Abstract
The past 20 years has seen rapid development of value-added food products. Using largely wasted fruit by-products has created a potential for sustainable use of these edible materials. The high levels of antioxidant activity, phenolic compounds, dietary fibres and resistant starch in banana pulp and peel have made this tropical fruit an outstanding source of nutritive ingredient for enrichment of foodstuffs. Accordingly, processing of separate banana parts into flour has been of interest by many researchers using different methods (oven drying, spouted bed drier, ultrasound, pulsed vacuum oven, microwave, spray drying and lyophilization). Regarding the high level of bioactive compounds, especially resistant starch in banana flour, the application of its flour in starchy foods provides a great opportunity for product development, even in gluten free foods. This review aims to provide concise evaluation of the health benefits of banana bioactive components and covers a wide range of literature conducted on the application of different parts of banana and the flour produced at various ripeness stages in the food industry. Of particular interest, the impact of drying methods on banana flour properties are discussed.
Keywords: Banana, Flour, Gluten free, Sensory, Starch, Rheology
Introduction
Banana is a tropical climacteric fruit and universally comprises a number of species in the genus Musa of the family Musaceae. It is one of the most favored fruits in the world and the fourth most important crop produced globally (Aurore et al. 2009). Nearly all of the identified cultivars derived from two diploid species, Musa acuminata and Musa balbisiana, in which the Cavendish variety is the most common. Plantain is related to the hybrid triploid cultivars of banana and is longer, more angular and diverse in shape. Even in the mature state, plantain is firmer than Cavendish and thus it is less valued as a fresh product (Zhang et al. 2005). According to the latest FAO statistics, Asia is the largest producer of banana with a share of 54.4% of the world’s banana production. With an average banana consumption of 12 kg per capita (FAOSTAT 2017), banana is amongst the world’s major food crops, after rice, wheat and maize.
Banana fruit consists of two parts: peel and pulp. Peel, which is the main by-product of banana, is about 40% of total weight of the fruit. Until recently, banana peel (Bpe) had no useful applications and was dumped as waste, contributing massive amounts of organic materials to be managed. Since researchers have begun to focus on studying the composition of BPe, several possible applications have emerged (Agama-Acevedo et al. 2016). Banana pulp (BP), which is the edible part of the fruit, has an abundant amount of nutrients. Studies conducted on BP have investigated different aspects ranging from its use as an ingredient for food enrichment to extraction and isolation of many health-beneficial components, such as different types of starch, cellulose and bioactive compounds (Singh et al. 2016). As stated by Kitts (1994), bioactive compounds are constituents with extra nutritional advantages that are naturally occurring in plants and foods in small amounts. They exert their beneficial biological effects by stimulating the probiotic growth and help in the prevention of cardiovascular disease and cancer (Kris-Etherton et al. 2002). Phenolics, carotenoids, flavonoids, biogenic amines, phytosterols, and other phytochemicals can be found in BP and peel (Pereira and Maraschin 2015). Due to the presence of these compounds, bananas have a higher antioxidant capacity than some berries, herbs and vegetables (Moongngarm et al. 2014). Bioactive compounds in different cultivars of banana and their health benefits were reviewed in details by Singh et al. (2016).
The increased attention to functional food products and health and wellbeing of consumers in the last decade led to an increased interest in vitamins, minerals, unsaturated fatty acids, bioactive compounds and fibre in food products. The utilization of by-products of fruits, especially banana, has become a trend as of late and many studies are in progress to evaluate their effects on food properties (Chávez-Salazar et al. 2017; Kaur et al. 2014). As approximately one-third of banana is lost due to the public tendency to consume only ripened fruit, utilization of different parts of the banana at different ripening stages has also gained interest over the past years (Sheikh et al. 2017).
This review discusses the health benefits of banana bioactive compounds and utilization of different parts of the banana and the flour produced at various ripeness stages in food applications. Of particular interest, various methods for producing banana flour are evaluated to highlight processing options and their influence on flour nutritional and functional properties.
Composition of banana
Banana pulp is a rich source of essential phytonutrients, including phenolic compounds and vitamins (B3, B6, B12, C and E). It also contains carotenoids, flavonoids, amine compounds and dietary fibre (DF).
Dietary fibres are indigestible carbohydrate polymers that are classified based on their water solubility into two types, soluble fibres (pectin and some hemicelluloses) and insoluble fibres (cellulose, lignin and resistant starch) (Alba et al. 2018). In general, it has been reported that Bpe contains more DF than BP (Garcia-Amezquita et al. 2018). Extracting pectin from the peels could increase their added value. In addition, BPe has high amount of lignin, cellulose and hemicelluloses fractions which can be extracted as a formed complex substrate named lignocellulosic biomass which could be used to produce bioethanol (Happi Emaga et al. 2008). Also Khamsucharit et al. (2018) signified that the extracted pectin from Bpe could be an alternative source for commercial pectin.
One of the most important DF which has gained a lot of attention in recent years is resistant starch (RS). It is mainly composed of the linear part of starch (amylose) which is fermented by probiotics in the colon, specifically Bifidobacterium and Lactobacillus species (Kale et al. 2002). This brings about the production of short chain fatty acids, mainly butyric acid, which has a key role in prevention of colorectal cancer (Amini et al. 2015). There are five types of RS introduced up to now: starch that is physically inaccessible in crop’s cell walls (RS1), granular native starch with high crystalline structure (RS2), retrograded starch achieved by heating and cooling of starchy foods (RS3), chemically modified starch (RS4) and amylose-lipid complex (RS5) (Amini Khoozani et al. 2019). Fractionation of the DF and RS is different parts of banana in different levels of maturation is shown in Table 1.
Table 1.
Composition of banana flour produced from different ripening stage (dry basis percentage)
| Banana maturity | Flour base | Starch | RS | DF | Ash | Lipid | Protein | References |
|---|---|---|---|---|---|---|---|---|
| Green (stage 1–2) | Pulp | 64–75 | 17.5–48 | 7.5–15 | 2.6–4.7 | 0.4–2.7 | 6.5–14.3 | Haslinda et al. (2009), Juarez-Garcia et al. (2006), Menezes et al. (2011) |
| Peel | 10.1–11.7 | 8.2–8.6 | 43–50 | 1.2–9.6 | 6.1–9 | 4.1–8.1 | Agama-Acevedo et al. (2016), Eshak (2016), Haslinda et al. (2009), Nasrin et al. (2015) | |
| Yellow (stage 6–7) | Pulp | 56–63 | 11–17 | 17–18 | 3.3–6.9 | 0.6–1.2 | 3.76 | Aurore et al. (2009), Da Mota et al. (2000), Segundo et al. (2017b) |
| Peel | 3.5–6.3 | 2.3–2.5 | 47–53 | 9–11 | 3.8–11 | 5–8 | Kurhade et al. (2016), Ramli et al. (2009), Zhang et al. (2005) |
RS resistant starch, DF dietary fiber
Unripe banana is rich in RS2, which is beneficial to colon health, while ripe banana contains more digestible starch and protein (Singh et al. 2016). Banana peel is a rich source of minerals, bioactive compounds and DF (Kusuma et al. 2018). Several studies reported the use of banana peel flour (BPeF) as a functional food source (Agama-Acevedo et al. 2016; Ramli et al. 2009; Ramli et al. 2010; Türker et al. 2016). According to some reports, both pulp and peel have high antioxidant activity (Agama-Acevedo et al. 2016; González-Montelongo et al. 2010). As lipid oxidation in food components is one of the unwanted reactions causing rancidity, food producers rely on synthetic antioxidants to minimize lipid deterioration. Potential health risks, however, is a limiting factor of using these preservatives extensively in food products, especially staple ones (Pathak et al. 2017). Given that BPe extract has been found to be non-toxic to human cells, more information has become available on using it as an inexpensive fruit by-product source of antioxidants (Segundo et al. 2017b). The amount of ash, protein, crude fibre and digestible starch of BPeF was reported to be significantly higher than that of pulp, which makes the BPeF more effective as a functional additive (Nasrin et al. 2015). For instance, the higher quantity of ash can be valuable in treating deficiencies of minerals caused by celiac disease (Presutti et al. 2007). Additionally, several studies have shown the application of BPe as a low-cost precursor for producing materials such as anionic dye and heavy metal adsorbents (Mahindrakar and Rathod 2018; Munagapati et al. 2018; Oyewo et al. 2018; Singh et al. 2018; Vilardi et al. 2018), recovering phenolic compounds (Vu et al. 2018), producing cellulose nanofibers (Costa et al. 2018; Harini et al. 2018; Tibolla et al. 2018), as well as bioethanol (Berawi and Bimandama 2018; Prakash et al. 2018) and pectin extract (Khamsucharit et al. 2018). In the following sections, some of the exclusive added value components in banana are introduced.
Health effects of banana bioactive compounds
Carotenoids are natural antioxidants which contribute to the stability of foods during storage. Previous studies documented the existence of various carotenoids in banana fruit (Davey et al. 2006). Although some suggested that the cultivars genotype specifies the quantity of carotenoids, they mostly concurred that the amount of trans-alpha and trans-beta carotene comprised the majority of pro-vitamin A compounds (Yan et al. 2016). Another significant carotenoid reported was lutein, which exhibited antioxidant properties and an inhibitory effect on the age-related macular degeneration. Interestingly, it has been identified that green banana peel (GBPe) has substantially higher carotenoids than the pulp (Davey et al. 2006).
Phytochemicals, especially phenolic acids, are the main bioactive compounds known for exerting health benefits. Unexpectedly, the percentage of phenolic compounds have been reported to be greater in peel than in pulp (Kanazawa and Sakakibara 2000). Similarly, it was demonstrated that the quantity of gallocatechins in peel was five times greater than pulp. With regard to presented results, BPe extract was found to inhibit lipid oxidation better than pulp extract (Someya et al. 2002). Recently, it has been shown that gallocatechin extracted from GBPe was effective in the healing of surgical wounds in rats (Von Atzingen et al. 2015). Correspondingly, a unique flavonoid named leucocyanidin was found in aqueous extract of unripe plantain pulp, which is now known to be effective in the treatment of gastric diseases (Lewis et al. 1999).
Biogenic amines play a key role in the prevention of depression. Catecholamines, dopamine, norepinephrine (noradrenaline) and epinephrine (adrenaline) are the best-known examples of these bioactive compounds which regulate hormones in glycogen metabolism (González-Montelongo et al. 2010). Results of dopamine levels in different ripening stages of banana revealed an inverse relation between its concentration and fruit’s maturity, noting that BPe contained more dopamine than pulp (Kanazawa and Sakakibara 2000). The possibility of BPe application as a dopamine biomass source for the prevention of Parkinson’s disease was also discussed (Pereira and Maraschin 2015). Furthermore, in comparison to other plant residual biomasses, dopamine has exerted higher antioxidant capacity in vitro (Babbar et al. 2011).
There is a wide range of DF in banana fruit, including pectin, cellulose, lignin and hemicellulose which can be found naturally in banana flour (BF). Amongst them, RS is the most notable one which provides bioactive effects (Thebaudin et al. 1997). Resistant starch, which is mainly composed of the linear part of starch (amylose), is resistant to digestion in the small intestine after 2 h incubation (Homayouni et al. 2014). After it reaches the colon undigested, it will be fermented by membrane microbiota (mainly probiotics) and cause pH reduction. Therefore, the environment will be undesirable for the growth of pathogenic microbiota and formation of carcinogenic cells (Khalili and Amini 2015).
A considerable amount of literature has been published on the direct relationship between RS intake and reduction of the large bowel cancer risks either in vitro or in vivo; it seems that RS may be a major protective factor against colorectal cancer (Panebianco et al. 2017; Yin and Zhao 2017).
It was shown that the amount of RS decreased from 8 to 2% with progression in peel ripeness. However, the percentage of total dietary fibre (TDF) slightly increased in yellow banana peel (YBPe) (Ramli et al. 2010). In a parallel report, the RS and TDF content of green banana pulp flour (GBPF) reported 49.9% and 7.2%, respectively (Menezes et al. 2011). In general, it can be concluded that because approximately 70% (dry basis) of the peeled green banana comprises RS2, the unripe pulp is a remarkable source of this kind of bioactive compound (Wang et al. 2017a).
It is well-known that phytosterols act as immune system modulators and exert cholesterol-lowering and anticancer properties in the intestine (González-Montelongo et al. 2010).
As reported by (Marangoni and Poli 2010), a daily intake of phytosterols up to 3 grams/day significantly reduced total and LDL cholesterol. A study pointed to a high amount of phytosterols, mainly beta-sitosterol, campesterol and stigmasterol, which can be found in whole green banana flour (GBF). After they investigated the effect of pre-treatments on BF and although citric acid treatment gave rise to phytosterol reduction in flour obtained from the peel, no change was detected between phytosterols of acidified and control samples from the pulp (Bertolini et al. 2010). Consequently, the known properties of phytosterols suggest these flours could be used as functional food components.
Methods for banana flour preparation
Due to the climacteric nature of the banana, it is highly perishable and requires drying during processing for preserving for a longer period of time. Banana flour is a product with high storability potential and long shelf life and can be readily applied to food products. The proximate composition of the flour also depends on the origin, variety, time of harvest, and drying procedure of bananas (Haslinda et al. 2009). Table 1 depicts a summary of the proximate composition of both green and yellow BF made from pulp and peel.
Processing steps used for flour preparation from banana pulp and peel are similar, except for the heating procedure used. Pretreatment process steps of banana flour preparation are summarized in Fig. 1.
Fig. 1.
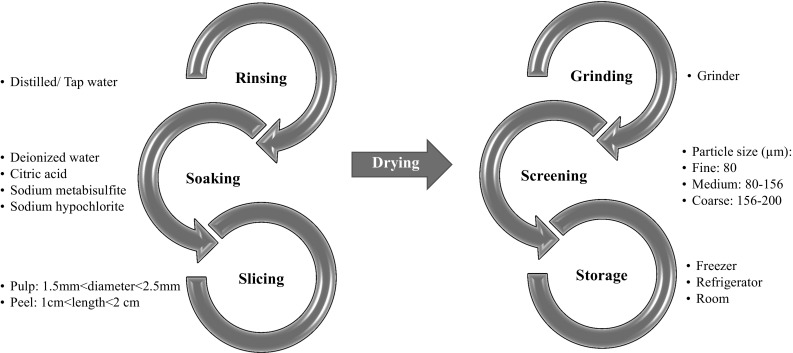
Pretreatment process steps of banana flour preparation
While most researchers applied oven drying for banana fruit (Gomes et al. 2016; Kurhade et al. 2016; Nasrin et al. 2015; Segundo et al. 2017a, b; Türker et al. 2016), spouted bed drying (Bezerra et al. 2013a, b) and lyophilization (Da Mota et al. 2000; Türker et al. 2016; Wang et al. 2012) were also applied in previous studies. In order to minimize enzymatic browning, soaking in sodium metabisulfite, sodium hypochlorite or citric acid solutions was a general pretreatment and considered as the first step after rinsing bananas with water.
The composition of samples obtained by lyophilization and spouted drying techniques showed a significant increase in phenolic acid content, heat sensitive vitamins and minerals compared to traditional drying methods such as solar or hot air-oven drying. Using a spouted drier for producing GBF resulted in high DF and RS content with an average of 21.91% and 68.02%, respectively. This technique did not alter the RS content; however, this effect was consistently reported since a similar study reported lower values, 13.89% and 40.14% for DF and RS, respectively (Bezerra et al. 2013a).
Likewise, post-treatments could affect the composition of BF produced. For example, smaller particle sizes of GBPF (less than 80 µm diameter) had a higher amount of RS, while flour particles bigger than 156 µm had more TDF, ash, protein and phenolic compounds (Segundo et al. 2017a). Briefly, depending on the enrichment purpose, selecting a proper procedure is imperative. Table 2 demonstrates the effect of various drying process on produced banana flour properties regarding the type and part of banana used.
Table 2.
The effect of different drying methods on banana flours
| Method | Conditions | Banana flour type | Remarks | Reference |
|---|---|---|---|---|
| Room drying | 23 °C 6 days |
GBPeF | Minimum or no maillard reaction Long preparation time |
Eshak (2016) |
| Oven drying | 50–60 °C 12–24 h |
GBPF | ↑ Phenolic acids with in higher temperatures ↓50% of RS in final flour |
Alkarkhi et al. (2011), Juarez-Garcia et al. (2006), Segundo et al. (2017a), Yangilar (2015) |
| Air flow drying | 55 °C, 6 h 1.4 m/s |
GBPF | RS content remained constant between GBPF and banana paste Low dispersibility and solubility in water |
Tribess et al. (2009) |
| Spouted bed with hot air flow | 80 °C 50 m3/h |
GBF GBPF | No need for grinding process after drying Maintaining RS to 35% in GBF and 42% in GBPF RS content remained constant between GBPF and banana paste Low solubility under cold conditions |
Bezerra et al. (2013b) |
| Pulsed-fluidized bed agglomeration | 95 °C, 0.3 m/s 10 Hz 1 m/s (air flow) |
GBPF | Lower wetting time Dispersible in cold water Higher level of RS achieved in GBPF |
Rayo et al. (2015) |
| Ultrasound and pulsed-vacuum followed by air drier | 20–25 min (ultrasound) 60 min under 50 kPa pressure (vacuum convective drier) 50 °C (air drying) |
GBPF | ↓RS in US pretreatment followed by 60°C heating in GBPF No significant increase in RS amount |
La Fuente et al. (2017) |
| Microwave heating | 960 W for 6 min | YBPeF | ↓Lightness more than other drying methods Lowest drying time Minimum loss of bioactive compounds, total phenolic and antioxidant activity Maximum moisture content compared to other drying methods (but below the safe limit) |
Vu et al. (2017) |
| Freeze drying | − 50°C, 12–48 h | GBPeF GBPF GBPF YBPF |
Maintained the original colour regarding the least browning reaction Highest value of total flavonoids reported in YBPeF |
Da Mota et al. (2000), Türker et al. (2016), Vu et al. (2017), Wang et al. (2012) |
| Spray drier | 27,000 rpm (atomizer rotation) 50% (biomass concentration) 30 °C (biomass temperature) 40 mL/min (biomass flow rate) |
GBPF | Moisture content of 9.17% Fastest method after microwave No analytical measurements |
Oi et al. (2013) |
| Dehumidified drier | 60 °C, 13 h | YBPeF | Highest flour lightness after freeze drying Lowest phenolic compounds |
Vu et al. (2017) |
GBPeF green banana peel flour, GBPF green banana pulp flour, YBPeF yellow banana peel flour, YBPF yellow banana pulp flour, RS resistant starch
Banana flour applications
The high dietary fibre content of GBPF and high levels of mentioned bioactive compounds have enabled the production of BF foodstuffs with remarkable functionalities. Moreover, discovering high nutrition value of BPeF represents a low-cost by-product for industrial application. As discussed earlier, depending on the ripening stage, there are four flour products produced from banana with different chemical composition. Due to the structure of BF, cereal-based products have gained more attention than other food products. The main starch-based foods targeted for enrichment with BF products are as follows.
Bread
In a study by Juarez-Garcia et al. (2006), GBF was obtained from a Mexican species (Musa paradisiacal L.) to develop a high gluten bread using 37% GBF in the formulation. Chemical composition analysis showed an increase in ash, protein, TDF and starch percentage of banana bread compared to wheat bread. Even though RS was decreased from 17.5% in flour to 6.7% in banana bread, it was significantly higher than the control sample. The authors also reported a significant decrease in predicted Glycemic Index (pGI) and Hydrolysis Index (HI) of the final product; a result that was in accordance with a higher value of total indigestible fraction (TIF), the main ingredients unavailable for digestion in the small intestine. However, rheological and sensory properties and shelf life of banana bread were not determined. In another study, with more attention to the sensory characteristics, GBF substitution with wheat flour resulted in lower sensory scores in 20% enriched-samples (Gomes et al. 2016). Moreover, darker colour, higher hardness and lower specific volume compared to control sample indicated negative effects of this enrichment. While TDF and ash content increased significantly in 20% substitution, the 10% substituted samples was regarded the best as it had no significant technological defects (Gomes et al. 2016).
In a study on gluten-added bread, substitution of 25% of ready freeze-dried banana powder (maturity level was not mentioned) with wheat flour resulted in increased volume and viscosity of leavened bread (Mohamed et al. 2010). Banana bread was darker than the control due to excess sugar in the BF that caused the Maillard Reaction to occur between reducing sugars and proteins. Regarding the evaluation of shelf life, while bread staling (firmness) increased in higher BF concentration regardless of storage temperature (25 °C, 4 °C and − 20 °C), the stiffness of the control was higher than BF bread (Mohamed et al. 2010). By comparing storage temperature, it was stated that bread stored at − 20 °C up to 7 days, experienced lower firmness compared to other samples (Mohamed et al. 2010). Similar findings reported by (Ho et al. 2013) who prepared a steam bread with 30% GBPF wheat flour substitution. In terms of mineral evaluation, the percentage of Mg, K, Na and Ca were higher than the control, and yielded consequently higher ash content. Increased TDF and RS were also reported to a level of 9% and 5%, respectively. However, the adverse impact of GBPF and added gluten caused an increase in hardness and adhesiveness in the produced bread. The cohesiveness, elasticity and chewiness of bread supplemented with 30% GBPF were decreased due to the lack of consistency in gluten structure. With reference to higher specific volume in banana bread samples containing 8% gluten, researchers explained that GBPF could affect the gluten network and attenuate the gas holding capability of the dough, which leads to low elasticity and expansion in leavened bread. These findings were in agreement with (Steel et al. 2013) and consistent with those reported by (Gomes et al. 2016; Kurhade et al. 2016) which focused on yellow banana peel flour (YBPeF) and GBF, correspondingly.
Considering nutritional properties, a reduction in TDF level and a slight increase in RS level (2.6%) were observed in a freeze-dried tortilla bread containing 40% GBPF substituted with corn flour. Moreover, higher values of pGI and HI than control showed that GBPF addition may not be a proper enrichment strategy for the purpose of producing a low-calorie tortilla bread (Aparicio-Saguilan et al. 2013). Additionally, the high amount of RS2 in corn flour, should lead to a major increase in RS amount. It can be concluded that RS2 is sensitive to the cooking process and most of it gelatinizes during the process (Robles-Ramírez et al. 2012).
A comprehensive research was carried out on composite bread with 10% peeled yellow banana pseudo-stem flour substitution with wheat flour. Due to the dilution of gluten in substituted samples, crumb and crust lightness improved in the banana bread. A significant increase in total phenolic content, ash and TDF demonstrated the fact that peeled mature banana pseudo-stem flour could be effective in producing a functional composite bread. Besides, no significant difference was observed between samples and control, except for colour and softness in a sensory analysis (Ho et al. 2013). In their following study, researchers also found an interaction between gum type and BF effect on volume, in which the addition of 0.8% sodium carboxymethyl cellulose (NaCMC) improved specific volume, minerals (Na, K, Mg and Ca) and RS content (14.98%) more than the same amount of xanthan. NaCMC, which is a soluble DF, acts like a sponge and absorbs water in the intestine. Therefore, it helps in mixing with the starchy food’s structure to form a dense structure that results in slowing down the rate of digestion (Ho et al. 2015). As a result, it can be a suitable additive together with peeled yellow banana pseudo-stem flour.
In two separate studies, different types of flat bread were enriched with the addition of BPeF in two stages of ripeness. In another report, (Kurhade et al. 2016) found that with the substitution of YBPeF at any ratio with wheat flour in chapatti bread, lightness will decline. Because of the increased water absorption, chapatti containing 10% YBPeF was found to be softer owing to a decrease in the tear force. In terms of antioxidants, total phenolic content, flavonoids and free radical scavenging activity were higher to than that of the control; which justified the high concentration of these elements in YBPeF. Though, overall acceptability score was not distinguished by panelists up to 10% of substitution. Nevertheless, when green banana peel flour (GBPeF) was added to a level of 10% in balady bread in another study, the control sample scored better in all sensory parameters, except taste and chewiness. Contrary to previous findings (Eshak 2016) also reported a higher amount of protein in banana bread (12.52%) which was ascribed to the slightly higher amount of protein in GBPeF (8.74%) compared to wheat flour (8.68%). As there was no textural analysis of balady bread in this research, further work is needed to address the technological quality of GBPeF enriched bread. Similarly, increased protein and TDF content were reported in leavened bread containing 20% and 30% fermented green banana slurry substituted with wheat flour. However, in accordance with the application of GBPeF in previous studies, sensory scores diminished for all treatments with more than 10% substitution (Adebayo-Oyetoro et al. 2016). Again, textural analysis and firmness during storage were not considered, therefore it is hard to validate the quality of bread produced.
It follows from the above that there are contrary results on BF usage in bread products. Also, more information of bread enrichment with GBPeF and its technological effect on the final product is required.
Pasta
Pasta products are foodstuffs with an important role in diets. In addition to being easily produced with a long shelf life, pasta products also have a lower glycemic index (GI) in comparison with white bread or rice (Nilsson et al. 2008). Hence, enrichment of pasta products with different DF-enriched flours and micronutrients has been considered in the last decade (Filipović et al. 2010).
In 2009, the properties of spaghetti enriched with different substitution ratios of GBPF with semolina flour was investigated in two studies. Following a similar trend in most of the banana bread products, a reduction in lightness, protein and fat content of spaghetti containing 20, 35 and 40% GBPF was reported (Ovando-Martinez et al. 2009). However, textural results indicated increased adhesiveness and chewiness compared to the control, which was because of the release of amylose from starch granules during cooking. This also caused a rise in cooking loss (less than 7%) with increasing GBPF in the formulation. Yet, since a cooking loss below than 8% is considered as acceptable for semolina-based pasta products, it can be said it was not a negative effect. On account of the high amount of RS (12%) and incomplete gelatinization of starch granules, enriched samples showed lower values in vitro digestion tests.
In a study conducted by Agama-Acevedo et al. (2009), produced GBPF comprised 42.54% RS2 (dwb). With regard to a higher percentage of polyphenols and antioxidant capacity of banana pasta, it was concluded that enrichment of spaghetti with 30% GBPF could provide a product without off-flavours (Agama-Acevedo et al. 2009; Ovando-Martinez et al. 2009). These results were confirmed by Krishnan and Prabhasankar (2010) findings a year later. Besides, they found a synergic relation between sprouted Ragi Flour and GBPF; in which a combination of 15% sprouted Ragi Flour and 15% GBPF proved to be the best for nutritional and technological attributes.
Incorporation of green banana parts, individually, has also been considered in pasta products.
A separate substitution of 10% pulp and peel (obtained from two different varieties) with wheat flour was conducted to produce alkaline noodles. Since the GBPeF was higher in TDF but lower in RS content than the GBPF, the low pGI of banana peel noodles was primarily because of its high TDF. Whilst pulp enriched noodles originated from the Cavendish variety, exhibited higher elasticity, enriched samples with GBPeF from the Dream variety showed the lowest values. In discussion, it was declared that higher sugar content was responsible for high levels of total solid content which led to a rise in the density of the molecular structure (Ramli et al. 2009).
In a very recent study, banana flour was prepared from whole green banana and added to tagliatelle pasta up to 30% substitution with wheat flour. Contrary to previous findings, there was no evidence of darker colour in banana pasta which possibly was due to the use of wheat flour instead of semolina flour. But in support of previous researches, banana pasta presented higher ash, TDF and total phenolic content than the control, though 15% substitution showed more ash content. Likewise, the mentioned sample showed better sensory attributes by panelist scores (Zheng et al. 2016). Because rheological properties and some important physical properties, such as cooking loss, were not assessed, it is hard to determine the effect of GBF on quality of the final pasta product.
Confectionaries
Due to its high sugar content, utilization of BF in confectionaries has been heightened by the food industry, specifically cereal-based ones. The growth of pathogens in a cake premix made with 60% GBPF instead of wheat flour over 4 months of storage was investigated. Despite a high sugar concentration, the pre-mixture remained significantly unaltered in pH and pathogenic growth, fungus or yeasts (Borges et al. 2010). In another study, foaming stability and overall acceptability increased in the presence of 10% BPF. Also, with increased content of BPF to 20% of the formulation, higher hardness was reported, although chewiness and adhesiveness were not significantly different amongst samples. The reason behind this phenomenon was the lower amount of moisture content in banana cakes (Park et al. 2010).
With the aim of increasing DF and RS in layer and sponge cakes, GBPF was added at different particle sizes; ranging from 80 µm (fine) to 200 µm (coarse) in diameter. Researchers showed that the fine flour comprised 40% RS compared to 25% RS in the coarse flour. This fact specified higher RS content (about 3%) in 30% replacement samples with fine flours in both layer and sponge cakes. However, the percentage of TDF, protein, ash, lipid, phenolic compounds and amylose was higher in the coarse flour. In terms of technological properties, sponge cakes were noticeably worsened with the presence of banana flours (lower specific volume, inferior sensory characteristics and higher hardness), which was diminished at the 15% ratio; except for cohesiveness that showed a dramatic decrease in all samples compared to the control. The authors accounted for different gelatinization and retrogradation behaviour of banana starch compared to wheat starch for textural changes and decreased sensory scores in banana cakes. Still, samples made by fine particle sizes of GBPF showed better nutritional properties without negatively affecting textural attributes (Segundo et al. 2017a). The same results were reported in a similar study by selection of YBPF in 40% substitution with sugar. Both sponge and layer cakes depicted enhancement in DF, polyphenols and antioxidant capacity values. In concurrence with their previous work, increased hardness and decreased volume led to the decline of the acceptability of cakes by panelists, especially in 40% banana cakes. Considering the correlation between volume and hardness was more significant in sponge than in layer cakes, the maturity of banana was not correlated to improvements of textural properties (Segundo et al. 2017b). The same behavior was observed by (Oliveira de Souza et al. 2018), even though they used a higher concentration of GPB puree instead of flour for pound cakes.
Cookies containing 20% of YBPeF were rich in TDF and were categorized as a low-calorie product (Agama-Acevedo et al. 2012). The same pattern was reported by Elaveniya and Jayamuthunagai (2014) in which banana blossom powder was added at 5 g in 100 g of biscuit formulation. However, consumer acceptance of samples for colour, crispiness and taste decreased with the addition of BPe, while it was acceptable at 5% substitution.
In another study on biscuit, YBPeF was substituted up to 75% with wheat flour and caused a decline in hardness. According to the discussions, prior treatments of mashed peels together with DF led to an increase in softness of the product. While a high level of consistency and crispiness is required in a biscuit product, dilution of gluten in higher concentrations of YBPeF led to insignificant decrease of these attributes in all enriched samples. Organoleptic results were not significantly different in terms of colour, flavour, after taste and mouthfeel at even the 75% level of substitution (Joshi 2007). These findings were consistent with those reported by Carvalho and Conti-Silva (2018), where enrichment of cereal bars with 14% YBPeF exerted no negative effect on aroma or taste, while further incorporation caused a darker colour, more hardness and adhesiveness together with a bitter aftertaste.
Overall, replacement of BF, regardless of ripeness degree, was feasible up to 15% for achieving optimal quality and maximum 30% for functional purposes in confectionaries. Still, quality control of produced different types of cakes with BPeF is needed to be instigated.
Gluten-free products
Because of the rise of the gluten related disorders, such as celiac disease and dermatitis herpetiformis, it has been essential to expand the gluten-free (GF) food market. Moreover, considering that untreated celiac disease contributes to intestinal cancer, nutrient deficiencies and oxidative stress, developing GF products with the consideration of additional nutritional values is highly important (Wang et al. 2017b). In this regard, bioactive compounds are a prominent ingredient in GF foods. As most of GF starchy products do not provide proper technological qualities, application of different types of BF has been considered as of late (Torres et al. 2017).
By incorporation of 47% GBPF in 100 g pasta formulation, Zandonadi et al. (2012) produced a GF pasta with an additional proportion of egg white and hydrocolloids. Regarding excess amount of water absorption during cooking, increased stickiness of the product led to a weakened structure. Another negative effect was the poor nutritional value of the product that was exhibited by a reduction in TDF and protein content. On the other hand, improvements in ash content and sensory scores were reported. These findings were in agreement to Radoi et al. (2015), who reported a growth in total phenolic acids, cinnamic acids and minerals (mainly Fe, Cu, Zn, Mn, Ni) with presence of 30–40% dried banana. Yet, textural and cooking properties of GF pasta were missing important assessments.
Gluten free bread has also been produced by the use of BF. In a study conducted by Sarawong et al. (2014), while crumb firmness increased in 15% of green plantain flour addition, bread volume improved by 25% addition at a lower baking temperature over a longer time. An elevated proportion of green plantain flour in GF bread contributed to higher water binding capacity and a reduction in starch retrogradation owing to the presence of extra water. In addition to darker crumb at 5% addition level, RS increased only up to 2.5% compared to the control sample.
In sweetened bread, both green and yellow banana GF bread showed lower volume, lesser height and darker colour, but, when black banana pulp flour was utilized at 20% of the formulation, those variables improved to a significant level. However, sensory evaluation, shelf life and textural properties of bread produced were overlooked (Seguchi et al. 2014).
The substitution of 15% of GBPeF was with rice flour in GF cake gave rise to a decrease in GF cake volume. Due to an increase in viscosity, the density of banana GF cakes rose (Türker et al. 2016). Once more, without considering textural, sensory and storability properties of the final product in the later study, it is of importance to note that more studies are needed to be done in GF starch-based food products with the aim of considering of high nutritional value and technological quality of final product.
Conclusion
It is desirable to find proper food applications for banana peel for achieving two goals; first, helping the environment through sustainability by utilizing secondary processing products and second, creating a new outlook for consumers and producers for generating value-added food products. Besides adding nutritional value to food products, GBF also stands out for not creating production waste, thus representing the complete use of the fruit, increasing the yield and reducing manpower costs due to peeling which is not required.
In gluten-added starchy products, such as bread, cake and pasta, textural properties were not negatively affected by banana flour addition to a certain extent. However, in gluten-free products, more research on improving rheological and cooking properties need to be undertaken; especially investigation on the application of green and yellow banana peel.
As discussed in this review, there are several methods for producing banana flour. Comparing new drying technologies with existing methods and their effect on bioactive components of produced products would be an important subject for future research. Also, further studies, especially clinical trials are needed to be considered in order to confirm the health benefits of banana flour enriched food products.
Acknowledgements
We would like to show our gratitude to the University of Otago for their support of this study.
Compliance with ethical standards
Conflict of interest
The authors declare that they do not have any conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amir Amini Khoozani, Email: amir.amini@postgrad.otago.ac.nz.
John Birch, Phone: 64 3 479 7566, Email: john.birch@otago.ac.nz.
Alaa El-Din Ahmed Bekhit, Email: aladin.bekhit@otago.ac.nz.
References
- Adebayo-Oyetoro AO, Ogundipe OO, Adeeko KN. Quality assessment and consumer acceptability of bread from wheat and fermented banana flour. Food Sci Nutr. 2016;4:364–369. doi: 10.1002/fsn3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agama-Acevedo E, Islas-Hernandez JJ, Osorio-Díaz P, Rendón-Villalobos R, Utrilla-Coello RG, Angulo O, Bello-Pérez LA. Pasta with unripe banana flour: physical, texture, and preference study. J Food Sci. 2009;74:S263–S267. doi: 10.1111/j.1750-3841.2009.01215.x. [DOI] [PubMed] [Google Scholar]
- Agama-Acevedo E, Islas-Hernández JJ, Pacheco-Vargas G, Osorio-Díaz P, Bello-Pérez LA. Starch digestibility and glycemic index of cookies partially substituted with unripe banana flour LWT - Food. Sci Technol. 2012;46:177–182. [Google Scholar]
- Agama-Acevedo E, Sañudo-Barajas JA, Vélez De La Rocha R, González-Aguilar GA, Bello-Peréz LA. Potential of plantain peels flour (Musa paradisiaca L.) as a source of dietary fiber and antioxidant compound CYTA. J Food. 2016;14:117–123. [Google Scholar]
- Alba K, MacNaughtan W, Laws AP, Foster TJ, Campbell GM, Kontogiorgos V. Fractionation and characterisation of dietary fibre from blackcurrant pomace Food Hydrocoll. 2018;81:398–408. [Google Scholar]
- Alkarkhi AFM, Ramli SB, Yong YS, Easa AM. Comparing physicochemical properties of banana pulp and peel flours prepared from green and ripe fruits. Food Chem. 2011;129:312–318. doi: 10.1016/j.foodchem.2011.04.060. [DOI] [PubMed] [Google Scholar]
- Amini Khoozani A, Birch J, El-Din Ahmed Bekhit A. Resistant starch preparation methods. In: Melton L, Shahidi F, Varelis P, editors. Encyclopedia of food chemistry. Oxford: Academic Press; 2019. pp. 390–394. [Google Scholar]
- Amini A, Khalili L, Keshtiban AK, Homayouni A (2015) Resistant starch as a bioactive compound in colorectal cancer prevention. In: Probiotics, prebiotics, and synbiotics: bioactive foods in health promotion. Elsevier Inc., pp 773–780. 10.1016/b978-0-12-802189-7.00058-7
- Aparicio-Saguilan A, Osorio-Díaz P, Agama-Acevedo E, Islas-Hernández JJ, Bello-Perez LA. Tortilla added with unripe banana and cassava flours: chemical composition and starch digestibility CYTA J Food. 2013;11:90–95. [Google Scholar]
- Aurore G, Parfait B, Fahrasmane L. Bananas, raw materials for making processed food products. Trends Food Sci Technol. 2009;20:78–91. [Google Scholar]
- Babbar N, Oberoi HS, Uppal DS, Patil RT. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res Int. 2011;44:391–396. [Google Scholar]
- Berawi KN, Bimandama MA. The effect of giving extract etanol of kepok banana peel (Musa Acuminata) toward total cholesterol level on male mice (Mus Musculus L.) strain deutschland-denken-yoken (ddy) Obese. Biomed Pharmacol J. 2018;11:769–774. [Google Scholar]
- Bertolini AC, Bello-Pérez LA, Méndez-Montealvo G, Almeida CAS, Lajolo F. Rheological and functional properties of flours from banana pulp and peel Starke. 2010;62:277–284. [Google Scholar]
- Bezerra CV, Amante ER, de Oliveira DC, Rodrigues AMC, da Silva LHM. Green banana (Musa cavendishii) flour obtained in spouted bed—effect of drying on physico-chemical, functional and morphological characteristics of the starch. Ind Crop Prod. 2013;41:241–249. [Google Scholar]
- Bezerra CV, Rodrigues AMC, Amante ER, da Silva LHM. Nutritional potential of green banana flour obtained by drying in spouted bed. Rev Bras Frutic. 2013;35:1140–1146. [Google Scholar]
- Borges AM, Pereira J, Silva Júnior A, de Lucena EMP, de Sales JC. Stability of cake pre-mixture made with 60% of green banana flour. Cienc Agrotecnol. 2010;34:173–181. [Google Scholar]
- Carvalho VS, Conti-Silva AC. Cereal bars produced with banana peel flour: evaluation of acceptability and sensory profile. J Sci Food Agric. 2018;98:134–139. doi: 10.1002/jsfa.8447. [DOI] [PubMed] [Google Scholar]
- Chávez-Salazar A, Bello-Pérez LA, Agama-Acevedo E, Castellanos-Galeano FJ, Álvarez-Barreto CI, Pacheco-Vargas G. Isolation and partial characterization of starch from banana cultivars grown in Colombia. Int J Biol Macromol. 2017;98:240–246. doi: 10.1016/j.ijbiomac.2017.01.024. [DOI] [PubMed] [Google Scholar]
- Costa ALR, Gomes A, Tibolla H, Menegalli FC, Cunha RL. Cellulose nanofibers from banana peels as a pickering emulsifier: high-energy emulsification processes. Carbohydr Polym. 2018;194:122–131. doi: 10.1016/j.carbpol.2018.04.001. [DOI] [PubMed] [Google Scholar]
- Da Mota RV, Lajolo FM, Ciacco C, Cordenunsi BR. Composition and functional properties of banana flour from different varieties. Starke. 2000;52:63–68. [Google Scholar]
- Davey MW, Keulemans J, Swennen R. Methods for the efficient quantification of fruit provitamin A contents. J Chromatogr A. 2006;1136:176–184. doi: 10.1016/j.chroma.2006.09.077. [DOI] [PubMed] [Google Scholar]
- Elaveniya E, Jayamuthunagai J. Functional, physicochemical and anti-oxidant properties of dehydrated banana blossom powder and its incorporation in biscuits. Int J Chemtech Res. 2014;6:4446–4454. [Google Scholar]
- Eshak NS. Sensory evaluation and nutritional value of balady flat bread supplemented with banana peels as a natural source of dietary fiber AOAS. 2016;61:229–235. [Google Scholar]
- FAOSTAT (2017) Top production-bananas-2016. Food and Agriculture Organisation of the United Nations. http://www.fao.org/economic/est/est-commodities/bananas/en/
- Filipović J, Filipović N, Filipović V. The effects of commercial fibres on frozen bread dough. J Serb Chem Soc. 2010;75:195–207. [Google Scholar]
- Garcia-Amezquita LE, Tejada-Ortigoza V, Heredia-Olea E, Serna-Saldívar SO, Welti-Chanes J. Differences in the dietary fiber content of fruits and their by-products quantified by conventional and integrated AOAC official methodologies. J Food Compost Anal. 2018;67:77–85. [Google Scholar]
- Gomes AAB, Ferreira ME, Pimentel TC. Bread with flour obtained from green banana with its peel as partial substitute for wheat flour: physical, chemical and microbiological characteristics and acceptance. Int Food Res J. 2016;23:2214–2222. [Google Scholar]
- González-Montelongo R, Gloria Lobo M, González M. Antioxidant activity in banana peel extracts: testing extraction conditions and related bioactive compounds. Food Chem. 2010;119:1030–1039. [Google Scholar]
- Happi Emaga T, Robert C, Ronkart SN, Wathelet B, Paquot M. Dietary fibre components and pectin chemical features of peels during ripening in banana and plantain varieties. Bioresour Technol. 2008;99:4346–4354. doi: 10.1016/j.biortech.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Harini K, Ramya K, Sukumar M. Extraction of nano cellulose fibers from the banana peel and bract for production of acetyl and lauroyl cellulose. Carbohydr Polym. 2018;201:329–339. doi: 10.1016/j.carbpol.2018.08.081. [DOI] [PubMed] [Google Scholar]
- Haslinda WH, Cheng LH, Chong LC, Aziah AAN. Chemical composition and physicochemical properties of green banana (Musa acuminata × balbisiana Colla cv. Awak) flour. Int J Food Sci Nutr. 2009;60:232–239. doi: 10.1080/09637480902915525. [DOI] [PubMed] [Google Scholar]
- Ho LH, Abdul Aziz NA, Azahari B. Physico-chemical characteristics and sensory evaluation of wheat bread partially substituted with banana (Musa acuminata X balbisiana cv. Awak) pseudo-stem flour. Food Chem. 2013;139:532–539. doi: 10.1016/j.foodchem.2013.01.039. [DOI] [PubMed] [Google Scholar]
- Ho LH, Tan TC, Abdul Aziz NA, Bhat R. In vitro starch digestibility of bread with banana (Musa acuminata X balbisiana ABB cv. Awak) pseudo-stem flour and hydrocolloids. Food Biosci. 2015;12:10–17. [Google Scholar]
- Homayouni A, Amini A, Keshtiban AK, Mortazavian AM, Esazadeh K, Pourmoradian S. Resistant starch in food industry: a changing outlook for consumer and producer. Starke. 2014;66:102–114. [Google Scholar]
- Joshi RV. Low calorie biscuits from banana peel pulp. J Solid Waste Technol Manag. 2007;33:142–147. [Google Scholar]
- Juarez-Garcia E, Agama-Acevedo E, Sáyago-Ayerdi SG, Rodríguez-Ambriz SL, Bello-Pérez LA. Composition, digestibility and application in breadmaking of banana flour. Plant Foods Hum Nutr. 2006;61:131–137. doi: 10.1007/s11130-006-0020-x. [DOI] [PubMed] [Google Scholar]
- Kale CK, Kotecha PM, Chavan JK, Kadam SS. Effect of processing conditions of bakery products on formation of resistant starch. J Food Sci Technol. 2002;39:520–524. [Google Scholar]
- Kanazawa K, Sakakibara H. High content of dopamine, a strong antioxidant, in cavendish banana. J Agric Food Chem. 2000;48:844–848. doi: 10.1021/jf9909860. [DOI] [PubMed] [Google Scholar]
- Kaur A, Kaur S, Singh M, Singh N, Shevkani K, Singh B. Effect of banana flour, screw speed and temperature on extrusion behaviour of corn extrudates. J Food Sci Technol. 2014;52:4276–4285. doi: 10.1007/s13197-014-1524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili L, Amini A (2015) Resistant starch in food industry. In: Polysaccharides: bioactivity and biotechnology. pp 663–673. 10.1007/978-3-319-16298-0_42
- Khamsucharit P, Laohaphatanalert K, Gavinlertvatana P, Sriroth K, Sangseethong K. Characterization of pectin extracted from banana peels of different varieties. Food Sci Biotechnol. 2018;27:623–629. doi: 10.1007/s10068-017-0302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitts DD. Bioactive substances in food: identification and potential uses. Can J Physiol Pharmacol. 1994;72:423–434. doi: 10.1139/y94-062. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113:71S–88S. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- Krishnan M, Prabhasankar P. Studies on pasting, microstructure, sensory, and nutritional profile of pasta influenced by sprouted finger millet (Eleucina coracana) and green banana (Musa paradisiaca) flours. J Texture Stud. 2010;41:825–841. [Google Scholar]
- Kurhade A, Patil S, Sonawane SK, Waghmare JS, Arya SS. Effect of banana peel powder on bioactive constituents and microstructural quality of chapatti: unleavened Indian flat bread. J Food Meas Charact. 2016;10:32–41. [Google Scholar]
- Kusuma SAF, Febrianti M, Saraswati A. Comparison of unripe banana peel of kepok (Musa paradisiaca L.) and klutuk (Musa balbisiana colla): phytochemical and anti- dysenteriae activity. J Pharm Sci Res. 2018;10:911–914. [Google Scholar]
- La Fuente CIA, Zabalaga RF, Tadini CC. Combined effects of ultrasound and pulsed-vacuum on air-drying to obtain unripe banana flour. Innov Food Sci Emerg Technol. 2017;44:123–130. [Google Scholar]
- Lewis DA, Fields WN, Shaw GP. A natural flavonoid present in unripe plantain banana pulp (Musa sapientum L. var. paradisiaca) protects the gastric mucosa from aspirin-induced erosions. J Ethnopharmacol. 1999;65:283–288. doi: 10.1016/s0378-8741(99)00005-7. [DOI] [PubMed] [Google Scholar]
- Mahindrakar KV, Rathod VK. Utilization of banana peels for removal of strontium (II) from water. Environ Technol Innov. 2018;11:371–383. [Google Scholar]
- Marangoni F, Poli A. Phytosterols and cardiovascular health. Pharmacol Res. 2010;61:193–199. doi: 10.1016/j.phrs.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Menezes EW, et al. Chemical composition and nutritional value of unripe banana flour (Musa acuminata, var. Nanicão) Plant Foods Hum Nutr. 2011;66:231–237. doi: 10.1007/s11130-011-0238-0. [DOI] [PubMed] [Google Scholar]
- Mohamed A, Xu J, Singh M. Yeast leavened banana-bread: formulation, processing, colour and texture analysis. Food Chem. 2010;118:620–626. [Google Scholar]
- Moongngarm A, Tiboonbun W, Sanpong M, Sriwong P, Phiewtong L, Prakitrum R, Huychan N. Resistant starch and bioactive contents of unripe banana flour as influenced by harvesting periods and its application. Am J Agric Biol Sci. 2014;9:457–465. [Google Scholar]
- Munagapati VS, Yarramuthi V, Kim Y, Lee KM, Kim DS. Removal of anionic dyes (reactive black 5 and congo red) from aqueous solutions using banana peel powder as an adsorbent. Ecotoxicol Environ Saf. 2018;148:601–607. doi: 10.1016/j.ecoenv.2017.10.075. [DOI] [PubMed] [Google Scholar]
- Nasrin TAA, Noomhorm A, Anal AK. Physico-chemical characterization of culled plantain pulp starch, peel starch, and flour. Int J Food Prop. 2015;18:165–177. [Google Scholar]
- Nilsson A, Östman E, Preston T, Björck I. Effects of GI vs content of cereal fibre of the evening meal on glucose tolerance at a subsequent standardized breakfast. Eur J Clin Nutr. 2008;62:712–720. doi: 10.1038/sj.ejcn.1602784. [DOI] [PubMed] [Google Scholar]
- Oi RK, Santana JCC, Tambourgi EB, Júnior M (2013) Feasibility study for production of green banana flour in a spray dryer, vol 32. 10.3303/cet1332305
- Oliveira de Souza NC, de Lacerda de Oliveira L, Rodrigues de Alencar E, Moreira GP, Santos Leandro ED, Ginani VC, Zandonadi RP. Textural, physical and sensory impacts of the use of green banana puree to replace fat in reduced sugar pound cakes. LWT Food Sci Technol. 2018;89:617–623. [Google Scholar]
- Ovando-Martinez M, Sáyago-Ayerdi S, Agama-Acevedo E, Goñi I, Bello-Pérez LA. Unripe banana flour as an ingredient to increase the undigestible carbohydrates of pasta. Food Chem. 2009;113:121–126. [Google Scholar]
- Oyewo OA, Onyango MS, Wolkersdorfer C. Lanthanides removal from mine water using banana peels nanosorbent. Int J Environ Sci Technol (Tehran) 2018;15:1265–1274. [Google Scholar]
- Panebianco C, et al. Engineered resistant-starch (ERS) diet shapes colon microbiota profile in parallel with the retardation of tumor growth in in vitro and in vivo pancreatic cancer models. Nutrients. 2017 doi: 10.3390/nu9040331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Lee YJ, Chun SS. Quality characteristics of sponge cake added with banana powder. J Korean Soc Food Sci Nutr. 2010;39:1509–1515. [Google Scholar]
- Pathak PD, Mandavgane SA, Kulkarni BD. Fruit peel waste: characterization and its potential uses. Curr Sci. 2017;113:444–454. [Google Scholar]
- Pereira A, Maraschin M. Banana (Musa spp) from peel to pulp: ethnopharmacology, source of bioactive compounds and its relevance for human health. J Ethnopharmacol. 2015;160:149–163. doi: 10.1016/j.jep.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Prakash H, Chauhan PS, General T, Sharma AK. Development of eco-friendly process for the production of bioethanol from banana peel using inhouse developed cocktail of thermo-alkali-stable depolymerizing enzymes. Bioproc Biosyst Eng. 2018;41:1003–1016. doi: 10.1007/s00449-018-1930-3. [DOI] [PubMed] [Google Scholar]
- Presutti RJ, Cangemi JR, Cassidy HD, Hill DA. Celiac disease. Am Fam Phys. 2007;76:1795–1802+1809–1810. [PubMed] [Google Scholar]
- Radoi PB, Alexa E, Radulov I, Morvay A, Mihai CSS, Trasca TI, Trasca TI. Total phenolic, cinnamic acids and selected microelements in gluten free pasta fortified with banana. Rev Chim. 2015;66:1162–1165. [Google Scholar]
- Ramli S, Alkarkhi AFM, Shin Yong Y, Min-Tze L, Easa AM. Effect of banana pulp and peel flour on physicochemical properties and in vitro starch digestibility of yellow alkaline noodles. Int J Food Sci Nutr. 2009;60:326–340. doi: 10.1080/09637480903183503. [DOI] [PubMed] [Google Scholar]
- Ramli S, Ismail N, Alkarkhi AFM, Easa AM. The use of principal component and cluster analysis to differentiate banana peel flours based on their starch and dietary fibre components. Trop Life Sci Res. 2010;21:91–100. [PMC free article] [PubMed] [Google Scholar]
- Rayo LM, Chagurie Carvalho L, Sardá FAH, Dacanal GC, Menezes EW, Tadini CC. Production of instant green banana flour (Musa cavendischii, var. Nanicão) by a pulsed-fluidized bed agglomeration. LWT Food Sci Technol. 2015;63:461–469. [Google Scholar]
- Robles-Ramírez MC, Flores-Morales A, Mora-Escobedo R (2012) Corn tortillas: physicochemical, structural and functional changes. In: Maize: cultivation, uses and health benefits. Nova Science Publishers, Inc., pp 89–111
- Sarawong C, Gutiérrez ZR, Berghofer E, Schoenlechner R. Effect of green plantain flour addition to gluten-free bread on functional bread properties and resistant starch content. Int J Food Sci Technol. 2014;49:1825–1833. [Google Scholar]
- Seguchi M, Tabara A, Iseki K, Takeuchi M, Nakamura C. Development of gluten-free bread baked with banana (musa spp.) flour. Food Sci Technol Res. 2014;20:613–619. [Google Scholar]
- Segundo C, Román L, Gómez M, Martínez MM. Mechanically fractionated flour isolated from green bananas (M. cavendishii var. nanica) as a tool to increase the dietary fiber and phytochemical bioactivity of layer and sponge cakes. Food Chem. 2017;219:240–248. doi: 10.1016/j.foodchem.2016.09.143. [DOI] [PubMed] [Google Scholar]
- Segundo C, Román L, Lobo M, Martinez MM, Gómez M. Ripe banana flour as a source of antioxidants in layer and sponge cakes. Plant Foods Hum Nutr. 2017;72:365–371. doi: 10.1007/s11130-017-0630-5. [DOI] [PubMed] [Google Scholar]
- Sheikh BY, Sarker MMR, Kamarudin MNA, Ismail A. Prophetic medicine as potential functional food elements in the intervention of cancer: a review. Biomed Pharmacother. 2017;95:614–648. doi: 10.1016/j.biopha.2017.08.043. [DOI] [PubMed] [Google Scholar]
- Singh B, Singh JP, Kaur A, Singh N. Bioactive compounds in banana and their associated health benefits—a review. Food Chem. 2016;206:1–11. doi: 10.1016/j.foodchem.2016.03.033. [DOI] [PubMed] [Google Scholar]
- Singh S, Parveen N, Gupta H. Adsorptive decontamination of rhodamine-B from water using banana peel powder: a biosorbent. Environ Technol Innov. 2018;12:189–195. [Google Scholar]
- Someya S, Yoshiki Y, Okubo K. Antioxidant compounds from bananas (Musa Cavendish) Food Chem. 2002;79:351–354. [Google Scholar]
- Steel CJ, Da Silva CB, Ormenese RCSC, Clerici MTPS, Cáceres MC, Chang YK (2013) Technological evaluation of pan bread produced with the addition of commercial resistant starch or unripe banana flour. In: Bread consumption and health. Nova Science Publishers, Inc., pp 107–119
- Thebaudin JY, Lefebvre AC, Harrington M, Bourgeois CM. Dietary fibres: nutritional and technological interest. Trends Food Sci Technol. 1997;8:41–48. [Google Scholar]
- Tibolla H, Pelissari FM, Martins JT, Vicente AA, Menegalli FC. Cellulose nanofibers produced from banana peel by chemical and mechanical treatments: characterization and cytotoxicity assessment. Food Hydrocoll. 2018;75:192–201. [Google Scholar]
- Torres MD, Arufe S, Chenlo F, Moreira R. Coeliacs cannot live by gluten-free bread alone—every once in awhile they need antioxidants. Int J Food Sci Technol. 2017;52:81–90. [Google Scholar]
- Tribess TB, Hernández-Uribe JP, Méndez-Montealvo MGC, Menezes EW, Bello-Perez LA, Tadini CC. Thermal properties and resistant starch content of green banana flour (Musa cavendishii) produced at different drying conditions. LWT Food Sci Technol. 2009;42:1022–1025. [Google Scholar]
- Türker B, Savlak N, Kaşikci MB. Effect of green banana peel flour substitution on physical characteristics of gluten-free cakes. Curr Res Nutr Food Sci. 2016;4:197–204. [Google Scholar]
- Vilardi G, Di Palma L, Verdone N. Heavy metals adsorption by banana peels micro-powder: equilibrium modeling by non-linear models. Chin J Chem Eng. 2018;26:455–464. [Google Scholar]
- Von Atzingen DANC, et al. Repair of surgical wounds in rats using a 10% unripe Musa sapientum peel gel. Acta Cir Bras. 2015;30:586–592. doi: 10.1590/S0102-865020150090000001. [DOI] [PubMed] [Google Scholar]
- Vu HT, Scarlett CJ, Vuong QV. Effects of drying conditions on physicochemical and antioxidant properties of banana (Musa cavendish) peels drying. Technol. 2017;35:1141–1151. [Google Scholar]
- Vu HT, Scarlett CJ, Vuong QV. Phenolic compounds within banana peel and their potential uses: a review. J Funct Foods. 2018;40:238–248. [Google Scholar]
- Wang Y, Zhang M, Mujumdar AS. Influence of green banana flour substitution for cassava starch on the nutrition, color, texture and sensory quality in two types of snacks. LWT Food Sci Technol. 2012;47:175–182. [Google Scholar]
- Wang J, Huang HH, Chen PS. Structural and physicochemical properties of banana resistant starch from four cultivars. Int J Food Prop. 2017;20:1338–1347. [Google Scholar]
- Wang K, Lu F, Li Z, Zhao L, Han C. Recent developments in gluten-free bread baking approaches: a review Food. Sci Technol. 2017;37:1–9. [Google Scholar]
- Yan L, Fernando WMADB, Brennan M, Brennan CS, Jayasena V, Coorey R. Effect of extraction method and ripening stage on banana peel pigments. Int J Food Sci Technol. 2016;51:1449–1456. [Google Scholar]
- Yangilar F. Effects of green banana flour on the physical, chemical and sensory properties of ice cream. Food Technol Biotechnol. 2015;53:315–323. doi: 10.17113/ftb.53.03.15.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin DT, Zhao XH. Impact of exogenous strains on in vitro fermentation and anti-colon cancer activities of maize resistant starch and xylo-oligosaccharides. Starke. 2017;69:15–20. [Google Scholar]
- Zandonadi RP, Botelho RBA, Gandolfi L, Ginani JS, Montenegro FM, Pratesi R. Green banana pasta: an alternative for gluten-free diets. J Acad Nutr Diet. 2012;112:1068–1072. doi: 10.1016/j.jand.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Zhang P, Whistler RL, Bemiller JN, Hamaker BR. Banana starch: production, physicochemical properties, and digestibility—a review. Carbohydr Polym. 2005;59:443–458. [Google Scholar]
- Zheng Z, Stanley R, Gidley MJ, Dhital S. Structural properties and digestion of green banana flour as a functional ingredient in pasta. Food Funct. 2016;7:771–780. doi: 10.1039/c5fo01156f. [DOI] [PubMed] [Google Scholar]


