Abstract
This study aimed to investigate the effect of in vitro digestion on the antioxidant activity and carbohydrate-digestive enzymes inhibitory potential of five edible mushrooms after subjected to four domestic cooking; namely, boiling, microwaving, steaming and pressure-cooking. The water extracts of raw (uncooked), cooked and in vitro digested mushrooms were compared for their water-soluble phenolic content (WPC), total flavonoid content (TFC), ferric reducing antioxidant power (FRAP), radical scavenging activity (TEAC and DPPH), anti-α-amylase and anti-α-glucosidase activities. Among the raw samples, Lentinula edodes possessed the highest antioxidant activities (FRAP, TEAC, DPPH) and WPC while Pleurotus sajor-caju displayed the highest TFC, anti-α-amylase and anti-α-glucosidase activities. The antioxidant and carbohydrate-digestive enzyme inhibitory activities significantly varied according to mushroom species and cooking methods applied. Short duration of microwaving (Agaricus bisporus and Flammulina velutipes), boiling (Auricularia polytricha) and pressure cooking (L. edodes and P. sajor-caju) yielded the best antioxidant and carbohydrate-digestive enzymes inhibition values in the mushroom extracts. TFC was positively correlated with the antioxidant activities and anti-α-glucosidase activity in the mushroom extracts. In vitro digestion significantly improved the total antioxidant and anti-α-glucosidase activities but decreased the anti-α-amylase activity in the cooked mushroom extracts. Principle component analysis showed that in vitro digestion and the cooking process accounted for respective 48.9% and 19.7% of variation in the observed activities. Domestic cooking and in vitro digestion could potentiate the total antioxidant and carbohydrate-digestive enzymes inhibitory activities in the selected water extract of edible mushrooms.
Keywords: Antioxidant, Carbohydrate-digestive enzymes, Cooking, Phenolic, In vitro digestion, Mushroom
Introduction
Mushrooms have been described as healthy foods due to their low calories, cholesterol, fat, and sodium content but rich in protein, carbohydrate, fibre, minerals and vitamins (Aida et al. 2009). Among the 2000 edible mushrooms, 35 species have gained much popularity worldwide due to their unique textures and aroma. Button mushroom (Agaricus bisporus) is the most widely cultivated species, followed by shiitake (Lentinula edodes), oyster (Pleurotus spp.), jelly ear (Auricularia spp.) and golden needle (Flammulina velutipe) (Aida et al. 2009). A. bisporus is well known to the Western countries since they are commonly found in Europe and North America. In contrast, Auricularia spp., L. edodes and F. velutipe are mostly cultivated in South Pacific and East Asian countries such as India, China, Taiwan, Singapore, Japan, Korea and Thailand (Ghorai et al. 2009).
In Asia, mushrooms are recognized as functional foods as they contain a variety of biological active compounds with therapeutic potential such as alkaloids, β-glucan, lectins, peptides, phenolics, sterols (ergosterols) and terpenes (Bach et al. 2017). The synergistic effect exerted by these compounds in the mushrooms could alleviate the development of many inflammatory diseases associated with oxidative stress such as type 2 diabetes mellitus (De Silva et al. 2012). For instance, a water-soluble polysaccharide-peptide complex from the fruiting body of Pleurotus spp. displayed significantly high antioxidant and hypoglycemic activities (Li et al. 2012).
Generally, mushrooms are either consumed raw as salad or cooked by microwaving, stir-frying, pressure-cooking, steaming or boiling. Thermal processing such as domestic cooking has been shown to cause differential effect on the phytochemicals in plant foods (Ng et al. 2011, 2014; Ribas-Agustí et al. 2017). The phytochemicals present in the mushrooms, particularly the antioxidants varied according to mushroom species, cell wall matrix, and the properties of phytochemicals such as their thermal stability (Ng and Tan 2017). More importantly, the bioaccessibility of phytochemicals in foods could be altered by drastic process such as gastrointestinal digestion due to the effect of different pH, enzymatic polymerization, and molecule hydrophobicity (Siracusa et al. 2011). Hence, it is essential to quantify the proportion of phytochemicals and their bioactivity available for absorption into the biological system after the digestion. This is especially important for mushrooms with significant amount of indigestible polysaccharides that might affect the extractability of phytochemicals (Soler-Rivas et al. 2009).
Although the nutritional profile of different edible mushrooms have been extensively studied, only a relatively small number of reports on the changes of antioxidant and carbohydrate-digestive enzymes inhibition activities in the cooked and in vitro digested mushrooms are available but limited to a few selected cultivars (Heleno et al. 2015; Soler-Rivas et al. 2009). The present study is aimed to address this by investigating the water soluble phenolic and flavonoid content, total antioxidant activity and carbohydrate-digestive enzymes inhibition potential of five edible mushrooms; namely, A. bisporus, A. polytricha, F. velutipes, L. edodes and P. sajor-caju after subjecting to four types of domestic cooking and followed by in vitro digestion. This study utilizes an in vitro digestion model which mimics human gastrointestinal digestive process to evaluate the digestive stability of phenolic contents on their reducing power and radical scavenging activities in the digested mushroom products.
Materials and methods
Chemicals
All chemicals used in the study were of analytical grade. 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one (quercetin), 2,4,6-tripyridyl-s-triazine (TPTZ), 3,4,5-trihydroxybenzoic acid (gallic acid), 3,5-dinitrosalicylic acid (DNS), 4-nitrophenyl-α-d-glucopyranoside (PNPG), 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), α-amylase (human saliva type IX-A, EC 3.2.1.1), α-amylase (porcine pancreatic Type IV-B, EC 3.2.1.1), α-glucosidase (Saccharomyces cerevisiae, EC 3.2.1.20), aluminium chloride (AlCl3), bile extract porcine, calcium chloride (CaCl2), iron(II) sulphate heptahydrate (FeSO4·7H2O), L-ascorbic acid (C6H8O6), pancreatin from porcine pancreas, pepsin from porcine gastric mucosa, potassium acetate (C2H3KO2), potassium chloride (KCl), sodium acetate trihydrate (C2H3NaO2·3H2O), starch and voglibose, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Absolute ethanol (CH3CH2OH), Folin-Ciocalteu’s reagent, fuming hydrochloric acid (HCl), glacial acetic acid (C2H4O2), iron(III) chloride hexahydrate (FeCl3·6H2O), potassium peroxodisulphate (K2O8S2), sodium carbonate (Na2CO3), sodium hydroxide (NaOH) and sodium phosphate (C2H6O) were purchased from Merck (Darmstadt, Germany). 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) was purchased from Roche (Baden-Wurttemberg, Germany). Ammonium carbonate (NH4)2CO3, magnesium chloride hexahydrate (MgCl2·6H2O), potassium dihydrogen phosphate (KH2PO4), sodium chloride (NaCl) and sodium bicarbonate (NaHCO3) and were purchased from Fisher Scientific (Loughborough, UK).
Equipments
800 W-microwave oven (Sanyo Electric Co., Ltd, Osaka, Japan), autoclave and sterilizer (Hirayama Manufacturing Corp., Saitama, Japan), centrifuge (Eppendorf AG, Hamburg, Germany), Epoch microplate spectrophotometer (Biotek Instrument Inc., Vermont, USA), electrical blender (Panasonic Corporation., Osaka, Japan), hot air oven (Memmert GmbH & Co. KG, Schwabach, Germany), hot plate stirrer (Corning Inc., New York, USA), stainless steel steam rack plate 26 cm (Satien Stainless Steel Public Co. Ltd., Pravet, Thailand), vortex mixer (IKA-Werke GmbH & Co. KG, Staufen, Germany), water bath (Memmert GmbH & Co. KG, Schwabach, Germany).
Sample collection
Five commercially-cultivated fresh mushroom species; namely, Agaricus bisporus (white button), Auricularia polytricha (black jelly), Flammulina velutipes (enokitake), Lentinula edodes (shiitake) and Pleurotus sajor-caju (grey oyster) were purchased from a local wholesale market in Semenyih, Malaysia. The selected mushrooms comprised of complete fruiting bodies and morphologically identified according to Huang (1998). Each mushroom sample (biological triplicates) was washed with distilled water and blot-dried. The edible portions were sliced into small pieces (approximate 1–2 cm3) and divided into 100 g portions for each cooking regime.
Moisture content
The moisture content of each mushroom sample was measured based on AOAC official gravimetric method 934.06 (AOAC International 2006).
Cooking and juice extraction
The cooking procedure was adapted from Ng et al. (2011) to mimic the domestic cooking styles. For pressure-cooking, each mushroom sample was autoclaved with 100 mL of distilled water at 121 °C under 2 MPa for 20 min; for steam cooking, the mushroom samples were cooked with steam on a steamer rack plate over 95 °C water in a closed water bath for 10 min; for microwave cooking, the mushroom samples were microwaved in 100 mL of distilled water with a 800 W microwave oven at high power for 10 min; for boiling, each mushroom sample was boiled in 100 mL of boiling distilled water on a hot plate at 100 °C for 10 min. The glass beakers were loosely covered to prevent evaporation during the cooking. All cooked mushroom samples and the cooking water were cooled rapidly on ice and homogenised with an electrical blender. For raw samples, one hundred grams of each mushroom (biological triplicates) were homogenised with 100 mL of distilled water. The homogenate were filtered and centrifuged at 3220×g for 20 min at 4 °C to obtain a clear supernatant. Five millilitre of each supernatant was subjected to in vitro digestion immediately while the remaining extracts were stored at − 20 °C for bioactivity analyses.
In vitro digestion
The in vitro digestion was performed according to the method by Minekus et al. (2014). In brief, five millilitre of simulated salivary fluid was mixed with equivalent volume of each mushroom extract (1 g/mL) cooked with different methods. Distilled water was used to replace the cooked mushroom extracts as digestion negative control. After the addition of 1500 U/mL α-amylase (human saliva type IX-A), the solution was adjusted to pH 7 and incubated for 2 min at 37 °C to complete the oral digestion phase. Ten millilitre of simulated gastric fluid with 25,000 U/mL pepsin was then added into the solution and adjusted to pH 3 with 1 M hydrochloric acid. The solution was incubated for 2 h at 37 °C in a shaking water bath (200 rpm) to complete the gastric digestion phase. The intestinal digestion phase was started with the addition of twenty millilitre of simulated duodenal fluid containing 800 U/mL porcine pancreatin and 160 mM bile, followed by the adjustment of pH to 7 with 1 M sodium hydroxide. The solution was then incubated for another 2 h at 37 °C in a shaking water bath (200 rpm). Lastly, the digested extracts were centrifuged at 3220×g for 15 min at 4 °C and the supernatants were stored at − 20 °C in dark. All sample extracts were analysed with three technical replicates in each of the bioassay within 2 weeks.
Ferric reducing antioxidant power (FRAP)
The ferric reducing power of the mushroom extracts (raw, cooked and digested) was measured according to Benzie and Strain (1996). FRAP reagent was prepared by mixing acetate buffer (300 mM, pH 3.6), TPTZ (10 mM in 40 mM HCl) and FeCl3·6H2O (20 mM) in the ratio of 10:1:1. Ten µL of each mushroom extract was incubated with 300 µL of FRAP reagent for 4 min at room temperature prior to the measurement of absorbance at 593 nm with a microplate spectrophotometer. Iron(III) sulphate (0–1000 µM) was used as the standard. The FRAP value was expressed as millimole iron(II) equivalent per 100 g fresh weight sample (mmol Fe2+/100 g FW).
Trolox equivalent antioxidant capacity (TEAC)
The ABTS radical scavenging activity in the mushroom extracts (raw, cooked and digested) was measured according to Re et al. (1999). ABTS radicals were generated by incubating ABTS diammonium salt (7 mM) with potassium persulfate solution (104 mM) for 12 to 16 h. The ABTS radical solution was diluted in ethanol to prepare a working ABTS reagent with an absorbance of 0.7 ± 0.02 at 734 nm. Ten µL of each mushroom extract was incubated with 100 µL of working ABTS reagent for 1 min at room temperature prior to the measurement of absorbance at 734 nm with a microplate spectrophotometer. Trolox (0–250 µg/ml) was used as the standard. The TEAC value was expressed as millimole trolox equivalent per 100 g fresh weight sample (mmol trolox/100 g FW).
DPPH radical scavenging capacity
The DPPH radical scavenging activity in the mushroom extracts (raw, cooked and digested) was measured according to Gerhauser et al. (2003). Briefly, 5 µL of each mushroom extract was incubated with 195 µL of DPPH reagent (100 µM in ethanol) at room temperature in dark. The change in the absorbance of the reaction mixture was monitored for 120 min, with 15 min interval, at 515 nm with a microplate spectrophotometer. Ascorbic acid (0–1000 µM) with half maximal inhibitory concentration (IC50) of 22.16 µM was used as the standard. The DPPH radical scavenging capacity was expressed as millimole ascorbic acid equivalent per 100 g fresh weight sample (mmol AA/100 g FW).
Water-soluble phenolic content (WPC)
The WPC in the mushroom extracts (raw, cooked and digested) was measured according to Singleton and Rossi (1965). Fifty µL of each mushroom extract was mixed with equal volume of Folin-Ciocalteu reagent (10% v/v) and incubated for 3 min at room temperature in dark, followed by the addition of 100 µL of Na2CO3 (10% w/v). The blue colour in the reaction was allowed to develop for 60 min prior to the measurement of absorbance at 750 nm with a microplate spectrophotometer. Gallic acid (0–100 µg/ml) was used as the standard. The WPC value was expressed as milligrams gallic acid equivalent per 100 g fresh weight sample (mg GAE/100 g FW).
Total flavonoid content (TFC)
The TFC in the mushroom extracts (raw, cooked and digested) was measured according to Chang et al. (2002). Twenty five µL of each mushroom extract was mixed with 75 µL ethanol, 10 µL C2H3KO2 (1 M), 10 µL AlCl3 (10% w/v) and 140 µL distilled water. After 30-min incubation at room temperature in dark, the absorbance of the reaction mixtures was measured at 415 nm with a microplate spectrophotometer. Sample blank was prepared by substituting the AlCl3 with the same amount of distilled water. Quercetin (0–1000 µg/ml) was used as the standard. The TFC value was expressed as milligrams quercetin equivalent per 100 g fresh weight sample (mg QE/100 g FW).
Anti-α-amylase activity
The anti-α-amylase activity of the mushroom extracts was measured according to Telagari and Hullatti (2015). Twenty µL of each mushroom extract was pre-incubated with 50 µL sodium phosphate buffer (100 mM, pH 6.8) and 10 µL α-amylase (2 U/mL) at 37 °C for 20 min, followed by the addition of 20 µL soluble starch (1% w/v) in sodium phosphate buffer. After 30-min incubation at 37 °C, 100 µL DNS reagent was then added and incubated for another 10 min at 95 °C. The absorbance of the reaction mixture was measured at 540 nm with a microplate spectrophotometer. Reagent blank (reaction without α-amylase) was used to exclude the background absorbance. Voglibose (0–40 mg/ml) with half maximal inhibitory concentration (IC50) of 19.33 mg/ml was used as the standard. The anti-α-amylase activity was expressed as percentage inhibition (%) as follows:
where Abs and blank refer to the absorbance and reagent blank respectively.
Anti-α-glucosidase activity
The anti-α-glucosidase activity of the mushroom extracts was measured according to Telagari and Hullatti (2015). Twenty µL of each mushroom extract was pre-incubated with 50 µL sodium phosphate buffer (100 mM, pH 6.8) and 10 µL α-glucosidase (1 U/mL) for 15 min at 37 °C. Following the addition of 20 µL PNPG substrate (5 mM), the reaction mixture was incubated for another 20 min at 37 °C prior to adding 50 µl Na2CO3 (0.1 M) to terminate the reaction. The absorbance of the reaction mixture was measured at 405 nm with a microplate spectrophotometer. Reagent blank (reaction without α-glucosidase) was used to exclude the background absorbance. Voglibose (0–40 mg/ml) with half maximal inhibitory concentration (IC50) of 10.47 mg/ml was used as the standard. The anti-α-glucosidase activity was expressed as percentage inhibition (%) as follows:
where Abs and blank refer to the absorbance and reagent blank respectively.
Antioxidant index (AI)
The AI of each mushroom extract was calculated as an average relative percentage value obtained through the five different antioxidant assays; namely, FRAP, DPPH, TEAC, WPC and TFC, It was used to relatively rank the mushroom sample extracts based on their overall antioxidant capacity according to Puttaraju et al. (2006). The highest value in each assay was considered as 100 while the remaining lower values were converted based on the numerical scale. The mushroom sample extracts were classified into high (76–100%), moderate (50–75%), low (25–49%), and very low (0–24%) AI species.
Statistical analysis
All data were expressed as mean of nine independent determinations ± standard deviation (SD). Statistical mean difference among the mushroom extracts was determined by using analysis of variance (ANOVA) with Tukey post hoc test. Pearson’s linear correlation and regression analyses were used to determine the association between the investigated activity parameters. Principle component analysis was used to determine the influence of cooking and digestion on the investigated variables in the whole data set. A p value of less than 0.05 was considered statistically significant. All data analysis was performed using GraphPad Prism 6 software (GraphPad Software, USA) and and XLSTAT 2014 software for Microsoft Excel program (Addinsoft Inc., New York, NY, USA).
Results and discussion
The moisture content, antioxidant and carbohydrate digestive enzyme inhibitory activities in raw mushrooms
All the five selected edible mushrooms in this study belonged to different genera. Their moisture content were significantly (p < 0.05) different from each other, ranging from 83.1 to 94.8%. A. bisporus showed the highest moisture content (94.8 ± 0.01%), followed by P. sajor-caju (92.5 ± 0.3%), F. velutipes (89.8 ± 0.1%), A. polytricha (84.9 ± 0.1%) and L. edodes (83.1 ± 0.2%).
The antioxidant activities in the mushroom extracts were estimated based on their ferric reducing power (FRAP) as well as radical scavenging potential (TEAC and DPPH) (Table 1). L. edodes showed the highest FRAP value, followed by A. bisporus, F. velutipes, A. polytricha and P. sajor-caju. The standard ascorbic acid used in DPPH radical scavenging assay scavenged 50% DPPH radicals at 22.16 µM as compared to 21.6 µM (Ng et al. 2011) shown in our previous study. L. edodes was the most effective radical scavengers among the raw mushrooms by showing the highest ABTS and DPPH radical scavenging activities. The TEAC values of A. bisporus, F. velutipes and P. sajor-caju were comparable while similar finding was found in the DPPH radical scavenging activities of A. polytricha, A. bisporus and F. velutipes. The WPC of raw mushroom extracts showed similar trend when compared to their antioxidant activities (Table 1). The decreasing WPC of raw mushrooms were in the following order: L. edodes > A. bisporus > F. velutipes > P. sajor-caju > A. polytricha. On the contrary, P. sajor-caju displayed the highest TFC, followed by A. bisporus, F. velutipes, L. edodes and A. polytricha.. The variation of WPC and TFC among the mushrooms suggests the presence of aglycone phenolics and their glycosides with different water-extraction efficiency. In addition, flavonoids were the major phenolic compounds for Pleurotus spp., particularly catechin, quercetin and chrysin (Mohamed and Farghaly 2014). This corresponds with the current finding that P. sajor-caju possessed the highest TFC among the five selected mushrooms. In tandem, FRAP, TEAC, WPC, and TFC values in the raw A. bisporus, F. velutipes and L. edodes samples were comparable to the values shown in previous studies (Gan et al. 2013; Ng and Tan 2017).
Table 1.
The total antioxidant activity and carbohydrate-digestive enzyme inhibition potential of raw (uncooked) mushroom samples
| Raw (uncooked) mushrooms | Antioxidant activity | Phenolic content | Carbohydrate digestive enzyme inhibitory activity | ||||
|---|---|---|---|---|---|---|---|
| FRAP (mmol Fe2+/100 g) | TEAC (mmol trolox/100 g) | DPPHe (mmol AA/100 g) | WPC (mg GAE/100 g) | TFC (mg QE/100 g) | Anti-α-amylase (% inhibition) | Anti-α-glucosidase (% inhibition) | |
| A. bisporus | 0.16 ± 0.02a | 0.08 ± 0.00a | 0.06 ± 0.00a | 26.33 ± 0.70a | 78.67 ± 8.80a | 30.58 ± 9.64a | 17.64 ± 1.33a |
| A. polytricha | 0.02 ± 0.01b | 0.01 ± 0.00b | 0.05 ± 0.00ac | 6.68 ± 0.07b | 18.78 ± 5.18b | 26.53 ± 3.30a | 13.35 ± 1.87b |
| F. velutipes | 0.12 ± 0.00a | 0.08 ± 0.01ac | 0.05 ± 0.00ac | 24.27 ± 0.10a | 41.44 ± 10.02c | 29.18 ± 2.18a | 22.64 ± 1.56c |
| L. edodes | 0.33 ± 0.02c | 0.11 ± 0.01a | 0.09 ± 0.00b | 36.44 ± 0.04c | 31.89 ± 7.29c | 33.49 ± 5.84a | 21.39 ± 1.56c |
| P. sajor-caju | 0.02 ± 0.00b | 0.06 ± 0.00c | 0.04 ± 0.01c | 14.72 ± 0.58d | 112.00 ± 5.51d | 76.38 ± 1.03b | 39.66 ± 2.08d |
Values represent mean ± standard deviations (SD) of nine independent determinations. Values within the same column followed by different superscript lower case letters (a, b, c, d) are significantly different at p < 0.05
eThe half maximal inhibitory concentration (IC50) for ascorbic acid standard was 22.16 µM
The management of diabetes mellitus is associated with the control of postprandial hyperglycemia. The two key rate-limiting carbohydrate-digestive enzymes are pancreatic α-amylase and intestinal α-glucosidase (Telagari and Hullatti 2015). The inhibition of these enzymes can delay the digestion of polysaccharide in human intestine and thus reduce the postprandial blood sugar. In this study, the half maximal inhibitory concentration (IC50) of positive control, voglibose used in the anti-α-amylase and anti-α-glucosidase assays were comparable to a previous finding (Telagari and Hullatti 2015). The anti-α-amylase activities (26.5–76.4%) in the mushroom extracts were generally stronger than their anti-α-glucosidase activities (13.4–39.7%) (Table 1) but weaker than the positive control. Interestingly, P. sajor-caju with the highest TFC also showed the highest anti-α-amylase and anti-α-glucosidase activities. Comparable anti-α-amylase and anti-α-glucosidase activities were found in L. edodes, A. bisporus and F. velutipes extracts while A. polytricha possessed the lowest activities.
Based on the AI value shown in Table 2, the raw edible mushroom samples were grouped into three categories: high, moderate and very low AI species. L. edodes was ranked as high AI mushroom species due to its consistent strong activity trend shown across the 4 antioxidant assays (FRAP, TEAC, DPPH and WPC). This was in agreement with Cheung et al. (2003) which reported high phenolic and radical scavenging activity in the water extract of L. edodes. Three mushrooms with AI between 51 and 66; namely, A. bisporus, F. velutipes and P. sajor-caju, were classified as moderate AI mushroom species. A. polytricha with its relatively low activity trend in three antioxidant assays, was labelled as very low AI mushroom species. When combined with other therapeutic properties found in the mushrooms such as their carbohydrate-digestive enzymes inhibition values, the AI could be an alternative approach to rank the potential health benefit of mushrooms.
Table 2.
The antioxidant index (AI) of five raw (uncooked) mushroom samples
| Raw sample | FRAP (mmol Fe2+/100 g) | WPC (mg GAE/100 g) | TFC (mg QE/100 g) | TEAC (mmol trolox/100 g) | DPPHa (mmol AA/100 g) | Relative % FRAP | Relative % WPC | Relative % TFC | Relative %TEAC | Relative %DPPH | AIa | Categoryb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. edodes | 0.33 | 36.44 | 31.89 | 0.11 | 0.09 | 100 | 100 | 29 | 100 | 100 | 86 | High |
| A. bisporus | 0.16 | 26.33 | 78.67 | 0.08 | 0.06 | 49 | 72 | 70 | 73 | 67 | 66 | Moderate |
| F. velutipes | 0.12 | 24.27 | 41.44 | 0.08 | 0.05 | 36 | 67 | 37 | 73 | 56 | 54 | Moderate |
| P. sajor-caju | 0.02 | 14.72 | 112.00 | 0.06 | 0.04 | 6 | 40 | 100 | 55 | 56 | 51 | Moderate |
| A. polytricha | 0.02 | 6.68 | 18.78 | 0.01 | 0.05 | 6 | 18 | 17 | 9 | 56 | 21 | Very low |
Relative percentage value calculated based on the highest value in each antioxidant assay
aAverage value of the relative percentages from five antioxidant assays
bAI species category: high (76–100%), moderate (50–75%), low (25–49%), and very low (0–24%)
Effect of domestic cooking on the antioxidant and carbohydrate-digestive enzyme inhibition activities in the mushrooms
Table 3 depicts the changes of antioxidant and carbohydrate-digestive enzyme inhibition activities in five edible mushrooms cooked with different methods. Regardless of cooking methods, the FRAP values of cooked F. velutipes and L. edodes samples were significantly (p < 0.05) decreased by at least 50% when compared to their raw counterparts. Pressure cooking with prolonged cooking time (20 min) exerted the strongest impact on F. velutipes (− 67%). However, boiling (150%) and steaming (350%) significantly (p < 0.05) increased the FRAP values of A. polytricha. Similar finding was found in the boiled (350%), microwaved (50%) and pressured cooked (200%) samples of P. sajor-caju. On the contrary, the FRAP value of A. bisporus was not affected after the cooking. The radical scavenging activities in the mushroom extracts were less affected by the cooking process when compared to their FRAP values. Pressure cooking generally decreased the TEAC values of A. bisporus (− 75%) and F. velutipes (− 38%). Similar trend was observed in L. edodes cooked by steam and microwave (− 45%) as well as in P. sajor-caju’s boiled (− 33%) and microwaved (− 17%) extracts. However, boiling (400%), microwaving (400%) and pressure cooking (300%) increased the TEAC value of A. polytricha. All cooking methods significantly improved the DPPH radical scavenging activities of F. velutipes (160–200%), L. edodes (33–150%) and P. sajor-caju (120–160%). Tan et al. (2015) reported a decrease of DPPH radical scavenging activity in Pleurotus spp. after the cooking process. This conflicting result could be attributed to the different mushroom species and cooking duration used in their study. The improvement of antioxidant activity in the cooked mushroom extracts could be contributed by the leeching of active antioxidants from the fibrous complexes of mushroom matrix into the cooking water. On the contrary, boiling, microwaving and steaming significantly decreased the DPPH radical scavenging activity in A. bisporus (− 33% to − 50%) and A. polytricha (− 60%) extracts.
Table 3.
The total antioxidant activity and carbohydrate-digestive enzyme inhibition potential of cooked mushroom samples
| Cooked mushrooms | Antioxidant activity | Phenolic content | Carbohydrate digestive enzyme inhibitory potential | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FRAP (mmol Fe2+/100 g) | ∆ (%) | TEAC (mmol trolox/100 g) | ∆ (%) | DPPH (mmol AA/100 g) | ∆ (%) | WPC (mg GAE/100 g) | ∆ (%) | TFC (mg QE/100 g) | ∆ (%) | Anti-α-amylase (% inhibition) | ∆ (%) | Anti-α-glucosidase (% inhibition) | ∆ (%) | |
| A. bisporus | ||||||||||||||
| b | 0.13 ± 0.00 | 0.11 ± 0.01 | 0.03 ± 0.00b | − 50 | 7.32 ± 0.97b | − 72 | 46.89 ± 1.25 | 7.95 ± 3.55a | − 74 | 16.73 ± 2.10a | − 5 | |||
| m | 0.11 ± 0.00 | 0.11 ± 0.00a | 38 | 0.01 ± 0.01b | − 83 | 9.85 ± 0.18d | − 63 | 43.39 ± 4.66 | 90.86 ± 5.91a | 197 | 19.42 ± 2.99 | |||
| s | 0.11 ± 0.00 | 0.11 ± 0.01 | 0.04 ± 0.01a | − 33 | 8.09 ± 0.38c | − 69 | 32.83 ± 4.51b | 55.81 ± 2.19 | 21.09 ± 3.65 | |||||
| p | 0.11 ± 0.00 | 0.02 ± 0.01b | − 75 | 0.04 ± 0.01 | 10.03 ± 0.11c | − 62 | 59.11 ± 10.30b | 88.87 ± 0.45a | 191 | 14.67 ± 2.01 | ||||
| A. polytricha | ||||||||||||||
| b | 0.05 ± 0.00a | 150 | 0.05 ± 0.00b | 400 | 0.18 ± 0.01d | 260 | 4.76 ± 0.05d | − 29 | 15.89 ± 7.32 | 9.43 ± 6.71 | 15.96 ± 0.52 | |||
| m | 0.02 ± 0.00 | 0.05 ± 0.01b | 400 | 0.16 ± 0.01d | 220 | 4.60 ± 0.03d | − 31 | 23.56 ± 7.22 | 41.57 ± 6.48 | 10.55 ± 3.14 | ||||
| s | 0.09 ± 0.00b | 350 | 0.05 ± 0.01 | 0.02 ± 0.00a | − 60 | 6.60 ± 0.48 | 41.11 ± 4.63b | 119 | 43.29 ± 4.63 | 0.88 ± 0.58a | − 93 | |||
| p | 0.01 ± 0.00 | 0.04 ± 0.01b | 300 | 0.17 ± 0.01d | 240 | 2.71 ± 0.09d | − 59 | 16.78 ± 7.43 | 76.70 ± 3.40b | 189 | 10.11 ± 2.69 | |||
| F. velutipes | ||||||||||||||
| b | 0.05 ± 0.00b | − 58 | 0.06 ± 0.01 | 0.13 ± 0.01b | 160 | 7.08 ± 0.14d | − 71 | 27.28 ± 3.13 | 34.07 ± 2.35a | 17 | 27.93 ± 3.46 | |||
| m | 0.06 ± 0.01a | − 50 | 0.06 ± 0.00 | 0.14 ± 0.01a | 180 | 6.83 ± 0.16d | − 72 | 31.06 ± 5.25 | 50.36 ± 1.19b | 73 | 15.13 ± 1.85 | |||
| s | 0.05 ± 0.00c | − 58 | 0.05 ± 0.00 | 0.15 ± 0.00d | 200 | 8.37 ± 0.34d | − 66 | 53.17 ± 8.59 | 42.64 ± 2.04a | 46 | 5.36 ± 1.12b | − 76 | ||
| p | 0.04 ± 0.00d | − 67 | 0.05 ± 0.00a | − 38 | 0.14 ± 0.01b | 180 | 6.82 ± 0.20d | − 72 | 47.00 ± 16.12 | 86.16 ± 2.84b | 195 | 11.62 ± 0.88a | − 49 | |
| L. edodes | ||||||||||||||
| b | 0.12 ± 0.00b | − 64 | 0.08 ± 0.01 | 0.08 ± 0.01 | 33 | 7.98 ± 0.62d | − 78 | 49.89 ± 6.80 | 34.38 ± 6.43 | 16.73 ± 2.10 | ||||
| m | 0.13 ± 0.00b | − 61 | 0.06 ± 0.01a | − 45 | 0.09 ± 0.00c | 50 | 8.15 ± 0.53d | − 78 | 46.72 ± 12.61 | 50.64 ± 5.62 | 6.27 ± 1.62a | − 71 | ||
| s | 0.12 ± 0.00b | − 64 | 0.06 ± 0.01b | − 45 | 0.08 ± 0.01 | 33 | 7.07 ± 0.21d | − 81 | 70.61 ± 11.17 | 5.33 ± 2.78a | 4.41 ± 2.17a | − 79 | ||
| p | 0.12 ± 0.00b | − 64 | 0.07 ± 0.01 | 0.15 ± 0.01a | 150 | 5.39 ± 0.04d | − 85 | 69.11 ± 2.55a | 117 | 67.98 ± 3.10a | 103 | 4.29 ± 1.78b | − 80 | |
| P. sajor - caju | ||||||||||||||
| b | 0.09 ± 0.01b | 350 | 0.04 ± 0.00a | − 33 | 0.12 ± 0.01a | 140 | 6.94 ± 0.22b | − 53 | 57.11 ± 11.11a | − 49 | 16.13 ± 3.70b | − 79 | 3.44 ± 3.46a | − 91 |
| m | 0.03 ± 0.00a | 50 | 0.05 ± 0.00a | − 17 | 0.13 ± 0.00b | 160 | 9.46 ± 0.23b | − 36 | 42.94 ± 5.23a | − 62 | 61.41 ± 2.78a | − 20 | 19.32 ± 1.75a | − 51 |
| s | 0.03 ± 0.00 | 0.05 ± 0.01 | 0.12 ± 0.01a | 140 | 7.98 ± 0.63a | − 46 | 38.78 ± 6.31a | − 65 | 58.40 ± 1.91c | − 24 | 24.14 ± 1.07b | − 39 | ||
| p | 0.06 ± 0.00b | 200 | 0.06 ± 0.01 | 0.11 ± 0.01b | 120 | 8.86 ± 0.39b | − 40 | 61.33 ± 12.09a | − 45 | 67.28 ± 1.84a | − 12 | 13.98 ± 2.01a | − 65 | |
The values are presented as mean ± standard deviations (SD) of nine independent determinations; ∆—Significant variation when compared to the respective raw (uncooked) sample; b—boiled samples; m—microwaved samples; p—pressure cooked samples; s—steamed samples; values within the same column followed by superscript lower case letters ‘a’ (p < 0.05), ‘b’ (p < 0.01), ‘c’ (p < 0.005) and ‘d’ (p < 0.001) were statistically different from their respective control species in Table 1
WPC in all mushroom extracts were significantly decreased by 29% to 85% after the cooking process. The decrease of WPC in all cooked mushroom extracts, particularly F. velutipes and L. edodes, were accompanied by the decrease of their FRAP values, suggesting the contribution of WPC to their antioxidant activity. However, this trend was absent in A. bisporus, A. polytricha and P. sajor-caju, possibly due to the presence of heat-resistant antioxidant compounds other than phenolic such as β-glucan and amino acid, ergothioneine (Soler-Rivas et al. 2009) as well as heat-stable superoxide dismutase (Cheng et al. 2012) and quinone oxidoreductase (Ng et al. 2014). Steaming and pressure-cooking improved the TFC in A. polytricha and L. edodes by 119% and 117% respectively but significant loss of TFC was observed in all the cooked P. sajor-caju extracts. Overall, cooking had lesser impact on the TFC in the mushroom extracts when compared to the WPC. This study hypothesizes that the thick and firm cell wall structure of mushrooms protected the flavonoids from degradation by cooking heat.
The anti-α-amylase activities in all cooked P. sajor-caju extracts were significantly reduced by 12% to 79%. However, opposite trend was found in F. velutipes extracts (17–195%). Interestingly, pressure-cooking significantly improved the anti-α-amylase activities of all mushrooms by as much as 103% to 195% except for P. sajor-caju. Cooking either decreased or caused no significant change in the anti-α-glucosidase activity in the mushrooms. Significant loss of anti-α-glucosidase activities were recorded in boiled A. bisporus (− 5%) and pressure-cooked samples of F. velutipes (− 49%) and L. edodes (− 80%). The decreasing effect of steam cooking on anti-α-glucosidase activity in the mushroom extracts was in the following order: A. polytricha (− 93%) > L. edodes (− 79%) > F. velutipes (− 76%) > P. sajor-caju (− 39%). All cooking methods significantly decreased the anti-α-glucosidase activity in P. sajor-caju, possibly due to the formation of phenol–protein complex with impaired activity (Jimenez-Monreal et al. 2009). The differential effects observed on the carbohydrate–digestive enzyme inhibition activity in the cooked mushroom extracts were likely due to the presence of phytochemicals with different thermal stability that influenced their binding with the carbohydrate-digestive enzymes (Silva et al. 2018). This study is in agreement with previous similar findings (Ng and Tan 2017; Sánchez 2017) that domestic cooking could either increase, decrease or cause no significant change to the antioxidant and carbohydrate-digestive enzyme inhibitory activities in the mushrooms.
Effect of in vitro digestion on the antioxidant and carbohydrate digestive enzyme inhibition activities in the cooked mushrooms
The antioxidant and carbohydrate-digestive enzyme inhibition activities of in vitro digested mushroom extracts were compared to their respective cooked counterparts (Table 4). In vitro digestion significantly increased the ferric reducing power of all cooked mushroom extracts by 80% to 1350% except for pressure-cooked L. edodes (− 75%). Among the digested samples, microwaved A. polytricha extract showed the highest increment in FRAP value. Similar trend was observed for the TEAC (45–2550%), DPPH (42–1400%) and TFC (437–3097%) in all cooked mushroom extracts after the digestion process. Pressure-cooked and microwaved A. bisporus extracts recorded the highest increment in TEAC and DPPH radical scavenging values respectively after the digestion. In vitro digestion also caused the highest increment in both TFC (3097%) and WPC (281%) of pressure-cooked A. polytricha extract.
Table 4.
The total antioxidant activity and carbohydrate-digestive enzyme inhibition potential of cooked and in vitro digested mushroom samples
| Cooked and digested mushrooms | Antioxidant activity | Phenolic content | Carbohydrate digestive enzyme inhibitory potential | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FRAP (mmol Fe2+/100 g) | ∆ (%) | TEAC (mmol trolox/100 g) | ∆ (%) | DPPH (mmol AA/100 g) | ∆ (%) | WPC (mg GAE/100 g) | ∆ (%) | TFC (mg QE/100 g) | ∆ (%) | Anti-α-amylase (% inhibition) | ∆ (%) | Anti-α-glucosidase (% inhibition) | ∆ (%) | |
| A. bisporus | ||||||||||||||
| b & d | 0.32 ± 0.01b | 146 | 0.23 ± 0.06b | 109 | 0.28 ± 0.00d | 833 | 15.16 ± 1.30a | 107 | 476.44 ± 14.75d | 916 | 27.26 ± 6.74a | 243 | 28.40 ± 7.77 | |
| m & d | 0.27 ± 0.01c | 145 | 0.39 ± 0.11b | 255 | 0.15 ± 0.00d | 1400 | 5.16 ± 1.06a | − 48 | 384.00 ± 16.71c | 785 | 9.77 ± 3.91b | − 89 | 24.70 ± 12.85 | |
| s & d | 0.29 ± 0.02b | 163 | 0.16 ± 0.06b | 45 | 0.24 ± 0.02b | 500 | 10.96 ± 1.76 | 225.78 ± 33.77a | 588 | 53.15 ± 5.52 | 20.75 ± 1.21 | |||
| p & d | 0.20 ± 0.00b | 82 | 0.53 ± 0.07b | 2550 | 0.33 ± 0.02b | 725 | 9.32 ± 0.73 | 317.33 ± 6.67c | 437 | 5.82 ± 2.40 | 15.44 ± 1.76 | |||
| A. polytricha | ||||||||||||||
| b & d | 0.14 ± 0.02a | 180 | 0.21 ± 0.03c | 320 | 0.32 ± 0.01d | 78 | 2.82 ± 1.20 | 479.56 ± 14.87c | 2918 | 44.36 ± 10.23 | 17.47 ± 3.00 | |||
| m & d | 0.29 ± 0.00d | 1350 | 0.44 ± 0.05d | 780 | 0.35 ± 0.02b | 119 | 11.76 ± 0.73b | 156 | 416.00 ± 10.07d | 1666 | 0.35 ± 0.07a | − 99 | 76.88 ± 6.50b | 629 |
| s & d | 0.19 ± 0.01b | 111 | 0.20 ± 0.09b | 300 | 0.16 ± 0.03a | 700 | 7.82 ± 1.30 | 32.89 ± 35.51 | 24.52 ± 1.59a | − 43 | 11.89 ± 0.48b | 1251 | ||
| P & d | 0.05 ± 0.00b | 80 | 0.34 ± 0.07a | 750 | 0.36 ± 0.00c | 112 | 10.32 ± 0.47c | 281 | 536.44 ± 35.81b | 3097 | 69.75 ± 13.61 | 13.69 ± 0.95 | ||
| F. velutipes | ||||||||||||||
| b & d | 0.05 ± 0.01 | 0.32 ± 0.03c | 433 | 0.32 ± 0.06a | 146 | 8.45 ± 1.30 | 279.56 ± 15.40b | 925 | 15.32 ± 3.33a | − 55 | 19.99 ± 9.51 | |||
| m & d | 0.44 ± 0.05b | 633 | 0.36 ± 0.05c | 500 | 0.59 ± 0.05b | 321 | 8.35 ± 0.66 | 496.89 ± 10.78d | 1500 | 28.81 ± 6.41 | 23.94 ± 9.06 | |||
| s & d | 0.07 ± 0.03 | 0.29 ± 0.03d | 480 | 0.22 ± 0.01a | 47 | 7.22 ± 1.29 | 290.67 ± 36.97b | 447 | 24.92 ± 10.90 | 22.48 ± 1.56a | 319 | |||
| p & d | 0.12 ± 0.00d | 200 | 0.18 ± 0.06b | 260 | 0.31 ± 0.03a | 121 | 7.55 ± 0.39 | 260.44 ± 15.91a | 454 | 10.05 ± 3.69d | − 88 | 38.87 ± 18.18 | ||
| L. edodes | ||||||||||||||
| b & d | 0.30 ± 0.01d | 150 | 0.26 ± 0.04c | 225 | 0.29 ± 0.01c | 263 | 8.45 ± 2.06 | 451.11 ± 13.62c | 804 | 23.15 ± 4.55 | 51.60 ± 10.27 | |||
| m & d | 0.46 ± 0.01d | 254 | 0.48 ± 0.04c | 700 | 0.29 ± 0.01b | 222 | 17.58 ± 2.52a | 116 | 440.00 ± 31.44b | 842 | 10.97 ± 9.60a | − 78 | 32.15 ± 4.68b | 413 |
| s & d | 0.28 ± 0.02a | 133 | 0.34 ± 0.08b | 467 | 0.29 ± 0.02a | 263 | 11.60 ± 2.14 | 498.22 ± 30.41b | 606 | 45.47 ± 7.14a | 753 | 11.46 ± 0.84 | ||
| p & d | 0.03 ± 0.00d | − 75 | 0.37 ± 0.05c | 429 | 0.28 ± 0.01a | 87 | 9.85 ± 1.93 | 477.33 ± 2.67d | 591 | 11.77 ± 4.74b | − 83 | 18.60 ± 2.40a | 334 | |
| P. sajor - caju | ||||||||||||||
| b & d | 0.20 ± 0.01c | 122 | 0.48 ± 0.03b | 1100 | 0.24 ± 0.02b | 100 | 3.99 ± 0.85a | − 43 | 460.31 ± 12.16c | 706 | 52.76 ± 27.29 | 17.48 ± 4.59 | ||
| m & d | 0.16 ± 0.03a | 433 | 0.36 ± 0.05c | 620 | 0.29 ± 0.01b | 123 | 3.80 ± 1.40a | − 60 | 789.33 ± 21.17d | 1738 | 8.82 ± 5.52b | − 86 | 12.39 ± 0.55a | − 36 |
| s & d | 0.12 ± 0.00b | 300 | 0.31 ± 0.07b | 520 | 0.17 ± 0.00a | 42 | 5.51 ± 1.24 | 48.89 ± 30.30 | 57.10 ± 28.85 | 4.05 ± 2.76a | − 83 | |||
| p & d | 0.10 ± 0.02 | 0.41 ± 0.03d | 583 | 0.36 ± 0.02d | 227 | 5.96 ± 1.32 | 405.33 ± 23.36b | 561 | 16.13 ± 2.34b | − 76 | 10.91 ± 8.37 | |||
The values are presented as mean ± standard deviations (SD) of nine independent determinations; ∆—Significant variation when compared to the respective cooked sample; b & d—boiled and digested samples; m & d—microwaved and digested samples; p & d– pressure cooked and digested samples; s & d—steamed and digested samples; Values within the same column followed by superscript letters ‘a’ (p < 0.05), ‘b’ (p < 0.01), ‘c’ (p < 0.005) and ‘d’ (p < 0.001) were statistically different from the respective cooked species in Table 3
In general, in vitro digestion improved the overall total antioxidant activity in the cooked mushroom extracts and this is in agreement with Chohan et al. (2012). The further breakdown of mushroom’s cell wall matrix during the digestion process could facilitate the release of compounds with radical scavenging properties from the vacuole cells (Chandrasekara and Shahidi 2012). Besides, cooking has been shown to enhance the stability of antioxidant compounds under extreme pH condition through the formation of secondary structures (Kim et al. 2010). This study depicts the differential effect of digestion on WPC in the boiled and microwaved mushroom extracts. For instance, among the digested microwaved samples, significant increase in WPC was observed for A. polytricha (156%) and L. edodes (116%) but opposite trend was found in A. bisporus (− 48%) and P. sajor-caju (− 60%).
The effect of in vitro digestion on the carbohydrate-digestive enzymes inhibition activity varied according to the mushroom species and cooking methods applied. Digestion significantly decreased the anti-α-amylase activity in four microwaved mushroom extracts (A. bisporus, A. polytricha, L. edodes and P. sajor-caju) and three pressured-cooked mushroom extracts (F. velutipes, L. edodes and P. sajor-caju). Among these digested extracts, microwaved A. polytricha recorded the highest loss (− 99%) in activity. The decreased of anti-α-amylase activity in the cooked mushroom extracts after the intestinal digestion phase could be caused by the proteolytic action of trypsin on enzyme inhibitors (Frels and Rupnow 1985). In contrast, boiled A. bisporus (243%) and steamed L. edodes (753%) extracts showed significant improvement in anti-α-amylase activity after the digestion process. In vitro digestion also improved the anti-α-glucosidase activity in the microwaved (629%) and steamed (1251%) extracts of A. polytricha, steamed extract of F. velutipes (319%), microwaved (413%) and pressure-cooked (334%) extracts of L. edodes. Tadera et al. (2006) showed that gastrointestinal digestion could induce the release of aglycone phenolic acids with higher enzyme inhibition activity from the glycosides. However, the anti-α-glucosidase activities in the microwaved (− 36%) and steamed (− 83%) P. sajor-caju extracts were significantly decreased after the digestion process. Nevertheless, all cooked A. bisporus extracts were able to maintain their anti-α-glucosidase activities after the digestion.
Linear correlation and regression analysis
In this study, mushroom extracts with high ferric reducing power and radical scavenging activity were accompanied by high WPC and TFC, implying the contribution of phenolic compounds to the observed total antioxidant activity. This was supported by the significant positive correlation found between the total antioxidant activities (FRAP, TEAC, DPPH) and the phenolic contents (WPC & TFC) in the mushrooms (Table 5). Besides, the FRAP value was positively correlated with both TEAC (r = 0.546, p < 0.005) and DPPH radical scavenging values (r = 0.472, p < 0.05). It is pertinent to suggest the antioxidant compounds in the selected mushrooms were not only good reducing agents but also potent radical scavengers.
Table 5.
Linear correlation of seven activity variables in the five edible mushroom samples
| Variables | FRAP | WPC | TFC | TEAC | DPPH | Anti-α-amylase activity | Anti-α-glucosidase activity |
|---|---|---|---|---|---|---|---|
| FRAP | 1.000 | ||||||
| WPC | 0.372a | 1.000 | |||||
| TFC | 0.499c | 0.848b | 1.000 | ||||
| TEAC | 0.546c | 0.378 | 0.818d | 1.000 | |||
| DPPH | 0.472a | 0.159 | 0.792d | 0.744d | 1.000 | ||
| Anti-α-amylase activity | − 0.339a | − 0.059 | − 0.363a | − 0.442b | − 0.358a | 1.000 | |
| Anti-α-glucosidase activity | 0.385b | 0.241 | 0.323a | 0.320a | 0.299b | 0.184 | 1.000 |
The values denote the Pearson’s correlation coefficient of 45 pairs of samples. The level of significance was expressed as p < 0.05 (a); p < 0.01 (b); p < 0.005 (c) and p < 0.001 (d)
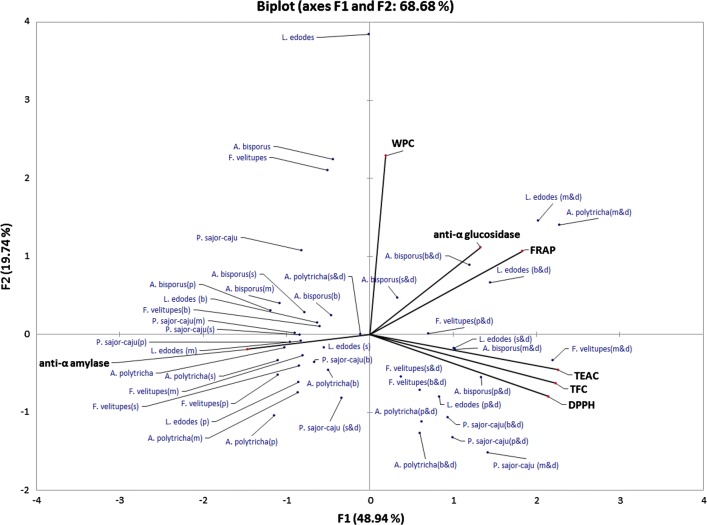
TFC in the mushroom extracts showed a weak but significant positive correlation with anti-α-glucosidase activity (r = 0.323, p < 0.05), possibly due to the effect of digestion which altered the flavonoid structural stability and rendered their binding to the enzymes. This explains the partial contribution of flavonoids to the anti-α-glucosidase activity in the mushroom extracts. The galloyl groups of plant flavonoids have been shown as potent inhibitors for yeast α-glucosidase (Tadera et al. 2006). However, TFC was found to be inversely correlated with anti-α-amylase activity (r = − 0.363, p < 0.05). Although WPC showed similar finding with both carbohydrate-digestive enzyme inhibition activities, they were not significant. The weak positive correlation found between the various antioxidant values (FRAP, r = 0.385, p < 0.01; TEAC, r = 0.320, p < 0.05; DPPH, r = 0.299, p < 0.01) and anti-α-glucosidase activity may suggest labile compounds other than antioxidants contributed to the carbohydrate-digestive enzymes inhibition activities in the mushroom extracts. For instance, the active form of β-glucan such as lentinan and pleuran found in L. edodes and Pleurotus spp. respectively have been shown to possess strong hypoglycaemic activity (Bach et al. 2017). This was further supported by the PCA result in this study that the anti-α-amylase activity was independent from the other activity groups (Fig. 1).
Fig. 1.
Principal component analysis of mushroom samples. DPPH 2,2-diphenyl-1-picryl-hydrazyl radical scavenging activity, FRAP ferric reducing antioxidant power, TEAC ferric reducing antioxidant power-ascorbic acid, TFC total flavonoid content, WPC water-soluble phenolic content; b—boiled samples; d—in vitro digested samples; m—microwaved samples; p—pressure cooked samples; s—steamed samples
Principle component analysis (PCA)
PCA was used to provide an overview on the source of activity variation in the edible mushroom extracts subjected to different cooking methods and in vitro digestion process (Fig. 1). The two principle components shown in the biplot collectively represented the largest cumulative variance of 68.6% found in the data set. The first sub-component F1 accounted for 48.9% variation while the second sub-component F2 predicted another 19.7% variation. The x-axis (sub-component F1) separated the raw and cooked mushroom water extracts (negative area plot) from their digested counterparts (positive area plot), suggesting the 48.9% of variation corresponded to the antioxidant and carbohydrate-digestive enzymes inhibition activities in the mushroom extracts was caused by the in vitro digestion process.
A clear separation of bioactivities in different mushroom extracts was observed in the second sub-component F2. The positive area on F2 axis was associated with WPC, ferric reducing power and anti-α-glucosidase activity while the negative area was linked with TFC, radical scavenging and anti-α-amylase parameters. These activity variables could be further separated into 3 activity groups with each of them located in different quadrants of the score plot. The first group consisted of WPC, FRAP and anti-α-glucosidase variables; the second group was made up of TFC and radical scavenging (DPPH and TEAC) variables while the last group was solely occupied by anti-α-amylase variable. The similar vector direction displayed by the variables in each of three activity groups indicated a strong relationship among them. For instance, the anti-α-amylase variable was closely associated with the different domestic cooking applied to the selected mushrooms. On the contrary, digestion was found to be closely associated with the antioxidant (WPC, TFC, FRAP, TEAC and DPPH) and anti-α-glucosidase activities in the mushroom extracts. The above findings confirmed the influence of digestion and cooking process, at least partly, on the antioxidant and carbohydrate-digestive enzyme inhibition activities in the selected edible mushroom species.
Conclusion
In summary, the five edible mushrooms selected in this study could act as alternate food source for antioxidant and carbohydrate-digestive enzymes inhibitors. This study shows the potential use of heat treatment such as domestic cooking to potentiate the antioxidant and carbohydrate-digestive enzymes inhibition activities in the mushroom water extracts. To achieve the best antioxidant and carbohydrate-digestive enzymes inhibition values, A. bisporus and F. velutipes were best cooked with microwave while boiling was preferred for A. polytricha. Pressure cooking was shown as the preferred cooking method for L. edodes and P. sajor-caju. In vitro digestion could produce mushroom products with high anti-α-glucosidase but low anti-α-amylase activities for the control of postprandial hyperglycaemia with minimal side effect. This study did not compare the bioavailability of bioactive compounds liberated from the mushroom extracts and absorbed through the intestinal borders. Further investigation with an in vivo model is required to provide a more comprehensive nutritional bioavailability profile across different mushroom species. Considering the vast consumption of mushrooms among the folks, the knowledge of heat treatment and in vitro digestion on the bioaccessibility of nutritional compounds in the mushrooms will benefit both the consumers and food manufacturers who aim for healthy food preparation, labelling and production.
Acknowledgements
This study was supported by University of Nottingham Faculty of Sciences Pump Priming research Grant P071/17.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhi Xiang Ng, Phone: +6(03) 8725 3616, Email: ngzx_86@yahoo.com, Email: ZhiXiang.Ng@nottingham.edu.my.
Nureen Farhana Rosman, Email: khyy5nfr@nottingham.edu.my.
References
- Aida FMNA, Shuhaimi M, Yazid M, Maaruf AG. Mushroom as a potential source of prebiotics: a review. Trends Food Sci Technol. 2009;20(11–12):567–575. [Google Scholar]
- AOAC (2006) Official methods of analysis proximate analysis and calculations moisture (M) fruits, vegetables, and their products - item 107. In: Horwitz W, Latimer GW (eds) Food analysis methods, vol 4, 18th edn. Association of Analytical Communities International, Gaithersburg, MD, p 2
- Bach F, Helm CV, Bellettini MB, Maciel GM, Haminiuk CWI. Edible mushrooms: a potential source of essential amino acids, glucans and minerals. Int J Food Sci Technol. 2017;52(11):2382–2392. [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Chandrasekara A, Shahidi F. Bioaccessibility and antioxidant potential of millet grain phenolics as affected by simulated in vitro digestion and microbial fermentation. J Funct Foods. 2012;4(1):226–237. [Google Scholar]
- Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10(3):178–182. [Google Scholar]
- Cheng GY, Liu J, Tao MX, Lu CM, Wu GR. Activity, thermostability and isozymes of superoxide dismutase in 17 edible mushrooms. J Food Compos Anal. 2012;26(1–2):136–143. [Google Scholar]
- Cheung L, Cheung PC, Ooi VE. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003;81(2):249–255. [Google Scholar]
- Chohan M, Naughton DP, Jones L, Opara EI. An investigation of the relationship between the anti-inflammatory activity, polyphenolic content, and antioxidant activities of cooked and in vitro digested culinary herbs. Oxid Med Cell Longev. 2012;2012:627843. doi: 10.1155/2012/627843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva DD, Rapior S, Hyde KD, Bahkali AH. Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Divers. 2012;56(1):1–29. [Google Scholar]
- Frels JM, Rupnow JH. Characterization of two α-amylase inhibitors from black bean (Phaseolus vulgaris) J Food Sci. 1985;50(1):72–77. [Google Scholar]
- Gan C, Amira NB, Asmah R. Antioxidant analysis of different types of edible mushrooms (Agaricus bisporous and Agaricus brasiliensis) Int Food Res J. 2013;20(3):1095. [Google Scholar]
- Gerhauser C, Klimo K, Heiss E, Neumann I, Gamal-Eldeen A, Knauft J, Liu GY, Sitthimonchai S, Frank N. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat Res. 2003;523–524:163–172. doi: 10.1016/s0027-5107(02)00332-9. [DOI] [PubMed] [Google Scholar]
- Ghorai S, Banik SP, Verma D, Chowdhury S, Mukherjee S, Khowala S. Fungal biotechnology in food and feed processing. Food Res Int. 2009;42(5–6):577–587. [Google Scholar]
- Heleno SA, Barros L, Martins A, Morales P, Fernández-Ruiz V, Glamoclija J, Sokovic M, Ferreira IC. Nutritional value, bioactive compounds, antimicrobial activity and bioaccessibility studies with wild edible mushrooms. LWT Food Sci Technol. 2015;63(2):799–806. [Google Scholar]
- Huang NL. Colored illustrations of macrofungi (mushrooms) of China. Beijing: China Agriculture Press; 1998. [Google Scholar]
- Jimenez-Monreal AM, Garcia-Diz L, Martinez-Tome M, Mariscal M, Murcia MA. Influence of cooking methods on antioxidant activity of vegetables. J Food Sci. 2009;74(3):H97–H103. doi: 10.1111/j.1750-3841.2009.01091.x. [DOI] [PubMed] [Google Scholar]
- Kim T, Silva J, Kim M, Jung Y. Enhanced antioxidant capacity and antimicrobial activity of tannic acid by thermal processing. Food Chem. 2010;118(3):740–746. [Google Scholar]
- Li N, Li L, Fang JC, Wong JH, Ng TB, Jiang Y, Wang CR, Zhang NY, Wen TY, Qu LY. Isolation and identification of a novel polysaccharide–peptide complex with antioxidant, anti-proliferative and hypoglycaemic activities from the abalone mushroom. Biosci Rep. 2012;32(3):221–228. doi: 10.1042/BSR20110012. [DOI] [PubMed] [Google Scholar]
- Minekus M, Alminger M, Alvito P, Ballance S, Bohn T, Bourlieu C, Carriere F, Boutrou R, Corredig M, Dupont D, Dufour C, Egger L, Golding M, Karakaya S, Kirkhus B, Le Feunteun S, Lesmes U, Macierzanka A, Mackie A, Marze S, McClements DJ, Menard O, Recio I, Santos CN, Singh RP, Vegarud GE, Wickham MS, Weitschies W, Brodkorb A. A standardised static in vitro digestion method suitable for food—an international consensus. Food Funct. 2014;5(6):1113–1124. doi: 10.1039/c3fo60702j. [DOI] [PubMed] [Google Scholar]
- Mohamed EM, Farghaly FA. Bioactive compounds of fresh and dried Pleurotus ostreatus mushroom. Int J Biotechnol Wellness Ind. 2014;3(1):4–14. [Google Scholar]
- Ng ZX, Tan WC. Impact of optimised cooking on the antioxidant activity in edible mushrooms. J Food Sci Technol. 2017;54(12):4100–4111. doi: 10.1007/s13197-017-2885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng ZX, Chai JW, Kuppusamy UR. Customized cooking method improves total antioxidant activity in selected vegetables. Int J Food Sci Nutr. 2011;62(2):158–163. doi: 10.3109/09637486.2010.526931. [DOI] [PubMed] [Google Scholar]
- Ng ZX, Chua KH, Kuppusamy UR. Proteomic analysis of heat treated bitter gourd (Momordica charantia L. var. Hong Kong Green) using 2D-DIGE. Food Chem. 2014;148:155–161. doi: 10.1016/j.foodchem.2013.10.025. [DOI] [PubMed] [Google Scholar]
- Puttaraju NG, Venkateshaiah SU, Dharmesh SM, Urs SM, Somasundaram R. Antioxidant activity of indigenous edible mushrooms. J Agric Food Chem. 2006;54(26):9764–9772. doi: 10.1021/jf0615707. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Ribas-Agustí A, Martín-Belloso O, Soliva-Fortuny R, Elez-Martínez P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit Rev Food Sci Nutr. 2017;13:1–18. doi: 10.1080/10408398.2017.1331200. [DOI] [PubMed] [Google Scholar]
- Sánchez C. Reactive oxygen species and antioxidant properties from mushrooms. Synth Syst Biotechnol. 2017;2(1):13–22. doi: 10.1016/j.synbio.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C, Sampaio G, Freitas R, Torres E. Polyphenols from guaraná after in vitro digestion: evaluation of bioacessibility and inhibition of activity of carbohydrate-hydrolyzing enzymes. Food Chem. 2018;267:405–409. doi: 10.1016/j.foodchem.2017.08.078. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16(3):144–158. [Google Scholar]
- Siracusa L, Kulisic-Bilusic T, Politeo O, Krause I, Dejanovic B, Ruberto G. Phenolic composition and antioxidant activity of aqueous infusions from Capparis spinosa L. and Crithmum maritimum L. before and after submission to a two-step in vitro digestion model. J Agric Food Chem. 2011;59(23):12453–12459. doi: 10.1021/jf203096q. [DOI] [PubMed] [Google Scholar]
- Soler-Rivas C, Ramírez-Anguiano AC, Reglero G, Santoyo S. Effect of cooking, in vitro digestion and Caco-2 cells absorption on the radical scavenging activities of edible mushrooms. Int J Food Sci Technol. 2009;44(11):2189–2197. [Google Scholar]
- Tadera K, Minami Y, Takamatsu K, Matsuoka T. Inhibition of α-glucosidase and α-amylase by flavonoids. J Nutr Sci Vitaminol. 2006;52(2):149–153. doi: 10.3177/jnsv.52.149. [DOI] [PubMed] [Google Scholar]
- Tan Y-S, Baskaran A, Nallathamby N, Chua K-H, Kuppusamy UR, Sabaratnam V. Influence of customized cooking methods on the phenolic contents and antioxidant activities of selected species of oyster mushrooms (Pleurotus spp.) J Food Sci Technol. 2015;52(5):3058–3064. doi: 10.1007/s13197-014-1332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telagari M, Hullatti K. In-vitro alpha-amylase and alpha-glucosidase inhibitory activity of Adiantum caudatum Linn. and Celosia argentea Linn. extracts and fractions. Indian J Pharmacol. 2015;47(4):425–429. doi: 10.4103/0253-7613.161270. [DOI] [PMC free article] [PubMed] [Google Scholar]