Abstract
The sensory, chemical (based on the thiobarbituric acid, total volatile basic nitrogen and trimethylamine), and microbial quality (based on the total viable count and lactic acid bacteria count) of the rainbow trout stored under modified atmosphere packaging (MAP) conditions was evaluated. Four different gas combinations, including P1 (80% CO2, 10% N2, 10% O2), P2 (60% CO2, 20% N2, 20% O2), P3 (60% CO2, 40% N2, 0% O2), and P4 (40% CO2, 30% N2, 30% O2), were used. Also, the fish packages were stored at four constant temperatures (including 0, 5, 10, and 15 °C) for 12 days. The absence of oxygen in P3 and high concentration of carbon dioxide in P1 extended the shelf life by delaying the chemical, microbial, and sensory spoilage. Over the storage time of trout fillets in MAP, the rate of chemical reactions significantly increased while the sensory scores decreased. Based on the Arrhenius kinetic modeling for the spoilage reactions of the sensory (total acceptance) and chemical (total volatile basic nitrogen) indices, the shelf life was extended for P3 and succeedingly, for P1 packaging.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3521-3) contains supplementary material, which is available to authorized users.
Keywords: Arrhenius model, Modified atmosphere packaging, Rainbow trout, Shelf life kinetics
Introduction
Rainbow trout is one of the most important species of Salmonidae in Iran. Immediately after death, the fish muscle is exposed to several biochemical and enzymatic changes (Masniyom et al. 2013). Bacteria are the most important cause of seafood spoilage. In addition, the oxygen of the atmosphere can attack lipids and result in rancidity, off-flavor, and off-odor. This is especially important in fatty fishes such as trout, salmon, and mackerel.
Since the quality of seafood is unsystematic, the freshness of fish is always assessed by trained panelists based on sensory evaluations of freshness-associated features (such as appearance, texture, smell, and color). Moreover, the microbial (e.g. total viable count), physical (e.g. tissue and electrical properties), and chemical (e.g. K, K1, total volatile basic nitrogen, trimethylamine, and lipid oxidation index) evaluations are also useful for that purpose (Kerry and Butler 2008).
Reddy et al. (1992) tabulated the shelf-life extensions reported by several researchers. They demonstrated that the modified atmosphere packaging (MAP) can effectively change and delay the spoilage process. For example, the shelf-life of fresh cod fillets, packaged under 100% CO2 at the storage temperature of 8.0 °C, was increased 280% compared to the packaging with 50% CO2 at 26.0 °C.
Although the effect of MAP on the chemical, microbial, and sensory properties of many fishes such as salmon (Sone et al. 2012; Macé et al. 2011; Fernández et al. 2010; Fagan et al. 2004), sardines (Özogul et al. 2004), cod (Debevere and Boskou 1996), seabass (Torrieri et al. 2006), gilt-head seabream (Tsironi and Taoukis 2010), whiting (Hassoun and Karoui 2016; Fagan et al. 2004), mackerel (Fagan et al. 2004), and stellate sturgeon (Hedayatifard and Aroujalian 2010) were widely evaluated by researchers, still there is no comprehensive study on the shelf-life kinetics of the rainbow trout with MAP. Therefore, in this work, we studied the effect of MAP (with the CO2 concentration of 40–80%, at the temperature range 0–15 °C) on chemical, microbial, and sensory properties. Furthermore, the shelf life of rainbow trout fillets with MAP was predicted based on the sensory and chemical indices.
Materials and methods
Preparing the samples
Rainbow trout, with the weight of 500 ± 10 g, was purchased from a fish breeding pool. The fish were brought to the packaging laboratory for about 30 min while they were stored in a refrigerator and were covered with ice. At the laboratory, after rinsing each fish, its head, tail, and bones were manually removed to obtain two fillets with the dimensions of 10 × 20 cm and weight of 170 ± 5 g. After draining the fillets by using sterile clothes, they were individually placed in 80 µm thick plastic bags with three LDPE/EVOH/LDPE layers (where LDPE and EVOH stand for low-density polyethylene and ethylene vinyl alcohol, respectively) without any coatings. Then, samples were packed by using the HENKELMAN vacuum system 200 A, in four different modified atmospheres with the gas combinations of 80% CO2/10% N2/10% O2 (P1), 60% CO2/20% N2/20% O2 (P2), 60% CO2/40% N2/0% O2 (P3), and 40% CO2/30% N2/30% O2 (P4). The gas composition contained in the packages was measured and analyzed by the gas analyzer OXYBABY (Wittgas) at the specified intervals (i.e. immediately after packaging and after every 3 days, up to 12 days). All the packages were kept in the controlled isothermal conditions (constant temperature) at 0, 5, 10, and 15 °C in a GT-7005-T Desk Type Temperature and Humidity Tester (GOTECH TESTING MACHINES INC.). The specimens were also sampled at the same intervals for analyzing chemical and sensory spoilage.
Chemical evaluation
Measurement of lipid oxidation
To measure lipid oxidation, the evaluation of 2-thiobarbituric acid reactive substances (TBARS) was conducted based on the Loovas’s method (Loovas 1992; Tsironi et al. 2009). TBARS concentration was calculated from the standard curve prepared using 1,1,3,3-tetraethoxypropane and expressed in mg of Malonaldehyde diethyl acetal (MDA) per kg of muscle (Robles-Martínez et al. 1982).
Measurement of total volatile basic nitrogen (TVB-N)
The extract, used for determining the total volatile bases, was obtained by mixing 100 g of fish sample, 200 ml of aqueous solution, and 7.5% trichloroacetic acid (TCA), in a high-speed homogenizer at 60 s. Then, the homogenized mixture was centrifuged for 3 min at 3000 (1200×g) and the supernatant solution was filtered by using Wattman No. 1 filter paper. Next, the volatile basic nitrogen was measured by steam distillation of the TCA-fish extract, based on the modified method proposed by Malle and Tao (1987). All results were reported in mg of TVB-N per 100 g of the fish muscle.
Measurement of trimethylamine (TMA)
The method used for measuring TVB-N was also applied to determine TMA, except that 20 ml of a 35% formaldehyde solution (v/v) was added to the distillation tube for remaining only the reactions of tertiary amines by blocking the primary and secondary amines (Malle and Poumeyrol 1989). The results were expressed in mg of nitrogen per 100 g of fish sample.
Microbial evaluation
For microbial enumeration, 10 g of the fish muscle was mixed with 90 ml of the sterile Ringer solution. Then, it was transferred to the sterile stomacher bag and homogenized for 60 s by the stomacher Seward, 4 N, England. The samples (0.1 ml) of 10-fold diluted homogenized muscle fish were pipetted onto the surface of plate-count-agar plates (in duplicate) to count colonies. The total-viable-count (TVC) was obtained after 72 h incubation at 25 °C (Tsironi and Taoukis 2010). The lactic acid bacteria (LAB) counts were also determined by using the pour plate method and De Man–Rogosa–Sharpe (MRS) agar, after incubation for 96 h at 25 °C. Two replicates were counted from at least three appropriate dilutions by colony counters (Tsironi and Taoukis 2010). All counts were expressed as log10 cfu/g.
Sensory evaluation
The fish samples were evaluated by 20 trained panelists. The appearance and smell of raw fish, as well as odor, taste and overall acceptance of cooked fish fillets were evaluated. The sensory parameters, reported by panelists in appropriate forms, reflect the organoleptic evaluation of quality loss. To assess the cooked fish, the samples were separately cooked in an aluminum foil at 180 °C for 20–30 min. Scoring of each parameter is performed based on the descriptive hedonic scale from 1 to 9. For sensory evaluations, the score of 5 was considered as the least acceptance (Tsironi et al. 2008).
Kinetic study of data
The curves of the chemical (TVB-N) and sensory (total acceptance) indices were plotted as a function of time for all the studied temperatures. The rate of quality loss was determined based on the least-squares statistical interpolation. Also, the temperature dependence of the constant rate of quality loss (k) was modeled by the Arrhenius equation (Nuin et al. 2008).
Calculating the shelf-life
The shelf-life was calculated based on the Arrhenius kinetics of the studied sensory and chemical indices of the rainbow trout fillets. Moreover, the values of chemical parameters were reported at the sensory rejection time of samples (Koutsoumanis and Nychas 2000).
Data analysis
All factorial experiments were carried out in a randomized complete block design with at least two replicates. The variance statistical analysis was performed using SPSS software (IBM SPSS Statistics 23). Also, Duncan’s multi-domain test with the confidence level of 95% was used to determine the significant difference between different means. The graphs were plotted using Excel (2010), SPSS, and MATLAB (2016). All models were obtained by using the MATLAB software.
Results and discussion
Chemical evaluation
Evaluating the lipid oxidation changes
TBARS (as an indicator of oxidation) is widely used to determine the secondary lipid oxidation products in the oxidation process. Results showed that there was a significant difference between different storage temperatures (P < 0.05). Generally, TBARS was augmented by increasing the storage temperature. The TBARS of samples stored at 0–15 °C was in the range of from 0.84 ± 0.11 to 3.35 ± 0.36 mg MDA/kg.
The oxidation reaction produces a range of substances, among which some have undesirable taste and odor. This phenomenon is commonly performed in the fishes with high percentage of polyunsaturated fatty acids like trout (Muela et al. 2014). Also, some of those substances may contribute in texture changes due to creation of covalent bonds with fish muscle proteins. Generally, due to quicker oxidation reactions in fatty fishes, the spoilage rate increases, especially, at high temperatures.
TBARS index of fish samples in different MAP packages was in the range from 1.30 ± 0.22 to 2.90 ± 0.35 mg MDA/kg. The highest TBARS was obtained for P4 (with 40% CO2 and 30% O2), while the least TBARS was given by P3 (with 60% CO2 and 0% O2) and succeedingly, P1 (with 80% CO2 and 10% O2). For more details, see Fig. Sup-1 in Supplementary Notes. Therefore, by increasing CO2 and decreasing O2 in the MAP packaging of trout, the TBARS index decreases. Hassoun and Karoui (2016) showed that by increasing CO2 in whiting fillets, TBARS was decreased. Similar results were also reported by Masniyom et al. (2013) for the Tilapia fish packed in the CO2-rich atmosphere. Sone et al. (2012) found that the TBARS index of salmon in the MAP packaging was much less than those in the air and vacuum packaging at the same temperature. Furthermore, Muela et al. (2014) reported that the lipid oxidation of Thunnus obesus (measured by TBARS) was activated in the presence of O2 since oxygen leads to the release of free radicals. Indeed, in the MAP packaging, O2 is replaced by N2 to delay oxidative rancidity based on preventing the growth of aerobic microorganisms.
TBARS index increased from 0.33 ± 0.01 to 4.03 ± 0.39 mg MDA/kg during 12 days of storage. The TBARS index showed significant differences at different sampling times (with the significance level of α = 0.05). This increase can be due to the decomposition of hydroxides into secondary oxidation products, such as Malone aldehyde (Goulas and Kontominas 2007). Sone et al. (2012) found that the lipid oxidation increased during storage of salmon in the air, vacuum, and MAP (with 60% CO2 and 40% N2) at 4 °C. The increase in TBARS for whiting fillets (packed in air and two modified atmospheres) also showed the same result (Hassoun and Karoui 2016). The TBARS increase during fish storage was also reported in other studies (Cosansu et al. 2013; Goulas and Kontominas 2007).
Masniyom et al. (2002) reported the increase of lipid oxidation during storage in seabass slices stored even under CO2-rich packaging. Obviously, the carbonic acid formed in the samples under the MAP packaging may result in the protein denaturation of the muscle and lead to the release of heme free iron (i.e. a potential pro-oxidant in the muscular system).
According to Connell (1990); once the amount of TBARS in fish fillets reaches to about 1–2 mg MDA/kg, an unpleasant odor is smelled. The maximum TBARS for the optimal fish quality (frozen, chilled, or stored-in-ice) is 5 mg MDA/kg. However, the fish with the TBARS of less than 8 mg MDA/kg can still be consumed.
Evaluation of TVB-N changes
TVB-N is a part of the non-protein nitrogen fraction of the fish muscle which contains dimethylamine, TMA, ammonia, and other nitrogen basic compounds. TVB-N and TMA were reported in many studies as the indicative compounds of fish spoilage (Malle and Poumeyrol 1989; Dalgaard et al. 1993; Koutsoumanis and Nychas 2000).
The results showed that the average TVB-N of samples stored at 0–15 °C is in the range from 15.49 ± 0.55 to 24.75 ± 1.59 in mg per 100 g sample (mg/100 g). By increasing the temperature, the amount of TVB-N was augmented. The bacterial catabolism of amino acids in the fish muscle results in the accumulation of ammonia and other volatile bases. In other words, the increase in TVB-N is associated with the activity of spoilage bacteria and internal enzymes. Therefore, by reducing the fish storage temperature in MAP, the trend of the TVB-N increase slows down (Koutsoumanis and Nychas 2000) due to decreasing the bacterial and enzymatic activities.
The usage of MAP showed significant effect on TVB-N of trout fillets (P < 0.05). Average TVB-N of samples in the four studied packaging types ranged from 17.387 ± 0.98 to 22.08 ± 1.54 mg/100 g. The highest amount of TVB-N was given for the P4 packaging while the lowest values were obtained for P1 and P3 with insignificant differences (see Fig. Sup-2 in Supplementary Notes). Therefore, the amount of TVB-N in trout is reduced by increasing the concentration of CO2. This fact was previously reported in a number of other researches (Hedayatifard and Aroujalian 2010; Hassoun and Karoui 2016). Indeed, higher-order CO2 concentrations may potentially prevent the growth of gram-negative aerobic bacteria including the microorganisms which produce volatile compounds.
Storage time, similar to the storage temperature and packaging type, showed significant effect on the TVB-N content (P < 0.05). The amount of TVB-N was augmented with increase in storage time; such that it rose from 10.46 ± 0.10 mg/100 g (in the first sampling) to 27.08 ± 8.11 mg/100 g at the end of the storage period. Increase of TVB-N fractions, during storage time, actually occurs due to some autolysis reactions (Debevere and Boskou 1996). It was reported for different fish types, such as cod fish (Debevere and Boskou 1996), Stellate sturgeon fillet (Hedayatifard and Aroujalian 2010), and whiting (Hassoun and Karoui 2016), under MAP conditions.
There is no standard limit for the acceptance of rainbow trout based on the TVB-N value. However, Arashisar et al. (2004), after studying the trout packed under MAP conditions, proposed 25 mg/100 g as the highest acceptable level of TVB-N for this type of fish. Therefore, according to our results, the P2 and P4 packagings were spoiled after around 10th and 7th day, respectively.
Evaluation of TMA changes
TMA is used as an indicator of the microbial activity for evaluating quality loss. Based on our results, by increasing the storage temperature from 0 to 15 °C, TMA raised from 0.86 ± 0.17 to 3.76 ± 0.40 in mgN/100 g. Generally, TMA has a direct relationship with the bacterial accumulation in fish (Connell 1990). Indeed, by increasing the storage temperature, the level of TMA is augmented, because of rising the bacterial accumulation and activity.
According to our results, the packing type had no significant effect on TMA in trout (P > 0.05). The highest amount of TMA was obtained for P4 while the lowest values belonged to the P1 and, succeedingly, P3 packagings (see Fig. Sup-3 in Supplementary Notes). Therefore, by increasing the concentration of CO2 in the MAP packaging, the amount of TMA was decreased. However, there were no significant differences between the P4 and P1 packagings.
The trimethylamine oxide (TMAO) as a part of the osmotic system is one of the main components of the non-protein nitrogen fractions in the aquatic tissue. This compound improves the growth of microaerophilic and anaerobic microorganisms. In other words, once the level of oxygen reduces, TMAO is used as the final receptor by microaerophilic microorganisms to reduce to TMA-N (Debevere and Boskou 1996).
Therefore, it has been observed that high levels of oxygen in MAP can delay the production of TMA (Debevere and Boskou 1996). In this manner, the low amounts of TMA in the P1, P3, and P4 packagings and their insignificant differences can be understood. In more detail, the reduction of TMAO to TMA-N was possible in the P1 and P3 packagings since they contained low amounts of O2. On the other hand, more access to oxygen leads to lower CO2 levels, which consequently, reduces the antimicrobial activity (Sivertsvik et al. 2002). Hence, in the P4 packaging, TMA also remained comparable with the other specified packaging types.
TMA content in fish samples increased over 12 days, from 0 to 4.43 ± 0.35 mgN/100 g. In more detail, in the first sampling of trout, TMA was not found. In this regard, Chytiri et al. (2004) reported that TMA concentration in trout is very low and also, there is no significant increase during ice storage. It indicates the very low level of TMA oxide in these types of fish muscles. TMAO is also a common compound found in marine fish. For example, for the salmon in MAP, no TMA concentration was found before the third day (Macé et al. 2011).
Özogul et al. (2004) demonstrated that in the sardine samples stored in the vacuum, MAP (60% CO2, %40 N2), and air packagings at 4 °C, TMA concentration was increased by increasing the storage time. Also, they reported that the highest concentration of TMA was observed for the samples kept in air while the lowest concentration was for the MAP conditions. This is probably due to the effect of MAP on the bacterial growth and, consequently, reduction of TMA formation (Debevere and Boskou 1996).
Although there is no rule related to the TMA amount, generally, in contents over 12 mg TMA-N/100 g, the product quality is damaged (Macé et al. 2011). Commonly, by using a grading system based on comparative assessment of sensory quality, some levels have been proposed for TMA. For example, at the time of sensory rejection, the TMA limit for trout was considered as 5 mgN/100 g (Jouki 2014), which can be employed as the TMA index and limit.
Microbial evaluation
Although immediately after packaging, TVC was the same in all the samples (i.e. 1.6 log cfu/g), there was an increasing trend for each of the four packaging types during storage (see Fig. Sup-4 in Supplementary Notes). Also, by increasing CO2 from 40 to 80% in packages, TVC was reduced. This indicates that CO2 can directly or indirectly reduce the microbial load. The P4 packaging (with the smallest amount of CO2) provided the highest TVC which reached to 7 log cfu/g on the 12th day of storage. According to the International Commission on Microbiological Specifications for Foods (ICMSF), the maximum permitted level of the microbial load in fresh and frozen fish is 107 cfu/g or 7 log cfu/g (ICMSF 1986). The smallest TVC on the 12th day was related to the P3 packaging which contained 60% CO2 and 0% oxygen. Indeed, in this study, the P3 and P1 packagings (with the highest CO2 and lowest O2 concentrations) showed the best performance in terms of the TVC level and inhibiting bacterial growth.
The presence of CO2 in the MAP fish causes the dominant microflora to be gram-positive organisms (e.g. mainly, LAB). We found that the mean initial count of LAB was 1.5 log cfu/g. Fig. Sup-5 in Supplementary Notes shows the LAB growth curves of rainbow trout fillets at different storage temperatures (including 0, 5, 10 and 15 °C) in different MAP packages. Our results showed that at the end of the storage period, the LAB counts in both the P1 and P3 packagings were approximately less than those of P2 and P4 for all the four specified temperatures. Thus, the former spoiled later compared to the latter.
Sensory evaluation
The first sensory changes during storage occur in appearance and texture of the fish (Huss 1988). Our results showed that the storage temperature had a significant effect on the sensory characteristics of fish (P < 0.05). As reported in Table Sup-1 of Supplementary Notes, by increasing the temperature from 0 to 15 °C, the scores of all the considered sensory parameters were reduced. The lowest scores were related to the raw fillet smell since, undoubtedly, the smell is the most common indicator of fish freshness. In fact, the untrained consumer (without any knowledge about evaluation of the fish quality) assesses the freshness and safety of fish based on the odor and aroma, just after opening the fish package. Therefore, it can be expected that the first sensory parameter affecting the panelist is the smell of fish with the lowest score after sensory changes. In contrast, the score of the appearance of raw fillets provided the lowest changes in the different studied temperatures.
Analysis of our data indicated that the packaging type (i.e. modified atmosphere composition) had a significant effect on the sensory scores (P < 0.05). According to Table Sup-2 of Supplementary Notes, the highest score was for the P3 packaging (with 60% CO2 and 40% N2), following by the P1 packaging (with 80% CO2, 10% N2, and 10% O2).Thus, non- or low-oxygen packagings were more acceptable for panelists.
In the presence of high amounts of oxygen in the package, the lipid rancidity may be rapidly increased, which can cause fishy odor and off-flavors. Oily fish such as maatjes herring, mackerel, and trout are susceptible to rancidity which results in the ammonia flavor. Since the smell of fish is very influential on the total acceptance, the highest overall acceptance scores were obtained for the P1 and P3 packagings while the lowest scores were reported for the P4 packaging with the highest oxygen content.
Table Sup-3 of Supplementary Notes shows a decreasing trend over the time (from the beginning to the 12th day) for the total acceptance and sensory scores of the samples (i.e. the appearance and smell of the raw fillets and taste and smell of the cooked fillets).
At the starting time of the off-flavor phase, especially in fatty fishes, the fish may be slightly sour, bitter, and fruity. During different stages of this phase, various smells such as sickly sweet, cabbage-like, ammonia, sulfuric, and bitter may be developed. Also, at this phase, the tissue may be either soft-watery or rigid-dry (which was soft in the case of rainbow trout). By continuing this phase, the fish becomes spoiled and corrupted (Huss 1988).
In fatty fishes such as salmon and trout, which are susceptible to lipid destruction, the first parameter of fish spoilage is the oxidative rancidity. During the advanced stages of fat oxidation, the breakage of hydroperoxides results in the formation of low molecular weight carbonyls and alcoholic compounds. It can further lead to changes in color, texture, taste, smell, and consequently, in the total food quality. Moreover, the reaction between the protein and oxidized fat of fish creates yellow color products.
Enzymes also change the taste of the fish. They change the sweet taste of the fish muscle (which is different in various species) into a flavorless taste. The continuous activity of enzymes also leads to the formation of other important compounds like hypoxanthine, which is not typically found in the fresh fish and can make the bitter taste (Akhondzadeh et al. 2000).
Meaty foods stored in the atmospheres with more than 25% CO2, based on the content of myoglobin, show different levels of surface fading. This phenomenon is harmful, especially in the fresh red meat, which contains higher levels of myoglobin compared to fish and poultry (Torrieri et al. 2006).
Torrieri et al. (2006) showed that in seabass kept under modified atmosphere conditions, by raising the oxygen percentage content, the amount of primary red color of the fish is reduced while the yellowness is increased. Similar results were also obtained by Hassoun and Karoui (2016) for whiting. They also found that the best firmness score was obtained for samples stored in 70% CO2/20% O2/10% N2 (close to the P1 packaging in this study).
Kinetic study of data
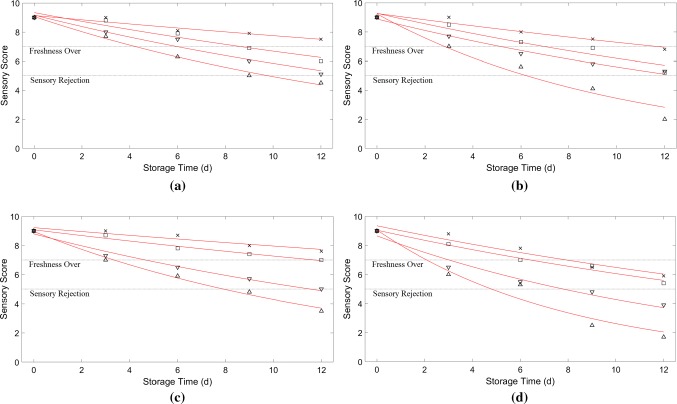
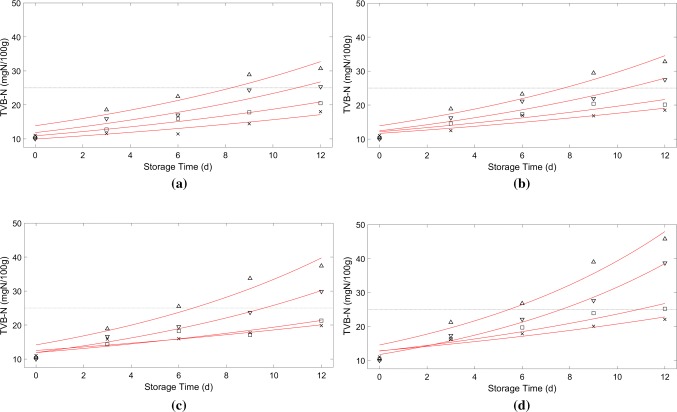
After plotting the variation charts of the chemical (TVB-N) and sensory (total acceptance) indices versus time, the rate constant of quality loss was interpolated by using the least squares error for each studied temperature (Figs. 1 and 2). Then, the dependency of the rate constant of the quality loss (i.e. k) on the temperature was modeled by the Arrhenius equation:
| 1 |
where R is the universal gas constant, T indicates the temperature in Kelvin, kref is the rate constant of quality index changes at the reference temperature Tref (e.g. Tref = 4 °C for chilled foods), and Ea determines the activation energy of the quality index for indicating its dependency on the temperature.
Fig. 1.
The total acceptance of rainbow trout as a function of time for the packages a P1, b P2, c P3 and d P4, at temperatures 0 °C (multiplication symbol), 5 °C (open square), 10 °C (open inverted triangle), and 15 °C (open triangle)
Fig. 2.
Amount of TVB-N in rainbow trout, as a function of time for the packages a P1, b P2, c P3 and d P4, at temperatures 0 °C (multiplication symbol), 5 °C (open square), 10 °C (open inverted triangle), and 15 °C (open triangle)
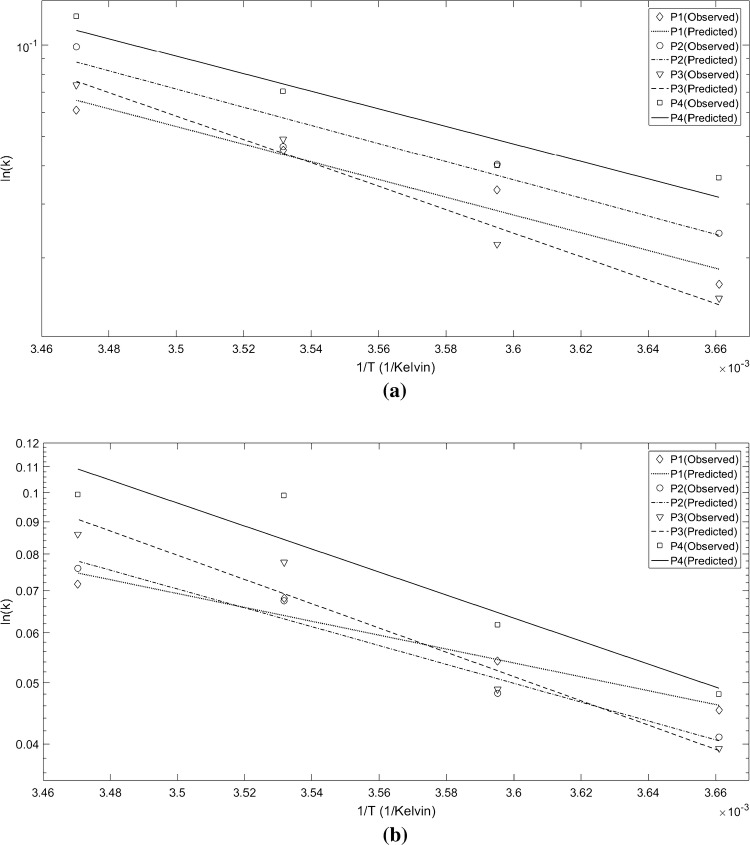
According to Fig. 3, the Ea of the chemical/sensory index was obtained as the slop of the Arrhenius line ln(k) versus (1/Tref − 1/T), by using linear regression. The estimated values of Ea and reference rate constants (i.e. Kref at the reference temperature 4 º C) for chemical and sensory indices are reported in Table 1. In more detail, the activation energy value of the sensory (chemical) index for all the packaging types varied from 55.12 (21.037) to 73.55 (36.874) kJ/mol. Also, for both the quality indices, the highest activation energy and the lowest rate constant were obtained for the P3 packaging. As stated in Sect. 3.1.2, the low level or absence of oxygen in the MAP packaging causes a slower rate of TVB-N increase. Moreover, in the P3 packaging, more energy was required for starting the chemical reaction. Therefore, its activation energy should be higher than the other packagings. Regarding the total acceptance assessed in Sect. 3.2, the lowest sensory changes during the storage time were obtained for the P3 packaging which leads to the lowest sensory loss rate.
Fig. 3.
Arrhenius curves of a the total acceptance and b TVB-N, in four types of the modified atmosphere packaging
Table 1.
The activation energy value (Ea) and reaction rate constant [at the reference temperature Kref(4 °C)] of the chemical (TVB-N) and sensory (total acceptance) indices for the trout fillets packed in four types of MAP at four constant temperatures 0, 5, 10 and 15 °C
| Packaginga | Total acceptance | TVB-N (mg/100 g) | ||
|---|---|---|---|---|
| Ea (kJ/mol) | Kref(4 °C)(d−1) | Ea (kJ/mol) | Kref(4 °C)(d−1) | |
| P1 | 55.623 | 0.026 | 21.037 | 0.053 |
| P2 | 57.044 | 0.034 | 28.587 | 0.049 |
| P3 | 73.550 | 0.022 | 36.874 | 0.047 |
| P4 | 55.120 | 0.045 | 34.870 | 0.061 |
aAtmosphere composition of the packaging P1:80%CO2/10%N2/10%O2, P2:60%CO2/20%N2/20%O2, P3:60%CO2/40%N2/20%O2, and P4:40%CO2/30%N2/30%O2
By considering the results reported in Table 1, the activation energy of sensory loss was almost twice higher than the chemical degradation caused by TVB-N production. Consequently, it can be said that the dependency of the total acceptance loss on the temperature was higher than the dependency of the chemical loss. In other words, by raising the temperature, the rate of sensory loss increases more rapidly compared to the rate of chemical loss.
Shelf life calculation
In this study, sensory quality was evaluated along with the evaluation of chemical indices. The values of chemical parameters (including TVB-N, TMA, and TBARS) were reported in Table 2. Since based on the evaluated indices, no spoilage occurred at temperatures 0 °C and 5 °C; the sensory rejection time and parameter values were reported only for temperatures 10 °C and 15 °C. In more detail, at these temperatures, the TVB-N concentration at the end of the shelf life (sensory rejection) was in the range of from 22.3 ± 0.98 to 27 ± 1.59 mgN/100. Koutsoumanis and Nychas (2000) also reported the similar range of 22–25 mgN/100 for TVB-N in fish. Moreover, the sensory rejection time coincided with the TMA level of 4 ± 0.27–5.4 ± 0.41 mg/100 g. Jouki (2014) also reported 5 mgN/100 g as the TMA limit of trout for sensory rejection time. The concentration of TBARS at the end of the shelf life (sensory rejection) was in the range of from 3.19 ± 0.29 to 4.92 ± 0.39 mg MDA/kg. Based on these suggestions, the maximum TBARS index, that indicates the optimal quality of the fish (frozen, chilled or kept in ice), is 5 mg MDA/kg.
Table 2.
The values of chemical parameters (TVB-N, TMA, and TBARS) at the time of sensory rejection (rejection score = 5) for trout fillets packed in four types of MAP at the temperatures 10 and 15 °C
| Packaginga | Temperature (°C) | TVB-N (mg/100 g) | TMA (mg/100 g) | TBARS (mg MDA/kg) |
|---|---|---|---|---|
| P1 | 10 | 25.5 ± 1.15 | 4.8 ± 0.38 | 4.92 ± 0.39 |
| 15 | 26.9 ± 1.48 | 5.4 ± 0.41 | 4.65 ± 0.38 | |
| P2 | 10 | 26.6 ± 1.5 | 4.6 ± 0.33 | 4.51 ± 0.35 |
| 15 | 22.3 ± 0.98 | 4.5 ± 0.34 | 4.18 ± 0.3 | |
| P3 | 10 | 26.1 ± 1.18 | 5 ± 0.33 | 3.19 ± 0.29 |
| 15 | 27 ± 1.59 | 5.3 ± 0.4 | 3.49 ± 0.32 | |
| P4 | 10 | 25.4 ± 1.23 | 4.7 ± 0.34 | 4.76 ± 0.39 |
| 15 | 23.4 ± 0.99 | 4 ± 0.27 | 4.06 ± 0.34 |
aAtmosphere composition of the packaging P1:80%CO2/10%N2/10%O2, P2:60%CO2/20%N2/20%O2, P3:60%CO2/40%N2/20%O2, and P4:40%CO2/30%N2/30%O2
Generally, by considering the values presented in Table 2 and permissible limits of studied parameters, it can be stated that in all cases, the values obtained for different parameters at the sensory rejection time, were in accordance with the values proposed in previous studies and supported them. It can also be claimed that in all cases, spoilage based on chemical parameters occurs later than sensory deterioration. In other words, in the trout fillet packed in the modified atmosphere, when the consumer rejects the sample based on the sensory evaluation, it is the beginning of chemical spoilage in that sample.
According to the Arrhenius kinetics given in Sect. 3.3, the shelf-life can be calculated, based on the sensory (total acceptance) and chemical (TVB-N) indices, by using Eqs. (2) and (3), respectively (Tsironi and Taoukis 2010):
| 2 |
| 3 |
where tSL is the shelf-life of the trout fillet (in day), CTVB-N indicates the concentration limit of TVB-N (25–30 mgN/100 g), and CTVB-N0 determines the initial concentration of TVB-N (in this study, 10–11 mgN/100 g). Also, S0 is the initial sensory score of the total acceptance while S1 is its corresponding rejection limit (S1 = 5). Moreover, kref is the reaction rate constant for each index (reported in Table 1) at the reference temperature (Tref = 4 °C) in d−1.
Table 3 reports the shelf life of each packaging type, based on the TVB-N and sensory evaluations at different storage temperatures. In both the examined indices, the highest shelf lives were obtained for P3 (with 60% CO2, 40% N2, and 0% O2) and succeedingly, P1 (with 80% CO2, 10% N2, and 10% O2). Moreover, the minimum shelf life was for the P4 packaging (with 40% CO2, 30% N2, and 30% O2).
Table 3.
The shelf-life (in day) of trout fillets packed in 4 types of MAP at different storage temperatures based on the total acceptance (sensory tSL) and TVB-N (chemical tSL) for comparing the relative error (RE %) of shelf-lives (sensory rejection score = 5, TVB-N permissible limit = 25–30 mg/100 g)
| Packaginga | Storage temperature (°C) | Sensory shelf-life | Chemical shelf-life | RE % |
|---|---|---|---|---|
| P1 | 0 | 13.9 | 11.4 | − 18.4 |
| 5 | 9 | 9.6 | 7.4 | |
| 10 | 5.9 | 6.9 | 18.6 | |
| 15 | 3.9 | 5.9 | 53.1 | |
| P2 | 0 | 10.8 | 9.8 | − 9.1 |
| 5 | 6.9 | 7.8 | 13.9 | |
| 10 | 4.4 | 5 | 13.3 | |
| 15 | 2.9 | 5.1 | 74.5 | |
| P3 | 0 | 18.2 | 14.5 | − 20.3 |
| 5 | 10.2 | 10.8 | 6.5 | |
| 10 | 5.8 | 7 | 20.4 | |
| 15 | 3.4 | 5.3 | 57.8 | |
| P4 | 0 | 8.1 | 9.5 | 17.8 |
| 5 | 5.2 | 4.9 | − 5.1 | |
| 10 | 3.4 | 3.8 | 10.7 | |
| 15 | 2.3 | 3.4 | 48.4 |
aAtmosphere composition of the packaging P1:80%CO2/10%N2/10%O2, P2:60%CO2/20%N2/20%O2, P3:60%CO2/40%N2/20%O2, and P4:40%CO2/30%N2/30%O2
Clearly, the percent differences between the shelf-lives of the sensory and chemical indices remained limited (almost smaller than 20%) at the storage temperatures 0, 5, and 10 °C. Therefore, there would be a high correlation between the sensory degradation and TVB-N production. It means that chemical spoilage in fish samples at those temperatures could be predicted based on sensory evaluation and shelf life. However, at the temperature 15 °C, the sensory shelf life was much lower than the chemical shelf life. In other words, sensory spoilage occurs much earlier than chemical spoilage at that temperature, and thus, sensory evaluation cannot be used as an indicator for chemical spoilage.
Conclusion
According to the experimental results, we can conclude that in various temperatures, the values obtained for different chemical parameters (including TVB-N, TMA, and TBARS) at the sensory rejection time are in accordance with the allowed limits proposed in previous studies, and approve them. It can also be declared that in all cases, chemical spoilage occurs later than sensory degradation. In other words, in the trout fillet packed in the modified atmosphere, when the consumer rejects the sample based on the sensory evaluation, it would be the beginning of chemical spoilage in that sample. Furthermore, P3 and P1 (in an ordered manner) were the best packagings in terms of inhibiting bacterial growth and LAB levels.
Based on our results, in both the TVB-N and sensory evaluations, the highest shelf life was obtained for P3 (with the sensory shelf-life of 10.2 and chemical shelf-life of 10.8 days at 5 °C) and succeedingly, P1 (with the sensory shelf-life of 9.0 and chemical shelf-life of 9.6 days at 5 °C). Moreover, the minimum shelf life was related to the P4 packaging (with the sensory shelf-life of 5.2 and chemical shelf-life of 4.9 days at 5 °C). Finally, the percent difference between the chemical and sensory shelf lives remained limited at the temperatures below 10 °C. Therefore, in this case, the chemical spoilage can be predicted based on the sensory evaluation and shelf life.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author Contributions Statement
This paper has been built based on VN’s PhD thesis in Ferodwsi University of Mashhad, Mashhad, Iran. NS was the associate supervisor of the project; and FTY and HBG were enrolled as the associate advisors. MS-T was partially contributed in the project for statistical analysis of experimental results and answering to reviewers’ comments.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Contributor Information
Vajihe Nikzade, Email: vajihe.nikzade@mail.um.ac.ir.
Naser Sedaghat, Phone: +98 5138788110, Email: sedaghat@um.ac.ir, Email: sedaghat@ferdowsi.um.ac.ir.
Farideh Tabatabaei Yazdi, Email: tabatabai@um.ac.ir.
Hamid Bahador Ghoddusi, Email: h.ghoddusi@londonmet.ac.uk.
Mahdi Saadatmand-Tarzjan, Email: saadatmand@um.ac.ir.
References
- Akhondzadeh A, Bokaie S, Zahraie ST. Comparative study of trimethylamine, total volatile nitrogen and bacterial total count in quality control of frozen bony fish. Iran J Vet Res. 2000;55:71–74. [Google Scholar]
- Arashisar S, Hisar O, Kaya M, Yanik T. Effects of modified atmosphere and vacuum packaging on microbiological and chemical properties of rainbow trout (oncorhynchus mykiss) fillets. Int J Food Microbiol. 2004;97:209–214. doi: 10.1016/j.ijfoodmicro.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Chytiri S, Chouliara I, Savvaidis IN, Kontominas MG. Microbiological, chemical and sensory assessment of iced whole and filleted aquacultured rainbow trout. Food Microbiol. 2004;21:157–165. doi: 10.1016/S0740-0020(03)00059-5. [DOI] [Google Scholar]
- Connell JJ. Methods of assessing and selecting for quality. In control of Fish Quality. 3. Oxford: Fishing News Books; 1990. [Google Scholar]
- Cosansu S, Mol S, Ucok Alakavuk D, Ozturan S. The effect of lemon juice on shelf life of sous vide packaged whiting. Food Bioprocess Technol. 2013;6:283–289. doi: 10.1007/s11947-011-0572-0. [DOI] [Google Scholar]
- Dalgaard P, Gram L, Huss HH. Spoilage and shelf-life of cod fillets packed in vacuum or modified atmospheres. Int J Food Microbiol. 1993;19:283–294. doi: 10.1016/0168-1605(93)90020-H. [DOI] [PubMed] [Google Scholar]
- Debevere J, Boskou G. Effect of modified atmosphere packaging on the TVB/TMA—producing microflora of cod fillets. Int J Food Microbiol. 1996;31:221–229. doi: 10.1016/0168-1605(96)01001-X. [DOI] [PubMed] [Google Scholar]
- Fagan JD, Gormley TR, Mhuircheartaigh MM. Effect of modified atmosphere packaging with freeze-chilling on some quality parameters of raw whiting, mackerel and salmon portions. Innov Food Sci Emerg. 2004;5:205–214. doi: 10.1016/j.ifset.2004.01.001. [DOI] [Google Scholar]
- Fernández K, Aspé E, Roeckel M. Scaling up parameters for shelf life extension of Atlantic Salmon (Salmo salar) fillets using super chilling and modified atmosphere packaging. Food Control. 2010;21:857–862. doi: 10.1016/j.foodcont.2009.11.016. [DOI] [Google Scholar]
- Goulas AE, Kontominas MG. Effect of modified atmosphere packaging and vacuum packaging on the shelf life of refrigerated chub mackerel (Scomber japonicus): biochemical and sensory attributes. Eur Food Res Technol. 2007;224:545–553. doi: 10.1007/s00217-006-0316-y. [DOI] [Google Scholar]
- Hassoun A, Karoui R. Monitoring changes in whiting (Merlangius merlangus) fillets stored under modified atmosphere packaging by front face fluorescence spectroscopy and instrumental techniques. Food Chem. 2016;200:343–353. doi: 10.1016/j.foodchem.2016.01.028. [DOI] [PubMed] [Google Scholar]
- Hedayatifard M, Aroujalian AR. Improvement of shelf life for stellate sturgeon fillet, Acipenser stellatus, under modified atmosphere packaging (MAP) and vacuum conditions. Iran Sci Fish J. 2010;19:127–139. [Google Scholar]
- Huss HH. Fresh fish quality and quality changes. FAO Fish Ser. 1988;29:20–24, 43–52 and 61–67. [Google Scholar]
- International Commission on Microbiological Specifications for Foods (ICMSF) (1986) Sampling plans for fish and shellfish. In: ICMSF, microorganisms in foods. Sampling for microbiological analysis: principles and scientific applications, 2nd ed., vol. 2. University of Toronto Press, Toronto, Canada, 181–196
- Jouki M (2014) Production of antimicrobial edible films based on quince seed mucilage containing oregano or thyme essential oil and their effects on shelf life extension of rainbow trout fillets. Ph.D. Dissertation. Ferdowsi University of Mashhad Faculty of Agriculture
- Kerry J, Butler P. Smart packaging technologies for fast moving consumer goods. Hoboken: Wiley; 2008. [Google Scholar]
- Koutsoumanis K, Nychas GJE. Application of a systematic experimental procedure to develop a microbial model for rapid fish shelf-life predictions. Int J Food Microbiol. 2000;60:171–184. doi: 10.1016/S0168-1605(00)00309-3. [DOI] [PubMed] [Google Scholar]
- Loovas EA. Sensitive spectrophotometric method for lipid hydroperoxide determination. J Am Oil Chem Soc. 1992;69:777–783. doi: 10.1007/BF02635914. [DOI] [Google Scholar]
- Macé S, Cornet J, Chevalier F, Cardinal M, Pilet MF, Dousset X, Joffraud JJ. Characterisation of the spoilage microbiota in raw salmon (Salmo salar) steaks stored under vacuum or modified atmosphere packaging combining conventional methods and PCReTTGE. Food Microbiol. 2011;30:164–172. doi: 10.1016/j.fm.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Malle P, Poumeyrol M. A new chemical criterion for the quality control of fish- trimethylamine total volatile basic nitrogen (percent) J Food Prot. 1989;52:419–423. doi: 10.4315/0362-028X-52.6.419. [DOI] [PubMed] [Google Scholar]
- Malle P, Tao SH. Rapid quantitative-determination of trimethylamine using steam distillation. J Food Prot. 1987;50:756–760. doi: 10.4315/0362-028X-50.9.756. [DOI] [PubMed] [Google Scholar]
- Masniyom P, Benjakul S, Visessanguan W. Shelf life extension of refrigerated seabass slices under modified atmosphere packaging. J Sci Food Agric. 2002;82:873–880. doi: 10.1002/jsfa.1108. [DOI] [Google Scholar]
- Masniyom P, Benjama O, Maneesri J. Effect of modified atmosphere and vacuum packaging on quality changes of refrigerated tilapia (Oreochromis niloticus) fillets. Int Food Res J. 2013;20:1401–1408. [Google Scholar]
- Muela E, Alonso V, Morago P, Calanche JB, Roncalés P, Beltrán JA. Effect of gas packaging conditions on thawed Thunnus obesus preservation. Food Control. 2014;46:17–24. doi: 10.1016/j.foodcont.2014.05.022. [DOI] [Google Scholar]
- Nuin M, Alfaro B, Cruz Z, Argarate N, George S, Marc YL, Olley J, Pin C. Modeling spoilage of fresh turbot and evaluation of a time–temperature integrator (TTI) label under fluctuating temperature. Int J Food Microbiol. 2008;127:193–199. doi: 10.1016/j.ijfoodmicro.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Özogul F, Polat A, Özogul Y. The effects of modified atmosphere packaging and vacuum packaging on chemical, sensory and microbiological changes of sardines (Sardina pilchardus) Food Chem. 2004;85:49–57. doi: 10.1016/j.foodchem.2003.05.006. [DOI] [Google Scholar]
- Reddy NR, Armstrong DJ, Rhodehamel EJ, Kauter DA. Shelf-life extension and safety concerns about fresh products packaged under modified atmosphere: a review. J Food Saf. 1992;12:87–118. doi: 10.1111/j.1745-4565.1991.tb00069.x. [DOI] [Google Scholar]
- Robles-Martínez C, Cervantes E, Ke PJ (1982) Recommended method for testing the objective rancidity development in fish based on TBARS formation. In: Issue 1089 of Canadian Technical Report of Fisheries and Aquatic Sciences
- Sivertsvik M, Jeksrud WK, Rosnes JT. A review of modified atmosphere packaging of fish and fishery products—significance of microbial growth, activities and safety. Int J Food Sci Technol. 2002;37:107–127. doi: 10.1046/j.1365-2621.2002.00548.x. [DOI] [Google Scholar]
- Sone I, Olsen RL, Sivertsen AH, Eilertsen G, Heia K. Classification of fresh Atlantic salmon (Salmo salar L.) fillets stored under different atmospheres by hyperspectral imaging. J Food Eng. 2012;109:482–489. doi: 10.1016/j.jfoodeng.2011.11.001. [DOI] [Google Scholar]
- Torrieri E, Cavella S, Villani F, Masi P. Influence of modified atmosphere packaging on the chilled shelf-life of gutted farmed bass (Dicentrarchus labrax) J Food Eng. 2006;77:1078–1086. doi: 10.1016/j.jfoodeng.2005.08.038. [DOI] [Google Scholar]
- Tsironi TN, Taoukis PS. Modeling microbial spoilage and quality of Gilthead Seabream fillets: combined effect of osmotic pretreatment, modified atmosphere packaging, and nisin on shelf life. J Food Sci. 2010;75:243–251. doi: 10.1111/j.1750-3841.2010.01574.x. [DOI] [PubMed] [Google Scholar]
- Tsironi T, Tsevdou M, Velliou E, Taoukis P. Modeling the effect of temperature and CO2 on microbial spoilage of chilled gilthead seabream fillets. Acta Hortic. 2008;802:345–350. doi: 10.17660/ActaHortic.2008.802.45. [DOI] [Google Scholar]
- Tsironi T, Dermesonlouoglou E, Giannakouroud M, Taoukis P. Shelf life modeling of frozen shrimp at variable temperature conditions. LWT Food Sci Technol. 2009;42:664–671. doi: 10.1016/j.lwt.2008.07.010. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.