Abstract
Crude glutenin of commercial Indian wheat varieties was fractionated into high molecular weight glutenin subunits (HMW-GS) and low molecular weight glutenin subunits (LMW-GS) by employing size-exclusion chromatography (SEC). The SEC profile of glutenins obtained with different buffers were discriminated effectively with respect to the quality of the proteins eluted in each peak. The most efficient separation of LMW-GS was achieved using 3 M urea (pH 5.5) buffer under unreduced conditions. The chromatogram was segregated predominantly into three peaks with varied molecular weights as determined by SDS-PAGE. Peak I corresponded to a mixture of HMW-GS and LMW-GS (Mw 100–30 kDa). Peak II enclosed LMW-GS specifically with molecular weights in the range of 45–35 kDa. Lastly, a mixture of proteins associated with LMW-GS (Mw < 35 kDa) were eluted in peak III. SEC proved to be a valuable tool in purifying LMW-GS in a functionally active state.
Keywords: Size-exclusion chromatography, LMW-GS, SDS-PAGE
Introduction
The baking quality of wheat is primarily a function of the composition and interactions among gluten proteins (gliadins and glutenins). On hydration of flour, gliadins and glutenins associate through non-covalent linkages to form a three-dimensional viscoelastic mass called gluten. Glutenin polymers, being large in size, provides continuity to the dough system. Besides this, gliadin acts as a plasticizer that diminishes interactions between glutenin chains, by that booming dough viscosity. The ratio of monomeric gliadin to polymeric glutenin regulates the harmony between dough viscosity and elasticity. Inadequate elastic gluten prompts low bread loaf volume, moreover, increased elasticity leads to a higher loaf volume, but a too elastic gluten impedes the expansion of gas cells leading again to lower loaf volume (Kasarda 1994). The glutenin proteins are the polymeric wheat storage prolamins linked by intermolecular disulphide associations with molecular weight ranging from few hundred thousand to millions. Glutenins are composed of LMW-GS and HMW-GS, representing approximately 40% and 10%, respectively of the gluten proteins. On the basis of SDS-PAGE mobility, the molecular mass of LMW-GS and HMW-GS was identified as 30–45 kDa and 70–90 kDa, respectively (Wieser 2007). HMW-GS and LMW-GS occur majorly in the polymerized, aggregated form being shaped through hydrogen bonds, hydrophobic and ionic interactions, and disulphide linkages, thus possessed a more extended conformation which impart elasticity (or strength) to the dough (Khatkar et al. 1996).
Pioneer research work on glutenins was approached towards HMW-GS specifically, assuming that HMW-GS were the sole determinants of dough peculiar characteristics (Khatkar 2006). They were known to be responsible for 45–70% of the variation in breadmaking performance within European wheats. Subsequently, it was recognized that, besides HMW-GS composition, the ration of HMW-GS/LMW-GS affected the baking quality of wheat substantially (Gupta et al. 1989; Dhaka and Khatkar 2015; Dangi and Khatkar 2017a). LMW-GS, in comparison to HMW-GS, are postulated as fundamentally more heterogeneous and less well-characterized group of glutenin. They are recognized as the second most abundant class of storage proteins after the gliadins. A single wheat variety is expected to consist of 7–16 different types of LMW-GS. These can be classified into typical LMW-GS (also known as B-subunits) and gliadin-like LMW-GS (C- and D-subunits) which are structurally similar to gliadins, but functionally they are glutenins due to their ability to form intermolecular disulphide bonds (D’Ovidio and Masci 2004). Based on SDS-PAGE, LMW-GS were classified as B-subunits (MW = 40–50 kDa), C-subunits (MW = 30–40 kDa) and D-subunits (MW = 55–70 kDa). LMW-GS composition was supposed to affect the glutenin quality in a wheat cultivar, where the major difference strike out from the qualitative differences being emerged from individual B-type and C-type LMW-GS, in addition, to the quantity of D-type LMW-GS (Veraverbeke and Delcour 2002).
Numerous attempts in the past had centralized on the efficient separation of HMW-GS, LMW-GS and gliadins by utilizing chromatography and electrophoresis using variety of solvent systems. Based on the selective precipitation technique, dimethyl sulfoxide (DMSO) (Gupta and MacRitchie 1991), 1-propanol in alliance with sodium iodide (DuPont et al. 2005), acetone (Hou and Ng 1995), ethanol (Wieser et al. 1990), sodium dodecyl sulphate (SDS) (Singh et al. 1990) were few among the diverse range of solvents used for their separation. RP-HPLC technique has also been utilized by Burnouf and Bietz (1984) to separate LMW-GS from HMW-GS depending on the differences in the hydrophobicity of the protein fractions. However, clear separation of monomeric and polymeric proteins could not be achieved so far owing to the (1) poor solubility of glutenin which was attributed to its large size and extensive disulphide linkages and (2) overlapping of LMW-GS and gliadins due to overlying molecular weight range (Veraverbeke and Delcour 2002). Furthermore, they concluded that the heterogeneity in glutenin structure emerged from variation in its structure, size distribution and subunit composition. Rhazi et al. (2009) emphasized on knowledge of glutenin subunit composition which could be adapted as a successful marker for predicting the genetic potential of breeding lines.
LMW-GS are receiving increasing interest since correlations have been found between the presence of certain components and the technological quality of the wheat flour. The elementary objective of this investigation was to design a simple LMW-GS purification protocol with minimal cross-contamination, which can further be utilized for understanding the structure and interactions of LMW-GS at molecular level. The availability of purified glutenin subunits will improve the biochemical characterization of single components and, then, will be of help in the elucidation of their involvement in wheat gluten functionality. The divergence among glutenin molecules is noticeable in substance to molecular size instead of mass–charge ratio which recommended the use of SEC technique to fractionate glutenins and purify LMW-GS.
Materials and methods
Selection of wheat variety and flour extraction
Commercial Indian wheat varieties viz. C 306, HI 977, HW 2004 and PBW 550 of diverse origin and baking quality were chosen for the study. The varieties were cleaned, tempered to an appropriate moisture level overnight and passed to a Chopin laboratory mill (Model CD1, Villeneuve la Garenne, France) for milling. The wheat flour obtained was stored at 5 °C in an air-tight container for further analysis, respectively (Dangi and Khatkar 2017b).
Extraction of wheat gluten and its fractionation
Gluten was isolated from flour using Glutomatic instrument (AACC 2000). Flour (10 g) was accurately weighed and placed into the washing chamber of Glutomatic system. The flour was washed vigorously with 2% NaCl solution at 15 °C for 5 min and subsequently with distilled water. This removed starch and other solubles from the flour and the residual viscoelastic mass so obtained was collected and referred as gluten. Powdered freeze dried gluten sample (50 g) was dissolved in 200 ml of 70% ethanol and the mixture was stirred on a magnetic stirrer for 3 h at 25 °C followed by centrifugation for 30 min at 1000×g, 4 °C. The whole process was repeated thrice. The resultant pellet, rich in glutenin was freeze dried, powdered in pestle and mortar and was stored at 5 °C.
Acetone precipitation method for obtaining HMW-GS and LMW-GS fractions
The fractions of HMW-GS and LMW-GS were recovered using a method based on selective precipitation by acetone as demonstrated by Melas et al. (1994). Crude glutenin fraction was isolated from flour by repeated washing with 50% (v/v) propan-2-ol (Singh et al. 1991). The extracted glutenins were resuspended in 1.5 ml of 50% (v/v) propan-2-ol containing 0.08 M Tris-HCl buffer, pH 8 and 1% (w/v) DTT. The reduction process was performed at 60 °C for 30 min, followed by centrifugation for 5 min at 40,000×g, 20 °C. The supernatant was collected and acetone was added to it to give a final concentration of 40% (v/v) so as to precipitate whole of HMW-GS from the sample. Finally, the concentration of supernatant was increased to 80% (v/v) acetone, which successfully precipitated LMW-GS specifically.
Purification of LMW-GS
Sample preparation
Wide varieties of buffer systems were selected to extract urea-solubilized glutenin, so as to obtain a rapid, high resolution separation of LMW-GS (Khan and Bushuk 1979; Skerritt et al. 1996). 20 mg crude glutenin was dissolved in each buffer (10 ml) maintained at pH 5.5. (a) 2 M urea, (b) 3 M urea, (c) 4 M urea, (d) 5 M urea, (e) 6 M urea, (f) 3 M urea, 1% DTT, and (g) 3 M urea, 1% SDS. The samples were then sonicated in an ultrasonic chamber for 1 h and centrifuged for 30 min at 12,000×g, − 15 °C. Sample extract (2 ml) was filtered through 0.22 μm Millipore syringe filter (HV Millipore, DuraPore) and used for injection.
Size-exclusion chromatography
A Sephacryl S-200 column (HI Prep™ 16/60 Sephacryl S-200 HR, GE Healthcare) connected to a GE Healthcare and Lifesciences Fast Protein Liquid Chromatography system, comprising a model Äktaprime plus was used for size-exclusion separations. The eluting buffers used were respective buffers containing 0.15 M NaCl (pH 5.5) at a flow rate of 0.5 ml/min. The elution buffers were filtered through 0.45 μm filter (HV Millipore, DuraPore) and degassed under vacuum prior to use. The fractions were collected with an automated fraction collector, concentrated, dialyzed against 1% acetic acid and freeze-dried (Chaudhary et al. 2016b, c).
SDS polyacrylamide gel electrophoresis
SDS-PAGE was carried out for 40% and 80% acetone precipitated fractions and SEC eluted protein fractions by using electrophoretic assembly of M/S Genetix Scientific (India) as described previously by Chaudhary et al. (2016a, 2017). Electrophoresis was performed on 12% separating gel and 4% stacking gel. SEC eluted protein fractions were concentrated and dialyzed with 50 mM Tris/HCl (pH 8). Sample (10 µl) was taken and mixed with 5 µl sample buffer containing 62.5 mM Tris/HCl (pH 6.8), 2% (w/v) SDS, 10% (v/v) glycerol, 0.001% (w/v) bromophenol blue and 5% (v/v) 2-mercaptoethanol. The protein samples were boiled for 3 min in a boiling water bath and vortexed for 2 min. 15 μl of the protein extract, thus obtained was loaded into the wells and gel was run at a constant current of 18 mA. The gels were stained for overnight in the staining solution (60% distilled water, 30% methanol, 10% acetic acid and 0.1% G-250 Coomassie Brilliant Blue). Completely stained gels were kept for destaining using a destaining solution (60% distilled water, 30% methanol and 10% acetic acid) which was changed every 2 h till the gels become colourless.
Results and discussion
Fractionation of glutenins using different buffers on SEC
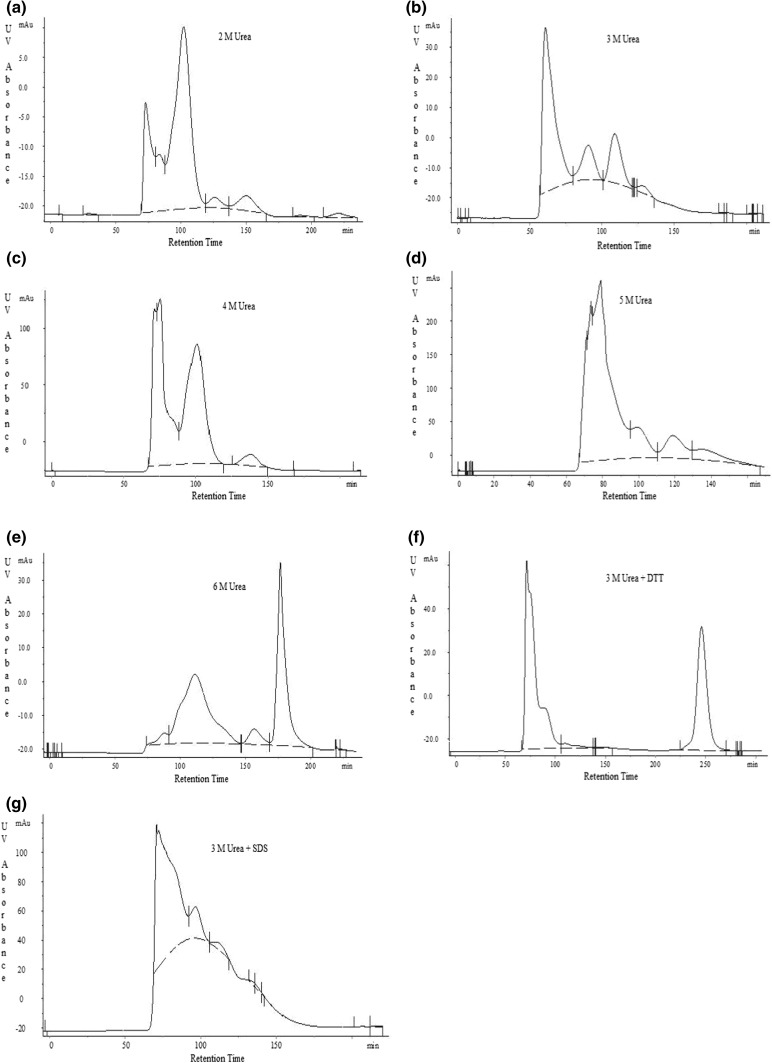
The polymeric proteins were fractionated on Sephacryl S-200 size-exclusion column using number of buffers. The chromatograms were plotted in terms of UV absorbance (mAu) vs retention time (min) as presented in Fig. 1. Glutenins exhibited contrasting behaviour in different buffer systems as reflected from their chromatographic profiles. It can be speculated from SEC profiles, that the least extraction of glutenins was resulted from 2 M urea. A sharp increment in the extraction of glutenins was noticeable as higher concentrations of urea were used in the buffers (2 M < 3 M < 4 M < 5 M < 6 M). The results pinpoint that urea evidently disaggregated more of the lower molecular weight glutenin. The pure peaks without any shoulder can be identified from using 3 M urea, 6 M urea and 3 M urea + DTT buffers. Preceding work accomplished by Khan and Bushuk (1979) summarized that with the upsurge in the dissociating ability of the solvent, the percentage of protein eluted in latter peaks (peak II and III) increased successively. Urea, being a hydrogen-bond breaking agent, minimizes protein aggregation which is principally due to hydrogen bonding. However, the resolution of the chromatogram decreased suggesting a high level of cross-contamination of different peaks (as in 4 M and 5 M urea buffers). The single peak isolated by the use of 6 M urea buffer, carried a blend of LMW-GS and other lower molecular weight proteins, which was not desirable. It is believed that DTT and SDS aids in solubilizing glutenin molecules, yet no significant results were observed. One of the pure peak, isolated from SEC using DTT, a chemical reductant, did not match the interest of the study as the fraction corresponds to lower molecular weight proteins. This may be due to the action of DTT, which cleaves disulphide bond linkages of glutenin subunits and resulted in the smaller protein subunits. The addition of reductant to the buffer system resulted in a higher magnitude of protein being eluted with a transition in the allocation of the profile approaching later elution times, as evident from the greater area under the SEC profile which corresponds to lower molecular weight species (Larre et al. 1997; Lindsay and Skerritt 1998). Skerritt et al. (1996) suggested a dramatically decrease in molecular size of proteins upon addition of reducing agents such as DTT and 2-mercaptoethanol by bringing about intensive disulphide-bond cleavage. This limits the use of reducing agents in the exact determinations of molecular size of glutenins. On the contrary, SDS is effective in dissociating a large proportion of glutenin molecules by disrupting the hydrophobic interactions (Khan and Bushuk 1979). In an effect, it can also not be chosen due to the relative mixing of peaks. Conclusively, amongst the range of solvents used, the most efficient fractionation of glutenins was recovered using 3 M urea under non-reduced conditions, thereby maintaining the native state of LMW-GS. In addition, Wu et al. (1967) adjudged that the denaturing response of 3 M urea on gluten proteins is imperceptible in view of their intrinsic viscosities and helix content which remain unaltered in 3 M urea solution and urea-free solutions at the same ionic strengths. This strengthened the fact that LMW-GS can be purified in their native functional state using 3 M urea buffer system.
Fig. 1.
Size-exclusion chromatograms of glutenin of HI 977 wheat variety executed under different buffer conditions a 2 M urea, b 3 M urea, c 4 M urea, d 5 M urea, e 6 M urea, f 3 M urea, 1% DTT, and g 3 M urea, 1% SDS. All the buffers were maintained at a pH of 5.5
Identification of eluted fractions on SDS-PAGE
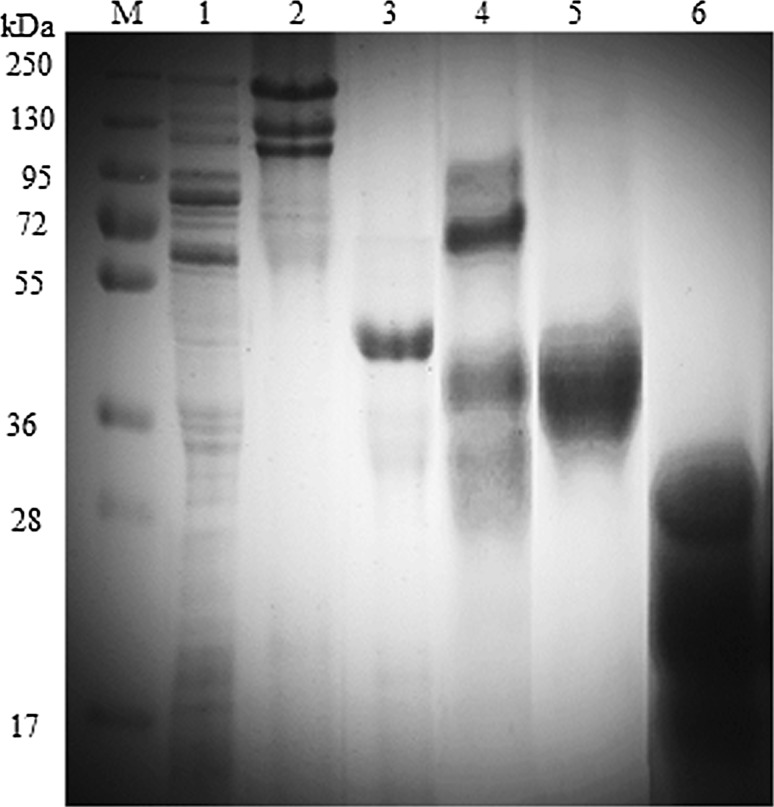
Figure 2 displays the electrophoretic profile of purified glutenin fractions from acetone precipitation method and SEC eluted peaks. Primarily, three peaks were resolved from the chromatogram when crude glutenin was run on SEC using 3 M urea buffer system. These peaks were collected, dialyzed and freeze-dried. Peak I eluted at a retention time of 72.18 min, apparently consists of a mixture of HMW-GS and LMW-GS with molecular size ranging from 100 to 30 kDa. Peak II was eluted at 99.20 min, which appeared to contain a single band in the range of molecular weight 45–35 kDa. This particular band is specific to purified fraction of LMW-GS. The intensity of the band was quite high, which was particularly due to the abundant concentration of LMW-GS in this region. The protein eluted in peak III corresponds to mixture of proteins that were present in association with LMW-GS. This fraction has a retention time of 118.72 min and a molecular weight of < 35 kDa.
Fig. 2.
Electrophoretic profile of purified glutenin fractions. Lane M: marker; Lane 1: flour protein; Lane 2: HMW-GS (40% acetone precipitated); Lane 3: LMW-GS (80% acetone precipitated); Lane 4–6: SEC eluted peaks I, II and III, respectively
A comparative analysis of proteins eluted in SEC peaks was contrived with HMW-GS and LMW-GS fractions of those isolated by Melas et al. (1994) method. The LMW-GS fraction obtained by both the methods showed clear analogy in terms of their molecular size, which were in congruency with the flour protein profile, with slight deviations in molecular weight. This is in view to the use of dissociating and reducing agents in the two methods which caused the fluctuations to a low level in the molecular weight of these fractions. Kaur et al. (2015); Katyal et al. (2018) examined the glutenin fraction of durum wheats on SDS-PAGE and classified the polypeptides with molecular weight range of 42–52 kDa as LMW-B and those between 23 and 39 kDa as LMW-C type. The molecular weight estimation of HMW-GS and LMW-GS from SDS-PAGE were in agreement with the studies of Khatkar and Schofield (1997), Lindsay and Skerritt (1998), Kaur et al. (2016) and Katyal et al. (2017) which further strengthened the fact that isolated fraction was LMW-GS only. Thus, the determination of molecular weight of LMW-GS obtained from SEC with its counterparts, validated the method for purifying LMW-GS using SEC.
Conclusion
LMW-GS were successfully separated from associated gliadins, despite their overlapping molecular weights, using Sephacryl S-200 size-exclusion column on FPLC system. From the variety of buffers selected for study, the most efficient separation was achieved under unreduced conditions with 3 M urea (pH 5.5) with regard to obtaining adequate amounts of LMW-GS with least cross-contamination. SDS and DTT were not proven to be an effective solvent for the dissolution of glutenin as they impaired their native states and fractions of lower molecular weight range were observed. Precisely, the results showed the efficiency of the optimized FPLC protocol in the purification of LMW-GS, which can be utilized further to explore their structural and techno-functional importance.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AACC . Approved methods of the American Association of Cereal Chemists. St. Paul: American Association of Cereal Chemists; 2000. [Google Scholar]
- Burnouf T, Bietz JA. Reversed-phase high-performance liquid chromatography of reduced glutenin, a disulfide-bonded protein of wheat endosperm. J Chromatogr. 1984;229:185–199. [Google Scholar]
- Chaudhary N, Dangi P, Khatkar BS. Assessment of molecular weight distribution of wheat gluten proteins for chapatti quality. Food Chem. 2016;199:28–35. doi: 10.1016/j.foodchem.2015.11.106. [DOI] [PubMed] [Google Scholar]
- Chaudhary N, Dangi P, Khatkar BS. Evaluation of molecular weight distribution of unreduced wheat gluten proteins associated with noodle quality. J Food Sci Technol. 2016;53:2695–2704. doi: 10.1007/s13197-016-2241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N, Dangi P, Khatkar BS. Relationship of molecular weight distribution profile of unreduced gluten protein extracts with quality characteristics of bread. Food Chem. 2016;210:325–331. doi: 10.1016/j.foodchem.2016.04.043. [DOI] [PubMed] [Google Scholar]
- Chaudhary N, Dangi P, Khatkar BS. Fractionation of unreduced gluten proteins on SEC and their relationship with cookie quality characteristics. J Food Sci Technol. 2017;54:342–348. doi: 10.1007/s13197-016-2467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangi P, Khatkar BS. Effect of glutenin addition on dough mixing characteristics of wheat varieties. Int J Innov Res Sci Eng Technol. 2017;6:13444–13447. [Google Scholar]
- Dangi P, Khatkar BS. Physicochemical and gluten quality characteristics of commercial wheat varieties. Int J Innov Res Sci Eng Technol. 2017;6:13448–13454. [Google Scholar]
- Dhaka V, Khatkar BS. Effects of gliadin/glutenin and HMW-GS/LMW-GS ratio on dough rheological properties and bread-making potential of wheat varieties. J Food Qual. 2015;38:71–82. [Google Scholar]
- D’Ovidio R, Masci S. The low-molecular-weight glutenin subunits of wheat gluten. J Cereal Sci. 2004;39:321–339. [Google Scholar]
- DuPont FM, Chan R, Lopez R, Vensel WH. Sequential extraction and quantitative recovery of gliadins, glutenins, and other proteins from small samples of wheat flour. J Agric Food Chem. 2005;53:1575–1584. doi: 10.1021/jf048697l. [DOI] [PubMed] [Google Scholar]
- Gupta RB, MacRitchie F. A rapid one-step one-dimensional SDS-PAGE procedure for analysis of subunit composition of glutenin in wheat. J Cereal Sci. 1991;14:105–109. [Google Scholar]
- Gupta RB, Singh NK, Shepherd KW. The cumulative effect of allelic variation in LMW and HMW gluten subunits on dough properties in the progeny of two bread wheats. Theor Appl Genet. 1989;77:57–64. doi: 10.1007/BF00292316. [DOI] [PubMed] [Google Scholar]
- Hou G, Ng PKW. Quantification of glutenin subunits by sequential acetone precipitation and by SDS-PAGE coupled with densitometry using a known quantity of glutenins as a standard. Cereal Chem. 1995;72:545–551. [Google Scholar]
- Kasarda DD. Glutenin structure in relation to wheat quality. In: Pomeranz Y, editor. Wheat is unique. St. Paul: AACC; 1994. pp. 73–106. [Google Scholar]
- Katyal M, Singh N, Virdi AS, Kaur A, Chopra N, Ahlawat AK, Singh AM. Extraordinarily soft, medium-hard and hard Indian wheat varieties: composition, protein profile, dough and baking properties. Food Res Int. 2017;100:306–317. doi: 10.1016/j.foodres.2017.08.050. [DOI] [PubMed] [Google Scholar]
- Katyal M, Virdi AS, Singh N, Kaur A, Rana JC, Kumari J. Diversity in protein profiling, pasting, empirical and dynamic dough rheological properties of meal from different durum wheat accessions. J Food Sci Technol. 2018;55:1256–1269. doi: 10.1007/s13197-018-3036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A, Singh N, Kaur S, Katyal M, Virdi AS, Kaur D, Ahlawat AK, Singh AM. Relationship of various flour properties with noodle making characteristics among durum wheat varieties. Food Chem. 2015;188:517–526. doi: 10.1016/j.foodchem.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Kaur A, Shevkani K, Katyal M, Singh N, Ahlawat AK, Singh AM. Physicochemical and rheological properties of starch and flour from different durum wheat varieties and their relationships with noodle quality. J Food Sci Technol. 2016;53:1–12. doi: 10.1007/s13197-016-2202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K, Bushuk W. Studies of glutenin XIII. Gel filtration, isoelectric focusing and amino acid composition studies. Cereal Chem. 1979;56:505–512. [Google Scholar]
- Khatkar BS. Effect of high Mr glutenin subunits on dynamic rheological properties and bread making qualities of wheat glutenin. J Food Sci Technol. 2006;43:382–387. [Google Scholar]
- Khatkar BS, Schofield JD. Molecular and physicochemical basis of bread making properties of wheat gluten proteins. J Food Sci Technol. 1997;34:85–103. [Google Scholar]
- Khatkar BS, Bell AE, Schofield JD. A comparative study of the inter-relationships between mixograph parameters and bread- making qualities of wheat flours and glutens. J Sci Food Agric. 1996;72:71–85. [Google Scholar]
- Larre C, Nicolas Y, Desserme C, Courcoux P, Popineau Y. Preparative separation of high and low molecuar weight subunits of glutenin from wheat. J Cereal Sci. 1997;25:143–150. [Google Scholar]
- Lindsay MP, Skerritt JH. Examination of the structure of the glutenin macropolymer in wheat flour and doughs by stepwise reduction. J Agric Food Chem. 1998;46:3447–3457. [Google Scholar]
- Melas V, Morel M, Autran J, Feillet P. Simple and rapid method for purifying low molecular weight subunits of glutenin from wheat. Cereal Chem. 1994;71:234–237. [Google Scholar]
- Rhazi L, Bodard AL, Fathollahi B, Aussenac T. High throughput microchip-based separation and quantitation of high-molecular-weight glutenin subunits. J Cereal Sci. 2009;49:272–277. [Google Scholar]
- Singh NK, Donovan GR, Batey IL, MacRitchie F. Use of sonication and size-exclusion high performance liquid chromatography in the study of wheat flour proteins. I. Dissolution of total proteins in the absence of reducing agents. Cereal Chem. 1990;67:150–162. [Google Scholar]
- Singh NK, Shepherd KW, Cornish GB. A simplified SDS-PAGE procedure for separating LMW subunits of glutenin. J Cereal Sci. 1991;14:203–208. [Google Scholar]
- Skerritt JH, Bekes F, Murray D. Isolation treatments and effects of gliaidin and glutenin fractions on dough mixing properties. Cereal Chem. 1996;73:644–649. [Google Scholar]
- Veraverbeke WS, Delcour JA. Wheat protein composition and properties of wheat glutenin in relation to breadmaking functionality. Crit Rev Food Sci Nutr. 2002;42:179–208. doi: 10.1080/10408690290825510. [DOI] [PubMed] [Google Scholar]
- Wieser H. Chemistry of gluten proteins. Food Microbiol. 2007;24:115–119. doi: 10.1016/j.fm.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Wieser H, Seilmeier W, Belitz HD. Characterization of ethanol-extractable reduced subunits of glutenin separated by reversed-phase high-performance liquid chromatography. J Cereal Sci. 1990;12:63–71. [Google Scholar]
- Wu YV, Cluskey JE, Sexson KR. Effect of ionic strength on the molecular weight and conformation of wheat gluten proteins in 3 M urea solutions. Biochim Biophys Acta BBA Protein Struct. 1967;133:83–90. doi: 10.1016/0005-2795(67)90040-2. [DOI] [PubMed] [Google Scholar]