Abstract
The viability of probiotics in the development of functional tropical fruit beverages is a technological challenge that may benefit from the addition of prebiotics, due to the synergistic (synbiotic) interaction. This study evaluated the viability of a commercial probiotic (Lactobacillus casei) in a blended red fruit beverage (RFB; 20% strawberry, 10% blackberry and 5% papaya), enriched with three separate prebiotics: inulin (IN), fructooligosaccharides (FOS) and galactooligosaccharides, added at 1 and 5%. The consumer preference for the beverages was also examined. The inoculum was produced in MRS broth supplemented with 10% RFB, which reached the exponential phase (9.96 log CFU mL−1) after incubation at 37 °C for 24 h. In search of the probiotic strain’s adaptation and viability in the presence of the different prebiotics (measured by optical density, OD600 nm), the prebiotics were added to MRS broth at 1 and 5%. Since 1% IN (OD = 3.99 ± 0.36) and 1% FOS (OD = 3.48 ± 0.28) were the most significant, these treatments, without inoculation of probiotics, were assessed by the sensory panel. Although neither RFB was significantly preferred the RFB with 1% IN received the greatest number of responses (n = 33/60). Its effect on the viability of L. casei inoculated in the RFB was monitored by the growth kinetics at 37 °C for 50 h. The findings indicated that fortification with 1% IN could have a possible protective effect on the stability of L. casei in RFBs, highlighting the use of tropical fruits as potential carriers of probiotics.
Keywords: Prebiotic, Lactobacillus casei, Inulin, Red fruit, Fruit beverage
Introduction
Extensive research and development activity are currently centered around food products formulated with biologically active compounds (Mylona et al. 2018). Products with natural functionality, such as Oriental and African fermented products of vegetable origin (e.g., kimchi), for instance, are particularly attractive, owing to the broad appeal among modern consumers for “all natural”, fruit/vegetable nutrition and functional products that help promote health (El Sheikha and Montet 2014; Ray et al. 2014).
Beverages are an excellent vehicle for supplying bioactive compounds, such as vitamins, minerals, antioxidants, fiber, prebiotics and probiotics (Corbo et al. 2014), and represent one of the fastest growing markets for product development. Fruit and vegetable juices are among the most rapidly advancing sectors in the beverages industry, due to their convenience and associated health benefits. In 2016, the global fruit and vegetable juices market size was valued at USD 154.18 billion and is expected to grow at a compound annual growth rate of 5.93% during 2018–2025 (Grand View Research 2018).
A beverage containing mixtures of red berries (strawberry and blackberry) could be an attractive option for consumers, as products derived from these fruits are a significant source of bioactive components, such as polyphenols, flavonoids, ellagic acid, tannins, ellagitannins, gallic acid, benzoic acid and anthocyanins (Gaurav and Tiwari 2017; Yahia 2018). Some interesting sources of these compounds are Colombian tropical fruits, as many of these fruits present a high phenolic content (1011–1018 mg gallic acid equivalents 100 g−1 fresh weight for Brazilian guava, guava apple, banana passion fruit and cashew, for example) and antioxidant activity (Yahia 2018).
Likewise, these blended products have been proposed as novel and appropriate vehicles for the inclusion of probiotic strains because of their essential micronutrient content, allowing to produce lactic acid and biomass under fermentative conditions (Perricone et al. 2015; Yoon et al. 2005). At the same time, lactic acid fermentation of fruits and vegetables enhances their organoleptic and nutritional quality and retains the nutrients and colored pigments (El Sheikha 2018a, b). In addition, fruit-based beverages have a healthy image and present a wide variety of sensory profiles (Pereira et al. 2011; Sheehan et al. 2007).
Probiotics are non-pathogenic microorganisms that when consumed in adequate amounts, confer a health benefit on the host (Hill et al. 2014). Although probiotics have traditionally been consumed in fermented dairy products, like yogurt, the consumption of these products is limited by the rise in various diet types (e.g., lactose-free, reduced-cholesterol), leading to the development of non-dairy alternatives. In this context, inclusion matrices based on vegetables and fruits are a promising healthy option, aimed at consumers allergic to dairy products, as well as vegetarians (Corbo et al. 2014).
The fruits most commonly used in commercial juice preparations inoculated with probiotics include cranberry, apple, blackcurrant, cherry, guarana, mango, grapes, kiwifruit, strawberries, peaches and plums, and some of the typical probiotic strains used in formulating new products are Lactobacillus acidophilus, L. casei, L. plantarum, L. rhamnosus and Bifidobacterium lactis (Martins et al. 2013). However, maintaining the recommended viability of probiotics in juices is technologically challenging, mainly because of the presence of insufficient amounts of peptides and free amino acids required for the metabolism of probiotic strains, as well as the susceptibility of probiotics to extreme acidic conditions (pH 2.5–3.6), and the potential for additional undesired fermentation and alterations to the sensory characteristics of food products, which may lead to poor microbiological quality and low consumer acceptability (Perricone et al. 2015; Ray et al. 2014). It is important to adapt probiotic bacteria to these kinds of products, for large-scale industrial production and processing, and that they maintain good activity during storage (El Sheikha 2018a, b).
The addition of prebiotics, substances capable of exerting a protective effect and selectively stimulating the growth of probiotics in the human intestine, is a strategy in the design of probiotic juices (Perricone et al. 2015; Shori 2016). The inclusion of prebiotics in beverages fulfills multiple functions beyond the probiotic strains’ survival, such as the improved sensory and physicochemical characteristics of the drinks, as sweetening agents for fruits drinks, and as stabilizers to avoid liquefaction processes (Munir et al. 2016). Inulin (IN), fructooligosaccharides (FOS) and galactooligosaccharides (GOS) are well-recognized prebiotics found naturally in fruits, vegetables, milk, honey and bamboo, among various other foods (Singh et al. 2015). Mixtures of probiotics and prebiotics (synbiotics) are often used in functional foods to exploit their synergistic interactions (Al-Sheraji et al. 2013). The consumption of synbiotic functional food products/beverages is a current global trend (Shori 2016).
Despite the numerous reports about the use of fruits to formulate functional beverages, studies focusing on the innovation of juices based on tropical and Andean mixtures of red fruits containing probiotic commercial lactobacilli strain and prebiotics, such as IN, are still scarce. Therefore, this research determined the influence of the addition of IN, FOS and GOS on the viability of a commercial probiotic strain (L. casei) in a red fruit beverage (RFB).
Materials and methods
Raw materials, culture medium and commercial prebiotics
Commercially-mature blackberry, strawberry and papaya fruits were purchased from a local market. The fruits were transformed into pulps and stored at − 14 °C before the formulation of the beverage. Culture medium and broth (de Man–Rogosa–Sharpe, MRS; Oxoid®) were prepared according to the manufacturer’s instructions. The prebiotic fibers, namely, FOS (96.4% purity; Nutraflora®, Ingredion), IN [Orafti® HSI (high soluble IN powder) with 88% IN] and GOS [57% minimum GOS content (solids basis); Bioigo™, Ingredion] were purchased, as indicated. For the preparation of the blended fruit beverage, sucrose (food grade) was used.
Formulation and production of the red fruit beverage (RFB)
The RFBs were prepared by blending 20% (w/v) of strawberry (Fragaria × ananassa), 10% (w/v) blackberries (Rubus glaucus Benth) and 5% (w/v) papaya (Carica papaya) pulps. Papaya was chosen as a pH stabilizer and additional fiber source (1.7 g 100 g−1) (Braga and Conti-Silva 2015). The pH was 3.36 ± 0.02, and the total soluble solids were 10 ± 0.01 °Brix. The beverages were poured into Schott bottles (250 mL) and sterilized (120 °C, 15 psi, 15 min) before inoculation, to avoid the interaction of the probiotic with the microbiota of the native fruits, which could lead to possible alterations in the beverage (Sheehan et al. 2007). The inoculation was done at 18–20 °C under aseptic conditions. All procedures were carried out in compliance with good manufacturing practices (Ministerio de Salud y Protección Social de Colombia 2013).
Culture and growth conditions
A commercial lyophilized probiotic culture of L. casei subsp. rhamnosus (Mediterranea Biotecnologie®, Termoli, Italy) was used. The strain was activated by rehydration in 5 mL of MRS broth at 37 °C for 72 h, and the purity was checked by Gram staining.
Production of inoculum in a synthetic medium supplemented with a red fruit beverage (RFB): adaptation of the strain
For the inoculum standardization, the time of maximum biomass production of the probiotic microorganism in the synthetic medium, which simulated the formulation of the RFBs, was used as a reference. The synthetic medium consisted of MRS broth supplemented with 10% RFB, as recommended by Perricone et al. (2014). The strain was active in MRS broth at 37 °C for 72 h. Afterward, 2% of the pre-inoculum was added to 50 mL of synthetic medium. Cells were centrifuged (4000 rpm, 10 min) and suspended in 10 mL of sterile saline solution.
Evaluation of the effect of the prebiotics on the survival of Lactobacillus casei in modified de Man–Rogosa–Sharpe (MRS)
The prebiotics viability was evaluated in an MRS supplemented with 5% sucrose (which was added to the beverage) and the prebiotic substances: IN, FOS and GOS. The culture broth was modified to simulate the conditions of the RFB, and the viability of the L. casei strain in the presence of the prebiotics was assessed. Each treatment (20 mL) was inoculated with 2% (400 mL) (7–8 log CFU mL−1) of the studied microorganism. The controls included an MRS broth without the addition of prebiotic, and an MRS supplemented with 5% sucrose. Biomass was determined at 0, 10, 24 and 48 h of incubation at 37 °C, by measuring the optical density at 600 nm (OD600nm) (Adebola et al. 2014). Triplicate analyses were undertaken.
Sensory analysis of the red fruit beverages (RFBs) incorporated with prebiotics
Treatments (1% IN and 1% FOS) were selected according to the results obtained from the analysis of the prebiotics in the modified MRS. The RFBs were refrigerated (4–8 °C, for no longer than 2 days) until the development of the test. Beverages were not inoculated with probiotic bacteria. A preference test (paired comparison) was conducted with a panel of 60 untrained male and female evaluators, aged between 19 and 60 years. Two treatments, namely, RFBs with 1% IN and 1% FOS, were evaluated. Samples (7–10 °C) were provided to the panelists as 10 mL of non-inoculated RFB coded with three random digits, and the test was performed as described elsewhere (Nuñez Hinostroza and Brumovsky 2010).
Survival of Lactobacillus casei in the red fruit beverages (RFBs): growth kinetics
Treatment with 1% IN was selected according to the viability assays in the modified MRS broth and sensorial preference. Growth kinetics were assayed in the RFBs containing 1% IN (200 mL) and inoculated with 2% (4 mL) of L. casei from the inoculum designed in the MRS supplemented with 10% of the RFB. Samples were incubated at 37 °C for 50 h. The number (CFU mL−1) of lactobacilli was determined on MRS agar at 0, 10, 24 and 50 h. In addition, physicochemical parameters, including acidity and pH (AOAC 942.15) and the total soluble solids (°Brix) (AOAC 932.12) were measured (AOAC 2012). The controls included an RFB without IN and an uninoculated RFB. Triplicate analyses were conducted.
Statistical analysis
For the interpretation of the results of the preference tests, the table provided by Rosesler et al. (1978), reprinted in Stone and Sidel (1993), was used. Analyses of the prebiotic evaluation assays and growth kinetics in RFBs with 1% IN were conducted using a generalized model test in the Minitab® statistical package. For each parameter evaluated (viability and physicochemical measures), the mean (n = 3) and standard deviation were calculated.
Results and discussion
Formulation and production of the red fruit beverage (RFB)
In the development of food with probiotic microorganisms, factors, such as the bacterial strain, inoculum preparation method, physiological state of the cells, oxygen levels and presence of prebiotics, are considered (Vandenplas et al. 2015). These products should maintain the survival of microorganisms at levels between 106 and 107 CFU mL−1 over the product´s shelf life. Therefore, considering its effectiveness as a vehicle for L. casei, the RFB was formulated to maintain a minimum cell density of 106 CFU mL−1, without altering the sensorial characteristics of the product. To achieve this, the beverage formulation was established with a pH around 3.3. Studies on RFBs with probiotics show that the composition of the drink and the adjustment of the pH in the final product are key to the viability of the microorganism in the product (Nematollahi et al. 2016). Therefore, it was necessary to add 5% papaya to the strawberry and blackberry mixture to stabilize the pH values, achieving average pH values of 3.36 ± 0.02, in which the viability was maintained. A low pH may have an antagonistic effect against pathogenic microorganisms. At the same time, the total soluble solids, pH and percentage of fruits were established, in compliance with current Colombian regulations for this type of product (Resolution 3929 of the Ministry of Health and Social Protection 2013). Red fruits, such as cherries, cranberries, blackberries and raspberries, contain components with antimicrobial properties (Perricone et al. 2015; Sheehan et al. 2007; Yahia 2018). Other factors that may influence the viability of probiotics in this type of matrix are the high content of phenols, as exemplified by the low viability of L. plantarum in lemon, pomegranate and cranberry juices, respectively (Nualkaekul and Charalampopoulos 2011).
Production of inoculum in a synthetic medium supplemented with a red fruit beverage (RFB): adaptation of the strain
Inoculum production with adaptation and scaling of the probiotic directly into the RFB was key to adapting the microorganism to the matrix, as a fermentation substrate allowing microbiological stability (Pereira et al. 2011). The inoculum production in the synthetic medium reached the exponential phase (9.96 log CFU mL−1) after incubation at 37 °C for 24 h. These conditions allowed the adaptation of L. casei, slightly inducing its resistance to the pH (3.36 ± 0.02) of the RFB and obtaining the highest cell density, based on the method suggested by Perricone et al. (2014).
Strain adaption proved effective in a previous study, evaluating the viability of L. reuteri in various fruit juices, in which the type of juice strongly affected the viability of the probiotic: surviving in pineapple, orange and apple juices while exhibiting a strong reduction in juice prepared from red fruits (Perricone et al. 2014). Supplementation with 10% of the drink in MRS significantly increased the initial reduction time of 0.47 days out to 9.28–11.20 days (Perricone et al. 2014).
Evaluation of the effect of the prebiotics on the survival of Lactobacillus casei in the modified de Man–Rogosa–Sharpe (MRS) medium
Table 1 describes the viability of L. casei in the modified MRS broth, in the presence of the prebiotics. Significant differences (p < 0.05) were found between the treatments with 1 and 5% GOS, as compared to the controls (MRS broth and 5% sucrose MRS broth), with a favorable effect on the viability of L. casei at low levels (1%). The protective effect may be because, as reported previously, prebiotics could maintain the residual water levels necessary to preserve cell structures, preventing adverse effects on microorganisms by creating a barrier against environmental factors. Prebiotics can be considered as protective molecules (Tymczyszyn et al. 2011). For example, the microencapsulation of probiotic microorganisms with prebiotic agents has been used to increase the survival of these microorganisms in synbiotic foods (Yonekura et al. 2014). In another study, IN acted as protective agent in the formation of synbiotic microcapsules, to improve the heat resistance of probiotics (Karimi et al. 2015).
Table 1.
Effect of prebiotics on the viability of L. casei (DO600 nm) in an MRS broth modified with 5% sucrose
| Treatment | Time (h) | |||
|---|---|---|---|---|
| 0 | 10 | 24 | 50 | |
| T1: MRS Broth + 5% sucrose + 1% IN | 0.08 ± 0.03ab | 0.71 ± 0.04ab | 3.43 ± 0.30ab | 3.99 ± 0.36ab |
| T2I: MRS Broth + 5% sucrose + 5% IN | 0.09 ± 0.01c | 0.75 ± 0.07c | 2.31 ± 0.04c | 3.15 ± 0.40c |
| T3: MRS Broth + 5% sucrose + 1% FOS | 0.06 ± 0.03abc | 0.82 ± 0.05abc | 2.99 ± 0.05abc | 3.48 ± 0.24abc |
| T4: MRS Broth + 5% sucrose + 5% FOS | 0.07 ± 0.00bc | 0.64 ± 0.03bc | 2.69 ± 0.15bc | 3.34 ± 0.34bc |
| T5: MRS Broth + 5% sucrose + 1% GOS | 0.06 ± 0.06abc | 0.80 ± 0.07abc | 2.42 ± 0.18abc | 4.02 ± 0.11abc |
| T6: MRS Broth + 5% sucrose + 5%GOS | 0.03 ± 0.03bc | 0.65 ± 0.10bc | 2.67 ± 0.20bc | 3.67 ± 0.16bc |
| C1: MRS Broth | 0.08 ± 0.07abc | 0.75 ± 0.03abc | 3.23 ± 0.13abc | 3.83 ± 0.06abc |
| C2: MRS Broth + 5% sucrose | 0.04 ± 0.01a | 0.86 ± 0.03a | 2.94 ± 0.08a | 4.95 ± 0.30a |
Each value is the mean ± standard deviation of the mean of three replications. Absorbance values with the same letter in the treatments do not differ significantly from each other at a significance level of 95% according to the Tukey test
The highest absorbance value at 50 h of incubation was obtained in the treatment with 1% GOS (OD600nm = 4.02 ± 0.11), followed by the treatment with 1% IN (OD600nm = 3.99 ± 0.36) and 1% FOS (OD600nm = 3.48 ± 0.24). However, excessive caramelization was observed in the culture medium with the addition of GOS at both levels (1 and 5%) after sterilization, which led to disregarding this prebiotic because of its possible effect on the sensory qualities of the beverage (Braga and Conti-Silva 2015). It was observed that the treatment with 1% IN was more viable than the control with MRS (OD600nm = 3.83 ± 0.06), indicating that IN might have exerted a protective influence on L. casei, improving its survival. It has been reported that IN has more pronounced prebiotic effects than FOS under fermentative conditions (37 °C), in simulators of the human intestinal microbial ecosystem (Shoaib et al. 2016).
Sensory analysis of the red fruit beverages (RFBs) incorporated with prebiotics
Considering the results above, sensory analysis of the RFBs fortified with 1% IN and 1% FOS, respectively, without inoculation of the probiotic bacteria was performed using a preference test. Although the number of similar responses in the test indicated that there was no tacit sensory outcome on the beverage preference for the prebiotic type (Table 2, p > 0.05), the highest number of responses was for the beverage enriched with 1% IN (Karimi et al. 2015).
Table 2.
Sensory analysis: preference test for the prebiotics in the RF beverages
| Type of beverage | Number of product acceptance responses |
|---|---|
| RF drink with 1% IN | 33a |
| RF drink with 1% FOS | 27a |
Other authors have reported similar results, in which there was no evidence of preference differences between beverages with prebiotics and their traditional counterparts (Braga and Conti-Silva 2015; Cassani et al. 2017). One study examined the sensory impact of prebiotics (FOS and IN) and sugar substitutes on papaya nectars and found that the preference was similar to the nectars with sugar (Braga and Conti-Silva 2015). Additionally, in optimization processes for the formulation of strawberry juices with probiotics, the addition of IN demonstrated no negative impacts on sensory quality (Cassani et al. 2017). However, the magnitude of the effect of IN on the sensory acceptability depends on the blend of the short- and long-chain IN, as well as the total IN content (Shoaib et al. 2016).
Survival of Lactobacillus casei in the red fruit beverages (RFBs): growth kinetics
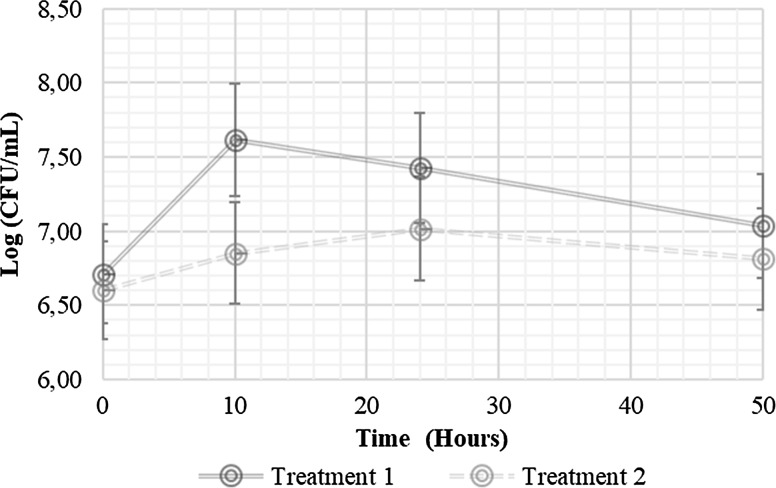
According to the probiotic viability in the modified MRS broth, and considering that the prebiotic type did not alter the sensory response, the viability of L. casei on the RFB incorporated with1% IN was selected to determine the growth kinetics under fermentation conditions that simulate the normal temperature of the human body, i.e., 37 °C (Fig. 1). Inoculation of L. casei into the RFB with 1% IN and a control (RFB without 1% IN) significantly affected (p < 0.05) the viability and physicochemical properties of the RFB. The plate counts were 7.03 and 6.81 log CFU mL−1 in the treatments with and without IN, respectively. It suggests that the addition of 1% IN may improve the viability of the probiotic microorganism. The stimulation of the metabolism of lactic acid bacteria (Lactobacillus) by IN may be due to partial hydrolysis and subsequent metabolization of fructose, as an additional source of carbon and energy (De Souza Oliveira et al. 2012).
Fig. 1.
Growth kinetics of L. casei in the RF beverages. Treatment 1: red fruit beverage with 1% IN. Treatment 2: red fruit beverage without the addition of prebiotics
Some studies on juices prepared from red fruits, like blueberries, blackcurrants and blackberries, have reported a drastic decrease in viability because of the presence of certain organic acids (benzoic acid) in red fruits that may have toxic effects on microorganisms and considerably affect the viability of the probiotic culture during processing and in its future useful life (Sheehan et al. 2007). When various Lactobacillus and Bifidobacterium strains were added to orange, pineapple and cranberry juices, a large difference in the acid resistance was observed, even at the same pH, and all strains survived least in the cranberry juice, possibly because of the high content of benzoic acid (34 mg L−1) (Sheehan et al. 2007). Therefore, authors, such as Perricone et al. (2015), have recommended strategies for the adaptation of the strains and the use of the prebiotics, such as those used in the current investigation.
In the absence of added prebiotics, Di Cagno et al. (2011) identified lactic acid bacteria (L. plantarum, Lactobacillus sp. and L. pentosus) from several fruits (blackberries, prunes and raisins) and developed a protocol for processing functional smoothies (without prebiotics) with acceptable nutritional, antioxidant and sensory attributes, despite the hostile environment (pH about 3.51 and high polyphenol content). Conversely, 1% IN improved the viability of a L. plantarum CECT 220 in blended carrot and orange juice, presenting 9.2 and 5.8 log CFU mL−1 in juices with and without the added prebiotic, respectively, and a higher monosaccharide concentration was retained relative to the juice without IN (40% lower) (Valero-Cases and Frutos 2017). The level of 1% IN is beneficial for sensory attributes and the microbiology stability of the RFB, possibly because the blend of short- and long-chain IN at 50:50 ratios provides various additional advantages in enhancing prebiotic effectiveness (Shoaib et al. 2016).
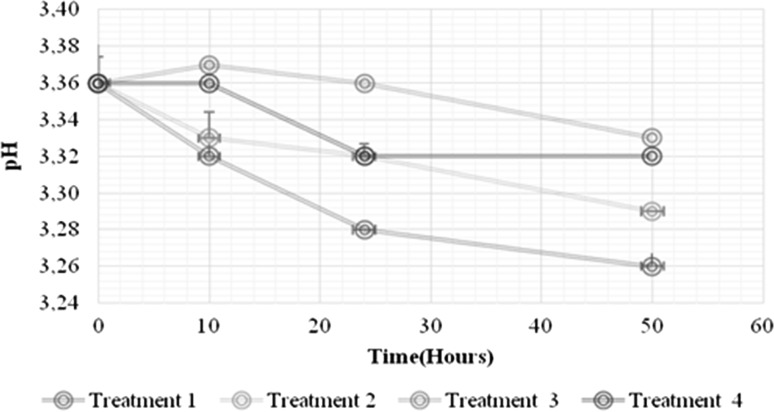
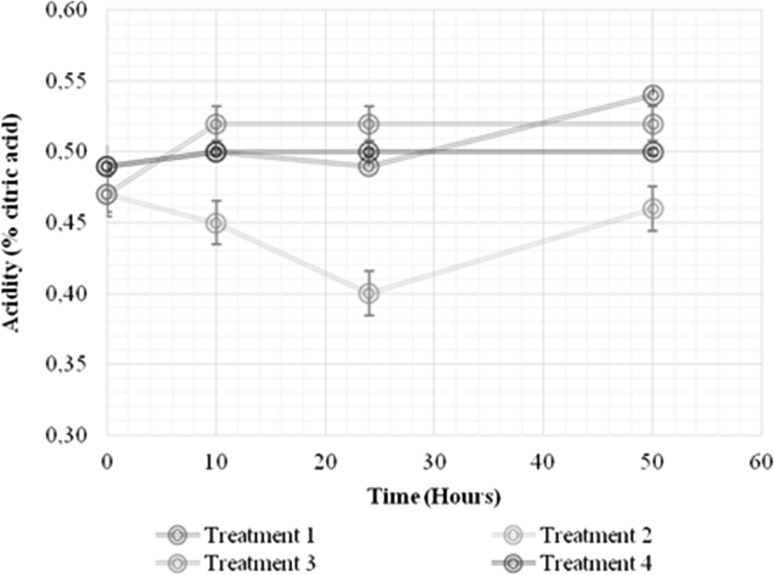
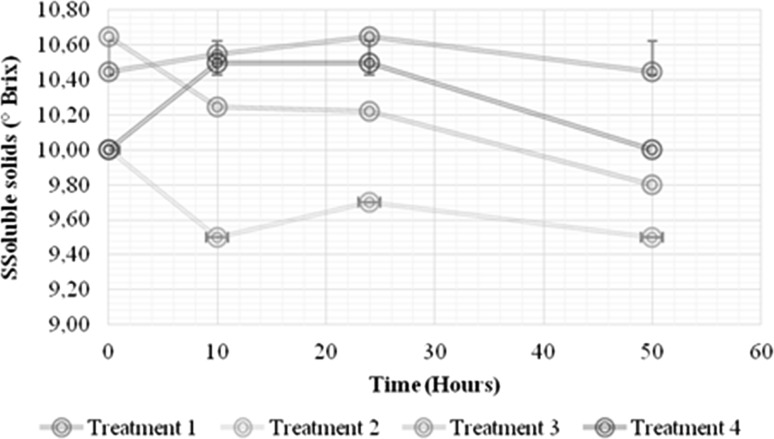
As already mentioned above, the incorporation of prebiotics into fruit juices presents several technical challenges. The compatibility of these ingredients with the products, regarding physicochemical, sensory and nutritional properties, must be well established (Fonteles and Rodrigues 2018). The current research demonstrated significant impacts of the growth of L. casei on the physicochemical parameters (pH, titratable acidity and soluble solids; Figs. 2, 3 and 4, respectively) of the RFBs, including the non-inoculated controls (p < 0.05). Decreases in pH of 3.36 ± 0.02 to 3.26 ± 0.01 occurred for both the 1% IN and no prebiotic treatment (Fig. 2), possibly because of the conversion of sugars to organic acids (e.g., malic and lactic) (Valero-Cases and Frutos 2017), and concomitant decrease in the initial pH values. Lactic acid bacterial cultures have been reported to be characterized by acid production that lowers pH and increases the acidity of the medium, creating an unfavorable environment for pathogens and altering microorganisms (Pereira et al. 2011). The acidity, although showing significant differences between the treatments with and without IN, did not exhibit marked variations in the fermentation time when compared to the non-inoculated controls (Fig. 3). Figure 4 depicts the effect of 1% IN on the total soluble solids in the RFBs (p < 0.05). An increase in the soluble solids content (°Brix) of the RFBs with IN (treatments 1 and 3) was evidenced relative to those that did not contain the prebiotic (treatments 2 and 4), independent of the L. casei growth. It likely indicates that the main sources of carbon and energy for L. casei were glucose and fructose while IN was fermented relatively more slowly (Valero-Cases and Frutos 2017). Similar trends in the total soluble solids have been noted in papaya nectars with added IN (Orafti®P95) and FOS that were appreciated for their taste and general acceptance in equal measure as nectars containing only sugar (Braga and Conti-Silva 2015).
Fig. 2.
Effect of the 1% IN on the pH of the growth kinetics of L. casei in the RF beverages. Treatment 1: RF beverage with 1% IN and L. casei. Treatment 2: fed fruit beverage without the addition of 1% IN with L. casei. Treatment 3: red fruit beverage control with 1% IN and without inoculation. Treatment 4: red fruit beverage without 1% IN and without inoculation
Fig. 3.
Effect of 1% IN on the titratable acidity in the growth kinetics of L. casei in a RF beverage. Treatment 1: red fruit beverage with the addition of 1% IN and L. casei. Treatment 2: red fruit beverage without 1% IN and with L. casei. Treatment 3: red fruit beverage control with 1% IN and without inoculation. Treatment 4: red fruit beverage without 1% IN and without inoculation. The acidity is expressed as a percentage of citric acid in the sample
Fig. 4.
Effect of 1% IN on the soluble solids in the growth kinetics of L. casei in a RF beverage. Treatment 1: red fruit beverage with the addition of 1% IN and L. casei. Treatment 2: red fruit beverage without 1% IN and with L. casei. Treatment 3: red fruit beverage control with 1% IN and without inoculation. Treatment 4: red fruit beverage without 1% IN and without inoculation
The inclusion and viability of a commercial-type probiotic (L. casei) in an RFB at 35% w/v fruit (blackberry and strawberry) was achieved while addressing several biotechnological factors. First, papaya (5% w/v) was added as a natural pH stabilizer. Second, L. casei was adapted to the acidic conditions by supplementing the MRS broth with the RFB (10%), for the production of the inoculum. Third, prebiotics (1% IN) were added to enhance the viability of the microorganism under fermentative conditions. This research permitted the design of a method for the formulation of an RFB-type blend of tropical origin with functional characteristics: addition of probiotic and prebiotic substances. This formulation and findings could advance the generation of fruit-based synbiotic products.
Conclusion
This study showed that red and tropical fruits from Colombia, such as strawberry, blackberry and papaya, are good fruit-derived matrices for incorporation of a commercial type probiotic (L. casei). Adaptation of the strain (L. casei) by supplementing the culture medium with the RFB (10%) to produce the inoculum and the addition of prebiotics (1% IN) were key technological strategies that could be implemented to design and formulate these functional products at an industrial scale. Moreover, IN significantly affected the viability of L. casei and physicochemical parameters of the RFBs under fermentative conditions, without impacting on the sensory quality of the RFBs, highlighting the suitability of this prebiotic in the final formulation of novel functional drinks prepared from red fruits.
Acknowledgements
The authors express their gratitude to the project “Uso de bacterias ácido lácticas para biopreservación y generación de nuevos productos con características probióticas”, financiad by the Dirección de Investigación, Sede Bogotá of the Universidad Nacional de Colombia; the Jardín Botánico de Bogotá, for its contribution to the Convocatoria Permanente in stimulating the research “Thomas Van Der Hammen”; and the staff of the Laboratorio de Biotecnología y Calidad Microbiologia de Alimentos, and the Planta de Investigación en Procesos Vegetales of the Instituto de Ciencia y Tecnología en Alimentos (ICTA).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adebola OO, Corcoran O, Morgan WA. Synbiotics: the impact of potential prebiotics inulin, lactulose and lactobionic acid on the survival and growth of lactobacilli probiotics. J Funct Foods. 2014;10:75–84. [Google Scholar]
- Al-Sheraji SH, Ismail A, Manap MY, Mustafa S, Yusof RM, Hassan FA. Prebiotics as functional foods: a review. J Funct Foods. 2013;5(4):1542–1553. [Google Scholar]
- AOAC International (AOAC) (2012) Latimer G (ed) Official methods of analysis, 19th ed, Chapt. 37, p 336
- Braga HF, Conti-Silva AC. Papaya nectar formulated with prebiotics: chemical characterization and sensory acceptability. LWT Food Sci Technol. 2015;62:854–860. [Google Scholar]
- Cassani L, Tomadoni B, Moreira MR, Ponce A, Agüero MV. Optimization of inulin: oligofructose proportion and non-thermal processing to enhance microbiological and sensory properties of fiber-enriched strawberry juice. LWT Food Sci Technol. 2017;80:446–455. [Google Scholar]
- Corbo MR, Bevilacqua A, Petruzzi L, Casanova FP, Sinigaglia M. Functional beverages: the emerging side of functional foods. Compr Rev Food Sci Food Saf. 2014;13(6):1192–1206. [Google Scholar]
- De Souza Oliveira RP, Perego P, de Oliveira MN, Converti A. Effect of inulin on the growth and metabolism of a probiotic strain of Lactobacillus rhamnosus in co-culture with Streptococcus thermophilus. LWT Food Sci Technol. 2012;47(2):358–363. [Google Scholar]
- Di Cagno R, Minervini G, Rizzello CG, De Angelis M, Gobbetti M. Effect of lactic acid fermentation on antioxidant, texture, color and sensory properties of red and green smoothies. Food Microbiol. 2011;28(5):1062–1071. doi: 10.1016/j.fm.2011.02.011. [DOI] [PubMed] [Google Scholar]
- El Sheikha A. Revolution in fermented food: from artisan household technology to era of biotechnology. In: El Sheikha AF, Levin RE, Xu J, editors. Molecular techniques in food biology: safety, biotechnology, authenticity and traceability. Chichester: Wiley; 2018. pp. 241–260. [Google Scholar]
- El Sheikha A. Molecular Techniques and Lactic Acid-Fermented fruits’ and Vegetables: Why and How? In: El Sheikha AF, Levin RE, Xu J, editors. Molecular techniques in food biology: safety, biotechnology, authenticity and traceability. Chichester: Wiley; 2018. pp. 285–308. [Google Scholar]
- El Sheikha A, Montet D. African fermented foods: historical roots and real benefits. In: Ray RC, Montet D, editors. Microorganisms and fermentation of traditional foods. Food biology series. Boca Raton: Science Publishers Inc.; 2014. pp. 248–282. [Google Scholar]
- Fonteles TV, Rodrigues S. Prebiotic in fruit juice: processing challenges, advances, and perspectives. Curr Opin Food Sci. 2018;22:55–61. [Google Scholar]
- Gaurav R, Tiwari BK (2017) Fruits juices: extraction, composition, quality and analysis, 1 edn. Academic Press, Inc
- Grand View Research (2018) Fruit and vegetable juice market size analysis report by product (fruit juices, fruit & vegetable blends, vegetable juices), by region and segment forecasts, 2018–2025. https://www.grandviewresearch.com/industry-analysis/fruit-vegetable-juice-market. Accessed 30 Oct 2018
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein D, Pot B, Sanders ME. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Karimi R, Azizi MH, Ghasemlou M, Vaziri M. Application of inulin in cheese as prebiotic, fat replacer and texturizer: a review. Carbohydr Polym. 2015;119:85–100. doi: 10.1016/j.carbpol.2014.11.029. [DOI] [PubMed] [Google Scholar]
- Martins EMF, Ramos AM, Vanzela ESL, Stringheta PC, de Oliveira Pinto CL, Martins JM. Products of vegetable origin: a new alternative for the consumption of probiotic bacteria. Food Res Int. 2013;51(2):764–770. [Google Scholar]
- Ministerio de Salud y Protección Social de Colombia (2013) Resolución 3929 de 2013. Colombia, p 29
- Munir MB, Hashim R, Chai YH, Marsh TL, Nor SAM. Dietary prebiotics and probiotics influence growth performance, nutrient digestibility and the expression of immune regulatory genes in snakehead (Channa striata) fingerlings. Aquaculture. 2016;460:59–68. [Google Scholar]
- Mylona K, Maragkoudakis P, Miko L, Bock AK, Wollgast J, Caldeira S, Ulberth F. Viewpoint: future of food safety and nutrition: seeking win-wins, coping with trade-offs. Food Policy. 2018;74:143–146. [Google Scholar]
- Nematollahi A, Sohrabvandi S, Mortazavian AM, Jazaeri S. Viability of probiotic bacteria and some chemical and sensory characteristics in cornelian cherry juice during cold storage. Electron J Biotechnol. 2016;21:49–53. [Google Scholar]
- Nualkaekul S, Charalampopoulos D. Survival of Lactobacillus plantarum in model solutions and fruit juices. Int J Food Microbiol. 2011;146(2):111–117. doi: 10.1016/j.ijfoodmicro.2011.01.040. [DOI] [PubMed] [Google Scholar]
- Nuñez Hinostroza R, Brumovsky LA. Evaluación sensorial de jugos de uva turbios y límpidos. Rev Cienc Tecnol. 2010;13:43. [Google Scholar]
- Pereira ALF, Maciel TC, Rodrigues S. Probiotic beverage from cashew apple juice fermented with Lactobacillus casei. Food Res Int. 2011;44(5):1276–1283. [Google Scholar]
- Perricone M, Corbo MR, Sinigaglia M, Speranza B, Bevilacqua A. Viability of Lactobacillus reuteri in fruit juices. J Funct Food. 2014;23(10):421–426. [Google Scholar]
- Perricone M, Bevilacqua A, Altieri C, Sinigaglia M, Corbo MR. Challenges for the production of probiotic fruit juices. Beverages. 2015;1:95–103. [Google Scholar]
- Ray R, El Sheikha A, Kumar S. Oriental fermented functional (probiotic) foods. In: Ray RC, Montet D, editors. Microorganisms and fermentation of traditional foods. Food biology series. Boca Raton: Science Publishers Inc.; 2014. pp. 283–311. [Google Scholar]
- Rosesler EB, Pangborn RM, Sidel JL, Stone H. Expanded statistical tables for estimating significance in paired—preference, paired–difference, duo–trio and triangle tests. J Food Sci Technol. 1978;43:940–947. [Google Scholar]
- Sheehan VM, Ross P, Fitzgerald GF. Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices. Innov Food Sci Emerg Technol. 2007;8(2):279–284. [Google Scholar]
- Shoaib M, Shehzad A, Omar M, Rakha A, Raza H, Sharif HR, Niazi S. Inulin: properties, health benefits and food applications. Carbohydr Polym. 2016;147:444–454. doi: 10.1016/j.carbpol.2016.04.020. [DOI] [PubMed] [Google Scholar]
- Shori AB. Influence of food matrix on the viability of probiotic bacteria: a review based on dairy and non-dairy beverages. Food Biosci. 2016;13:1–8. [Google Scholar]
- Singh RD, Banerjee J, Arora A. Prebiotic potential of oligosaccharides: a focus on xylan derived oligosaccharides. Bioact Carbohydr Diet Fiber. 2015;5(1):19–30. [Google Scholar]
- Stone H, Sidel J (1993) Sensory evaluation practices, 2nd edn. Academic Press, Inc
- Tymczyszyn E, Gerbino E, Illanes A, Gómez-Zavaglia A. Galacto-oligosaccharides as protective molecules in the preservation of Lactobacillus delbrueckii subsp. bulgaricus. Cryobiology. 2011;62(2):123–129. doi: 10.1016/j.cryobiol.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Valero-Cases E, Frutos MJ. Effect of inulin on the viability of L. plantarum during storage and in vitro digestion and on composition parameters of vegetable fermented juices. Plant Foods Hum Nutr. 2017;72:1–7. doi: 10.1007/s11130-017-0601-x. [DOI] [PubMed] [Google Scholar]
- Vandenplas Y, Huys G, Daube G. Probiotics: an update. J Pediatr (Rio J) 2015;91(1):6–21. doi: 10.1016/j.jped.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Yahia E (ed) (2018) Fruit and vegetable phytochemicals, 2nd edn. Wiley Blackwell
- Yonekura L, Sun H, Soukoulis C, Fisk I. Microencapsulation of Lactobacillus acidophilus NCIMB 701748 in matrices containing soluble fiber by spray drying: technological characterization, storage stability and survival after in vitro digestion. J Funct Foods. 2014;100:205–214. doi: 10.1016/j.jff.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KY, Woodams EE, Hang YD. Fermentation of beet juice by beneficial lactic acid bacteria. LWT Food Sci Technol. 2005;38(1):73–75. [Google Scholar]