Abstract
The presence of bioactive peptides has already been reported in many foods such as milk, fermented products, plant and marine proteins. Bioactive peptides are sequences between 2 and 20 amino acids that can inhibit chronic diseases by modulating and improving physiological functions, so these peptides contribute in holding the consumer health. Also, bioactive peptides can affect pro-health or functional properties of food products. Fractionation of the protein hydrolysate revealed a direct relationship between their structure and functional activity. So, this review focuses on different factors effecting on bioactive peptide structures, biological and functional properties such as antihypertensive, antioxidative, hypocholesterolemic, water-holding capacity, foaming capacity, emulsifying properties and solubility. Also, this review looks at the identified bioactive peptides from food protein sources as potential ingredients of health promoting functional foods.
Keywords: Bioactive peptides, Food protein hydrolysis, Functional properties, Human health, Peptides structure
Introduction
In recent years the number of studies on novel functional ingredients with various food sources especially phenolic compounds, bioactive peptides, etc. increased considerably (Bhat et al. 2015; Gu et al. 2015; Irshad et al. 2015; Karami et al. 2015). Particularly, there is more attention on bioactive peptides due to their nutritional capabilities and health effects. Bioactive peptides are inactive within the sequences of their parent protein, but can be released by enzymatic, chemical and microbial hydrolysis (Di Bernardini et al. 2011; Kim and Wijesekara 2010). By far, the most effective and dependable method to produce peptides with the intended functionalities is enzymatic digestion (Kim and Wijesekara 2010). Bioactive peptides usually contain 2–20 amino acid residues per molecule, but in some cases may consist of more than 20 amino acids, and molecular masses of less than 6000 Da (Sarmadi and Ismail 2010). After digestion, bioactive peptides can be absorbed in intestine and enter the blood stream directly, which ensures their bioavailability in vivo and a physiological effect at the target site (Erdmann et al. 2008). Biologically active peptides have a very important contribution in metabolic regulation and modulation. These peptides can be used as functional food ingredients, or nutraceuticals and pharmaceuticals to improve human health and prevent disease. Some of the reported activities include: anti-hypertension, opioid agonists or antagonists, immunomodulatory, antithrombotic, antioxidant, anti-cancer, and antimicrobial activities, in addition to nutrient utilization (Elias et al. 2008). The replacement of synthetic antioxidants with natural antioxidants, synthetic ACE inhibitors with natural ACE inhibitor or synthetic antimicrobial with natural antimicrobial could have beneficial effects in terms of health implications and functionality in food systems such as increasing the solubility of emulsions containing oil and water.
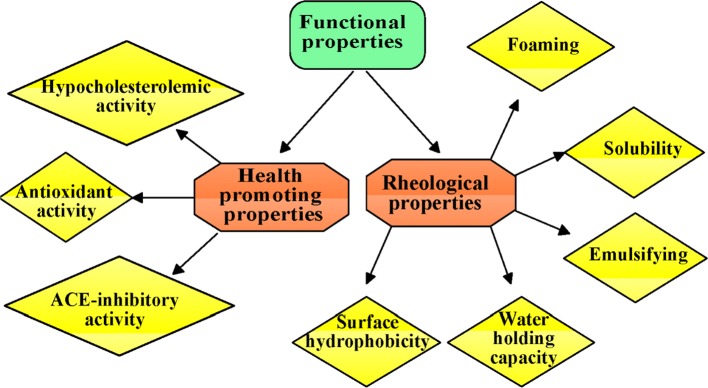
The functionality of bioactive peptides can be directly affected by various factors such as process conditions, protein sources, sequence and amino acid composition, molecular weight and charge distribution, pH and certain chemical treatments (de Castro and Sato 2015). Thus, this paper provides an overview from structural aspects on various factors governing functional properties of bioactive peptides (Fig. 1).
Fig. 1.
Functional properties of bioactive peptides and hydrolysates
Correlation between peptides structure and functional properties
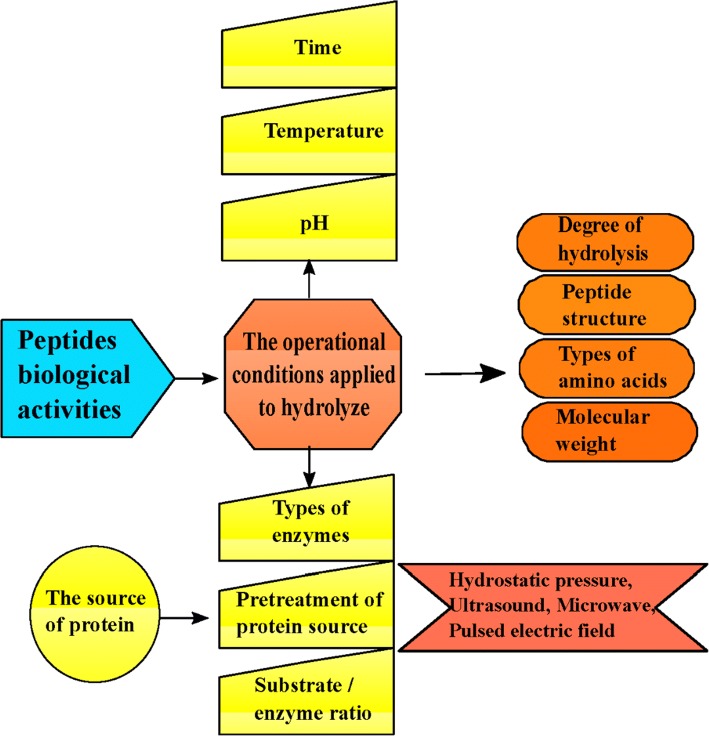
Numerous studies have been exhibited that food-derived bioactive peptides based on their structural properties and their amino acid composition and sequences, possess several physiological functions including antioxidant, antimicrobial, angiotensin-I-converting enzyme (ACE) inhibition activities and when added to food, they can cause good functional properties and can contribute to water holding, texture, gelling, whipping and emulsification properties (Liu et al. 2008). Various factors such as the source of proteins, the protein substrate pretreatment, the type of peptidases, and the hydrolysis conditions, could modify the structure and efficacy of protein hydrolysates and types of peptides produced (Ambigaipalan et al. 2015; Xiong et al. 2013) (Fig. 2). It is obvious that the nature of residues in a peptide affect its activity.
Fig. 2.
Key factors influencing the peptides biological activities
Structural factors governing health promoting properties
Relation between peptides structure and the antioxidant activity
It has been shown that hydrolysates exert higher antioxidant activity than purified peptides (Xiong et al. 2013). Many researches have been conducted to evaluate antioxidant properties of bioactive peptides from plant or animal sources (Girgih et al. 2014). The antioxidant or biological activities can be affected by the peptide structure (Saito et al. 2003) that it can affect by the operational conditions applied to isolate proteins, the source of protein, degree of hydrolysis, type of protease (Wang et al. 2010), molecular weight (MW) of peptides (Li et al. 2008), conditions of the hydrolysis process involved (e.g. peptidases, substrate/peptidases ratio, temperature, pH, reaction time etc.) (Shahidi and Zhong 2008). Ambigaipalan et al. (2015) suggested that the type of peptidases used for hydrolysis affects the antioxidant properties; they claimed their efficacy differences are due to endo versus exo-peptidase. It has been shown that the pretreatment of proteins before enzymatic hydrolysis such as heat (HT), hydrostatic pressure (HP), ultrasound (US), microwave (MW) and pulsed electric field (PEF) treatments improved the release of bioactive peptides from various proteins and functionality of peptides (Uluko et al. 2015). Uluko et al. (2015) revealed that pretreatment using ultrasound has the potential to improve antioxidant capacity of aggregated proteins such as milk protein concentrate during enzymatic hydrolysis with digestive enzymes. Particular structural features of the peptides have been asserted to influence antioxidant capacity. For instance, it has been demonstrated that certain amino acids can display higher antioxidative properties when they are incorporated in dipeptides structure (Nagasawa et al. 2001). In contrast, other findings suggested that its structural conformation can reduce the antioxidant activity of the constituent amino acids. In fact, it is stated that peptide conformation is capable of showing both synergistic and antagonistic effects, as far as the antioxidant activity of free amino acids is concerned (Hernández-Ledesma et al. 2005). Chen et al. (1995) stated that substitution of l-Histidine by d-Histidine in a peptide can reduce the antioxidant activity. They reported that the correct positioning of imidazole group is the key factor influencing the antioxidant activity. It has been reported that the antioxidant activity of corn gluten meal hydrolysates was related to the concentration and molecular weight of hydrolysates. Antioxidant activity of peptides with molecular weight 500–1500 Da was stronger than that of peptides above 1500 Da and peptides below 500 Da (Li et al. 2008). Moreover, other factors can influence antioxidant activity of bioactive peptides; it has been stated that the antioxidant activity of a peptide depends on which amino acids are in the sequence and also on the position that they occupy in the sequence. For example, tri-peptides with tryptophan and tyrosine at their C-terminus showed strong radical-scavenging activity, and different combinations of amino acids in tri-peptide chains also showed different antioxidant activities (Cumby et al. 2008). Saito et al. (2003) have reported that any change in the arrangements of amino acid sequence in tripeptides resulted in different antioxidant activities. Mendis et al. (2005) indicated that hydrophobic amino acids present in the peptides structure affect antioxidant activity of a peptide in inhibiting lipid peroxidation, they reported that hydrophobicity property leads to high interactions between the peptide and the fatty acids, concluding in protection against oxidation. Amino acids of tyrosine, tryptophan, methionine, lysine, cysteine, and histidine can cause antioxidant activity (Mundi and Aluko 2014). Antioxidative activity of Histidine -containing peptides has attributed to the hydrogen-donating, lipid peroxyl radical trapping and/or the metal ion-chelating ability of the imidazole group (Rajapakse et al. 2005). SH group in cysteine due to its direct interaction with radicals has an independently crucial antioxidant action (Qian et al. 2008). Amino acids with aromatic residues improve the radical-scavenging properties of the peptides because they can donate protons to electron deficient radicals (Rajapakse et al. 2005). Mundi and Aluko (2014) showed the higher DPPH inhibitory activity for < 1 and 5–10 kDa peptide fractions due to higher contents of hydrophobic aliphatic (valine, isoleucine and leucine) and hydrophobic aromatic (phenylanaline and tyrosine) amino acid residues in the two fractions, when compared to the 1–3 and 3–5 kDa fractions.
It has reported that hydrolysates categorized into three main groups based on their degree of hydrolysis (DH) that determine their application: 1. hydrolysates with broad DH (mostly used as nutritional supplements and in special medical diets), 2. hydrolysates with various DHs (generally used as flavorings), 3. hydrolysates with low DH and improved functional features (Vioque et al. 2001).
Type of peptidase, as previously mentioned, affect functional properties of peptides. Cumby et al. (2008) suggested canola sample hydrolyzed by Alcalase had similar degrees of hydrolysis (20.6% and 18.9%, respectively) with that hydrolyzed by a combination of Alcalase and Flavourzyme while the hydrolysate prepared with Flavourzyme alone had the lowest degree of hydrolysis (6.33%). This was unexpected as the combination of Alcalase and Flavourzyme was thought to produce a more extensively hydrolyzed product than using one peptidases alone. They reported Flavourzyme hydrolysates of canola protein with a lower DH exhibited higher antioxidant efficiency than hydrolysates produced using Alcalase. Also, they indicated scavenging capacity increased with increasing concentration. The hydrolysates prepared using Flavourzyme had the highest scavenging activity at all concentrations, while similar values were obtained for hydrolysates prepared using Alcalase or the combination of the two peptidases. Table 1 indicates some antioxidant peptides derived from food sources. Ambigaipalan et al. (2015) indicated that hydrolysis condition employed to produce peptides potentially affect antioxidant activities of date’s seed protein hydrolysates. Raikos and Dassios (2014) findings indicated that peptides with sequences of tyrosine–glycine–tyrosine–threonine–glycine–alanine and isoleucine–serine–glutamate–leucine–glycine–tryptophan released from human milk after digestion with pepsin and pancreatin could be exhibited high radical scavenging activity, and this property was related to the occurrence of the amino acid tryptophan which can break radical chain reactions thanks to the donation of the hydrogen attached to the nitrogen of its indole ring.
Table 1.
Food derived bioactive peptides with antioxidant activity
| Protein | Process | Bioactive peptide | References |
|---|---|---|---|
| Royal jelly protein | Protease N | Ala-Leu, Phe-Lys, Phe-Arg, Ile-Arg, Lys-Phe, Lys-Leu, Lys-Tyr, Arg-Tyr, Tyr-Asp,Tyr-Tyr, Leu-Asp-Arg, Lys-Asn-Tyr-Pro | Guo et al. (2009) |
| Rice | Alcalase | Thr-Gln-Val-Tyr | Li et al. (2007) |
| Palmaria palmata protein | Corolase PP | Ser-Asp-Ile-Thr-Arg-Pro-Gly-Gly-Asn-Met | Harnedy et al. (2017) |
| Wheat germ | Bacillus licheniformis alkaline protease | Ile-Val-Tyr | Matsui et al. (1999) |
| Soy (β-conglycinin) | protease | Leu-Leu-Pro-His-His | Chen et al. (1995) |
| Egg (egg white) | Pepsin- | Tyr-Ala-Glu-Glu-Arg-Tyr-Pro-Ile-Leu | Davalos et al. (2004) |
| Corn gluten meal | Alkaline protease and Flavourzyme | Leu-Pro-Phe, Leu-Leu-Pro-Phe, Phe-Leu-Pro-Phe | Zhuang et al. (2013) |
| Hemp seed protein | Pepsin | Trp-Val-Tyr-Tyr, Pro-Ser-Leu-Pro-Ala | Girgih et al. (2014) |
| Sweet potato protein green tender | Alcalase | Tyr-Tyr-Ile-Val-Ser | Zhang et al. (2014) |
| Sorghum | Alcalase | Leu-Asp-Ser-Cys-Lys-Asp-Tyr-Val-Met-Glu | Agrawal et al. (2017) |
| Sea squirt (Halocynthia roretzi) protein | Pepsin | Leu-Glu-Trp, Met-Thr-Thr-Leu, and Tyr-Tyr-Pro-Tyr-Gln-Leu | Kim et al. (2018) |
| Wheat germ protein | Alcalase | Gly-Asn-Pro-Ile-Pro-Arg-Glu-Pro-Gly-Gln-Val-Pro-Ala-Tyr | Karami et al. (2018) |
Chen et al. (1995) reported that the antioxidant properties of the isolated peptides from soy varied depending on their structure and the assay systems used because investigation of the inhibition of linoleic acid peroxidation assay showed peptides containing two tyrosine residues had higher antioxidant activity than the corresponding peptides containing two His residues, and tyrosine–(histidine–leucine–arginine)–tyrosine exhibited the highest activity (Saito et al. 2003). Also amino acids, such as tryptophan, tyrosine, methionine, lysine, histidine–tyrosine–proline, proline, alanine and cysteine, occurred in the sequence of antioxidant peptides are supposed to be responsible for the reported antioxidant activity (Cumby et al. 2008). The antioxidant activity of the two aromatic amino acids tryptophan and phenylalanine has been related to their capacity to act as radical scavengers, and the antioxidant activity of tyrosine is attributed to the special capability of phenolic groups to act as hydrogen donors (Ren et al. 2008). Six antioxidant peptides from the proteolytic hydrolysis of soybean protein have been detected by Chen et al. (1995). They discovered that the active peptides included 5–16 amino acid residues with hydrophobic valine or leucine amino acids at the N-terminal position and proline, histidine or tyrosine in the sequence. Upon comparing the antioxidant capacity of 28 short-chain peptides structurally attributed to leucine–leucine–proline–histidine–histidine, the tripeptide unit proline–histidine–histidine was found to be an active center responsible for the antioxidant activity of soy protein hydrolysate (Shahidi and Zhong 2010). It was hypothesized that histidine-containing peptides can act as metal chelator, active oxygen quencher and hydroxyl radical scavenger, so it affects the antioxidant activity of the protein hydrolysate (Chen et al. 1995).
Relation between peptides structure and the ACE-inhibitory activity of peptides
Angiotensin I-converting enzyme (ACE) plays a crucial role in the regulation of blood pressure as it promotes the conversion of angiotensin I to the potent vasoconstrictor angiotensin II as well as inactivates the vasodilator bradykinin. ACE-inhibitory substances are used to decrease the blood pressure of hypertensive patients (Rho et al. 2009). By inhibiting these processes, synthetic ACE inhibitors such as captopril, enalapril, alacepril, lisinopril and ramipril have been widely used for the effective clinical treatment of hypertension and heart failure in humans (Boye et al. 2010). These synthetic drugs, however, have several side effects including diarrhea, coughing, allergies, taste disturbances, skin rashes, impaired renal function, and in some cases excessively low blood pressure, i.e. hypotension (Tenenbaum et al. 2000). Therefore, search for natural ACE inhibitors as alternatives to synthetic ones is one of the great interests for safe and economical using them as pharmaceuticals. Although the effectiveness of the ACE-inhibitory activity may not be as high as those of synthetic drugs, many natural ACE-inhibitory peptides isolated from different food proteins could be applied in the prevention of hypertension and in the initial treatment of mildly hypertensive individuals (Li et al. 2008).
Cheung et al. (1980) indicated that the ACE inhibitor activity by peptides depended on the affinity of N- or C- terminal amino acid residues for the ACE active site. From this point of view, Matsui et al. (1999) have separated 12 ACE inhibitor peptides from sardine muscle hydrolysate, and displayed that the di-peptide, valine- tyrosine with the IC50 value of 5.2 mM, acted as a key ACE inhibitor. It has been reported that most of the ACE inhibitor peptides are short structure peptides with only two to nine amino acids. It is known that di- or tri peptides, especially those with C-terminal proline or hydroxyl proline residues, are generally resistant to degradation by digestive enzymes (Vermeirssen et al. 2004). Moreover, short peptides consisting of two or three amino acids are absorbed more rapidly than free amino acids (Erdmann et al. 2008). Also, findings in spontaneously hypertensive rats (SHR) showed that di-peptides with a C-terminal tyrosine residue caused a slow and prolonged decrease in systolic blood pressure compared to di-peptides with phenylalanine at the C-terminal. In contrast, di-peptides with C-terminal phenylalanine produced a more rapid reduction and a shorter duration of action (Erdmann et al. 2008).
Mundi and Aluko (2014) suggested that peptide fractions with molecular masses less than 1 kDa and 5–10 kDa have higher renin-inhibitory activities that it may be attributed to the increased levels of hydrophobic (valine, Isoleucine and leucine) and aromatic (phenylalanine and tryptophan) amino acids when compared to the 1–3 and 3–5 kDa peptide fractions. It is also well known that smaller size peptides may be more bioactive because of the higher probability for increased rate of intestinal absorption and entry into cells when compared with the bigger size peptides. Udenigwe et al. (2012) stated that nature and position of the amino acid play a major role in enhancing renin inhibition rather than size of peptides. Mundi and Aluko (2014) also reported the peptide fractions of Kidney Bean Protein Hydrolysate (KBH) have higher ACE-inhibitory activity than mung bean hydrolysate prepared with Neutrase. They concluded that the differences in these findings may be attributed by differences in the peptidase type, the concentration of the sample used, as well as the peptidase-to-substrate ratio, all of which could affect the type of peptides produced. Moreover, Matsui and Tanaka (2010) reported that peptides with antioxidant and ACE-inhibitory activities are usually rich in hydrophobic amino acids, which enhance absorption and interaction with target enzymes or free radicals. Milk derived bioactive peptides play important roles in human health and nutrition (Sánchez and Vázquez 2017). Some of these peptides have several functional properties, for instance peptides from the sequence 60–70 of β-casein exhibit ACE-inhibitory activities. This sequence is maintained from proteolysis due to its high hydrophobicity and the presence of proline residues (Sharma et al. 2011). Muguerza et al. (2006) have isolated ACE inhibitory peptides (e.g., valine–arginine–tyrosine–leucine) from the enzymatic hydrolysis of milk proteins. Recently three new ACE inhibitory peptides with IC50 values ranging from 316 to 354 µmol/L have been determined from goat milk hydrolysate and also exhibited antihypertensive effect in spontaneously hypertensive rats (Erdmann et al. 2008).
Raikos and Dassios (2014) revealed a potent ACE inhibitor in human milk and corresponded to the β-casein fragment f (125–129) with the sequence histidine–leucine–proline–leucine–proline. In this case, it was supposed that the presence of the amino acid proline at the C-terminal end could be crucial in terms of the activity observed (Raikos and Dassios 2014). Table 2 indicates some anti-hypertensive peptides derived from food sources.
Table 2.
Food derived bioactive peptides with anti-hypertensive activity
| Protein | Process | Bioactive peptide | References |
|---|---|---|---|
| Wheat (gliadin) | Acid protease | Leu-Ala-Pro | Motoi and Kodama (2003) |
| Fermented soybean | Enzymatic hydrolysis | Leu-Val-Gln-Gly-Ser | Rho et al. (2009) |
| αS1-Casein | pepsin | Arg-Tyr-Leu-Gly-Tyr and Ala-Tyr-Phe-Tyr-Pro-Glu-Leu | Hernández-Ledesma et al. (2013) |
| Whey | Trypsin | Tyr-Leu in N terminus | Sharma et al. (2011) |
| Wakame | Pepsin | Tyr-Asn-Lys-Leu | Suetsuna and Nakano (2000) |
| Hemp seed protein | Pepsin | Trp-Val-Tyr-Tyr and Pro-Ser-Leu-Pro-Ala | Girgih et al. (2014) |
| Rapeseed protein | Alcalase | Leu-Tyr and Arg-Ala-Leu-Pro | He et al. (2013) |
| Peach seed protein | Thermolysin | Leu-Tyr-Ser-Pro-His, Leu-Tyr-Thr-Pro-His and His-Leu-Leu-Pro | Vásquez-Villanueva et al. (2015) |
| Walnut protein | Pepsin | Tyr-Glu-Pro | Gu et al. (2015) |
Relation between peptides structure and the hypocholesterolemic activity of peptides
Various studies have shown that several dietary proteins can improve blood lipid profile. To date, hypocholesterolemic properties have been reported for, soy (Hori et al. 2001), whey (Nagaoka et al. 1992) and fish protein (Wergedahl et al. 2004), capable of changing the plasma profile from atherogenic to cardioprotective. In contrast, bovine casein tends to cause species-dependent hypercholesterolemia and atheromatous plaques in animal studies (Van Der Meer et al. 1988). The exact mechanisms responsible for the hypocholesterolemic effects are not clear, but evidence indicates that the specific amino acid composition in dietary proteins and peptides structure influences the effect of the protein source on plasma cholesterol levels. It has been reported that dietary proteins with low ratios of methionine–glycine and lysine–arginine, such as soy and fish protein, favor a hypocholesterolemic effect. In contrast, bovine casein tends to raise cholesterol levels probably because of its high ratios of methionine–glycine and lysine–arginine (Erdmann et al. 2008).
It has been reported that soy protein hypocholesterolemic effects has received the most attention recently (Erdmann et al. 2008). Several researches suggested a range of possible mechanism of action in soy protein’s ability to reduce total plasma cholesterol including induction of LDL receptor expression, enhance of bile acid synthesis and excretion as well as decline in steroid absorption from the intestine. Moreover, changes in the endocrine status such as alteration in the insulin–glucagon ratio and in thyroid hormone concentrations have also been reported (Erdmann et al. 2008). It has also been suggested that soy protein hydrolysates reduce total cholesterol levels more effectively than intact soy protein (Zhang and Beynen 1993). A peptide derived from soy glycinin, leucine–proline–tyrosine–proline–arginine, was discovered to produce serum cholesterol-lowering effects (Yoshikawa et al. 2000). Peptide leucine–proline–tyrosine–proline–arginine is structurally homologous to enterostatin (valine–proline–aspartate–proline–arginine), an endogenous peptide showing hypocholesterolemic and anorectic effects (Takenaka et al. 2003). Peptides isoleucine–alanine–valine–proline–glycine–glutamate–valine–alanine is another glycinin-derived peptide with cholesterol-lowering activity (Pak et al. 2005). It has been reported that both peptides leucine–proline–tyrosine–arginine–proline and isoleucine–alanine–valine–proline–glycine–lutamate–valine–alanine inhibited 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) which is a known key enzyme in cholesterol biosynthesis. Investigations on the structure–activity relationship indicated that the hydrophobic region of both peptides is a required structural element for their biological activity. The maximum length of the hydrophobic sequence was stated to be four amino acids. In addition, the proline residue seems to be a key component and can be located at both the C-terminus and in any other position of the amino acid sequence except the N-terminus (Pak et al. 2005). Table 3 indicates some hypocholesterolemic peptides derived from food sources.
Table 3.
Some bioactive peptides with hypocholesterolemic activity
| Protein | Process | Bioactive peptide | References |
|---|---|---|---|
| β-Lactoglobulin | Trypsin, chymotrypsin | Ile-Ile-Ala-Glu-Lys | Hernández-Ledesma et al. (2013) |
| β-Lactoglobulin | Trypsin | Ile-Ala-Glu-Lys | Zhong et al. (2007) |
| Soy | Alcalase | Trp-Gly-Ala-Pro-Ser-Leu, Leu-Pro-Tyr-Pro | Zhong et al. (2007) |
| Glycinin | Enzymatic hydrolysis | LPYPR, IAVPGEVA | Erdmann et al. (2008) |
| Brassica carinata protein | Trypsin, chymotrypsin, and carboxypeptidase A | His-Tyr-Val-Ile-Leu-Pro-Met-Lys-Trp(1800–1400 Da) | Pedroche et al. (2007) |
Structural factors governing rheological properties
Structural factors affecting on the solubility of peptides
The degradation of proteins to peptides and smaller structures leads to more soluble products (Gbogouri et al. 2004). It has also been reported that hydrolysates have an excellent solubility at high degrees of hydrolysis (Shahidi et al. 1995). The balance of hydrophilic and hydrophobic forces occurred in peptides structure is another crucial influence on solubility increments (Gbogouri et al. 2004). It was said that in addition to polar and ionizable groups of protein hydrolysates, enzymatic hydrolysis affects the molecular size and hydrophobicity and so, these factors switch peptides structure and thereby they leads to change in solubility of peptides (Klompong et al. 2007). The smaller peptides from myofibrillar proteins are expected to have proportionally more polar residues, with the ability to form hydrogen bonds with water and augment solubility (Gbogouri et al. 2004). Concluding, hydrolysates with smaller peptides, i.e. higher DH, were more soluble. The pH affects the charge on the weakly acidic and basic side chain groups and hydrolysates generally show low solubility at their isoelectric points (Linder et al., 1996). Solubility variations depend on net charge of peptides and surface hydrophobicity in the peptides structure. Solubility increase when pH moves away from pI (Isoelectric point) and surface hydrophobicity promotes the aggregation via hydrophobic interaction (Sorgentini and Wagner 2002).
Structural factors effecting on the emulsifying properties of peptides
The mechanism to generate the emulsion system is attributed to the adsorption of peptides on the surface of freshly formed oil droplets during homogenization and the formation of a protective membrane that inhibits coalescence of the oil droplet (Klompong et al. 2007). Hydrolysates are surface-active materials and promote oil-in-water emulsion because of hydrophilic and hydrophobic groups with their associated charges in their structure (Gbogouri et al. 2004). Klompong et al. (2007) findings regarding protein hydrolysates from yellow stripe trevally (Selaroides leptolepis) meat by Alcalase 2.4L (HA) and Flavourzyme 500L (HF) showed that Emulsifying Activity Index (EAI) and Emulsion Stability Index (ESI) for both HA and HF decreased (p < 0.05) with increasing DH. At low DH level (5%), hydrolysates showed strong emulsifying properties. With a limited degree of hydrolysis, the hydrolysates have special emulsifying activity and stability (Kristinsson and Rasco 2000). In the stability of the emulsion, higher contents of peptides with larger molecular weight peptides or more hydrophobic peptides have critical role (Klompong et al. 2007). On the other hand, extreme hydrolysis could cause the loss of emulsifying properties (Gbogouri et al. 2004; Kristinsson and Rasco 2000). The peptides with low molecular weight may not be amphiphilic enough to exhibit proper emulsifying properties (Klompong et al. 2007). Due to their small peptide size, hydrolysates with a higher DH had poorer EAI and ESI. Small peptides show less efficiency in decreasing the interface tension because they cannot unfold and reorient at the interface similar to large peptides to stabilize emulsions but they migrate rapidly and adsorb at the interface (Gbogouri et al. 2004; Rahali et al. 2000). Apart from peptide size, amphiphilicity of peptides is important for interfacial and emulsifying properties. Rahali et al. (2000) analyzed amino acid sequence at an oil/water interface and concluded that amphiphilic character was more important than peptide length for emulsion properties. The flexibility of protein or peptide structure may also be a vital factor governing the emulsifying properties (Klompong et al. 2007). When considering the effect of pH on EAI and ESI, it depends on peptides solubility. For instance, Klompong et al. (2007) found the lowest EAI and ESI at pH 4, with coincidental decrease in solubility. Since the lowest solubility occurred at pH 4, peptides could not move rapidly to the interface. Moreover, the net charge of peptide might be minimized at pH 4. The higher EAI of hydrolysates accompanied their higher solubility (Mutilangi et al. 1996). Hydrolysates with high solubility can rapidly diffuse and adsorb at the interface. Also, emulsifying properties were influenced by specificity of peptidase. Klompong et al. (2007) suggested at the same DH, hydrolyzed by Flavourzyme (HF) had a better EAI than had hydrolyzed by Alcalase (HA). So, HA showed a higher ESI than did HF. In general, EAI and ESI increased when the pH is moved away from the pH 4. Thus indicating that the sequence and composition of amino acids in peptide between HA and HF might be different, leading to varying charge of the resulting peptides at a particular pH (Klompong et al. 2007).
Structural factors effecting on the foaming properties of peptides
Foam formation is conducted by three factors, including transportation, penetration and reorganization of molecules at the air–water interface. In order to have good foaming, a protein must be capable of migrating rapidly to the air–water interface, unfolding and re-arranging at the interface (Klompong et al. 2007). Foam stability to correlate with the nature of the film and reflects the extent of protein–protein interaction within the matrix (Mutilangi et al. 1996). Authors expressed that flexible protein section by enhancing viscosity of the aqueous phase, protein concentration and film thickness can enhance foam stability (Klompong et al. 2007). Mutilangi et al. (1996) suggested that the foaming capacity of protein was improved by making it more flexible, exposing more hydrophobic residues and increasing capacity to decrease surface tension. For the adsorption at the air–water interface, molecules should contain hydrophobic regions in their structure (Mutilangi et al. 1996).
In general, high molecular weight peptides have direct effect on foam stability of protein hydrolysates (van der Ven et al. 2002). Hydrophobicity of structurally unfolded proteins has been known in relation to foaming properties (Townsend and Nakai 1983). Excessive hydrolysis may reduce the foaming stability because the more microscopic peptides do not have the strength needed to maintain stable foam (Shahidi et al. 1995). Klompong et al. (2007) stated as DH increased, both hydrolyzed by Alcalase and hydrolyzed by Flavourzyme displayed a lower foaming capacity and foam stability. Shahidi et al. (1995) reported good foaming properties for capelin protein hydrolysates at low DH (12%). Klompong et al. (2007) showed that pH affected the foaming properties of both hydrolyzed by Alcalase and hydrolyzed by Flavourzyme hydrolysates. Foaming capacity was declining at pH 4. The foaming capacity of HA was maximum at pH 6 with a slight decrease at alkaline pH. So, net charge must influence the adsorption of the proteins at the air–water interface. The foaming property was increased when the net charge was enhanced (Sorgentini and Wagner 2002; Townsend and Nakai 1983). The foaming characteristics of proteins reached to minimum at the lowest solubility, so these properties intend to reduce at their isoelectric pH (Klompong et al. 2007). Protein solubility plays an important role to the foaming behavior of protein. The pH of the dispersing medium dramatically influences foaming properties, especially foam stability (Townsend and Nakai 1983). Foam stability depends on the nature of the film and reflects the extent of protein–protein interaction within the matrix (Mutilangi et al. 1996). It has been reported the decreased foam stability at very acidic or alkaline pH may be due to the repulsion of peptides via ionic repulsion. It is known that the size and charge of peptides may be different for hydrolysates produced by different peptidases. Klompong et al. (2007) reported that hydrolysis by Flavourzyme (HF) could form flexible films around the air bubbles because HF most likely contained larger peptides. Kristinsson and Rasco (2000) indicated that fish protein hydrolysates (FPH) can use as emulsifying and emulsion stabilizing ingredients in a variety of products as well as aid in the formation and stabilization of foam-based ones due to the good foaming and emulsifying characteristics.
Structural factors effecting on the water holding capacity property of peptides
Water holding is important factor that influences basic quality characteristics and yield of protein products because it is significant in the determination of mechanical strength, elasticity, plasticity, and flow of food materials and was critical for desirable functions of plant protein materials, such as swelling, wet ability, water holding capacity, gelation and surface properties (Ge et al. 2000). Protein hydrolysates from seal meat were found to improve water-holding capacity in meat products (Cumby et al. 2008).
The water holding capacity property is affected by the concentration, pH and by the peptidases employed during the hydrolysis process influencing on peptides structure (Ge et al. 2000). Also, composition of the peptides in each of the fractions may also play an important role. Cumby et al. (2008) reported that Flavourzyme hydrolysate was most effective in decreasing the drip volume, followed by hydrolysates prepared by combination of Alcalase and Flavourzyme and least effective was the one prepared by Alcalase alone. It is clear that lower-molecular-weight peptides are more effective in holding water than larger-size peptides because smaller fragments of peptides would be more hydrophilic. Also, it has been reported that Flavourzyme has a good tendency to produce low-molecular-weight peptide fragments. Moreover, studies have shown that hydrolysate would cause a better water-holding capacity if hydrolysis via enzyme preserved most of the hydrophilic amino acid residues (Cumby et al. 2008). Also, they stated that canola protein hydrolysates improved water-holding capacity of meat products and also, they indicated hydrolysates prepared using Flavourzyme were superior in terms of their water-holding capacity.
Structural factors effecting on the surface hydrophobicity property of peptides
Since the hydrophobic interactions are the driving forces for manifestation of the physiological functions of peptides, information on the hydrophobic character of bioactive peptides could contribute to further understanding of their mechanism of action. The surface hydrophobic site is partly responsible for formation and maintenance of the spatial structures as well as protein interactions, including binding to cell membranes, protein–protein recognition, and formation of complexes with biologically active compounds (Voronov et al. 2002). When protein is hydrolyzed, protein globular structure may cause alter in as the hydrophobic regions hidden within the native protein are exposed (Mundi and Aluko 2014).
Mundi and Aluko (2014), observed a decrease in the surface hydrophobicity of smaller Kidney Bean Protein Hydrolysate (KBH) prepared by Alcalase hydrolysis compared to the larger peptides. This is probably because there are more oligopeptides available as the size of the membranes increased with larger surface containing the exposed hydrophobic residues. On a similar note Molina Ortiz and Wagner (2002) also reported that smaller chain peptides had less surface hydrophobicity. In contrast, Wang et al. (2007) indicated that permeate with a molecular weight cut-off of 5 kDa had higher surface hydrophobicity than the hydrolysate. Table 4 indicates some recently studies on structural factors governing rheological properties of peptides and hydrolysates.
Table 4.
Studies on structural factors governing rheological properties of peptides and hydrolysates
| Peptide or protein hydrolysate | Treatment | Outcome | References |
|---|---|---|---|
| Soy protein peptides | Proteolytic enzymatic (papain) modification of soy protein isolates (SPI), followed by ultrafiltration was used to fractionate of proteins into peptides with controlled molecular size | Soy protein peptides prepared from SPI by papain modification and ultrafiltration had lower molecular weight, higher solubility, and higher emulsifying properties | Wu et al. (1998) |
| Rice endosperm protein hydrolysates | Controlled enzymatic hydrolysis was used. The optimum degree of hydrolysis (DH) was determined for acid, neutral, and alkaline type proteases | The optimum DH was 6–10% for good emulsifying properties of rice protein, depending on enzyme specificity. High hydrophobic and sulfhydryl disulfide (SH-SS) interactions contributed to protein insolubility even at high DH | Paraman et al. (2007) |
| Rice bran protein hydrolysate (RBPH) | Rice bran protein (RBP) was hydrolyzed by trypsin and the DH values were set to be 1, 3, and 6% | Emulsions prepared with 3% DH RBP were much more stable than the emulsions prepared with RBPH with other DH values, and this RBP has potential as an emulsifier in the food industry | Zang et al. (2018) |
| Peanut protein hydrolysate | Peanut protein isolate (PPI) was modified with extrusion pretreatment and papain-induced proteolysis | Extrusion pretreatment conducted at 130 °C enhanced the protease accessibilities of the main constitutive proteins in EPPI (extrudates of PPI), concluding in a remarkable increase in DH and protein solubility for the hydrolysates. Extrusion pretreatment cause to a noticeable enhancement in the emulsification property of hydrolysates | Chen et al. (2018) |
| Rice glutelin hydrolysate | Rice glutelin was hydrolyzed by trypsin at an optimized enzyme/substrate ratio and the influence of the degree of hydrolysis (DH) on the structure, solubility, rheology, and emulsifying properties of rice glutelin hydrolysate was investigated | Protein hydrolysis changed molecular weight, increased flexibility, altered surface hydrophobicity, and increased solubility. Emulsions prepared with 2% DH rice glutelin were stable over a range of environmental conditions: pH 7–9; NaCl < 100 mM (pH 7); temperatures < 90 °C (pH 7, 0 mM NaCl) studied | Xu et al. (2016) |
| Soy protein hydrolysate | Soy protein isolate was treated by high-pressure micro-fluidization and pancreatin hydrolysis | Micro fluidization increased the accessibility of some subunits in SPI to pancreatin hydrolysis, resulting in changes in protein solubility (PS), surface hydrophobicity (H0) for hydrolysates. Emulsion systems formed by control SPI and SPIH (SPI hydrolysates) were unstable because of fast coalescence and bridging flocculation during homogenization, while that formed by MSPIH (micro fluidization pretreated SPIH) with 5.8% DH was more stable and indicated smaller mean droplet size. Compared with SPIH, MSPIH showed a stronger increase in PS and a more moderate change in H0 during pancreatin hydrolysis, indicating the production of more surface-active soluble peptides, which may explain their markedly improved emulsifying capabilities | Chen et al. (2016) |
| Acylated rapeseed (Brassica napus L.) peptides | A peptide fraction having an average size of 5.6 amino acids has been purified from a rapeseed hydrolyzate, acylated using C10–C14 acyl chlorides | Emulsions produced from acylated peptides were more stable to phase separation than those prepared from SDS. The C14 acylated peptides were more effective for generating emulsions than the C10 and C12 derivatives, especially concerning the stability of emulsions against coalescence and phase separation, which was better than SDS and close to BSA | Sánchez-Vioque et al. (2004) |
Conclusion
In this review, we revealed that some types of food proteins-derived bioactive peptides are a potential modulator in the body. Bioactive peptides with various structures show functional activities such as: antioxidant activity, ACE inhibitor activity, hypocholesterolemic activity and also, they were found to improve water-holding capacity, foaming capacity, emulsifying properties and solubility in products. This review stated that biological and functionality aspects of protein hydrolysates were mainly influenced by peptide structure and its amino acid sequence. Thereby, in order to maximize the functionality and biological activities of the protein hydrolysates, the determination of variables that exerts significant impact on hydrolysis processes is necessary. Also, further researches are needed on the safety and quality of the foods containing bioactive peptides and to develop modern techniques to enrich active peptide from food protein and to facilitate the production of these peptides in huge quantities for the market and to stabilize of chemical structures and biological activity of peptides in different food matrices new technique are needed. It is possible that encapsulation improve stability of peptides in various food products and also during their digestion.
Acknowledgements
The authors acknowledge the technical support from the food and drug reference laboratories in health holding and health promoting food scheme.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agrawal H, Joshi R, Gupta M. Isolation and characterisation of enzymatic hydrolysed peptides with antioxidant activities from green tender sorghum. Lwt-Food Sci Technol. 2017;84:608–616. [Google Scholar]
- Ambigaipalan PS, Al-Khalifa A, Shahidi F. Antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities of date seed protein hydrolysates prepared using Alcalase, Flavourzyme and Thermolysin. J Funct Foods. 2015;18:1125–1137. [Google Scholar]
- Bhat ZF, Kumar S, Bhat HF. Bioactive peptides of animal origin: a review. J Food Sci Technol. 2015;52:5377–5392. doi: 10.1007/s13197-015-1731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye JI, Roufik S, Pesta N, Barbana C. Angiotensin I-converting enzyme inhibitory properties and SDS-PAGE of red lentil protein hydrolysates. Lwt-Food Sci Technol. 2010;43:987–991. [Google Scholar]
- Chen HM, Muramoto K, Yamauchi F. Structural analysis of antioxidative peptides from soybean. J Agric Food Chem. 1995;43:574–578. [Google Scholar]
- Chen L, Chen J, Yu L, Wu K. Improved emulsifying capabilities of hydrolysates of soy protein isolate pretreated with high pressure microfluidization. Lwt-Food Sci Technol. 2016;60:1–8. [Google Scholar]
- Chen L, Chen J, Yu L, Wu K, Zhao M. Emulsification performance and interfacial properties of enzymically hydrolyzed peanut protein isolate pretreated by extrusion cooking. Food Hydrocoll. 2018;77:607–616. [Google Scholar]
- Cheung HS, Wang FL, Ondetti MA, Sabo EF, Cushman DW. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. Importance of the COOH-terminal dipeptide sequence. J Biol Chem. 1980;25:401–407. [PubMed] [Google Scholar]
- Cumby N, Zhong Y, Naczk M, Shahidi F. Antioxidant activity and water-holding capacity of canola protein hydrolysates. Food Chem. 2008;109:144–148. doi: 10.1016/j.foodchem.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Davalos A, Miguel M, Bartolome B, Lopez-Fandino R. Antioxidantactivity of peptides derived from egg white proteins by enzymatic hydrolysis. J Food Prot. 2004;67:1939–1944. doi: 10.4315/0362-028x-67.9.1939. [DOI] [PubMed] [Google Scholar]
- De Castro RJS, Sato HH. A response surface approach on optimization of hydrolysis parameters for the production of egg white protein hydrolysates with antioxidant activities. Biocatal Agric Biotechnol. 2015;4:55–62. [Google Scholar]
- Di Bernardini R, Harnedy P, Bolton D, Kerry J, O’Neill E, Mullen AM, Hayes M. Antioxidant and antimicrobial peptidic hydrolysates from muscle protein sources and by-products. Food Chem. 2011;124:1296–1307. [Google Scholar]
- Elias RJ, Kellerby SS, Decker EA. Antioxidant activity of proteins and peptides. Crit Rev Food Sci Nutr. 2008;48:430–441. doi: 10.1080/10408390701425615. [DOI] [PubMed] [Google Scholar]
- Erdmann K, Cheung BW, Schröder H. The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J Nutr Biochem. 2008;19:643–654. doi: 10.1016/j.jnutbio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Gbogouri GA, Linder M, Fanni J, Parmentier M. Influence of hydrolysis degree on the functional properties of salmon byproduct hydrolysates. J Food Sci. 2004;69:615–622. [Google Scholar]
- Ge Y, Sun A, Ni Y, Cal T. Some nutritional and functional properties of defatted wheat germ protein. J Agric Food Chem. 2000;48:6215–6218. doi: 10.1021/jf000478m. [DOI] [PubMed] [Google Scholar]
- Girgih AT, He R, Malomo S, Offengenden M, Wu J, Aluko RE. Structural and functional characterization of hemp seed (Cannabis sativa L.) protein-derived antioxidant and antihypertensive peptides. J Funct Foods. 2014;6:384–394. [Google Scholar]
- Gu X, Hou Y-K, Li D, Wang J-Z, Wang F-J. Separation, purification, and identification of angiotensin I-converting enzyme inhibitory peptides from Walnut (Juglans regia L.) hydrolyzate. Int J Food Prop. 2015;18:266–276. [Google Scholar]
- Guo H, Kouzuma Y, Yonekura M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009;113:238–245. [Google Scholar]
- Harnedy PA, O’Keeffe MB, FitzGerald RJ. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Food Res Int. 2017;100:416–422. doi: 10.1016/j.foodres.2017.07.037. [DOI] [PubMed] [Google Scholar]
- He R, Malomo SA, Alashi A, Girgih AT, Ju X, Aluko RE. Purification and hypotensive activity of rapeseed protein-derived renin and angiotensin converting enzyme inhibitory peptides. J Funct Foods. 2013;5:781–789. [Google Scholar]
- Hernández-Ledesma B, Dávalos A, Bartolomé B, Amigo L. Preparation of an enzymatic hydrolysates from a-Lactalbumin and b-Lactoglobulin. Identification of active peptides by HPLC-MS/MS. J Agric Food Chem. 2005;53:588–593. doi: 10.1021/jf048626m. [DOI] [PubMed] [Google Scholar]
- Hernández-Ledesma B, García-Nebot MJ, Fernández-Tomé S, Amigo L, Recio I. Dairy protein hydrolysates: peptides for health benefits. Int Dairy J. 2013;38:82–100. [Google Scholar]
- Hori G, Wang MF, Chan YC, Komatsu T, Wong Y, Chen TH, et al. Soy protein hydrolyzate with bound phospholipids reduces serum cholesterol levels in hypercholesterolemic adult male volunteers. Biosci Biotechnol Biochem. 2001;65:72–78. doi: 10.1271/bbb.65.72. [DOI] [PubMed] [Google Scholar]
- Irshad I, Kanekanian A, Peters A, Masud T. Antioxidant activity of bioactive peptides derived from bovine casein hydrolysate fractions. J Food Sci Technol. 2015;52:231–239. [Google Scholar]
- Karami Z, Emam-Djomeh Z, Mirzaee HA, Khomeiri M, Sadeghi Mahoonak A, Aydani E. Optimization of microwave assisted extraction (MAE) and soxhlet extraction of phenolic compound from licorice root. J Food Sci Technol. 2015;52:3242–3253. doi: 10.1007/s13197-014-1384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami Z, Peighambardoust SH, Hesari J, Akbari-adergani B (2018) Response surface methodology to optimize hydrolysis parameters in production of the antioxidant peptides from wheat germ protein by Alcalase digestion. Identification of antioxidant peptides by LC-MS/MS. J Agric Sci Technol (in press)
- Kim S-K, Wijesekara I. Development and biological activities of marine-derived bioactive peptides: a review. J Funct Foods. 2010;2:1–9. [Google Scholar]
- Kim SS, Ahn C-B, Moon SW, Je J-Y (2018) Purification and antioxidant activities of peptides from sea squirt (Halocynthia roretzi) protein hydrolysates using pepsin hydrolysis. Food Biosci (in press)
- Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. [Google Scholar]
- Kristinsson HG, Rasco BA. Fish protein hydrolysates: production, biochemical, and functional properties. Crit Rev Food Sci Nutr. 2000;40:43–81. doi: 10.1080/10408690091189266. [DOI] [PubMed] [Google Scholar]
- Li GH, Qu MR, Wan JZ, You JM. Antihypertensive effect of rice protein hydrolysate with in vitro angiotensin I-converting enzyme inhibitory activity in spontaneously hypertensive rats. Asia Pac J Clin Nutr. 2007;16:275–280. [PubMed] [Google Scholar]
- Li X, Han L, Chen L. In vitro antioxidant activity of protein hydrolysates prepared from corn gluten meal. J Sci Food Agric. 2008;88:1660–1666. [Google Scholar]
- Linder M, Fanni J, Parmentier M. Functional properties of veal bone hydrolysates. J Food Sci. 1996;61:712–716. [Google Scholar]
- Liu Z, Dong S, Xu J, Zeng M, Song H, Zhao Y. Production of cysteine-rich antimicrobial peptide by digestion of oyster (Crassostrea gigas) with alcalase and bromelin. Food Control. 2008;19:231–235. [Google Scholar]
- Matsui T, Tanaka M. Antihypertensive peptides and their underlying mechanisms. In: Mine Y, Li-Chan ECY, Jiang B, editors. Bioactive proteins and peptides as functional foods and nutraceuticals. New York: Wiley; 2010. pp. 43–53. [Google Scholar]
- Matsui T, Li CH, Osajima Y. Preparation and characterization of novel bioactive peptides responsible for angiotensin I-converting enzyme inhibition from wheat germ. J Pept Sci. 1999;5:289–297. doi: 10.1002/(SICI)1099-1387(199907)5:7<289::AID-PSC196>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Mendis E, Rajapakse N, Byun HG, Kim SK. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005;77:2166–2178. doi: 10.1016/j.lfs.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Molina Ortiz SE, Wagner JR. Hydrolysates of native and modified soy protein isolates: structural characteristics, solubility and foaming properties. Food Res Int. 2002;35:511–518. [Google Scholar]
- Motoi H, Kodama T. Isolation and characterization of angiotensin I-converting enzyme inhibitory peptides from wheat gliadin hydrolysate. Food/Nahrung. 2003;47:354–358. doi: 10.1002/food.200390081. [DOI] [PubMed] [Google Scholar]
- Muguerza B, Ramos M, Sánchez E, Manso MA, Miguel M, Aleixandre A, Delgado MA, Recio I. Antihypertensive activity of milk fermented by Enterococcus faecalis strains isolated from raw milk. Int Dairy J. 2006;16:61–69. [Google Scholar]
- Mundi S, Aluko RE. Inhibitory properties of kidney bean protein hydrolysate and its membrane fractions against renin, angiotensin converting enzyme, and free radicals. Austin J Nutr Food Sci. 2014;2:1–11. [Google Scholar]
- Mutilangi WAM, Panyam D, Kilara A. Functional properties of hydrolysates from proteolysis of heat-denatured whey protein isolate. J Food Sci. 1996;61:270–274. [Google Scholar]
- Nagaoka S, Kanamaru Y, Kojima T, Kuwata T. Comparative studies on the serum cholesterol lowering action of whey protein and soybean protein in rats. Biosci Biotechnol Biochem. 1992;56:1484–1485. [Google Scholar]
- Nagasawa T, Yonekura T, Nishizawa N, Kitts DD. In vitro and in vivo inhibition of muscle lipid and protein oxidation by carnosine. Mol Cell Biochem. 2001;225:29–34. doi: 10.1023/a:1012256521840. [DOI] [PubMed] [Google Scholar]
- Pak VV, Koo M, Lee N, Kim MS, Kwon DY. Structure-activity relationships of the peptide Ile-Ala-Val-Pro and its derivatives revealed using the semi-empirical AM1 method. Chem Nat Compd. 2005;41:454–460. [Google Scholar]
- Paraman I, Hettiarachchy NS, Schaefer CI, Beck M. Hydrophobicity, solubility, and emulsifying properties of enzyme-modified rice endosperm protein. AACC Int. 2007;84:343–349. [Google Scholar]
- Pedroche J, Yust MM, Lqari H, Megias C, Girón-Calle J, Alaiz M, Vioque J, Millan F. Obtaining of Brassica carinata protein hydrolysates enriched in bioactive peptides using immobilized digestive proteases. Food Res Int. 2007;40:931–938. [Google Scholar]
- Qian Z-J, Jung W-K, Kim S-K. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Rana catesbeiana Shaw. Bioresour Technol. 2008;99:1690–1698. doi: 10.1016/j.biortech.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Rahali V, Chobért JM, Haértle T, Gueguen J. Emulsification of chemical and enzymatic hydrolysates of β-lactoglobulin: characterization of the peptides adsorbed at the interface. Nahrung. 2000;44:89–95. doi: 10.1002/(SICI)1521-3803(20000301)44:2<89::AID-FOOD89>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Raikos V, Dassios T. Health-promoting properties of bioactive peptides derived from milk proteins in infant food: a review. Dairy Sci Technol. 2014;94:91–101. doi: 10.1007/s13594-013-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse N, Mendis E, Jung W-K, Je J-Y, Kim S-K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res Int. 2005;38:175–182. [Google Scholar]
- Ren J, Zhao M, Shi J, Wang J, Jiang Y, Cui C, Kakuda Y, Xue SJ. Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2008;108:727–736. doi: 10.1016/j.foodchem.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Rho SJ, Lee JS, Chung Y, Kim YW, Lee HG. Purification and identification of an angiotensin I-converting enzyme inhibitory peptide from fermented soybean extract. Process Biochem. 2009;44:490–493. [Google Scholar]
- Saito K, Jin DH, Ogawa T, Muramoto K, Hatakeyama E, Yasuhara T, Nokihara K. Antioxidative properties of tripeptide libraries prepared by the combinatorial chemistry. J Agric Food Chem. 2003;51:3668–3674. doi: 10.1021/jf021191n. [DOI] [PubMed] [Google Scholar]
- Sánchez A, Vázquez A. Bioactive peptides: a review. Food Qual Saf. 2017;1:29–46. [Google Scholar]
- Sánchez-Vioque R, Bagger C, Larré C, Guéguen J. Emulsifying properties of acylated rapeseed (Brassica napus L.) peptides. J Colloid Interface Sci. 2004;271:220–226. doi: 10.1016/j.jcis.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Sarmadi BH, Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;31:1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Zhong Y. Bioactive peptides. J AOAC Int. 2008;91:914–931. [PubMed] [Google Scholar]
- Shahidi F, Zhong Y. Novel antioxidants in food quality preservation and health promotion. Eur J Lipid Sci Technol. 2010;112:930–940. [Google Scholar]
- Shahidi F, Han X-Q, Synowiecki J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus) Food Chem. 1995;53:285–293. [Google Scholar]
- Sharma S, Singh R, Rana S. Bioactive peptides: a review. Int J Bio Autom. 2011;15:223–250. [Google Scholar]
- Sorgentini DA, Wagner JR. Comparative study of foaming properties of whey and isolate soy bean proteins. Food Res Int. 2002;35:721–729. [Google Scholar]
- Suetsuna K, Nakano T. Identification of an antihypertensive peptide frompeptic digest of wakame. J Nutr Biochem. 2000;11:450–454. doi: 10.1016/s0955-2863(00)00110-8. [DOI] [PubMed] [Google Scholar]
- Takenaka A, Annaka H, Kimura Y, Aoki H, Igarashi K. Reduction of paraquat-induced oxidative stress in rats by dietary soy peptide. Biosci Biotechnol Biochem. 2003;67:278–283. doi: 10.1271/bbb.67.278. [DOI] [PubMed] [Google Scholar]
- Tenenbaum A, Grossman E, Shemesh J, Fisman EZ, Nosrati I, Motro M. Intermediate but not low doses of aspirin can suppress angiotensin-converting enzyme inhibitor-induced cough. Am J Hypertens. 2000;13:776–782. doi: 10.1016/s0895-7061(00)00268-5. [DOI] [PubMed] [Google Scholar]
- Townsend AA, Nakai S. Relationships between hydrophobicity and foaming characteristics of food proteins. J Food Sci. 1983;48:588–594. [Google Scholar]
- Udenigwe CC, Adebiyi AP, Doyen A, Li H, Bazinet L, et al. Low molecular weight flaxseed protein-derived arginine-containing peptides reduced blood pressure of spontaneously hypertensive rats faster than amino acid form of arginine and native flaxseed protein. Food Chem. 2012;132:468–475. doi: 10.1016/j.foodchem.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Uluko H, Zhang S, Liu L, Tsakama M, Lu J, Lv J. Effects of thermal, microwave, and ultrasound pretreatments on antioxidative capacity of enzymatic milk protein concentrate hydrolysates. J Funct Foods. 2015;18:1138–1146. [Google Scholar]
- Van Der Meer R, De Vries HT, Van Tintelen G. The phosphorylation state of casein and the species-dependency of its hypercholesterolaemic effect. Br J Nutr. 1988;59:467–473. doi: 10.1079/bjn19880056. [DOI] [PubMed] [Google Scholar]
- Van der Ven C, Gruppen H, de Bont DBA, Voragen AGJ. Correlations between biochemical characteristics and foam-forming and -stabilizing ability of whey and casein hydrolysates. J Agric Food Chem. 2002;50:2938–2946. doi: 10.1021/jf011190f. [DOI] [PubMed] [Google Scholar]
- Vásquez-Villanueva R, Luisa Marina M, García M. Revalorization of a peach (Prunus persica (L.) Batsch) byproduct: extraction and characterization of ACE-inhibitory peptides from peach stones. J Funct Foods. 2015;18:137–146. [Google Scholar]
- Vermeirssen V, Van Camp J, Verstraete W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br J Nutr. 2004;92:357–366. doi: 10.1079/bjn20041189. [DOI] [PubMed] [Google Scholar]
- Vioque J, Clemente A, Pedroche J, Yust MM, Millán F. Obtention and uses of protein hydrolysates. Grasas Aceites. 2001;52:132–136. [Google Scholar]
- Voronov SV, Skirgello OE, Troshina NN, Orlova MA, Kost OA. A hydrophobic site on the surface of the angiotensin-converting enzyme molecule. Biochem (Mosc) 2002;67:553–557. doi: 10.1023/a:1015598228545. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhao M, Zhao Q, Jiang Y. Antioxidant properties of papain hydrolysates of wheat gluten in different oxidation systems. Food Chem. 2007;101:1658–1663. [Google Scholar]
- Wang L, Mao X, Cheng X, Xiong X, Ren F. Effect of enzyme type and hydrolysis conditions on the in vitro angiotensin I-converting enzyme inhibitory activity and ash content of hydrolysed whey protein isolate. Int J Food Sci Technol. 2010;45:807–812. [Google Scholar]
- Wergedahl H, Liaset B, Gudbrandsen OA, Lied E, Espe M, Muna Z, et al. Fish protein hydrolysate reduces plasma total cholesterol, increases the proportion of HDL cholesterol, and lowers acyl-CoA: cholesterol acyltransferase activity in liver of Zucker rats. J Nutr. 2004;134:1320–1327. doi: 10.1093/jn/134.6.1320. [DOI] [PubMed] [Google Scholar]
- Wu WU, Hettiarachchy NS, Qi M. Hydrophobicity, solubility, and emulsifying properties of soy protein peptides prepared by papain modification and ultrafiltration. J Am Oil Chem Soc. 1998;75:845–850. [Google Scholar]
- Xiong S, Yao X, Li A. Antioxidant properties of peptide from cowpea seed. Int J Food Prop. 2013;16:1245–1256. [Google Scholar]
- Xu X, Liu W, Liu C, Luo L, Chen J, Luo S, McClements DJ, Wu L. Effect of limited enzymatic hydrolysis on structure and emulsifying properties of rice glutelin. Food Hydrocoll. 2016;61:251–260. [Google Scholar]
- Yoshikawa M, Fujita H, Matoba N, Takenaka Y, Yamamoto T, Yamauchi R, Tsuruki H, Takahata K. Bioactive peptides derived from food proteins preventing lifestyle related diseases. BioFactors. 2000;12:143–146. doi: 10.1002/biof.5520120122. [DOI] [PubMed] [Google Scholar]
- Zang X, Yue C, Wang Y, Shao M, Yu G (2018) Effect of limited enzymatic hydrolysis on the structure and emulsifying properties of rice bran protein. J Cereal Sci. In press
- Zhang X, Beynen AC. Lowering effect of dietary milk-whey protein v. casein on plasma and liver cholesterol concentrations in rats. Br J Nutr. 1993;70:139–146. doi: 10.1079/bjn19930111. [DOI] [PubMed] [Google Scholar]
- Zhang M, Mu T-H, Sun M-J. Purification and identification of antioxidant peptides from sweet potato protein hydrolysates by Alcalase. J Funct Foods. 2014;7:191–200. [Google Scholar]
- Zhong F, Zhang X, Ma J, Shoemaker CF. Fractionation and identification of a novel hypocholesterolemic peptide derived from soy protein Alcalase hydrolysates. Food Res Int. 2007;40:756–762. [Google Scholar]
- Zhuang H, Tang N, Yuan Y. Purification and identification of antioxidant peptides from corn gluten meal. J Funct Foods. 2013;5:1810–1821. [Google Scholar]