Abstract
The aim of this study was to evaluate the effects of process conditions on the solid–liquid extraction of bioactive compounds from the Alicante Bouschet grape skin from the semi-arid region of Brazil. The influence of temperature (23–57 °C), ethanol concentration (16–84%) and citric acid concentration (0–4%) on the total phenolic content, monomeric anthocyanin content and on the antioxidant capacity of the extracts measured by oxygen radical absorbance capacity and cation radical scavenging activity (ABTS·+) methods was investigated. Ethanol concentration and temperature had a significant influence on total phenolic compounds extraction and antioxidant capacity while monomeric anthocyanins extraction was only affected by ethanol concentration. The conditions selected as the most adequate for the extraction were: temperature of 40 °C, 50% v/v ethanol and 2% m/v citric acid.
Keywords: Solid–liquid extraction, Bioactive compounds, Antioxidant capacity, Grape skin, Alicante Bouschet
Introduction
Alicante Bouschet var. (Vitis vinifera L.) grape, also known as Garnacha Tintorera is one of the few V. vinifera cultivars that have red flesh and red skin (Castillo-Muñoz et al. 2009). In Brazil, the genetic characteristics of the Alicante Bouschet grape, such as high anthocyanin content, together with the stress conditions of the semi-arid climate where this vine has been grown, such as intense UV radiation, results in higher concentration of secondary metabolites in grapes.
Wine production implies generating a large amount of by-products, mainly grape pomace, with a high content of phenolic compounds that remain in the skins and seeds due to an incomplete extraction during winemaking process (Brazinha et al. 2014; Caldas et al. 2018; Rockenbach et al. 2011). Those compounds are evaluated as potential antioxidants (Kumar et al. 2015) and colorants for foods (Giusti and Wrolstad 2003). Therefore, their extraction represents an alternative to take advantage of the winemaking by-products, resulting in low cost ingredients with high added value.
The studies reported in literature generally focus on the phenolic composition of the Alicante Bouschet grape grown in temperate climate regions (Castillo-Muñoz et al. 2009; Pinelo et al. 2005). To the best of our knowledge, just the study by Pinelo et al. (2005) assessed the extraction of phenolic compounds from the Alicante Bouschet grape pomace grown in temperate climate. The extraction of phenolic compounds from by-products of this variety grown in semi-arid climate of northeastern Brazil have not yet been reported, despite the importance to determine the influence of the regional climate in the phenolic content of grape by-product and a possible technological application. In this context, the objective of this study was to evaluate the effect of temperature, ethanol concentration and citric acid concentration in the solid–liquid extraction from Alicante Bouschet grape skin and select the best extraction conditions, aiming at obtaining the highest contents of total phenolic compounds and monomeric anthocyanins and the maximum antioxidant capacity of the extracts.
Materials and methods
Raw material
Alicante Bouschet grape pomace from red winemaking was supplied by the Rio Sol winery (Lagoa Grande, PE, Brazil). The pomace was dried in a convective tray dryer at 60 °C for 24 h. The dried material was processed in a Bonina 0.25 df (Itametal, Itabuna, Brasil) depulper to separate the seeds from the skins. The skins were grinded in a disc type mill (Perten Instruments AB, Huddinge, Sweden), resulting in grape skin flour.
Extraction process
The grape skin flour was subjected to solid–liquid extraction process in a thermostatic bath (Marconi—MA 093, São Paulo, Brazil) using a hydroethanolic solution acidified with citric acid. The solid: liquid ratio (1:10), stirring speed (200 rpm) and extraction time (1 h), were fixed according to Caldas et al. (2018). After removing the suspended solids by filtration, the extracts were stored at − 20 °C in amber recipients for subsequent analysis.
Analytical methods
Total phenolic content (TPC)
TPC in the extracts was determined with the Folin–Ciocalteu reagent according to the method described by Georgé et al. (2005). The absorbance was read at 760 nm in a UV-1800 spectrophotometer (Shimadzu Corp., Kyoto, Japan). The results were expressed in mg of gallic acid equivalent (GAE) per 100 g of grape skin flour.
Monomeric anthocyanin content (MAC)
MAC determination in the extracts was carried out by the pH differential method as described by Giusti and Wrolstad (2001). The absorbance was measured at 520 and 700 nm using SPECORD 205 UV–Vis spectrophotometer (Analytik Jena AG, Germany). The results were expressed in mg of monomeric malvidin 3-O-glucoside equivalents (molar extinction coefficient of 29,500 L cm−1 mol−1 and molecular weight of 562.5 g mol−1) per 100 g of grape skin flour.
Antioxidant capacity by the ORAC method
Determination of the ORAC value was based on the method described by Zulueta et al. (2009) using a TECAN Infinite® 200 Series (Tecan, Männedorf, Switzerland) fluorescence reader. Fluorescein was used as reference and 2, 2′-Azobis (2-amidinopropane) dihydrochloride (AAPH) as peroxyl radical generator. Fluorescence intensity (λexc = 485 nm, λem = 535 nm) was monitored every minute, in kinetic mode until obtaining a fluorescence value 5% below the initial reading. The results were expressed in µmol Trolox equivalents (TE) per g of grape skin flour.
Antioxidant capacity by the ABTS·+ method
The antioxidant capacity of the extracts was also assessed by the method described by Re et al. (1999). Absorbance was measured at 734 nm in UV-1800 spectrophotometer (Shimadzu Corp., Kyoto, Japan). The results were expressed in µmol of Trolox equivalents (TE) per g of grape skin flour.
Experimental design
Response Surface Methodology (RSM) was used to evaluate the influence of temperature, ethanol concentration and citric acid concentration on the recovery of bioactive compounds from grape skin. The major effects, quadratic effects and effects of interaction were assessed using a 23 Rotatable Central Composite Design with 17 assays (Table 1). A second order polynomial equation was used to express the responses as a function of the independent variables or factors (Eq. 1):
| 1 |
where is the response: TPC, MAC and antioxidant capacity (ORAC and ABTS·+); are the regression coefficients and , and are the independent variables (temperature, ethanol concentration and citric acid concentration, respectively).
Table 1.
Real and coded values of the independent variables in the rotatable central composite design and observed results
| Assay | Temperature (°C) | Ethanol (%) | Citric acid (%) | TPCa | MACb | ORACc | ABTS·+d |
|---|---|---|---|---|---|---|---|
| 1 | 30 (− 1) | 30 (− 1) | 0.8 (− 1) | 1387.2 ± 66.2 | 65.8 ± 1.8 | 142.5 ± 15.2 | 70.7 ± 1.2 |
| 2 | 30 (− 1) | 30 (− 1) | 3.2 (+ 1) | 1606.9 ± 43.2 | 76.1 ± 5.9 | 120.9 ± 0.7 | 80.1 ± 1.7 |
| 3 | 30 (− 1) | 70 (+ 1) | 0.8 (− 1) | 1873.6 ± 9.5 | 144.1 ± 2.6 | 236.0 ± 8.7 | 103.2 ± 2.4 |
| 4 | 30 (− 1) | 70 (+ 1) | 3.2 (+ 1) | 2319.0 ± 43.2 | 150.61 ± 6.5 | 213.3 ± 5.2 | 98.6 ± 2.4 |
| 5 | 50 (+ 1) | 30 (− 1) | 0.8 (− 1) | 2287.2 ± 50.1 | 88.2 ± 3.3 | 202.2 ± 7.3 | 103.2 ± 3.0 |
| 6 | 50 (+ 1) | 30 (− 1) | 3.2 (+ 1) | 2382.6 ± 25.0 | 107.0 ± 6.6 | 200.2 ± 11.5 | 115.9 ± 3.2 |
| 7 | 50 (+ 1) | 70 (+ 1) | 0.8 (− 1) | 2657.0 ± 68.8 | 163.2 ± 3.0 | 250.2 ± 6.1 | 136.0 ± 1.8 |
| 8 | 50 (+ 1) | 70 (+ 1) | 3.2 (+ 1) | 2962.0 ± 103.4 | 161.2 ± 2.9 | 340.3 ± 9.9 | 140.0 ± 2.7 |
| 9 | 23 (− 1.68) | 50 (0) | 2 (0) | 2378.8 ± 103.9 | 142.4 ± 7.2 | 267.4 ± 7.6 | 122.1 ± 2.0 |
| 10 | 57 (+ 1.68) | 50 (0) | 2 (0) | 4038.9 ± 116.8 | 149.3 ± 9.0 | 285.5 ± 17.8 | 180.9 ± 1.2 |
| 11 | 40 (0) | 16 (− 1.68) | 2 (0) | 951.8 ± 51.2 | 46.4 ± 0.8 | 129.1 ± 15.2 | 45.6 ± 1.5 |
| 12 | 40 (0) | 84 (+ 1.68) | 2 (0) | 1510.8 ± 64.1 | 143.8 ± 0.7 | 204.2 ± 13.1 | 83.7 ± 3.6 |
| 13 | 40 (0) | 50 (0) | 0 (− 1.68) | 2109.1 ± 71.9 | 158.4 ± 11.2 | 264.5 ± 14.3 | 136.5 ± 3.8 |
| 14 | 40 (0) | 50 (0) | 4 (+ 1.68) | 2848.3 ± 30.9 | 154.2 ± 1.4 | 233.6 ± 9.3 | 137.9 ± 4.2 |
| 15 | 40 (0) | 50 (0) | 2 (0) | 2638.0 ± 162.2 | 163.6 ± 4.4 | 261.4 ± 7.4 | 148.3 ± 6.4 |
| 16 | 40 (0) | 50 (0) | 2 (0) | 2422.9 ± 139.2 | 164.5 ± 5.9 | 256.9 ± 9.6 | 152.7 ± 4.6 |
| 17 | 40 (0) | 50 (0) | 2 (0) | 2513.5 ± 130.6 | 141.9 ± 10.7 | 271.8 ± 12.7 | 149.7 ± 3.3 |
Results expressed as mean ± standard deviation (n = 3)
aTPC: total phenolic content (mg of gallic acid equivalents [GAE]·100 g−1 skin flour)
bMAC: monomeric anthocyanin content (mg of malvidin-3-O-glucoside [Mv3gl]·100 g−1 skin flour)
cORAC: oxygen radical absorbance capacity (µmol Trolox equivalents [TE]·g−1 skin flour)
dABTS·+: cation radical scavenging activity (µmol Trolox equivalents [TE]. g−1 skin flour)
The multiple response optimization method was used as strategy to obtain an extract rich in phenolic compounds and anthocyanins and also, with high antioxidant capacity. Optimum operation conditions were obtained by using the desirability function (Candioti et al. 2014). The mathematical models were validated carrying out confirmatory assays of TPC and MAC and also in relation to antioxidant capacity (ORAC and ABTS·+) in the extract obtained under the conditions selected as the most adequate. Finally, experimental and predicted values were compared.
Statistical analysis
All results were reported as mean ± standard deviation (SD) (n = 3). Analysis of variance (ANOVA) and F test (Fischer) were performed to check model fitness. Regression and determination coefficients were also obtained generating both, response surfaces and profiles of the desirability function for response optimization. Statistica 10.0 (StatSoft, Tulsa, USA) was used for all statistical analysis with a level of significance of 5%.
Results and discussion
Table 1 presents the results of TPC and MAC as well as the antioxidant capacity measured by the ORAC and ABTS·+ methods of the extracts obtained under different extraction conditions. From these results, the regression coefficients coded for the second order polynomial equation with p values for each response were obtained. To check model fitness and adjustment, the equations were evaluated by ANOVA. All models showed a good fit for predicting the behavior of the response variables in relation to the independent variables: the lack of adjustment of the model was non-significant (p > 0.05), and the coefficients of determination were satisfactory (R2adj > 0.76).
Total phenolic content (TPC)
TPC in the extracts ranged from 951.8 to 4038.9 mg GAE/100 g of grape skin (Table 1), lower than those values obtained by Tournour et al. (2015) (6930–13,170 mg GAE/100 g of grape pomace), who assessed the extraction of phenolic compounds from the pomace of different grape cultivars using ethanol/water (80% v/v) as solvent under mechanical stirring, and with much longer extraction time (48 h) than the one used in the present study.
Singh et al. (2017) studied the extraction of polyphenols from whole mung bean, hull and cotyledon by using conventional solvent extraction. Using hydroethanolic solution (56% v/v) and temperature of 54 °C during 63 min, the authors reported high TPC values from the hull flour (3774 mg GAE/100 g) close to 4038.9 mg GAE/100 g obtained from grape skin flour with similar conditions in the present work (Table 1).
Several authors have reported higher yields in phenolic compound extraction when using pure methanol (Pinelo et al. 2005), and methanol solution acidified with HCl (Rockenbach et al. 2011). However, due to the toxicity of this solvent, we preferred to use ethanol, a non-toxic solvent that enables the subsequent use of the extracts for food purposes (Fontana et al. 2013). For the same reason, the acidifying agent used was citric acid, a low cost additive widely used in the food industry (Brazinha et al. 2014).
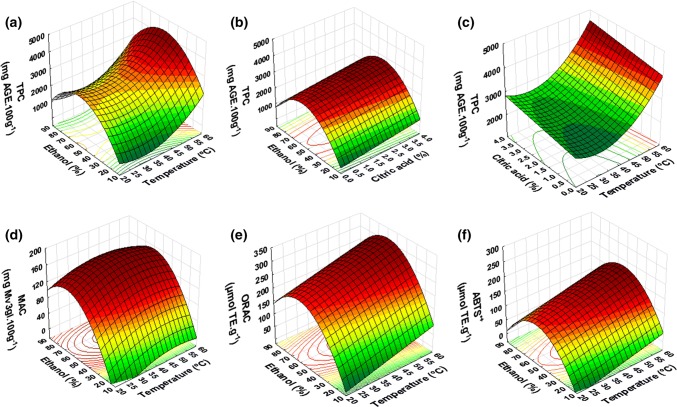
The influence of the process variables on TPC is presented in Fig. 1a–c. This response was significantly (p < 0.05) affected by all factors. The use of high temperatures favored extraction process due to the increase of both, solute solubility and the diffusion coefficient of phenolic compounds. The higher the temperature, the lower the dynamic viscosity coefficient and the higher the mass transfer coefficient (Cacace and Mazza 2003b; Pinelo et al. 2005). Furthermore, the use of higher acid citric concentrations slightly increases the extraction of total phenolic compounds.
Fig. 1.
Response surface graph for the influence of the extraction process on total phenolic content (TPC) (a–c), monomeric anthocyanin content (MAC) (d) and antioxidant capacity measured by the ORAC (e) and ABTS·+ (f) methods
The highest phenolic content (Fig. 1a, b) were found when using intermediate values of ethanol concentration in the studied range (50% v/v). This behavior was also reported by Caldas et al. (2018) in the extraction of phenolic compounds from the skins of Syrah grape variety. The authors attributed this trend to the diverse structure and thus polarity of the different groups of phenolic compounds present in grape skin. The polar nature of polyphenols (presence of hydroxyl groups) favors their solubilization in polar media such as hydroalcoholic solutions (Fontana et al. 2013).
According to the works by Cacace and Mazza (2003a) and Ćujić et al. (2016), binary solvent system containing hydro-organic solvents (ethanol/water) was superior to mono-component solvent system (ethanol or pure water) in the extraction of phenolic compounds from frozen black currants and dried chokeberry, respectively. Mixture of water and ethanol as solvent agent shows synergistic effect: water swells plant tissues and increase the permeability of cell tissues whereas ethanol disrupts the bonding between the solutes and plant matrix which increases mass transfer by molecular diffusion and facilitates phenolic extraction (Ćujić et al. 2016; Fontana et al. 2013; Singh et al. 2017).
Cacace and Mazza (2003b) also found that yields of phenolic compounds from milled frozen black currants were affected by the concentration of ethanol in the solvent. For nonpolar covalent molecules, for example flavonols which have very low solubility in water, it was necessary to increase the ethanol concentration in order to obtain a lower dielectric constant and a lower value of energy required to break the water arrangement and increase their solubility (Cacace and Mazza 2003a).
Monomeric anthocyanin content (MAC)
MAC ranged from 46.4 to 164.5 mg/100 g of grape skin (Table 1). Anthocyanin extraction was significantly influenced (p < 0.05) only by ethanol concentration (Fig. 1d). The highest MAC was obtained when 50% ethanol solution was used. Similar behavior was observed in the extraction of total phenolic compounds since MAC constitute a predominant group among total phenolic compounds which could certainly contribute to increase the total phenolic content in this variety.
In the extraction of anthocyanins from black currants, Cacace and Mazza (2003a) also observed the maximum yield at approximately 50% ethanol. According to the authors, anthocyanins might be considered as ionic molecules since these compounds would remain in the flavilium cation (AH+) form at acidic pH. Therefore, these molecules require lower concentration of ethanol than other groups of phenols to dissolve in the extraction media.
Antioxidant capacity by the ORAC method
The antioxidant capacity determined by the ORAC method was significantly (p < 0.05) affected by temperature and ethanol concentration. According to Fig. 1e, the antioxidant capacity increased when the temperature was increased. High temperatures enabled a higher extraction of compounds with antioxidant activity, which could be explained by the increase in solute solubility, as already discussed. This trend was similar to that observed for TPC and MAC extraction suggesting that these compounds are probably responsible for the antioxidant capacity against peroxyl and hydroxyl radicals.
The antioxidant capacity obtained by the ORAC method ranged from 120.9 to 340.3 µmol TE/g grape skin (Table 1), higher than those results reported by Yilmaz and Toledo (2006) when analyzing grape skin extracts from Chardonnay (102.8 µmol TE/g) and Merlot (69.8 µmol TE/g) varieties using water and acetone as solvents.
The ORAC values observed in the present work are 5.4-folds lower than those obtained by Tournour et al. (2015) (906–1579 µmol TE/g residue) in extracts obtained using ethanol/water 80% v/v under orbital agitation at 300 rpm for 48 h from whole grape pomace (skins and seeds) of different red grape cultivars (Vitis vinifera L.). As for TPC and MAC, the highest ORAC values were observed for ethanol concentrations about 50% v/v.
Antioxidant capacity by the ABTS·+method
The antioxidant capacity determined by the ABTS·+ method was also significantly influenced (p < 0.05) by ethanol concentration and extraction temperature. The extracts exhibited higher scavenging capacity of ABTS·+ radicals when extracted with 50% v/v hydroethanolic solutions and at higher temperatures (Fig. 1f). The similarities between the response surfaces obtained for bioactive compounds extraction and the antioxidant capacity suggest that the phenolic compounds and monomeric anthocyanins present in grape skins act as ABTS·+ radical scavengers. This behavior was previously reported by Caldas et al. (2018), who also studied the influence of ethanol concentration on the extraction of total phenolic compounds from grape skins by-products of sparkling wine production.
Rockenbach et al. (2011) assessed the antioxidant capacity measured by the ABTS·+ method in different red grapes pomaces using acidified (0.1% HCl) methanol. The extracts presented values between 193.4 and 485.4 µmol TE/g dry pomace, higher than those obtained in the present study (45.6–180.9 µmol TE/g skin flour) but with the disadvantage of using methanol and HCl, toxic reagents that limits subsequent extract application in foods.
Selection of the best conditions and model validation
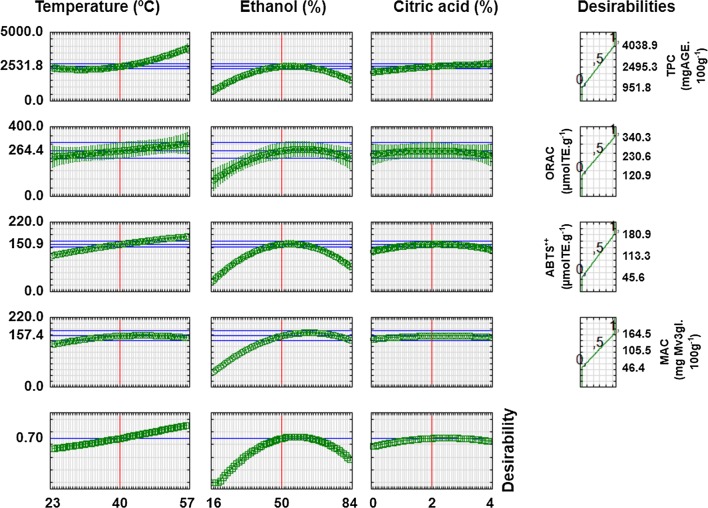
According to the response surfaces obtained, the best conditions for each response are located in different regions of the experimental space. Therefore, we used the constrained optimization method, which assesses individual and overall desirability functions (Candioti et al. 2014). Thus, all responses were maximized to obtain the most adequate extraction conditions within the studied range.
Figure 2 shows individual and overall desirability profiles in the extraction conditions. The overall desirability reached was equal to 0.70 (close to the maximum value of 1), corresponding to the operation conditions that generate the maximum response. In this way, the most adequate values found for the extraction were: temperature of 40 °C, 50% v/v ethanol and 2% m/v citric acid.
Fig. 2.
Profiles of the values predicted for individual and overall desirability in extraction optimization
In these conditions, the experimental values of TPC (2678.5 ± 256.6 mg GAE·100 g−1 skin), MAC (148.6 ± 9.6 mg of Mv3gl·100 g−1 skin), antioxidant capacity by ORAC (296.9 ± 2.0 µmol TE·g−1 skin) and by ABTS·+ method (144.2 ± 3.5 µmol TE·g−1 skin) were close to the values predicted by the experimental design: TPC (2480.0 mg GAE·100 g−1 skin), MAC (148.4 mg Mv3gl·100 g−1 skin), antioxidant capacity by ORAC (256.9 µmol TE·g−1 skin) and by ABTS·+ method (143.0 µmol TE·g−1 skin), with coefficients of variation less than 10%. The results indicate that the quadratic models were adequate to predict the responses assessed, within the ranges studied.
Conclusion
Extraction of bioactive compounds and the antioxidant capacity were significantly affected by the process variables studied in this work, mainly by temperature and ethanol concentration. The conditions defined as the most adequate for the extraction of bioactive compounds with antioxidant capacity were: temperature of 40 °C, 50% v/v ethanol and 2% m/v citric acid. Because of its high content of phenolic compounds and anthocyanins, Alicante Bouschet grape skin extract can be used as a promising natural additive (colorant and/or antioxidant) replacing synthetic additives in food formulations.
Acknowledgements
The authors are grateful to Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (Process Number E-26/203294/2016) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Process Number 408330/2016-3) for the financial support. The work by Yineth Ruíz-García was supported by Programa Estudantes-Convênio de Pós-Graduação (PEC-PG) CAPES-Brazil (Process Number 88882.195654/2018-01). The authors would like to thank Agnelli Hollanda Oliveira for the assistance during the laboratory work and Rio Sol winery of the ViniBrasil group for kindly providing grape pomace.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yineth Ruíz-García, Email: yineth.ruiz86@gmail.com.
Carolina Beres, Email: carolberes@gmail.com.
Davy W. H. Chávez, Email: davyhw76@gmail.com
Erika F. Souza, Email: erika.fraga@embrapa.br
Renata V. Tonon, Email: renata.tonon@embrapa.br
Lourdes M. C. Cabral, Phone: +55 21 3622-9601, Email: lourdes.cabral@embrapa.br
References
- Brazinha C, Cadima M, Crespo JG. Optimization of extraction of bioactive compounds from different types of grape pomace produced at wineries and distilleries. J Food Sci. 2014;79(6):1142–1149. doi: 10.1111/1750-3841.12476. [DOI] [PubMed] [Google Scholar]
- Cacace J, Mazza G. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J Food Sci. 2003;68(1):240–248. doi: 10.1111/j.1365-2621.2003.tb14146.x. [DOI] [Google Scholar]
- Cacace JE, Mazza G. Mass transfer process during extraction of phenolic compounds from milled berries. J Food Eng. 2003;59:379–389. doi: 10.1016/S0260-8774(02)00497-1. [DOI] [Google Scholar]
- Caldas TW, Mazza KEL, Teles ASC, Mattos GN, Brígida AIS, Conte-Junior CA, Borguini RG, Godoy RLO, Cabral LMC, Tonon RV. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind Crops Prod. 2018;111:86–91. doi: 10.1016/j.indcrop.2017.10.012. [DOI] [Google Scholar]
- Candioti LV, De Zan MM, Cámara MS, Goicoechea HC. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta. 2014;124:123–138. doi: 10.1016/j.talanta.2014.01.034. [DOI] [PubMed] [Google Scholar]
- Castillo-Muñoz N, Fernández-González M, Gómez-Alonso S, García-Romero E, Hermosín-Gutiérrez I. Red-color related phenolic composition of Garnacha Tintorera (Vitis vinifera L.) grapes and red wines. J Agric Food Chem. 2009;57(17):7883–7891. doi: 10.1021/jf9002736. [DOI] [PubMed] [Google Scholar]
- Ćujić N, Šavikin K, Janković T, Pljevljakušić D, Zdunić G, Ibrić S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016;194:135–142. doi: 10.1016/j.foodchem.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Fontana AR, Antoniolli A, Bottini RN. Grape pomace as a sustainable source of bioactive compounds: extraction, characterization, and biotechnological applications of phenolics. J Agric Food Chem. 2013;61:8987–9003. doi: 10.1021/jf402586f. [DOI] [PubMed] [Google Scholar]
- Georgé S, Brat P, Alter P, Amiot MJ. Rapid determination of polyphenols and vitamin C in plant-derived products. J Agric Food Chem. 2005;53:1370–1373. doi: 10.1021/jf048396b. [DOI] [PubMed] [Google Scholar]
- Giusti MM, Wrolstad RE. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In: Giusti MM, Wrolstad RE, editors. Current protocols in food analytical chemistry. Hoboken: Wiley; 2001. pp. F1.2.1–F1.2.13. [Google Scholar]
- Giusti MM, Wrolstad RE. Acylated anthocyanins from edible sources and their applications in food systems. Biochem Eng J. 2003;14:217–225. doi: 10.1016/S1369-703X(02)00221-8. [DOI] [Google Scholar]
- Kumar V, Chatli MK, Wagh RV, Mehta N, Kumar P. Effect of the combination of natural antioxidants and packaging methods on quality of pork patties during storage. J Food Sci Technol. 2015;52(10):6230–6241. doi: 10.1007/s13197-015-1734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinelo M, Rubilar M, Jerez M, Sineiro J, Núñez MJ. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J Agric Food Chem. 2005;53:2111–2117. doi: 10.1021/jf0488110. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rockenbach II, Rodrigues E, Gonzaga LV, Caliari V, Genovese MI, Gonçalves AESS, Fett R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011;127:174–179. doi: 10.1016/j.foodchem.2010.12.137. [DOI] [Google Scholar]
- Singh B, Singh N, Thakur S, Kaur A. Ultrasound assisted extraction of polyphenols and their distribution in whole mung bean, hull and cotyledon. J Food Sci Technol. 2017;54(4):921–932. doi: 10.1007/s13197-016-2356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournour HH, Segundo MA, Magalhães LM, Barreiros L, Queiroz J, Cunha LM. Valorization of grape pomace: extraction of bioactive phenolics with antioxidant properties. Ind Crops Prod. 2015;74:397–406. doi: 10.1016/j.indcrop.2015.05.055. [DOI] [Google Scholar]
- Yilmaz Y, Toledo RT. Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. J Food Compost Anal. 2006;19:41–48. doi: 10.1016/j.jfca.2004.10.009. [DOI] [Google Scholar]
- Zulueta A, Esteve MJ, Frígola A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009;114:310–316. doi: 10.1016/j.foodchem.2008.09.033. [DOI] [Google Scholar]