Abstract
Fermented sausages have a long tradition originating from China. In this study, three starter microorganisms including Pediococcus pentosaceus (P), Staphylococcus xylosus (S), and a combination of P. pentosaceus and S. xylosus (P + S) were conducted for the manufacture of traditional Xiangxi (a city in China) fermented sausages. The physicochemical changes of the above three kinds of sausages during fermentation were studied and discussed, and also compared with these properties on the natural fermented sausage (N, i.e., control). The results revealed that five kinds of bacterial phases were existed at different fermentation stages in N, P, S and P + S fermented sausages, respectively. The microbiological data showed that an initial enterobacteria count of approximately 5.3 log CFU/g for all four batches of sausages. The enterobacteria count in the inoculated sausages of P and P + S groups decreased significantly to about 1 log CFU/g whereas group N and S had a count of about 3.3 log CFU/g after fermentation. In the early stages of fermentation, the pH rapidly decreased below 5.3. FAA and FFA were significantly increased in all groups and TBARS value in group P was higher than that of the other three groups. In conclusion, starter cultures can be used to improve the hygiene level of Xiangxi sausages without significant effects on pH, AW, and nitrite residue.
Keywords: Xiangxi sausage, Pedicoccus pentosaceus, Staphylococcus xylosus, Microorganism and physicochemical characteristics, Free amino acid, Free fatty acid
Introduction
In recent years, improvement of living standards request greater demand for new meat products that are high in nutrition and quality, and have unique flavors and health benefits. These requirements can be partially met by processing meat products through fermentation. Consumers in foreign countries are favorable for fermented meat products for their nutritious, delicious, flavors richness, safe, no refrigeration requirement, and ready-to-eat properties (Rantsiou and Cocolin 2008). In China, fermented meat products such as Jinhua ham, Cantonese-style sausages, Sichuan sausages and Hunan bacon have a long history and are received great attention around the word. However, the prepared methods used in the production of these traditional fermented meat products exhibited many technological challenges, such as unstable product quality, long fermentation time, and seasonal constraints, which seriously limited the development of these fermented meat products (Zhang and Wang 2010). Fermented meat products have become a hot investigation topic in foreign meat processed industry in the last decades, however, the production of fermented meat products in China is still in its primary stages and have not received great attention. Therefore, how to effectively combine traditional fermentation technology with modern fermentation technology is the key to producing high-quality fermented meat.
During the fermentation process, microorganisms play an important role on the product’s flavor, color, and texture properties (Rantsiou et al. 2005). For instance, microorganisms can break down protein into polypeptides and amino acids that are easily absorbed by the human body, and also degrade fat into short-chain fatty acids and esters that contribute to the food’s flavors (Gøtterup et al. 2008). In addition, meat products contain active prebiotics and probiotics that can improve the nutritional and health value of fermented meat products (Dawood and Koshio 2016; Jofré et al. 2015; Candogan et al. 2009). During the fermentation process, an environment with a low pH, AW, and oxygen level were created, which could inhibits the growth of pathogens and bacteria that cause spoilage, thus improving the stability and safety of the produced products (Muguerza and Gimeno 2004). Therefore, microorganisms acted as a crucial role on the improvement of the nutrition, safety, quality, and flavor properties of fermented meat products.
Xiangxi sausages is a kind of fermented meat product with local characteristics, which is known to be fragrant, crisp, and peppery. The special taste of Xiangxi sausages is mainly due to the use of local sourced ingredients, chilli, Sichuan pepper, and pepper, and cooking with wood smoke. However, the production of Xiangxi sausages can only be carried out in winter, and the production of Xiangxi sausages is mainly based on family manual workshops using natural fermentation methods due to the climatic restrictions. Therefore, the quality of the processed sausage product is usually unstable, such as high salt content and susceptible to microbial contamination, thus severely limited their scale production in food industry. In order to provide theoretical information for the production of safe, stable, and nutritious standardized Xiangxi sausages, in this study, pure starter cultures were inoculated into traditionally-made Xiangxi sausages and investigated their effect on the physicochemical and nutritional properties of the sausages. In addition, microorganisms changes of sausages during the fermentation process was also evaluated.
Materials and methods
Raw material
The raw meat was purchased from a supermarket in Changsha, Hunan. The meat was separated into four batches and stored at − 20 °C until they were used for the manufacture of sausages.
Starter culture preparation
Two starter cultures of P. pentosaceus and S. xylosus which separated from traditional Xiangxi fermented meat products were provided by Microbial Preservation Center of Chinese Academy Sciences. P. pentosaceus and S. xylosus were cultivated in the medium of MRA and MSA at 30 °C for 48 h, then the cells were harvested by centrifuged at 10,000g for 5 min and then resuspended in sterile water to obtain the concentration of 108 CFU/mL.
Xiangxi sausage preparation
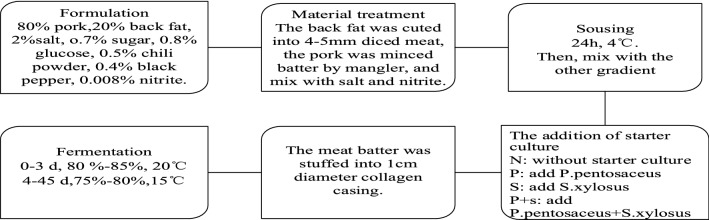
Pretreatment: the tendon and blood clot of the meat was removed and then pre-cooling at − 20 °C for half an hour to reduce the inner meat temperature. The basic traditional sausage formulation contained 80% lean pork meat, 20% pork backfat, 2% salt, 0.7% sugar, 0.8% glucose, 0.5% chili powder, 0.4% black pepper, 0.008% nitrite (the proportion of the ingredients was calculated in light of meat weight). The Xiangxi sausage was manufactured as follows: First of all, the back fat was cut into 1 × 1 × 1 cm cubes and the pork was minced by mangler, then completely mixed with salt and nitrite. After sousing at 4 °C for 24 h, the other ingredients were added into the mixture and soused. After that, the samples were carried out by the following four treatments: N (meat with no added starter culture); P (10 g P. pentosaceus liquid per 1 kg meat); S (10 g S. xylosus liquid per 1 kg meat); and P + S (10 g mixed bacteria liquid of P. pentosaceus and S. xylosus per 1 kg meat). All treatments were triplicated for further analysis.
For each of treatment, meat batter were stuffed in a natural porcine (30 mm diameter) by using a sausage machine to obtain each piece of 200 g weight and 20 cm length, and then tightly sealed for fermentation. Thereafter sausages were fermented for 3 days (20 °C, 80–85% RH), followed by 42 days of dry-curing (15 °C, 75–80% RH) in a controlled dry-cured chamber (Xinmiao Medical equipment, SPX-250B5-II, Shanghai).
The superficial antifungal activity, sensory, lipolytic and proteolytic characteristics of the processed sausages was analyzed at different fermentation times. Duration of the fermentation time was 45 days, and three sausages from each batch were randomly withdrawn at 0, 3, 7, 15, 25, 35 and 45 days then stored at − 80 °C for further analyses. The experiments were carried out in triplicates. The schematic representation of processing and fermentation of Xiangxi sausage are shown in Fig. 1.
Fig. 1.
The schematic representation of processing and fermentation of Xiangxi sausage
Microbial analysis
The sausage casing was removed in a sterile environment and 10 g of the sausage was cut out and placed in a flask containing 90 mL of sterilized physiological saline. The flask was then sealed and shaken for 40 min. Ten-fold serial dilutions were prepared with sterilized physiological saline according to the standard of GB/T 4789.35-2003 (China).
The total viable counts were enumerated using Plate Count Agar (PCA) and incubated at 30 °C for 48 h; the lactic acid bacteria were enumerated on the de Man, Rogosa, and Sharpe (MRS) Agar (pH 5.6) after being incubated at 37 °C for 48 h; staphylococci were enumerated on Mannitol Salt Agar (MSA) after being incubated at 37 °C for 48–72 h; enterobacteria was enumerated on Violet Red Bile Glucose (VRBG) Agar after being incubated at 37 °C for 24 h, and yeast was enumerated on Rose Bengal Agar after being incubated at 28 °C for 72 h. After incubation, the plates with 30–300 colonies were counted. The microbiological data were transformed into logarithms of the number of colony forming units (CFU/g).
pH, moisture, AW, nitrite and thiobarbituric acid reactive substances (TBARS) analysis
The pH of the sausages was measured using a digital pH meter (testo) equipped with a penetration probe. Moisture content was determined using the method of direct drying described by GB5009.3-2010 (China). Water activity was determined using Kang Wei plate diffusion method described by GB23490-2009. Nitrite was determined using the spectrophotometry method described by GB5009.33-2010 (China). The TBARS value was determined using the methods described by Olivares et al. (2010) with tricloroacetic acid as a solvent. The results were expressed as mg malonaldehyde (MDA) per 100 g sample.
Free amino acid analysis
A small amount of the sausage sample (1–5 g) without casing taken in a 50 mL conical flask was mixed with 40 mL of 0.01 mol/L HCl for 5 min. After extracted for 2 min in an ultrasonic bath and stand for 2 h in the dark at room temperature, the mixture was centrifuged at 4000 rpm for 10 min. 1 mL of supernatant was collected and mixed with 1 mL of 6–8% sulfosalicylic acid for 1 min. The mixture was then centrifuged at 15,000g for 15 min after it stand for 1 h in the dark. 500 μL of supernatant was then collected in a 5 mL plastic centrifuge tube. The supernatant was mixed with 250 μL of 1 mol/L triethylamine acetonitrile and 250 μL of 0.1 mol/L phenyl isothiocyanate acetonitrile at room temperature (25 °C). After the obtained mixture stand for 1 h, 2 mL of hexane was then added into the mixture and stand for 10 min. The substratum was filtered with a syringe filter (0.22 μm) and analyzed using an automatic amino acid analyzer HITACHI L-8900 (Hitachi Ltd., Japan) (GB/5009.124-2003). The total free amino acid was expressed in mg/kg.
Free fatty acid analysis
Gas chromatography and mass spectrometry were used to determine the free fatty acids. Sample (90 mg) was mixed with 2 mL of 5% HCl methanol solution and 3 mL of chloroform methanol solution (v/v, 1:1), 100 μL of methyleneoctanoate was used as an internal standard. The mixture was incubated in a water bath at 85 °C for 1 h, and then 1 mL of hexane was added into the mixture after cooling down to room temperature (25 °C). The mixture was shaken for 2 min and stand for 1 h at room temperature until the mixture separated into layers. 100 μL of supernatant was extracted and the volume was adjusted to 1 Ml with 900 μL of n-hexane. The solution was filtered through a 0.45 micron membrane and analyzed by a GC–MS (Trace 1310 ISQ). The results were expressed in FAA mg/kg.
Statistical analysis
The experiments were carried out in triplicates and the results were presented as means ± standard deviation (SD). All statistical analysis was performed using IBM SPSS Statistics 19.0 software (IBM, Chicago, IL, USA). After verification of normal distribution and constant variance of data, significant differences were determined by Analysis of Variance (ANOVA) with two factors: time (levels: days 0, (3), (7), 15, 25, 35 and 45) and treatments (levels: N (meat with no added starter culture); P (added P. pentosaceus to meat); S (added S. xylosus to meat); P + S (added mixed fermentation to meat)). A Duncan’s test was performed to compare the mean values for processing time at a significance level of P < 0.05.
Results and discussion
Microbial analysis
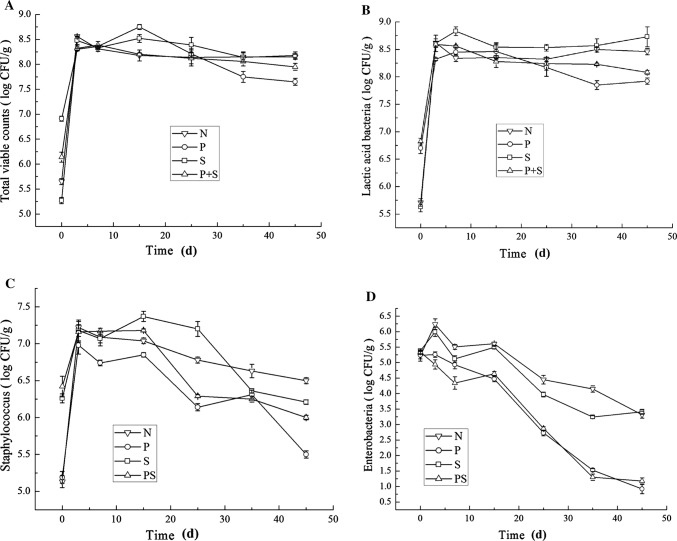
The traditional method of making fermented sausages is carried out by the fermentation of raw meat materials with microorganisms (e.g., lactic acid bacteria, Staphylococcus, and yeas) which existed in the environment (Kozačinski et al. 2008). A quantitative analysis of the microorganisms showed that the total count of bacteria, lactic acid bacteria, Staphylococcus, yeast, and enterobacteria during the fermentation process of the four batches of sausages were significantly different (P < 0.05) as shown in Fig. 2. The differences in the total count of lactic acid and Staphylococcus were attributed to the addition of starter cultures at the beginning of fermentation.
Fig. 2.
The changes of bacteria with different starters during the fermentation process. a Total viable counts, b lactic acid bacteria, ctaphylococcus, d enterobacteria
Figure 2a, b shows a similar trend of change between the total count of bacteria and lactic acid bacteria. The total count of lactic acid bacteria reached a high of 8.62 log CFU/g on day 3 in the N batch while the other three batches P, S, and P + S reached a high of 8.45 log CFU/g, 8.83 log CFU/g, and 8.59 log CFU/g, respectively, on day 7. During the fermentation period of 7–25 days, the total lactic acid bacteria count showed no significant change in the four batches. This result is consistent with the findings reported by Santiago et al. (2011) and Arief et al. (2014). At the later period of fermentation, the growth of lactic acid bacteria was inhibited and its count number began to decrease slowly due to the reduction of nutrients and water activity, as well as the increase of NaCl concentration. During the entire fermentation process, lactic acid bacteria played a dominant role among all the microorganisms which inhibited the growth of spoilage and pathogenic bacteria, thereby improving the stability and safety of the produced sausages. From the degree of change of lactic acid bacteria, lactic acid bacteria (raw material meat and environment lactic acid bacteria) in N and S groups grow more rapidly than those of P and P + S (mainly Pediococcus pentosaceus).
The changes of staphylococcus of the four sausages were similar to those of the lactic acid bacteria during the fermentation process (first increased, then stabilized, finally decreased), but the amount of staphylococcus was much lower than lactic acid bacteria (Fig. 2c). Staphylococcus in all four batches grew most rapidly during the first 3 days of fermentation, which reaching 7.12 log CFU/g for the N batch, 6.98 log CFU/g for the P batch, 7.18 log CFU/g for the S batch, and 7.16 log CFU/g for the P + S batch. However, the total staphylococcus count decreased during the middle stages of the fermentation process due to the rapid growth of lactic acid bacteria and the production of lactic acid (Gonzales-Barron et al. 2015). After fermentation, the staphylococcus count decreased to 6.5 log CFU/g for the N batch, 5.5 log CFU/g for the P batch, 6.12 log CFU/g for the S batch, and 6.0 log CFU/g for the P + S batch. In addition, it was found that the total staphylococcus count in the P batch was significantly lower (P < 0.005) than that in the other three batches. The phenomenon observed may be due to the initial lower competitiveness of staphylococcus in the P batch compared to lactic acid bacteria. In addition, staphylococci are acid-sensitive bacteria, which suggest that the lowering of pH from the lactic acid bacteria during the fermentation process lowered the competitiveness of staphylococci and led to its death (Ravyts et al. 2010).
The enterobacteria count in the four batches of sausages was 5.3 log CFU/g on day 0 (Fig. 2d). The magnitude of this value depends primarily on the hygienic quality of the raw meat and the processing conditions of the sausage. The enterobacteria found in the P and P + S batches showed a downward trend throughout the fermentation process, reaching 0.92 log CFU/g and 1.08 log CFU/g, respectively. On day 15, the enterobacteria count fell sharply (P < 0.001) while the N and S batches had a count of 3.37 log CFU/g at the end of the process (Casquete et al. 2011). Therefore, ii can infer that the bacteriocin produced by P. pentosaceus can effectively inhibit the growth of enterobacteria, which was reported by Lorenzo et al. (2014) and Simion et al. (2014). Both spoilage bacteria and pathogenic bacteria in fermented meat products are belong to enterobacteria that could produce bioamines to reduce the safety of the products, thereby enterobacteria are generally considered as harmful microorganisms. This study shows that addition of starter culture can inhibited the growth of the enterobacteria during the fermentation process, thus improving the safety of the product (Lorenzo et al. 2014; Simion et al. 2014).
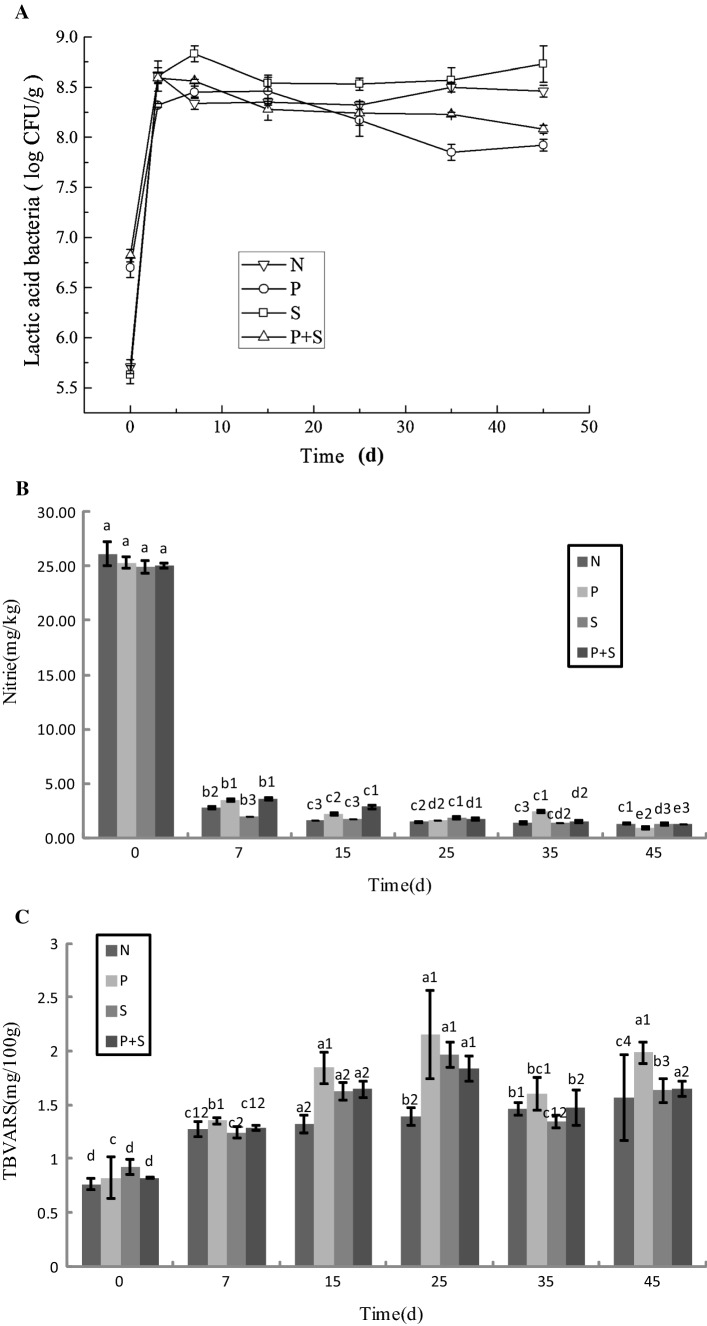
Yeast is an aerobic bacterium that can consume the oxygen remaining in the meat, and also produced proteases and lipases which contribute to the improvement of product’s flavor. Figure 3A shows that there was an increase (by about 2 log) followed by a slight decrease for yeast during the initial and middle stages of fermentation. After the process ended, the yeast count found in all four batches was slightly higher than the initial count. Compared with lactic acid bacteria and staphylococcus, there was a larger decrease in yeast in the later stages, and the descent also started at a later time. This indicates that the yeast has stronger resistance to stress, namely low water activity, acid resistance and anaerobic resistance.
Fig. 3.
The changes of substances with different starters during the fermentation process. A Yeast, B nitrite and C TBARS. a, b, c, d represent in-group comparisons, P < 0.005.1, 2, 3 represent intergroup comparisons, P < 0.005
pH, moisture, AW analysis
Table 1 shows that the pH level of all four batches of sausages decreased significantly (P < 0.005) in the first week of fermentation process. The pH value decreased to 5.20, 5.16, 5.01 and 5.08 for N, S, P and P + S batches, respectively, after 1 week of fermentation. This finding was consistent with the investigation of Zhao et al. (2011). This result obtained was mainly due to the consumption of sugar by lactic acid bacteria, which contributed to the production of organic acids (mainly lactic acid). At the middle stages of fermentation, it was found that the pH level increased as shown in Table 1. These results may correspond to the production of some alkaline peptides, amino acids, and other substances by the proteolysis degradation during fermentation. The microorganisms used in the production of fermented sausages are generally considered to possess a developed peptidase system, which could hydrolyze the meat proteins to smaller peptides and amino acids. After fermentation, the pH values of N, P, S and P + S batches were 5.47, 5.33, 5.44, and 5.49, respectively. The lower pH level at the initial stages of the fermentation process is essential as it inhibits the growth of undesirable microorganisms, thus ensuring the safety of the product. The pH levels in the four batches of sausages increased earlier and the final pH values were higher (5.4 as compared to an average of 5) when compared with previous studies conducted by Wang et al. (2013) and Olivares et al. (2010). This is related to the addition of starter cultures and the fermentation temperatures, which serves as the basis for the study of low acid fermented sausages.
Table 1.
The changes of pH, AW and moisture content with different starter cultures during the fermentation process
| Batch | Time (days) | Sign | |||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 15 | 25 | 35 | 45 | ||
| pH | |||||||
| N | 5.86 ± 0.01a | 5.20 ± 0.06c1 | 5.34 ± 0.08bc1 | 5.43 ± 0.06bc1 | 5.39 ± 0.0bc1 | 5.47 ± 0.06b1 | *** |
| P | 5.87 ± 0.01a | 5.08 ± 0.01de2 | 5.01 ± 0.04e3 | 5.14 ± 0.04d2 | 5.37 ± 0.04c1 | 5.33 ± 0.028b1 | *** |
| S | 5.86 ± 0.04a | 5.16 ± 0.04c1 | 5.14 ± 0.07c2 | 5.16 ± 0.04c2 | 5.38 ± 0.05b1 | 5.44 ± 0.03b1 | *** |
| P + S | 5.86 ± 0.01a | 5.08 ± 0.01bc2 | 5.14 ± 0.05bc2 | 5.21 ± 0.01bc12 | 5.38 ± 0.06c1 | 5.49 ± 0.03abc1 | *** |
| Sign | ns | ** | ** | * | ns | ns | n.s |
| Water activity | |||||||
| N | 0.95 ± 0.003a1 | 0.93 ± 0004a2 | 0.87 ± 0.018b2 | 0.76 ± 0.019c12 | 0.73 ± 0.035d12 | 0.72 ± 0.006d12 | *** |
| P | 0.95 ± 0.006a1 | 0.93 ± 0.004a2 | 0.94 ± 0.001a1 | 0.78 ± 0.015b12 | 0.75 ± 0.017c1 | 0.71 ± 0.013d1 | *** |
| S | 0.95 ± 0.006a1 | 0.93 ± 0.002a2 | 0.92 ± 0.048a1 | 0.79 ± 0.040b1 | 0.70 ± 0.073c12 | 0.77 ± 0.024c23 | *** |
| P + S | 0.96 ± 0.013a1 | 0.94 ± 0.012a1 | 0.92 ± 0.531a12 | 0.72 ± 0.044b2 | 0.71 ± 0.043c2 | 0.71 ± 0.011c3 | *** |
| Sign | ns | ns | * | * | * | ** | |
| Water content | |||||||
| N | 51.21% ± 0.004a2 | 49.46% ± 0.024b1 | 38.05% ± 0.007c2 | 31.34% ± 0.005d2 | 28.12% ± 0.011e2 | 27.34% ± 0.004e12 | *** |
| P | 56.26% ± 0.004a1 | 46.25% ± 0.008b1 | 38.43% ± 0.022b1 | 32.03% ± 0.012c2 | 24.51% ± 0.003e3 | 25.38% ± 0.002d1 | *** |
| S | 57.32% ± 0.006a1 | 48.09% ± 0.036b1 | 39.46% ± 0.007c1 | 36.64% ± 0.011d1 | 30.48% ± 0.012e1 | 26.74% ± 0.004f12 | *** |
| P + S | 55.35% ± 0.031a1 | 48.41% ± 0.083b1 | 35.61% ± 0.005c2 | 31.18% ± 0.006d2 | 27.34% ± 0.004d2 | 25.75% ± 0.016d2 | *** |
| Sign | ns | ns | ** | ns | *** | ns | |
N, meat with no added starter culture; P, added P. pentosaceus to meat; S, added S. xylosus to meat; P + S, added mixed fermentation to meat
a–dValues with different numbers within the same treatment are significantly different (P < 0.05); 1–3values with different letters within the same day of fermentation are significantly different (P < 0.05); significant levels: ns: no significance;*P < 0.05; **P < 0.01; ***P < 0.001
After fermentation, the average water content and AW were reduced from 55 to 25% and from 0.95 to 0.73, respectively, for all the four batches of sausages (P < 0.001). The sausages could be considered as dry fermented sausages based on the loss of 30% water. Table 1 shows that the rate of water loss was very fast during the beginning and middle stages of fermentation. The intestinal filling contains a lot of free fluid, and since the pH value had neared the isoelectric point (pI = 5.2), the water-holding capacity of the muscle proteins weakens, thus accelerating the rate of water loss. During the later fermentation stages, the rate of water loss slowed down as the surface of the sausages became slightly hard and it took more time for the water content from the inner parts to be drawn to the surface.
In the early stage of fermentation, the lost water is mainly derived from the water added during the production process, so there were no significant changes in the AW (P > 0.05). However, AW changed significantly during the middle stages of fermentation, which had a great influence on the growth of microorganisms. AW is a key indicator of the deterioration of fermented meat products and shelf life of products. A low AW is detrimental to microbial growth, which is conducive to the safety of products. Thus, the low average AW value of 0.73 in the four batches of sausages indicates that the products are safe for consumption and have a good shelf life.
Nitrite and TBARS analysis
50 mg/kg of nitrite was used in this experiment for the production of sausages. However, there were only 25 mg/kg of it on day 0 of the fermentation process as the reactions between nitrite and the meats during the curing process resulted in its reduction. Therefore, the nitrite’s antibacterial properties and reacts with the myoglobin to form the cured meat color is able to reduce the formation of nitrosamines during the curing process (nitrites are decomposed into NO2 which can react with secondary amine to form nitrosamines). Figure 3B shows that the nitrite content fell drastically after fermentation of 1 week and then remained quite stable at the later period of fermentation. At the end of fermentation, the nitrite content found in all four batches was relatively low, which is only 1.2 mg/kg. This finding is similar to the research conducted by Wang et al. (2013), whereas it is contrary to the result obtained by Essid and Hassouna (2013) who noted that the fermentation increased the content of nitrite. According to the results of observed in this experiment, it could be concluded that nitrite played an important role on the development of color and inhibition of bacteria during the initial stages of the fermentation process, which effectively enhances the safety of the product.
The entire meat process (from the slaughter of pigs into trimming, mashing and pickling) usually exposed to air that could cause meat oxidation, therefore led to a TBARS value of 0.84 mg/kg at the beginning of fermentation (Fig. 3C). There was a rapid growth of lactic acid bacteria during the early stages of the fermentation process, which led to the consumption of oxygen and contributed to the production of large amount of lactic acid. Accordingly, a hypoxic and hypoacidic environment was created during the fermentation process, which decreased the oxidation of saturated fatty acids, and inhibited the decomposition of aldehydes, acids and other compounds, leading to a slow increment of TBARS value. At the early to middle stages of the process, the fat oxidation rate and TBARS value increased due to the gradual recovery of the pH level and the reduction of sodium nitrite. During the later stages of fermentation, the changes in the pH level and microbial distribution in the sausages may have led to chemical reactions in the aldehydes that were produced during fat oxidation, resulting in a fall in TBARS value. As the process progressed, fat continued to be oxidized and the TBARS value rose again (Alicia et al. 2011). The N batch had the lowest TBARS value among all four batches of sausages probably due to the highest yeast count, which could inhibit the production of lipid peroxides (Flores et al. 2015). The TBARS value decreased suddenly during the 35 days of fermentation, which might be attributed to the changes of environmental factors such as microbial count, pH level, and AW.
Free amino acid analysis
Proteins are degraded into small molecules such as peptides and amino acids by exogenous enzymes produced by microorganisms and endogenous enzymes. Table 3 shows that glutamate and phenylalanine are the main amino acids found in the sausage. After the fermentation process, the amino acids composition of the meat product has been changed, glutamic acid, phenylalanine, alanine, and lysine become the main amino acids. The amino acid content between day 0 and day 45 was ranked in descending order: Glu > Phe > Ala > Gly > Leu > Lys, Glu > Phe > Ala > Lys > Leu > Ser > Gly.
Table 3.
The content of the different FFA of the four sample batches at days 0, 15, 25 and 45 (mean values ± standard deviation)
| FFA | Sausage batches | Fermentation period (days) | ||||
|---|---|---|---|---|---|---|
| 0 | 15 | 25 | 45 | Sign. | ||
| C10.0 | N | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.02 | n.s |
| P | 0.02 ± 0.01b | 0.01 ± 0.01b | 0.02 ± 0.01b | 0.04 ± 0.02a | * | |
| S | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.02 | n.s | |
| P + S | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.03 ± 0.01 | n.s | |
| Sign. | ns | ns | ns | ns | ||
| C14.0 | N | 0.13 ± 0.03c | 0.18 ± 0.01bc | 0.21 ± 0.01b | 0.35 ± 0.03a | * |
| P | 0.14 ± 0.03d | 0.20 ± 0.01c | 0.32 ± 0.02b | 0.45 ± 0.01a | *** | |
| S | 0.15 ± 0.03d | 0.20 ± 0.01c | 0.23 ± 0.01b | 0.36 ± 0.02a | *** | |
| P + S | 0.14 ± 0.04d | 0.17 ± 0.02c | 0.22 ± 0.01b | 0.33 ± 0.01a | *** | |
| Sign. | ns | ns | ns | ns | ||
| C16.0 | N | 16.64 ± 0.19d2 | 26.29 ± 0.25c2 | 25.89 ± 0.19b3 | 30.50 ± 0.19a3 | *** |
| P | 16.66 ± 0.45d2 | 27.59 ± 0.34c1 | 29.95 ± 0.03b1 | 35.35 ± 0.46a1 | *** | |
| S | 16.70 ± 0.34d1 | 20.08 ± 0.52c4 | 26.86 ± 0.53b2 | 32.62 ± 0.63a2 | *** | |
| P + S | 16.65 ± 0.82d2 | 20.28 ± 0.61c3 | 22.99 ± 0.83b4 | 30.30 ± 0.33a4 | *** | |
| Sign. | ns | *** | *** | *** | ||
| C16.1 | N | 1.16 ± 0.07d12 | 1.42 ± 0.01c2 | 2.52 ± 0.03a2 | 2.44 ± 0.02b3 | *** |
| P | 1.18 ± 0.17d1 | 1.42 ± 0.01c2 | 2.76 ± 0.02a1 | 2.47 ± 0.01b2 | *** | |
| S | 1.12 ± 0.18d3 | 1.45 ± 0.01c1 | 2.53 ± 0.01a2 | 2.44 ± 0.02b3 | *** | |
| P + S | 1.15 ± 0.43d2 | 1.41 ± 0.02c2 | 2.48 ± 0.01b3 | 2.54 ± 0.03a1 | *** | |
| Sign. | * | ns | * | * | ||
| C17.0 | N | 0.04 ± 0.01b | 0.05 ± 0.01b | 0.05 ± 0.01b | 0.15 ± 0.04a | ** |
| P | 0.04 ± 0.01c | 0.05 ± 0.03bc | 0.07 ± 0.02b | 0.16 ± 0.06a | *** | |
| S | 0.04 ± 0.03b | 0.04 ± 0.01b | 0.05 ± 0.02b | 0.14 ± 0.02a | ** | |
| P + S | 0.04 ± 0.02b | 0.04 ± 0.01b | 0.05 ± 0.01b | 0.12 ± 0.03a | ** | |
| Sign. | ns | ns | ** | ns | ||
| C17.1 | N | 0.02 ± 0.01c | 0.03 ± 0.01bc2 | 0.05 ± 0.01ab2 | 0.07 ± 0.01a | *** |
| P | 0.02 ± 0.02d | 0.04 ± 0.01c2 | 0.07 ± 0.01b1 | 0.09 ± 0.03a | *** | |
| S | 0.02 ± 0.01c | 0.06 ± 0.02b1 | 0.05 ± 0.02ab2 | 0.07 ± 0.03a | *** | |
| P + S | 0.02 ± 0.01b | 0.02 ± 0.01b3 | 0.03 ± 0.02b3 | 0.07 ± 0.02a | *** | |
| Sign. | ns | *** | *** | ns | ||
| C18.0 | N | 5.26 ± 0.15d4 | 5.96 ± 0.14c1 | 6.58 ± 0.01b2 | 6.80 ± 0.50a3 | *** |
| P | 5.36 ± 0.41d21 | 6.81 ± 0.77c2 | 6.72 ± 0.10b1 | 7.24 ± 0.06a1 | *** | |
| S | 5.39 ± 0.27d1 | 6.73 ± 0.07c3 | 6.42 ± 0.21b3 | 6.53 ± 0.06a2 | *** | |
| P + S | 5.33 ± 0.09d23 | 5.71 ± 0.10c4 | 6.00 ± 0.02b4 | 6.67 ± 0.04a4 | *** | |
| Sign. | *** | *** | *** | *** | ||
| C18.1N9C | N | 9.54 ± 0.70d3 | 11.36 ± 0.27c3 | 15.52 ± 0.02b3 | 25.54 ± 0.40a4 | *** |
| P | 10.37 ± 0.32d2 | 11.4 ± 0.59c2 | 18.35 ± 0.12b1 | 28.05 ± 0.56a3 | *** | |
| S | 10.52 ± 0.56d2 | 12.11 ± 0.71c1 | 17.49 ± 0.17b2 | 29.42 ± 0.22a2 | *** | |
| P + S | 10.84 ± 0.44d1 | 11.09 ± 0.79c4 | 13.06 ± 0.68b4 | 31.58 ± 0.40a1 | *** | |
| Sign. | *** | *** | *** | *** | ||
| C18.1N9T | N | 0.00 ± 0.00c | 0.55 ± 0.03a3 | 0.27 ± 0.30b4 | 0.00 ± 0.00c | *** |
| P | 0.00 ± 0.00c | 0.82 ± 0.22b1 | 1.37 ± 0.58a1 | 0.00 ± 0.00c | *** | |
| S | 0.00 ± 0.00c | 0.71 ± 0.06b2 | 0.96 ± 0.06a2 | 0.00 ± 0.00c | *** | |
| P + S | 0.00 ± 0.00c | 0.54 ± 0.04b3 | 0.58 ± 0.04a3 | 0.00 ± 0.00c | *** | |
| Sign. | ns | *** | *** | ns | ||
| C18.2N6C | N | 4.94 ± 0.21d2 | 13.25 ± 0.90c2 | 14.30 ± 0.41b4 | 27.59 ± 0.18a3 | *** |
| P | 4.88 ± 0.16d3 | 13.67 ± 0.80c1 | 18.69 ± 0.47b3 | 28.42 ± 0.12a2 | *** | |
| S | 4.82 ± 0.06d4 | 11.46 ± 0.33c3 | 19.94 ± 0.11b2 | 25.59 ± 0.69a4 | *** | |
| P + S | 4.99 ± 0.07d1 | 10.71 ± 0.60c4 | 21.11 ± 0.53b1 | 29.37 ± 2.10a1 | *** | |
| Sign. | *** | *** | *** | *** | ||
| C20.0 | N | 0.12 ± 0.01b | 0.22 ± 0.01b | 0.29 ± 0.01b | 0.37 ± 0.02a2 | *** |
| P | 0.16 ± 0.01c | 0.23 ± 0.01bc | 0.24 ± 0.10b | 0.39 ± 0.02a1 | *** | |
| S | 0.14 ± 0.01b | 0.19 ± 0.01b | 0.23 ± 0.01b | 0.37 ± 0.02a2 | *** | |
| P + S | 012 ± 0.01b | 0.22 ± 0.01b | 0.22 ± 0.01b | 0.35 ± 0.01a3 | *** | |
| Sign. | ns | ns | ns | *** | ||
| C20.1 | N | 0.07 ± 0.02d1 | 0.17 ± 0.02c | 0.27 ± 0.01b2 | 0.50 ± 0.10a3 | *** |
| P | 0.06 ± 0.02d1 | 0.18 ± 0.04c | 0.37 ± 0.07b1 | 0.48 ± 0.05a4 | *** | |
| S | 0.04 ± 0.01d2 | 0.19 ± 0.06c | 0.25 ± 0.09b3 | 0.67 ± 0.06a1 | *** | |
| P + S | 0.08 ± 0.03d1 | 0.17 ± 0.07c | 0.20 ± 0.09b4 | 0.65 ± 0.03a2 | *** | |
| Sign. | * | ns | *** | |||
| C20.2 | N | 0.08 ± 0.01d | 0.09 ± 0.01c2 | 0.10 ± 0.03b3 | 0.33 ± 0.03a2 | *** |
| P | 0.09 ± 0.01d | 0.13 ± 0.04c1 | 0.17 ± 0.05b1 | 0.35 ± 0.02a1 | *** | |
| S | 0.07 ± 0.04d | 0.10 ± 0.06c2 | 0.13 ± 0.04b2 | 0.32 ± 0.02a2 | *** | |
| P + S | 0.08 ± 0.03d | 0.09 ± 0.01c2 | 0.08 ± 0.03b3 | 0.22 ± 0.01a3 | *** | |
| sign. | ns | * | *** | *** | ||
| C20.3N3 | N | 0.01 ± 0.01b | 0.00 ± 0.00c2 | 0.00c2 | 0.03 ± 0.01a | *** |
| P | 0.01 ± 0.01b | 0.00 ± 0.00c2 | 0.01 ± 0.01b1 | 0.03 ± 0.01a | *** | |
| S | 0.01 ± 0.01b | 0.00 ± 0.00c2 | 0.00c2 | 0.04 ± 0.03a | *** | |
| P + S | 0.01 ± 0.01b | 0.01 ± 0.01b1 | 0.01 ± 0.02b1 | 0.03 ± 0.01a | * | |
| Sign. | ns | *** | *** | ns | ||
| C20.3N6 | N | 0.01 ± 0.01c | 0.03 ± 0.01b | 0.01 ± 0.02c | 0.06 ± 0.01a1 | ** |
| P | 0.01 ± 0.01b | 0.02 ± 0.01b | 0.02 ± 0.01b | 0.07 ± 0.01a1 | ** | |
| S | 0.01 ± 0.01b | 0.02 ± 0.01b | 0.02 ± 0.01b | 0.07 ± 0.01a1 | * | |
| P + S | 0.01 ± 0.01b | 0.01 ± 0.01b | 0.01 ± 0.01b | 0.04 ± 0.01a2 | * | |
| Sign. | ns | ns | ns | * | ||
| C20.4N6 | N | 0.06 ± 0.02b1 | 0.00 ± 0.00d2 | 0.01 ± 0.02c1 | 0.13 ± 0.02a | *** |
| P | 0.06 ± 0.01b1 | 0.01 ± 0.01c1 | 0.00 ± 0.00d2 | 0.11 ± 0.04a | *** | |
| S | 0.07 ± 0.02b1 | 0.00 ± 0.00c2 | 0.00 ± 0.00c2 | 0.12 ± 0.01a | *** | |
| P + S | 0.05 ± 0.02b12 | 0.00 ± 0.00c2 | 0.00 ± 0.00c2 | 0.13 ± 0.04a | *** | |
| Sign. | * | * | * | ns | ||
| C22.6N3 | N | 0.02 ± 0.01c | 0.01 ± 0.01c | 0.04 ± 0.01b | 0.06 ± 0.01a1 | *** |
| P | 0.02 ± 0.01b | 0.02 ± 0.01b | 0.02 ± 0.01b | 0.06 ± 0.02a | * | |
| S | 0.02 ± 0.01b | 0.03 ± 0.01b | 0.03 ± 0.01b | 0.07 ± 0.02a | * | |
| P + S | 0.02 ± 0.01b | 0.02 ± 0.01b | 0.03 ± 0.01b | 0.07 ± 0.02a | * | |
| Sign. | ns | ns | ns | ns | ||
| MUFA | N | 10.79 ± 0.06d | 13.54 ± 0.45c2 | 18.63 ± 0.36b2 | 28.55 ± 0.04a3 | *** |
| P | 11.636 ± 0.03d | 13.86 ± 0.28c2 | 22.92 ± 0.06b1 | 31.09 ± 0.07a23 | *** | |
| S | 11.70 ± 0.05d | 14.52 ± 0.17c1 | 21.03 ± 0.15b12 | 32.60 ± 0.16a2 | *** | |
| P + S | 12.09 ± 0.01d | 12.14 ± 0.11c2 | 16.35 ± 0.14b3 | 34.86 ± 0.64a1 | *** | |
| Sign. | ns | * | *** | **** | ||
| PUFA | N | 5.04 ± 0.03c | 13.38 ± 0.32bc1 | 14.47 ± 0.32b4 | 27.87 ± 0.44a12 | *** |
| P | 5.07 ± 0.09d | 13.84 ± 0.76c1 | 18.91 ± 0.24b3 | 29.04 ± 0.02a12 | *** | |
| S | 4.93 ± 0.15d | 11.61 ± 0.11c2 | 19.99 ± 0.72b12 | 29.64 ± 0.55a3 | *** | |
| P + S | 5.16 ± 0.13d | 10.83 ± 0.33c2 | 21.25 ± 0.02b1 | 29.86 ± 0.47a1 | *** | |
| Sign. | ns | ** | *** | *** | ||
| SFA | N | 22.11 ± 0.19c | 32.53 ± 0.43b1 | 32.57 ± 0.02b2 | 37.91 ± 0.30a2 | *** |
| P | 22.25 ± 0.56d | 34.70 ± 0.27c1 | 37.13 ± 0.17b1 | 38.35. ± 0.23a1 | *** | |
| S | 22.32 ± 0.33d | 27.10 ± 0.09c2 | 33.61 ± 0.58b2 | 39.76 ± 0.01a1 | *** | |
| P + S | 22.20 ± 0.25c | 26.24 ± 0.51bc2 | 29.29 ± 0.29b3 | 39.52 ± 0.57a2 | *** | |
| Sign. | ns | ** | *** | ** | ||
| UFA/SFA | N | 0.72 ± 0.06 cd | 0.83 ± 0.05c12 | 1.02 ± 0.03ab3 | 1.41 ± 0.03a2 | *** |
| P | 0.75 ± 0.07c | 0.80 ± 0.12b12 | 1.13 ± 0.03a23 | 1.39 ± 0.02a3 | *** | |
| S | 0.75 ± 0.06d | 0.96 ± 0.04c1 | 1.27 ± 0.01b2 | 1.57 ± 0.02a2 | *** | |
| P + S | 0.78 ± 0.02d | 0.88 ± 0.42c1 | 1.28 ± 0.01b1 | 1.70 ± 0.03a1 | *** | |
| Sign. | ns | * | ** | *** | ||
N, meat with no added starter culture; P, added P. pentosaceus to meat; S, added S. xylosus to meat; P + S, added mixed fermentation to meat
MUFA monounsaturated fatty acids, PUFA polyunsaturated fatty acids, SFA saturated fatty acids, UFA unsaturated fatty acids
a–dValues with different numbers within the same treatment are significantly different (P < 0.05); 1–3values with different letters within the same day of fermentation are significantly different (P < 0.05); significant levels: ns: no significance;*P < 0.05; **P < 0.01; ***P < 0.001
Table 2 also reveals that the total amino acids and free amino acids in the four batches increased significantly during fermentation. The total amino acids increased by 10.77 g/kg for the N batch, 12.33 g/kg for the P batch, 11.45 g/kg for the S batch, and 11.71 g/kg for the P + S batch. It is noted in Table 3 that the free amino acids increased by 4.72 g/kg, 5.48/kg, 4.96 g/kg, and 5.04 g/kg for N, P, S and P + S batches, respectively. This enhancement was mainly attributed to the hydrolysis of myosin and myofibrillar proteins by endogenous and microbial enzymes during the fermentation process, which significantly improved the product’s nutrition. After the fermentation, the total free amino acids in batches P and P + S were significantly higher than those in batches N and S, this result was similar with Nie et al. (2014), mainly due to the enhancement of enzyme activity at that pH level (Hierro et al. 1999). The observed result in this experiment is different from with previous studies, which mainly attributed to the different metabolic activities of the microorganisms at different fermentation temperature and fermentation period, thereby affecting the composition and content of the amino acids.
Table 2.
The content of FAA with different starters during the fermentation process
| FAA | 0 (mg/kg) | N-45 (mg/kg) | P-45 (mg/kg) | S-45 (mg/kg) | P + S-45 (mg/kg) |
|---|---|---|---|---|---|
| Asp | 12 | 220 | 290 | 220 | 250 |
| Glu | 1620 | 5900 | 6300 | 6200 | 6300 |
| Ser | 40 | 370 | 400 | 380 | 380 |
| Gly | 58 | 320 | 380 | 330 | 350 |
| Thr | 27 | 250 | 330 | 270 | 280 |
| His | 16 | 150 | 220 | 180 | 200 |
| Ala | 170 | 780 | 890 | 810 | 810 |
| Arg | 42 | 63 | 74 | 70 | 74 |
| Tyr | 36 | 110 | 120 | 130 | 100 |
| Val | 33 | 280 | 370 | 280 | 310 |
| Met | 22 | 140 | 190 | 140 | 160 |
| Phe | 1300 | 4500 | 4700 | 4700 | 4600 |
| lle | 17 | 200 | 250 | 200 | 220 |
| Leu | 48 | 390 | 510 | 380 | 420 |
| Lys | 45 | 450 | 620 | 480 | 540 |
| Pro | 37 | 140 | 190 | 180 | 220 |
| Total | 3503 | 14,263 | 15,834 | 14,950 | 15,214 |
| EAA | 1492 | 6210 | 6970 | 6450 | 6530 |
N, meat with no added starter culture; P, added P. pentosaceus to meat; S, added S. xylosus to meat; P + S, added mixed fermentation to meat
FAA free amino acids, EAA essential amino acid
Free fatty acid analysis
Table 3 shows that the free fatty acids increased significantly during the end of the fermentation process. The main free fatty acids are: C16:0, C18:2, and C18:1. There were significant differences (P < 0.05) between the saturated fatty acids and unsaturated fatty acids in all four batches of sausages, with the P batch having a significantly larger difference than the other batches. C18:2 and C18:1, two essential free fatty acids for humans, were detected both in the P and P + S batches.
As depicts in Table 3, after the fermentation process, the monounsaturated fatty acids (MUFA) increased by 2.7 times, polyunsaturated fatty acids (PUFA) increased by 5.8 times, and saturated fatty acids (SFA) increased by 1.6 times, and the ratio of unsaturated fatty acids to saturated fatty acids rose from 0.92 to 1.5. The MUFA, PUFA and MUFA/PUFA in all four batches displayed significant differences (P < 0.05) during the later stages of the fermentation process, with the P + S batch displaying a larger difference (P < 0.05) than the other batches. The results demonstrated that endogenous enzymes played a vital role during lipolysis in the initial to middle stages of the fermentation while the exogenous enzymes produced by microorganisms played an important role in the later stages. The relative dissociation rate of fatty acid was: PUFA > MUFA > SFA, which corresponded to results obtained by Juan et al. (1999).
Conclusion
In this study, the pH value, Aw, and water content of all the prepared four kinds of Xiangxi sausages (i.e., N, P, S and P + S) were significantly decreased during the fermentation process. The rapid reduction of pH and low Aw achieved during fermentation could contribute to the safety and stability of the sausages. In addition, low pH value can improve the hardness and quality of the sausages. Furthermore, this study also showed that addition of starter culture was able to improve the quality of fermented sausages when compared to naturally fermented sausages, and the combination of two starter cultures could further improve its quality. The P. pentosaceus has the ability to produce acid and bacteriocin, which could effectively inhibit the growth of enterobacteria. The nitrite content found in all four batches decreased significantly during the fermentation process and the residual amount (1.3 mg/kg) was under the national standards. Besides, the TBARS value, free amino acids and free fat content were significantly increased during fermentation, and this effect is more pronounced after P + S was inoculated into the sausages Therefore, this study demonstrated that the flavor, safety, and nutrition of Xiangxi fermented sausages could be enhanced by the inoculation of starter culture, and mixed fermentation technology could further enhance the quality of the fermented sausage product. According to the above results, it could be concluded that P + S sausages can be considered to be safer, more nutritious, and longer preservation shelf-life than the other fermented sausages.
Acknowledgements
Authors are grateful for financial supports from the Department of Agriculture of Hunan Province, China, and Core Research Program 1515, Hunan Agricultural University.
Abbreviations
- N
Natural fermentation
- P
Pediococcus pentosaceus
- S
Staphylococcus xylosus
- P + S
Mixed fermentation
- FAA
Free amino acid
- FFA
Free fatty acid
- TBARS
Thiobarbituric acid reactive substances
- MUFA
Mono-unsaturated fatty acids
- PUFA
Polyunsaturated fatty acids
Contributor Information
Chuan-hua Wang, Phone: +86-13874943101, Email: 870421475@qq.com.
Yuan-Liang Wang, Phone: +86-73184617007, Email: wangyuanliang@hunau.edu.cn.
References
- Alicia O, José Luis N, Mónica F. Effect of fat content on aroma generation during processing of dry fermented sausages. Meat Sci. 2011;87(3):264–273. doi: 10.1016/j.meatsci.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Arief II, Wulandari Z, Aditia EL, et al. Physicochemical and microbiological properties of fermented lamb sausages using probiotic Lactobacillus plantarum, IIA-2C12 as starter culture. Procedia Environ Sci. 2014;20:352–356. [Google Scholar]
- Candogan K, Wardlaw FB, Acton JC. Effect of starter culture on proteolytic changes during processing of fermented beef sausages. Food Chem. 2009;116(3):731–737. [Google Scholar]
- Casquete R, Benito MJ, Martín A, et al. Role of an autochthonous starter culture and the protease EPg222 on the sensory and safety properties of a traditional Iberian dry-fermented sausage “salchichón”. Food Microbiol. 2011;28(8):1432–1440. doi: 10.1016/j.fm.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Dawood MAO, Koshio S. Recent advances in the role of probiotics and prebiotics in carp aquaculture: a review. Aquaculture. 2016;454(28):243–251. [Google Scholar]
- Essid I, Hassouna M. Effect of inoculation of selected Staphylococcus xylosus, and Lactobacillus plantarum, strains on biochemical, microbiological and textural characteristics of a Tunisian dry fermented sausage. Food Control. 2013;32(2):707–714. [Google Scholar]
- Flores M, Corral S, Cano-García L, et al. Yeast strains as potential aroma enhancers in dry fermented sausages. Int J Food Microbiol. 2015;212:16–24. doi: 10.1016/j.ijfoodmicro.2015.02.028. [DOI] [PubMed] [Google Scholar]
- Gonzales-Barron U, Cadavez V, Pereira AP, et al. Relating physicochemical and microbiological safety indicators during processing of linguiça, a Portuguese traditional dry-fermented sausage. Food Res Int. 2015;78:50–61. doi: 10.1016/j.foodres.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Gøtterup J, Olsen K, Knøchel S, et al. Colour formation in fermented sausages by meat-associated staphylococci with different nitrite- and nitrate-reductase activities. Meat Sci. 2008;78(4):492–501. doi: 10.1016/j.meatsci.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Hierro E, de la Hoz LHL, Ordóñez JA. Contribution of the microbial and meat endogenous enzymes to the free amino acid and amine contents of dry fermented sausages. J Agric Food Chem. 1999;47(3):1156–1161. doi: 10.1021/jf980834p. [DOI] [PubMed] [Google Scholar]
- Jofré A, Aymerich T, Garriga M. Probiotic fermented sausages: myth or reality? Procedia Food Sci. 2015;5:133–136. [Google Scholar]
- Juan AO, Eva MH, Jose MB, et al. Changes in the components of dry-fermented sausages during ripening. Crit Rev Food Sci Nutr. 1999;39(4):329–367. doi: 10.1080/10408699991279204. [DOI] [PubMed] [Google Scholar]
- Kozačinski L, Drosinos E, Čaklovica F, Cocolin L, Gasparikreichardt J, Vesković S. Investigation of microbial association of traditionally fermented sausages. Food Technol Biotechnol. 2008;46(1):93–106. [Google Scholar]
- Lorenzo JM, Gómez M, Fonseca S. Effect of commercial starter cultures on physicochemical characteristics, microbial counts and free fatty acid composition of dry-cured foal sausage. Food Control. 2014;46:382–389. [Google Scholar]
- Muguerza E, Gimeno O, Ansorena D, et al. New formulations for healthier dry fermented sausages: a review. Trends Food Sci Technol. 2004;15(9):452–457. [Google Scholar]
- Nie X, Lin S, Zhang Q. Proteolytic characterisation in grass carp sausage inoculated with Lactobacillus plantarum, and Pediococcus pentosaceus. Food Chem. 2014;145(7):840–844. doi: 10.1016/j.foodchem.2013.08.096. [DOI] [PubMed] [Google Scholar]
- Olivares A, Navarro JL, Salvador A, Flores M. Sensory acceptability of slow fermented sausages based on fat content and ripening time. Meat Sci. 2010;86(2):251–257. doi: 10.1016/j.meatsci.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Rantsiou K, Cocolin L. Fermented meat products. Food Microbiol Food Saf. 2008;27(94):91–118. [Google Scholar]
- Rantsiou K, Urso R, Iacumin L, Cantoni C, Cattaneo P, Comi G, Cocolin L. Culture-dependent and -independent methods to investigate the microbial ecology of Italian fermented sausages. Appl Environ Microbiol. 2005;71(4):1977–1986. doi: 10.1128/AEM.71.4.1977-1986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravyts F, Steen L, Goemaere O, et al. The application of staphylococci with flavour-generating potential is affected by acidification in fermented dry sausages. Food Microbiol. 2010;27(7):945–954. doi: 10.1016/j.fm.2010.05.030. [DOI] [PubMed] [Google Scholar]
- Santiago RM, Alberto M, María José B, et al. Application of Lactobacillus fermentum HL57 and Pediococcus acidilactici SP979 as potential probiotics in the manufacture of traditional Iberian dry-fermented sausages. Food Microbiol. 2011;28(5):839–847. doi: 10.1016/j.fm.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Simion AMC, Vizireanu C, Alexe P, et al. Effect of the use of selected starter cultures on some quality, safety and sensorial properties of Dacia sausage, a traditional Romanian dry-sausage variety. Food Control. 2014;35(1):123–131. [Google Scholar]
- Wang XH, Ren HY, Liu DY, et al. Effects of inoculating Lactobacillus sakei, starter cultures on the microbiological quality and nitrite depletion of Chinese fermented sausages. Food Control. 2013;32(2):591–596. [Google Scholar]
- Zhang Y, Wang C. Research on fermented meat products of domestic progress. Jiangxi Food Ind. 2010;2:46–48. [Google Scholar]
- Zhao L, Jin Y, Ma C, et al. Physico-chemical characteristics and free fatty acid composition of dry fermented mutton sausages as affected by the use of various combinations of starter cultures and spices. Meat Sci. 2011;88(4):761–766. doi: 10.1016/j.meatsci.2011.03.010. [DOI] [PubMed] [Google Scholar]