Abstract
Recently, unconventional methods especially microwave-assisted hydrodistillation extraction (MAHE) is being used as an alternative technique for extracting bioactive compounds from plant materials due to its advantages over conventional methods such as Soxhlet extraction (SE). In this study, bioactive compounds were extracted from Vernonia cinerea leaf using both MAHE and SE methods. In addition, the kinetic study of MAHE and SE methods were carried out using first- and second-order kinetic models. The results obtained showed that MAHE can extract higher yield of bioactive compounds from V. cinerea leaf in a shorter time and reduced used of extracting solvent compared with SE method. Based on the results obtained, second-order kinetic models can actually describe the extraction of bioactive compounds from V. cinerea leaf through MAHE with extraction rate coefficient of 0.1172 L/gmin and extraction capacity of 1.0547 L/g as compared to SE with 0.0157 L/gmin and 1.1626 L/g of extraction rate coefficient and extraction capacity, respectively. The gas chromatography–mass spectrometry analysis of the oil showed the presence of numerous heavy fractions in the oil obtained through MAHE as compared with the SE method. Moreover, the electric consumption and environmental impacts analysis of the oil suggested that MAHE can be a suitable green technique for extracting bioactive compounds from V. cinerea leaf.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3512-4) contains supplementary material, which is available to authorized users.
Keywords: Microwave-assisted hydrodistillation, Bioactive compounds, Soxhlet, Kinetic model, Extraction capacity, Extraction coefficient
Introduction
An essential oil is one of the indispensable agroindustry commodities that can boost the economy. These oils are mainly from natural plant extracts of grains, flowers, seeds, flower buds, and leaves. Statistics had shown that about 5–10% of essential oils and their derivatives are consumed annually (Putri et al. 2017). The demands might be due to growth in food and pharmaceutical establishments. Even though several types of bioactive compounds are produced in Malaysia, a little variety are being commercialized.
Malaysia rainforests are endowed with different varieties of flowering and non-flowering plants from which larger percentages are being used for the essential wellsprings of traditional medications. United Nations Environment Program (UNEP) has enlisted Malaysia to be one of the 12 mega-diverse nations that possess the world’s species of plants which might have unmeasurable advantages for future eras (Ibrahim et al. 2014). In approximate, 16% of around 10,000 species higher plants and 2000 species lower plants found in Peninsular Malaysia have been explored for medical purposes (Chua et al. 2005). However, only a few numbers have been investigated fully for their potentials. As the Malaysian flora diverse greatly, well diverse secondary metabolites can be anticipated from these medicinal plants.
Vernonia cinerea, known to be purple fleabane, are mostly distributed in Africa, Bangladesh, Sri Lanka, India, and Malaysia (Alara et al. 2018a; Prabha 2015). Bioactive compounds from V. cinerea leaves are mostly enriched with different phytochemical compounds such as sesquiterpenes, terpenoids, tannins, polyphenols, alkaloids, flavonoids, and glycosides which are used as natural ingredients as antidandruff, antifungal, antioxidant, anti-diabetes, anti-inflammatory, anti-cancer, and gastrointestinal disorder medicines (Alara et al. 2018b, c; Arivoli et al. 2011; Shelar et al. 2014; Soma et al. 2017; Somasundaram et al. 2010). Moreover, the extracts had been traditionally utilized for healing wound, cold, asthma, diarrhoea, and dysentery. It is being used for reducing the smoking rate in Thailand (Youn et al. 2014). From the previous findings, it has been seen that V. cinerea leaf possesses several pharmacological activities (Arivoli et al. 2011; Shelar et al. 2014; Soma et al. 2017; Somasundaram et al. 2010).
Several methods of extraction are being employed in the recovery of oil from plant samples, which include conventional and modern methods. The use of conventional methods such as Soxhlet, hydro-distillation, maceration, soaking, and others generally limit the production capacity of oil, degradation of volatile chemical compounds resulting from thermal effects during the long duration of extraction process (Akhbari et al. 2018). Moreover, conventional methods have shown low extraction yields and toxic solvent residues (Alara et al. 2018d; Raut et al. 2015). Therefore, the use of improved extraction methods (modern) is imperative for increasing crop productivity through efficient production and improvement of post-harvest handling (Kusuma and Mahfud 2017). In the recent time, microwave-assisted hydrodistillation extraction (MAHE) has been the most used method for recovering oil from plant matrix because of its effective heating, faster start-up, reduction in process steps, faster energy transfer, and improved production (Kusuma and Mahfud 2015). In addition, MAHE is cost-effective and possess higher yield of bioactive compounds at lower extraction time compare to the conventional methods (Alara et al. 2018d; Kusuma and Mahfud 2015). In order to investigate the efficiencies of conventional and modern extraction methods, bioactive compounds were extracted from V. cinerea leaf by comparing the yields from MAHE and SE in this study.
Therefore, this study focused on the extraction of bioactive compounds from V. cinerea leaf using microwave-assisted hydrodistillation extraction as compared with Soxhlet extraction method. The kinetic studies of these extraction methods and the chemical compositions of the oils were evaluated. Moreover, the efficiencies of both extraction methods were evaluated in terms of the environmental impact and electric consumption.
Materials and methods
Plant sample and chemicals
The plant sample used in this study was purple fleabane leaves (V. cinerea) obtained from the premises of Universiti Malaysia Pahang, Gambang, Malaysia. The sample was checked by Prof. Abdurahman. All the samples were washed with tap water, shade-dried until constant weight was achieved, blended with a grinder and sieved to achieve the average particle size of 105 µm. The moisture content was 0.01 ± 0.04 g water/g dry before stored in an air-tight container at room temperature. Ethanol (99.5% purity), dichloromethane and anhydrous sodium sulphate were purchased from Sigma Aldrich Sdn Bhd, Selangor. Distilled water used was obtained from the Faculty of Chemical and Natural Resources Engineering laboratory.
Microwave-assisted hydrodistillation extraction method
An ethos microwave extractor (1000 W, Frequency 2450 MHz, Milestone, Italy) was used for extracting oil from V. cinerea leaf. The sample (20 g) was placed in a 250 mL conical flask containing distilled water of 200 mL. The flask was placed in the extractor cavity and a condenser was mounted to collect the extracted oil (Fig. S1). The microwave was set at a power level of 500 W for a period of 90 min at 15 min intervals (15, 30, 45, 60, 75, and 90 min). This irradiation was enough to extract the oil in the plant sample. Thereafter, separating funnel was used to separate the oil by adding few drops of dichloromethane. The oil was dried over anhydrous sodium sulphate and stored at 4 °C until further use. The yield of essential oil from V. cinerea leaf was calculated using Eq. (1).
| 1 |
Soxhlet extraction method
The V. cinerea leaf sample (20 g) was loaded into a thimble. A 200 mL of distilled water was placed into a round-bottom flask that was attached to a Soxhlet extractor equipped with a condenser (BST/SXM-6A, Delhi) on a mantle heater (Fig. S2). The sample was placed inside the Soxhlet extractor and glass wool was used to lag the sidearm. As the solvent was being heated using the mantle heater, it began to evaporate by moving through the apparatus to the condenser. The condensate dripped into the reservoir containing the thimble with plant matrix. Once the level of solvent reached the siphon, it was refluxed back into the round bottom flask and the recycling began again. The experiment was carried out for 3 h at an interval of 30 min (60, 90, 120, 150, and 180 min) before the mixture was allowed to cool down. The separating funnel was used to separate the oil from water by adding few drops of dichloromethane. To remove any trace of water, the oil was dried over anhydrous sodium sulphate and stored at 4 °C until further use. The yield of V. cinerea leaf was calculated using Eq. (1).
Kinetic modelling for the extraction methods
The first- and second-order kinetic models have been commonly employed to explain adsorption results obtained under non-equilibrium situations. However, the kinetics of extraction is similar to adsorption as previously explained. Therefore, the adsorption equations are applied to extraction (Harouna-Oumarou et al. 2007). In this study, first- and second-order kinetic models were used for the extraction of bioactive compounds from V. cinerea leaves by considering MAHE and Soxhlet extraction methods. The rate of leaching is proportional to a driving force, which is assumed as (Cs-Ct), where Ct is the extraction capacity at different extraction time t and Cs is the concentration of water-soluble compounds of V. cinerea leaf at saturation.
First order kinetic model
The equation proposed by Lagergren and modified by Kusuma and Mahfud (2017) for first-order extraction kinetic model can be written as shown in Eq. (2). It is assumed that the first-order rate equation is correlated with the idea of a linear driving force (Liu and Shen 2008).
| 2 |
where Ct is the extraction capacity at different extraction time t, k1 is the first-order extraction rate coefficient in per minute (min−1) and Cs is the concentration of water-soluble compounds of V. cinerea leaf at saturation.
Equation (2) was further integrated at the boundary conditions of Ct = 0 and Ct = Ct to obtain Eq. (3).
| 3 |
Then, Eq. (3) was expressed in a linear form as shown in Eq. (4) whereby log10 (Cs − Ct) was plotted against t to obtain a slope and intercept that are used in determining the first-order extraction rate and extraction capacity.
| 4 |
Second-order kinetic model
In the same way, a second-order kinetic model for determining the extraction rate of oil from V. cinerea leaf can be written in the differential form as described by Kusuma and Mahfud (2017). The mechanism of a second-order kinetic model indicates that the extraction occurs in two simultaneous processes. The quantity of extracted oil improves with time in the beginning and decline gradually with time until the extraction process ends (Man et al. 2012). Thus, the rate of dissolution of oil present in the plant sample to a solvent can be described as:
| 5 |
where k2 is the second-order extraction rate coefficient in litre per gram per minute (L g−1 min−1).
Equation (5) was further integrated using the boundary conditions Ct = 0 at t = 0 and Ct = Ct at t = t to obtain final Eq. (8) through Eqs. (6)–(7).
| 6 |
By rearranging Eq. (6) to obtain Eqs. (7) and (8), respectively as follows:
| 7 |
| 8 |
Equation (8) was then rearranged in linearize form to through Eqs. (9)–(10) obtain Eq. (11) as follows:
| 9 |
| 10 |
By taking the inverse of Eqs. (10), (11) will be obtained as follows:
| 11 |
Thus, extraction rate (Ct/t) can be evaluated from Eq. (11). If the initial extraction rate is represented as m with Ct = t as t approaches zero. Then,
| 12 |
By substituting Eq. (12) in Eq. (10), the equation becomes:
| 13 |
Therefore, the initial extraction rate coefficient (m), second-order extraction rate coefficient (k2) and extraction capacity can be calculated through experimental procedure from the slope and intercept of a plot between t/Ct and t.
Chemical analysis of the oils using GC–MS
The components identification and their percentage of abundance from oils of V. cinerea leaves were evaluated using gas chromatography–mass spectrometry analysis. A Hewlett-Packard 6890 coupled with MS 5973A, equipped with a capillary column Agilent 19091S-433 HP-5MS (30 m tubular column length × 0.25 mm i.d. × 0.25 µm film thickness) and autosampler was used. The spectra were obtained using the following operating conditions: carrier helium at a flow rate of 1.0 mL/min; split ratio 25:1; the ionization voltage of 70 eV; run time 67.5 min; sample injection volume was 1 µL solution of oil (5 mg/mL); injection temperature of 320 °C; and oven temperature between 50 and 340 °C at 10 °C/min. The components were identified by relating the spectra with those on NIST 05a library database and the percentages of abundance were calculated with the total ion chromatogram. A 1 mL plant sample was prepared by diluting the oil with analytical absolute ethanol at a ratio of 1:20 (w/v).
Electric consumption
The electric consumptions in both extraction methods were evaluated based on the effects of extraction time and power consumption. Equation (14) described the general equation for expressing electric consumption.
| 14 |
where Ec is the electric consumption expressed as kilowatt-hour per gram (kWh/g), P is the power in watt, and t is the extraction time in minute.
Furthermore, the relative electric consumption was determined for both extraction methods as shown in Eq. (15).
| 15 |
where denotes relative electric consumption and m represents mass of the extracted essential oil from V. cinerea leaf expressed in gram (g).
Carbon IV oxide emission
The carbon IV oxide emitted from both extraction methods were determined using the procedure as stated by Ferhat et al. (2006). To evaluate 1 kWh of energy from fossil fuels during combustion, an equivalent of 800 g CO2 will be emitted into the atmosphere. Therefore, Eq. (16) is used to determine carbon IV oxide emission.
| 16 |
where denotes CO2 emission in kilogram and Ec represents electric consumption in kilowatt-hour (kWh).
Moreover, the relative carbon IV oxide emission of the two extraction methods can be determined using Eq. (17).
| 17 |
where represents relative CO2 emission expressed in kilogram per gram (kg/g) and m represents mass of essential oil extracted in gram (g).
Results and discussion
Comparison between the yield of oil from V. cinerea leaf using microwave-assisted hydrodistillation and Soxhlet extraction methods
There is a distinct difference between the yield of oil from V. cinerea leaf using MAHE and SE method as clearly illustrated in Fig. 1. In both methods, as the extraction time increases, the yields of oil continue to increase at a nearly stable value until no further increase in the yields at longer extraction time. The yields obtained from MAHE was higher than Soxhlet method. This might be due to the effect of localize heating in SE at a longer period of extraction which tends to reduce the yield from plant matrix (Alara et al. 2018d; Karabegovic et al. 2014). Moreover, the highest yields of 2.92% and 1.38% oil were obtained at 30 and 120 min for MAHE and SE method, respectively. The working principle of MAHE is totally different from conventional methods in that the extraction occurs due to changes in the cell wall caused by electromagnetic waves. In MAHE, faster extraction time with higher yield might be due to a synergy that results from the combined effect of mass and heat transfer operating in the same direction (Putri et al. 2017). Based on the results obtained, there was a significant difference between the two considered extraction methods. The similar result had been obtained for the extraction of aloe-emodin from aloe leaf whereby optimal yield was obtained using MAHE as compared with Soxhlet and ultrasonic extraction (Wang et al. 2011).
Fig. 1.
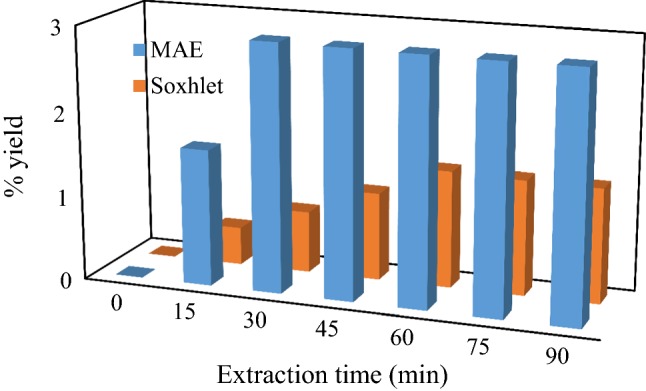
The extraction yields of oil from Vernonia cinerea leaf at varied time using microwave-assisted hydrodistillation and Soxhlet extraction methods
Kinetics modelling of the oil from V. cinerea leaf using microwave-assisted and Soxhlet extraction methods
Based on Fick’s law, the diffusion of soluble components during the extraction process relies on the concentration gradient that exists between the liquid (less concentrated) and solid phase (more concentrated). This concentration gradient tends to generate a balance between the two phases, the diffusion becomes insignificant even if the extraction time is increased infinitely under the fixed extraction conditions (Harouna-Oumarou et al. 2007). Nevertheless, if the liquid phase is being renewed continuously, the diffusion progresses until equilibrium is attained between the liquid and solid phase. In the extraction of oil from V. cinerea leaf using MAHE and SE methods, the yields of oil increases at the beginning of the extraction processes and thereafter remain constant as extraction time increases (Fig. 1). This behaviour can be utilized in evaluating the kinetic study of V. cinerea leaf oil extraction. Both the first and second order kinetic models were considered for the extraction of oil from V. cinerea leaf using MAHE and SE methods. Generally, it has been observed that the extraction rate is fast at the inception of the process and remains steady as an equilibrium is being reached (Harouna-Oumarou et al. 2007; Kusuma and Mahfud 2017; Seikova et al. 2004).
For the first-order kinetic model, plots of log (Cs − Ct) versus t was used for the study. Based on the derived equations, linearization of the individual plot made it easy to determine the values of coefficient of determination (R2), k1 and Cs. These were done by minimizing the sum of square errors of the predicted data from the first-order kinetic model and experimental data using Solver. The first-order kinetic model parameters had been determined as illustrated in Table 1. On an overall, it can be observed that the coefficient of determination obtained for the extraction of oil from V. cinerea leaf using MAHE was relatively low. Thus, a first-order kinetic model does not well describe the experimental results obtained from MAHE of V. cinerea leaf.
Table 1.
First-order kinetic model for the extraction of oil from V. cinerea leaf using microwave-assisted hydrodistillation extraction and Soxhlet extraction methods
| Extraction methods | Calculation methods | Slopea | k1 (min−1) | Intercepta | Cs (L/g) | R2a |
|---|---|---|---|---|---|---|
| MAHE | Linear regression | − 0.0094 | 0.0216 | 0.5648 | 3.6711 | 0.7487 |
| Solvera | 0.0216 | 3.6711 | ||||
| SE | Linear regression | − 0.0016 | 0.0037 | 0.3353 | 2.1642 | 0.9948 |
| Solvera | 0.0037 | 2.1642 |
aRepresent the result obtained from Solver of Microsoft Excel®
However, the higher value of a coefficient of determination obtained through a Soxhlet extraction method indicates that the first-order kinetic model can be used to study the extraction of oil from V. cinerea leaf. The results obtained are similar to the previous reports whereby first-order kinetic model can best describe conventional hydrodistillation as compared with microwave air-hydrodistillation and microwave hydrodistillation extraction methods (Kusuma and Mahfud 2017). Moreover, the report had shown that first-order kinetic model cannot be used to describe all processes, thus, it can only explain processes that have a single mechanism (Covelo et al. 2004; Ho and McKay 1999).
In addition, it can be observed in Table 1 that the results obtained for extraction capacity and first-order kinetic model constant (k1) for the extraction of oil from V. cinerea leaf using MAHE is higher compared to Soxhlet extraction technique. This is the rationale for faster recovery of yield from the plant matrix when using MAHE as compared to SE method. There are two general mechanisms involved when using a microwave for extraction of plant sample. The first mechanism involves a change in a plant’s cell wall due to the electromagnetic waves from a microwave. During this process, water molecules from the solvent used and microwave energy interacts, then, the plant matrix swollen, expand and rupture which leach out the components of bioactive compounds. In the second mechanism, organic compounds in the oil absorb microwave energy, thus this organic compounds can be extracted with specific contents (Kusuma and Mahfud 2017).
Table 2 illustrates the results obtained using a second-order kinetic model to represent the experimental results from the extraction of oil from V. cinerea leaf using both MAHE and Soxhlet extraction method. It can be observed that the value of R2 obtained for the SE method is higher which is closer to that of the first-order kinetic model. Second-order kinetic was studied by plotting t/Ct against time (t). Slope and intercept were obtained from this plot whereby the extraction rate (k2), extraction capacity and coefficient of determination were evaluated. In comparing the linearization with Solver in excel, the sum of the squared error was minimized. On an overall, the coefficient of determination is higher which implies that the second-order kinetic model can best describe the extraction of oil from V. cinerea leaf using both MAHE and Soxhlet extraction methods. However, the extraction capacity for the V. cinerea leaf oil obtained for SE is higher than MAHE. Nevertheless, the higher value obtained for extraction constant using MAHE justified why this extraction method is faster in achieving oil as compared to the SE method. In addition, Eq. (11) supported the fact that the value of extraction capacity (Cs) possesses higher effect than extraction rate. The similar outcome had been reported by Kusuma and Mahfud (2017).
Table 2.
Second-order kinetic model for the extraction of essential oil from V. cinerea leaf using microwave-assisted hydrodistillation extraction and Soxhlet extraction methods
| Extraction methods | Calculation methods | Slopea | Cs (L/g) | k2 (L/gmin) | Intercepta | R2a |
|---|---|---|---|---|---|---|
| MAHE | Linear regression | 0.9481 | 1.0547 | 0.1172 | 9.4899 | 0.9959 |
| Solvera | 1.0547 | 0.1172 | ||||
| SE | Linear regression | 0.8602 | 1.1626 | 0.0157 | 47.0221 | 0.9993 |
| Solvera | 1.1625 | 0.0157 |
aRepresent the result obtained from Solver of Microsoft Excel®
Chemical properties of oil from V. cinerea leaf using microwave-assisted hydrodistillation and Soxhlet extraction methods
Although, the chemical properties are not certainly related to the aroma of an oil but determine the quality (Kusuma and Mahfud 2017). The chemical composition of the extracted oil from V. cinerea leaf using MAHE and SE methods were analyzed to check the quality of the extracted oil. The present chemical compounds in the oil were identified using GC–MS analysis.
The chemical compositions of V. cinerea leaf oil using MAHE and SE method has been listed in Table 3. The identified compounds are the fatty acid ester, sesquiterpenes, palmitic acids, ether, caprylic, aromatic, phenolic, myristic acid, plasticizer, terpene alcohol, linoleic acid, and triterpene. The main compounds in the oils are 9,12,15-Octadecatrienoic acid (Z,Z,Z) (27.55 and 20.95%); 13-Docosenoic acid methyl ester (20.02 and 17.26%); n-Hexadecanoic acid (8.55 and 12.35%); 12,15-Octadecatrienoic acid methyl ester (Z,Z,Z) (7.82 and 5.15%); Squalene (4.53 and 2.15%); 1,2-Benzenedicarboxylic acid diisooctyl ester (6.13 and 15.12%); Phenol-2,4-bis(1-phenylethyl) (4.19 and 3.73%); Hexadecanoic acid ethyl ester (4.18 and 0.85%); 9,12-Octadecadienoic acid methyl ester (4.10 and 3.88%); 8,11,14-Eicosatrienoic acid (Z,Z,Z) (4.05 and 3.99%); and 11-Eicosenoic acid methyl ester (3.55 and 2.75%). It can be clearly seen that the oil has a larger percentage of fatty acids which were 71.49 and 56.66% for MAHE and SE, respectively. Reports had shown that essential fatty acids are the main components of modulating gene transcription, cell membrane structure, energy sources, and cytokine precursors (Connor 2000; Glick and Fischer 2013). Moreover, they have stronger antiarrhythmic action on the heart, anti-inflammatory, inhibition of mitogens and cytokines, antithrombotic, inhibition of atherosclerosis, and stimulate endothelial-derived nitric oxide (Connor 2000).
Table 3.
Chemical composition of V. cinerea leaf oil obtained from using microwave-assisted hydrodistillation and Soxhlet extraction methods
| S/N | Compound name | Molecular formula | Molecular weight | Nature | Area (%) | |
|---|---|---|---|---|---|---|
| MAHE | SE | |||||
| 1 | α-Bulnesene | C15H24 | 204 | Sesquiterpene | 0.03 | 0.16 |
| 2 | α-Caryophyllene | C15H24 | 204 | Sesquiterpene | 0.02 | – |
| 3 | α-Guaiene | C15H24 | 204 | Sesquiterpene | 0.22 | 0.17 |
| 4 | Azulene, 1,4-dimethyl-7-(1-methylethyl)- | C15H18 | 198 | Hydrocarbon | 0.26 | 1.15 |
| 5 | Benzene, 1,4-dichloro- | C6H4Cl2 | 146 | Aromatic compound | 0.11 | 0.32 |
| 6 | Caryophyllene | C15H24 | 204 | Sesquiterpene | 0.15 | 0.63 |
| 7 | Caprylic anhydride | C16H30O3 | 270 | Caprylic compound | 0.01 | – |
| 8 | Dodecanoic acid | C12H24O2 | 200 | Lauric acid | 0.06 | 0.24 |
| 9 | Hexadecanoic acid ethyl ester | C18H36O2 | 284 | Fatty acid ester | 4.18 | 0.85 |
| 10 | n-Hexadecanoic acid | C16H32O2 | 256 | Palmitic acid | 8.55 | 12.35 |
| 11 | Methanone, [1,4-dimethyl-7-(1-methylethyl)-2-azulenyl]pheny | C22H22O | 302 | Ketone compound | 0.15 | 1.02 |
| 12 | Phenol-2,4-bis(1-phenylethyl) | C22H22O | 302 | Phenolic compound | 2.98 | 1.28 |
| 13 | Tetradecanoic acid | C14H28O2 | 228 | Myristic acid | 0.01 | 0.17 |
| 14 | 1-Monolinoleoylglycerol trimethylsilyl ether | C27H54O4Si2 | 498 | Ether compound | 0.07 | 0.67 |
| 15 | 1,2-Benzenedicarboxylic acid diisooctyl ester | C24H38O4 | 390 | Plasticizer compound | 6.13 | 15.12 |
| 16 | 2-Isopropylidene-5-methylhex-4-enal | C10H16O | 152 | Alkene compound | 0.02 | 0.58 |
| 17 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O | 296 | Terpene alcohol | 1.01 | 3.59 |
| 18 | 8,11,14-Eicosatrienoic acid (Z,Z,Z) | C20H34O2 | 306 | Fatty acid ester | 4.05 | 3.99 |
| 19 | 9,12-Octadecadienoic acid methyl ester | C19H34O2 | 294 | Linoleic acid | 4.10 | 3.88 |
| 20 | 9,12,15-Octadecatrienoic acid methyl ester (Z,Z,Z) | C19H32O2 | 292 | Fatty acid ester | 7.82 | 5.15 |
| 21 | 9,12,15-Octadecatrienoic acid, (Z,Z,Z) | C18H30O2 | 278 | Linolenic acid | 27.55 | 20.95 |
| 22 | 11-Eicosenoic acid methyl ester | C21H40O2 | 324 | Fatty acid ester | 3.55 | 2.75 |
| 23 | 13-Docosenoic acid methyl ester | C23H44O2 | 352 | Fatty acid ester | 20.02 | 17.26 |
| 24 | 15-Tetracosenoic acid, methyl ester | C25H48O2 | 380 | Fatty acid ester | 0.22 | 1.83 |
| 25 | Phenol, 2,4-bis(1-phenylethyl)- | C22H22O | 302 | Phenolic compound | 4.19 | 3.73 |
| 26 | Squalene | C30H50 | 410 | Triterpene | 4.53 | 2.15 |
Furthermore, it can be clearly observed that 26 chemical components are identified from MAHE oil of V. cinerea leaf, whereas 24 chemical components were identified for Soxhlet extraction method. Thus, MAHE can extract heavy fractions components present in the cell membrane of the plant matrix. Reports had shown that the presence of heavy fraction components can actually predict the aroma of an oil because they indicate the presence of oxygenated terpenes (Kusuma and Mahfud 2017). The presence of a larger portion of oxygenated compounds in the MAHE oils might be due to reduced hydrolytic and thermal effects as compared to SE that uses a larger volume of extracting solvent which requires higher energy consumption (Ferhat et al. 2006). It can be said that V. cinerea leaf oil obtained using MAHE can have better aroma than that obtained from the Soxhlet extraction method.
Electric consumption and environmental impact of V. cinerea extraction using microwave-assisted hydrodistillation and Soxhlet extraction methods
The results have shown that higher yields of oil were obtained from V. cinerea leaf in shorter extraction time using MAHE as compared to SE method. Extraction time is relatively associated with energy and cost required for the process. Thus, in order to determine these, the electric consumption and environmental impact of V. cinerea leaf oil were evaluated for both extraction methods as illustrated in Table 4.
Table 4.
Electric consumption and environmental impact of V. cinerea leaf oil extraction using microwave-assisted hydrodistillation and Soxhlet extraction methods
| Extraction method | Extraction time (h) | Power consumption (W) | Electric consumption (kWh) | Relative electric consumption (kWh/g) | CO2 emission (kg) | Relative CO2 emission (kg/g) |
|---|---|---|---|---|---|---|
| MAHE | 0.5 | 500 | 0.0694 | 0.1197 | 0.0555 | 0.0957 |
| SE | 2.0 | 450 | 0.2500 | 0.8929 | 0.2000 | 0.7143 |
The results showed that electric consumption in the extraction of oil from V. cinerea leaf using MAHE and SE method were 0.0694 and 0.25 kWh, respectively. Thus, the electricity required for the Soxhlet extraction of oil from V. cinerea leaf was about 3.6 times that of MAHE. The electric required to obtain 1 g of V. cinerea leaf oil using MAHE was 0.1197 kWh, whereas, for Soxhlet extraction, it was 0.8929 kWh. This implies that SE requires about 7.5 times more electricity than MAHE in order to achieve 1 g of oil. Therefore, it can be concluded that extraction of oil from V. cinerea leaf requires lower operating cost as compared with SE method.
In the same way, the environmental effect of the extracted oil was determined from the quantity of carbon IV oxide emission generated. It has been reported that the 800 g of carbon IV oxide will be released into the atmosphere whenever 1 kWh of energy from coal is consumed (Putri et al. 2017). For the MAHE, the carbon IV oxide emitted was 0.0555 kg, whereas it was 0.2 kg in the SE method. Thus, it can be assumed that extraction using the Soxhlet method emitted higher CO2 into the atmosphere as compared with MAHE. Moreover, to obtain 1 g of oil from V. cinerea leaf, CO2 that will be emitted from MAHE and SE methods will be 0.0957 and 0.7143 kg, respectively. Thus, it can be assumed that Soxhlet extraction will emit a higher amount of carbon IV oxide to the atmosphere as compared to MAHE.
Conclusion
This study reflected that MAHE is a better method for extracting higher yield of oil from V. cinerea leaf in a shorter extraction time and reduced use of solvent as compared to SE. The kinetic study had shown that second-order model can better describe the MAHE with extraction rate coefficient of 0.1172 L/gmin and extraction capacity of 1.0547 L/g as compared to Soxhlet that has 0.0157 L/gmin and 1.1626 L/g of extraction rate coefficient and extraction capacity, respectively. The presence of higher amount of heavy fractions, lower consumption of electricity and reduced emission of carbon IV oxide into the atmosphere confirmed the efficacy of MAHE in achieving a quality yield of oil from V. cinerea leaf.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflict of interest
The authors of this manuscript has confirmed that there is no conflict of interest.
Contributor Information
O. R. Alara, Phone: +601116543727, Email: ruthoalao@gmail.com
N. H. Abdurahman, Email: abrahman@ump.edu.my
References
- Akhbari M, Masoum S, Aghababaei F, Hamedi S. Optimization of microwave assisted extraction of essential oils from Iranian Rosmarinus officinalis L. using RSM. J Food Sci Technol. 2018;55:2197–2207. doi: 10.1007/s13197-018-3137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alara OR, Abdurahman NH, Ukaegbu CI. Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J Appl Res Med Aromat Plants. 2018;11:12–17. [Google Scholar]
- Alara OR, Abdurahman NH, Ukaegbu CI, Azhari NH, Kabbashi NA. Metabolic profiling of flavonoids, saponins, alkaloids, and terpenoids in the extract from Vernonia cinerea using LC–Q–TOF–MS. J Liq Chromatogr Relat Technol. 2018 [Google Scholar]
- Alara OR, Abdurahman NH, Ukaegbu CI, Azhari NH. Vernonia cinerea leaves as the source of phenolic compounds, antioxidants, and anti-diabetic activity using microwave-assisted extraction technique. Ind Crop Prod. 2018;122:533–544. doi: 10.1016/j.indcrop.2018.06.034. [DOI] [Google Scholar]
- Alara OR, Abdurahman NH, Olalere OA. Optimization of microwave-assisted extraction of flavonoids and antioxidants from Vernonia amygdalina leaf using response surface methodology. Food Bioprod Process. 2018;107:36–48. doi: 10.1016/j.fbp.2017.10.007. [DOI] [Google Scholar]
- Arivoli S, Tennyson S, Jesudoss Martin J. Larvicidal efficacy of Vernonia cinerea (L.) (asteraceae) leaf extracts against the filarial vector culex quinquefasciatus say (Diptera: Culicidae) J Biopestic. 2011;4:37–42. [Google Scholar]
- Chua LSL, Kirton LG, Saw LG (eds) (2005) Status of biological diversity in Malaysia and threat assessment of plant species in Malaysia. Forest Research Institute of Malaysia, pp 1–298
- Connor WE. Importance of n-3 fatty acids in health and disease 1–3. Am J Clin Nutr. 2000;71:1–5. doi: 10.1093/ajcn/71.1.21. [DOI] [PubMed] [Google Scholar]
- Covelo EF, Andrade ML, Vega FA. Heavy metal adsorption by humic umbrisols: selectivity sequences and competitive sorption kinetics. J Colloid Interface Sci. 2004;280:1–8. doi: 10.1016/j.jcis.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Ferhat MA, Meklati BY, Smadja J, Chemat F. An improved microwave clevenger apparatus for distillation of essential oils from orange peel. J Chromatogr A. 2006;1112:121–126. doi: 10.1016/j.chroma.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Glick NR, Fischer MH. The role of essential fatty acids in human health. J Evid Based Complement Altern Med. 2013;18:268–289. doi: 10.1177/2156587213488788. [DOI] [Google Scholar]
- Harouna-Oumarou HA, Fauduet H, Porte C, Ho YS. Comparison of kinetic models for the aqueous solid-liquid extraction of Tilia sapwood a continuous stirred tank reactor. Chem Eng Commun. 2007;194:537–552. doi: 10.1080/00986440600992511. [DOI] [Google Scholar]
- Ho YS, McKay G. The sorption of lead(II) ions on peat. Water Res. 1999;33:578–584. doi: 10.1016/S0043-1354(98)00207-3. [DOI] [Google Scholar]
- Ibrahim R, Shaari AR, Faris G (2014) Overview of medicinal plants spread and their uses in Asia, pp 1–6
- Karabegovic IT, Stojicevic SS, Velickovic DT, Todorovic ZB, Nikoli NC, Lazic ML. The effect of different extraction techniques on the composition and antioxidant activity of cherry laurel (Prunus laurocerasus) leaf and fruit extracts. Ind Crops Prod. 2014;54:142–148. doi: 10.1016/j.indcrop.2013.12.047. [DOI] [Google Scholar]
- Kusuma HS, Mahfud M. Preliminary study: kinetics of oil extraction from sandalwood by microwave-assisted hydrodistillation. ASEAN J Chem Eng. 2015;15:62–69. [Google Scholar]
- Kusuma HS, Mahfud M. The extraction of essential oils from patchouli leaves (Pogostemon cablin Benth) using a microwave air-hydrodistillation method as a new green technique. RSC Adv. 2017;7:1336–1347. doi: 10.1039/C6RA25894H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shen L. From Langmuir kinetics to first- and second-order rate equations for adsorption. Langmuir. 2008;24:11625–11630. doi: 10.1021/la801839b. [DOI] [PubMed] [Google Scholar]
- Man HC, Hamzah MH, Jamaludin H, Abidin ZZ. Preliminary study: kinetics of oil extraction from citronella grass by ohmic heated hydro distillation. APCBEE Procedia. 2012;3:124–128. doi: 10.1016/j.apcbee.2012.06.057. [DOI] [Google Scholar]
- Prabha JL. Therapeutic uses of Vernonia cinerea—a short review. Int J Pharm Clin Res. 2015;7:323–325. [Google Scholar]
- Putri DK, Kusuma HS, Syahputra ME, Parasandi D, Mahfud M. The extraction of essential oil from patchouli leaves (Pogostemon cablin Benth) using microwave hydrodistillation and solvent-free microwave extraction methods. IOP Conf Ser Earth Environ Sci. 2017;101:1–7. doi: 10.1088/1755-1315/101/1/012013. [DOI] [Google Scholar]
- Raut P, Bhosle D, Janghel A, Deo S, Verma C, Kumar SS, Agrawal M, Amit N, Sharma M, Giri T. Emerging microwave assisted extraction (MAE) techniques as an innovative green technologies for the effective extraction of the active phytopharmaceuticals. Res J Pharm Technol. 2015;8:655–666. doi: 10.5958/0974-360X.2015.00104.3. [DOI] [Google Scholar]
- Seikova I, Simeonov E, Ivanova E. Protein leaching from tomato seed-Experimental kinetics and prediction of effective diffusivity. J Food Eng. 2004;61:165–171. doi: 10.1016/S0260-8774(03)00083-9. [DOI] [Google Scholar]
- Shelar D, Tikole S, Kakade T. Vernonia cinerea: a review. J Curr Pharma Res. 2014;4:1194–1200. [Google Scholar]
- Soma A, Sanon S, Gansané A, Ouattara LP, Ouédraogo N, Nikiema J-B, Sirima SB. Antiplasmodial activity of Vernonia cinerea Less (Asteraceae), a plant used in traditional medicine in Burkina Faso to treat malaria. Afr J Pharm Pharmacol. 2017;11:87–93. doi: 10.5897/AJPP2016.4703. [DOI] [Google Scholar]
- Somasundaram A, Velmurugan V, Senthilkumar GP. In vitro antimicrobial activity of Vernonia cinerea (L.) Less. Pharmacol. 2010;2:957–960. [Google Scholar]
- Wang G, Su P, Zhang F, Hou X, Yang Y, Guo Z. Comparison of microwave-assisted extraction of aloe-emodin in aloe with Soxhlet extraction and ultrasound-assisted extraction. Sci China Chem. 2011;54:231–236. doi: 10.1007/s11426-010-4017-9. [DOI] [Google Scholar]
- Youn UJ, Miklossy G, Chai X, Wongwiwatthananukit S, Toyama O, Songsak T, Turkson J, Chang LC. Bioactive sesquiterpene lactones and other compounds isolated from Vernonia cinerea. Fitoterapia. 2014;93:194–200. doi: 10.1016/j.fitote.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


