Abstract
Blueberry belongs to the genus vaccinium, which is rich in a variety of biologically active components beneficial to the human body. Drying of blueberry is a slow and energy-intensive process because of its waxy skin, which has low permeability to moisture. Therefore, chemical pretreatment of ethyl oleate (AEEO) was adopted to accelerate moisture diffusivity. The results showed that the drying rate of blueberries was increased significantly by AEEO treatment, and the drying time can be shortened by 17.17–40.70%. After AEEO dipping, the effective diffusion coefficient increased from 5.461 × 10−9 to 1.067 × 10−8 m2/s at 60 °C. Six semi-theoretical thin-layer models were used to estimate the curves of air-drying of blueberry, and Wang–Singh model was found to perform better than other models. Besides, the rehydration and retention of nutritional contents were also improved by AEEO dipping. The total phenolics, total flavonoids, total anthocyanin content, and ABTS*+ scavenging activity of blueberry were increased by 37.74%, 21.01%, 47.83%, and 30.75%, respectively. The result of SEM observation and cell-membrane permeability indicated that AEEO could break down the wax layer of blueberry, change the crystal structure of wax layer, and increase cell permeability, which resulted in shorter drying time and higher quality of blueberry.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3538-7) contains supplementary material, which is available to authorized users.
Keywords: Ethyl oleate, Wax layer, Anthocyanins, Cell membrane permeability, Modelling
Introduction
Blueberry (Vaccinium corymbosum L.) belongs to the genus Vaccinium of the Ericaceae family. It is known to have high antioxidant capacity, which is related to the phenolic compounds and anthocyanins they contain (Kalt et al. 2007), thus has many protective properties against diseases such as memory loss, cancer, heart disease, urinary disease, vision problems and ageing (Shi et al. 2008). However, blueberries have a short growing season, and fresh fruit quickly deteriorate after they are picked and have a shelf life of less than 2 weeks under refrigeration (Feng et al. 1999). Therefore, seasonal availability may limit the consumption of fresh blueberries and further processing including freezing, canning, or drying is often used.
Drying is one of the most common and important methods of food preservation (Tunde-Akintunde and Afolabi 2010). By removing water from the food, microbial growth and moisture-meditated deteriorative reactions are minimized (Sacilik 2007). At present, the most applicable drying methods include: hot air, freeze, vacuum, heat pump, microwave drying, cabinet or tray, fluidized bed, spouted bed, and combinations thereof. However, drying of blueberry is very slow process, due to the peculiar structure of blueberry peel, that is covered by a waxy layer. The wax layer is a hydrophobic layer composed of lipophilic compounds, including very-long-chain aliphatic series and their derivatives, which greatly influences the rate of water loss during natural and artificial drying. This was also proved by Shi et al. (2008), who reported the main problem during drying of blueberries is a waxy hydrophobic layer that impedes water transport from the interior to its surface, especially at relatively low air temperature.
Pre-treatments play an important role in quality attributes and drying rate in many fruits and vegetables, such as chemical dipping (Doymaz 2006; Ergünes and Tarhan 2006), freezing/thawing treatment (Zielinska et al. 2015) and high pulsed electric field (HPEF) (Yu et al. 2016). Among these treatments, chemical dipping (sodium hydroxide, potassium carbonate, methyl and ethyl ester emulsions) is the simplest and most efficient method (Doymaz and Pala 2002; Deshmukh et al. 2013). Doymaz (2004a) reported treating fresh plum with alkali solution containing ethyl oleate could reduce the drying time by 29.4%. Srimagal et al. (2017) stated that after ethyl oleate treatment the bitter gourd showed lower shrinkage ratio and higher diffusivity (increased from 2.65 × 10−8 m2/s to 3.86 × 10−8 m2/s). Ergünes and Tarhan (2006) observed dipping red peppers in the solution of 2% ethyl oleate + 2% NaOH + 4% K2CO3 at 60 °C could result in the best color retention. There were many studies about the effects of ethyl oleate on the drying kinetics and quality attributes of waxy fruits, such as grapes, plum, apricot and mulberry etc., but little literature was about the effect of ethyl oleate on drying kinetics and antioxidant activity of blueberry. Besides, there was no study show how the ethyl oleate changed the wax layer structure and cell membrane permeability of blueberry.
Thus, the main objective of this study was to investigate the effect of ethyl oleate dipping on drying kinetics, quality characteristics (color, rehydration property, total phenols, total flavonoids, total anthocyanin, and antioxidant activity) and changes in microstructure of wax layer of blueberry.
Materials and methods
Samples
Blueberries (Vaccinium corymbosum L.) were obtained from a local farm (Heyuan, Guangdong, China), which generally in uniform size of 1.0–1.5 g. The initial moisture content of blueberries varied from 87.3 to 89.6%, with soluble solid content of 12.5 ± 0.2%. The fruits were delivered and stored at 4 °C until pretreatments.
Reagent and chemicals
Chemicals used for dipping grape were technical grade. Ethyl oleate (AEEO) was obtained from Macklin Biochemical Corporation (Shanghai China). Analytical grade chemicals: Folin–Ciocalteu reagent; gallic acid; ascorbic acid; 2,2-diphenyl-1-picrylhydrazyl (DPPH); 2,2′-azinobis (3-ethylbenzo thiazoline-6-sulfonic acid) diammonium salt (ABTS); 2,4,6-tripyridyl-s-triazine (TPTZ) were procured from Sigma-Aldrich (St. Louis, MO, USA). Other analytical grade reagents like potassium carbonate and sodium carbonate were from National Pharmaceutical Corporation (Beijing, China).
AEEO dipping and drying procedure
Blueberries were washed and then dipped into alkali emulsion of AEEO which were prepared by potassium carbonate (3% w/w) and AEEO (1% w/w) at room temperature. The time for dipping treatment was 1 min and 5 min, respectively. After dipping in AEEO, the blueberries were dried as a single layer in a cabinet dryer (Shanghai Keheng Corporation, China) with air velocity of 1.2 m/s. The drying tray had an area of size 0.3 m × 0.4 m. The dryer was adjusted to the selected temperature for about half an hour before the start of experiment to achieve the steady state conditions. Each sample utilized in the experiment weighed 200 g. The final moisture content was 0.2 ± 0.02 g/g d.w. Each treatment was performed in triplicate.
Mathematical modelling
The air-drying curves obtained were fitted with six semi-theoretical thin layer-drying models namely, the Newton model, the Henderson and Pabis model, the Page model, the logarithmic model, the two- term exponential model and the Wang and Singh model (Table S1). The moisture ratio (MR) can be simplified to M/M0 instead of (M − Me)/(M0 − Me) for long drying times (Thakor et al. 1999), where M is the moisture content at any time t, M0 is the initial moisture content, and Me is the equilibrium moisture content. For model evaluation, a nonlinear regression procedure was used. The correlation coefficient (R2), root-mean-square error (RMSE), and Chi square (χ2) were used to determine the quality of the fit. RMSE, and χ2 are defined as follows:
| 1 |
| 2 |
where MRpre is a predictive value of MR, MRpre,i an experimental value of MR; N the number of observation times, and n the number of constant terms in the regression model (Roselló et al. 1992).
Effective moisture diffusivity
The most widely investigated theoretical model in the thin layer drying of different foods is given by the solution of Fick’s second law. The assumptions of moisture migration being by diffusion, constant temperature and diffusion coefficients, negligible shrinkage and for sphere is (Mahmutoglu et al. 1996):
| 3 |
For long drying periods, Eq. (3) can be further simplified to only first term of the series (Ramesh et al. 2001):
| 4 |
The effective moisture diffusivity was calculated using the method of slopes. Diffusion coefficients are typically determined by plotting experimental drying data in terms of lnMR versus time (as given in Eq. (4)) (Tutuncu and Labuza 1996). From Eq. (4), a plot of lnMR versus time gives a straight line with a slope of:
| 5 |
Rehydration
Rehydration is used to test the injury degree of drying on cell structure. It was determined by soaking a known weight of dried blueberries in a sufficient volume of water at 25 °C according to Santos-Sánchez et al. (2012). After reaching a constant weight, dried samples were weighed after removing excess water with the help of absorbent paper.
| 6 |
Preparation of extract for the determination of antioxidant activity
Extracts were prepared according to the procedure described by Fracassetti et al. (2013). 0.5 g blueberry powder (5 g blueberry puree) was dissolved in 20 mL of methanol acidified with 1% HCl and sonicated for 10 min. The suspension was centrifuged at 3000 g for 15 min, then the supernatant was collected. The precipitate was extracted with acidic methanol multiple times until the color became clear, and the collected supernatant was transferred to a 100 mL volumetric flask and made up to the volume by methanol acidified with 1% HCl.
Total phenol content (TPC)
Total polyphenolics of plum was determined using the Folin–Ciocalteu assay according to Velioglu et al. (1998) with modifications. Samples (400 μL) were introduced into test tubes followed by 2.0 mL of Folin–Ciocalteu reagents. After 5 min, 3.0 mL of sodium carbonate (7.5% w/v) solution was added. The absorbance was measured at 765 nm using a spectrophotometer (UV-1800, Shimadzu, Japan) after 2 h reaction in darkness with prepared blank. Total phenolics in plums were expressed as gallic acid equivalents (GAE, mg/g of dry sample).
Total flavonoid contents (TFC)
Total flavonoids were measured according to Kim et al. (2003) with slight modifications. Diluted extracts 2 mL were put in a 10 mL volumetric flask. Initially, 5% NaNO2 0.3 mL was added, after 6 min, 0.3 mL of 10% AlCl3 was added, then after 6 min, 2 mL of 4% NaOH was added. At last, distilled water (5.4 mL) was added to fill up the flask after 15 min. Absorbance of the reaction mixture was read at 510 nm. 2 mL 1% HCl-80% methanol was taken as blank instead of sample. TFC was determined as rutin equivalents (mg/g of dry weight).
Total anthocyanins content (TAC)
The quantification of total anthocyanins was evaluated by the pH differential method of Lee et al. (2005). Phenolic extracts of plums in 0.025 M potassium chloride buffer (pH 1.0) and 0.4 M sodium acetate buffer (pH 4.5) were measured at 510 and 700 nm after 15 min of incubation at 23 °C. The content of total anthocyanins was expressed as cyanidin 3-glucoside equivalent (CGE mg/g d.w.). A molar absorptivity of 26,900 was used for cyanidin 3-glucoside (molecular weight 449.2).
ABTS·+ scavenging activity
The ABTS antioxidant activity was carried out according to the method of Kim et al. (2002). ABTS stock solution was dissolved in sodium acetate-acetic acid buffer (20 Mm pH = 4.5) to make a 7 mM ABTS stock solution. 7 mM ABTS solution and 2.45 mM potassium persulfate were mixed in 1:1 ratio and allowed to stand in the dark for 12–16 h to produce ABTS+ working solution. This solution was further diluted with 80% methanol to reach the absorbance of 0.70 ± 0.02 at 734 nm. 0.2 mL of extract was mixed with 2 mL ABTS+ working solution and measured at 734 nm after 30 min in the dark. The blank was run with 80% methanol. A standard curve was prepared using Trolox solution (30–90 μg/mL).
Cell-membrane permeability
Cell membranes permeability was estimated by relative electrolyte leakage (REL) according to Galindo et al. (2005). Disks from both peels and flesh of blueberry were cut with diameter of 8 mm and thickness of 2 mm. 8–10 disks were put into deionized water and treated under vacuum condition for 10 min, then shaken (120 rpm) for 1 h. The electrical conductivity was measured using a conductivity meter (CPC-505, smarttester, Germany). Tissues were later destroyed by heating in boiling water for 10 min. After cooling, the conductivity of the bathing medium was measured. REL was calculated as the ratio of conductivity before and after tissue destruction.
| 7 |
where Le indicates the ratio of electric conductivity of blueberry tissue extract of samples (L1) to the electric conductivity of the extract after killing (L0).
Optical microscope and scanning electron microscope
Microstructure changes of waxy layer were analyzed using an optical microscope (DMI3000B, LEICA, Germany) and a scanning electron microscope (JSM-6360LV, JEOL, Japan). Optical microscope observation was according to Katepalli et al. (2017) with modifications. The skin of blueberry treated with AEEO solution was spread on the glass slide, avoiding bubbles without staining. Scanning-electron-microscopy (SEM) observation of wax layer was according to Bain and McBean (1967) and An et al. (2018). The wax layer near the equator was selected for testing, slices with central area of (0.5–1) cm × (0.5–1) cm and thickness of 1 mm were cut. Each specimen was glued on the metal stub, coated with a very thin layer of gold and observed.
Statistical analysis
Experiment data were analyzed using Origin 8.0 (Microcal Software, Inc., Northampton, USA) and Spss18.0 (Chicago, IL, USA). Significant differences between samples were analyzed using Duncan’s multiple-range test (P < 0.05). All experiments were run in triplicate, and data were reported as the mean ± standard deviation (SD).
Results and discussion
Drying characteristics of blueberry after AEEO treatment
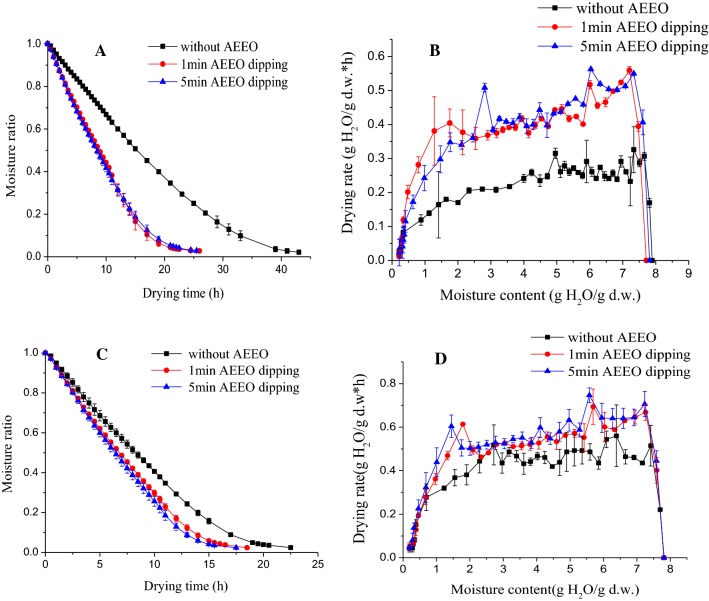
Moisture ratio (MR) curves were observed to be reduced exponentially with drying time (Fig. 1a, c), and curves exhibited a steeper slope, indicating difference in drying rate. The curve of 5 min AEEO dipping showed the steepest slope, followed by 1 min AEEO dipping, and samples without AEEO dipping. It indicated that 5 min AEEO treated samples had the fastest drying rate.
Fig. 1.
The effect of ethyl oleate pretreatments on drying kinetics of blueberry. a Moisture ratio of blueberry under 60 °C, b drying rate of blueberry under 60 °C, c moisture ratio kinetics under 70 °C, d drying rate of blueberry under 70 °C
It took 43 h for blueberry to reach the final moisture content at 60 °C. After AEEO dipping for 1 min, the drying time was reduced by 39.53%, after 5 min dipping, the drying time was shortened by 43.02%. At 70 °C, the drying time of blueberry was 22.5 h, after AEEO dipping, the drying time can be shortened by 17.78–22.22%. Therefore, the effect of AEEO dipping was more significant at lower temperature.
The curves of drying rate are illustrated in Fig. 1b, d which can be identified in three distinct periods: a warming-up, constant rate, and falling rate period. This was attributed to the different roles of capillary diffusion and moisture evaporation playing in moisture transfer during drying (Wang and Chen 2000). At the beginning of drying, the inside moisture was sufficient for moisture evaporation on the surface. Therefore, the inner moisture diffusion rate was higher than its surface evaporation rate, which showed a warming-up period. With the drying proceed, the internal moisture diffusion rate was equal to the rate of moisture evaporation, which presented a constant rate period. As drying progressed further, the amount of capillary liquid available inside became inadequate to maintain the moisture evaporation on the surface and the rate declined rapidly. This is the falling rate period. The falling-rate period determines the whole drying efficiency which depends on the nature of the material and difficulty of capillary diffusion. As it was shown in Fig. 1b, d, AEEO treatment could significantly increase the drying rate of blueberry, especially in the constant rate and falling rate periods, indicating AEEO dipping could reduce the difficulty of capillary diffusion.
Effective moisture diffusivity (Deff)
The effective moisture diffusivity (Deff) of a food material characterizes its intrinsic moisture mass transport property. Effective moisture diffusivity could describe all possible mechanisms of moisture movement within the foods, such as liquid diffusion, vapour diffusion, surface diffusion, capillary flow and hydrodynamic flow (Karathanos et al. 1990). As shown in Table 1, the moisture diffusivity increased from 5.461 × 10−9–1.067 × 10−8 to 1.092 × 10−8–1.390 × 10−8 as the air temperature increased from 60 to 70 °C. It indicated that the increase of temperature resulted in the rapid moisture evaporation of blueberry. The improved vapor pressure differences on the surface could speed up the inner moisture diffusion. This was proved by many researchers (Sharma and Prasad 2004; Doymaz and Pala 2003).
Table 1.
Effective diffusion coefficient values obtained for blueberry with different pretreatments
| Pretreatment | Effective diffusion coefficient (m2/s) | |
|---|---|---|
| 60 °C | Control | 5.461 × 10−9 |
| Treated for 1 min | 1.043 × 10−8 | |
| Treated for 5 min | 1.067 × 10−8 | |
| 70 °C | Control | 1.092 × 10−8 |
| Treated for 1 min | 1.340 × 10−8 | |
| Treated for 5 min | 1.390 × 10−8 | |
The effective moisture diffusivity of blueberries without AEEO dipping was 5.46 × 10−9 m2/s at 60 °C. After 1 min and 5 min dipping, it increased to 1.04 × 10−8 m2/s, and 1.07 × 10−8 m2/s respectively. The same trend was also found in samples dried at 70 °C (Table 1). This showed that the effective diffusion coefficient was increased with AEEO dipping time. Similar result was also described by Bingol et al. (2012). This is because longer dipping time could cause severer damage to wax layer and membrane permeability of blueberry, which considerably reduce the mass transfer resistance of moisture diffusion. But longer dipping time could cause serious damage to epidermis and weakened the surface color of blueberry. So 5 min-dipping time was recommended for blueberries.
Modelling of drying curves
The profiles of experimental moisture ratio as function of time during drying of blueberry for various dipping time are shown in Fig. 1. Drying curves were fitted to six thin layer drying models. The higher value of correlation coefficient (R2), lower value of root-mean-square error (RMSE) and Chi square (χ2) indicate better fitness of drying models. As the statistical analysis values summarized in Table 2, the R2 values of the Page, logarithmic, and Wang–Singh models were greater than 0.99, χ2 was less than 2.05 × 10−5, and the RMSE was less than 0.0264, suggesting that the three models were well fit. Among these, Wang–Singh model had the highest R2 and lowest RMSE and χ2, indicating it can best represent the thin layer drying behavior of the blueberry.
Table 2.
Curve-fitting criteria for various models and parameters
| Temperature | Pretreatment | Model number | Equation coefficient | R2 | χ2 | RMSE | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | k | n | b | c | g | ||||||
| 60 °C | Control | 1 | 0.0481 | 0.9657 | 3.10 × 10−3 | 0.0557 | |||||
| 2 | 1.0721 | 0.0537 | 0.9770 | 2.02 × 10−3 | 0.0450 | ||||||
| 3 | 0.0185 | 1.3476 | 0.9966 | 3.03 × 10−4 | 0.0174 | ||||||
| 4 | 1.4757 | 0.0287 | − 0.4510 | 0.9974 | 2.34 × 10−4 | 0.0151 | |||||
| 5 | 0.5361 | 0.0536 | 0.5361 | 0.0536 | 0.9757 | 2.02 × 10−3 | 0.0450 | ||||
| 6 | − 0.0375 | 3.26 × 10−4 | 0.9980 | 1.78 × 10−4 | 0.0132 | ||||||
| Treated for 1 min | 1 | 0.0903 | 0.9570 | 4.23 × 10−3 | 0.0650 | ||||||
| 2 | 1.0861 | 0.0999 | 0.9674 | 3.12 × 10−3 | 0.0558 | ||||||
| 3 | 0.0341 | 1.4233 | 0.9926 | 7.04 × 10−4 | 0.0265 | ||||||
| 4 | 1.3834 | 0.0576 | − 0.3522 | 0.9912 | 8.36 × 10−4 | 0.0285 | |||||
| 5 | 0.5430 | 0.0999 | 0.5430 | 0.0999 | 0.9653 | 3.12 × 10−3 | 0.0558 | ||||
| 6 | − 0.0691 | 0.0012 | 0.9941 | 7.16 × 10−4 | 0.0264 | ||||||
| Treated for 5 min | 1 | 0.0924 | 0.9691 | 2.94 × 10−3 | 0.0542 | ||||||
| 2 | 1.0784 | 0.1014 | 0.9780 | 2.03 × 10−3 | 0.0451 | ||||||
| 3 | 0.0417 | 1.3487 | 0.9961 | 1.57 × 10−3 | 0.0396 | ||||||
| 4 | 1.3061 | 0.0639 | − 0.2765 | 0.9960 | 3.75 × 10−4 | 0.0191 | |||||
| 5 | 0.5392 | 0.1014 | 0.5392 | 0.1014 | 0.9765 | 2.03 × 10−3 | 0.0451 | ||||
| 6 | − 0.0715 | 0.0013 | 0.9982 | 2.11 × 10−4 | 0.0145 | ||||||
| 70 °C | Control | 1 | 0.0945 | 0.9544 | 4.31 × 10−3 | 0.3364 | |||||
| 2 | 1.0970 | 0.1060 | 0.9684 | 2.89 × 10−3 | 0.0538 | ||||||
| 3 | 0.0348 | 1.4451 | 0.9947 | 4.87 × 10−2 | 0.2206 | ||||||
| 4 | 1.5253 | 0.0535 | − 0.4898 | 0.9961 | 3.57 × 10−4 | 0.0186 | |||||
| 5 | 0.5485 | 0.1060 | 0.5484 | 0.1060 | 0.9662 | 2.89 × 10−3 | 0.0538 | ||||
| 6 | − 0.0707 | 0.0011 | 0.9963 | 3.70 × 10−4 | 0.0189 | ||||||
| Treated for 1 min | 1 | 0.1180 | 0.9532 | 4.39 × 10−3 | 0.0663 | ||||||
| 2 | 1.0966 | 0.1311 | 0.9658 | 3.10 × 10−3 | 0.0557 | ||||||
| 3 | 0.0459 | 1.4481 | 0.9932 | 5.00 × 10−4 | 0.0224 | ||||||
| 4 | 1.5657 | 0.0624 | − 0.5359 | 0.9958 | 3.84 × 10−4 | 0.0193 | |||||
| 5 | 0.5483 | 0.1311 | 0.5483 | 0.1311 | 0.9633 | 3.10 × 10−3 | 0.0557 | ||||
| 6 | − 0.0863 | 0.0017 | 0.9965 | 3.66 × 10−4 | 0.0188 | ||||||
| Treated for 5 min | 1 | 0.1251 | 0.9535 | 4.29 × 10−3 | 0.0655 | ||||||
| 2 | 1.0970 | 0.1390 | 0.9664 | 2.98 × 10−3 | 0.0546 | ||||||
| 3 | 0.0498 | 1.4517 | 0.9939 | 5.02 × 10−4 | 0.0224 | ||||||
| 4 | 1.5466 | 0.0684 | − 0.5121 | 0.9949 | 4.54 × 10−4 | 0.0209 | |||||
| 5 | 0.5484 | 0.1390 | 0.5484 | 0.1390 | 0.9638 | 2.98 × 10−3 | 0.0546 | ||||
| 6 | − 0.0922 | 0.0019 | 0.9956 | 4.04 × 10−4 | 0.0198 | ||||||
Similar findings were reported by Doymaz (2004a), who found Wang and Singh model represents drying characteristics better than other models for pretreated plums. However, Sawhney et al. (1999) found the Page model was more suitable for describing air drying of grapes pretreated with chemical solutions. Doymaz (2004b) found exponential model was better than Page model in representing drying characteristics of mulberry. Therefore, different fruit varieties, drying methods and dipping conditions are applicable to different fitting models.
Effect of AEEO dipping on rehydration, polyphenols and antioxidant activity
Rehydration can be considered as a measure of injuries to the material caused by treatments of dehydration and drying (Lewicki 1998). Higher rehydration ratio indicated less injury to the fruit caused by drying process. As shown in Table 3, AEEO pre-treatment generally made the rehydration ratio (RR) of blueberry increase. Besides, with the increase of dipping time, the value of RR increased. This is because AEEO could significantly decrease the drying time, reducing the damage to blueberry caused by high temperature. The maximum value of RR occurred at 70 °C with 5 min AEEO dipping and minimum RR occurred at 60 °C without AEEO dipping also proved this.
Table 3.
Effects of ethyl oleate pretreatments on Rehydration ratio, total phenolics, flavonoids, anthocyanin content, and ABTS+ radical scavenging activity
| Fresh sample | 60 °C | Dipping for 1 min 60 °C | Dipping for 5 min 60 °C | 70 °C | Dipping for 1 min 70 °C | Dipping for 5 min 70 °C | |
|---|---|---|---|---|---|---|---|
| Rehydration ratio | 0 | 0.75 ± 0.028e | 0.91 ± 0.013d | 1.01 ± 0.02c | 1.07 ± 0.01c | 1.24 ± 0.03b | 1.35 ± 0.04a |
| Total phenolics (mg/g d.w.) | 33.41 ± 7.97a | 9.54 ± 0.25f | 11.86 ± 0.80e | 13.14 ± 0.17d | 14.22 ± 0.15c | 15.03 ± 0.15b | 15.60 ± 0.36b |
| Total flavonoids (mg/g d.w.) | 44.59 ± 1.86a | 26.08 ± 0.47d | 30.71 ± 1.39c | 31.56 ± 0.31c | 33.19 ± 1.82b | 33.44 ± 0.72bc | 34.30 ± 0.16b |
| Total anthocyanin (mg/g d.w.) | 5.70 ± 0.34a | 1.84 ± 0.04e | 2.20 ± 0.02d | 2.72 ± 0.08c | 2.87 ± 0.06c | 3.11 ± 0.05b | 3.31 ± 0.08b |
| ABTS+ scavenging activity (mg Trolox/g d.w) | 57.49 ± 1.32a | 25.07 ± 0.19f | 30.20 ± 0.33e | 32.78 ± 0.07d | 36.04 ± 0.28c | 38.02 ± 0.18b | 39.74 ± 0.12b |
For a, b, c, and d, the same letter indicates no significant difference; different letters indicate a significant difference, P < 0.05
The content of total phenols (TPC), total flavonoids (TFC), total anthocyanin (TAC), and ABTS*+ scavenging activity of fresh blueberry were 33.41, 44.59, 5.70, and 57.49 mg/g d.w., respectively. After drying, TPC, TFC, TAC and ABTS*+ scavenging activity were decreased significantly, and the samples dried at 60 °C had lower TPC, TFC, TAC and ABTS*+ scavenging activity than samples dried at 70 °C, which indicated longer drying process could lead to more serious loss of nutritional components (Table 3). After AEEO dipping, TPC, TFC, TAC and ABTS*+ scavenging activity were improved significantly. The retention rate of TPC, TFC, TAC and ABTS*+ scavenging activity were increased by 24.32–37.74%, 17.75–21.01%, 19.57–47.83%, and 20.47–30.75% at 60 °C and increased by 5.70–9.70%, 0.75–3.34%, 8.36–15.33%, and 5.49–10.27% at 70 °C. Therefore, AEEO pretreatment also showed significant effect in preserving nutritional components.
Effect of AEEO pretreatment on cell membrane permeability
Cell membrane permeability of blueberry was expressed by the change of relative electrolyte leakage (REL). The higher value of REL indicated the higher permeability of cell membrane. As shown in Fig. 2, the RELs of fresh peel and flesh were 58.76 ± 2.93% and 93.08 ± 0.67%. After AEEO dipping, the RELs were increased. The REL of peel was increased from 67.04 ± 1.00% to 75.02 ± 1.58% with the increase of dipping time. The same trend was occurred in REL of flesh after AEEO dipping. It indicated that AEEO could increase cell membrane permeability of both peel and flesh of blueberry and the effect of AEEO on peel was more significant than that on flesh.
Fig. 2.
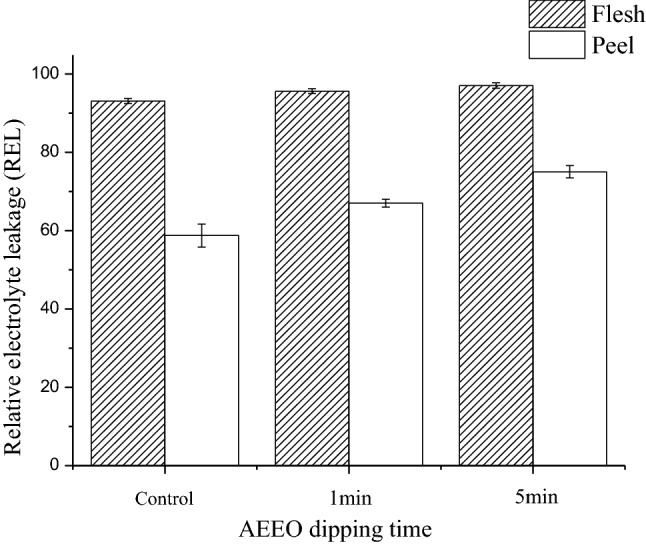
The effect of ethyl oleate pretreatments on cell permeability of peel and flesh of blueberry
According to Parrish and Leopold (1978), when structure and function of cell membrane were disturbed decrease in cell membrane activity, and increase in membrane permeability was observed. It suggested that AEEO could cause certain damage to peel and flesh. The damage to peel was greater than to flesh. This may because AEEO, an aliphatic ester, can dissolve the epicuticular wax, as well as alkaline solution could cause formation of tiny cracks, which considerably reduce the mass transfer resistance and improve drying efficiency.
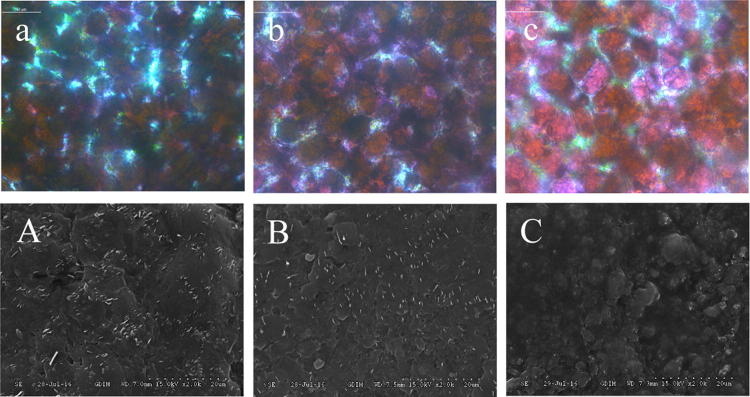
Effect of ethyl oleate pretreatment on skin and wax layer structure
The optical microscope and SEM observation can show the influence of AEEO dipping on structures of epidermis and epicuticular wax of blueberry. As shown in Fig. 3a–c, after treated by AEEO, the compactness of intracellular spaces decreased, and the transmittance of blueberry skin was increased, suggesting that AEEO could destroy the epidermis and improve the cell permeability of the fruit skin. As shown in the SEM morphology (Fig. 3a), the crystalline microstructure of epicuticular wax on blueberry can be described as small thin tubes, after AEEO dipping, the tubular crystalline structure decreased, especially after 5 min AEEO dipping, the tubular crystal structure almost disappeared, and there were tiny cracks in the amorphous site (Fig. 3b, c).
Fig. 3.
The effect of ethyl oleate pretreatments on wax layer structure of blueberry: light microscopy image (× 400). a Untreated samples, b samples treated for 1 min, c samples treated for 5 min; Scanning electron micrographs (× 2000)
The wax layer is a continuous hydrophobic membrane outside the skin, which is the first barrier to prevent water loss effectively and protect against environmental damage. It can be divided into intracuticular wax and epicuticular wax. The epicuticular wax exhibits crystalline morphology and can be observed by SEM. The epicuticular wax crystalline structure presents different shapes depending on wax composition. According to Gao et al. (2014), the tubular crystal morphology of blueberry is related to long-chain fatty acid (C31 and C33) and its derivatives. Ethyl oleate is an aliphatic ester, which can dissolve the long-chain fatty acids and the alkaline solution could also cause formation of tiny cracks. Therefore, dipping with ethyl oleate in alkaline solution (AEEO) could destroy the wax layer of blueberry and increase its cell permeability, which is beneficial to internal moisture diffusion and evaporation. The higher drying efficiency and shorter drying time could also lead to a better nutritional quality.
Conclusion
Ethyl oleate pretreatment could significantly improve drying rate, shorten drying time, and increase the water effective diffusion coefficient of blueberry. Meanwhile, the rehydration ratio, contents of total phenols, total flavonoids, total anthocyanin, and ABTS*+ scavenging activity were also improved. This could be attributed to removal of crystalline structure of wax layer, destruction of the epidermis and increase of cell permeability.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the financial support of the National Key R&D Program of China (2017YFD0400900, 2017YFD0400904), National Natural Science Foundation of China (31501500), and Province Natural Science Fund of Guangdong (2015A030312001).
References
- An KJ, Wu JJ, Tang DB, Wen J, Fu MQ, Xiao GS, Xu YJ. Effect of carbonic maceration (CM) on mass transfer characteristics and quality attributes of Sanhua plum (Prunus Salicina Lindl.) LWT Food Sci Technol. 2018;87:537–545. [Google Scholar]
- Bain JM, McBean DM. The structure of the cuticular wax of prune plums and its influence as a water barrier. Aust J Biol Sci. 1967;20:895–900. [Google Scholar]
- Bingol G, Roberts JS, Balaban MO, Devres YO. Effect of dipping temperature and dipping time on drying rate and color change of grapes. Dry Technol. 2012;30:597–606. [Google Scholar]
- Deshmukh AW, Varma MN, Yoo CK, Wasewar KL. Effect of ethyl oleate pretreatment on drying of ginger: characteristics and mathematical modelling. J Chem. 2013;10:1–6. [Google Scholar]
- Doymaz I. Effect of dipping treatment on air drying of plums. J Food Eng. 2004;64:465–470. [Google Scholar]
- Doymaz I. Pretreatment effect on sun drying of mulberry fruits (Morus alba L.) J Food Eng. 2004;65:205–209. [Google Scholar]
- Doymaz I. Drying kinetics of black grapes treated with different solutions. J Food Eng. 2006;76:212–217. [Google Scholar]
- Doymaz I, Pala M. The effects of dipping pretreatments on air-drying rates of the seedless grapes. J Food Eng. 2002;52(4):413–417. [Google Scholar]
- Doymaz I, Pala M. Effect of ethyl oleate on drying characteristics of mulberries. Nahrung/Food. 2003;47(5):304–308. doi: 10.1002/food.200390071. [DOI] [PubMed] [Google Scholar]
- Ergünes G, Tarhan S. Color retention of red peppers by chemical pretreatments during greenhouse and open sun drying. J Food Eng. 2006;76:446–452. [Google Scholar]
- Feng H, Tang JM, Mattinson DS, Fellman JK. Microwave and spouted bed drying of frozen blueberries: the effect of drying and pretreatment methods on physical properties and retention of flavor volatiles. J Food Process Preserv. 1999;23(6):463–479. [Google Scholar]
- Fracassetti D, Bo CD, Simonetti P, Gardana C, Klimis-Zacas D, Ciappellano S. Effect of time and storage temperature on anthocyanin decay and antioxidant activity in wild blueberry (Vaccinium angustifolium) powder. J Agric Food Chem. 2013;61:2999–3005. doi: 10.1021/jf3048884. [DOI] [PubMed] [Google Scholar]
- Galindo FG, Toledo RT, Sjöholm I. Tissue damage in heated carrot slices: comparing mild hot water blanching and infrared heating. J Food Eng. 2005;67:381–385. [Google Scholar]
- Gao HY, Yang S, Chen HJ, Chu WJ, Mu HL, Ge LM. Epicuticular Wax’s effect on fruit softening of blueberry. J Chin Inst Food Sci Technol. 2014;14(2):103–108. [Google Scholar]
- Kalt W, Joseph JA, Shukitt-Hale B. Blueberries and human health: a review of current research. J Am Pomol Soc. 2007;61:151–160. [Google Scholar]
- Karathanos VT, Villalobos G, Saravacos GD. Comparison of two methods of estimation of the effective moisture diffusivity from drying data. J Food Sci. 1990;55(1):218–231. [Google Scholar]
- Katepalli H, John VT, Tripathi A, Bose A. Microstructure and rheology of particle stabilized emulsions: effects of particle shape and interparticle interactions. J Colloid Interface Sci. 2017;485:11–17. doi: 10.1016/j.jcis.2016.09.015. [DOI] [PubMed] [Google Scholar]
- Kim D-O, Lee KW, Lee HJ, Lee CY. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J Agric Food Chem. 2002;50:3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- Kim D-O, Chun OY, Kim YJ, Moon HY, Lee CY. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J Agric Food Chem. 2003;51:6509–6515. doi: 10.1021/jf0343074. [DOI] [PubMed] [Google Scholar]
- Lee J, Durst RW, Wrolstad RL. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88:1269–1278. [PubMed] [Google Scholar]
- Lewicki PP. Some remarks on rehydration of dried foods. J Food Eng. 1998;36(1):81–87. [Google Scholar]
- Mahmutoglu T, Emir F, Saygi YB. Sun/solar drying of differently treated grapes and storage stability of dried grapes. J Food Eng. 1996;29:289–300. [Google Scholar]
- Parrish DJ, Leopold CA. On the mechanism of aging in soybean seeds. Plant Physiol. 1978;61:365–368. doi: 10.1104/pp.61.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh MN, Wolf W, Tevini D, Jung G. Influence of processing parameters on the drying spice paprika. J Food Eng. 2001;49:63–72. [Google Scholar]
- Roselló C, Cañellas J, Simal S, Berna A. Simple mathematical model to predict the drying rates of potatoes. J Agric Food Chem. 1992;40:2374–2376. [Google Scholar]
- Sacilik K. Effect of drying methods on thin-layer drying characteristics of hull-less seed pumpkin (Cucurbita pepo L.) J Food Eng. 2007;79:23–30. [Google Scholar]
- Santos-Sánchez NF, Valadez-Blanco R, Gómez-Gómez MS, Pérez-Herrera A, Salas-Coronado R. Effect of rotating tray drying on antioxidant components, color and rehydration ratio of tomato saladette slices. LWT Food Sci Technol. 2012;46:298–304. [Google Scholar]
- Sawhney RL, Pangavhane DR, Sarsavadia PN. Drying kinetics of single layer Thompson seedless grapes under heated ambient air conditions. Dry Technol. 1999;7(1–2):215–236. [Google Scholar]
- Sharma GP, Prasad S. Effective moisture diffusivity of garlic cloves undergoing microwave-convective drying. J Food Eng. 2004;65:609–617. [Google Scholar]
- Shi J, Pan Z, McHugh TH, Wood D, Hirschberg E, Olson D. Drying and quality characteristics of fresh and sugar-infused blueberries dried with infrared radiation heating. LWT Food Sci Technol. 2008;41:1962–1972. [Google Scholar]
- Srimagal A, Mishra S, Pradhan RC. Effects of ethyl oleate and microwave blanching on drying kinetics of bitter gourd. J Food Sci Technol. 2017;54(5):1192–1198. doi: 10.1007/s13197-017-2518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakor NJ, Sokhansanj S, Sosulski FW, Yannacopoulosd S. Mass and dimensional changes of single canola kernels during drying. J Food Eng. 1999;40(3):153–160. [Google Scholar]
- Tunde-Akintunde TY, Afolabi TJ. Drying of chili pepper (Capscium frutscens) J Food Process Eng. 2010;33(4):649–660. [Google Scholar]
- Tutuncu AM, Labuza TP. Effect of geometry on the effective moisture transfer diffusion coefficient. J Food Eng. 1996;30:433–447. [Google Scholar]
- Velioglu YS, Mazza G, Gao L, et al. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46:4113–4117. [Google Scholar]
- Wang ZH, Chen GH. Heat and mass transfer in batch fluidized-bed drying of porous particles. Chem Eng Sci. 2000;55:1857–1869. [Google Scholar]
- Yu YS, Jin TZ, Fan XT, Xu YJ. Osmotic dehydration of blueberries pretreated with pulsed electric fields: effects on dehydration kinetics, and microbiological and nutritional qualities. Drying Technol. 2016;35(13):1543–1551. [Google Scholar]
- Zielinska M, Sadowski P, Błaszczak W. Freezing/thawing and microwave-assisted drying of blueberries (Vaccinium corymbosum L.) LWT Food Sci Technol. 2015;62:555–563. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.