under anaerobiosis to promote culture adaptation. The inocula were plated prior to the inoculation for standardization and comparative purposes of the assays. To achieve the equivalent cellular concentration of 8 log CFU/mL, it were necessary to add 0.3% (v/v) of L. reuteri DSM 17938 or 1% of L. reuteri LR 92 to the fermentation medium.
Counts of Lactobacillus reuteri
The viability of L. reuteri LR 92 and L. reuteri DSM 17938 in the fermented coconut milk was determined by the plate count method. Aliquots of 1 mL of each sample were diluted with 9 mL of sterile peptone water in serial decimal dilutions and it was plated in MRS agar (Himedia) and incubated under anaerobic conditions at 37 °C for 48 h. Anaerobiosis was created by using anaerobic jars and microaerophilic generators (Anaerobac, Probac, Brazil). The results were expressed as log of colony forming units per mL (log CFU/mL).
Experimental design
To optimize and study the coconut milk fermentation process by L. reuteri LR 92 or DSM 17938, the face-centered central composite design (FCCCD) was conducted. The design 22 had face-centered star points (α = ± 1) and 2 center point repetitions. The repetitions at the central point provided a measure of the pure error and stabilization of the expected variance response (Barros Neto et al. 2010).
The independent variables were the temperature (°C) and concentration of coconut pulp in water (w/v) and the response variable was the count of viable cells of L. reuteri (CFU/mL). The values of the independent variables were established as described in the literature or according to preliminary tests, with temperatures ranging from 31 to 43 °C (Tobajas et al. 2007) and the concentration of coconut pulp in water of 1:9–1:3 (w/v) for the growth of the microorganisms. The time of 15 h for the end of the fermentation was established according to preliminary tests, in which L. reuteri had already reached the stationary phase of bacterial growth.
The volume of 40 mL of pasteurized coconut milk was added to falcon tubes (50 mL) and incubated with the addition of 100 mM of sterile glycerol. Fermentation was carried in anaerobic jars and microaerophilic generators (Anaerobac, Probac, Brazil) to promote reuterin synthesis. The initial pH value in the matrix was 6.4 ± 0.1. The following second-order polynomial regression model was used to correlate the experimental data obtained:
where y = response variable to cell viability (log CFU/mL); X1 and X2 = coded independent variables (temperature and concentration of coconut pulp); β = estimated coefficients of each term of the response surface model. The impact and meaning of each term (linear, quadratic, and interactions) in the regression equation was evaluated by analysis of variance (ANOVA).
Fermentation process
0.3% (v/v) of L. reuteri DSM 17938 or 1% (v/v) of L. reuteri LR 92 was added to the pasteurized coconut milk and incubated at the optimum temperature determined in the experimental design for 48 h in anaerobiosis. All fermentations started with 6 log CFU/mL of L. reuteri. Samples were collected during fermentation (0, 2, 4, 8, 10, 12, 14, 16, 20, 24, 32 and 48 h) and analysis of cell viability, pH, sugars and organic acids were conducted.
pH and titratable acidity
pH measurements were made with a digital pH meter (Kasvi K39-2014B), and acidity measurements were made via titration with a 0.1 M NaOH solution. The results were expressed in g of lactic acid/100 mL of sample (AOAC 1990).
Sugars and organic acids
Sample preparation
The samples were homogenized and ultracentrifuged (Hitachi, Ibaraki, Japan) at 230,600×g for 20 min at 4 °C. The supernatant was collected and filtered on a 0.22 μm PVDF membrane (Millex®) to result in a sample for determination of sugars and organic acids. Standard solutions of glucose, fructose and sucrose (purity > 99% for HPLC, Sigma-Aldrich) were prepared in ultrapure water at a concentration of 2 g/L, as well as the standard solutions of lactic, malic, citric, acetic and succinic acids (purity > 98% for HPLC, Sigma-Aldrich).
Sugars and organic acids
The chromatographic system employed was a Shimadzu Prominence LC-20A series HPLC (Shimadzu, Kyoto, Japan) consisting of a pump (LC-20AT) with solvent organizer and degas module (DGU-20A5), autosampler (SIL-20AC), column oven (CTO-20A), refractive index (RID-10A) and a photodiode array detector (SPD-M20A). The data processing was performed using Shimadzu LC Solutions Software.
For the determination of glucose, fructose and sucrose, ultra pure water was used as the mobile phase, due to the improvement of the chromatographic resolution and the symmetry of peaks. The mobile phase flow rate was 1.0 mL min−1, with an ion-exchange column Aminex HPX-87P (7.8 × 300 mm in ionic form Pb + 2, BioRad, CA, USA) kept at a constant temperature of 85 °C, with 20.0 µL injection volume. The detection was conducted by refractive index (RID-10A).
For organic acids determination, a chromatographic column Shiseido Capcell Pak C18 MG (4.6 × 250 mm, 5 µm) was employed. The mobile phase consisted of 25.0 mmol L−1 of sodium phosphate buffer solution, with pH 2.4 and flow rate of 1.0 mL min−1. The column temperature was maintained at 30 °C and 20.0 µL was injected. Detection was performed simultaneously in a Refractive Index Detector (RID-10A) and a Photodiode array detector (SPD-M20A) (Pauli et al. 2011).
Stability during refrigerated storage
After the fermentation process analysis, L. reuteri DSM 17938 and LR92 were cultured in coconut milk until pH 4.5 ± 0.1, the usual pH range for the production of dairy products (Yildiz 2009). Then, 40 mL of the fermented coconut milk were stored in falcon flasks for each time determination and were kept at 4 °C for 30 days. Analysis of cell viability, pH and titratable acidity were conducted in triplicate at time 0 (end of fermentation), 1, 15 and 30 days of storage.
Reuterin content
The determination of reuterin was performed according to the photometric method described by Tobajas et al. (2007). The samples were fermented until pH 4.5 ± 0.1, homogenized and centrifuged at 12,000×g for 20 min at 4 °C. The supernatants were filtered (0.22 μm, Millex®) and used to quantify the presence of reuterin in triplicate. Acrolein (Sigma-Aldrich) was used for calibration in 50 mM phosphate buffer pH 7.5. The volume of 1 mL of sample was added to 0.75 mL of 10 mM tryptophan dissolved in 0.05 N HCl. After addition of 3 mL 37% HCl, the mixture was incubated at 37 °C for 20 min, and the absorbance at 560 nm was recorded. From the standard curve obtained between 0.05 and 6 mM concentrations of acrolein in buffer-phosphate solution, molar quantification of reuterin was possible.
Statistical analysis
Data were submitted to Analysis of Variance (ANOVA) and Tukey’s test, to compare means at the 5% level of significance, using program STATISTICA 8.0. The graphs were developed in GraphPad Prism 5.
Results and discussion
Optimization of L. reuteri viability in coconut milk
The effects of temperature and coconut pulp concentration on L. reuteri cell counts (log CFU/mL) are shown in Table 1. The experiments were conducted in two blocks (replicate 1 and 2), totaling 24 experiments. The response (viable cells) ranged from 6.98 to 8.95 log CFU/mL and 6.87–8.50 log CFU/mL for the strains DSM 17938 and LR 92, respectively.
Table 1.
FCCCD for fermentation by Lactobacillus reuteri DSM 17938 and Lactobacillus reuteri LR92 and response functions YP and YL
| Replicate | Assays | Coded variables | Uncoded variables | Response functions | |||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X1 | X2 | YP | YL | ||
| 1 | 1 | − 1 | − 1 | 31 | 1:9 | 8.55 | 7.74 |
| 2 | − 1 | 1 | 31 | 1:3 | 8.83 | 8.24 | |
| 3 | 1 | − 1 | 43 | 1:9 | 6.98 | 6.87 | |
| 4 | 1 | 1 | 43 | 1:3 | 7.59 | 7.94 | |
| 5 | − 1 | 0 | 31 | 1:6 | 8.58 | 7.92 | |
| 6 | 1 | 0 | 43 | 1:6 | 7.45 | 7.66 | |
| 7 | 0 | − 1 | 37 | 1:9 | 8.22 | 8.06 | |
| 8 | 0 | 1 | 37 | 1:3 | 8.89 | 8.48 | |
| 9 | 0 | 0 | 37 | 1:6 | 8.79 | 8.10 | |
| 10 | 0 | 0 | 37 | 1:6 | 8.83 | 7.95 | |
| 11 | 0 | 0 | 37 | 1:6 | 8.44 | 7.86 | |
| 12 | 0 | 0 | 37 | 1:6 | 8.51 | 7.84 | |
| 2 | 13 | − 1 | − 1 | 31 | 1:9 | 8.52 | 8.06 |
| 14 | − 1 | 1 | 31 | 1:3 | 8.87 | 8.16 | |
| 15 | 1 | − 1 | 43 | 1:9 | 7.46 | 6.90 | |
| 16 | 1 | 1 | 43 | 1:3 | 8.26 | 7.95 | |
| 17 | − 1 | 0 | 31 | 1:6 | 8.54 | 8.00 | |
| 18 | 1 | 0 | 43 | 1:6 | 7.43 | 7.70 | |
| 19 | 0 | − 1 | 37 | 1:9 | 8.52 | 8.16 | |
| 20 | 0 | 1 | 37 | 1:3 | 8.95 | 8.50 | |
| 21 | 0 | 0 | 37 | 1:6 | 8.78 | 7.84 | |
| 22 | 0 | 0 | 37 | 1:6 | 8.42 | 8.12 | |
| 23 | 0 | 0 | 37 | 1:6 | 8.79 | 7.83 | |
| 24 | 0 | 0 | 37 | 1:6 | 8.78 | 8.20 | |
X1 incubation temperature (°C), X2 coconut pulp concentration (w/v), YPL. reuteri DSM 17938 count, expressed in log CFU/mL of sample; YLL. reuteri LR 92 count, expressed in log CFU/mL of sample
These data indicate that both strains of L. reuteri were able to multiply in coconut milk without the need for sugar supplementation, using the sugars naturally present in the substrate as an energy source. Only in the 1:9 (w/v) condition at 43 °C was observed minor cell multiplication (6.87–7.46) log CFU/mL, since the initial concentration of viable cells in the fermentation medium was approximately 6 log CFU/mL.
From the data obtained, ANOVA was performed to verify the influence of the independent variables X1 (temperature) and X2 (coconut pulp concentration) on the response variables. The results showed that both variables had effects on the bacterial growth of L. reuteri (p < 0.05). The R2 values were 0.93 for L reuteri DSM 17938 and 0.83 for L reuteri LR 92. Both values were above 0.8 for the two parameters studied, indicating that the models can explain above 80% the values observed in the experiment and therefore, it can be used for predictive purposes. The experiments did not present significant variation between the blocks (replicate 1 and 2) (p < 0.05).
Considering only the significant variables, the models (Eqs. 1, 2) representing the viable cell counts of L. reuteri DSM 17938 (YP) and L. reuteri LR 92 (YL) in log CFU/mL were proposed in function of the independent variables:
| 1 |
| 2 |
The quadratic effects of temperature of the models presented negative values, indicating a point of maximum response for viable cells. The concentration of coconut pulp showed only the linear effect significant (p < 0.01).
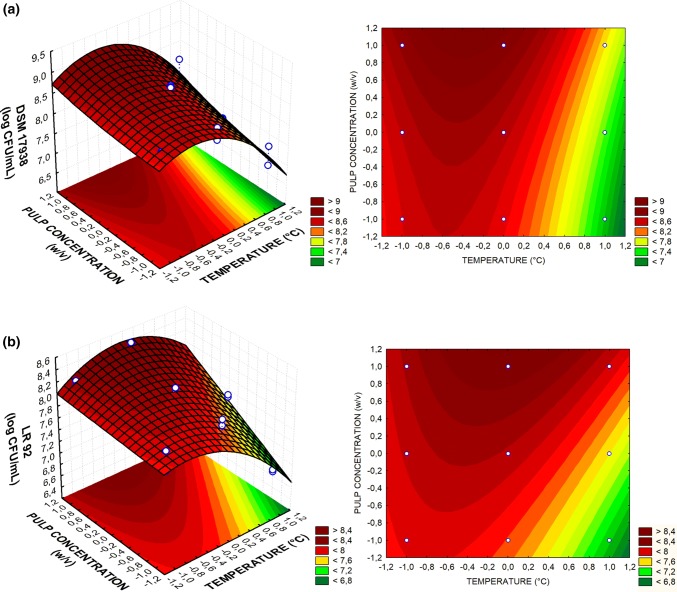
The surface response graphs for coconut milk fermented by L. reuteri LR 92 and DSM7938 are shown in Fig. 1a and b, respectively. The concentration of coconut pulp was studied to determine if the microorganisms would be able to multiply at lower concentrations of this substrate, since the optimum formulation should be defined as the one that requires minimum production costs. However, at the center point concentration (1:6 (w/v)), the number of viable cells of L. reuteri DSM 17938 and LR 92 was significantly below the number found at the highest concentration (1:3 (w/v)) and therefore, this was chosen as the optimal condition of cell multiplication.
Fig. 1.
Response surface 3D and 2D contour plots for L. reuteri LR 92 (a) and L. reuteri DSM 17938 (b) in coconut milk, according to temperature (X1) and coconut pulp concentration (X2) at its optimum point
The effect of the temperature for strain LR 92 reached the highest response at the central point, in which the temperature was 37 °C. This result is in agreement with Liu et al. (2014), which determined the ideal fermentation pH (5.7) and temperature of 37 °C for the production of viable cells of L. reuteri I5007 in MRS medium.
In the proposed model for DSM 17938, it was found that the optimum temperature (34 °C) was not in the tests conducted. However, as the configuration was part of the range considered, a new triplicate analysis was performed under the optimal conditions (34 °C, 1:3 (w/v)) for model validation. L. reuteri DSM 17938 growth reached counts of log 9.14 ± 0.02 CFU/mL, confirming the theoretical estimated response of 9.13 log CFU/mL in the matrix. The results of the optimal point for LR 92 were also validated after repetition of the experiments in triplicate and the means did not differ from the estimated Tukey test response (p < 0.05).
The optimal growth temperature of Lactobacillus spp. can vary between species and strains. In soymilk fermented by Lactobacillus plantarum BG 112, the optimum temperature was 37 °C, while Lactobacillus acidophilus LA3 presented better growth at 31 °C (Moraes Filho et al. 2016). When incubated at 37 °C and 45 °C, L. reuteri SD2112 exhibited rapid growth in milk (Østlie et al. 2003). However, in the present study using coconut milk as fermentative matrix, lower growth of both L. reuteri strains was observed when the temperature employed was 43 °C.
Fermentation process
Viability of Lactobacillus reuteri
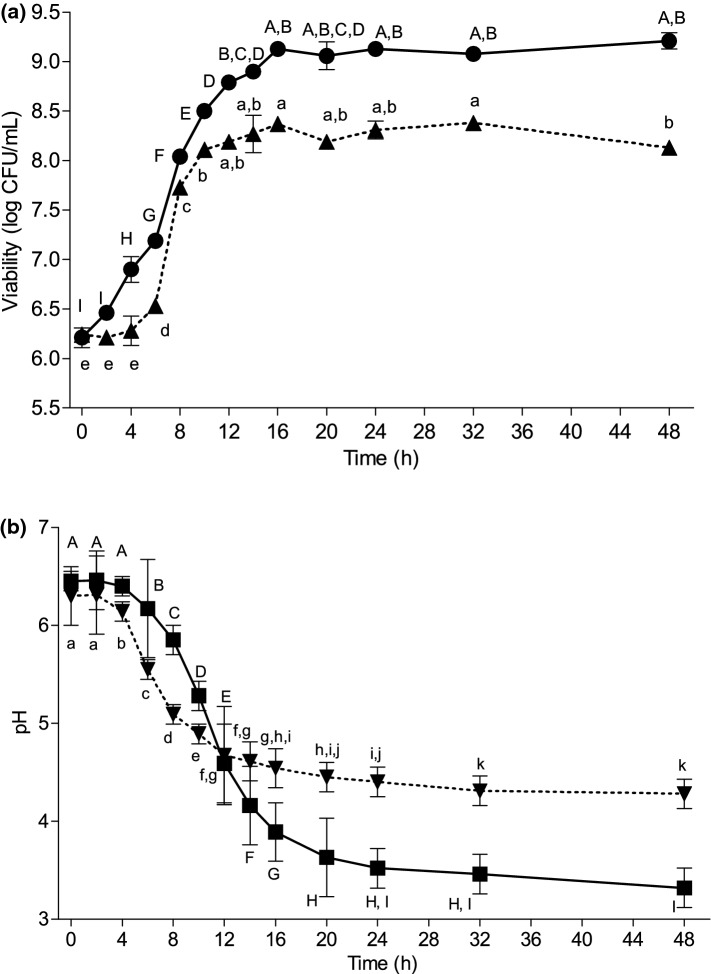
The optimal conditions obtained were used in the analysis of the fermentation process of L. reuteri in coconut milk. Figure 2a shows the viability of L. reuteri DSM 17938 and LR 92 during 48 h fermentation. For comparative purposes, the initial concentration (0 h) of viable cells was 6.24 ± 0.03 and 6.21 ± 0.10 log CFU/mL for DSM 17938 and LR 92, respectively.
Fig. 2.
a Viability of Lactobacillus reuteri DSM 17938 (●) at 34 °C and Lactobacillus reuteri LR 92 (▲) at 37 °C in coconut milk 1:3 (w/v) during fermentation process of 48 h. b pH of the coconut milk fermented by Lactobacillus reuteri DSM 17938 (■) and Lactobacillus reuteri LR 92 (▼). Different lowercase (LR 92) and uppercase (DSM 17938) letters show a significant difference (p < 0.05)
It can be observed that LR 92 presented 4 h of lag phase and counts above 8 log CFU/mL after 10 h of fermentation. The DSM 17938 presented lag phase of 2 h and consequently reached approximately the same number of viable cells in only 8 h of fermentation. The duration of the lag phase can be influenced by differences in the metabolism of the strains in adapting to ambient conditions. In this study, the reactivation of the inoculum in coconut milk was made to reduce the adaptation phase of the strains at the time of inoculation. However, as observed, this phase may still occur.
The DSM 17938 strain was better adapted to the coconut milk matrix, presenting superior performance in the fermentation process, with increase of 3 log cycles until reaching the stationary phase, while the LR 92 presented an increase of 2 log cycles. In a previous work, the development of a blend of carrot and blueberry fermented by the same strain of L. reuteri LR 92 demanded 40 h to reach viability of 10.26 ± 0.23 log CFU/mL, a value above the maximum found in the present study (Mauro et al. 2016).
The fermentation of oat milk by L. reuteri ATCC 55730 after 4 h reached maximum counts of 9 log CFU/mL of product. However, in this medium, glucose, fructose and inulin supplementation was performed, in addition to fermentation combined with Streptococcus thermophilus, to reach a higher number of viable cells in a shorter fermentation time (Bernat et al. 2015a). In this way, it can be affirmed that the metabolism of the bacteria undergoes interference of the fermentative matrix, because of differences in the content of fermentable sugars and nutrients available.
pH
The pH values during the growth of L. reuteri DSM 17938 and LR 92 are shown in Fig. 2b. The coconut milk fermented by DSM 17938 had initial pH 6.45 and final 3.32 and LR 92 had initial pH 6.36 and final pH 4.28.
The pH of the fermentative matrix reduced in the first 12 h until it reached close value for both strains. After this period, DSM 17938 presented lower pH values than LR 92. The pH of the fermented matrix by L. reuteri DSM 17938 stabilized after 24 h, whereas LR 92 showed stabilization after 32 h of the fermentation process. The fermentation in barley flour also revealed differences in pH between L. reuteri strains, and in the accordance with the results obtained, the pH reduction capacity was higher for L. reuteri DSM 17938 (Pallin et al. 2016).
Sugars profile
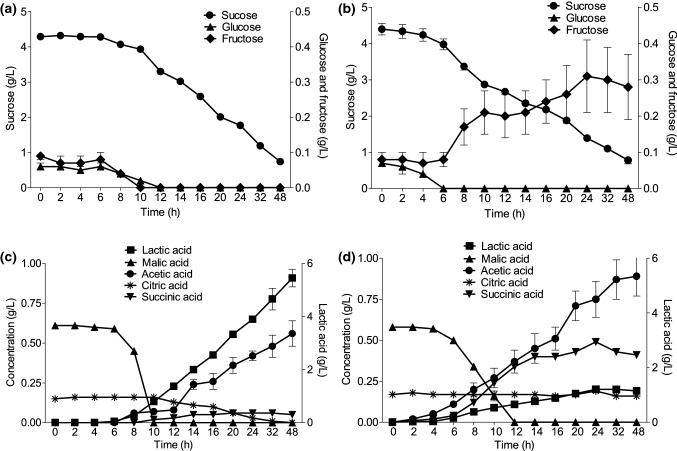
Figure 3 shows the sugars profile of coconut milk 1:3 (w/v) during L. reuteri DSM 17938 (a) and LR 92 (b) fermentation. The initial sugars concentrations are in agreement with the content found in mature coconut pulps, in which sucrose is the main sugar present (Santoso et al. 1996). The value found in coconut milk is proportional to the dilution used and processing. There was hydrolysis and sucrose consumption by both strains during 48 h. The initial sucrose content was 4.3–4.4 g/L, consumed to reach final values of 0.7 and 0.8 g/L, for L. reuteri DSM 17938 and LR 92, respectively.
Fig. 3.
Sugars profile (●—sucrose,▲—glucose and ■—fructose) of L. reuteri DSM 17938 (a) at 34 °C and LR 92 (b) at 37 °C in 1:3 (w/v) coconut milk for during 48 h of fermentation and organic acids profile (■—lactic acid, ●—acetic acid, ▲—malic acid,▼—succinic acid and —citric acid) by L. reuteri DSM 17938 (c) and LR 92 (d) during 48 h of fermentation
In the first hours of fermentation, DSM 17938 consumed fructose (0.09 ± 0.01 g/L) and glucose (0.06 ± 0.01 g/L), sugars that even in a small amount were used by microorganisms because they are easily fermentable monosaccharides. When depletion occurred, the DSM 17938 behaved differently, hydrolyzing the sucrose molecule. There was no detection of glucose and fructose after 12 h, suggesting the efficiency of sucrose hydrolysis and the simultaneous consumption of glucose and fructose by the microorganism. The preferred sources of carbon used by L. reuteri are maltose, sucrose and raffinose, with the use of pentoses specific to each strain (Zhao and Gänzle 2018).
The strain LR 92 showed hydrolysis of the sucrose molecule in fructose and glucose, but the bacteria did not consume fructose in the same way as DSM 17938. It was possible to observe that there was fructose accumulation in the fermentative matrix, reaching 0.28 ± 0.09 g/L after 48 h. In a study conducted by Pallin et al. (2016), L. reuteri did not consume fructose in medium containing only this sugar as a carbon source. However, the microorganism used fructose as an electron acceptor in the medium containing glucose.
Organic acids profile
Figure 3 shows the production of organic acids during the fermentation of L. reuteri DSM 17938 (c) and LR 92 (d). There is a natural occurrence of malic and citric acids in the mature coconut pulp, therefore they are detected at the initial time of coconut milk fermentation (Santoso et al. 1996).
The graphs show that there was malic acid consumption by the two strains, until total depletion between 10 and 12 h, indicating the occurrence of malolactic fermentation. In this fermentation performed by lactic acid bacteria, there is metabolic deviation with decarboxylation of malic acid in lactic acid, increasing cell growth in media containing malic acid as the carbon source. It has already been shown that Lactobacillus plantarum CECT 220 was able to degrade malic acid as a protection system at low pH, with energy production (ATP) resulting from this process (Garcia et al. 1992).
The coconut milk fermented by LR 92 showed synthesis of lactic, acetic and succinic acids below 1.2 g/L. However, coconut milk fermented by DSM 17938, showed higher production of lactic acid (5.45 ± 0.33 g/L) and acetic acid in a lower proportion (0.56 ± 0.08 g/L) after 48 h, and the succinic acid production was not detected. Similarly, Pallin et al. (2016) found lower concentrations of succinic acid in barley fermented by L. reuteri DSM 17938. In addition, the authors detected higher concentrations of lactic acid compared to the other strains of L. reuteri analyzed.
The synthesis of lactic acid by DSM 17938 reached a maximum concentration 4.7 times higher than by LR 92 at the end of 48 h of fermentation. Årsköld et al. (2008) found the presence of genes for both the homofermentative and heterofermentative pathways during the fermentation of L. reuteri ATCC 55730, with high production of lactic acid. The strain of the food supplement Provance (DSM 17938) is derived from the strain ATCC 55730, differing only in two plasmids containing antibiotic resistant genes (Rosander et al. 2008). Therefore, it is possible to explain the higher production of lactic acid by DSM 17938 in coconut milk due to the possible existence of the glycolytic pathway of homofermentative bacteria, which may have been used by the microorganism.
The concentration of citric acid naturally present in coconut milk remained stable and did not differ significantly during fermentation for LR 92 (p < 0.05). However, the initial citric acid concentration (0.16 ± 0.01 g/L) reduced to zero during 48 h fermentation for DSM 17938. It has been described that L. reuteri SD 2112 was able to metabolize citrate using the heterofermentative pathway, resulting in the production of carbon dioxide and acetic acid, which may explain the absence of citric acid in the matrix (Østlie et al. 2003).
Stability during refrigerated storage
The characteristics of the fermented coconut milk during storage for 30 days at 4 °C are presented in Table 2. The fermentation time was 12 h at 34 °C for DSM 17938 and 14 h at 37 °C for LR 92, until pH 4.5 ± 0.1 was reached.
Table 2.
pH, titratable acidity and viability of L. reuteri DSM 17938 and LR 92 in fermented coconut milk during 0, 1, 15 and 30 days of storage at 4 °C
| Time (days) | L.reuteri DSM 17938 | L. reuteri LR 92 | ||||
|---|---|---|---|---|---|---|
| Viability (log CFU/mL) | pH | Acidity (lactic acid g/100 mL) | Viability (log CFU/mL) | pH | Acidity (lactic acid g/100 mL) | |
| 0 | 8.64 ± 0.02b | 4.53 ± 0.06ª | 0.15 ± 0.01ª | 8.04 ± 0.07a | 4.59 ± 0.05ª | 0.13 ± 0.01ª |
| 1 | 8.91 ± 0.15a | 4.05 ± 0.01b | 0.18 ± 0.02ª | 8.10 ± 0.02a | 4.58 ± 0.01ª | 0.13 ± 0.02ª |
| 15 | 8.70 ± 0.07abc | 3.91 ± 0.02c | 0.22 ± 0.02b | 7.61 ± 0.06b | 4.44 ± 0.01b | 0.13 ± 0.02ª |
| 30 | 8.57 ± 0.09bc | 3.60 ± 0.01d | 0.46 ± 0.04c | 7.55 ± 0.07b | 4.33 ± 0.03c | 0.16 ± 0.01ª |
Means followed by the same lowercase letters in a column do not differ significantly by the Tukey test (p < 0.05)
L. reuteri DSM 17938 showed a significant increase of the viable cells in only 1 day after the end of fermentation, with consequent decrease of the pH. This characteristic denotes a growth capacity in refrigeration temperature and post acidification of this strain. However, a cell count reduction was observed after 15 days of storage.
Differently from DSM 17938, the LR 92 showed low post acidification and stable pH between days 0 and 1. Viable cells presented a decrease after 15 days and remained stable until the end of the 30 days of storage. The results found in this work after 30 days of storage show that there was a 0.8% reduction in the viability of L. reuteri DSM 17938 and a 6.1% reduction in the viability of L. reuteri LR 92.
Although information on minimum effective concentrations of probiotics is not established, it is generally accepted that probiotic products should have a minimum concentration of 106 CFU/mL or gram and that a total 109 CFU of probiotics should be present in the portion of the product at the moment of consumption (Kechagia et al. 2013; Hill et al. 2014). Therefore, the viability values of 8.57 ± 0.09 and 7.55 ± 0.07 log CFU/mL for L. reuteri DSM 17938 and LR 92, respectively, are in agreement with the cited parameters.
Langa et al. (2013) observed that different strains of L. reuteri did not multiply in yogurt during storage at 6 °C and had a reduction in the viability at the end of 28 days of storage (5.88–3.02 log CFU/mL) due to sensitivity to the storage temperature, which could accelerate the cell death process. In the storage of fermented oat milk, the viability of L. reuteri ATCC 55730 was 7.43 ± 0.06 log CFU/mL after 28 days at 4 °C (Bernat et al. 2015a). These viability results are below the values found in the fermented coconut milk.
The initial titratable acidity of fermented coconut milk was 0.15 ± 0.01 g/100 mL of lactic acid for DSM 17938 (12 h of fermentation), and 0.46 ± 0.04 g/100 mL at the end of 30 days. For LR 92 (14 h of fermentation), the initial value was 0.13 ± 0.01 g/100 mL and final, 0.16 ± 0.01 g/100 mL. In this way, a higher post acidification of DSM 17938 was observed. Very similar results were found by Bernat et al. (2015a) in oat milk fermented by L. reuteri ATCC 55730 and S. thermophilus CECT 986, in which the titratable acidity found was 0.17 g/100 mL in the product (pH 4.4–4.6) and increased to reach 0.5 ± 0.04 g/100 mL after 28 days of storage.
The titratable acidity, although pronounced in L. reuteri DSM 17938 resulting from post acidification during storage, is below the values reported for fermented milk. The results showed low acidifying characteristic of Lactobacillus reuteri in coconut milk, since the acidity for fermented milk products ranges from 0.7 to 0.9% of lactic acid (FDA 1982).
Santos et al. (2014) observed that pure cultures of lactic acid bacteria were not able to promote acidification similar to milk in peanut and soybean vegetable milks, reaching values below 0.2% (w/w) of acid lactic. These authors observed that in the combined cultivation of different strains, the titratable acidity increased up to 0.48% (w/w) after 24 h of fermentation.
Reuterin content
After addition of glycerol under anaerobic conditions, L. reuteri DSM 17938 produced 0.15 ± 0.03 mM of reuterin when the pH reached 4.5 ± 0.1 (12 h). Similarly, L. reuteri LR 92 produced 0.14 ± 0.04 mM of reuterin in the same pH range (14 h), not significantly differing from the other strain (p < 0.05).
Spinler et al. (2014) analyzed L. reuteri DSM 17938 reuterin production in MRS broth, in which it reached a concentration of 9 mM during the log phase (4 h) to approximately 140 mM during the stationary phase (12 h). In antimicrobial tests, the lowest concentration of reuterin capable of inhibit Escherichia coli DH5α in fermented milk was 0.9 mM (Ortiz-Rivera et al. 2017). These concentrations are much higher than those found in the present study, suggesting that coconut milk 1:3 (w/v) did not constitute a favorable matrix for reuterin prodution. This fact can be explained by the absence of carbohydrate supplementation in coconut milk, since the presence of glycerol in the culture medium with more usable sources of carbon and energy results in a higher growth rate of L. reuteri and reuterin production (Talarico et al. 1990).
Conclusion
The results showed that coconut milk provided nutrients for the fermentation of L. reuteri DSM 17938 and LR 92 without the need for supplementation. The viable cell counts after storage at 4 °C for 30 days was 8.57 ± 0.09 and 7.55 ± 0.07 log CFU/mL for L. reuteri DSM 17938 and LR 92, respectively, a suitable cell viability to exert beneficial effects to the consumers. The strain DSM 17938 presented better performance in the fermentation process and higher production of lactic acid, but showed a great decrease of pH and acidity increase (post acidification) until the end of the storage period. The strain LR 92 presented low post acidification when submitted to the same storage conditions. Therefore, it was possible to observe that L. reuteri LR 92 was the most suitable strain for the development of functional products using coconut milk.
Acknowledgements
This research was developed with the support of the State University of Londrina, which provided the infrastructure and facilities. This study was supported by a scholarship from the Coordination for the Improvement of Higher Education Personnel (CAPES).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Årsköld E, Lohmeier-Vogel E, Cao R, Roos S, Rådstrom P, Van Niel EWJ. Phosphoketolase pathway dominates in Lactobacillus reuteri ATCC 55730 containing dual pathways for glycolysis. J Bacteriol. 2008;190:206–212. doi: 10.1128/JB.01227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros Neto BB, Scarminio IS, Bruns RE. Como Fazer Experimentos: Pesquisa e desenvolvimento na ciência e na indústria. 4ª. Porto Alegre: Bookman, Campinas; 2010. [Google Scholar]
- Bernat N, Cháfer M, González-Martínez C, Rodríguez-Garcia J, Chiralt A. Optimisation of oat milk formulation to obtain fermented derivatives by using probiotic Lactobacillus reuteri microorganisms. Food Sci Technol Int. 2015;21:145–157. doi: 10.1177/1082013213518936. [DOI] [PubMed] [Google Scholar]
- Bernat N, Cháfer M, Chiralt A, González-Martínez C. Probiotic fermented almond “milk ” as an alternative to cow-milk yoghurt. Int J Food Stud. 2015;4:201–211. [Google Scholar]
- Dinleyici EC, Dalgic N, Guven S, Metin O, Yasa O, Kurugol Z, Turel O, Tanir G, Yazar AS, Arica V, Sancar M, Karbuz A, Eren M, Ozen M, Kara A, Vandenplas Y. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. J Pediatr (Rio J) 2015;91:392–396. doi: 10.1016/j.jped.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Fatheree NY, Liu Y, Taylor CM, Hoang TK, Cai C, Rahbar MH, HessabI M, Ferris M, McMurthy V, Wong C, Vu T, Dancsak T, Wang T, Gleason W, Bandla V, Navarro F, Tran DQ, Rhoads JM. Lactobacillus reuteri for infants with colic: a double-blind, placebo-controlled, randomized clinical trial. J Pediatr. 2017;191:170–178.e2. doi: 10.1016/j.jpeds.2017.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations, World Health Organization (FAO/WHO) (2001) Evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Córdoba, Argentina
- Food and Drug Administration (FDA) (1982) Cultured and acidified milks, cultured and acidified buttermilks, yogurts, and eggnog; confirmation of effective date and further amendments; and stay of effective date of certain provisions. Fed Regist 74(183):41522
- Garcia MJ, Zuniga M, Kobayashi H. Energy production from L-malic acid degradation and protection against acidic external pH in Lactobacillus plantarum CECT 220. J Gen Microbiol. 1992;138:2519–2524. doi: 10.1099/00221287-138-12-2519. [DOI] [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- IFT (2018) U.S. non-dairy milk sales surpass $2 billion in 2017. http://www.ift.org/Food-Technology/Daily-News/2018/January/05/us-non-dairy-milk-sales-surpass-$2-billion-in-2017.aspx. Accessed 11 Feb 2018
- Jeske S, Zannini E, Arendt EK. Past, present and future: the strength of plant-based dairy substitutes based on gluten-free raw materials. Food Res Int. 2018;110:42–51. doi: 10.1016/j.foodres.2017.03.045. [DOI] [PubMed] [Google Scholar]
- Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N, Fakiri EM. Health benefits of probiotics: a review. ISRN Nutr. 2013;2013:1–7. doi: 10.5402/2013/481651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft-Bodi E, Jørgensen MR, Keller MK, Kragelund C, Twetman S. Effect of probiotic bacteria on oral candida in frail elderly. J Dent Res. 2015;94:181S–186S. doi: 10.1177/0022034515595950. [DOI] [PubMed] [Google Scholar]
- Langa S, Landete JM, Martín-Cabrejas I, Rodríguez E, Arqués JL, Medina M. In situ reuterin production by Lactobacillus reuteri in dairy products. Food Control. 2013;33:200–206. doi: 10.1016/j.foodcont.2013.02.035. [DOI] [Google Scholar]
- Liu XT, Hou CL, Zhang J, et al. Fermentation conditions influence the fatty acid composition of the membranes of Lactobacillus reuteri I5007 and its survival following freeze-drying. Lett Appl Microbiol. 2014;59:398–403. doi: 10.1111/lam.12292. [DOI] [PubMed] [Google Scholar]
- Mäkinen OE, Uniacke-Lowe T, O’Mahony JA, Arendt EK. Physicochemical and acid gelation properties of commercial UHT-treated plant-based milk substitutes and lactose free bovine milk. Food Chem. 2015;168:630–638. doi: 10.1016/j.foodchem.2014.07.036. [DOI] [PubMed] [Google Scholar]
- Mauro CSI, Guergoletto KB, Garcia S. Development of blueberry and carrot juice blend fermented by Lactobacillus reuteri LR92. Beverages. 2016;2:37. doi: 10.3390/beverages2040037. [DOI] [Google Scholar]
- McCarthy KS, Parker M, Ameerally A, Drake SL, Drake MA. Drivers of choice for fluid milk versus plant-based alternatives: what are consumer perceptions of fluid milk? J Dairy Sci. 2017;100:6125–6138. doi: 10.3168/jds.2016-12519. [DOI] [PubMed] [Google Scholar]
- Moraes Filho ML, Busanello M, Garcia S. Optimization of the fermentation parameters for the growth of Lactobacillus in soymilk with okara flour. LWT: Food Sci Technol. 2016;74:456–464. doi: 10.1016/j.lwt.2016.08.009. [DOI] [Google Scholar]
- Ortiz-Rivera Y, Sánchez-Vega R, Gutiérrez-Méndez N, León-Félix J, Acosta-Muñiz C, Sepulveda DR. Production of reuterin in a fermented milk product by Lactobacillus reuteri: inhibition of pathogens, spoilage microorganisms, and lactic acid bacteria. J Dairy Sci. 2017;100:4258–4268. doi: 10.3168/jds.2016-11534. [DOI] [PubMed] [Google Scholar]
- Østlie HM, Helland MH, Narvhus JA. Growth and metabolism of selected strains of probiotic bacteria in milk. Int J Food Microbiol. 2003;87(1–2):17–27. doi: 10.1016/S0168-1605(03)00044-8. [DOI] [PubMed] [Google Scholar]
- Pallin A, Agback P, Jonsson H, Roos S. Evaluation of growth, metabolism and production of potentially bioactive components during fermentation of barley with Lactobacillus reuteri. Food Microbiol. 2016;57:159–171. doi: 10.1016/j.fm.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Pauli ED, Cristiano V, Nixdorf SL. Método para determinação de carboidratos empregado na triagem de adulterações do café. Quím Nova. 2011;34(4):689–694. doi: 10.1590/S0100-40422011000400023. [DOI] [Google Scholar]
- Rosander A, Connolly E, Roos S. Removal of antibiotic resistance gene-carrying plasmids from Lactobacillus reuteri ATCC 55730 and characterization of the resulting daughter strain, L. reuteri DSM 17938. Appl Environ Microbiol. 2008;74:6032–6040. doi: 10.1128/AEM.00991-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CCA do A, Libeck B da S, Schwan RF (2014) Co-culture fermentation of peanut-soy milk for the development of a novel functional beverage. Int J Food Microbiol 186:32–41. 10.1016/j.ijfoodmicro.2014.06.011 [DOI] [PubMed]
- Santoso U, Kubo K, Ota T, Tadokoro T, Maekawa A. Nutrient composition of kopyor coconuts (Cocos nucifera L.) Food Chem. 1996;57:299–304. doi: 10.1016/0308-8146(95)00237-5. [DOI] [Google Scholar]
- Seow CC, Gwee CN. Review coconut milk: chemistry and technology. Int J Food Sci Technol. 1997;32:189–201. doi: 10.1046/j.1365-2621.1997.00400.x. [DOI] [Google Scholar]
- Sethi S, Tyagi SK, Anurag RK. Plant-based milk alternatives an emerging segment of functional beverages: a review. J Food Sci Technol. 2016;53:3408–3423. doi: 10.1007/s13197-016-2328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriphanich J, Saradhuldhat P, Romphophak T, Krisanapook K, Pathaveerat S, Tongchitpakdee S. Coconut (Cocos nucifera L) Cambridge: Woodhead Publishing Limited; 2011. [Google Scholar]
- Spinler JK, Sontakke A, Hollister EB, Venable SF, Oh PL, Balderas MA, Saulnier DMA, Mistretta T, Deveraj S, Walter J, Versalovic J. From prediction to function using evolutionary genomics: human-specific ecotypes of Lactobacillus reuteri have diverse probiotic functions. Genome Biol Evol. 2014;6:1772–1789. doi: 10.1093/gbe/evu137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico TL, Axelsson LT, Novotny J, Fiuzat M, Dobrogosz WJ. Utilization of glycerol as a hydrogen acceptor by Lactobacillus reuteri: purification of 1,3-propanediol:NAD + oxidoreductase. Appl Environ Microbiol. 1990;56:943–948. doi: 10.1128/aem.56.4.943-948.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobajas M, Mohedano AF, Casas JA, Rodríguez JJ. A kinetic study of reuterin production by Lactobacillus reuteri PRO 137 in resting cells. Biochem Eng J. 2007;35:218–225. doi: 10.1016/j.bej.2007.01.017. [DOI] [Google Scholar]
- Yildiz F. Development and manufacture of yogurt and other functional dairy products. 1. Boca Raton: CRC Press; 2009. [Google Scholar]
- Zannini E, Jeske S, Lynch K, Arendt EK. Development of novel quinoa-based yoghurt fermented with dextran producer Weissella cibaria MG1. Int J Food Microbiol. 2018;268:19–26. doi: 10.1016/j.ijfoodmicro.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Zhao X, Gänzle MG. Genetic and phenotypic analysis of carbohydrate metabolism and transport in Lactobacillus reuteri. Int J Food Microbiol. 2018;272:12–21. doi: 10.1016/j.ijfoodmicro.2018.02.021. [DOI] [PubMed] [Google Scholar]