Abstract
The rheological, pasting, and gel textural properties of corn starch blended with date syrup (DS) or sugar (SG) were studied. The average amylose content of the starch was 27.8%. Corn starch gel is considered elastic since the elastic modulus (G′) was much greater than the viscous modulus (G″). Different effect between DS and SG on corn starch gel was observed, where SG addition and DS replacement experiments exhibited the highest G′. The tan δ of all samples was in the range of 0.02–0.20 indicating elastic behavior since it is less than unity. The hardness of starch gel ranged from 13 to 146 g and 212-145 for DS replacement and DS addition, respectively. Unlike the replacement experiment, the addition experiment exhibited significant increase in peak viscosity, setback and pasting temperature (p > 0.05). The magnitude of the effect of DS on corn starch gel was more evident compared to SG. This was apparent by looking at the slopes of the linear regression of the log of G′ or G″ versus the log of frequency. Based on the information provided here, date syrup application can expand to cover the baking and beverage industries.
Keywords: Date syrup, Corn starch, Pasting, Rheology, Texture
Introduction
The main purpose of adding sugars or other sweeteners to starch or starchy products is to reach certain taste requirements. Studies based on differential scanning calorimetry (DSC) showed that sugars increase starch gelatinization temperature and influence starch pasting properties (Ahmad and Williams 1999; Baek et al. 2004; Chang et al. 2014; Gunaratne et al. 2007; Maaruf et al. 2001; Sopade et al. 2004; Wang et al. 2009; Zhang et al. 2013). Using many different techniques, researchers proposed a mechanism of the effect of sugars and other polyhydroxy on starch gelatinization. The idea can be summarized in the reduction of water activity, sugar starch interaction, plasticizing effect of water on sugars, and the ability of sugars to restrict starch granules swelling and amylose leaching. All three possibilities are dependent on the type and the concentration of sugars (D’Appolonia 1972; Levine and Slade 1988; Chiotelli et al. 2000; Hoover and Senanayake 1996; Ahmad and Williams 1999).
Inconsistent information regarding the effect of sugars on starch properties is reported in the literature (Baek et al. 2004; Pongsawatmanit et al. 2007; Chang et al. 2014). Zhang et al. (2013) reported that sugars increased starch activation energy as a function of increased sugar content, however, decreased the rate constant and delayed starch gelatinization. They also reported that sucrose was more effective than glucose. Kim and walker (1992) reported significant increase in the peak temperature of wheat starch but not the ΔH. The same authors observed collapse of cakes containing sucrose esters right after removal from the oven because sucrose esters was found to increase starch gelatinization temperature. The effect of sugars on G′ of starch was ranked as: xylose > sucrose > glucose > control (Chang et al. 2014).
Tuber starches are known to be different from cereals. Sweet potato, for example, is a native of Africa used mostly in baked products and is a major source of starch. It is used as an ingredient in producing noodles and different sauces. As reported by other researchers, the granule of sweet potato starch range from 3 to 40 μm and contains 17–30% amylose. It has gelatinization temperature between 60 and 70 °C and a mixed X-ray diffraction pattern of A-and C-type (Noda et al. 1998). Compared to corn starch, sweet potato starch exhibits higher viscosity and paste clarity (Jung et al. 1991). Although the effect of date syrup and different sugars on the physicochemical and pasting properties of some starches was studied by other researchers (Jung et al. 1991; Chen et al. 2003; Jangchud et al. 2003), a comparison between the effect of date syrup and sugars on starch was not explicated.
Xanthan gum was reported to increase the G′, G″, and η* as a function of increase in xanthan gum, while tan δ decreased (Choi and Yoo 2009). The pasting temperature of starch was increased due to date syrup addition, whereas the G′ was increased only at specific ratio of starch to date syrup (Mohamed and Babucurr 2015). The same authors suggested that date syrup can be effective in reducing starch retrogradation.
One of the reasons for gelatinization temperature increase in the presence of sugars was reported to be due to penetration of small sugars into the amorphous regions of starch granules instigating complex formation between sugars and starch components (amylose or amylopectin). Obviously, this penetration occurs after heating starch in adequate amount of water (Chiotelli et al. 2000). Sugars penetration evidence was provided by comparing diffractograms of starch and starch–sugar systems and how sugars cause disorders in the starch granules structure thereby increase the gelatinization temperature (Tomasik et al. 1995; Sikora et al. 1999).
The elastic (G′) response of starch gel can be attributed to the physical entanglement of neighbouring molecules or to the intermolecular association of amylose chains released from the swollen granules via hydrogen bonding (Miles et al. 1985). Sudden increase in G′ was observed during cooling of pure starch gel (no sugar) which reaches the maximum values at 4 °C. Nonetheless, corn starch–sugar composite showed no significant variations in the pasting temperature but sharp increase in G′ was observed regardless of the type of sugar or sugar concentration. A drop in G′ was observed at high sugar concentrations which indicate that the addition of sugars decreased the rate of preliminary conformational organization of amylose during cooling (Evageliou et al. 2000). The effect of date syrup on the functional properties of starch has been reported in the literature. However, a comparison between the effect of date syrup and sugars on starch gelatinization has not been done to the best of our knowledge. The objective of this work was to determine and compare the effect of date syrup and sugars on the rheological and pasting properties of corn starch. In this work the concentration of glucose/fructose solutions were selected to mimic sugars concentrations found naturally in date syrup to facilitate for meaningful comparison. The novelty of this work is based on replacing processed sugars with a natural source which can find application in the food industry such as the baking, beverage, and snack industry.
Materials and methods
Materials
The ingredients used in this study were purchased locally. Fresh date syrup (DS) 75% solid content was supplied by (Esten date and fresh date E.st). Glucose and fructose were supplied by Wells Lawrence House (126 Back Church lane, London, EI IFH, UK), whereas Corn starch (27.8% amylose) was donated by ARASCO (Riyadh, Saudi Arabia).
High pressure liquid chromatographic analysis (HPLC)
Glucose and fructose content of the date syrup was determined according to AOAC standard method 16.12 (AOAC 1990) using HPLC system (LC-10A HPLC Series, Shimadzu, Kyoto, Japan) equipped with a refractive index detector (RID-10A) and a UV/Vis detector (SPD-20A) monitored at 210 nm. Sugars analysis was done using Aminex HPX-87H column (300 × 7.8 mm) (Bio-Rad) at 55 °C at 0.3 mL/min flow. Isocratic solvent mixture of 0.045 N H2SO4 with 6% acetonitrile solvent (v/v) was designated as the mobile phase. An aliquot of date syrup diluted with 100 mL of distilled water, filtered through 0.45 mm membrane. A volume of 20 μL was injected and the total running time was 10 min.
Blends preparation
Blends were prepared in two ways. A replacement or addition, where portion of the starch was replaced with DS or SG or added to a fixed amount of starch. Sugar solutions were prepared at a ratio of 55:45 glucose: fructose which represents the ratio of glucose to fructose in the date syrup determined by HPLC analysis. The total amount of starch used was 2.8 g for the addition study, but varying amounts were used for the replacement experiment. Blends were prepared at 0, 5, 15, 20, 30, and 50% DS of the starch weight (14% moisture content). For example, in the replacement experiment the 5% DS or SG is equal to 0.14 g solids of DS or SG in the total weight of 2.8 g and the remaining 2.66 g was starch. Since DS is 75% solids, 0.14 g solids are in 0.187 g of DS. That means for the 5% DS blend, 2.66 g of starch + 0.187 g of DS. The remaining blends were calculated in the same manner.
Rapid visco analyzer measurements (RVA)
Pasting properties of the blends were determined using a rapid visco analyzer (Newport Scientific, Sydney, Australia). Corn starch/syrup or sugars blends (2.8 g at 14% moisture basis) were directly weighed into aluminum RVA containers and the total weight of 28 g was reached by distilled water. The obtained slurry was held at 50 °C for 30 s, heated to 95 °C in 4.40 min (at 10.23 °C/min) and held at 95 °C for 4 min. It was then cooled to 50 °C in 2 min (at 22.5 °C/min) and held for 2 min. The speed of the paddle was 960 rpm for the first 10 s and then reduced to 160 rpm throughout the rest of the experiment. All measurements were done in three replicates. The pasting parameters were obtained using Thermocline window software supplied by the manufacturer.
Rheological measurements
Dynamic rheology measurements were carried out on starch paste cooked in RVA and tested using DHR-Hybrid Rheometer (TA Instruments, DE, USA). Prior to the shear measurements, a strain-sweep was conducted to determine the linear viscoelastic region. Small amplitude oscillatory shear tests were done over a frequency sweep range of 0.1–100 (rad/s) at 5% strain produced shear storage modulus (G′), loss modulus (G″) and complex viscosity (η* Pa s). The frequency sweep range used here is typically used so that G′, G″, and η* were within the linear region. The storage modulus represents the non-dissipative element of mechanical properties, whereas the loss modulus symbolizes the dissipative component of mechanical properties of the material. Elastic behavior indicates that G′ is independent of frequency and greater than G″. Materials can be described as solid with perfect elasticity when the phase shift angle (δ) is zero, liquid with impeccable viscosity when δ = 90, or somewhere in the middle. The phase angle is defined as δ = tan−1(G″/G′).
Gel texture
Gels prepared in RVA were used for gel texture parameters determination. The testing was done after transferring gels into 25 mL beakers (35 mm in height) with internal diameters of 30 mm and stored overnight at room temperature. Gels compression using Brookfield CT3 Texture Analyzer (Brookfield Engineering Laboratories, Inc. Middleboro, USA) was performed in two penetration cycles at a speed of 0.5 mm/s to a distance of 10 mm using 12.7 mm wide and 35 mm high cylindrical probe. For every run, gel hardness, springiness, cohesiveness, and adhesiveness were recorded directly from the instrument.
Statistics
Four separate regression equations were obtained consisting of corn starch treatment with DS or SG levels (0, 5, 15, 20, 30, and 50%). The form of the dependent Y-variable and the form of the independent X-variable are as defined in equation Y = aX + b, where Y represents the peak viscosity or setback and X is the level of the added or replaced DS or SG. The GLM F-tests for determining peak viscosity or setback equation differences (Y) uses a full model.
Results and discussion
HPLC
The HPLC profile of date syrup sugar analysis revealed two distinct peaks at 8.42 and 8.83 min representing glucose and fructose, respectively. Based on the area under the curves, these results can be interpreted as 55 g glucose and 45 g of fructose/100 g of date syrup. Automatically date syrup sugar content can be expressed as 55% glucose and 45% fructose. These results were not significantly different from what Ahmed and Ramaswamy (2006) reported, even though they used specific date varieties and a blend of different varieties was used in the study.
Rapid visco analyzer measurements (RVA)
Based on 55:45 glucose:fructose of DS, sugar solutions were prepared for either replacement or addition experiments. In order to determine the effect of the source of the total solids (starch, date syrup or sugars), this study was done as a replacement of starch or addition. The replacement study was done by simply replacing portion of the starch with DS or SG, whereas the addition studies via adding DS or SG to the fixed amount of starch. This is important because, if we ignore the source of the total solids, the comparison will be meaningless, because starch contributes directly to the viscosity of the gel, unlike the sugars. The RVA profile in Fig. 1a, b illustrates the effect of date syrup (DS) on the peak viscosity (PV) and setback (SB) of corn starch. The theoretical value of PV or SB was calculated according to the percent reduction in starch content when the starch is replaced by date syrup (DS) or sugars (SG). For example, when starch was reduced by 5% it will theoretically reduce the peak viscosity by 5%. In Fig. 1a, b, the profile compares the theoretical value of PV and SB to the actual value obtained from the RVA. The profile showed that the replacement or addition of DS or SG reduced PV and SB below the theoretical profile. Multiple regression equation for predicting PV and SB data is shown in Table 1. All equations were statistically significant, as were all slope and interaction coefficients for the models (p < 0.0001), indicating true, non-zero contributions to the prediction equations. Although DS and SG reduced the PV and SB, the reduction in PV and SB in the presence of SG was more apparent. This could be due to the introduction of carboxyl and carbonyl groups which resulted in lower swelling power as reported by (Sandhu et al. 2008). The actual PV value dropped by threefolds at 50% DS replacement compared to the theoretical value. The SB exhibited similar drop as well. This indicates that the reduction on the amount of starch was not the main cause for the drop on PV or SB; otherwise the theoretical and actual data of the samples with reduced starch content should have been the same or at least similar. Possibly, the effect of DS on the gelatinization temperature and the delay of granules swelling are the cause of the lower PV (Eliasson 1992). The effect of Sugars (SG) on PV and SB of corn starch was similar to that of the DS (Fig. 1c, d). In both situations the effect of DS and SG was more intense on the PV than the SB as shown by the slope of the lines representing the actual PV or SB for both DS and SG (Fig. 1a–d). Sugary maize was reported to exhibit a broad peak and higher peak temperature compared to common starches, which indicates that gel destruction is slower in sugary than common starches (Singh et al. 2006).
Fig. 1.
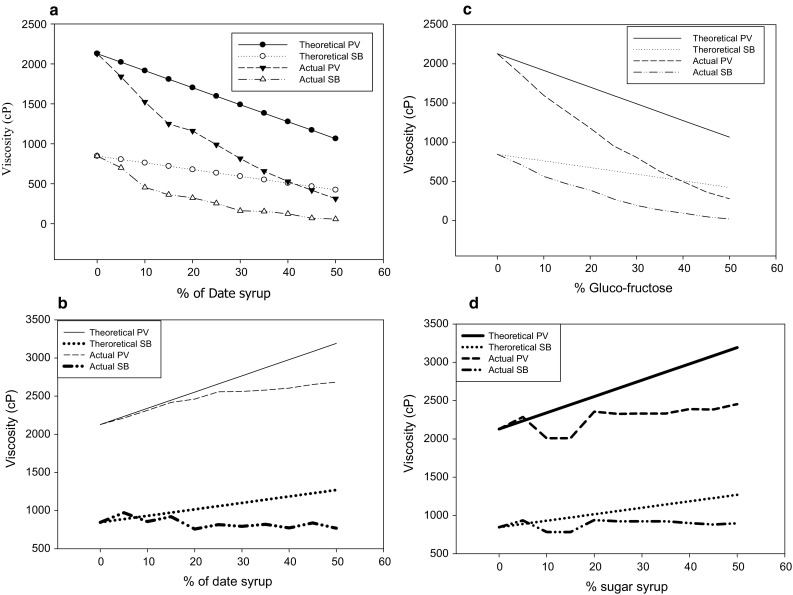
Effect of date syrup (DS) and glucose/fructose sugar blend (SG) on the peak viscosity and the setback of corn starch: a replacement of starch with DS. b Addition of DS to fixed amount of starch. c Replacement of starch with SG. d Addition of SG to fixed amount of starch
Table 1.
Multiple regression data for predicting corn starch peak viscosity and setback
| Treatment | Equations | R2 | P value |
|---|---|---|---|
| Peak viscosity | |||
| DS replacement | Y = 681.83 − 731.14X | 0.97 | < 0.0001 |
| DS addition | Y = 2728.28 + 214.92X | 0.82 | < 0.0001 |
| SG replacement | Y = 640.83 − 740.26X | 0.96 | < 0.0001 |
| SG addition | Y = 2435.69 − 137.69X | 0.96 | < 0.0001 |
| Setback | |||
| DS replacement | Y = 137.26 − 276.26X | 0.99 | < 0.0001 |
| DS addition | Y = 854.79 − 45.22X | 0.74 | < 0.0001 |
| SG replacement | Y = 133.78 − 328.03X | 0.99 | < 0.0001 |
| SG addition | Y = 921.78 + 16.636X | 0.83 | < 0.0001 |
DS date syrup, SG sugars solution
The capacity of DS to reduce starch retrogradation was reported by Mohamed and Babucurr (2015). Although the sugar content of DS and SG was similar, the data showed that DS was more effective in reducing starch retrogradation. The DS and SG significantly increased the pasting temperature of corn starch by around 15 °C (data is not shown). This is consistent with the delay in starch granules swelling theory mentioned above. This theory is based on the ability of additives either to mask starch granules and slow down water penetration or interact with the water molecules and make water less available (Alamri et al. 2013). It is essential to mention that starch granules structure and compactness could play important role on the effect of DS and SG. Singh et al. (2006) reported that starches low in short chain amylopectin fraction and high amylose content requires more time to reach peak viscosity than those with greater amount of short chain amylopectin. The data reported by the same authors showed that high amylose content is confirmed to reduce swelling power and intrinsic viscosity.
The data presented in Fig. 1a–d reflect the capacity of DS to influence corn starch pasting properties compared to SG at equal total solids content. This data showed that, DS and SG can impact starch pasting properties in a dissimilar way, such as inducing higher peak viscosity. Other researchers looked at the molecular structure in order to explain some starch properties. Swelling power, water holding capacity, and solubility are considered a measure of the degree of interaction between amylose and amylopectin chains within the amorphous and crystalline regions of the granule. Starch swelling occurs concurrently with loss of birefringence and comes before solubilization (Singh et al. 2004). Water holding capacity is a measure of the degree of availability of water binding sites in the granule and may contribute to the variation among different starches (Singh et al. 2004; Sandhu and Singh 2007).
Rheological measurements
Starch gels were prepared in RVA then loaded on the DHR-Hybrid Rheometer at 25 °C. The illustrative pattern of the storage modulus (G′) plotted as a function of time is indicative of elastic property of the gels. Figure 2a–d shows the G′ as a function of time at three different DS or SG levels. Figure 2a, b represents the partial replacement of starch with DS or SG which illustrates the significant drop on G′ at higher SG or DS, whereas the 50% replacement showed the lowest G′ value. Starch dilution with DS or SG can be the cause of the drop in G′ which resulted in softer less-elastic gel. The effect of DS appeared to be significantly more than SG because SG replacement experiment exhibited gradual G1 drop at higher SG content compared to DS (Fig. 2a). Unlike 30 and 50% DS replacement, the 20% showed significant G′ increase (Fig. 2b). This is a clear indication of the contrasting effect of DS on the elastic properties of the gel compared to SG. The difference between replacement and addition was obvious. The 20% SG addition experiment revealed little effect of SG on G′, whereas significant sharp increase on G′ by the 30 and 50% was detected. Little difference between the influence of 30 and 50% on G′ was observed (Fig. 2a). On the other hand, DS addition experiment exhibited no difference in G′ between the control and 20 and 30% DS (Fig. 2d). Therefore, this can be considered another confirmation of the diverse elastic behavior of corn starch gel due to DS compared with SG. This is consistent with the RVA data discussed above, where lower peak viscosity and setback were observed due to DS, which is in agreement with the findings of Mohamed and Babucurr (2015). The change in G′ can be considered a two steps process, where G′ increase from 7 to 20 min as the first step and from 20 to 26 min as the second (Fig. 2). Therefore, the increase in G′ was slower at the beginning of the experiment, after 20 min a sharp increase was observed. This was true for both DS and SG samples. These results indicate that DS or SG as a replacement has similar effect on the G′ pattern by virtue of low structural development rate with one exception of the 20% DS replacement experiment. It appears that the similarity on the slow increase on G′ at the beginning of the experiment is due to the fact that DS and SG limit amylose leaching out during the first steps of starch gelatinization (Willett et al. 1995; Ahmad and Williams 1999). This is critical because starch gel strength is mostly dependent on the free amylose.
Fig. 2.
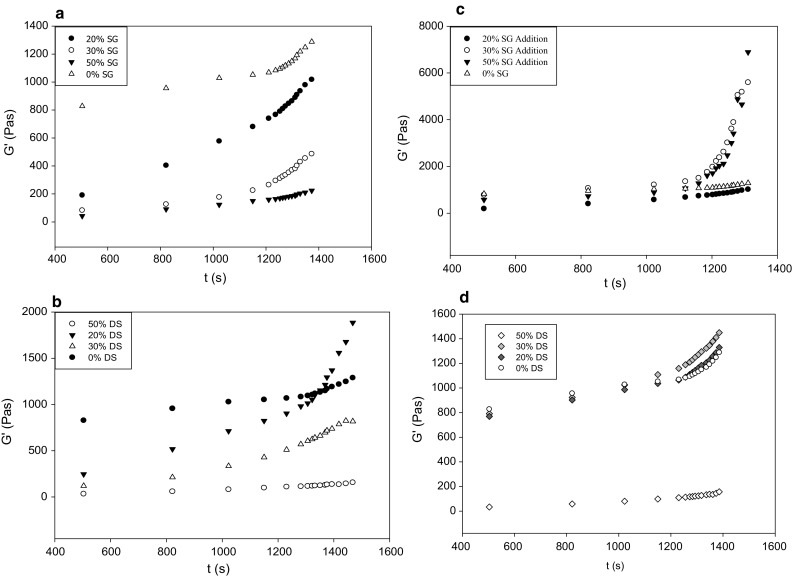
Effect of date syrup (DS) and glucose/fructose sugar blend (SG) on the G′ of corn starch gels: a replacement of starch with SG. b Replacement of starch DS. c Addition of SG to fixed amount of starch. d Addition of DS to fixed amount of starch
Generally, DS or SG reduced the G′ especially at higher concentrations. This can be attributed to the increase in amylose content in the continuous phase due to the sugars interaction with the aqueous phase (Alloncle et al. 1989). This occurrence indicates thermodynamic incompatibility between starch components (amylose and amylopectin) and DS or SG which commonly leads to co-existing phases; one is less rich in amylose (Dickinson and McClements 1996; Tolstoguzov 1986). Therefore, amylose network formation is weakened because of the phase separation. Nonetheless, a balance can be maintained by lowering the temperature of the gel and offset the effect of thermodynamic incompatibility (Bansil 1993; Tromp et al. 1995).
The effect of DS and SG addition on the gel properties did not exhibit specific order with respect to concentration. Gels with SG demonstrated Slight increase in G′ of the 50% SG addition, whereas the remaining concentrations followed similar profile as the replacement experiment (Fig. 2c). Conversely, the addition of DS showed sharp increase in G′ after 20 min without significant difference between 0, 20 and 30% addition (Fig. 2d). In this case, the effect of SG seems to be the opposite of DS where all concentrations appear to have similar effect on G′ until 20 min (Fig. 2c), but after that the 20 and 30% DS exhibited threefolds increase in G′ compared to the 50%. The addition of 50% DS gel can be considered soft, whereas 50% SG gels were a lot more elastic due to the higher G′ (Fig. 2c). This could be attributed to the possible interaction of DS with amylose causing weaker network and extended phase separation. Such behavior is consistent with previous reports on gels containing incompatible mixtures of food polymers (Abdulmola et al. 2000). However, no conclusive relationship between gel strength and degree of phase separation can be inferred from this set of data.
In general, the addition of sugars is known to increase gelatinization temperature of starches and limits starch gelatinization as shown by the RVA data discussed above. This phenomenon was observed at the beginning of starch gelatinization, but once the gelatinization starts its progress is identical to that in pure water (Parry and Donald 2002). In addition, the gelatinization temperature is dependent on the molecular weight or the concentration of the solute which is directly related to starch granules swelling as a precursor to full gelatinization. Although G′ pattern in starch-gum system was shown to increase at the beginning of the aging process, reached plateau after some storage time (Choi and Yoo 2009). This signifies the end of the gel structure formation during ageing.
The plot of G′ and G″ of corn starch showed that starch gel exhibited elastic property because the magnitude of G′ was much higher than G″. The same behavior was observed when G′ and G″ was plotted against frequency showing insignificant frequency dependence. This was not in complete agreement with Singh et al. (2006) because they reported that G′ and G″ were depend on frequency, but G′ was observed to be less dependent on frequency. Both moduli increased with increasing frequency, which is in agreement with literature reports. Starch gels can be considered true gel-like because it exhibits much higher G′ values than G″ (Chang et al. 2014).
The linear regression of the log of G′ or G″ versus log of frequency as shown in Eqs. 1 and 2, can be used to calculate n′ and n″ or k′ or k″ where n is the slope of the line and k is the Y-intercept for both n′ and n″ or K′ and k″ which represents G′ or G″.
| 1 |
| 2 |
The slope and the Y-intercept of corn starch gels addition and replacement of DS and SG experiment are shown in Table 2. Overall, the k′ for samples with DS or SG at all concentrations was much higher than k″. For instance, K′ of the gel containing DS ranged from 4.50 to 6.67, whereas K″ range 1.63–3.73. In other words, K′ dropped by 33% and K″ by 56% relative to the control. Higher DS or SG reduced the k′ value except for the SG addition samples (Table 1). These results indicate that the elastic property of corn starch gel is reduced due to DS or SG. Such reduction in elastic property can simply be related to the disruption of the amylose network which is the main architect of the gel formation. These findings were in line with what was discussed above.
Table 2.
Effect of date syrup and sugars (glucose + fructose) on the n′, k′, n″, and k″ representing G′ and G″ respectively according to Eq. 2; log G′ = log k′ + n′ log ω
| DS1 or SG2 | G′ | G″ | ||||
|---|---|---|---|---|---|---|
| n′ | K′ (Pas) | R2 | n″ | K″ (Pas) | R2 | |
| 0% (control) | 0.04 | 6.95 | 0.84 | 0.25 | 3.88 | 0.90 |
| 20% DS Re | 0.20 | 6.67 | 0.79 | 0.44 | 3.73 | 0.92 |
| 30% DS Re | 0.19 | 5.42 | 0.80 | 0.32 | 2.34 | 0.95 |
| 50% DS Re | 0.14 | 4.50 | 0.67 | 0.29 | 1.63 | 0.91 |
| 20% DS Ad | 0.05 | 6.94 | 0.87 | 0.24 | 3.51 | 0.99 |
| 30% DS Ad | 0.06 | 7.00 | 0.84 | 0.25 | 3.51 | 0.98 |
| 50% DS Ad | 0.05 | 7.04 | 0.88 | 0.25 | 3.49 | 0.99 |
| 20% SG Re | 0.14 | 6.40 | 0.60 | 0.27 | 3.50 | 0.64 |
| 30% SG Re | 0.19 | 5.42 | 0.80 | 0.32 | 2.34 | 0.95 |
| 50% SG Re | 0.14 | 4.87 | 0.61 | 0.25 | 2.01 | 0.78 |
| 20% SG Ad | 0.14 | 7.40 | 0.86 | 0.42 | 4.39 | 0.91 |
| 30% SG Ad | 0.27 | 7.42 | 0.99 | 0.69 | 4.86 | 0.95 |
| 50% SG Ad | 0.31 | 7.21 | 0.97 | 0.46 | 5.41 | 0.93 |
DS date syrup, SG glucose/fructose
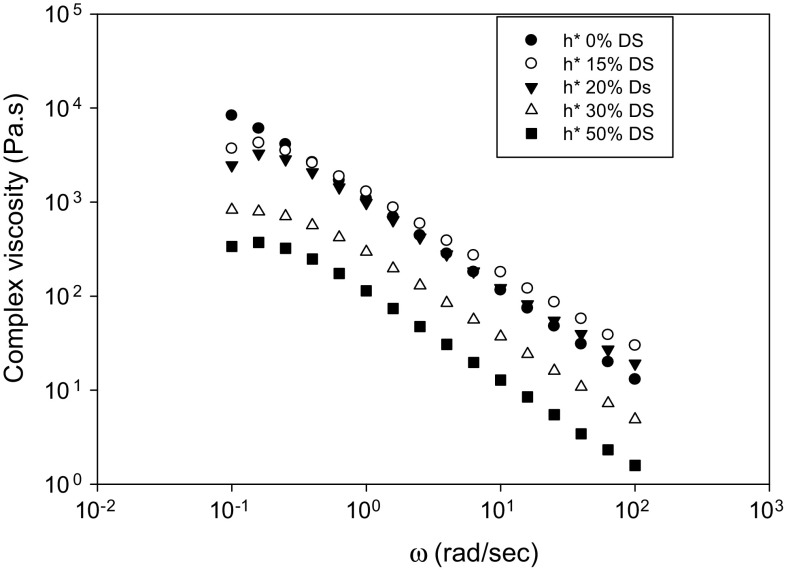
The frequency dependence of the complex viscosity (η*) is illustrated in Fig. 3. Corn starch gels exhibited shear thinning, but samples with DS or SG behaved differently relative to the η* of the control. For both DS and SG, the 15% replacement showed lower shear thinning (higher η*) compared to the control, whereas 30 and 50% presented higher shear thinning than the control. Unlike the 20% DS replacement, the η* of the 20% SG was lower than the control. Once again, the behavior of the starch in the presence of the DS was different from SG according to the data presented in Table 2, where the K′ and the K″ dropped as a function of increase in DS or SG.
Fig. 3.
Complex viscosity of corn starch partially replaced with date syrup
Conversely, the DS addition experiment showed no difference between the control and the samples regardless of the amount of DS, whereas the K′ and K″ were increased (Table 2). This data showed the significant difference between the effect of sugars on the rheological properties of starch gels compared to gums, where galactomannan gum have a tendency to increase the K′ and K″ as reported by Dong and Yoon (2012). Even though, DS and SG negatively influenced the G′ of corn starch gels, the magnitude of their effect was different. These differences between the effect of gums, DS and SG can be attributed to the difference in molecular weight and probabilities of interaction with amylose or water. Obviously, more interaction with water or amylose will reduce starch gel strength thus affecting the G′. This was apparent in the data presented in Table 2.
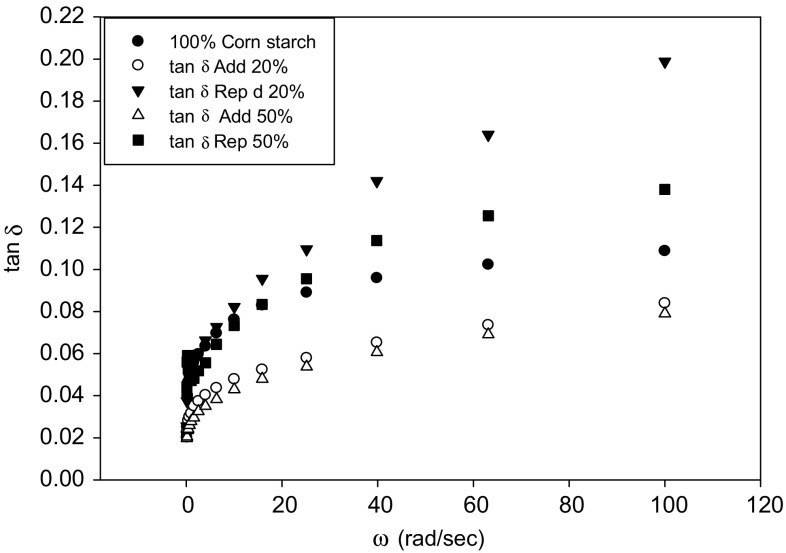
The tan δ of all samples was in the range of 0.02–0.20 indicating elastic behavior since it is less than unity, however, lower tan δ (high G′) indicates more elastic starch gel. The high tan δ (low G′) is a result of less structured more viscous and softer gels. In Fig. 4, unlike the replacement, the profile of the addition of DS exhibited lower tan δ compared to the control. Therefore, starch replacement reduced the G′ and the opposite is true. Although tan δ increased at lower frequencies but it was leaning toward plateau at higher frequencies (Fig. 4). For frequencies lower than 15 (rad/s), samples exhibited similar tan δ. These variations in tan δ were not observed when starch is blended with xanthan gum because xanthan gum tends to have more control over the aqueous component of the blend (Dong and Yoon 2012). Once again the different effect of DS and SG on the rheological properties of corn starch gel is shown by tan δ data. Based on these findings, it is safe to say that adding DS to starch is more effective than replacing starch.
Fig. 4.
Tan δ of corn starch gel replaced with 0, 20 and 50% date syrup (Rep) and date syrup added to a fixed amount of starch (Add)
The effect of sugars on starch physicochemical properties was explained by different researchers as the penetration of small sugars into the amorphous regions of starch granules instigating complex formation between sugars and amylose. This will cause delay in starch swelling, amylose leaching, and limit the start of starch gelatinization. Other investigators reported that the effect is attributable to concentration of sugars on the continuous phase of the system (Tomasik et al. 1995; Sikora et al. 1999; Chiotelli et al. 2000). Regarding the rate of amylose network formation, the addition of sugars caused large decrease in the rate of preliminary conformational organization of amylose network (retrogradation) during cooling, which is corroborated by the decrease in G′ (Evageliou et al. 2000). Either of the theories can be used to explain the change in G′ and the peak viscosity of the starch caused by DS or SG.
Gel texture
The gel hardness of corn starch replaced with DS or SG was lower at higher replacement level (Table 3). The low hardness is predominantly due to the absence of amylose (less starch) which is consistent with low G′. However, linear increase in gel hardness was observed at higher DS addition compared to SG (Table 3). This can be attributed to the faster amylose retrogradation in the presence of DS (addition) due to the interaction of DS with the water allowing amylose molecules to come closer and retrograde. Regarding SG, sugars appear to interact with amylose and partially restricted retrogradation. The slope of decrease in hardness for DS or SG replacement was pretty close − 2.72 and − 2.84 for the DS and SG, respectively, but the addition experiment exhibited 1.87 and − 2.52 (the negative slope here because hardness decreased) for DS and SG, respectively (Table 3).
Table 3.
Corn starch gels textural characteristics
| % DS1 or SG2 | Hardness | Cohesiveness | Adhesiveness | |||
|---|---|---|---|---|---|---|
| DS | SG | DS | SG | DS | SG | |
| Replacement | ||||||
| 0 | 146 | 146 | 0.41 | 0.41 | 0.40 | 0.40 |
| 15 | 120 | 127 | 0.44 | 0.42 | 1.00 | 0.40 |
| 20 | 105 | 116 | 0.41 | 0.37 | 0.90 | 0.90 |
| 30 | 76 | 63 | 0.35 | 0.42 | 0.70 | 0.70 |
| 50 | 13 | 13 | 0.71 | 0.57 | 0.20 | 0.10 |
| Addition | ||||||
| 15 | 145 | 165 | 0.47 | 0.38 | 0.30 | 1.50 |
| 20 | 156 | 148 | 0.52 | 0.51 | 2.00 | 0.70 |
| 30 | 161 | 105 | 0.45 | 0.48 | 0.90 | 0.80 |
| 50 | 212 | 76 | 0.46 | 0.50 | 0.40 | 0.50 |
DS date syrup, SG sugar solutions
The gel cohesiveness exhibited higher values only at 50% DS or SG replacement where the slope of increase was 5.39 and 3.21 for DS and SG, respectively. Whereas the addition samples exhibited − 2.43 and 3.29 slopes for DS and SG, respectively (Table 3). Principally, the flow properties of starch gels are dependent on several factors such as the rheological features of the formed amylose network, the volume fraction and the rigidity of the swollen starch granules in addition to the interactions between the dispersed and the continuous phases of the gel, represented here by the DS and SG (Biliaderis 1998). These factors are dependent on the amylose content and the structure of amylopectin in terms of degree of branching (Yamin et al. 1999). Doublier et al. (1987) proposed that the main structural restrictions of a starch gel are the deformability of swollen starch particles (amylose and amylopectin) and the amylose concentration in the continuous phase (network). Other researchers reported that the increase in gel hardness of wheat and potato starch could be attributed to the formation of a strong amylose matrix by changing the conformation of the gel-structure and the intermolecular interaction between amylose–amylose molecules. However, decreased gel hardness at a concentration higher than 40% sugars could be attributed to the greater reduction in the leached amylose which reduces the amylose concentration in the continuous phase, thus weakening the gel matrix (Gunaratne et al. 2007; Doublier et al. 1987). This behavior of starch gels at high sugar content is true for this work only for SG addition and not for DS as shown in Table 3. In addition, this was also correct for the reduced values of K′ except for DS addition (Table 2).
Conclusion
Date syrup (DS) and sugars (SG) reduced the peak viscosity and the setback of corn starch which could be attributed to the limited starch granules swelling. This effect was more obvious at higher DS and SG. The effect of DS and SG was observed on the properties of the gel, where G′ dropped significantly at higher sugar or date syrup content, particularly at the 50% replacement. In spite of the difference in testing conditions between RVA and rheometry, both tests indicated interference of DS and SG with starch paste network-formation by limiting amylose capacity to form strong network. The influence of DS on starch pasting properties was more obvious than that of SG, especially the results obtained from the addition experiment. This was apparent in the conclusion of the RVA, rheometry, and texture testing. Changes in tan δ values were a good example of the dissimilarities between DS and SG regarding starch pasting and visco-elastic properties.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research Group No RGP-114.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdulmola NA, Richardson RK, Morris ER. Effect of oxidized starch on calcium pectinate gels. Food Hydrocoll. 2000;14:569–577. doi: 10.1016/S0268-005X(00)00038-2. [DOI] [Google Scholar]
- Ahmad FB, Williams PA. Effect of sugars on the thermal and rheological properties of sago starch. Biopolymers. 1999;50:401–412. doi: 10.1002/(SICI)1097-0282(19991005)50:4<401::AID-BIP6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Ahmed J, Ramaswamy HS. Physico-chemical properties of commercial date pastes (Phoenix dactylifera) J Food Eng. 2006;76:348–352. doi: 10.1016/j.jfoodeng.2005.05.033. [DOI] [Google Scholar]
- Alamri MS, Mohamed AA, Hussain S, Almania HA. Legume starches and okra (Abelmoschus esculentus) gum blends: pasting, thermal, and viscous properties. Food Sci Technol Res. 2013;19:381–392. doi: 10.3136/fstr.19.381. [DOI] [Google Scholar]
- Alloncle M, Lefebvre J, Llamas G, Doublier J. A rheological characterization of cereal starch—galactomannan mixtures. Cereal Chem. 1989;66:90–93. [Google Scholar]
- AOAC (1990) Official methods (16.12) of analysis of AOAC international. 16th edn, Edited by Association of Official Analytical Chemists, Washington
- Baek MH, Yoo B, Lim ST. Effects of sugars and sugar alcohols on thermal transition and cold stability of corn starch gel. Food Hydrocoll. 2004;18:133–142. doi: 10.1016/S0268-005X(03)00058-4. [DOI] [Google Scholar]
- Bansil R. Phase separation in polymer solutions and gels. J de Phys. 1993;3:225–235. [Google Scholar]
- Biliaderis CG. Structures and phase transitions of starch polymers. In: Walker RH, editor. Polysaccharide association structures in food. New York: Marcel Dekker Inc; 1998. pp. 57–168. [Google Scholar]
- Chang YH, Lim ST, Yoo B. Dynamic rheology of corn starch–sugar composites. J Food Eng. 2014;64:521–527. doi: 10.1016/j.jfoodeng.2003.08.017. [DOI] [Google Scholar]
- Chen Z, Schols HA, Voragen AGJ. Physicochemical properties of starches obtained from three varieties of Chinese sweet potatoes. J Food Sci. 2003;68:431–437. doi: 10.1111/j.1365-2621.2003.tb05690.x. [DOI] [Google Scholar]
- Chiotelli E, Role’e A, Le Meste M. Effect of sucrose on the thermo-mechanical behavior of concentrated wheat and waxy corn starch–water preparations. J Agric Food Chem. 2000;48:1327–1339. doi: 10.1021/jf990817f. [DOI] [PubMed] [Google Scholar]
- Choi HM, Yoo B. Steady and dynamic shear rheology of sweet potato starch–xanthan gum mixtures. Food Chem. 2009;116:638–643. doi: 10.1016/j.foodchem.2009.02.076. [DOI] [Google Scholar]
- D’Appolonia BL. Effect of bread ingredients on starch gelatinization properties as measured by the amylograph. Cereal Chem. 1972;49:532–543. [Google Scholar]
- Dickinson E, McClements DJ. Advances in food colloids. Glasgow: Blackie/Chapman & Hall; 1996. pp. 81–101. [Google Scholar]
- Dong WC, Yoon HC. Steady and dynamic rheological properties of buckwheat starch-galactomannan mixtures. Prev Nutr Food Sci. 2012;17:192–196. doi: 10.3746/pnf.2012.17.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doublier JL, Llamas G, Le Meur M. A rheological investigation of cereal starch pastes and gels. Effects of pasting procedures. Carbohydr Polym. 1987;7:251–275. doi: 10.1016/0144-8617(87)90063-4. [DOI] [Google Scholar]
- Eliasson A. Calorimetric investigation of the effect of sucrose on starch gelatinization. Carbohydr Polym. 1992;18:131–138. doi: 10.1016/0144-8617(92)90135-D. [DOI] [Google Scholar]
- Evageliou V, Richardson RK, Morris ER. Effect of sucrose, glucose, and fructose on gelation of oxidised starch. Carbohydr Polym. 2000;42:261–272. doi: 10.1016/S0144-8617(99)00158-7. [DOI] [Google Scholar]
- Gunaratne A, Ranaweera S, Corke H. Thermal, pasting, and gelling properties of heat and potato starches in the presence of sucrose, glucose, glycerol, and hydroxypropyl b-cyclodextrin. Carbohydr Polym. 2007;70:112–122. doi: 10.1016/j.carbpol.2007.03.011. [DOI] [Google Scholar]
- Hoover R, Senanayake N. Effect of sugars on the thermal and retrogradation properties of oat starches. J Food Biochem. 1996;20:65–83. doi: 10.1111/j.1745-4514.1996.tb00585.x. [DOI] [Google Scholar]
- Jangchud K, Phimolsiripol Y, Haruthaithanasan V. Physicochemical properties of sweet potato flour and starch as affected by blanching and processing. Starch/Starke. 2003;55:258–264. doi: 10.1002/star.200390053. [DOI] [Google Scholar]
- Jung SH, Shin GJ, Choi CU. Comparison of physicochemical properties of corn, sweet potato, potato, wheat and mungbean starches. Korean J Food Sci Technol. 1991;23:272–275. [Google Scholar]
- Kim CS, Walker CE. Effect of sugars and emulsifiers on starch gelatinization using deferential scanning calorimetry. Cereal Chem. 1992;69:212–217. [Google Scholar]
- Levine H, Slade L. Non-equilibrium behavior of small carbohydrate water system. Pure Appl Chem. 1988;60:1841–1847. doi: 10.1351/pac198860121841. [DOI] [Google Scholar]
- Maaruf AG, Che Man YB, Asbi BA, Junainah AH, Kennedy JF. Effect of water content on the gelatinisation temperature of sago starch. Carbohydr Polym. 2001;46:331–337. doi: 10.1016/S0144-8617(00)00335-0. [DOI] [Google Scholar]
- Miles MJ, Morris VJ, Orford PD, Ring SG. The roles of amylose and amylopectin in the gelation and retrogradation of starch. Carbohydr Res. 1985;135:271–281. doi: 10.1016/S0008-6215(00)90778-X. [DOI] [Google Scholar]
- Mohamed IO, Babucurr J. Effect of date syrup on pasting, rheological, and retrogradation properties of corn starch gels. Starch/Stärke. 2015;67:709–715. doi: 10.1002/star.201500062. [DOI] [Google Scholar]
- Noda T, Tkahata T, Sato T, Kumagai T, Yamakawa O. Starch properties and cell-wall material contents in sweet potatoes as affected by flesh color, cultivation method and year. J Appl Glycosci. 1998;45:1–9. [Google Scholar]
- Parry PA, Donald AM. The effect of sugars on the gelatinization of starch. Carbohydr Polym. 2002;49:155–165. doi: 10.1016/S0144-8617(01)00324-1. [DOI] [Google Scholar]
- Pongsawatmanit R, Temsiripong T, Suwonsichon T. Thermal and rheological properties of tapioca starch and xyloglucan mixtures in the presence of sucrose. Food Res Int. 2007;40:239–248. doi: 10.1016/j.foodres.2006.10.013. [DOI] [Google Scholar]
- Sandhu KS, Singh N. Some properties of corn starches II: physicochemical, gelatinization, retrogradation, pasting and gel textural properties. Food Chem. 2007;101:1499–1507. doi: 10.1016/j.foodchem.2006.01.060. [DOI] [Google Scholar]
- Sandhu KS, Kaur M, Singh N, Lim ST. A comparison of native and oxidized normal and waxy corn starches: physicochemical, thermal, morphological and pasting properties. LWT Food Sci Technol. 2008;41:1000–1010. doi: 10.1016/j.lwt.2007.07.012. [DOI] [Google Scholar]
- Sikora M, Mazurkiewicz J, Tomasik P, Pielichowski K. Rheological properties of some starch–water–sugar systems. Int J Food Sci Technol. 1999;34:371–383. doi: 10.1046/j.1365-2621.1999.00283.x. [DOI] [Google Scholar]
- Singh N, Sandhu KS, Kaur M. Characterization of starches separated from Indian chickpea (Cicer arietinum) cultivars. J Food Eng. 2004;63(4):441–449. doi: 10.1016/j.jfoodeng.2003.09.003. [DOI] [Google Scholar]
- Singh N, Inouchi N, Nishinari K. Structural thermal and viscoelastic characteristics of starches separated from normal, sugary and waxy maize. Food Hydrocoll. 2006;20:923–935. doi: 10.1016/j.foodhyd.2005.09.009. [DOI] [Google Scholar]
- Sopade PA, Halley PJ, Junming LL. Gelatinisation of starch in mixtures of sugars. II. Application of differential scanning calorimetry. Carbohydr Polym. 2004;58:311–321. doi: 10.1016/j.carbpol.2004.07.007. [DOI] [Google Scholar]
- Tolstoguzov VB. Functional properties of protein–polysaccharide mixtures. In: Mitchell JR, Ledward DL, editors. Functional properties of food macromolecules. London: Elsevier; 1986. pp. 385–415. [Google Scholar]
- Tomasik P, Wang Y, Jane J. Complexes of starch with low molecular saccharides. Starch/Starke. 1995;47:185–189. doi: 10.1002/star.19950470506. [DOI] [Google Scholar]
- Tromp RH, Rennie AR, Jones RAL. Kinetics of simultaneous phase separation and gelation in solutions of dextran and gelatin. Macromolecular. 1995;28:4129–4138. doi: 10.1021/ma00116a012. [DOI] [Google Scholar]
- Wang B, Wang L-J, Li D, Ozkan N, et al. Rheological properties of waxy maize starch and xanthan gum mixtures in the presence of sucrose. Carbohydr Polym. 2009;77:472–781. doi: 10.1016/j.carbpol.2009.01.017. [DOI] [Google Scholar]
- Willett JL, Jasberg BK, Swanson CL. Rheology of thermoplastic starch: effects of temperature, moisture content, and additives on melt viscosity. Polym Eng Sci. 1995;35:202–210. doi: 10.1002/pen.760350214. [DOI] [Google Scholar]
- Yamin FF, Lee M, Pollak LM, White PJ. Thermal properties of starch in corn variants isolated after chemical mutagenesis of inbred line B73. Cereal Chem. 1999;76:175–181. doi: 10.1094/CCHEM.1999.76.2.175. [DOI] [Google Scholar]
- Zhang X, Tong Q, Zhu W, Ren F. Pasting, rheological properties and gelatinization kinetics of tapioca starch with sucrose or glucose. J Food Eng. 2013;114:255–261. doi: 10.1016/j.jfoodeng.2012.08.002. [DOI] [Google Scholar]