Abstract
Mushrooms are known to mankind since early human civilization and are gaining importance because of their nutritional and medicinal properties. Till date 37 wild edible mushrooms are reported from Nagaland. In this study, nutritional analysis including total phenolics, flavonoids and antioxidant activity was done for ten popular WEM species. Total protein content was found to be high which ranged from 62.27 g/100 g (Lentinus sajor-caju) to 18.77 g/100 g (Lentinus squarrosulus var. squarrosulus); total carbohydrate content ranged from 38.44 g/100 g (Lentinula edodes) to 5.31 g/100 g (Schizophyllum commune); reducing sugar content ranged from 7.81 g/100 g (Termitomyces heimii) to 2.33 g/100 g (S. commune). Crude fiber ranged from 11.1% (A. auricula-judae) to 1.71% (L. squarrosulus) and ash content ranged from 10.66% (L. squarrosulus) to 3.12% (L. squarrosulus var. squarrosulus). The highest phenolic content was observed in L. squarrosulus (18.7 g/100 g) and highest flavonoid content was observed in L. sulphureus (9.3 g/100 g). All the ten mushroom species exhibited antioxidant activity against DPPH free radical, but highest activity was recorded in L. tigrinus (47.5 μg/ml, IC50). Hence, mushrooms are valuable natural resource to mankind and should be exploited judiciously for the betterment of society.
Keywords: Antioxidant properties, Nagaland, Nutritional value, Wild edible mushrooms
Introduction
Mushrooms have long been known for nutritional and medicinal values and considered to be nutraceuticals due to its antioxidant, antitumor and antimicrobial properties (Barros et al. 2007; Ferreira et al. 2007). Edible mushrooms are considered to be health food as they are rich in proteins, minerals, vitamins, fibers, low/no calories and cholesterol lowering properties (Wani et al. 2010). Barros et al. (2008) considered wild mushrooms to be richer source of proteins and low fat content as compared to commercial mushrooms. The presence of secondary metabolites in mushrooms like polyphenols, terpenes, steroids and polyketides which have antioxidant properties and pharmacological applications play important role in maintaining the health of consumers (Barros et al. 2007). Patel and Goyal (2013) reported that mushrooms act as anti-cancer compounds, plays crucial role as reactive oxygen species inducer, mitotic kinase inhibitor, anti-mitotic, angiogenesis inhibitor, topoisomerase inhibitor, leading to apoptosis and also checking cancer proliferation. Most organisms possess antioxidant defense and repair systems for protection against free radical damage by oxidative enzymes like superoxide dismutase (SOD) and catalase (CAT) and by chemical compounds like ascorbic acid, carotenoids, α-tocopherol, glutathione and polyphenolic compounds (Mau et al. 2001), yet these systems are not sufficient. Natural sources of antioxidants are extensively studied for their properties to protect organisms and cells from damage brought about by oxidative stress (Cazzi et al. 1997).
Nagaland lies in the North Eastern part of India with 16,579 sq km geographical area. In Nagaland, WEMs are considered as traditionally important nutritious food which forms a vital part of the food culture of all the ethnic tribes. Recently, it is reported that Nagaland is home for 37 WEM species and the number is likely to increase as study progresses (Ao et al. 2016). In this study, the local markets were surveyed to gather information on the wild edible varieties consumed by the locals. During the mushrooming season, A. auricula-judae, A. polytricha, L. edodes, L. squarrosulus, L. sajor-caju, L. tigrinus, S. commune and T. heimii are some of the popularly sold WEM species at the local markets. Presently, the world is dominated by those edible mushrooms which have medicinal properties. The objective of the present work was to evaluate the nutritional compositions and antioxidant properties of ten popular WEM from Nagaland viz., Auricularia auricula-judae, A. polytricha, Lactifluus piperatus, Laetiporus sulphureus, Lentinula edodes, Lentinus squarrosulus, L. squarrosulus var. squarrosulus, L. sajor-caju, L. tigrinus, Schizophyllum commune, Termitomyces heimii and also to set up a nutritional database of these mushrooms with the aim to retain the information which will be unique for each species as the climatic factors play a major role in the biochemical composition of mushrooms.
Materials and method
Sample collection and extraction
The mushroom samples were collected from local markets and forest areas during the season of availability. Oven dried samples at 70 °C for 6–12 h was used for biochemical and antioxidant analysis. The samples were extracted according to the methods and procedure followed for each estimation.
Biochemical analysis
Moisture content
For calculation of moisture content, pre-weighed mushrooms were maintained in over (100 ± 5 °C) and weighed till a constant weight was achieved.
Dry matter content
Dry matter content was taken as the final weight obtained after the sample have been dried in the oven at 100 ± 5 °C for 12–48 h.
Total protein
Total protein content was determined following Lowry’s protocol (Lowry et al. 1951).
Total carbohydrate
Total carbohydrate was determined following Phenol Sulphuric Acid method of Dubois et al. (1956).
Reducing sugar
Reducing sugar was determined following DNS method (Miller 1972).
Crude fiber and ash content
Crude fiber and ash content was determined following the methods of AOAC (2000).
2, 2-Diphenyl-1-picrylhydazyl (DPPH) radical scavenging assay
The DPPH stable free radical method of Aoshima et al. (2004) was used to determine the antioxidant activity. Inhibition of free radical by DPPH was calculated by the following equation:
where Ao is the absorbance of control reaction and A1 is the absorbance in the presence of sample.
Total phenolic content
Total phenolic content was determined by Folin–Ciocalteu method (Singleton and Rossi 1965).
Total flavonoid content
Total flavonoid content was estimated by colorimetric method of Sahreen et al. (2010).
Statistical analysis
All the experimental results were the mean of three replicates and the data expressed as Mean ± standard deviation.
Results and discussion
The biochemical composition of the ten WEM species is presented in Table 1. It has been reported by past workers that the nutritional values of mushrooms are directly related to moisture and environmental conditions (Colak et al. 2009; Kalac 2009). In this study, the highest moisture content was observed in A. auricula-judae (88.9%) followed by L. squarrosulus (87.3%), L. squarrosulus var. squarrosulus (86.2%), L. sajor-caju (85.1%), L. edodes (82.8%), A. polytricha (82.01%), T. heimii (81.1%), L. piperatus (80.03%), L. tigrinus (73.7%), S. commune (69.8%) and L. sulphureus (49.8%). The dry matter content was determined from the final weight obtained after the WEM samples have been dried in the oven at 100 ± 5 °C for 12–48 h. The dry matter content ranged from 50.2% in L. sulphureus to 11.1% in A. auricula-judae.
Table 1.
Biochemical composition of the ten popular wild edible mushrooms from Nagaland, India
| Mushroom species | Moisture (%) | Dry matter (%) | Total protein (g/100 g DW) | Total carbohydrate (g/100 g DW) | Reducing sugar (g/100 g DW) | Non-reducing sugar (g/100 g DW) | Crude fiber (%) | Ash (%) |
|---|---|---|---|---|---|---|---|---|
| Auricularia auricula-judae | 88.9 ± .02 | 11.1 ± .02 | 56.92 ± .01 | 18.67 ± .01 | 2.51 ± .01 | 16.16 ± .01 | 11.1 ± .08 | 3.15 ± .3 |
| Auricularia polytricha | 82.01 ± .04 | 17.9 ± .04 | 42 ± .02 | 16.03 ± .02 | 4.82 ± .02 | 11.21 ± .02 | 10.45 ± .3 | 8.44 ± .8 |
| Lactifluus piperatus | 80.03 ± .02 | 19.97 ± .02 | 19.33 ± .02 | 9.2 ± .07 | 3.12 ± .02 | 6.08 ± .05 | 5.09 ± .1 | 5.38 ± .6 |
| Laetiporus sulphureus | 49.8 ± .02 | 50.2 ± .02 | 22.73 ± .01 | 7.65 ± .01 | 4.32 ± .01 | 3.33 ± .01 | 6.09 ± .08 | 4.81 ± .5 |
| Lentinula edodes | 82.8 ± .01 | 17.2 ± .01 | 43.81 ± .02 | 38.44 ± .01 | 6.19 ± .06 | 32.25 ± .07 | 3.6 ± .07 | 5.59 ± .3 |
| Lentinus sajor-caju | 85.1 ± .02 | 14.9 ± .02 | 62.27 ± .02 | 6.81 ± .01 | 5.08 ± .03 | 1.73 ± .02 | 7.02 ± .05 | 8.41 ± .2 |
| Lentinus squarrosulus | 87.3 ± .02 | 12.7 ± .02 | 27.86 ± .01 | 9.32 ± .01 | 6.05 ± .02 | 3.27 ± .02 | 1.71 ± .2 | 10.66 ± .4 |
| Lentinus squarrosulus var. squarrosulus | 86.2 ± .01 | 13.8 ± .01 | 18.77 ± .02 | 19.14 ± .01 | 5.39 ± .08 | 13.75 ± .06 | 6.1 ± .1 | 3.12 ± .2 |
| Lentinus tigrinus | 73.7 ± .04 | 26.3 ± .04 | 31.85 ± .03 | 16.09 ± .3 | 5.76 ± .02 | 10.33 ± .2 | 7.09 ± .08 | 3.41 ± .2 |
| Schizophyllum commune | 69.8 ± .02 | 30.2 ± .02 | 24.42 ± .02 | 5.31 ± .01 | 2.33 ± .02 | 2.98 ± .02 | 11.01 ± .08 | 6.02 ± .6 |
| Termitomyces heimii | 81.1 ± .02 | 18.9 ± .02 | 60.53 ± .01 | 22.74 ± .01 | 7.81 ± .04 | 14.93 ± .03 | 8.11 ± .04 | 5.66 ± .02 |
DW dry weight
Protein is an essential component of dry matter of mushrooms. In this study, the protein content of dried mushrooms was observed to be richer than carbohydrate which is similar to previous reports (Manzi et al. 1999). The total protein content ranged from 62.27 g/100 g (DW) in L. sajor-caju to 18.77 g/100 g in L. squarrosulus var. squarrosulus. The protein content of mushrooms is generally regarded to be higher than green vegetables (Jonathan 2002). Bioactive proteins or fungal immuno-modulatory proteins are a new class of bioactive compounds isolated from mushrooms which has shown potential application in suppressing tumor invasion and metastasis (Lin et al. 2010).
A considerable part of carbohydrates occur in the form of polysaccharides represented by starch, glycogen and fibers which is necessary for proper functioning of the alimentary tract (Manzi et al. 2001). Studies have reported edible mushrooms to be rich source of carbohydrates which range from 28.38 to 82.8% (DW) (Thatoi and Singdevsachan 2014). In the present study, the total carbohydrate content ranged from 38.44 g/100 g (DW) in L. edodes to 5.31 g/100 g (DW) in S. commune and is found as an abundant macronutrient. The reducing sugar content ranged from 7.81 g/100 g (DW) in T. heimii to 2.33 g/100 g (DW) in S. commune. The non-reducing sugar was calculated as the difference between total carbohydrate and reducing sugar and ranged from 52.04 g/100 g (DW) in L. edodes to 1.73 g/100 g (DW) in L. sajor-caju. β-glucans are the main polysaccharides found in mushrooms which are responsible for antioxidant, anti-tumor, anti-cancer, anti-cholesterolemic, immunomodulating and neuroprotective activities of many mushrooms (Khan et al. 2013).
Fiber is also a part of carbohydrate and the presence of fibers is an important characteristic in mushrooms as fibers are important in maintaining healthy human diet. The amount of crude fiber was found to be 11.1% in A. auricula-judae, 11.01% in S. commune, 10.45% in A. polytricha, 8.11% in T. heimii, 7.09% in L. tigrinus, 7.02% in L. sajor-caju, 6.1% in L. squarrosulus var. squarrosulus, 6.09% in L. sulphureus, 5.09% in L. piperatus, 3.6% in L. edodes, and 1.71% in L. squarrosulus.
In the present study, the highest ash value was observed in L. squarrosulus (10.66%) followed by A. polytricha (8.44%), L. sajor-caju (8.41%), S. commune (6.02%), T. heimii (5.66%), L. edodes (5.59%), L. piperatus (5.38%), L. sulphureus (4.81%), L. tigrinus (3.41%), A. auricula-judae (3.15%) and L. squarrosulus var. squarrosulus (3.12%). The value of ash content is important because it determines the minerals present in the food. Similar ash values in the studied WEM species have been reported by some previous workers (Ayaz et al. 2011; Enas et al. 2016).
The current study shows that all the mushrooms are rich in its nutritional compositions but there are wide variations in the macronutrient profiles even for the same species by different workers. During the present investigation, L. squarrosulus and L. squarrosulus var. squarrosulus collected from different locations showed variations in their macronutrient compositions and the remaining mushrooms also showed variations in their nutritional profiles. The differences in the nutritional compositions of mushrooms is related with factors like different species, mycelium and fructifications, climatic conditions, soil conditions, level of pollution, metal accumulations etc. which overall affects the nutritional composition of mushrooms (Rudawska and Leski 2005; Kalac 2009).
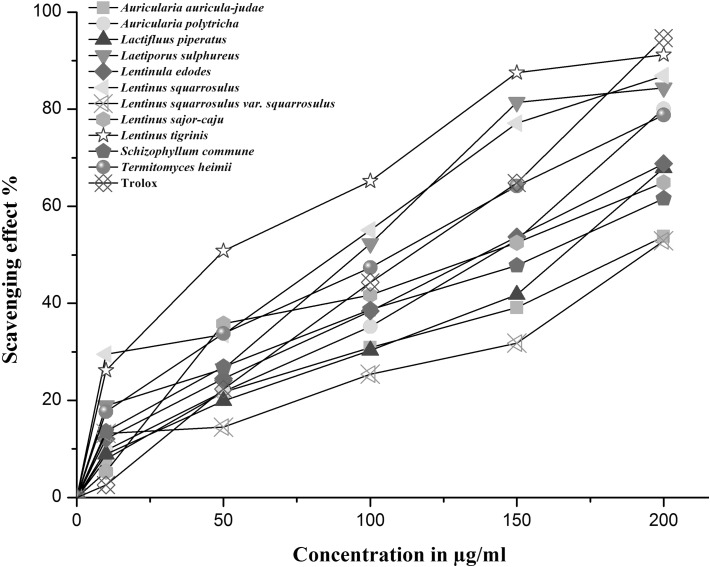
The antioxidant capacity of the mushrooms studied was determined by DPPH scavenging assay and the methanolic extracts of the mushrooms showed significant antioxidant activity. The IC50 value was calculated to study the inhibition ability of the mushrooms against DPPH radicals. IC50 is the quantity of sample required to scavenge 50% of the radicals. The lower the IC50 value, higher is the antioxidant activity of the samples. Among the ten WEM species studied, L. tigrinus (47.5 μg/ml, IC50) exhibited the highest scavenger of DPPH radicals followed by L. squarrosulus (82.6 μg/ml, IC50). The scavenging effect (%) of the ten WEM at different extract concentrations against Trolox on DPPH radicals is shown in Fig. 1 and the IC50 values are shown in Table 2.
Fig. 1.
The scavenging effect (%) of the ten wild edible mushrooms at different extract concentrations against Trolox on DPPH radicals
Table 2.
Total phenolic (TPC g GAE/100 g DW), total flavonoid (TFC g QE/100 g DW) and IC50 (μg/ml) values of the ten wild edible mushrooms
| Mushroom species | TPC g GAE/100 g DW | TFC g QE/100 g DW | IC50 (μg/ml) |
|---|---|---|---|
| Auricularia auricula-judae | 7.3 ± .03 | 1.5 ± .04 | 187.8 ± .05 |
| Auricularia polytricha | 9.9 ± .04 | 1.8 ± .02 | 130.1 ± .06 |
| Lactifluus piperatus | 8.1 ± .04 | 2.1 ± .04 | 157.7 ± .03 |
| Laetiporus sulphureus | 16.1 ± .02 | 9.3 ± .03 | 95.1 ± .04 |
| Lentinula edodes | 10.2 ± .03 | 3.2 ± .03 | 137.4 ± .05 |
| Lentinus sajor-caju | 17.2 ± .04 | 6.6 ± .05 | 137.9 ± .07 |
| Lentinus squarrosulus | 18.7 ± .03 | 4.5 ± .04 | 82.6 ± .03 |
| Lentinus squarrosulus var. squarrosulus | 7.7 ± .02 | 1.7 ± .02 | 212.8 ± .06 |
| Lentinus tigrinus | 10.1 ± .02 | 3.7 ± .08 | 47.5 ± .03 |
| Schizophyllum commune | 16.4 ± .04 | 5.6 ± .02 | 153.1 ± .02 |
| Termitomyces heimii | 17.4 ± .05 | 4.7 ± .03 | 107.2 ± .05 |
| Trolox | – | – | 111.1 ± .04 |
Generally, the presence of phenols and flavonoids indicates mushrooms as source of biologically active compounds and exhibits antioxidant, anticancer, anti-inflammatory, immunomodulatory etc. properties which play an important in pharmacology. The phenolic and flavonoid content of the studied mushrooms are shown in Table 2. The highest phenolic content was observed in L. squarrosulus (18.7 g/100 g DW) and lowest in A. auricula-judae (7.3 g/100 g DW). The flavonoid content was found to be lower as compared to phenolic content in the present study and ranged from 9.3 g/100 g in L. sulphureus to 1.5 g/100 g in A. auricula-judae. Antioxidants play an essential role in maintaining human health due to their ability to scavenge free radicals in the bodies reducing oxidative damage (Elmastas et al. 2007; Ferreira et al. 2007) and mushrooms are rich sources of antioxidants (Puttaraju et al. 2006). The antioxidant potential, phenolic and flavonoid content of various mushrooms have been reported by many workers (Boonsong et al. 2016; Sanchez 2017) and it is said that the antioxidant potential of mushrooms is higher than most vegetables and fruits (Sanchez 2017).
There has been tremendous progress in mushroom cultivation and research because of its nutritional and medicinal properties. These wild edible mushrooms are healthy food supplements required to maintain balanced diets as it contains macro as well as micro nutrients and functional minerals. It is highly popular as a low caloric diet food especially for cancer, diabetic and cardiac patients. Moreover, mushrooms are gaining importance as a protein supplementary food for the people especially living in under developed countries. The State of Food Security and Nutrition in the World (SOFI) has called upon all countries and stakeholders to act together to end hunger and prevent all forms of malnutrition by 2030 as the present scenario is that the number of undernourished people in the world is increasing alarmingly. Thus, the present study creates awareness about the nutritional and medicinal importance of mushrooms. This study also shows the potential for cultivation and production of mushrooms in Nagaland and the rest of the world as mushroom farming is eco-friendly and cost-effective. This will provide alternative means of income to the people. Moreover, a thorough screening of the wild mushrooms is of necessity to study its medicinal properties and bioactivity. The work realizes the need to conserve and manage this natural resource as the present environmental crisis like climate change and global warming adversely affects the survivability of mushrooms which require specific micro-climatic conditions for its growth.
Conclusion
Mushrooms have the solution to some extent on many of the present day problems like food quality, unemployment, environmental pollutions and pharmaceutical applications. Mushrooms as food and medicines are solutions to many health problems. The present study highlights the potential commercial use of these wild mushrooms. However, extensive explorations and thorough screening of the wild mushrooms is the need of the hour as mushrooms are valuable natural resource to mankind with immense health benefits and should be exploited judiciously for the betterment of society.
Acknowledgements
Authors are thankful to Department of Biotechnology, Ministry of Science & Technology, Govt. of India, New Delhi for financial support through Institutional Biotech Hub vide order No. BT/22/NE/2011. Toshinungla Ao is thankful to University Grants Commission, New Delhi, India for UGC-BSR fellowship for her Ph.D. programme. Facilities used from the UGC-SAP (DRS-III) programme are duly acknowledged.
Compliance with ethical standards
Conflict of interest
All authors declare that there are no conflicts of interests among the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ao T, Deb CR, Khrumo N. Wild edible mushrooms of Nagaland, India: a potential food resource. J Exp Biol Agric Sci. 2016;4(1):59–65. [Google Scholar]
- AOAC (2000) Official methods of analysis, 17th edn. The Association of Official Analytical Chemists, Gaithersburg. Methods 925.10, 65.17, 974.24, 992.16
- Aoshima H, Tsunoue H, Koda H, Kiso Y. Ageing of whiskey increases 1, 1-diphenyl-2-picryl hydrozyl radical scavenging activity. J Agric Food Chem. 2004;52(16):5240–5244. doi: 10.1021/jf049817s. [DOI] [PubMed] [Google Scholar]
- Ayaz FA, Torun H, Ozel A, Col M, Duran C, Sesli E, Colak A. Nutritional value of some wild edible mushrooms from the Black Sea region (Turkey) Turk J Biochem. 2011;36(4):384–393. [Google Scholar]
- Barros L, Baptista P, Correia DM, Casal S, Oliveira B, Ferreira ICFR. Fatty acid and sugar compositions and nutritional value of five wild edible mushrooms from Northeast Portugal. Food Chem. 2007;105:140–145. [Google Scholar]
- Barros L, Cruz T, Baptista P, Estevinho ML, Ferreira CFRI. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem Toxicol. 2008;46:2743–2747. doi: 10.1016/j.fct.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Boonsong S, Klaypradit W, Wilaipuna P. Antioxidant activities of extracts from five edible mushrooms using different extractants. Agric Nat Resources. 2016;50:89–97. [Google Scholar]
- Cazzi R, Ricardy R, Aglitti T, Gatta V, Petricone P, De SR. Ascorbic acid and b-carotene as modulators of oxidative damage. Carcinogenesis. 1997;18:223–228. doi: 10.1093/carcin/18.1.223. [DOI] [PubMed] [Google Scholar]
- Colak A, Faiz O, Sesli E. Nutritional composition of some wild edible mushrooms. Turk J Biochem. 2009;34:25–31. [Google Scholar]
- Dubois MKA, Gilles JK, Hamilton PA, Rebers Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- Elmastas M, Isıldak O, Turkekul I, Temur N. Determination of antioxidant activity and compounds in wild edible mushrooms. J Food Compos Anal. 2007;20(3–4):337–345. [Google Scholar]
- Enas AE, Sabahelkhier MK, Malaz MM. Nutritional composition and minerals content of five species of wild edible mushroom brought from UAE: mushroom considered as protein source. Int J Adv Res. 2016;4(2):1108–1112. [Google Scholar]
- Ferreira ICFR, Baptista P, Boas VM, Barros L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal. Food Chem. 2007;100:1511–1516. [Google Scholar]
- Jonathan SG (2002) Vegetable growth requirements and antimicrobial activities of some higher fungi in Nigeria. Ph.D. thesis, University of Ibadan
- Kalac P. Chemical composition and nutritional value of European species of wild growing mushrooms: a review. Food Chem. 2009;113:9–16. [Google Scholar]
- Khan MA, Tania M, Liu R, Rahman MM. Hericium erinaceus: an edible mushroom with medicinal values. J Complement Integr Med. 2013;10(1):1–6. doi: 10.1515/jcim-2013-0001. [DOI] [PubMed] [Google Scholar]
- Lin CH, Sheu GT, Lin YW, Yeh CS, Huang YH, Lai YC, Chang JG, Ko JL. A new immunomodulatory protein from Ganoderma microsporum inhibits epidermal growth factor mediated migration and invasion in A549 lung cancer cells. Process Biochem. 2010;45(9):1537–1542. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Manzi P, Gambelli L, Marconi S, Vivanti V, Pizzoferrato L. Nutrients in edible mushrooms: an inter-species comparative study. Food Chem. 1999;65:477–482. [Google Scholar]
- Manzi P, Aguzzi A, Pizzoferrato L. Nutritional value of mushrooms widely consumed in Italy. Food Chem. 2001;73:321–325. [Google Scholar]
- Mau JL, Chao GR, Wu KT. Antioxidant properties of methanolic extracts from several ear mushrooms. J Agric Food Chem. 2001;49:5461–5467. doi: 10.1021/jf010637h. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Anal Chem. 1972;31(3):426–428. [Google Scholar]
- Patel S, Goyal A. Recent developments in mushrooms as anti-cancer therapeutics. J Biotechnol. 2013;2:1–15. doi: 10.1007/s13205-011-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttaraju NG, Venkateshaiah SU, Dharmesh SM, Urs SM, Somasundaram R. Antioxidant activity of indigenous edible mushrooms. J Agric Food Chem. 2006;54:9764–9772. doi: 10.1021/jf0615707. [DOI] [PubMed] [Google Scholar]
- Rudawska M, Leski T. Macro and microelement contents in fruiting bodies of wild mushrooms from the Notecka forest in west-central Poland. Food Chem. 2005;92:499–506. [Google Scholar]
- Sahreen S, Khan M, Khan RA. Evaluation of antioxidant activities of various solvent extracts of Carisa apaca fruits. Food Chem. 2010;122:1205–1211. [Google Scholar]
- Sanchez C. Reactive oxygen species and antioxidant properties from mushrooms. Synth Syst Biotechnol. 2017;2:13–22. doi: 10.1016/j.synbio.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimerty of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–158. [Google Scholar]
- Thatoi H, Singdevsachan SK. Diversity, nutritional composition and medicinal potential of Indian mushrooms: a review. Afr J Biotechnol. 2014;13(4):523–545. [Google Scholar]
- Wani BA, Bodha RH, Wani AH. Nutritional and medicinal importance of mushrooms. J Med Plant Res. 2010;4(24):2598–2604. [Google Scholar]