Abstract
Information on the variability available in lignan and fatty acid content in the oilseed crop of Sesamum indicum has been limited. This article presents and discusses the composition, quantity, and variability available for the two traits in the sesame germplasm that are grown in diverse agro climatic regions of India. HPLC and GC analysis of sesame seeds harvested over a period of three crop seasons revealed a considerable amount of variability in lignan and fatty acids. The antioxidant lignans sesamol, sesamin and sesamolin were observed to be in the range of 0.16–3.24, 2.10–5.98 and 1.52–3.76 mg/g of seed, respectively. Similarly oleic and linoleic acids, respectively, have ranged from 34.71 to 45.61% and 38.49 to 49.60%. The black sesame seeds were found rich in sesamin, sesamolin, total lignan content and oleic acid and are thus identified nutritionally and pharmaceutically more important than white and brown seeds. Pearson statistics showed a strong correlation between the components within a particular trait and also some correlation was found between the traits. The study revealed promising cultivars for use in sesame breeding aimed at improving lignan and fatty acid contents, and can be thus directly used in human foods, nutrition, health and welfare.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-03564-x) contains supplementary material, which is available to authorized users.
Keywords: Sesamum indicum L., Seed coat colour, Lignans, Fatty acids, HPLC, GC, Pearson correlation
Introduction
Sesamum indicum L. commonly known as Gingelley, is the world’s most ancient oilseed crop (Bedigian 2004). Sesame is characterized by its high oil content containing up to 60% oil, 25% protein, 13.5% carbohydrates and about 1% as minerals (Dar et al. 2015). The bio chemical composition of sesame seed differs with variety, origin, colour and size. The crop is the major source of medicinally important lignans namely, sesamin and sesamolin that are known for their anti-proliferative, antihypertensive and neuroprotective properties (Cooney et al. 2001; Cheng et al. 2006; Yokota et al. 2007; Visavadiya and Narasimhacharya 2008; Dar and Arumugam 2013). Sesame oil is the most stable of all edible oils, due to the presence of the antioxidant lignans thus inciting a proposition to incorporate sesame lignans in other edible oils for improving their shelf life (Suja et al. 2004). Oleic acid and linoleic acid are the two major fatty acids of sesame oil constituting about 85% of the total fatty acids.
Despite being ancient and with high pharmacological significance and nutritional values, the crop remains neglected. There are hardly a few reports that describe genetic diversity of the crop for fatty acid composition and lignan content (Hemalatha and Ghafoorunissa 2004; Uzun et al. 2008; Rangkadilok et al. 2010; Mondal et al. 2010; Bhunia et al. 2015). Since lignans and unsaturated fatty acids are responsible for several pharmacological properties, information on the available variability for these compounds is essential, especially for identifying superior sesame genotypes for use as food ingredients. Such genotypes would also be highly useful in breeding programmes aimed at enhancing the content of the two metabolites (Uzun et al. 2008; Williamson et al. 2008; Rangkadilok et al. 2010). Understanding the variation in the content of such physiologically active constituents is desirable for their incorporation in functional foods. Therefore, the aim of this study was to determine the extent of variability for lignans and fatty acid contents in the sesame germplasm grown widely in India, and to find out the correlation between the two traits.
Materials and methods
Plant material
Forty-three different accessions of S. indicum used in this study were obtained from National Bureau of Plant Genetic Resources, New Delhi, is presented in Table 1. The germplasm was maintained by raising it once every year in the experimental garden of our Department at School of Life Sciences, Pondicherry University, India.
Table 1.
Variation in lignan content in seeds of different accessions of Sesamum indicum germplasm studied over a period of 3 years
| Variety | Seed colour | Lignan content (mg/g) | |||
|---|---|---|---|---|---|
| Sesamol | Sesamin | Sesamolin | Total | ||
| AKT64 | White | 0.62 | 3.99 | 2.39 | 7.00 |
| Amrit | Brown | 0.32 | 4.56 | 1.85 | 6.73 |
| Chandana | Brown | 0.46 | 2.10 | 1.58 | 4.14 |
| DS1 | White | 0.63 | 4.39 | 2.41 | 7.43 |
| E8 | White | 1.08 | 5.75 | 2.68 | 9.51 |
| FFAT0822 | White | 3.24 | 4.19 | 2.64 | 10.07 |
| GT1 | White | 2.27 | 3.32 | 2.46 | 8.05 |
| GT2 | White | 1.82 | 2.98 | 2.10 | 6.90 |
| JLT1 | Black | 0.74 | 3.33 | 2.91 | 6.98 |
| JLT7 | White | 0.81 | 5.33 | 2.57 | 8.71 |
| JLT26 | White | 0.98 | 4.10 | 1.72 | 6.80 |
| JT7 | White | 0.48 | 3.11 | 2.81 | 6.40 |
| JTS8 | White | 0.85 | 2.82 | 2.06 | 5.73 |
| Kallika | Brown | 0.23 | 3.93 | 2.95 | 7.11 |
| Krishna | Black | 0.42 | 4.49 | 3.76 | 8.67 |
| N8 | Brown | 0.17 | 4.36 | 2.18 | 6.71 |
| N32 | White | 0.68 | 3.51 | 2.07 | 6.26 |
| Nirmala | Brown | 0.63 | 3.44 | 1.74 | 5.81 |
| Phuletil | White | 1.21 | 5.51 | 2.85 | 9.57 |
| Prachi | Black | 0.43 | 4.97 | 2.95 | 8.35 |
| Praghti | White | 1.43 | 3.46 | 2.82 | 7.71 |
| Rajeswari | White | 0.35 | 4.99 | 3.47 | 8.81 |
| RT103 | White | 0.49 | 2.93 | 3.06 | 6.48 |
| RT125 | White | 0.52 | 4.38 | 3.52 | 8.42 |
| RT127 | White | 0.83 | 3.39 | 3.12 | 7.34 |
| SVPR1 | White | 0.63 | 5.86 | 3.37 | 9.86 |
| T12 | White | 1.50 | 4.62 | 2.93 | 9.05 |
| T13 | White | 2.30 | 3.26 | 2.31 | 7.87 |
| T78 | White | 1.69 | 3.32 | 1.96 | 6.97 |
| Tarun | White | 1.80 | 2.25 | 1.69 | 5.74 |
| TC25 | White | 1.02 | 4.57 | 2.78 | 8.37 |
| TC289 | White | 0.87 | 3.10 | 1.77 | 5.74 |
| TKG22 | White | 0.80 | 3.33 | 2.44 | 6.57 |
| TKG55 | White | 0.35 | 3.31 | 2.80 | 6.46 |
| TMV3 | Black | 0.30 | 4.89 | 3.08 | 8.27 |
| TMV4 | Brown | 0.25 | 4.97 | 2.90 | 8.12 |
| TMV5 | Brown | 0.24 | 5.73 | 3.43 | 9.40 |
| TMV6 | Brown | 0.28 | 4.87 | 3.24 | 8.39 |
| Uma | Brown | 0.16 | 3.91 | 1.52 | 5.59 |
| Vinayak | Brown | 0.47 | 4.00 | 3.28 | 7.75 |
| VRI1 | Brown | 0.29 | 5.98 | 3.31 | 9.58 |
| XLM19 | Brown | 0.22 | 3.92 | 3.02 | 7.16 |
| YLM17 | Brown | 0.46 | 3.90 | 2.75 | 7.11 |
| Mean | – | 0.82 | 4.07 | 2.63 | 7.53 |
| SD | – | 0.67 | 0.97 | 0.59 | 1.35 |
| CV | – | 81.67 | 23.76 | 22.43 | 17.89 |
| Minimum | – | 0.16 | 2.10 | 1.52 | 4.14 |
| Maximum | – | 3.24 | 5.98 | 3.76 | 10.07 |
Reagents and chemicals
Solvents used for extraction of lignans and fatty acyl methyl esters (FAME’s) were of analytical grade and HPLC grade, procured from Merck and Himedia, India. Reference standard of sesamin (Cat. No. S9314), sesamol (Cat. No. S8518), and naringenin (the internal standard—Cat. No. N5893) were procured from Sigma, USA. Pure sesamolin was isolated in-house from sesame oil, authenticated by NMR, and used as reference standard for quantification of sesamolin in the germplasm.
Lignan analysis
Extraction of lignans from seeds
Isolation of lignans from seeds of different sesame varieties was performed by following methodology described in Wang et al. (2012). 100 mg of sesame seed was homogenized in 5 ml of 80% ethanol using a pestle and mortar. The whole content was transferred into 30-ml Oakridge tube and centrifuged at 8000 rpm for 10 min at room temperature. The supernatant was transferred to a fresh tube and the residue was subjected to one more round of extraction. Ethanol was evaporated by rotary evaporation. To the residue, 1 ml of 80% methanol was added and filtered through 0.45 μm nylon membrane. The sample thus prepared was used for HPLC analysis.
Preparation of lignan standards and calculation of response factor (Rf)
A 0.0001 M stock solution of each of naringenin, sesamol, sesamin and sesamolin standards were prepared in HPLC grade methanol and filtered through 0.45 μm nylon membrane. Initially the lignan standards were analysed individually by HPLC to determine their specific RT (retention time). The response factor (Rf) of each of the compound, which is required for their quantification in the methanol extract, was determined by analysing a mixture of known concentration of the reference standards along with naringenin as internal standard by HPLC (Kupiec 2004). Calculation of Rf and estimation of lignans in the methanol extract of seeds were carried out by following Dar et al. (2015). Each sample was analysed thrice to ensure reproducibility.
Instrumentation and chromatographic conditions for HPLC of lignans
A Shimadzu-make HPLC (model LCATVP) equipped with a reverse phase C18 column (250 mm × 4.6 mm i.d) and UV detector (model SPD-10AVP) was used and the analysis was performed at 24 °C and 55% humidity. The mobile phase was isocratic and consisted of methanol and water (70:30), run at a flow rate of 0.7 ml/min and the peaks were detected at 290 nm.
Fatty acid analysis
Preparation of fatty acid methyl esters
Fatty acid methyl esters for GC analysis were prepared following the procedure described in Thies (1971). About 500 mg of dry clean seeds of each accession was made into a powder in a pestle and mortar and 200 mg of the powder was transferred into 12 × 75 mm size polystyrene tubes. About 1 ml of 0.5 M sodium methylate was then added and mixed using a vortex before it was left for incubation at room temperature. After 20 min, 100 μl of 5% aqueous NaHSO4 was added and mixed properly. Isooctane (300 μl) was added and left undisturbed for 20 min, thereby, allowing the diffusion of fatty acid methyl esters into the upper clear organic phase. The upper clear phase was taken out gently with the help of sterile syringe and transferred to 200 μl GC vial. The analysis was performed in a Perkin Elmer GC by following the procedure of Velasco et al. (1997).
Gas chromatograph conditions for fatty acid analysis
Perkin Elmer gas chromatograph (Model Autosystem XLGC, USA) equipped with a split liner (split ratio 15:1) injector and flame ionization detector (FID) was used. Analytical separation was achieved using capillary column (30 m × 0.25 mm i.d) with 0.25 μm film thickness. The detector and injector temperatures were maintained at 280 °C and 250 °C, respectively. The oven temperature was increased from 180 to 196 °C at the rate of 4 °C/min and kept at 196 °C for 1.5 min. Temperature was further ramped from 196 to 202 °C at the rate of 4 °C/min and kept at 202 °C for 1 min. Oven temperature was again increased from 202 to 230 °C at the rate of 6 °C/min and kept at 230 °C for 1.3 min. Nitrogen was used as carrier gas, whereas hydrogen and zero air were supplied to FID to maintain 280 °C during the analysis. Carrier flow rate of 2 ml/min, hydrogen flow rate of 40 ml/min and zero air flow rate of 400 ml/min were maintained during the analysis. A sample volume of 2 μl was injected for quantitation of different fatty acid in a sample. The FAME’s peaks in samples were identified by comparison with retention time of standard methyl esters (oil reference standard supplied by Sigma Aldrich) under identical GC conditions. The quantitative determination was performed by calculating the peak areas using Turbochrome Software. The peak areas were expressed as percentage of total fatty acids in a sample. Each sample was analysed thrice to ensure reproducibility.
Calculation of fatty acid desaturation ratios and oxidizing potential of the oil
Total saturated and unsaturated fatty acid content was directly read from the GC chromatogram as percentages of respective fatty acids. The GC data was used to compute oleic desaturation ratio (ODR), linoleic desaturation ratio (LDR) and oxidazibility potential (COX) of the oil by following Pleines and Friedt (1988) and Fatemi and Hammond (1980). The equations used were as follows:
SFA = (%Myristic acid + %Palmitic acid + %Stearic acid + %Arachidic acid)
MUFA = (%Oleic acid + %Gadoleic acid)
PUFA = (%Linoleic acid + %Linolenic acid)
Statistical data analysis
For statistical analysis, Microsoft Excel 2007 and SPSS statistical package version 16.0.2 were used. Pearson correlation coefficient (p < 0.01, p < 0.05) was calculated by using SPSS.
Results and discussion
Lignan analysis
HPLC of reference and internal standards of lignans
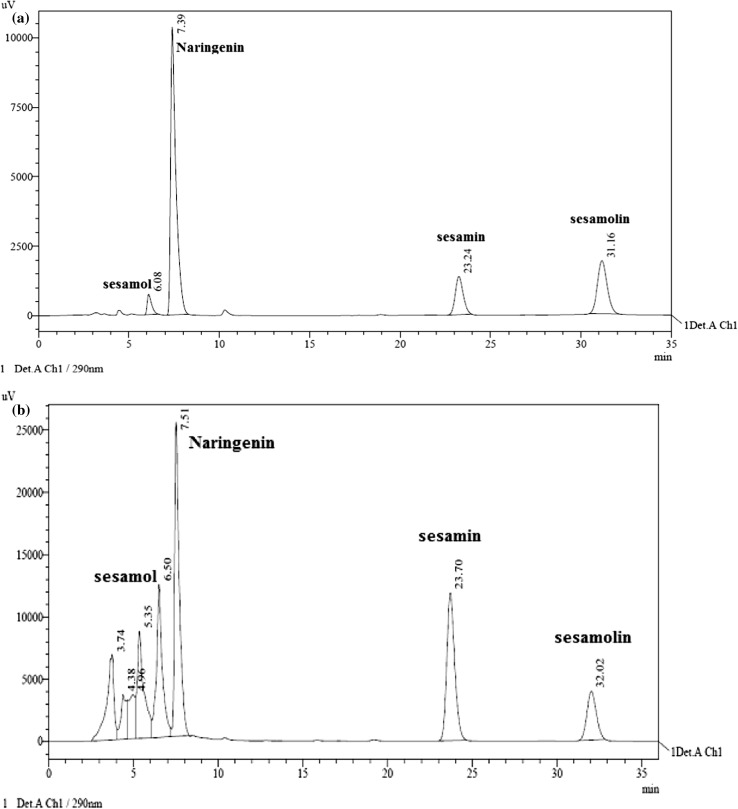
Independent HPLC of the reference standards of sesamin, sesamolin and sesamol revealed their retention time (RT) in the order of 23.7, 32.0 and 6.5 min, respectively. Similarly, for the internal standard naringenin, the RT was 7.5 min. This result was reproduced when a mixture of the four standards was run in the HPLC for the calculation of Rf (Fig. 1a). Response factor for sesamin, seamolin and sesamol with respect to the naringenin was found to be 0.11, 0.09 and 0.08, respectively, which were subsequently used for the quantification of the lignans in the germplasm.
Fig. 1.
HPLC of lignans of Sesamum indicum L. a A picture of detector response to HPLC of a mixture of reference standards sesamol, naringenin (internal standard), sesamin and sesamolin showing peaks at their corresponding retention times. b HPLC profile of ethanol extract of seeds of S. indicum var. T78 showing prominent peaks corresponding to major sesame lignans and the internal standard shown in a above indicating their presence in the extract
Quantification of major lignans in seeds
HPLC of ethanol extracts of sesame seeds under study revealed that sesamin and sesamolin are the major lignans present in sesame (Fig. 1b). In addition, there were up to eight other distinct peaks observed and one of them was identified as sesamol. Using TLC, it was reported earlier that there are about 15 different lignans present in sesame (Kamal-Eldin et al. 1994). Computation of lignan contents in the crude extracts of the accessions harvested over three consecutive crop seasons is presented in Table 1. An analysis of the table shows that there is considerable variation in lignan contents among the accessions tested indicating presence of divergence for the trait in the germplasm. Sesamol content on an average ranged from 0.16 to 3.24 mg/g of seed with minimum and maximum contents, respectively, in Uma and FFAT0822. Sesamin was in the range of 2.10–5.98 mg/g of seed with minimum and maximum contents, respectively, in Chandana and VRI1. Sesamolin came next to sesamin with an amount ranging from 1.52 to 3.76 mg/g seed with minimum content in Uma and maximum in Krishna. The coefficient of variation (CV) for total lignan content was 17.89%. Even though FFAT0822 ranked first in terms of total lignan content and considering sesamol as degradation product of sesamin, VRI1 remains the candidate of choice for highest sesamin content. One way ANOVA for lignan content revealed that sesamin content remained unchanged across the years but a significant seasonal variation was observed in case of the other two lignans (data not shown). Differences in secondary metabolite content among accessions with years may be attributed to micro environmental variation with respect to daily sunshine, temperature, rainfall and humidity as it has been reported in crops like soybean and also in sesame (Were et al. 2006; Cho et al. 2013; Kim et al. 2014).
While the total lignan content (for the Indian germplasm) reported here is higher than those reported for germplasm from Thailand (Rangkadilok et al. 2010), it agrees with those reported for certain exotic germplasm (Moazzami et al. 2007; Williamson et al. 2008; Bhunia et al. 2015). Our results also agree with an earlier study that showed sesamin followed by sesamolin as the major antioxidant lignans in sesame seeds (Moazzami et al. 2007; Bhunia et al. 2015). The covariance (CV) for sesamin and sesamolin were 23.76% and 22.43% respectively. Though sesamol showed highest variability, the mean sesamol content was the least among the three lignans. The difference in our observation on total lignan content from the recent report on Indian sesame could be due to exclusion of the variety VRI1 in their study (Bhunia et al. 2015). Moazzami and Kamal-Eldin (2006) reported sesamin in the range of 0.07–7.12 and sesamolin in the range of 0.21–2.9 mg/g in the 65 different sesame accessions maintained by Sesaco Corporation, USA. In European accessions, sesamin and sesamolin ranged from 1.67 to 8.0 mg and 0.5 to 2.8 mg, respectively, per gram of seed (Moazzami et al. 2007).
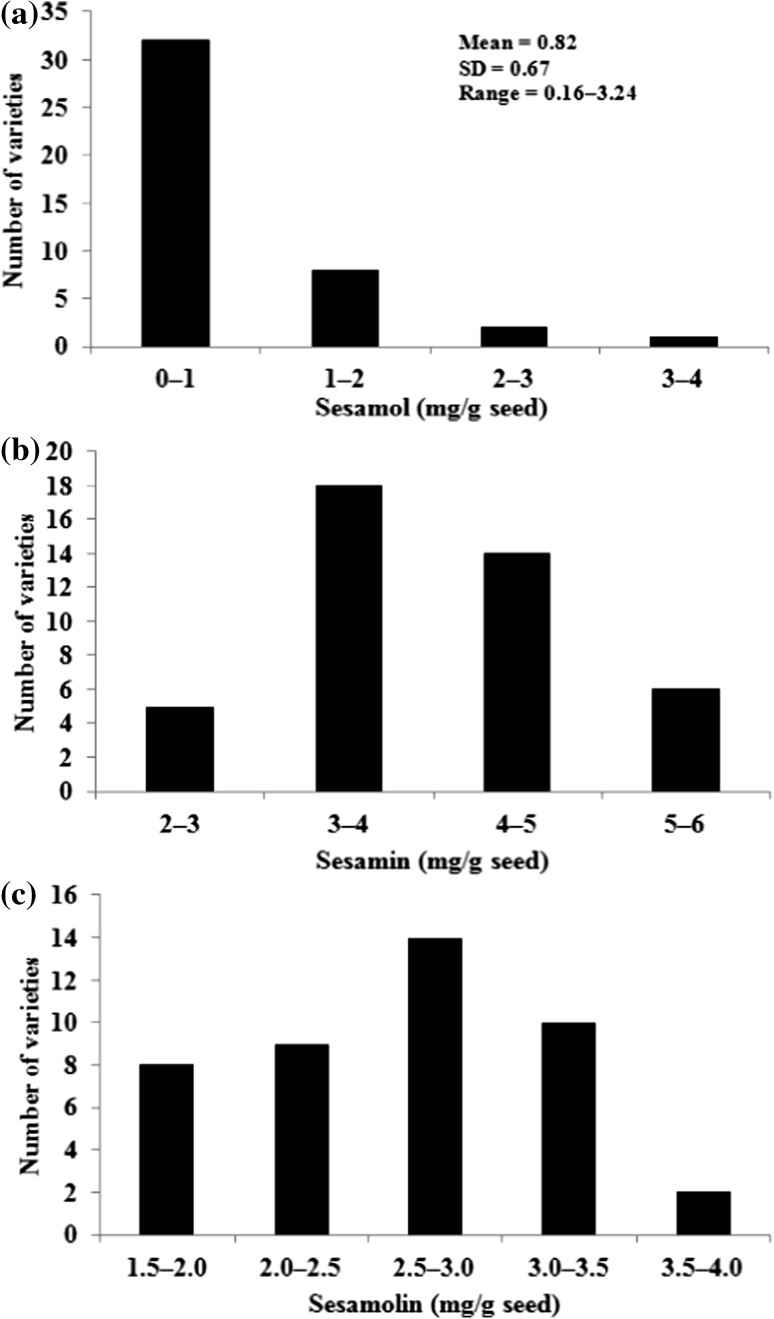
A comparison of seed color with respect to mean lignan content revealed that black seeded varieties contain highest sesamin, sesamolin and total lignan content, while white seeded varieties contain high sesamol content (Table S1). Similar observation was reported by Shi et al. (2017), where black sesame seeds were found rich in these types of lignans. But the results were contradictory with the report on Korean sesame in which white seeds were shown to have higher lignan content (Kim et al. 2014). The CV for total lignan content was found highest for white seeds followed by brown and black seed varieties (Table S1). The black Indian sesame with its higher lignan content could be considered nutrition rich and thus may be recommended for incorporation in human health foods and as supplements in nutraceuticals. A distribution plot of population size verses sesamol content showed skewness towards left side with majority of the accessions falling in the class range 0–1 mg/g of seed (Fig. 2a). In case of sesamin, the plot turned out to be Gaussian type with accessions having 3–4 mg/g of seed constituting the highest frequency class. The four frequency classes recognised here gives an impression that there could be multiple genes controlling this trait (Fig. 2b). Similar is the case for sesamolin where the population resolved into five major classes (Fig. 2c).
Fig. 2.
Frequency distribution of the accessions of Sesamum indicum L. for the seed lignan content. a sesamol, b sesamin and c sesamolin
Fatty acid analysis
Fatty acid profiling by gas chromatography
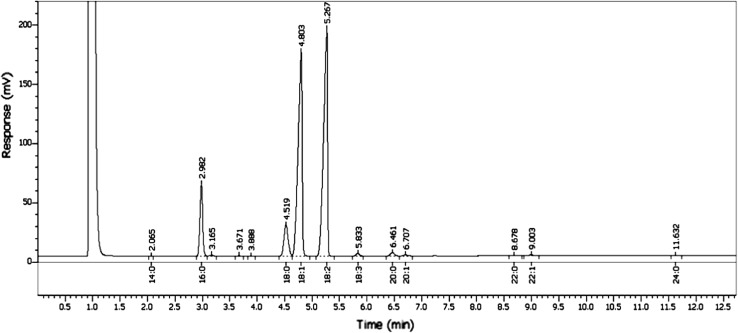
A picture of the chromatograph showing fatty acid profile of sesame seeds of variety Chandana by GC is presented in Fig. 3. It shows two major peaks for the unsaturated fatty acids, oleate (18:1) and linoleate (18:2). In addition, there are two distinct but smaller peaks representing saturated palmitate (16:0) and stearate (18:0). Mean percent fraction of such fatty acids in different accessions of S. indicum studied over a period of 3 years is presented in Table 2. Analysis of the data given in the table clearly shows that oleate and linoleate are the main fatty acid constituents of sesame seed, together constituting about 84% of the total fatty acids. Palmitate and stearate are the abundant saturated fatty acids constituting about 15% of total fatty acids. Linoleneate (18:3), arachidate (20:0) and gadoleate (20:1) were the other prominent FAs but were found in trace amounts in the germplasm. Palmitate ranged from 8.94 to 11.02% with minimum in Prachi and maximum in T12. Stearate ranged from 4.05 to 5.89% with lowest in AKT64 and highest in Prachi. The percentage of oleate was found minimum (34.71%) in DS1 but maximum (45.61%) in Prachi. However, linoleate was least (38.49%) in Prachi but highest (49.60%) in DS1. Unlike lignans, that showed considerable variation among the accessions, the fatty acid fractions did not change much and remained almost stable across the years. The results were consistent with the earlier reports on exotic and other germplasm (Were et al. 2006), but were contradictory with the report of Bhunia et al. (2015) where T12, DS1 showed a different percentages for FA content. The difference may be attributed to environmental or soil conditions in which the crops were raised. Yermanos et al. (1972) in an earlier report indicated that the sesame is characterised by 39.6% oleate, 46% linoleate, 9.5% palmitate and 4.4% stearate. Similarly Were et al. (2006) observed linoleate as the major component of fatty acids in East African sesame accessions. Similar results were also reported in Turkish germplasm as well (Uzun et al. 2008). From the studies outlined above, including the present study, it is evident that the linoleate is the major component of the total fatty acids in the sesame seeds. Mondal et al. (2010) and Bhunia et al. (2015) however found oleate to be the major fatty acid in certain Indian varieties of sesame. As far as variability is concerned, a maximum variability of 4% could be observed for both oleate and linoleate, indicating a possibility of marginal improvement that can be achieved for this trait by breeding.
Fig. 3.
GC of fatty acid methyl esters (FAME) prepared from seeds of Sesamum indicum L. The picture presents detector response to GC of the preparation from seeds of the sesame variety Chandana showing peaks corresponding to 12 different fractions of fatty acids (FA). Details of the prominent FA fraction are explained in the text
Table 2.
Mean data of fatty acid attributes estimated over a period of 3 years by GC in the germplasm of Sesamum indicum L.
| Variety name | Saturated fatty acid (%) | Unsaturated fatty acid (%) | SFA (%) | MUFA (%) | PUFA (%) | ODR | LDR | COX | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Palmitate | Stearate | Arachidate | Oleate | Linoleate | Linolinate | Gadoleate | |||||||
| AKT64 | 10.50 | 4.05 | 0.34 | 39.93 | 44.56 | 0.33 | 0.30 | 14.893 | 40.236 | 44.890 | 0.529 | 0.007 | 5.061 |
| Amrit | 10.08 | 5.19 | 0.70 | 39.21 | 44.45 | 0.38 | 0.09 | 15.966 | 39.299 | 44.823 | 0.533 | 0.008 | 5.052 |
| Chandana | 9.63 | 5.55 | 0.52 | 38.86 | 44.46 | 0.44 | 0.36 | 15.692 | 39.220 | 44.893 | 0.536 | 0.010 | 5.062 |
| DS1 | 9.58 | 4.86 | 0.42 | 34.71 | 49.60 | 0.35 | 0.33 | 14.862 | 35.033 | 49.951 | 0.590 | 0.007 | 5.532 |
| E8 | 9.55 | 5.06 | 0.43 | 39.31 | 44.81 | 0.34 | 0.37 | 15.042 | 39.677 | 45.142 | 0.535 | 0.007 | 5.081 |
| FFAT0822 | 10.39 | 4.25 | 0.50 | 41.83 | 42.35 | 0.39 | 0.19 | 15.144 | 42.021 | 42.744 | 0.505 | 0.009 | 4.865 |
| GT1 | 9.73 | 4.72 | 0.42 | 41.78 | 42.49 | 0.34 | 0.35 | 14.874 | 42.133 | 42.833 | 0.506 | 0.008 | 4.868 |
| GT2 | 9.81 | 4.61 | 0.53 | 40.54 | 44.32 | 0.43 | 0.23 | 14.952 | 40.776 | 44.749 | 0.525 | 0.010 | 5.063 |
| JLT1 | 10.81 | 4.73 | 0.48 | 40.48 | 42.93 | 0.31 | 0.29 | 16.016 | 40.769 | 43.232 | 0.516 | 0.007 | 4.892 |
| JLT7 | 10.43 | 4.61 | 0.50 | 39.88 | 43.97 | 0.39 | 0.19 | 15.536 | 40.073 | 44.363 | 0.527 | 0.009 | 5.013 |
| JLT26 | 10.62 | 4.50 | 0.39 | 41.71 | 42.50 | 0.36 | 0.34 | 15.516 | 42.052 | 42.863 | 0.507 | 0.008 | 4.873 |
| JT7 | 10.26 | 4.68 | 0.41 | 41.74 | 42.09 | 0.40 | 0.33 | 15.348 | 42.069 | 42.493 | 0.504 | 0.009 | 4.839 |
| JTS8 | 10.26 | 4.50 | 0.41 | 42.11 | 42.48 | 0.37 | 0.21 | 15.180 | 42.318 | 42.850 | 0.504 | 0.009 | 4.876 |
| Kallika | 9.42 | 4.84 | 0.40 | 40.51 | 44.36 | 0.35 | 0.24 | 14.663 | 40.743 | 44.704 | 0.525 | 0.008 | 5.049 |
| Krishna | 10.07 | 4.79 | 0.52 | 41.55 | 42.13 | 0.39 | 0.40 | 15.381 | 41.941 | 42.523 | 0.506 | 0.009 | 4.840 |
| N8 | 10.24 | 4.19 | 0.51 | 36.46 | 48.10 | 0.46 | 0.22 | 14.950 | 36.683 | 48.553 | 0.571 | 0.009 | 5.417 |
| N32 | 10.09 | 4.71 | 0.42 | 41.68 | 43.06 | 0.22 | 0.07 | 15.220 | 41.746 | 43.279 | 0.509 | 0.005 | 4.899 |
| Nirmala | 10.36 | 4.88 | 0.73 | 38.46 | 45.00 | 0.36 | 0.06 | 15.972 | 38.524 | 45.364 | 0.541 | 0.008 | 5.098 |
| Phuletil | 10.55 | 4.92 | 0.39 | 41.57 | 42.19 | 0.34 | 0.24 | 15.863 | 41.810 | 42.527 | 0.506 | 0.008 | 4.834 |
| Prachi | 8.94 | 5.89 | 0.91 | 45.61 | 38.49 | 0.41 | 0.08 | 15.733 | 45.688 | 38.893 | 0.460 | 0.010 | 4.508 |
| Praghti | 10.65 | 4.43 | 0.39 | 42.14 | 41.93 | 0.32 | 0.14 | 15.468 | 42.288 | 42.248 | 0.501 | 0.008 | 4.809 |
| Rajeswari | 10.30 | 4.53 | 0.37 | 40.89 | 43.26 | 0.44 | 0.18 | 15.196 | 41.068 | 43.701 | 0.517 | 0.010 | 4.960 |
| RT103 | 10.41 | 4.48 | 0.60 | 41.70 | 42.56 | 0.38 | 0.20 | 15.487 | 41.902 | 42.933 | 0.507 | 0.009 | 4.882 |
| RT125 | 9.33 | 5.01 | 0.61 | 40.57 | 43.95 | 0.38 | 0.24 | 14.953 | 40.802 | 44.327 | 0.522 | 0.009 | 5.014 |
| RT127 | 10.18 | 4.66 | 0.44 | 42.22 | 42.19 | 0.25 | 0.35 | 15.283 | 42.577 | 42.437 | 0.501 | 0.006 | 4.822 |
| SVPR1 | 9.99 | 5.14 | 0.50 | 41.17 | 42.90 | 0.36 | 0.23 | 15.629 | 41.404 | 43.256 | 0.512 | 0.008 | 4.908 |
| T12 | 11.02 | 4.78 | 0.39 | 40.73 | 42.61 | 0.35 | 0.22 | 16.192 | 40.954 | 42.963 | 0.513 | 0.008 | 4.872 |
| T13 | 10.57 | 4.80 | 0.44 | 40.68 | 43.57 | 0.33 | 0.12 | 15.803 | 40.800 | 43.899 | 0.519 | 0.007 | 4.965 |
| T78 | 9.98 | 4.40 | 0.57 | 40.15 | 44.57 | 0.28 | 0.21 | 14.947 | 40.364 | 44.856 | 0.528 | 0.006 | 5.054 |
| Tarun | 10.10 | 4.40 | 0.55 | 40.61 | 43.96 | 0.36 | 0.19 | 15.050 | 40.799 | 44.320 | 0.522 | 0.008 | 5.012 |
| TC25 | 9.61 | 4.41 | 0.37 | 42.99 | 42.29 | 0.36 | 0.22 | 14.388 | 43.201 | 42.644 | 0.498 | 0.008 | 4.863 |
| TC289 | 9.95 | 4.15 | 0.41 | 42.16 | 43.28 | 0.39 | 0.34 | 14.510 | 42.492 | 43.669 | 0.509 | 0.009 | 4.964 |
| TKG22 | 9.73 | 4.57 | 0.58 | 41.80 | 42.90 | 0.39 | 0.22 | 14.883 | 42.020 | 43.282 | 0.509 | 0.009 | 4.920 |
| TKG55 | 9.96 | 4.67 | 0.47 | 42.43 | 41.84 | 0.40 | 0.36 | 15.098 | 42.783 | 42.238 | 0.499 | 0.009 | 4.820 |
| TMV3 | 9.77 | 4.89 | 0.61 | 42.33 | 41.71 | 0.39 | 0.20 | 15.262 | 42.530 | 42.093 | 0.499 | 0.009 | 4.803 |
| TMV4 | 9.56 | 5.01 | 0.53 | 42.95 | 42.24 | 0.33 | 0.29 | 15.094 | 43.233 | 42.569 | 0.498 | 0.008 | 4.851 |
| TMV5 | 9.80 | 5.03 | 0.52 | 42.91 | 41.67 | 0.38 | 0.33 | 15.357 | 43.234 | 42.049 | 0.495 | 0.009 | 4.803 |
| TMV6 | 9.88 | 4.72 | 0.55 | 42.34 | 42.38 | 0.27 | 0.20 | 15.146 | 42.543 | 42.653 | 0.502 | 0.006 | 4.847 |
| Uma | 10.02 | 4.92 | 0.62 | 38.35 | 45.34 | 0.41 | 0.18 | 15.556 | 38.526 | 45.751 | 0.544 | 0.009 | 5.142 |
| Vinayak | 9.57 | 4.58 | 0.45 | 41.13 | 43.54 | 0.25 | 0.37 | 14.600 | 41.501 | 43.791 | 0.516 | 0.006 | 4.950 |
| VRI1 | 9.45 | 5.14 | 0.62 | 42.45 | 41.66 | 0.37 | 0.22 | 15.206 | 42.671 | 42.030 | 0.497 | 0.009 | 4.796 |
| XLM19 | 9.60 | 4.27 | 0.42 | 43.09 | 42.09 | 0.36 | 0.25 | 14.288 | 43.343 | 42.444 | 0.496 | 0.008 | 4.843 |
| YLM17 | 9.76 | 4.44 | 0.65 | 41.76 | 42.76 | 0.24 | 0.21 | 14.850 | 41.976 | 43.009 | 0.507 | 0.006 | 4.875 |
| Mean | 10.01 | 4.72 | 0.50 | 41.06 | 43.24 | 0.36 | 0.24 | 15.234 | 41.298 | 43.601 | 0.515 | 0.008 | 4.942 |
| SD | 0.44 | 0.36 | 0.11 | 1.85 | 1.77 | 0.05 | 0.09 | 0.446 | 1.849 | 1.772 | 0.021 | 0.001 | 0.166 |
| CV | 4.39 | 7.59 | 22.74 | 4.51 | 4.08 | 15.12 | 36.35 | 2.928 | 4.477 | 4.064 | 4.116 | 15.174 | 3.354 |
| Minimum | 8.94 | 4.05 | 0.34 | 34.71 | 38.49 | 0.22 | 0.06 | 14.288 | 35.033 | 38.893 | 0.460 | 0.005 | 4.508 |
| Maximum | 11.02 | 5.89 | 0.91 | 45.61 | 49.60 | 0.46 | 0.40 | 16.192 | 45.688 | 49.951 | 0.590 | 0.010 | 5.532 |
SFA saturated fatty acid, MUFA monounsaturated fatty acid, PUFA polyunsaturated fatty acid, ODR oleic desaturation ratio, LDR linoleic desaturation ratio, COX oxidizability value
As far as seed coat colour is concerned, oleic acid was found little higher in black seeds followed by white and brown seeds (Table S2). The distribution plot of major fatty acids showed that the highest frequency class for palmitic and stearic acids fell in the region of 9–11% and 4.5–5.0%, respectively (Fig. S1A and S1B). Highest frequency class for oleic and linoleic acids, fell in the region of 40–43% and 41–43%, respectively (Fig. S1C and S1D). The total saturated fatty acid content (SFA) ranged from 14.29 to 16.19% with maximum percentage in T12 and minimum in XLM19. Total saturated fatty acid content in sesame is in the levels that are recommended for human consumption. Polyunsaturated fatty acid (PUFA) and monounsaturated fatty acid (MUFA) contents are the attributes that determine the suitability of edible oil in human nutrition. The MUFA content was highest in Prachi (45.69%) and lowest in DS1 (35.03%). The high MUFA level is responsible for maintenance of oil quality and shelf life. As far as PUFA is concerned, it is maximum in DS1 (49.95%) and minimum in Prachi (38.89%). For stability of oil, a low level of PUFA is preferred (Table 2).
Desaturation efficiency and oxidizing potential of the oil
An estimate of relative efficiency of desaturation pathway operating in the crop is an important attribute to be considered before designing breeding programmes. The estimated oleic and linoleic acid desaturation efficiencies for sesame is presented in Table 2. The mean ODR estimated for sesame germplasm was 0.515 with a minimum ODR value of 0.460 found in Prachi and a maximum value of 0.590 in DS1. A high ODR indicates considerably higher conversion of oleic acid to linoleic acid and thereby implying that this conversion being efficient (Velasco et al. 1997; Mondal et al. 2010). However, the LDR was found to be very insignificant with the mean value of 0.008 implying that the linoleic desaturation pathway is lacking in the crop. Therefore, we conclude that the pathway converting oleic acid to linoleic is only operative in S. indicum.
An estimation of oxidizability (COX) helps to infer the capacity of the oil to resist oxidation and thereby its stability (Coni et al. 2004). In this regard, DS1 exhibited maximum oxidizability value of 5.532, while Prachi had the minimum value of 4.508 (Table 2). Oils with higher oxidizability value have higher tendency to undergo auto oxidation. The seed colour appeared to be non-influential on fatty acid content in the present germplasm (Table S2).
Correlation between lignans and fatty acids
Pearson analysis revealed a positive correlation of 0.55 (p < 0.01) between sesamin and sesamolin, and a negative correlation value for these two with that of sesamol (Table 3). Similar correlation was reported earlier in Indian sesame by Bhunia et al. (2015). Since, fatty acids are synthesized from FAS complex in a sequential manner, a correlation study would enable better understanding of the relationship in their biosynthesis. The data on Pearson analysis of correlation coefficient for the major fatty acids is presented in Table 3. Computation for the oleic and linoleic acid content revealed a strong negative correlation of − 0.960 (p < 0.01), as has been reported earlier in sesame (Were et al. 2006) and soybean (Patil et al. 2007). A negative correlation was also observed between palmitic and stearic acid (− 0.440, p < 0.01), palmitic and arachidic acid (− 0.396, p < 0.01), arachidic and gadoleic acid (− 0.459, p < 0.01). Such inverse relationships have been attributed to the combined influence of environment and the constituent genotype (Uzun et al. 2008). A positive correlation, however, was found between stearic and arachidic acid (0.531, p < 0.01). The elongation of 16-carbon acyl chains followed by desaturation plays a vital role in regulation of the relative amounts of palmitic acid and the other fatty acids derived from it (Carlsson et al. 2000). A deficiency in this step has lead to the reduction in the amounts of 18-carbon fatty acids and increase in the palmitic acid content of plant tissues. This has been considered as the cause observed in the correlations (Were et al. 2006).
Table 3.
Pearson correlation between different lignan and fatty acid components of the oilseed crop Sesamum indicum L.
| Palmitic acid | Stearic acid | Oleic acid | Linoleic acid | Linolenic acid | Arachidic acid | Gadoleic acid | Sesamol | Sesamin | |
|---|---|---|---|---|---|---|---|---|---|
| Stearic acid | − 0.440* | ||||||||
| Oleic acid | − 0.182 | 0.073 | |||||||
| Linoleic acid | 0.064 | − 0.209 | − 0.960* | ||||||
| Linolenic acid | − 0.063 | 0.160 | − 0.161 | 0.106 | |||||
| Arachidic acid | − 0.396* | 0.531* | 0.096 | − 0.164 | 0.166 | ||||
| Gadoleic acid | − 0.133 | − 0.101 | − 0.037 | 0.064 | 0.017 | − 0.459* | |||
| Sesamol | 0.336** | − 0.278 | − 0.028 | − 0.033 | − 0.094 | − 0.227 | − 0.102 | ||
| Sesamin | − 0.192 | 0.336** | 0.105 | − 0.133 | 0.033 | 0.093 | − 0.027 | − 0.268** | |
| Sesamolin | − 0.188 | 0.145 | 0.426* | − 0.438* | − 0.121 | − 0.052 | 0.219 | − 0.256** | 0.550* |
Bold values indicate the Pearson values for the highly correlating components
*Correlation is significant at the 0.01 level, **correlation is significant at the 0.05 level
Biosynthesis of lignans and fatty acids follows entirely different pathways but finally they are targeted towards the seeds. Therefore it would be worthwhile to analyse the data obtained to find if there is any relationship in the production and distribution of the two metabolites. Analysis of the results showed presence of some positive correlation between oleic acid and sesamolin (0.426, p < 0.01), palmitic acid and sesamol (0.336, p < 0.05), stearic acid and sesamin (0.336, p < 0.05). A negative correlation was observed for linoleic acid and sesamolin content (−0.438, p < 0.01). However, further studies are required to find out if there is any definite link between the two biosynthetic pathways. Modern plant breeding relies on integration of techniques such as classical breeding, induced mutations and transgenic technology for genetic enhancement of qualitative and quantitative traits in plants (Zhao et al. 2009). Crops like Safflower and soybean have been earlier improved through mutation breeding (Sahu et al. 1980; Rahman et al. 1996). Mutagenesis has also been reported in sesame with an improvement in the seed oil content and fatty acid composition (Savant and Kothekar 2011). Recent report on sesame genome sequencing has opened up a new face for researchers to study this orphan crop thoroughly for genetic improvement (Zhang et al. 2013). It is our endeavour that researches on lignan and fatty acid biosynthetic mechanisms in sesame would definitely lead to altering of genes for improvement of nutritional quality of edible oil.
Conclusion
The lignan and fatty acid profile of different varieties of S. indicum L. cultivated in diverse agroclimatic regions of India is reported. The data from the present study revealed presence of considerable variability among the accessions for lignan and fatty acid contents. A positive correlation found between lignans and fatty acids indicates possibility of a link between the lignan and fatty acid biosynthetic pathways. However, further study is required to establish this relationship between the two traits. Due to rich source of antioxidant lignans and unsaturated fatty acids, sesame seeds can be effectively used as nutraceuticals and as functional foods. The study has led to identification of varieties with desirable nutritional composition that can be recommended for nutraceuticals and medical applications. Such varieties include VRI1, SVPR1 and E8 for sesamin; Krishna, RT125 and Rajeswari for sesamolin; FFAT0822, SVPR1 and Phule til for total lignan content; Prachi and XLM19 for oleic acid; and DS1 and N8 for linoleic acid. These varieties may be considered as superior lines for the traits cited. The black seeded sesame being rich in sesamin, sesamolin, total lignan content and oleic acid have been identified to be nutritionally and pharmaceutically more important than white and brown sesame. The variability reported in this study would serve as base line for transferring favourable genes into desirable varieties by breeding to enhance the nutritional value and quality of the crop.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge NBPGR, New Delhi for providing the germplasm used in this study. We acknowledge University Grants Commission-Special Assistance Programme (UGC-SAP) New Delhi and DBT, Govt. of India, BUILDER program (BT/PR14554/INF/22/125/2010) for financial assistance.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bedigian D. History and lore of sesame in Southwest Asia. Econ Bot. 2004;58:329–353. doi: 10.1663/0013-0001(2004)058[0329:AR]2.0.CO;2. [DOI] [Google Scholar]
- Bhunia RK, Chakraborty A, Kaur R, Gayatri T, Bhat KV, Basu A, Maiti MK, Sen SK. Analysis of fatty acid and lignan composition of Indian germplasm of sesame to evaluate their nutrional merits. J Am Oil Chem Soc. 2015;92:65–76. doi: 10.1007/s11746-014-2566-3. [DOI] [Google Scholar]
- Carlsson AS, LaBrie ST, Kinney AJ, Wettstein-Knowles P, Browse JA. KAS2 cDNA complements the phenotypes of the Arabidopsis fab1 mutant that differs in a single residue bordering the substrate binding pocket. Plant J. 2000;29:761–770. doi: 10.1046/j.1365-313X.2002.01253.x. [DOI] [PubMed] [Google Scholar]
- Cheng FC, Jinn TR, Hou RC, Tzen JTC. Neuroprotective effects of sesamin and sesamolin on gerbil brain in cerebral ischemia. Int J Biomed Sci. 2006;2:284–288. [PMC free article] [PubMed] [Google Scholar]
- Cho KM, Ha TJ, Lee YB, Seo WD, Kim JY, Ryu HW, Jeong SH, Kang YM, Lee JH. Soluble phenolics and antioxidant properties of soybean (Glycine max L.) cultivars with varying seed coat colours. J Funct Foods. 2013;5:1065–1076. doi: 10.1016/j.jff.2013.03.002. [DOI] [Google Scholar]
- Coni E, Podesta E, Catone T. Oxidizability of different vegetables oils evaluated by thermogravimetric analysis. Thermochim Acta. 2004;418:11–15. doi: 10.1016/j.tca.2003.11.038. [DOI] [Google Scholar]
- Cooney RV, Custer LJ, Okinaka L, Franke AA. Effects of dietary sesame seeds on plasma tocopherol levels. Nutr Cancer. 2001;39:66–71. doi: 10.1207/S15327914nc391_9. [DOI] [PubMed] [Google Scholar]
- Dar AA, Arumugam N. Lignans of sesame: purification methods, biological activities and biosynthesis—a review. Bioorg Chem. 2013;50:1–10. doi: 10.1016/j.bioorg.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Dar AA, Verma NK, Arumugam N. An updated method for isolation, purification and characterization of clinically important antioxidant lignans—sesamin and sesamolin from sesame oil. Ind Crop Prod. 2015;64:201–208. doi: 10.1016/j.indcrop.2014.10.026. [DOI] [Google Scholar]
- Fatemi SH, Hammond EG. Analysis of oleate, linoleate and linolenate hydroperoxides in oxidized ester mixtures. Lipids. 1980;15:379–385. doi: 10.1007/BF02533555. [DOI] [Google Scholar]
- Hemalatha S, Ghafoorunissa Lignans and tocopherols in Indian sesame cultivars. J Am Oil Chem Soc. 2004;81:467–470. doi: 10.1007/s11746-004-0924-5. [DOI] [Google Scholar]
- Kamal-Eldin A, Appelqvist LA, Yousif G. Lignan analysis in seed oils from four Sesamum species: comparison of different chromatographic methods. J Am Oil Chem Soc. 1994;71:141–147. doi: 10.1007/BF02541548. [DOI] [Google Scholar]
- Kim JH, Seo WD, Lee SK, Lee YB, Park CH, Ryu HW, Lee JH. Comparative assessment of compositional components, antioxidant effects, and lignan extractions form Korean white and black sesame (Sesamum indicum L.) seeds for different crop years. J Funct Foods. 2014;7:495–505. doi: 10.1016/j.jff.2014.01.006. [DOI] [Google Scholar]
- Kupiec T. Quality-control analytical methods: high-performance liquid chromatography. Int J Pharm Compd. 2004;8:223–227. [PubMed] [Google Scholar]
- Moazzami AA, Kamal-Eldin A. Sesame seed is a rich of dietary lignans. J Am Oil Chem Soc. 2006;83:719–723. doi: 10.1007/s11746-006-5029-7. [DOI] [Google Scholar]
- Moazzami AA, Haese SL, Kamal-Eldin A. Lignan contents in sesame seeds and products. Eur J Lipid Sci Technol. 2007;109:1022–1027. doi: 10.1002/ejlt.200700057. [DOI] [Google Scholar]
- Mondal N, Bhat KV, Srivastava PS. Variation in fatty acid composition in Indian germplasm of sesame. J Am Oil Chem Soc. 2010;87:1263–1269. doi: 10.1007/s11746-010-1615-9. [DOI] [Google Scholar]
- Patil A, Taware SP, Oak MD, Tamhankar SA, Rao VS. Improvement of oil quality in soybean [Glycine max (L.) Merrill] by mutation breeding. J Am Oil Chem Soc. 2007;84:1117–1124. doi: 10.1007/s11746-007-1146-1. [DOI] [Google Scholar]
- Pleines S, Friedt W. Breeding for improved C18 fatty acid composition in rapeseed (Brassica napus L.) Eur J Lipid Sci Technol. 1988;90:167–171. [Google Scholar]
- Rahman SM, Takagi Y, Kinoshita T. Genetic control of high oleic acid content in the seed oil of two soybean mutants. Crop Sci. 1996;36:1125–1128. doi: 10.2135/cropsci1996.0011183X003600050009x. [DOI] [PubMed] [Google Scholar]
- Rangkadilok N, Pholphana N, Wongyai CMW, Saengsooksree K, Nookabkaew S, Satayavivad J. Variation of sesamin, sesamolin and tocopherols in sesame (Sesamum indicum L.) seeds and oil products in Thailand. Food Chem. 2010;122:724–730. doi: 10.1016/j.foodchem.2010.03.044. [DOI] [Google Scholar]
- Sahu GR, Mukerji P, Singh BB, Singh RB. Induced polygenetic variability in safflower (Carthamus tinctorius L.) J Cytol Genet. 1980;15:81–85. [Google Scholar]
- Savant KD, Kothekar VS. Induction of variability in fatty acid profile in sesame (Sesamum indicum L.) J Phytol. 2011;3:01–03. [Google Scholar]
- Shi L, Liu R, Jin Q, Wang X. The contents of lignans in sesame seeds and commercial sesame oils of China. J Am Oil Chem Soc. 2017 [Google Scholar]
- Suja KP, Jayalekshmy A, Arumughan C. Free radical scavenging behavior of antioxidant compounds of sesame (Sesamum indicum L.) in DPPH(*) system. J Agric Food Chem. 2004;52:912–915. doi: 10.1021/jf0303621. [DOI] [PubMed] [Google Scholar]
- Thies W. Schnelle und einfache Analysen der Fettsaurezusammensetzung in einzelnen RapsKotyledonen I. Gaschromatographische und papierchromatographische Methoden. Z. Pflanzenzuchtg. 1971;65:181–202. [Google Scholar]
- Uzun B, Arslan C, Furat S. Variation in fatty acid compositions, oil content and oil yield in a germplasm collection of sesame (Sesamum indicum L.) J Am Oil Chem Soc. 2008;85:1135–1142. doi: 10.1007/s11746-008-1304-0. [DOI] [Google Scholar]
- Velasco L, Martinez JMF, DeHaro A. Induced variability for C18 unsaturated fatty acids in Ethiopian mustard. Can J Plant Sci. 1997;77:91–95. doi: 10.4141/P96-025. [DOI] [Google Scholar]
- Visavadiya NP, Narasimhacharya AVRL. Sesame as a hypocholesteraemic and antioxidant dietary component. Food Chem Toxicol. 2008;46:1889–1895. doi: 10.1016/j.fct.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Y, Li P, Wang X, Zhang W, Wei W, Zhang X. HPLC analysis of seed sesamin and sesamolin variation in a sesame germplasm collection in China. J Am Oil Chem Soc. 2012;89:1011–1020. doi: 10.1007/s11746-011-2005-7. [DOI] [Google Scholar]
- Were BA, Onkware AO, Gudu S, Welander M, Carlsson AS. Seed oil content and fatty acid composition in East African sesame (Sesamum indicum L.) accessions evaluated over 3 years. Field Crops Res. 2006;97:254–260. doi: 10.1016/j.fcr.2005.10.009. [DOI] [Google Scholar]
- Williamson S, Morris JB, Quentin N, Pye QN, Chandrashekhar DK. A survey of sesamin and composition of tocopherol variability from seeds of eleven diverse Sesame (Sesamum indicum L.) genotypes using HPLC-PAD-ECDK. Phytochem Anal. 2008;19:311–322. doi: 10.1002/pca.1050. [DOI] [PubMed] [Google Scholar]
- Yermanos DM, Hemstreet S, Saleeb W, Huszar CK. Oil content and composition of the seed in the world collection of sesame introductions. J Am Oil Chem Soc. 1972;49:20–23. doi: 10.1007/BF02545131. [DOI] [Google Scholar]
- Yokota T, Matsuzaki Y, Koyama M, Hitomi T, Kawanaka M, Enoki-Konishi M, et al. Sesamin, a lignan of sesame, down-regulates cyclin D1 protein expression in human tumor cells. Cancer Sci. 2007;98:1447–1453. doi: 10.1111/j.1349-7006.2007.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Mia H, Wan L, Qu L, Liu H, Wang Q, Yue M. Genome sequencing of the important oilseed crop Sesamum indicum L. Genome Biol. 2013;14:401. doi: 10.1186/gb-2013-14-1-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Niu J, Dangs Z, Li Y, Xie X, Guan T, Tian C. Evaluation of lignan contents of newly bred flax varieties (lines) in China. Sci Agric Sin. 2009;42:454–459. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.