Abstract
Background
Chronic obstructive pulmonary disease (COPD) has been recognised as a global health concern, and one of the leading causes of morbidity and mortality worldwide. Projections of the World Health Organization (WHO) indicate that prevalence rates of COPD continue to increase, and by 2030, it will become the world's third leading cause of death. Depression is a major comorbidity amongst patients with COPD, with an estimate prevalence of up to 80% in severe stages of COPD. Prevalence studies show that patients who have COPD are four times as likely to develop depression compared to those without COPD. Regrettably, they rarely receive appropriate treatment for COPD‐related depression. Available findings from trials indicate that untreated depression is associated with worse compliance with medical treatment, poor quality of life, increased mortality rates, increased hospital admissions and readmissions, prolonged length of hospital stay, and subsequently, increased costs to the healthcare system. Given the burden and high prevalence of untreated depression, it is important to evaluate and update existing experimental evidence using rigorous methodology, and to identify effective psychological therapies for patients with COPD‐related depression.
Objectives
To assess the effectiveness of psychological therapies for the treatment of depression in patients with chronic obstructive pulmonary disease.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2018, Issue 11), and Ovid MEDLINE, Embase and PsycINFO from June 2016 to 26 November 2018. Previously these databases were searched via the Cochrane Airways and Common Mental Disorders Groups' Specialised Trials Registers (all years to June 2016). We searched ClinicalTrials.gov, the ISRCTN registry, and the World Health Organization International Clinical Trials Registry Platform (ICTRP) to 26 November 2018 to identify unpublished or ongoing trials. Additionally, the grey literature databases and the reference lists of studies initially identified for full‐text screening were also searched.
Selection criteria
Eligible for inclusion were randomised controlled trials that compared the use of psychological therapies with either no intervention, education, or combined with a co‐intervention and compared with the same co‐intervention in a population of patients with COPD whose depressive symptoms were measured before or at baseline assessment.
Data collection and analysis
Two review authors independently assessed the titles and abstracts identified by the search to determine which studies satisfied the inclusion criteria. We assessed two primary outcomes: depressive symptoms and adverse events; and the following secondary outcomes: quality of life, dyspnoea, forced expiratory volume in one second (FEV1), exercise tolerance, hospital length of stay or readmission rate, and cost‐effectiveness. Potentially eligible full‐text articles were also independently assessed by two review authors. A PRISMA flow diagram was prepared to demonstrate the decision process in detail. We used the Cochrane 'Risk of bias' evaluation tool to examine the risk of bias, and assessed the quality of evidence using the GRADE framework. All outcomes were continuous, therefore, we calculated the pooled standardised mean difference (SMD) or mean difference (MD) with a corresponding 95% confidence interval (CI). We used a random‐effects model to calculate treatment effects.
Main results
The findings are based on 13 randomised controlled trials (RCTs), with a total of 1500 participants. In some of the included studies, the investigators did not recruit participants with clinically confirmed depression but applied screening criteria after randomisation. Hence, across the studies, baseline scores for depressive symptoms varied from no symptoms to severe depression. The severity of COPD across the studies was moderate to severe.
Primary outcomes
There was a small effect showing the effectiveness of psychological therapies in improving depressive symptoms when compared to no intervention (SMD 0.19, 95% CI 0.05 to 0.33; P = 0.009; 6 studies, 764 participants), or to education (SMD 0.23, 95% CI 0.06 to 0.41; P = 0.010; 3 studies, 507 participants).
Two studies compared psychological therapies plus a co‐intervention versus the co‐intervention alone (i.e. pulmonary rehabilitation (PR)). The results suggest that a psychological therapy combined with a PR programme can reduce depressive symptoms more than a PR programme alone (SMD 0.37, 95% CI ‐0.00 to 0.74; P = 0.05; 2 studies, 112 participants).
We rated the quality of evidence as very low. Owing to the nature of psychological therapies, blinding of participants, personnel, and outcome assessment was a concern.
None of the included studies measured adverse events.
Secondary outcomes
Quality of life was measured in four studies in the comparison with no intervention, and in three studies in the comparison with education. We found inconclusive results for improving quality of life. However, when we pooled data from two studies using the same measure, the result suggested that psychological therapy improved quality of life better than no intervention. One study measured hospital admission rates and cost‐effectiveness and showed significant reductions in the intervention group compared to the education group. We rated the quality of evidence as very low for the secondary outcomes.
Authors' conclusions
The findings from this review indicate that psychological therapies (using a CBT‐based approach) may be effective for treating COPD‐related depression, but the evidence is limited. Depressive symptoms improved more in the intervention groups compared to: 1) no intervention (attention placebo or standard care), 2) educational interventions, and 3) a co‐intervention (pulmonary rehabilitation). However, the effect sizes were small and quality of the evidence very low due to clinical heterogeneity and risk of bias. This means that more experimental studies with larger numbers of participants are needed, to confirm the potential beneficial effects of therapies with a CBT approach for COPD‐related depression.
New trials should also address the gap in knowledge related to limited data on adverse effects, and the secondary outcomes of quality of life, dyspnoea, forced expiratory volume in one second (FEV1), exercise tolerance, hospital length of stay and frequency of readmissions, and cost‐effectiveness. Also, new research studies need to adhere to robust methodology to produce higher quality evidence.
Plain language summary
Are psychological therapies effective in treating depression in patients with COPD?
Chronic obstructive pulmonary disease (COPD) comprises two conditions: emphysema and chronic bronchitis. It has been recognised as a serious health problem and one of the main causes of death around the world. The World Health Organization (WHO) informs that the number of people with COPD continues to grow, and by 2030, COPD will become the world's third cause of death. Most of the people who have COPD also experience depression. Studies show that up to 80% of patients with more severe COPD can have symptoms of depression. Other findings show that patients with COPD are four times more likely to have depression than those without COPD.
Why is this review important?
The number of people living with COPD is increasing, rather than decreasing, around the world. Depression in this population is commonly unrecognised, and patients rarely receive appropriate treatment. Untreated depression increases the risk of death, hospitalisation, readmissions, and healthcare costs. Currently, there is no strong evidence showing which psychological therapy is most effective for patients with COPD and depression.
Who will be interested in this review?
People who have COPD and depression, respiratory physicians, mental health specialists, respiratory nurses, other healthcare professionals, and policy makers.
What questions does this review aim to answer?
Which psychological therapy (if any) is effective in reducing symptoms of depression in patients with COPD?
Which studies were included in the review?
This review included experimental studies, called randomised controlled trials (RCTs), with participants who had diagnosed COPD.
What does the evidence from the review tell us?
This review included 13 experimental studies (RCTs) with 1500 participants. Our main result shows that psychological therapies using cognitive‐behavioural therapy (CBT) approach may, potentially, be effective in reducing depressive symptoms in patients with COPD. However, the quality of this evidence is very low because of many limitations related to the way the studies were conducted.
What should happen next?
More experimental studies with larger numbers of participants are needed, to confirm beneficial effects of CBT for patients with COPD‐related depression. Future studies need to produce higher quality evidence and measure adverse events and other important outcomes, such as quality of life or cost‐effectiveness.
Summary of findings
Background
Description of the condition
Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) comprises primarily chronic bronchitis and emphysema, that is, conditions characterized by airway inflammation and destruction of pulmonary tissue. The main risk factor for COPD is smoking. Other causes include passive smoking, exposure to air pollutants, occupational dusts, fumes, and noxious substances. A population‐based study assessing the burden of COPD found that approximately 20% of individuals diagnosed with COPD never smoked (Lamprecht 2011). The COPD diagnosis is based on a ratio of post‐bronchodilator forced expiratory volume in one second divided by forced vital capacity (FEV1/FVC) that is less than 70% (Rabe 2007).
Chronic obstructive pulmonary disease has been recognised as a global health concern, and is one of the leading causes of morbidity and mortality (Lopez 2006). Worldwide, approximately 251 million people were affected by COPD in 2016, and projections by the World Health Organization (WHO) suggest that with continually rising prevalence rates, COPD will become the world's third leading cause of death by 2030 (Mathers 2006; WHO 2017). Across the world, COPD is one of the main causes of hospital admissions and potentially preventable hospitalisations. In Australia, in 2014, COPD accounted for costly 355,328 hospital bed days (NHPA 2015).
A number of recent studies have indicated that psychological comorbidities, i.e. untreated depression, contribute significantly to the risk of hospitalisation and premature death in COPD (Atlantis 2013; de Voogd 2009; Yohannes 2006).
Depression
Depressive illness can present in a variety of ways, which can differ in severity (Pignone 2002). According to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5), a diagnosis of major depressive disorder (MDD) is defined as experiencing at least five of the symptoms listed below, when at least one of the symptoms is depressed mood or loss of interest or pleasure:
depressed mood;
markedly diminished interest or pleasure in all, or almost all, activities most of the day;
significant weight loss or weight gain, decrease or increase in appetite;
insomnia or hypersomnia; fatigue or loss of energy;
feelings of worthlessness or excessive guilt;
diminished ability to think or concentrate;
indecisiveness;
recurrent suicidal ideation or a suicide attempt.
The symptoms must be present for at least two weeks, every day or nearly every day (APA 2013).
WHO's estimates indicate that by 2020, depression will be the second leading public health concern, proceeded only by cardiovascular disease (DeJean 2013).
Depression in patients with COPD
Depression is a major comorbidity in COPD, and is associated with higher rates of acute exacerbations, hospitalisations, 30‐day mortality, and worse compliance with a medical treatment plan (Abrams 2011; Dalal 2011; Pooler 2014). It is also a major determinant of COPD‐related quality of life (Jang 2018). Amongst the three chronic conditions that affect 60 million people in the US (diabetes, heart disease, and COPD), the population with COPD has the highest prevalence of depression (Maurer 2008; Panagioti 2014). Results from a systematic review show that the rates are equal to, or higher than the rates of depression amongst patients with cancer, AIDS, or heart disease (Solano 2006).
A number of epidemiological and clinical studies have investigated the high prevalence rates of depression among patients with COPD and the results indicate that depression in this population varies from 18% to 80% (Bentsen 2013; Di Marco 2006; DiNicola 2013; Fleehart 2014; Goodwin 2012; Karajgi 1990; Lecheler 2017; Maurer 2008; Smith 2014; van Manen 2002,). This variability in rates may be due to diverse measures and cut‐off scores, differences in sample populations, severity of COPD, or lack of standardisation of methodology. Results from a meta‐analysis that included 39,587 participants with COPD and 39,431 controls found that clinically significant depressive symptoms affected nearly 50% of the study sample (Zhang 2011). This is compared to a one‐year prevalence of 6.9% in the general population (Wittchen 2011). An evaluation by van Manen 2002 found that patients with severe COPD had a higher risk of depression compared to controls, with rates of depression up to 62% in oxygen‐dependent patients. Even after adjusting for demographic variables and comorbidities, the risk of depressive disorder was 2.5 times higher in patients with COPD compared to those without COPD (Omachi 2009; Zhang 2011). The 80% prevalence rates of depression in one study may have been elevated due to the greater physical and psychological disease burden in the studied population of US veterans (NCT00105911).
There are multiple aspects of COPD that contribute to the development of depression, such as restricted physical functioning, exacerbations, dyspnoea, oxygen dependence (in more severe stages of COPD), multiple comorbidities, irreversible and progressive nature of COPD, as well as disease‐specific psychobiological responses (Lecheler 2017). When combined with potential social isolation and self‐blame, it is hardly surprising that the prevalence of depression in the population with COPD is high.
Importantly, depression is a particularly strong predictor for mortality in COPD (Almagro 2002; Groenwegen 2003; Ng 2007); its predictive ability persists over and above the effects of other prognostic factors, including physiological and demographic factors, or disease severity (de Voogd 2009; Fan 2007). Atlantis 2013 showed that the presence of depression in patients with COPD increased the risk of mortality by 83% compared to the COPD patients without comorbid depression. Interestingly, a retrospective cohort study found a 30% decrease in mortality in patients with COPD who were using mental health services, compared with those who were not referred to specialist services but were treated in primary care (Hanania 2011). Despite the significant impact of COPD‐related depression on a patient's daily life and on healthcare costs, it remains largely untreated, or is treated ineffectively.
A more detailed description on the mechanisms of depression in patients with COPD can be found in our sibling review focusing on the effectiveness of pharmacological interventions for the treatment of depression in COPD (Pollok 2018).
Description of the intervention
The existing guidelines for management of depression in patients with COPD are based on a relatively limited evidence base. The studies cited in the Global Initiative for Chronic Obstructive Lung Disease are often small, single trials with low methodological quality (GOLD update 2018). Antidepressant medication aims to regulate the neurotransmitter systems in the brain that have been associated with depression, however, antidepressant medical treatment trials in patients with COPD are few; which makes it difficult to assess medication effectiveness (Fritzsche 2011; Pollok 2018; Yang 2018). Moreover, the use of antidepressants in this population can be risky due to serious respiratory complications (Vozoris 2018). Psychological therapies, with clearly established evidence, include cognitive and behavioural therapies, third wave CBT, psychodynamic therapy, humanistic therapy, integrative therapy, family therapy, narrative therapy, and more (Davison 2003; Fritzsche 2011; Hunot 2013; Rose 2002; Shinohara 2013). Importantly, the UK National Institute for Health and Care Excellence guidelines for COPD advise that patients should be offered psychological therapies before they are offered antidepressants (NICE 2009).
Psychological therapies are delivered by trained professionals, and are used to provide education and techniques that can assist people in increasing their ability to cope with life problems. The Australian Lung Foundation suggests a shift from reliance on pharmacological treatment for depression to therapies that include specific educational and self‐management elements, such as management of the cycle of dyspnoea (McKenzie 2003). Psychological therapies may be used by a range of practitioners, including psychologists, psychiatrists, marriage and family therapists, occupational therapists, licensed clinical social workers, counsellors, psychiatric nurses, and trained health professionals. Therapists may work with an individual, a couple, a group, or a family (NICE 2010).
How the intervention might work
Chronic obstructive pulmonary disease is an irreversible condition, therefore, treatment recommendations are aimed at improving the quality of life (Norweg 2005). Current evidence examining quality of life suggests that comorbid depression leads to a reduction in satisfaction above and beyond what would be expected by COPD disease severity or medical illnesses alone; indicating that psychological status plays an intrinsic role in the overall well‐being (Coventry 2007). Interventions specifically targeted at increasing the ability to cope with stressors should be expected to improve quality of life (Baraniak 2011; Ries 1995; Rose 2002). Moreover, side‐effects experienced with pharmacological therapies (Eiser 1997; MacGillivray 2003), and the patient’s reluctance to take ‘yet another medication’ (Yohannes 2001), are the main reasons why alternative ways to treat depression in patients with COPD should be evaluated.
There are a number of psychological therapies that may be effective in managing depression in patients with COPD. Cognitive behavioural therapy (CBT) is a psychological approach that aims to identify and correct unhelpful emotions, behaviours, and thoughts through a goal‐oriented, systematic procedure (Kaplan 2009). There is evidence for its efficacy in patients with major depression, therefore, it may be effective in managing concurrent depression in patients with COPD (Hynninen 2010; Tolin 2010). While it does not improve an individual's medical conditions, CBT may help increase their perceived self‐efficacy, and motivate them to manage their physical condition, thereby improving quality of life (Doyle 2013a; Kunik 2001; Lamers 2010). The learning about oneself that occurs in various psychotherapy approaches may influence brain function (Kandel 1998; Kring 2007) and have a positive impact on serotonin metabolism (Linden 2006; Viinamaki 1998).
'Third wave CBT' refers to behavioural psychological therapies that integrate mindfulness and acceptance of unwanted thoughts and feelings with a behavioural understanding of emotional suffering (NCT02042976). Its aim is to elicit change in the thinking processes (Hunot 2013).
Behaviour therapy does not focuse on thoughts and feelings that might be causing certain behaviour (Shinohara 2013). A behavioural approach to therapy assumes that behaviour associated with psychological problems develops through the same processes of learning as other behaviours (Antony 2003; Shinohara 2013).
Psychodynamic therapy focuses on unconscious processes as they are manifested in a patient's present behaviour. By making the unconscious aspects of one's life a part of one's present experience, psychodynamic therapy helps people understand how their behaviour and mood are affected by unconscious feelings (Shedler 2010).
Humanistic psychotherapy emphasises human uniqueness, positive qualities, and individual potential. It works by emphasising one's capacity to make informed and rational choices, and develop to one's maximum potential (Greening 2006).
Integrative therapies are approaches that combine components of different psychological therapy models, for example Interpersonal Psychotherapy (IPT) (Hayes 2005; Norcross 2005).
Family therapy (e.g. a systemic therapy) focuses more on the family system than an individual, and addresses problems of an individual within the context of their relationships with significant people or social networks. One of the most important aims of family therapy is to enhance the ability of family members to support each other, or to develop coping skills for various life situations, including long‐term illness. Therefore, it can be a useful therapy when addressing health problems, particularly chronic physical illness and mental health issues (Carr 2009).
Why it is important to do this review
Given the prevalence and the impact of depressive disorders in patients with COPD, it is essential that effective therapies are identified and implemented. Findings by Kim 2000 and NCT00105911 suggest that fewer than one third of patients with COPD receive appropriate treatment for depression. A number of other studies found that depression is often untreated (Cafarella 2012; Kim 2014), or inappropriately treated (Maurer 2008; van Manen 2002). This is associated with worse compliance with medical treatment (Yohannes 2008), poor quality of life, increased hospital readmissions, prolonged length of hospital stay (Coventry 2011; Ng 2007), and subsequently increased costs to the healthcare system (Felker 2010; Gudmundsson 2005; Maurer 2008; NCT00105911; Ng 2007; Pumar 2014). Evidence from systematic reviews shows that the presence of psychological problems inflates the cost of care for chronic conditions by at least 45%; this is after controlling for severity of the physical illness (Hutter 2010; Naylor 2012). It has been suggested that comorbid depression in an elderly population of patients with COPD is a significant predictor of frequent hospital admissions (Yohannes 2000; Yohannes 2006). Due to the increased health and economic burden associated with aging populations with chronic illness, it is paramount to promote treatment that integrates physical and psychological care. This approach may lead to improved patient outcomes, reduced hospitalisation, and healthcare costs (NICE 2009). Regrettably, available evidence to support the use of either antidepressant medication or psychological therapies for the treatment of COPD‐related depression is based on low‐quality studies (Hegerl 2013; Lecheler 2017; NCT00105911; Pollok 2018). As it is becoming more and more evident that the therapeutic focus in COPD should point to improvement of patient‐centred outcomes and their psychological health (Roche 2007; Spruit 2013), stronger evidence is required to make psychological therapies part of the training of health professionals involved in the care of patients with COPD.
Given the burden and high prevalence of untreated depression, it is important to evaluate and update existing experimental evidence using rigorous methodology, in order to identify effective psychological therapies. The findings may help clinicians, health professionals, or policy makers decide what type of therapies can be implemented as part of the guideline for routine treatment for depression in COPD. This review is one of four linked Cochrane reviews that address both pharmacological and psychological treatments for depression and anxiety in patients with COPD (Pollok 2016; Pollok 2018; Usmani 2011; Usmani 2013).
Objectives
To assess the effectiveness of psychological therapies for the treatment of depression in patients with chronic obstructive pulmonary disease (COPD).
Methods
Criteria for considering studies for this review
Types of studies
All the studies included in this review are randomised controlled trials (RCTs); with one study being a cluster RCT. We had intended to include cross‐over trials if identified by the search; however, we did not find any.
Types of participants
Participant characteristics
We included studies involving adults, 40 years of age and older, of either gender, and of any ethnicity. As most people with COPD begin experiencing COPD symptoms in their 40s, it is unlikely that individuals under 40 years of age would be diagnosed with clinically significant COPD (WHO 2018).
Diagnosis
As per protocol, studies were eligible to be included in this review if the participants were diagnosed with COPD (FEV1/FVC less than 70% predicted) and a recognised depressive disorder (or depressive symptoms) at the time of recruitment to the trial – assessed using standardised diagnostic criteria, or a formal, validated instrument (i.e. a questionnaire), or both. Considering that trials testing effectiveness of psychological therapies often apply diagnostic criteria for depression after randomisation and at baseline assessment (i.e. participants are not included in the trial on the basis of having a depressive diagnosis or depression symptoms), we allowed inclusion of these studies as well.
The COPD diagnosis must have been made by a medical professional, clinically, or by the Global Initiative for Chronic Obstructive Lung Disease criteria, or both (WHO 2018).
Depression diagnostic criteria included, but were not limited to, DSM‐3, DSM‐4 (APA 2000), DSM‐5 (APA 2013).
Comorbidities
As long as a comorbidity was not the primary focus, we included studies with participants with comorbid chronic physical conditions (e.g. hypertension, cardiovascular disease, metabolic disease, asthma), comorbid mental disorders (e.g. anxiety), or both. For example, we did not include interventions where anxiety was the primary focus.
Setting
All types of settings were eligible for inclusion, e.g. inpatient (psychiatric setting, inpatient treatment for COPD), outpatient, and primary care.
Subset data
As per protocol, studies containing subsets of eligible participants were permitted, providing 60% of the study population had clinically diagnosed COPD and a depressive disorder. However, participants with clinically diagnosed COPD and a depressive disorder were the primary population in all of the studies included in this review.
Types of interventions
Although gradually increasing, there is a limited number of experimental trials evaluating the effectiveness of psychological therapies as a treatment for depression in patients with COPD. Therefore, we included studies that assessed any form of psychological therapies for the treatment of COPD‐related depression. We prespecified three comparators, listed below. Studies in which a psychological therapy was delivered in combination with another intervention (co‐intervention) were included only if there was a comparison group that received the same co‐intervention alone. We included any mode of delivery, e.g. group or one‐on‐one sessions, phone, e‐mail, or internet‐based.
Listed below are psychological therapies that were eligible for inclusion in our review, and the examples of relevant therapeutic approaches, which fall into five broad categories (Kazdin 2000).
Experimental intervention
behaviour therapies (e.g. behavioural activation, relaxation training)
cognitive and cognitive behavioural therapies (e.g. cognitive behavioural therapy, rational emotive therapy, acceptance and commitment therapy, mindfulness‐based cognitive therapy)
psychoanalysis and psychodynamic therapies (e.g. functional analytic psychotherapy, insight‐oriented therapy, interpersonal psychotherapy, group therapy)
humanistic therapy (e.g. Gestalt therapy, person‐centred therapy)
integrative or holistic therapy (relaxation, hypnotherapy, imagery)
Other approaches included, for example, expressive therapy, family therapy, narrative therapy.
Comparator intervention
no intervention (i.e. attention placebo, psychological placebo, waiting list, and usual care)
education only (written or oral; i.e. information about physical and mental health issues provided during a medical consultation or during a visit with a nurse, where no formal counselling or psychological therapy was provided)
co‐intervention (only if the same co‐intervention was used in the intervention arm of the study). The co‐interventions may have been pharmacotherapy or pulmonary rehabilitation
Types of outcome measures
Primary outcomes
We accepted validated and commonly used depressive symptom measures, for example: Beck Depression Inventory (BDI; Beck 1961), Hamilton Depression Rating Scale (HDRS; Hamilton 1960), Patient Health Questionnaire‐9 (PHQ‐9; Spritzer 1999), Geriatric Depression Scale (GDS; Yesavage 1982), Depression Anxiety Stress Scales (DASS; Lovibond 1995), or another validated depression scale.
Change in depressive symptoms, measured by a standardised or validated measure (listed above)
-
Adverse events, separated into two subgroups
disease‐related adverse events (e.g. exacerbation of illness, breathlessness, respiratory infections, pulmonary hypertension)
mortality (30‐day and long‐term), measured by the total number of deaths
Secondary outcomes
Change in quality of life (measured by, for example: St George's Respiratory Questionnaire (SGRQ; Jones 1991), COPD Assessment Test (CAT; Jones 2009), COPD Respiratory Questionnaire (CRQ; Guyatt 1987), 36‐Item Short Form Health Survey (SF‐36; Ware 1993), and other validated tools (in this order, if authors reported multiple scales))
Change in dyspnoea (measured by the BORG scale (Borg 1982), or other validated tools)
Change in forced expiratory volume in one second (FEV1)
Change in exercise tolerance (measured by the six‐minute walk test (6MWT), twelve‐minute walk test (Butland 1982; ATS 2002), or other validated tools)
Hospital length of stay or readmission rate
Cost‐effectiveness (e.g. measured as reduction of costs of treatment, number of appointments with a health professional, use of additional treatments, or ability to work)
Timing of outcome assessment
We defined time frames as short‐term (less than six months), medium‐term (six to 12 months), and long‐term (12 months or longer) follow‐up assessment periods. In studies with multiple reported long‐term follow‐up time points, for example 12 and 24 months, we had planned to use the final follow‐up assessment reported. The primary time point reported in the 'Summary of findings' tables was the final follow‐up assessment.
Hierarchy of outcome measures
We considered most of the validated and commonly used depressive symptom measures as equivalent: e.g. BDI, HDRS, PHQ, GDS, or DASS. If a study used two or more scales to measure the same symptom, we used the scale that was used in the other trials included in the same comparison. If a study used more than one quality of life measure, we considered the following hierarchy of scales: (1) SGCRQ, (2) CAT, (3) CRQ, (4) SF‐36, (5) any other quality of life measure used. In case of other measures used, we planned to use them as defined and reported by the study authors.
Search methods for identification of studies
We conducted the following searches to identify all relevant published and unpublished RCTs, without applying any date or language restrictions. The last search performed in October 2018 identified a new study that met our eligibility criteria, and therefore, we included it in this review.
Electronic searches
Cochrane Specialised Registers
1. Cochrane Common Mental Disorders Controlled Trials Register (CCMDCTR)
The Cochrane Common Mental Disorders Group maintains a register of randomised controlled trials: the CCMDCTR. The register contains over 40,000 reference records (reports of RCTs) for depression, anxiety, and other common mental disorders. It is a partially studies‐based register with more than 50% of reference records tagged to approximately 12,500 individually PICO‐coded study records, which can help facilitate precision searching. Reports of trials for inclusion in the register are collated from weekly generic searches of MEDLINE, EMBASE and PsycINFO, quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), and review‐specific searches of additional databases. Reports of trials are also sourced from international trial registries, drug companies, the handsearching of key journals, conference proceedings, and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCMD's core search strategies can be found on the Group's website, with an example of the core MEDLINE search displayed in Appendix 1. This register is current to June 2016 only.
The search of the CCMDCTR was conducted on 13 June 2016.
The Group's Information Specialist searched the CCDMDCTR (studies and references register) using the following terms (all years to date):
#1 (depress* or dysthymi* or "mood disorder*" or "affective disorder*" or "affective symptom*"):ti,ab,kw,ky,emt,mh #2 ((obstruct* and (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)) or COPD or emphysema or (chronic* and bronchiti*)):ti,ab,kw,ky,emt,mh #3 (#1 and #2)
We screened records for psychological interventions for the treatment of depression in COPD.
2. Cochrane Airways Group Register (CAGR)
The Cochrane Airways Group's Specialised Register is also derived from systematic searches of bibliographic databases including: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature), AMED (Allied and Complementary Medicine), and PsycINFO, and handsearching of respiratory journals and meeting abstracts (details of the CAGR can be found on the Group's website).
The Group’s Information Specialist searched their register for records coded as ’COPD’ and ’depression’.
The search of the CAGR was conducted on 28 June 2016.
3. Other bibliographic database searches
In March 2017 and November 2018 the Information Specialist with the Cochrane Common Mental Disorders Group ran updated searches directly on the following bibliographic databases (Appendix 2). We did not request an additional search of the CAGR register at this time.
Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 11) in the Cochrane Library (searched November 2018).
MEDLINE, Embase, PsycINFO Ovid cross‐search (1 January 2016 to 20 March 2017);
MEDLINE Ovid (1 January 2016 to 26 November 2018);
Embase Ovid (1 January 2016 to 2018 Week 48);
Ovid PsycINFO (1 January 2016 to November Week 3 2018).
Searching other resources
We searched online clinical trial registers for ongoing and recently completed studies, including the ISRCTN Registry, US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (who.int/trialsearch/) (26 November 2018).
Grey literature
We searched sources of grey literature, including theses and dissertations, clinical guidelines, and reports from regulatory agencies, in order to reduce the risk of publication bias and to identify as much relevant evidence as possible (26 November 2018).
Open Grey (opengrey.eu)
Trove (trove.nla.gov.au)
The Agency for Healthcare Research and Quality (ahrq.gov)
Grey Literature Network Service (greynet.org)
Handsearching
We did not perform any handsearching for this review.
Reference lists
We searched the reference lists of all included studies and systematic reviews identified by the search to find studies that may have been missed from the original electronic searches (e.g. unpublished or in‐press citations). We also conducted a cited reference search on the Web of Science for reports of all included studies.
Correspondence
We contacted the authors of the following studies to request additional data: Howard 2014, Stanley 2005; ISRCTN59537391; NCT02499653.
Data collection and analysis
Selection of studies
Two review authors (JP and JA) independently assessed the titles, abstracts, and descriptors identified by the search strategies to determine potential eligibility. We obtained the full‐text articles of studies deemed potentially relevant and two of the three review authors (JP and JA, or JP and KCC) independently assessed the full‐texts, and made the final decision on inclusion. Any disagreements over eligibility were resolved through discussion, or by consulting with a third review author (KCC or AE) when consensus could not be reached.
The decision process was recorded in detail, allowing for completion of a PRISMA flow diagram (Figure 1).
1.
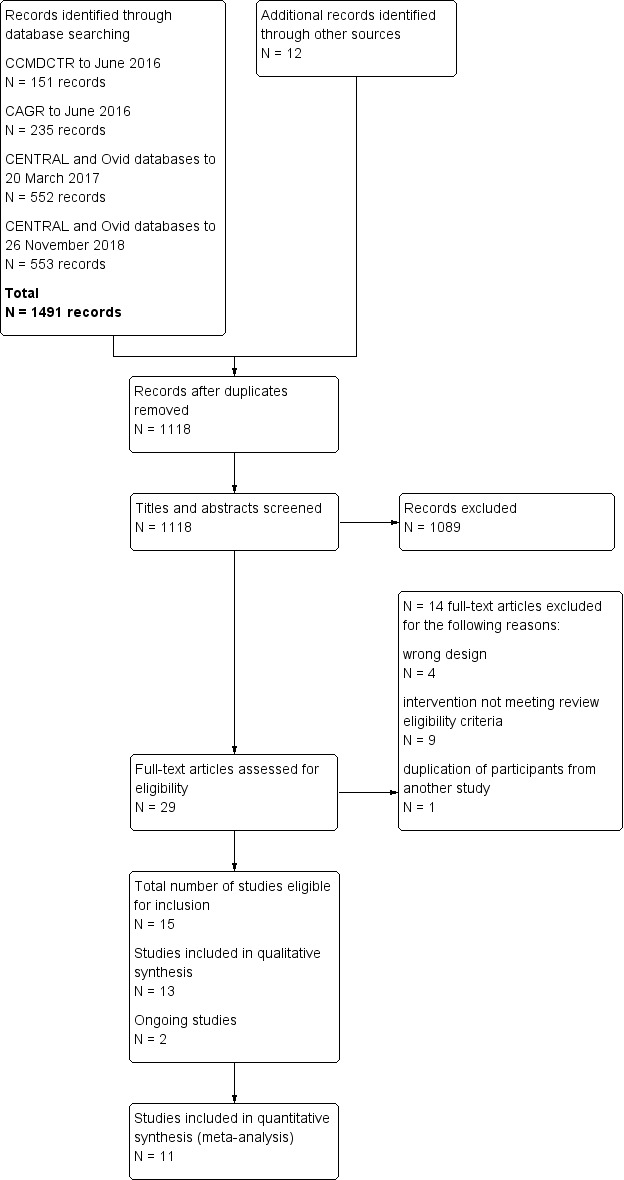
Study flow diagram
Data extraction and management
Two review authors (JP and JA, or JP and KCC) independently extracted data from each study onto a standardised and piloted data extraction form. We resolved disagreements through discussion, and if necessary, by consulting with the third investigator (KCC or AE). The following data were extracted:
Study Eligibility
General study information: authors, year of publication, country
Study design, population group, and description of psychological therapy
Participants
Number of participants, age, gender distribution, ethnicity, and other relevant information, e.g. comorbidities, severity of condition, eligibility criteria
Interventions
Type of psychological therapy, description and duration of intervention, intensity, type of comparison
Outcomes: primary and secondary outcome measures, time points, loss to follow‐up
Reported statistics (means and standard deviations, P value, or odds ratio)
Main comparisons
psychological therapies versus no intervention
psychological therapies versus education
psychological therapies and co‐intervention versus co‐intervention alone
We presented an overall pooled estimate for intervention effectiveness for each outcome in each of these comparisons.
Assessment of risk of bias in included studies
Two review authors (JP, JA) independently assessed the risk of bias for all the included studies, as per the Handbook of Systematic Reviews of Interventions guidelines, using a domain‐based evaluation. We assessed the risk of bias for each domain as 'low risk of bias', 'high risk of bias', and 'unclear risk of bias' (Higgins 2011). We resolved conflicts in the assessment by consensus. The following domains were evaluated (Higgins 2011):
Sequence generation
Methods considered to be adequate included: random number table, computer random number generator, coin toss, shuffling cards or envelopes, throwing dice, and drawing lots.
Allocation concealment
We assessed the method used to conceal allocation to interventions prior to participant assignment. We rated trials where the allocation sequence was available to investigators before randomisation as having a high risk of bias (e.g. assignment envelopes used without appropriate safeguards).
We rated trials that adequately concealed allocation as having a low risk of bias. Methods considered to be adequate included: central allocation or serially‐numbered, sealed, opaque envelopes.
Where the information about the allocation concealment was insufficient, we rated the trial as having unclear risk of bias.
Blinding (of participants and personnel)
We considered blinding to be adequate if trial authors mentioned that participants and personnel were blinded to the intervention. However, we expected it to be unlikely, due to the nature of communication‐based interventions (i.e. talking therapies) where proper blinding is more difficult (or impossible) to establish and maintain. In general, most existing RCTs using talking therapies describe blinding poorly.
Blinding (of outcome assessors)
We considered blinding to be adequate if trial authors mentioned that outcome assessors were blinded to sequence allocation.
Incomplete outcome data
We assessed the risk of bias due to incomplete outcome data on the grounds of whether the incomplete outcome data were adequately addressed; as per Cochrane Handbook for Systematic Reviews of Interventions, Section 8.13 (Higgins 2011).
Selective outcome reporting
We considered studies to have minimal bias if a protocol was available and pre‐specified outcomes were reported accordingly; or in the absence of a protocol, if all expected outcomes were reported; as per recommendations in the Cochrane Handbook for Systematic Reviews of Interventions, Table 8.5.d. (Higgins 2011).
Other bias
We considered studies to be at low risk of 'other' bias if other influencing factors that could potentially affect the outcome were not evident. Examples of other bias included carry‐over effect in a cross‐over trial, or extreme baseline imbalance.
We considered studies with inadequate or unclear randomisation, allocation concealment or both at high risk of bias.
We presented the results of our assessment in the 'Risk of bias' table and described it in the text using a narrative synthesis.
Measures of treatment effect
Continuous data
For continuous outcomes, we entered data from validated depressive symptoms rating scales, quality of life questionnaires, and other clinical measures. We summarised available data by either mean difference (MD) or standardised mean difference (SMD), if various tools were used to measure the same outcome, with 95% confidence intervals (CI), using mean values and standard deviations (SD). We examined the change in primary and secondary outcomes and presented data based on the mean change scores between the groups, from baseline to the final follow‐up assessment.
Dichotomous data
For binary data, we had intended to present odds ratios with 95% CI; however, we did not use any binary data in this review.
Unit of analysis issues
Cluster randomised trials
Cluster‐randomised controlled trials (i.e. trials in which outcomes relate to individual participants whilst allocation to the intervention is by hospital, clinic, or practitioner) may introduce unit of analysis errors. Statistical methods which assume, for example, that all patients' chances of benefit are independent, ignore the possible similarity between outcomes for patients seen by the same provider. This may underestimate standard errors and give misleading, narrow confidence intervals, leading to the possibility of a type 1 error (Altman 1997). In this review, we performed analyses at the level of individuals, whilst accounting for clustering in the data by using estimates that had been adjusted for clustering by the study authors. We also prespecified that for studies not adjusting for clustering, the actual sample size was to be replaced with the effective sample size (ESS), calculated using a rho = 0.02, as per Campbell 2000; however, this proved unnecessary.
Cross‐over trials
We had planned to extract data from cross‐over studies only for the first phase (pre cross‐over), due to the potential for a significant carry‐over effect in psychological therapies. However, we did not include any randomised cross‐over trials in this review.
Studies with multiple treatment groups
We had intended to include multi‐arm trials, provided there was an intervention arm with any of the interventions of interest, and a control arm with any of the comparators mentioned above. We had intended to include each pair‐wise comparison separately, and to equally divide shared intervention groups among the comparisons. We also prespecified that if the intervention groups were deemed similar enough to be pooled, we would combine the groups using appropriate formulae from Chapters 16.5 and 16.6 (Higgins 2011). However, we did not include any trials with multiple treatment groups in this review.
Dealing with missing data
As prespecified in the protocol, we had planned to evaluate the missing participant information on an available case analysis basis, as recommended in Chapter 16.2.2 Higgins 2011. However, we deemed this unnecessary. We had intended to address missing standard deviations by imputing data from studies within the same meta‐analysis, or from a different meta‐analysis, but with studies that used the same measurement scales, had the same degree of measurement error, and had the same time periods between baseline and final value measurement (Chapter 16.1.3.2 Higgins 2011). However, we deemed this unnecessary. Where statistics essential to conduct the analyses were missing, and we could not calculate them from other available data (e.g. group means and standard deviations for both groups not reported), we had prespecified that we would attempt to contact the study authors to obtain data. However, this was not necessary.
We assumed that the loss of participants before baseline measurements were acquired, would not affect the outcome data. We discussed attrition in the 'Risk of bias' tables and in the main text. We reported a dropout rate higher than 20% qualitatively. Schulz and Grimes argue that attrition rates of 5% or lower may lead to low risk of bias, whereas a loss of 20% or greater may potentially lead to high risk of bias due to incomplete outcome data (Schulz 2002).
Assessment of heterogeneity
We expected this review to have some heterogeneity, contributed by factors such as baseline severity of depression, severity of underlying COPD, or varying measuring tools used to assess outcomes. We used Chi² and I² statistics to quantify inconsistency across studies, in combination with visual inspection of the data for differences between studies (e.g. types of interventions, participants, etc). Thresholds for the interpretation of I² can be misleading, due to the fact that inconsistency depends on several factors. The factors include, for example, magnitude and direction of the effect or strength of the evidence for heterogeneity. We had intended to investigate possible causes of an I² statistic that represented considerable heterogeneity by conducting subgroup analyses, as per Chapter 9.5.2 (Higgins 2011); however, we deemed this unnecessary. We examined the I² value, using the following overlapping bands provided in the Cochrane Handbook for Systematic Reviews of Intervenions (Higgins 2011).
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We had planned to assess potential publication bias using a visual inspection of a funnel plot. Potential asymmetry in the plot could be attributed to a publication bias, but may well be due to true heterogeneity or poor methodological design. In case of asymmetry, contour lines may correspond to perceived milestones of statistical significance (P = 0.01, 0.05, 0.1, etc.), which may help to differentiate between causes of asymmetry (Higgins 2011). However, we provided assessment of 'selective reporting' in the 'Risk of bias' table, as there were fewer than ten studies per comparison.
Data synthesis
We calculated pooled MD and SMD with 95% CI for continuous outcomes, as appropriate. We obtained continuous treatment effects from a random‐effects model to allow for expected heterogeneity in intervention types and populations. In relation to the trials reporting data at more than one assessment time point, we extracted data from the final follow‐up period reported by the study authors. We analysed data using Review Manager 5.3 (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We expected that the included studies would be heterogeneous due to multiple factors, such as baseline severity of depression, severity of underlying COPD, duration of intervention, and the use of multiple measuring tools to assess the same outcome. As such, in our protocol, we prespecified subgroups to investigate heterogeneity and reduce the likelihood of spurious findings. This procedure limits the number of subgroups investigated and prevents knowledge of the studies' results from dictating which subgroups should be analysed (Higgins 2011). We did not identify any studies with unexplained sources of heterogeneity; therefore, considering the small number of included studies, we did not conduct subgroup analyses.
In the protocol, we prespecified that we would perform the following subgroup analyses within each classification of psychological therapies:
age (40 to 55 years of age, above 55 to 70 years of age, above 70 years of age);
gender (male compared to female);
comorbidity (patients with (non‐psychological) comorbidity compared to patients with no comorbidity);
duration of the intervention (less than two months compared to two months or longer)
intervention setting (inpatient compared to outpatient);
severity of COPD (mild, moderate, severe);
severity of depression symptoms (mild, moderate, severe).
Sensitivity analysis
We performed sensitivity analyses to evaluate the impact of our methodology on final results. We had planned to test the validity and robustness of the findings by removing studies based on the following criteria:
inadequate sequence generation (unclear or high risk of bias)
inadequate allocation concealment (unclear or high risk of bias)
significant attrition of the study population (20% or higher attrition)
cluster‐randomised trials
cross‐over studies
studies containing data imputed by the review authors
quality of the studies (i.e. high risk of bias for: two or fewer domains, three or four domains, or five to seven domains)
As per protocol, sensitivity analyses were to be conducted only for primary outcomes; however, we also performed a sensitivity analysis for one secondary outcome (i.e. change in quality of life). Please see Differences between protocol and review.
Summary of findings table
We used the GRADE approach to evaluate the quality of evidence, as described in Chapter 12.8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used GRADEpro GDT software to prepare a 'Summary of findings' table for each comparison (GRADEpro GDT 2015). We assessed the following factors: risk of bias, indirectness of evidence, inconsistency of results, imprecision of results, and publication bias.
We included and assessed the following outcomes: 1) Change in depressive symptoms, 2) Adverse events, 3) Change in quality of life, 4) Change in dyspnoea scores, 5) Change in forced expiratory volume in one second (FEV1), 6) Change in exercise tolerance, 7) Hospital length of stay, or readmission rates.
In the 'Summary of findings' table, we reported data based on the mean change score between groups from baseline to the final follow‐up assessment.
Results
Description of studies
Results of the search
We conducted all searches to 26 November 2018, which identified a total of 1491 records. We identified additional 12 records through other sources, including the reference lists of included studies. After removing duplicates, we screened 1118 records for eligibility, 29 of which we selected for full‐text review. A total of 15 studies met the inclusion criteria, two of which were classified as ongoing studies (ISRCTN59537391, NCT02499653 ). We included a total of 13 studies in the qualitative synthesis, 11 of which contributed data for the meta‐analyses (Figure 1).
Included studies
In this review, we included 13 studies (excluding the two ongoing studies) with a total of 1500 participants. The studies had the following characteristics: (see also Characteristics of included studies and Table 4 for a brief summary of the characteristics of included studies).
1. Summary of the characteristics of included studies.
|
Study ID Country |
Study design/length of trial Last follow‐up time point |
Total N of participants randomised vs analysed as reported in the original studies | Severity of COPD | Outcome measure | Diagnosed depression required at study entry | Intervention type/length of sessions | Control group | Setting/ intervention delivery | Comments |
| 1. de Godoy 2003 Brazil | RCT
3 months 3 months |
randomised
N = 30 analysed N = 30 |
severe | BDI baseline mean scores: intervention 13.7 (SD 8.9); control 14.9 (SD 11.5) |
No | group psychotherapy (with cognitive therapy elements) embedded into pulmonary rehabilitation programme | pulmonary rehabilitation | pulmonary rehabilitation clinic/ group based setting | Participants were not required to have diagnosed depression at study entry; symptoms were measured at baseline. |
| 2. Doyle 2017 Australia | RCT
2 months 4 months |
randomised
N = 110 analysed N = 110 |
mild (20% in each arm); moderate (50% in each arm); severe (30% in each arm) |
PHQ‐9 baseline mean scores: intervention 12.6 (SD 6.0); control 11.2 (SD 16.8) |
Yes | phone‐based CBT/ 8 weekly phone sessions plus one introductory session | phone delivered befriending (attention placebo) | phone based; delivered by nurse | Participants were required to have diagnosed depression at study entry. 70% of the participants had attended or were attending pulmonary rehabilitation programmes at the time of the study; 19% of the participants were receiving other psychological or psychiatric services. |
| 3. Farver‐Vestergaard 2018 Denmark | RCT
2 months 6 months |
randomised
N = 84 analysed N = 84 |
severe | HADS‐D baseline mean scores: intervention 6.3 (SD 3.7); control 5.9 (SD 4.1) |
No | mindfulness‐based cognitive therapy embedded into pulmonary rehabilitation programme/ 8 weekly, 105‐min group sessions | pulmonary rehabilitation | pulmonary rehabilitation clinic/ group‐based setting | Participants were not required to have diagnosed depression at study entry; symptoms were measured at baseline. |
| 4. Howard 2014 UK | RCT
6 weeks 6 weeks |
randomised
N = 222 analysed N = 221 (final follow up for depression) |
moderate | HADS baseline mean scores: intervention 8.8 (SD 3.7); control 8.6 (SD 3.5) |
No | booklet based CBT; self‐guided/ 6 weeks of self‐help booklet based intervention with two phone sessions |
COPD information booklets | self‐help booklet; two phone sessions | Participants were not required to have diagnosed depression at study entry; symptoms were measured at baseline. A small proportion of participants were taking medication for depression and anxiety. Participants were recruited without screening for depression at study entry. Only N = 29 out of 110 participants in the intervention group had HADS score above threshold for depression. N = 32 out of 112 participants in the control group had HADS score above threshold for depression (>11). |
| 5. Hynninen 2010 Norway | RCT
2 months 6 months |
randomised
N = 51 analysed N = 51 |
moderate | BDI‐II baseline mean scores: intervention 20.7 (SD 8.6); control 20.5 (SD 9.7) |
Yes | booklet based, group CBT/ 7 weekly 2h group sessions |
standard care plus fortnightly phone call (5 to 10 min) | manual based, group setting | Participants were required to have diagnosed depression at study entry. There was no 'attention placebo' employed although the intervention group received four phone calls. |
| 6. Jiang 2012 China | RCT
10 months 10 months |
randomised
N = 100 analysed N = 96 |
moderate to severe | HADS‐D baseline mean scores: intervention 7.2 (SD 3.0); control 7.1 (SD 2.9) |
No | CBT with the focus on 'uncertainty management'/ Audio (CD), 90‐page self‐help manual, instructions booklet, 4 telephone contacts during first 4 weeks and then monthly phone calls for 10 months |
standard care | nurse‐led; booklet, CD and phone call once a week for 4 weeks (35 min) then monthly phone calls for 10 months | Participants were not required to have diagnosed depression at study entry; symptoms were measured at baseline. HADS‐D baseline scores were slightly above 7; scores for borderline depressive symptoms are from 8 to 10. |
| 7. Kunik 2001 USA | RCT
Two‐hour single session with weekly phone‐call for 6 weeks 6 weeks |
randomised
N = 53 analysed N = 48 |
moderate to severe | GDS baseline mean scores: intervention 11.5 (SD 7.3); control 7.7 (SD 5.4) |
No | CBT/ single 2h session plus self‐help workbooks and audio tapes for use after the session |
COPD education (2h) | small group in clinical setting; afterwards weekly phone follow up for 6 weeks to monitor compliance regarding using workbooks and audiotapes | Participants were not required to have diagnosed depression at study entry; symptoms were measured at baseline. No long‐term follow‐up assessment; primarily male participants; both groups reported to be similarly satisfied with the intervention. |
| 8. Kunik 2008 USA | RCT
8 one‐hour sessions 8 months‐12 months |
randomised
N = 238 analysed N = 238 |
moderate to severe | BDI‐II baseline mean scores: intervention 14.2 (SD13.7); control 14.5 (SD 13.6) |
Yes | CBT/ eight 1h‐sessions |
COPD education | group session/ veterans clinic |
Participants were required to have diagnosed depression at study entry. 53.2% of the study sample had a diagnosis of depression (based on DSM‐IV) at study entry. |
| 9. Lamers 2010 Netherlands | RCT
3 months 9 months |
randomised
N = 187 analysed N = 187 |
not reported | BDI baseline mean scores: intervention 17.1 (SD 6.5); control 18.3 (SD 7.2) |
Yes | CBT‐based, Minimal Psychological Intervention/ two to ten home visits depending on participants' progress for 3 months; on average four 1h contacts per participant |
standard care | delivered by nurse; home visits | Participants were required to have diagnosed depression at study entry. Despite home visit setting, there was 36% dropout rate and slightly higher in the intervention group (40%) compared to control (33%). However, the difference was not statistically significant. Participants who dropped out were on average older and had higher baseline BDI score. |
| 10. Lee 2015 South Korea | RCT
6 months 6 months |
randomised
N = 254 analysed N = 151 |
mild to severe | CES‐D baseline mean scores for subgroup of depressed participants (N = 25 out of 151): intervention 30.7 (SD 6.3); control 29.4 (SD 8.7) |
Yes | phone based CBT with the focus on problem solving/ 12 fortnightly phone sessions per participant over 6 months |
standard care | phone based; delivered by nurse | Participants were required to have diagnosed depression at study entry. Only 25 out of 151 participants who completed the study had clinically significant depressive symptoms (CES‐D >24). High dropout rate (41%) amongst older participants with more severe COPD and less mobility. |
| 11. Perkins‐Porras 2018 UK | RCT
3 days 3 days |
randomised
N = 80 analysed N = 50 |
not reported | HADS baseline mean scores: intervention 6.5 (SD 3.3); control 7.0 (SD 3.2) |
No | 10‐minute audio recording of a mindfulness‐based body scan | active control group listened to a 10‐minute audio recording of a natural history text | acute admissions hospital word/home | The intervention was delivered to participants admitted to a London hospital with an acute COPD exacerbation. Participants were not required to have diagnosed depression at study entry; the assessments were performed at Day 1 (pre‐intervention) at the hospital and Day 3 (post‐intervention) either at the hospital or at a patient's home. |
| 12. Rosser 1983 UK | RCT
2 months 6 months |
randomised
N = 65 analysed N = 65 |
mild to moderate | VAM‐D baseline median scores: intervention 8; control 20 |
No | one‐on‐one psychotherapy 8 weekly x 45 min sessions for 2 months |
standard care | one‐on‐one | Participants were not required to have diagnosed depression at study entry; symptoms were measured at baseline. |
| 13. Schüz 2015 Australia | cluster RCT
12 months 12 months |
randomised
N = 182 analysed N = 169 |
moderate | HADS baseline mean scores: intervention 4.6 (SD 3.1); control 5.1 (SD 3.5) |
No | CBT with the focus on self‐management skills/ up to 16 phone calls over 12 months |
standard care plus monthly phone call without specific advice or skill training | delivered by nurse; phone based | Participants were not required to have diagnosed depression at study entry; symptoms were measured at baseline. Only 39 participants (21.4%) of the study sample had clinically significant depressive symptoms (HADS‐D > 8). |
RCT ‐ Randomised Controlled Trial
BDI ‐ Beck Depresssion Inventory
PHQ‐9 ‐ Patient Health Questionnaire
HADS ‐ Hospital Anxiety and Depression Scale
GDS ‐ Geriatric Depression Scale
CES‐D ‐ Center for Epidemiological Studies ‐ Depression Scale
VAM‐D ‐ Visual Analogue Measure of Depression
CBT ‐ Cognitive‐Behavioural Therapy
Design
All the included studies used a randomised controlled trial (RCT) design. All the trials tested the effectiveness of psychological therapies, therefore, blinding of participants was not feasible. Eleven studies reported that a single blinding method was used, and study personnel completing assessments were blinded to the treatment allocation. One study reported that due to the nature of the intervention, neither participants nor therapists were blinded for allocation (Hynninen 2010), and one study reported that no blinding was used (Perkins‐Porras 2018).
Four studies were multi‐centred trials (Howard 2014; Lamers 2010; Lee 2015; Schüz 2015). Howard 2014 recruited participants from 10 general practices in north‐west London, England. In Lamers 2010, a total of 89 clinics in the south of the Netherlands participated in the recruitment of participants. In Lee 2015, the participants were recruited from three outpatient clinics in South Korea. Schüz 2015 recruited participants from 31 general practices in Tasmania, Australia. Two studies used cluster RCT design: Schüz 2015 and Farver‐Vestergaard 2018 who recruited participants from one pulmonary rehabilitation (PR) clinic, but they chose a cluster‐randomised design to avoid the risk of contamination between individual patients attending the PR groups.
The treatment components included, for example, psycho‐education, problem‐solving techniques, behavioural activation, exposure to feared situations, cognitive therapy, mindfulness‐based body scan, uncertainty management.
Sample sizes
The smallest sample sizes were reported in de Godoy 2003 (30 participants), Hynninen 2010 (51 participants randomised), and Perkins‐Porras 2018 (50 participants analysed). The largest sample was reported in Lee 2015 (254 participants randomised); however, the dropout rate was 41%, and as a result, only 151 participants completed the trial. Those who dropped out were older participants, with more severe COPD symptoms, and limited mobility. The next largest sample size was reported in Kunik 2008 (238 participants randomised); but at 12‐month follow‐up, 108 participants completed the assessments. Of the thirteen studies, the following nine had the sample sizes calculated: Doyle 2017, Farver‐Vestergaard 2018, Howard 2014, Hynninen 2010, Jiang 2012, Kunik 2008, Lamers 2010, Lee 2015, Perkins‐Porras 2018.
Setting
The studies were conducted in various countries: Australia (Doyle 2017; Schüz 2015), Brazil (de Godoy 2003), China (Jiang 2012), Denmark (Farver‐Vestergaard 2018), the Netherlands (Lamers 2010), Norway (Hynninen 2010), South Korea (Lee 2015), the UK (Howard 2014; Perkins‐Porras 2018; Rosser 1983), and the USA (Kunik 2001; Kunik 2008). The majority of them were conducted in university affiliated hospital outpatient clinics.
Five studies reported group settings for intervention delivery (de Godoy 2003; Farver‐Vestergaard 2018; Hynninen 2010; Kunik 2001; Kunik 2008); with an average of five participants in each session in Hynninen 2010, six to ten participants in a single two‐hour intervention session in Kunik 2001, and up to 10 participants in each session in Kunik 2008. The settings for de Godoy 2003 were not described in detail; however, the trial participants were part of an ongoing pulmonary rehabilitation programme at the outpatient university affiliated clinic in Brazil. Similarly, Farver‐Vestergaard 2018 recruited patients referred to pulmonary rehabilitation at a hospital in Denmark. Limited details were provided by Rosser 1983 and colleagues; however, basing on Appendices their intervention was delivered in one‐on‐one sessions.
Four studies used a telephone to deliver their interventions, with regularly scheduled telephone session of various length and frequency (Doyle 2017; Jiang 2012; Lee 2015; Schüz 2015). In Doyle 2017, one introductory group session was provided to the participants before the telephone delivered therapy commenced. Three trials used self‐guided, booklet‐based interventions, where contact with the participants and monitoring of their progress was maintained through group sessions, or telephone contacts, or both (Howard 2014; Hynninen 2010; Jiang 2012). Lamers 2010 delivered the intervention at each participant's home. Kunik 2001 in addition to the single, two‐hour CBT session, also used self‐guided booklets and follow‐up phone calls for six weeks. Five interventions were delivered by a nurse (Doyle 2017; Jiang 2012; Lamers 2010; Lee 2015; Schüz 2015). Table 5, Table 6 and Table 7 include the summary of the above setting details presented in three separate comparisons.
2. Intervention details ‐ Comparison 1.
| Study ID | Psychological therapy used | Intervention delivery | Intervention length |
| Doyle 2017 | CBT | phone based, nurse‐delivered | 8 weekly calls (2 months) |
| Hynninen 2010 | CBT | group based, plus self‐guided booklet | 7 weekly sessions (2 hours each) (2 months) |
| Jiang 2012 | CBT (focus on uncertainty management) | phone based, nurse‐delivered, plus self‐guided booklet with audio | 4 weekly phone calls, 9 monthly (10 months) |
| Lamers 2010 | CBT (minimal psychological intervention) | home visits, nurse‐delivered | 2 to 10 visits (on average 1 hour each) (3 months) |
| Lee 2015 | CBT (focused on problem solving) | phone based, nurse‐delivered | 12 fortnightly phone sessions (6 months) |
| Schüz 2015 | CBT (focused on self‐management) | phone based, nurse‐delivered | 16 phone sessions in 12 months |
| Perkins‐Porras 2018 | mindfulness based body scan | individual sessions | 10 minutes each session (3 days) |
| Rosser 1983 | psychotherapy (no description reported) | one‐on‐one psychotherapy (no description reported) | 8 weekly sessions (45 min each) (2 months) |
CBT ‐ Cognitive‐Behavioural Therapy
3. Intervention details ‐ Comparison 2.
| Study ID | Psychological therapy used | Intervention delivery | Intervention length |
| Howard 2014 | CBT | booklet based, self‐guided, plus 2 phone calls | 6 weeks |
| Kunik 2001 | CBT | group session, plus self‐guided booklets and follow‐up weekly phone calls | single 2 hour session; weekly phone calls for 6 weeks) |
| Kunik 2008 | CBT | group session | 8 weekly sessions (1 hour each) (8 weeks) |
CBT ‐ Cognitive‐Behavioural Therapy
4. Intervention details ‐ Comparison 3.
| Study Id | Psychological therapy used | Intervention delivery | Intervention length |
| de Godoy 2003 | psychotherapy with cognitive elements (as an add‐on to PR) | group session | 1 session per week (3 months) |
| Farver‐Vestergaard 2018 | Mindfulness Based Cognitive Therapy (as an add‐on to PR) | group session | 8 sessions per week (105 min each) (2 months) |
PR ‐ Pulmonary Rehabilitation
Participants
The lowest reported mean age across the 13 included studies was 58.8 years (standard deviation (SD) 11.8) in the control group in de Godoy 2003, and 59.3 years (SD 7.6) in the intervention group in Hynninen 2010. The highest reported mean age was 73.2 years (SD 11.4) in the control arm of Howard 2014.
Baseline COPD severity varied across the studies, with most reporting moderate to severe COPD stages and FEV1 between 30% and 80% (Doyle 2017; Farver‐Vestergaard 2018; Hynninen 2010; Jiang 2012; Lee 2015; Schüz 2015). In de Godoy 2003, 23 participants out of the total sample of 30 had severe COPD.
Depressive symptoms were not a required inclusion criterion in most of the trials, and the screening for depressive symptoms took place after randomisation, at baseline assessment stage. This meant that some studies with participants who had subthreshold depressive symptoms were considered eligible for this review. In total, there were only four trials where depressive symptoms were required as an inclusion criterion (Doyle 2017; Hynninen 2010; Kunik 2008; Lamers 2010). Therefore, baseline depression scores varied across the studies.
Four studies used Beck Depression Inventory (BDI) to assess the severity of depressive symptoms:
The mean baseline scores ranged from 13.7 (SD 8.9) to 20.7 (SD 8.6) in the intervention groups, and from 14.9 (SD 11.5) to 20.5 (SD 9.7) in the control groups.
One study used the Patinet Health Questionnaire‐9 (PHQ‐9):
Doyle 2017 reported that 60% of the participants had moderate to severe depressive symptoms, measured by the Hospital Anxiety and Depression Scale (HADS) and the PHQ‐9 at the recruitment stage. At baseline and follow‐up assessment time points, only the PHQ‐9 was used. The mean baseline score for depressive symptoms, measured by the PHQ‐9, was 12.6 (SD 6.0) in the intervention group, and 11.2 (SD 6.8) in the control group. Seventy per cent of the participants were attending pulmonary rehabilitation programmes at the time of the trial, and 19% were receiving other psychological or psychiatric services.
Five studies used Hospital Anxiety and Depression Scale (HADS):
The mean baseline scores ranged from 4.64 (SD 3.14) to 14.04 (SD 7.65) in the intervention groups, and from 5.12 (SD 3.55) to 13.46 (SD 7.77) in the control groups.
One study used the Geriatric Depression Scale (GDS):
In Kunik 2001, participants were included regardless of the presence or absence of depressive symptoms. The mean score was 11.5 (SD 7.3) for the intervention group, and 7.7 (SD 5.4) for the control group, out of a possible 15.
One study used the Center for Epidemiologic Studies – Depression scale (CES‐D):
The participants in Lee 2015 were screened for depressive symptoms after the randomisation process. The mean baseline score was 14.2 out of 60 for both the intervention and control groups; only 25 out of 151 participants scored above the cut‐off (CES‐D > 24).
One study used the General Health Questionnaire (GHQ)
In Rosser GHQ and Visual Analogue Measure of Depression (VAMD) were used to assess depressive symptoms. Only median scores for 'psychiatric symptoms' were reported for the whole study sample: 6.3 (GHQ) and 9 (VAMD).
Interventions
Ten of the included studies examined the effectiveness of therapies based on cognitive behavioural therapy, and compared the interventions to no intervention, education, or a co‐intervention. In two studies, the authors reported that they used psychotherapy sessions (one reported using elements of cognitive therapy and the second one did not specify the type of therapy), and one study used mindfulness‐based body scan.
Eight studies used standard care or attention placebo as a 'no intervention' comparator (psychological therapy versus no intervention comparison). Three studies compared a form of CBT to education (psychological therapy versus education comparison), and two studies compared a psychological therapy intervention plus a pulmonary rehabilitation programme to a pulmonary rehabilitation programme alone (psychological therapy versus co‐intervention).
Four interventions were delivered by phone (plus one study where the phone was used for booster sessions and monitoring purposes). Three trials with a self‐guided approach used booklets or workbooks. Five trials were classified as nurse‐led interventions, and another five used a group setting to deliver their intervention. Most studies used a combination of the above settings and delivery formats. Please refer to Table 4 for the summary of intervention types, format and delivery methods used in the trials.
Comparison with no intervention
In Doyle 2017, telephone‐delivered CBT was compared to attention placebo (the control arm received telephone calls, but the conversations focused on everyday topics). Hynninen 2010 and Jiang 2012 used manual‐based, group CBT sessions compared to standard care. Lamers 2010 and Schüz 2015 used nurse‐led, CBT‐based interventions compared to standard care. Lamers 2010 used a Minimal Psychological Intervention, based on principles of CBT and self‐management. Lee 2015 used Problem Solving Therapy with telephone‐based counselling. Both Lamers 2010 and Lee 2015 used COPD‐tailored interventions with a patient‐centred approach. One study used a 10‐minute mindfulness‐based body scan as an intervention for patients admitted to an acute respiratory hospital ward (Perkins‐Porras 2018). And in one study (Rosser 1983), the intervention was eight, 45‐minute psychotherapy sessions, compared to no psychotherapy with weekly lab tests for the control. There were no details reported in relation to the type or delivery of therapy used.
Table 5 includes the summary of intervention formats and delivery methods used in Comparison 1.
Comparison with education
Howard 2014, Kunik 2001 and Kunik 2008 used education as a comparator in their trials. Howard 2014 used a self‐guided booklet based CBT intervention versus COPD education in the control arm. The intervention group used self‐help booklets at home for five weeks, and the participants were contacted by phone in the third and sixth week of the intervention. The intervention duration was six weeks in total. Kunik 2001 administered CBT to the intervention group during a single two‐hour session; the control group received a two‐hour session of COPD education alone. All the participants who attended the two‐hour session, received self‐guided materials (workbooks and audio), and were contacted weekly by phone for six weeks, to allow questions and to monitor compliance. The intervention duration was six weeks in total. The intervention delivered by Kunik 2008 and the colleagues used CBT in eight one‐hour group sessions; the frequency of the sessions was not reported. The intervention duration was 8 weeks in total. In this study, the control group received COPD education. Table 6 includes the summary of intervention formats and delivery methods used in Comparison 2.
Comparison with co‐intervention (pulmonary rehabilitation)
de Godoy 2003 and Farver‐Vestergaard 2018 embedded psychological therapy into the pulmonary rehabilitation programme (exercise, breathing, education) and compared it to pulmonary rehabilitation programme alone. de Godoy 2003 used group therapy sessions which included cognitive elements; however, no specific details in relation to the type of psychotherapy were reported. It was a three month treatment program with one psychological therapy session per week. Farver‐Vestergaard 2018 used group mindfulness based cognitive therapy, with a two month treatment program, and eight weekly session (105 min per session). Table 7 includes summary of intervention formats and delivery methods used in Comparison 3.
Outcomes
Change in depressive symptoms
In the primary analysis, the mood was assessed with a number of questionnaires, including the Beck Depression Inventory (BDI and BDI‐II), Hospital Anxiety and Depression Scale (HADS), Patient Health Questionnaire‐9 (PHQ‐9), Geriatic Depression Scale (GDS), Center for Epidemiological Studies – Depression scale (CES‐D) and General Health Questionnaire‐30 (GHQ‐30).
Scores measuring depression symptoms that indicate the level of depression severity are categorised differently for each scale:
BDI
Scores between 17‐20 indicate symptoms for borderline depression; 21‐30 moderate depression; 31‐40 severe depression; over 40 extreme depression
HADS
Scores 0‐7 indicate no depressive symptoms, 8‐10 symptoms for borderline depression, 11‐21 clinical depression
PHQ‐9
Scores between 5‐9 indicate symptoms for mild depression; 10‐14 moderate depression; 15‐19 moderately severe depression; 20‐27 severe depression
GDS
Scores 0‐9 indicate no depressive symptoms; 10‐19 symptoms for mild to moderate depression; 20‐30 severe depression
CES‐D
Scores 0‐16 indicate no symptoms or symptoms for mild depression; 16‐23 moderate depression 24‐60 severe depression. A cutoff score of 16 or greater indicates risk for clinical depression
GHQ‐30
The overall total score is produced via a scoring system based on descriptions that range from a 'better/healthier than normal' option, through a 'same as usual' and a 'worse/more than usual' to a 'much worse/more than usual' option
Adverse events
None of the studies collected data on adverse events.
Change in quality of life
Four trials collected data on quality of life. Various measures were used, including the St George's Respiratory Questionnaire (SGRQ), the COPD Assessment Test (CAT), the Chronic Respiratory Disease Questionnaire (CRQ), and the 36‐item Short Form Health Survey (SF‐36).
Change in dyspnoea
Two studies reported data on dyspnoea scores, using CRQ as a measure.
Change in forced expiratory volume in one second (FEV1)
None of the studies collected data on FEV1.
Change in exercise tolerance
Two studies reported data on exercise tolerance, using the six‐minute walk distance test (6MWT).
Hospital length of stay or readmission rates
Only one study assessed hospital utilisation rates.
Cost‐effectiveness
Only one study assessed cost‐effectiveness.
Excluded studies
We excluded 14 studies following the review of full‐text reports. We listed the main reasons for exclusion in the 'Characteristics of excluded studies' table. While Alexopoulos 2016 and Emery 1998 assessed the change in depressive symptoms due to the effects of psychological and behavioural interventions, their comparator interventions did not meet our prespecified inclusion criteria. Emery 1998 randomised their participants to one of the three study arms: 1) exercise, education and stress management; 2) education and stress management; 3) waiting list. Alexopoulos 2016 used standard care or education as a co‐intervention called Problem Solving Adherence (PSA), rather than as a comparator. Therefore, an intervention (a Personalised Intervention for Depressed Patients with COPD (PID‐C)) was compared to the co‐intervention (PSA). We excluded the study, as our protocol prespecified that the same co‐intervention was to be included in both arms of the study.
We excluded four studies with the main focus on anxiety rather than depression (Bove 2016; Heslop‐Marshal 2015; Livermore 2010; Livermore 2015). In two studies, the subset of eligible participants with COPD comprised less than 60% of the study population (Blumenthal 2006; Stoop 2015). Luk 2017 is a prospective controlled trial and the participants were not randomised. Similaryly, Eiser 1997 did not randomise the participants. A study by de Godoy 2005 is a separate study that used the same sample as de Godoy 2003 and was excluded because we were unable to reconcile participation rates between intervention groups. Stanley 2005 did not provide comprehensive outcome data and it was a case study with 5 participants.
Ongoing studies
Two studies have not been completed (ISRCTN59537391; NCT02499653); please refer to the Characteristics of ongoing studies.
Studies awaiting classification
We identified no studies awaiting classification.
Risk of bias in included studies
For details of the risk of bias assessment for each study, see the 'Characteristics of included studies' table. A graphical representation of the overall risk of bias in included studies is presented in Figure 2 and Figure 3.
2.
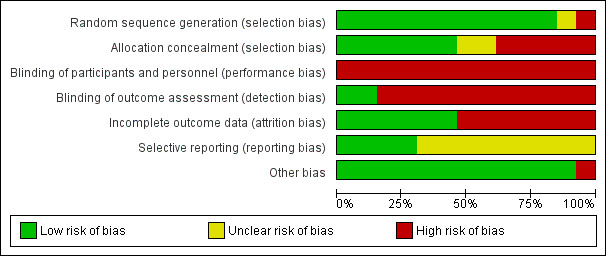
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
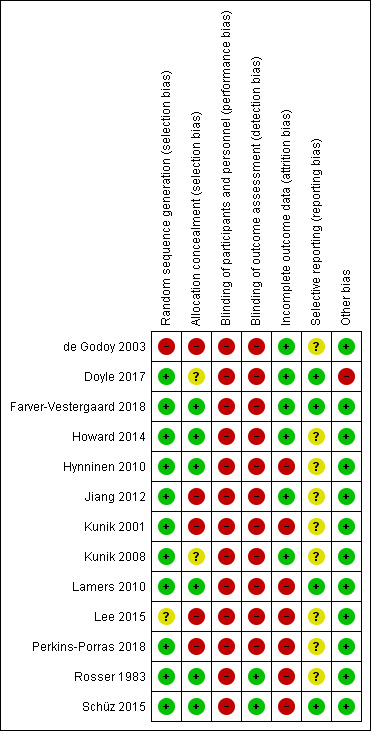
Risk of bias summary: review authors' assessment about each risk of bias item for each included study
Allocation
Out of the thirteen studies included in this review, we assessed eleven at low risk of bias for random sequence generation, as the randomisation methods were adequately described (Doyle 2017; Farver‐Vestergaard 2018; Howard 2014; Hynninen 2010; Jiang 2012; Kunik 2001; Kunik 2008; Lamers 2010; Perkins‐Porras 2018; Rosser 1983; Schüz 2015). de Godoy 2003 and Lee 2015 did not report their methods of randomisation.
Out of the thirteen studies, we assessed six studies at low risk for selection bias due to adequate reporting of allocation concealment (Farver‐Vestergaard 2018; Howard 2014; Hynninen 2010; Lamers 2010; Rosser 1983; Schüz 2015). The remaining seven studies either did not provide details, or provided insufficient information, on allocation concealment, therefore, we assessed them at unclear or high risk of selection bias.
Blinding
Thirteen studies included in this review assessed the effects of psychological therapies on depression. Due to the nature of psychological therapies, blinding of participants and personnel in pragmatic trials is often not feasible; therefore, we assessed all the included studies at high risk of bias due to inadequate blinding.
Eleven studies were at high risk of detection bias due to lack of blinding of outcome assessment. However, in two studies, we considered blinding of outcome assessors to be adequate (Rosser 1983; Schüz 2015).
Incomplete outcome data
We assessed seven studies at high risk of attrition bias due to high dropout rates and/or lack of relevant reporting on handling of the missing data (Hynninen 2010; Kunik 2001; Lamers 2010; Lee 2015; Perkins‐Porras 2018; Rosser 1983; Schüz 2015). The remaining six studies reported attrition rates, reasons and missing data per group (intervention versus control), and used adequate methods of imputation; therefore, we assessed these at low risk of attrition bias.
Selective reporting
Out of the thirteen studies, nine did not provide details on whether trial protocols had been published. Therefore, we assessed all nine studies at unclear risk of reporting bias (de Godoy 2003; Howard 2014; Hynninen 2010; Jiang 2012; Kunik 2001; Kunik 2008; Lee 2015; Perkins‐Porras 2018; Rosser 1983). Two studies provided references for published protocols for their trials (Doyle 2017; Lamers 2010), whereas the remaining two studies provided a statement about a trial registration (Farver‐Vestergaard 2018; Schüz 2015).
Other potential sources of bias
One study (Doyle 2017) received funding from Beyondblue and Bruce Wall Trust (Australia). Also, 70% of the study participants had attended, or were attending pulmonary rehabilitation programmes and 19%, at the time of the trial, were receiving other psychological or psychiatric services. We did not identify any other potential sources of bias for the remaining 12 studies studies included in this review.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Psychological therapies compared to no intervention for the treatment of depression in chronic obstructive pulmonary disease.
| Psychological therapies compared to no intervention for the treatment of depression in chronic obstructive pulmonary disease | |||||
| Patient or population: people with chronic obstructive pulmonary disease (COPD) Setting: outpatient, secondary care; intervention delivered in group, by telephone, or in home setting Intervention: psychological therapy Comparison: no intervention | |||||
| Outcomes | Anticipated absolute effects* (95% CI) for psychological therapies compared to no intervention | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|
Change in depressive symptoms Assessed with: BDI, PHQ‐9, HADS, CES‐D (scale from 0 to 60 (higher score = worse symptoms)) (final follow‐up: range 4 months to 12 months) |
The standardised mean improvement in depressive symptoms from baseline in the psychological therapies group was 0.19 higher (0.05 higher to 0.33 higher) compared to no intervention | ‐ | 764 (6 RCTs) | ⊕⊝⊝⊝ VERY LOW a,b,c | Most trials used CBT‐based therapy as an intervention. |
| Adverse events | None of the studies reported on adverse events. | ‐ | (6 RCTs) | ‐ | |
|
Change in quality of life (QoL) Assessed with: CAT, SGRQ Scale from: 0 to 100 (higher score = worse QoL) (final follow up: range 4 months to 9 months) |
The standardised mean improvement in QoL from baseline in the psychological therapies group was 0.15 higher (0.09 lower to 0.38 higher) compared to no intervention | ‐ | 348 (3 RCTs) | ⊝⊝⊝⊝ VERY LOW a,b,c,d | |
| Change in dyspnoea | None of the studies measured dyspnoea | ‐ | (0 studies) | ‐ | |
| Change in FEV1 | None of the studies measured FEV1 | ‐ | (0 studies) | ‐ | |
| Change in exercise tolerance | None of the studies measured exercise tolerance | ‐ | (0 studies) | ‐ | |
| Hospital length of stay or readmission rates | None of the studies measured hospital length of stay or readmission rates | ‐ | (0 studies) | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval, CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
a Downgraded one level due to risk of bias. Lack of blinding of participants and/or personnel. Also, blinding of outcome assessment was not reported in most of the studies, or the primary outcome was self‐rated by participants who were not blinded to treatment allocation. Allocation concealment and selective reporting were assessed at unclear risk of bias in most of the studies.
b Downgraded one level due to clinical heterogeneity. Although there was a low degree of statistical heterogeneity, factors that may have influenced the size of treatment effect include: variability in sample sizes, depression outcome measures, and baseline depression severity.
c Downgraded one level: in four studies out of six, the participants did not have baseline scores above threshold for depression; some were borderline.
d Downgraded one level due to a relatively small sample size for this continuous outcome (given that for continuous outcomes it is recommended that a total number of participants is at least 400).
BDI: Beck Depression Inventory
CAT: Chronic obstructive pulmonary disease Assessment Test
CBT: cognitive‐behavioural therapy
CES‐D: Center for Epidemiologic Studies Depression
FEV1: forced expiratory volume in 1 second
HADS: Hospital Anxiety and Depression Scale
PHQ‐9: Patient Health Questionnaire‐9
RCT: randomised controlled trial
SGRQ: Saint George's Respiratory Questionnaire
Summary of findings 2. Psychological therapies compared to education for the treatment of depression in chronic obstructive pulmonary disease.
| Psychological therapies compared to education for the treatment of depression in chronic obstructive pulmonary disease | ||||||
| Patient or population: people with chronic obstructive pulmonary disease (COPD) Setting: outpatient, secondary care; intervention delivered in group, by telephone, or in home setting Intervention: psychological therapy Comparison: education | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with education | Risk with psychological therapies | |||||
|
Change in depressive symptoms Assessed with: BDI, HADS, GDS (scale from: 0 to 40 (higher score = worse symptoms)) (final follow‐up: range 6 weeks to 12 months) |
The standardised mean improvement in depressive symptoms from baseline in the psychological therapies group was 0.23 higher (0.06 higher to 0.41 higher) compared to education | ‐ | 507 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW a,b,c | ||
| Adverse events | None of the studies reported on adverse events | ‐ | (0 studies) | ‐ | ||
|
Change in quality of life (QoL), emotional functioning Assessed with: CRQ (scale from 7 to 49 (lower score = worse emotional function)) (final follow‐up: range 6 weeks to 12 months) |
The mean change from baseline in QoL, emotional functioning in the education group ranged between ‐0.52 and 3 units CRQ | The mean change from baseline in QoL, emotional functioning in the psychological therapies group was 2.98 units CRQ higher (9.15 lower to 3.19 lower) compared to education | ‐ | 460 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW c,d,e | |
|
Change in dyspnoea Assessed with: CRQ (scale from: 5 to 35 (lower score = worse dyspnoea)) (final follow‐up: range 6 weeks to 12 months) |
The mean quality of life ‐ CRQ ‐ dyspnoea ranged from 3.6 to 12.8 units | The mean quality of life ‐ CRQ ‐ dyspnoea in the psychological therapies group was 1.84 units CRQ higher (5.94 lower to 2.26 higher) compared to education | ‐ | 458 (2 RCTs) | ⊝⊝⊝⊝ VERY LOW c,d,e,f | |
|
Change in exercise tolerance Assessed with: 6MWT (lower score = worse) (final follow‐up: range 6 months to 12 months) |
The mean change in exercise tolerance (final follow‐up) ranged from 1,058 feet to 1,207 feet | The mean exercise tolerance in the psychological therapies group improved by 16.98 feet (187.54 lower to 153.59 higher) compared to education | ‐ | 286 (2 RCTs) | ⊝⊝⊝⊝ VERY LOW a,b,c,g | |
| Change in hospital length of stay or readmission rates | At 12‐month follow‐up, hospital admissions were reduced by 42% in the intervention group and by 16% in the control group | 222 (1 RCT) |
⊕⊝⊝⊝ VERY LOWe,h,i |
|||
| Cost effectiveness | At 12‐month follow‐up, expenses related to hospital admissions were reduced by GBP 23,263 | ‐ | 222 (1 RCT) |
⊕⊝⊝⊝ VERY LOW e,h,i | ||
| *The risk in the intervention group (and its 95% confidence interval, CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
a Downgraded one level due to risk of bias. Lack of blinding of participants and/or personnel. Also, blinding of outcome assessment was reported in only one study or the primary outcome was self‐rated by participants who were not blinded to treatment allocation. Allocation concealment was was an issue. Selective reporting was assessed at unclear risk of bias in all studies.
b Downgraded one level due to clinical heterogeneity: variability in sample sizes (from 53 to 238 participants), depression outcome measures and baseline depression severity.
c Downgraded one level due to differences in length of follow‐up; from 6 weeks to 12 months.
d Downgraded one level due to risk of bias. Lack of blinding of participants and personnel. One study did not describe allocation concealment process. Unclear reporting bias in both studies.
e Downgraded one level due to no depressive symptoms or low score for depressive symptoms at baseline in one of the two studies.
f Downgraded one level due to large degree of statistical heterogeneity; I² = 91%
g Downgraded one level due to a relatively small sample size for this continuous outcome (given that for continuous outcomes it is recommended that a total number of participants is at least 400).
h Single study
i Risk of bias: blinding and selective reporting
BDI: Beck Depression Inventory
CRQ: Chronic Respiratory Questionnaire
GDS: Geriatric Depression Scale
HADS: Hospital Anxiety and Depression Scale
RCT: randomised controlled trial
6MWT: 6‐min walk test
Summary of findings 3. Psychological therapies and co‐intervention compared to co‐intervention alone for the treatment of depression in chronic obstructive pulmonary disease.
| Psychological therapies and pulmonary rehabilitation compared to pulmonary rehabilitation alone for the treatment of depression in chronic obstructive pulmonary disease | |||||
| Patient or population: people with chronic obstructive pulmonary disease (COPD) Setting: outpatient secondary care, pulmonary rehabilitation clinic Intervention: psychological therapy and pulmonary rehabilitation (PR) Comparison: pulmonary rehabilitation alone (PR) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| change in depressive symptoms Assessed with: HADS Scale from: 0 to 40 | The standardised mean change in depressive symptoms for participants treated with psychological therapies and pulmonary rehabilitation was 0.37 higher (0.00 lower to 0.74 higher) compared to pulmonary rehabilitation alone | ‐ | 114 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded one level due to risk of bias. Owing to the nature of the intervention, blinding of participants and research personnel, as well as blinding of outcome assessors was not feasible.
2 The smaller study did not provide details describing methods of randomisation, allocation concealment.
3 Downgraded one level due to the relatively small sample size (given that for continuous outcomes it is recommended that a total number of participants is at least 400).
HADS: Hospital Anxiety and Depression Scale
RCT: randomised controlled trial
Comparison 1: Psychological therapy versus no intervention
See also: Table 1.
Primary outcomes
1.1 Change in depressive symptoms
Eight studies (879 participants) measured change scores for this outcome (Doyle 2017; Hynninen 2010; Jiang 2012; Lamers 2010; Lee 2015; Perkins‐Porras 2018; Rosser 1983; Schüz 2015); however, we were able to include only six studies, with 764 participants, in the meta‐analysis. Various scales were used to measure depressive symptoms, including the BDI, PHQ‐9, HADS, and CES‐D, therefore, we calculated a standardised mean difference (SMD). The CBT‐based psychological therapy intervention reduced depressive symptoms better than 'no intervention' (SMD 0.19; 95% CI 0.05 to 0.33; P = 0.009; I² = 0%; six RCTs, 764 participants; Analysis 1.1; Figure 4) in the experimental group compared to the control group and the difference was statistically significant. Although there was no statistical heterogeneity across the studies, we rated the quality of evidence as very low for two main reasons, 1) the interventions used in the studies stemmed from different methodological approaches, 2) they were delivered using different formats (e.g. telephone versus group). Also, the risk of performance and detection bias was high across the studies. These factors may have influenced the size of the treatment effect.
1.1. Analysis.
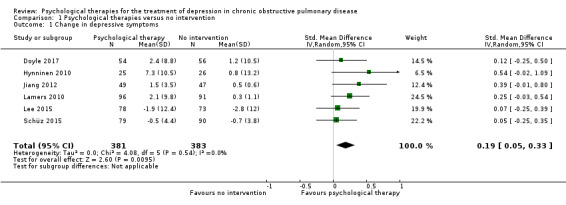
Comparison 1 Psychological therapies versus no intervention, Outcome 1 Change in depressive symptoms.
4.
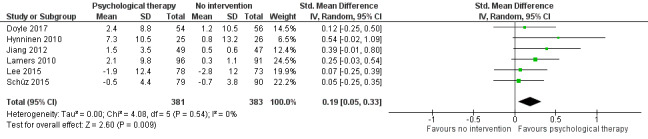
Forest plot of comparison 1. Psychological therapies versus no intervention, outcome: 1.1 Change in depressive symptoms
We were unable to include data from two studies in this meta‐analysis; one study with 65 participants reported only median scores (Rosser 1983), and the second study with 50 participants used a 10‐minute intervention in the form of a mindful body‐scan (Perkins‐Porras 2018). We considered it methodologically inappropriate to combine the results from a 10‐minute trial with the results from trials that lasted, for example, for a few months. Rosser 1983 used the GHQ to measure psychiatric symptoms, and the Visual Analogue Measure of Depression (VAMD) to measure depressive symptoms specifically. The VAMD results indicated that depression scores were reduced in the control group at the final six‐month follow‐up assessment, but the result was not statistically significant (P = 0.09). The increase in depression in the psychotherapy group was significant compared to the control group (P > 0.05). The results in Perkins‐Porras 2018 (a feasibility study) did not show any improvement in depressive symptoms (as measured by HADS); however, it is important to note that the baseline scores in both groups were below the cut‐off for depression.
Sensitivity analysis
We conducted a sensitivity analysis for depression by removing the studies with participants who did not have clinically significant depressive symptoms on intake, or the severity of depression was unknown. When we pooled data from the four studies that recruited participants with clinically significant depressive symptoms, results showed that psychological therapy was more effective than standard care or attention placebo in reducing depressive symptoms (SMD 0.20, 95% CI 0.02 to 0.37; P = 0.03; I² = 0%; four studies, 499 participants; Analysis 1.2; Figure 5). We rated the quality of the evidence as very low; and there was no statistical heterogeneity across the studies (Doyle 2017; Hynninen 2010; Lamers 2010; Lee 2015).
1.2. Analysis.
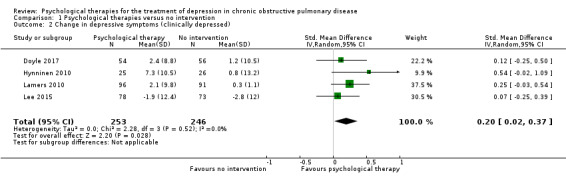
Comparison 1 Psychological therapies versus no intervention, Outcome 2 Change in depressive symptoms (clinically depressed).
5.
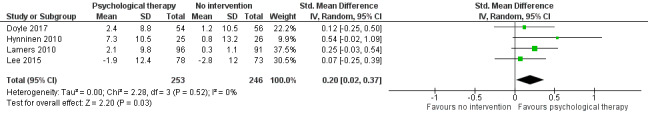
Forest plot of comparison 1. Psychological therapies versus no intervention, outcome: 1.2 Change in depressive symptoms (clinically depressed)
1.2 Adverse events
None of the studies measured adverse events.
Secondary outcomes
1.3 Change in quality of life
Four studies measured quality of life (Doyle 2017; Hynninen 2010; Jiang 2012; Lamers 2010).
Three studies contributed useable data for this outcome (Doyle 2017; Hynninen 2010; Lamers 2010). Two studies used the St. George's Respiratory Quesionnaire (SGRQ) to measure changes in health‐related quality of life, and one study used the COPD Assessment Test (CAT). Using a random‐effects model, we found inconclusive results between the psychological therapy and no intervention groups for quality of life (SMD 0.15, 95% CI ‐0.09 to 0.38; P = 0.22; I² = 15%; three RCTs, 348 participants; Analysis 1.3), as the difference between groups was not statistically significant. We rated the quality of evidence as very low, due to clinical and methodological variability across the studies.
1.3. Analysis.
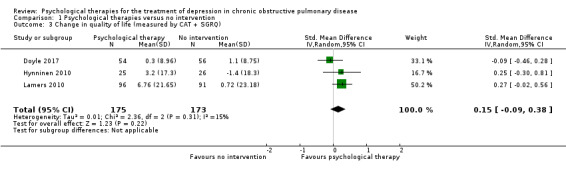
Comparison 1 Psychological therapies versus no intervention, Outcome 3 Change in quality of life (measured by CAT + SGRQ).
We did not include one study, with 96 participants, in the meta‐analysis, as it was the only study in the comparison using the SF‐36 to measure quality of life ‐ a measure that does not calculate a single quality of life total score (Jiang 2012). The results showed that on average, a psychological therapy, based on cognitive and behaviour strategies, did not improve the scores in the SF‐36 physical or mental health domains (Analysis 1.5), as the change score difference between groups did not reach statistical significance.
1.5. Analysis.
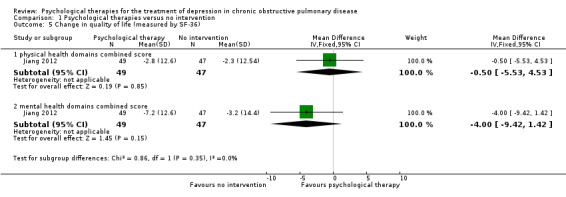
Comparison 1 Psychological therapies versus no intervention, Outcome 5 Change in quality of life (measured by SF‐36).
We rated the quality of evidence as very low, due to a small number of participants and a potential risk of selection, detection, and reporting bias, as well as a high risk of performance bias.
Sensitivity analysis
We prespecified in our protocol that we would only conduct sensitivity analyses for the primary outcomes. However, we decided to conduct sensitivity analysis for a secondary outcome, quality of life (QoL), by pooling results from two trials that used a single measure of QoL, that is the SGRQ (Hynninen 2010; Lamers 2010). Our results found that a psychological therapy was more effective than standard care or attention placebo in improving QoL (MD 5.60, 95% CI 0.23 to 10.98; P = 0.04; I² = 0%; two studies, 238 participants; Analysis 1.4). The small sample size was relatively small and the study suffered from a potential performance and detection bias. There was no statistical heterogeneity across these two studies.
1.4. Analysis.
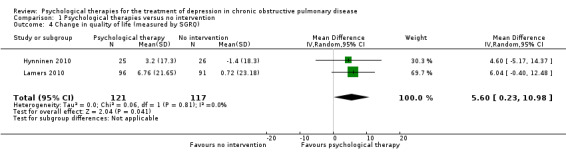
Comparison 1 Psychological therapies versus no intervention, Outcome 4 Change in quality of life (measured by SGRQ).
1.4 Change in dyspnoea
Only one study reported data on dyspnoea, measured by an ordinal Fletcher rating of self‐reported breathlessness as well as Visual Analogue of Dyspnoea (VAD) (Rosser 1983). Median values were compared for both groups at baseline, post‐treatment, and six‐month follow‐up. They did not find a significant reduction in dyspnoea scores at the final follow‐up for the whole sample, however, there was a significant improvement in dyspnoea at post‐intervention for the whole sample (P < 0.05). We rated the quality of evidence as very low due to the small sample size, high risk of performance bias, and potential reporting and attrition biases.
1.5 Change in forced expiratory volume in one second (FEV1)
None of the studies measured change in FEV1.
1.6 Change in exercise tolerance
Only one study reported data on exercise tolerance outcome, measured by bicycle or walk tests (distance walked). Rosser 1983 compared median values at baseline, post‐intervention, and at six‐month follow‐up. They did not find a significant improvement in exercise tolerance at the final follow‐up in the intervention group. We rated the quality of the evidence as very low due to the small sample size, high risk of performance bias, and potential reporting and attrition biases.
1.7 Hospital length of stay or readmission rate
None of the studies measured hospital length of stay or readmission rates.
1.8 Cost effectiveness
None of the studies measured cost effectiveness.
Comparison 2: Psychological therapies versus education
See also: Table 2.
Primary outcomes
2.1 Change in depressive symptoms
Three studies (507 participants) measured this outcome (Howard 2014; Kunik 2001; Kunik 2008). Three scales were used, BDI, HADS, and GDS, therefore we calculated the standardised mean difference (SMD). Psychological therapies reduced depressive symptoms more than education (SMD 0.23, 95% CI 0.06 to 0.41; three studies, 507 participants; Analysis 2.1).
2.1. Analysis.
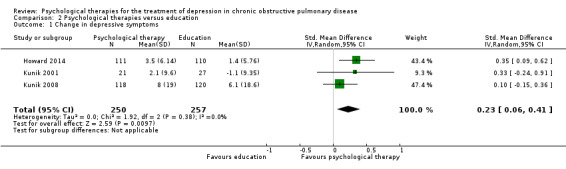
Comparison 2 Psychological therapies versus education, Outcome 1 Change in depressive symptoms.
We assessed the quality of evidence as very low due to clinical and methodological heterogeneity, including high risk of performance, detection, reporting, and selection bias.
2.2 Adverse events
None of the studies reported data on adverse events.
Secondary outcomes
2.3 Change in quality of life
Four studies, with 744 participants, measured this outcome.
Two studies, with 458 participants, used the Chronic Respiratory Disease Questionnaire (CRQ) to measure health‐related quality of life, assessing four domains: dyspnoea, fatigue, emotional functioning, and mastery (Howard 2014; Kunik 2008). The results of the mean difference in change scores from baseline were inconclusive between the psychological therapy and education groups for any of the domains (Analysis 2.2). We assessed the quality of evidence as very low, due to clinical and methodological heterogeneity across the studies, and high risk of bias, due to performance and reporting bias.
2.2. Analysis.
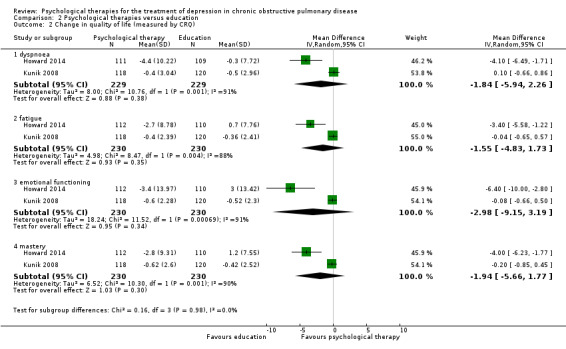
Comparison 2 Psychological therapies versus education, Outcome 2 Change in quality of life (measured by CRQ).
Two studies with 286 participants used the SF‐36 to measure quality of life across eight domains (Kunik 2001; Kunik 2008). The results of the mean difference in change scores from baseline were inconclusive between the psychological therapy and education groups for any of the eight subgroups (Analysis 2.3). We assessed the quality of evidence as very low due to a relatively small number of participants, high performance bias, and a potential for selection and reporting bias.
2.3. Analysis.
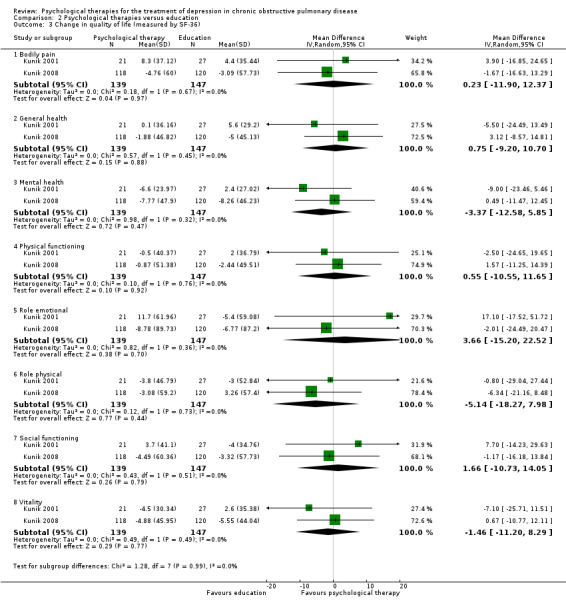
Comparison 2 Psychological therapies versus education, Outcome 3 Change in quality of life (measured by SF‐36).
2.4 Change in dyspnoea
Two studies, with 458 participants, measured dyspnoea with the CRQ (Howard 2014; Kunik 2008), The results of the mean difference in change scores from baseline between the psychological therapy and education groups were inconclusive (MD ‐1.84, 95% CI ‐5.94 to 2.26; P = 0.38; I² = 91%; two studies, 458 participants; Analysis 2.2). We assessed the quality of evidence as very low due to clinical and methodological variability. A large degree of heterogeneity indicated inconsistency in the results across studies.
2.5 Change in forced expiratory volume in one second (FEV1)
None of the studies measured FEV1.
2.6 Change in exercise tolerance
Two studies, with 286 participants, measured exercise tolerance with the six‐minute walk distance test (Kunik 2001, Kunik 2008). The mean difference showed inconsistent results between the psychological therapy and education groups (MD ‐16.98 feet, 95% CI ‐187.54 to 153.59; P = 0.85; I² = 0; two studies, 286 participants; Analysis 2.4). We rated the quality of evidence as very low. We found no statistical heterogeneity, indicating consistency in the results across studies.
2.4. Analysis.
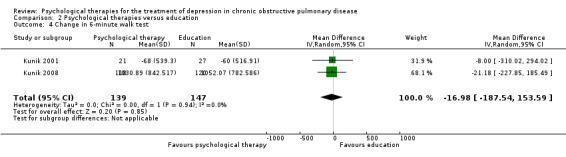
Comparison 2 Psychological therapies versus education, Outcome 4 Change in 6‐minute walk test.
2.7 Hospital length of stay or readmission rate
One study, with 224 participants, measured this outcome (Howard 2014). At 12‐month follow‐up, hospital admissions were reduced by 42% in the group who participated in psychological therapy, and by 16% in those who received education. The total number of bed days reduced by 61% in the psychological therapy group, and increased by 18% in the education group. We rated the quality of evidence as very low due to a high risk of bias related to lack of blinding of participants and personnel, and potential risks of bias related to blinding of outcome assessment, and selective reporting.
2.8 Cost effectiveness
One study, with 224 participants, measured cost effectiveness (Howard 2014). At 12‐month follow‐up, expenses related to hospital admissions were reduced by £23,263.00 in the psychological therapy group, and by £7,439.00 in the education group, due to bed days categorised as 'reduced short stay emergency tariff'. The total savings at 12‐month follow‐up in the psychological therapy group was £30,197.12 (or £269.62 per participant). We rated the quality of evidence as very low due to a high risk of bias related to lack of blinding of participants and personnel, and potential risks of bias related to blinding of outcome assessment, and selective reporting.
Comparison 3: Psychological therapies and pulmonary rehabilitation versus pulmonary rehabilitation alone
See also: Table 3.
Primary outcomes
3.1 Change in depressive symptoms
Two studies (114 participants) measured depressive symptoms with the BDI and HADS‐D (de Godoy 2003, Farver‐Vestergaard 2018). Psychological therapy combined with pulmonary rehabilitation was more effective than pulmonary rehabilitation alone in reducing symptoms of depression (SMD 0.37, 95% CI ‐0.00 to 0.74; P = 0.05; I² = 0%; two studies, 114 participants; Analysis 3.1). We assessed the quality of evidence as very low. There was no heterogeneity, indicating consistency in the results across studies. Randomisation and allocation concealment processes were not described, and we assessed the blinding of participants, personnel and outcome assessment at high risk of bias in de Godoy 2003.
3.1. Analysis.

Comparison 3 Psychological therapies + pulmonary rehabilitation (PR) versus PR alone, Outcome 1 Change in depressive symptoms.
3.2 Adverse events
The studies did not measure adverse events.
Secondary outcomes
3.3 Change in quality of life
The studies did not measure quality of life.
3.4 Change in dyspnoea
The studies did not measure dyspnoea.
3.5 Change in forced expiratory volume in one second (FEV1)
The studies did not measure FEV1.
3.6 Change in exercise tolerance
Exercise tolerance was measured with the 6MWT in de Godoy 2003 (30 participants). The scores in the pulmonary rehabilitation with psychotherapy group improved nearly twice as much as the pulmonary rehabilitation group alone, based on the change scores between baseline and post‐intervention. However, the difference in the change score between the groups was not statistically significant (P = 0.11). We rated the quality of evidence as very low due to a high risk of bias and a very small sample size, potentially affecting the precision of the results. We assessed the risk of bias as high due to an unclear randomisation process, concerns with allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and selective reporting.
3.7 Hospital length of stay or readmission rate
None of the studies measured hospital length of stay or readmission rate.
3.8 Cost effectiveness
None of the studies measured cost effectiveness.
Subgroup analysis
We did not perform any subgroup analyses.
Reporting bias
Two studies provided references to published protocols with pre‐specified methods (Doyle 2017; Lamers 2010). Two other studies reported trial registration details (Farver‐Vestergaard 2018; Schüz 2015).
Discussion
Summary of main results
This systematic review examined the effectiveness of psychological therapies for the treatment of depression in patients with moderate to severe chronic obstructive pulmonary disease (COPD). The findings are based on 13 randomised controlled trials (RCTs) with 1500 participants. Ten trials, with a total of 1355 participants, used cognitive behavioural therapy (CBT)‐based interventions; two trials, with 95 participants, reported using psychotherapy sessions (one reported using elements of cognitive therapy and the second one did not provide any details); and one trial used mindfulness‐based body scan (50 participants). Therefore, 12 trials fit the category of cognitive and cognitive‐behavioural therapies by Kazdin 2000.
Psychological therapy compared to no intervention
The pooled analysis of six trials showed that a CBT‐based intervention, compared to standard care or attention placebo, was associated with a significant, but small treatment effect for improvement in depressive symptoms (Figure 4). We rated the quality of this evidence as very low, due to clinical heterogeneity and high risk of bias. The major cause of clinical diversity was the difference in the participants' baseline levels of depression severity, variability in the types of outcome measurements used, and intervention characteristics (e.g. diversity in therapy techniques and settings). A significant treatment effect was also found for a subgroup of trials that included only participants with clinically significant depressive symptoms (Figure 5). Again, we considered the quality of evidence for this outcome as very low.
This review did not find evidence that psychological therapies (using mainly CBT approach) improved the quality of life compared with no treatment. There were three trials, and we rated the quality of evidence as very low, due to a small number of studies, high risk of bias, and clinical diversity. The sensitivity analysis of the two studies that used a single measure of quality of life, the St. George's Respiratory Questionnaire (SGRQ), found that CBT‐based intervention was more effective than 'no intervention'.
None of the studies measured the important outcomes of adverse events, change in dyspnoea, forced expiratory volume in one second (FEV1), exercise tolerance, hospital admission rates, or cost‐effectiveness. Please refer to Table 1 for further details.
Psychological therapy compared to education
The pooled analysis of three trials in this comparison showed that CBT‐based interventions were more effective in reducing depressive symptoms than educational interventions. None of the studies collected data on adverse events. In terms of the secondary outcomes, results of the effect of CBT‐based therapy were inconclusive for improvement in quality of life, dyspnoea, and exercise tolerance. None of the studies measured FEV1, and only one study measured cost‐effectiveness and hospital utilisation rates, showing more significant reductions in the intervention group than in the control group. Please refer to Table 2 for further details.
Psychological therapy and pulmonary rehabilitation compared to pulmonary rehabilitation alone (PR)
This review did not find enough evidence for this comparison as only two studies were included. The findings suggested that a psychological therapy combined with a PR programme reduced depressive symptoms more significantly than the PR programme alone. However, the type and delivery of therapies used differed markedly, the sample size was relatively small (N = 114 participants), and the smaller study (with 30 participants) had a number of methodological limitations.
Overall completeness and applicability of evidence
This review's objective was to evaluate the effectiveness of psychological therapies in reducing symptoms of depression in patients with COPD. Thriteen RCTs from the global scientific literature search met our inclusion criteria and were available for investigation of the research question. Aside from two trials that examined group and one‐on‐one psychotherapy, the included studies used various therapeutic techniques of CBT. The pooled analysis showed that a psychological therapy (predominantly CBT‐based) may be effective in reducing COPD‐related depression when compared to 'no intervention', 'education' interventions, or added to and compared against a 'co‐intervention' (in this case, pulmonary rehabilitation). The quality of this evidence, however, is low. Our results are relevant to patients with moderate to severe COPD.
In terms of the applicability of CBT for depression in the general population, the evidence is strong (David 2018; Hofmann 2012), despite a debate on the potential decline of CBT's anti‐depressive effects (Cristea 2017; Johnsen 2015; Waltman 2016). There is also evidence for the effectiveness of CBT for patients with comorbid depression and chronic illnesses (Tyrer 2014). Meta‐analytic literature on the efficacy of cognitive and behavioural approaches for treatment of depression in the COPD population provides results in line with our findings, indicating that its psychotherapeutic approach is promising, and has antidepressant effects (Fritzsche 2011; von Leupoldt 2012). Coventry and colleagues, in a systematic review and meta‐regression of 74 RCTs, found that interventions that used psychological therapies (alone or in a combination with antidepressant treatment) were associated with greater reduction in depressive symptoms compared to studies that included antidepressant therapy only (Coventry 2014).
Out of the 13 included studies, eight did not include depression diagnosis or a cut‐off score for depressive symptoms in their participant eligibility criteria. As a consequence, some data came from participants who did not have clinically significant depressive symptoms (or had borderline symptoms) at their study entry. Out of the eight trials, only one study performed post‐hoc analysis to provide data for the subset of those participants who had clinically significant depressive symptoms. In order to improve applicability of this review, we performed sensitivity analysis with the four trials that tested their interventions only with participants who had clinically confirmed depressive symptoms.
The use of different instruments to measure depressive symptoms may have contributed to clinical heterogeneity across the studies: Beck Depression index (BDI (Hynninen 2010; Kunik 2008; Lamers 2010)); Hospital Anxiety and Depression Scale (HADS (Howard 2014; Jiang 2012; Schüz 2015)); Geriatic Depression Scale (GDS (Kunik 2001)); Patient Health Questionnaire‐9 (PHQ‐9 (Doyle 2017)); Center for Epidemiological Studies – Depression scale (CES‐D (Lee 2015)), Center for Epidemiological Studies–Depression scale (VAMD (Rosser 1983)). Although self‐reported screening tools for depression are validated and convenient to use, investigators question their suitability for chronically ill (older) patients and stress their inability to distinguish between overlapping symptoms of depression and physical illness (Meader 2011; O'Connor 2009), or between depression and anxiety (Norton 2013).
Also, it is important to note that the differences in disease patterns between males and females hold implications for suitable treatment type (Camp 2009; Morrow 2015).Traditionally, COPD was more commonly found in men (Buist 2007), but the condition is becoming increasingly prevalent in women, and manifests itself differently in disease mechanisms, comorbidities, and survival rates (Aryal 2013; Barnes 2016). Similarly, gender differences exist when it comes to manifestation of depression, and can be observed in prevalence rates, symptom profile, and treatment response (Altemus 2014; Johnsen 2015; Van de Velde 2010). Although in total, there were more male than female participants included in this review, the applicability of our results is not limited only to males, as a total of four studies had a greater (or equal) number of female participants compared to the number of males. The trials originate from various countries and cultural backgrounds, and different healthcare settings. Therefore, generalisability of the results is not a concern and the findings can be applied to various COPD populations and settings.
Despite the results showing improvement for depressive symptoms in the intervention groups, our review provides insufficient evidence to support the claim that CBT‐based psychological therapy is an effective treatment for COPD‐related depression. Given the variability in intervention delivery, it is not possible to provide conclusive, robust evidence supporting a specific therapy approach. Considering the low number of studies in each comparison and limited reporting for each of the secondary outcomes, further research is required to improve the overall completeness and acceptability of the evidence found.
Quality of the evidence
We included thirteen studies with 1500 participants in the review, out of which, 11 studies were used in the meta‐analyses. Given the variability in the designs of included studies, relatively small sample sizes, and overall high risk of performance, selection (allocation concealment) and detection bias, we rated the quality of evidence as very low. For this reason, confidence in the results is limited. High quality interventional experiments are designed to control for sources of bias (systematic errors) by using adequate randomisation, blinding, or allocation concealment. In the current review, the blinding of the participants, or personnel (or both) was often not feasible due to the nature of psychological therapies (Foster 2012). Lack of blinding or allocation concealment may inflate the effect size and produce (erroneously) a statistically significant result (Patsopoulos 2011). In a study that replicated the meta‐analysis of 70 systematic reviews, randomised trials without adequate allocation concealment have been shown to impact the conclusions drawn by overestimating the effectiveness of the interventions. Results from 48 of 70 pooled analyses no longer showed effectiveness when only the trials with adequate allocation concealment were included (Pildal 2007).
In terms of methodological designs, although we included only randomised controlled trials, and ten studies used CBT‐based interventions as a psychological therapy, the interventions varied. Therefore, the quality of evidence was downgraded due to inconsistency related to study designs, setting, means of delivery, and lengths of follow‐up.
Variability in baseline depression scores was a significant concern in this review. In Schüz 2015, only 21% of the sample had a score of 8 or higher on the HADS at baseline; in Kunik 2008, 53% of the sample had clinically diagnosed depression; and in Jiang 2012, the baseline mean for depression measured by the HADS was 7.16 in the intervention group and 7.08 in the control group. This indicates merely borderline depressive symptoms in the recruited participants. Also, Howard 2014 reported that only 32 out of 112 intervention participants had a score above 11 on the HADS, which means that only a small subgroup of the sample had clinically significant depressive symptoms. Moreover, a small proportion of these participants were taking medication for depression and anxiety, which may have confounded the results notably.
Aside from the fact that a number of studies included participants with unknown, borderline, or no depression, there are other noteworthy limitations reported across the studies that further explain the very low quality of the evidence. The fact that 70% of the participants in Doyle 2017 attended pulmonary rehabilitation programmes at the time of the trial, and 19% used mental health services, questions the reliability of their evidence. The dropout rate in Lee 2015 was 41% and was observed in older, less mobile participants, with more severe COPD. Also, the nurses who delivered the intervention via Skype reported difficulty in controlling the participants' environments, and reported that the participants were distracted by the circumstances around them. In Lamers 2010, the dropout rate was also high, despite the intervention being delivered via home visits. Again, the attrition was higher in older participants, with higher baseline BDI scores.
For the second primary outcome of adverse events, there were no data collected by any of the included studies. Duggan 2014 and colleagues examined 82 trials for the reporting of adverse events, and concluded that adverse events from experimental interventions were insufficiently documented in clinical trials in general, and in those assessing psychological treatments in particular. In the current review, four studies reported Patient Satisfaction scores, which may serve as useful feedback in the context of adverse events (Doyle 2017; Howard 2014; Hynninen 2010; Kunik 2001). The results from Kunik 2001 suggested that the participants had been equally satisfied with a two‐hour group session of CBT and a two‐hour group session of COPD education. In Hynninen 2010, the collected feedback indicated higher satisfaction with a manual‐based, group CBT intervention than with standard care and a brief telephone contact. In Howard 2014, where self‐guided, manual‐based CBT intervention was compared to information booklets, 78% of the participants were satisfied with the intervention, and found telephone conversations helpful. Also, group sessions were described as useful for improving the social aspects of life. In Doyle 2017, the participants were more satisfied with a telephone‐delivered CBT‐based intervention than with a telephone‐delivered attention placebo (befriending).
Few studies reported data on our secondary outcomes: quality of life, dyspnoea, lung function, exercise capacity, health service use, and cost effectiveness. Our results did not indicate improvement in the quality of life when CBT‐based intervention was compared to 'no intervention' or education. However, the sensitivity analysis of two studies that used the same quality of life measure, namely the SGRQ, showed that a CBT‐based intervention was more effective than 'no intervention'. However, this result is limited by a relatively small sample size (238 participants) since for continuous outcomes the sample size is not large enough to calculate a precise effect estimate if a total number of participants is smaller than 400 (a 'rule of thumb').
Paucity of trials reporting on dyspnoea, lung function, exercise tolerance, health service use, and cost‐effectiveness is a considerable gap in knowledge. Howard 2014 included health service use as their primary outcome, and compared a manual‐based CBT intervention to education (COPD booklets). Their results showed a reduction in hospital admissions (by 42% in the intervention group and by 16% in the control group) with significantly higher savings related to treatment costs in the intervention group compared to the control group. According to one Cochrane Review, it appears that interventions based on self‐management and education prevent hospital admissions, but do not have much effect on respiratory outcomes, and consequently, quality of life outcomes (Effing 2007). Another systematic review and meta‐analysis found that depressed patients with COPD were more frequently admitted and re‐admitted to hospital (Atlantis 2013), and that the costs associated with hospitalisation in this population are substantial (Torabipour 2016).
Two studies compared a psychological therapy added to a pulmonary rehabilitation programme, with the pulmonary rehabilitation alone, and showed a positive effect for the intervention. Current COPD guidelines basing on studies, such as Tselebis 2013, suggest that multidisciplinary pulmonary rehabilitation reduces symptoms of depression in this patient population. However, it is difficult to determine the extent to which cognitive and/or behavioural components benefit patients in pulmonary rehabilitation. Further investigation is needed to determine the most effective and efficient mode of delivery.
In summary, the reliability of our final results is limited, primarily due to indirectness related to diverse outcome measures, and inconsistency related to different designs of the studies.
Potential biases in the review process
The studies included in this review suffered from different degrees of bias. Most were at a high risk of performance and detection bias, due to lack of blinding of participants, personnel or both. In terms of outcome assessment, where the primary outcome was self‐rated by participants who were not blinded to treatment allocation, outcome assessor blinding was not achieved in these studies. Also, high risk of selection bias was present, due to lack of allocation concealment.
The inclusion of non‐depressed and depressed participants in a number of studies may have also led to biased outcomes and distorted results. Most of the self‐report rating scales for detecting depressive symptoms are biased by a large number of items addressing somatic symptoms, which may be the cause for confounded results.
Potential biases related to the selection of studies, data extraction, or data analyses were unlikely to be a concern, as we followed Cochrane guidelines. We obtained the searches from the registers of two Cochrane groups: Common Mental Disorders Group and Airways Group. We also searched trial registers and reference lists of relevant published manuscripts. Decisions about inclusion and exclusion of potential studies were made independently by two review authors, according to prespecified inclusion criteria defined in the protocol. Publication bias was not detected; however, there is a possibility of negative studies being missed before the publication of protocols became mandatory.
Agreements and disagreements with other studies or reviews
Other systematic reviews on comborbid COPD and depression based their findings on similar or a higher number of studies, however, they applied different eligibility criteria, and allowed the inclusion of randomised as well as non‐randomised trials (Baraniak 2011; Tselebis 2016). Moreover, most of the available systematic reviews published data related to the effect of psychological therapies on both depression and anxiety, rather than focusing exclusively on depression to minimise confounding of the results (Baraniak 2011; Tselebis 2016). The evidence from the current review is in line with the results from another systematic review focusing on the treatment of depression in COPD (Smith 2014). Our main finding indicates that a CBT‐based intervention, when compared to 'no intervention', 'education' interventions, and combined with a pulmonary rehabilitation compared to the pulmonary rehabilitation alone, may be effective in reducing symptoms of depression, but the effect sizes are small. This finding aligns with the findings from a systematic review and meta‐analysis that also reported only a small effect of CBT on improvement of depressive symptoms among patients with COPD (Coventry 2013). Meta‐analyses attempting to examine the impact of psychological interventions for depression in people with COPD have consistently identified a lack of methodologically rigorous evidence to support treatment efficacy (Baraniak 2011; Mikkelsen 2004). A recent review, assessing the management of depression and anxiety in COPD, highlighted the need for further research on CBT in this medical population (Panagioti 2014).
It is important to note that COPD is associated with cognitive dysfunction, regardless of the severity of disease; therefore, lower intensity and more respiratory symptom‐tailored therapies may be the best choice in the presence of COPD (Baraniak 2011). Studies investigating effectiveness of pulmonary rehabilitation in combination with a psychological therapy seem to support this hypothesis. Minimal interventions with a potential to avoid transport issues are of particular importance to patients with COPD. In this review, a number of included studies used phone‐based, nurse‐led, or minimal intervention approaches, with self‐help manuals. Telephone‐administered psychotherapy aiming to reduce depression was found to be effective in the general population (Stiles‐Shields 2014), as well as in patients with chronic illness such as cancer (Watson 2013). Nurse‐led programmes that do not require fully trained psychologists to administer the therapy have been shown to improve health‐related quality of life, and reduce hospital readmission rates (Wood‐Baker 2012). Admittedly, none of the trials included in this review investigated internet‐based interventions which have been shown to be effective for depression in the general population (Cuijpers 2010).
Future research is needed to more reliably determine the potential benefits of CBT approach for patients with COPD and comorbid depression. There is no published research evaluating the effectiveness of other psychological therapies in patients with COPD (for example Interpersonal Therapy, which is an evidence‐based therapy (de Mello 2005)). A study evaluating Supportive Therapy (ST) for oxygent‐dependant patients found that the ST enabled patients to participate in support groups and other social activities, while reducing the impact of COPD, and improving quality of life (Petty 1998). In another trial, pooled results showed statistically significant beneficial effects for both psychological well‐being and dyspnoea when using Relaxation Therapy in participants with COPD (Devine 1996).
Authors' conclusions
Implications for practice.
The eleven studies included in the meta‐analyses assessed the effectiveness of cognitive behaviour therapy (CBT)‐based interventions for treatment of depression in chronic obstructive pulmonary disease, compared to no intervention, education or when combined with pulmonary rehabilitation programme (PRP) and compared to PRP alone. We did not find enough evidence to show that, compared to all three types of control groups, a CBT‐based treatment for patients with COPD and depression can effectively reduce depressive symptoms. Although the results from pooled analyses show potential effectiveness, there was a considerable clinical heterogeneity among the studies. The differences in intervention delivery methods and format, high risk of bias, and lack of data on important secondary outcomes (such as dyspnoea, hospital utilisation, and cost‐effectiveness) make it difficult to determine a precise effect size, and to ascertain whether CBT‐based psychological therapies are a viable long‐term treatment solution for patients who have COPD‐related depression.
Given that none of the studies reported data on adverse events, there is no evidence on participants' well‐being during a given intervention, or seriousness or severity of potential adverse events.
As for the secondary outcomes, our results show that interventions using a CBT approach (compared to 'no intervention') did not improve the quality of life in participants with COPD, as measured by COPD Assessment Test (CAT) and St George's Respiratory Questionnaire (SGRQ). This result, however, is based on only three studies with 348 participants and low quality evidence. The sensitivity analysis with the two studies (238 participants) using only the SGRQ measure, suggested that the intervention might effectively improve the quality of life compared to 'no intervention' but the limited number of studies does not allow conclusive recommendations. Similarly, there was insufficient evidence to support the effectiveness of CBT‐based psychological therapies compared to 'education' in improving quality of life or exercise tolerance.
The quality of evidence was evaluated as very low due to clinical heterogeneity and methodological limitations of the included studies. For this reason the findings need to be interpreted with caution.
Implications for research.
There is a need for more rigorous, adequately powered randomised controlled trials to evaluate the effectiveness of psychological therapies in the treatment of COPD‐related depression. A number of trials used a minimal CBT‐ based intervention, often consisting of telephone sessions, self‐guided booklets or nurse visits at home. Future studies are required to compare these minimal interventions with conventional psychological therapy interventions to provide data, not only on efficacy, but also on cost‐effectiveness. It is also important to evaluate the methods of delivery, and acceptability of CBT among this medical population. COPD is associated with cognitive dysfunction, breathlessness, and limited motivation to exercise. Therefore, future studies could investigate whether therapies tailored around COPD symptoms and patients' individual biopsychosocial needs may be more applicable.
In the trials included in this review, interventions were delivered via diverse methods; well‐powered rigorous trials are needed to show which mode of delivery produces strong therapeutic effects and is cost‐effective. Given the small sample sizes, future investigators might wish to consider multi‐centre studies to test the effectiveness of CBT (or other psychological therapies) for patients with COPD‐related depression. In our review, only one study reported data on frequency of health service use, readmission rates, and cost‐effectiveness. Studies focusing on these outcomes are required to show long‐term effect on improved mental health, physical health, and health‐related quality of life. New research should also address the issue of adverse events, as none of the studies included in this review collected data on this important outcome related to well‐being and safety of a patient.
Based on this review, a better description of conducting and adequately reporting the allocation concealment process is required. Moreover, most studies did not provide information on whether a protocol with prespecified methodology had been published or registered. Given that full blinding is often not feasible in pragmatic trials, a careful description of pre‐planned steps to be undertaken to minimise the risk of performance and detection bias is highly recommended. In general, providing sufficient information in relation to methodology allows a more accurate assessment of the quality of evidence, and potentially, higher confidence in the results.
New studies investigating the effectiveness of CBT‐based therapies for the treatment of depression in COPD need to prespecify whether the participants will be recruited with or without depression before randomisation. Consequently, prespecifying the cut‐off score for inclusion is crucial. Additionally, when recruiting participants, it is important to differentiate between depressive symptoms measured by self‐reporting measures (e.g. Beck Depression Inventory (BDI) or Patient Health Questionnaire‐9 (PHQ‐9) and a clinical interview, which allows accurate diagnosis of depression. Questionnaires are useful tools that can measure depressive symptoms, but they should not replace a clinical diagnostic interview, and therefore, depressive symptoms should not be confused with depression. As may be expected, results from a study where participants were recruited with mild depressive symptoms may not be relevant to a clinically depressed person. Future trials need to address this important issue of participant eligibility criteria, by carefully taking into consideration the implications of randomising participants with and without depressive symptoms. Screening is a useful method, but not fully reliable, and cannot be considered as a replacement for appropriate clinical assessment when diagnosis of depression is needed.
In summary, well‐powered future studies need to use methodologically robust designs to evaluate the effectiveness of psychological therapies (i.e. CBT‐based therapies) for management of depression in COPD. Future trials should address adverse events, quality of life, dyspnoea, exercise capacity, health service use and cost‐effectiveness outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 15 April 2019 | Amended | Minor correction made to abbreviations for Hospital Anxiety and Depression Scale (HADS). |
Acknowledgements
We acknowledge the assistance of the editors and staff of the Cochrane Common Mental Disorders Group.
We would like to thank Professor Anna Chur‐Hansen and Professor Gregory Crawford from the University of Adelaide, Faculty of Health and Medical Sciences, for their mentoring, clinical input, and support of this project.
CRG Funding Acknowledgement:
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Common Mental Disorders Group.
Disclaimer:
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health and Social Care.
Appendices
Appendix 1. CCMDCTR ‐ core MEDLINE search
Core search strategy used to inform the Cochrane Common Mental Disorders Group's specialised register: MEDLINE Ovid A weekly search alert based on condition + RCT filter only 1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/
2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf.
3. [RCT filter]: (controlled clinical trial.pt. or randomized controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomized controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)
4. (1 and 2 and 3)
Records are screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs are tagged to the appropriate study record. Similar weekly search alerts are also conducted on OVID EMBASE and PsycINFO, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource.
Appendix 2. Additional search strategies
Update Search‐1 (20‐March‐2017)
Ovid Cross‐search (2017‐03‐20) PsycINFO 1806 to March Week 2 2017, PsycARTICLES Full Text, Embase 1974 to 2017 Week 12, Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present 1 (depress* or dysthymi* or mood disorder* or affective disorder* or affective symptom*).ti,ab,kf,kw,hw,id. 2 ((obstruct* adj3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)) or COPD or emphysema or (chronic* and bronchiti*)).ti,ab,kf,kw,hw,id. (361994) 3 (random* or RCT or placebo or (control* adj3 (trial or study or group))).ti,ab,kf,kw,id,hw. (8561141) 4 (1 and 2 and 3) (3022) 5 (2016* or 2017*).yr,ed,an,dd. (4561633) 6 4 and 5 (548) 7 remove duplicates from 6 (473) Key to field tags: ti:title; ab:abstract; kf:author keywords(MEDLINE); kw:author keywords (Embase); id:key concepts (PsycINFO); hw:subject heading word; yr:year; ed:entry date (MEDLINE); dd:date delivered (Embase); AN:Accession No. PsycINFO; CENTRAL, Issue 4, 2017 #1 (depress* or dysthymi* or mood or "affective disorder*" or "affective symptom*") #2 ((obstruct* NEAR (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)) or COPD or emphysema or (chronic* NEAR bronchiti*)) Published in CENTRAL, Issue 1 2016 to Issue 4, 2017 n = 89
************************************************************************************************************
Update Search‐2 (26‐Nov‐2018) Ovid MEDLINE databases MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily <1946 to November 26, 2018> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Pulmonary Disease, Chronic Obstructive/ (50014) 2 ((obstruct* and (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)) or COPD or emphysema or (chronic* and bronchiti*)).ti,ab,kf. (141393) 3 1 or 2 (149656) 4 controlled clinical trial.pt. (92764) 5 randomized controlled trial.pt. (471777) 6 (randomi#ed or randomi#ation or randomi#ing).ti,ab,kf. (562415) 7 (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or control* or crossover or cross‐over or design* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*))).ti,ab,kf. (500722) 8 placebo*.ab,ti,kf. (200661) 9 (trial or study).ab,ti,kf. (7158218) 10 groups.ab. (1855391) 11 (control* and (trial or study or group*) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,kf,hw. (182660) 12 ((single or double or triple or treble) adj2 (blind* or mask* or dummy)).ti,ab,kf. (161164) 13 double‐blind method/ or random allocation/ or single‐blind method/ (259767) 14 or/4‐13 (8196580) 15 exp animals/ not humans.sh. (4518028) 16 14 not 15 (6774846) 17 Depression/ (104935) 18 depressive disorder/ or depressive disorder, major/ (94708) 19 (depress* or dysthymi* or mood? or affective disorder* or affective symptom*).ti,kf. (167428) 20 (depress* adj3 (acute or clinical* or diagnos* or disorder* or major or unipolar or illness or scale* or score* or adult* or patient* or participant* or people or inpatient* or in‐patient* or outpatient* or out‐patient*)).ab. (136135) 21 (depress* and (Beck* or BDI* or DSM* or (Statistical Manual adj2 Mental Disorders) or Hamilton or HAM‐D or HAMD or MADRS or (International Classification adj2 Disease?) or ICD‐10 or ICD‐9)).ab. (39235) 22 "with depressi*".ab. (23500) 23 or/17‐22 (297594) 24 3 and 16 and 23 (1214) 25 (2016* or 2017* or 2018*).yr,dp,dt,ep,ez. (3722698) 26 24 and 25 (355) Ovid Embase <1974 to 2018 Week 48> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 chronic obstructive lung disease/ (112753) 2 *obstructive airway disease/ (907) 3 ((obstruct* and (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)) or COPD or emphysema or (chronic* and bronchiti*)).ti,ab,kw. (211149) 4 or/1‐3 (241610) 5 randomized controlled trial/ (523926) 6 randomization.de. (80124) 7 controlled clinical trial/ and (Disease Management or Drug Therapy or Prevention or Rehabilitation or Therapy).fs. (252274) 8 *clinical trial/ (17544) 9 placebo.de. (326769) 10 placebo.ti,ab. (279306) 11 trial.ti. (256582) 12 (randomi#ed or randomi#ation or randomi#ing).ti,ab,kw. (799511) 13 (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*))).ti,ab,kw. (634066) 14 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$ or dummy)).mp. (281505) 15 (control* and (trial or study or group) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,kw,hw. (320621) 16 or/5‐15 (1546292) 17 ((animal or nonhuman) not (human and (animal or nonhuman))).de. (5264241) 18 16 not 17 (1408025) 19 (depress* or dysthymi* or mood or "affective disorder*" or "affective symptom*").ti,kw. (228590) 20 (depress* adj3 (acute or clinical* or diagnos* or disorder* or major or unipolar or illness or scale* or score* or adult* or patient* or participant* or people or inpatient* or in‐patient* or outpatient* or out‐patient*)).ab. (194627) 21 (depress* and (Beck* or BDI* or DSM* or (Statistical Manual adj2 Mental Disorders) or Hamilton or HAM‐D or HAMD or MADRS or (International Classification adj2 Disease?) or ICD‐10 or ICD‐9)).ab. (61054) 22 "with depressi*".ab. (33197) 23 depression/ or agitated depression/ or atypical depression/ or endogenous depression/ or involutional depression/ or late life depression/ or major depression/ or masked depression/ or minor depression/ or "mixed anxiety and depression"/ or post‐stroke depression/ or postoperative depression/ or reactive depression/ (374345) 24 or/19‐23 (490635) 25 4 and 18 and 24 (820) 26 (depress* adj3 ((obstruct* adj3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)) or COPD or emphysema or (chronic* and bronchiti*))).ti,ab,kw. (843) 27 18 and 26 (101) 28 25 or 27 (845) 29 (2016* or 2017* or 2018*).yr,dp,dc. (4830864) 30 28 and 29 (238) Ovid: PsycINFO <1806 to November Week 3 2018> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp chronic obstructive pulmonary disease/ (1270) 2 ((obstruct* and (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)) or COPD or emphysema or (chronic* and bronchiti*)).ti,ab,id. (4262) 3 1 or 2 (4387) 4 clinical trials.sh. (11146) 5 (randomi#ed or randomi#ation or randomi#ing).ti,ab,id. (76308) 6 (RCT or at random or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or recruit* or split or subsitut* or treat*))).ti,ab,id. (90941) 7 (control* and (trial or study or group) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,id,hw. (26617) 8 ((single or double or triple or treble) adj2 (blind* or mask* or dummy)).ti,ab,id. (24670) 9 trial.ti. (26899) 10 placebo.ti,ab,id,hw. (38105) 11 treatment outcome.md. (19203) 12 treatment effectiveness evaluation.sh. (22438) 13 mental health program evaluation.sh. (2052) 14 or/4‐13 (179672) 15 (depress* or dysthymi* or mood? or "affective disorder*" or "affective symptom*").ti,ab,id. (329755) 16 3 and 14 and 15 (102) 17 (2016* or 2017* or 2018*).yr,an. (525740) 18 16 and 17 (20) CENTRAL, Issue 11 of 12, November 2018 #1 (depress* or dysthymi* or mood or "affective disorder*" or "affective symptom*") #2 ((obstruct* NEAR (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)) or COPD or emphysema or (chronic* NEAR bronchiti*)) Date added to CENTRAL trials database: 1‐May‐2016 to 27‐Nov‐2018 n = 304
************************************************************************************************************
Data and analyses
Comparison 1. Psychological therapies versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in depressive symptoms | 6 | 764 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.05, 0.33] |
| 2 Change in depressive symptoms (clinically depressed) | 4 | 499 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.02, 0.37] |
| 3 Change in quality of life (measured by CAT + SGRQ) | 3 | 348 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.09, 0.38] |
| 4 Change in quality of life (measured by SGRQ) | 2 | 238 | Mean Difference (IV, Random, 95% CI) | 5.60 [0.23, 10.98] |
| 5 Change in quality of life (measured by SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 physical health domains combined score | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐5.53, 4.53] |
| 5.2 mental health domains combined score | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐9.42, 1.42] |
Comparison 2. Psychological therapies versus education.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in depressive symptoms | 3 | 507 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [0.06, 0.41] |
| 2 Change in quality of life (measured by CRQ) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 dyspnoea | 2 | 458 | Mean Difference (IV, Random, 95% CI) | ‐1.84 [‐5.94, 2.26] |
| 2.2 fatigue | 2 | 460 | Mean Difference (IV, Random, 95% CI) | ‐1.55 [‐4.83, 1.73] |
| 2.3 emotional functioning | 2 | 460 | Mean Difference (IV, Random, 95% CI) | ‐2.98 [‐9.15, 3.19] |
| 2.4 mastery | 2 | 460 | Mean Difference (IV, Random, 95% CI) | ‐1.94 [‐5.66, 1.77] |
| 3 Change in quality of life (measured by SF‐36) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Bodily pain | 2 | 286 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐11.90, 12.37] |
| 3.2 General health | 2 | 286 | Mean Difference (IV, Random, 95% CI) | 0.75 [‐9.20, 10.70] |
| 3.3 Mental health | 2 | 286 | Mean Difference (IV, Random, 95% CI) | ‐3.37 [‐12.58, 5.85] |
| 3.4 Physical functioning | 2 | 286 | Mean Difference (IV, Random, 95% CI) | 0.55 [‐10.55, 11.65] |
| 3.5 Role emotional | 2 | 286 | Mean Difference (IV, Random, 95% CI) | 3.66 [‐15.20, 22.52] |
| 3.6 Role physical | 2 | 286 | Mean Difference (IV, Random, 95% CI) | ‐5.14 [‐18.27, 7.98] |
| 3.7 Social functioning | 2 | 286 | Mean Difference (IV, Random, 95% CI) | 1.66 [‐10.73, 14.05] |
| 3.8 Vitality | 2 | 286 | Mean Difference (IV, Random, 95% CI) | ‐1.46 [‐11.20, 8.29] |
| 4 Change in 6‐minute walk test | 2 | 286 | Mean Difference (IV, Random, 95% CI) | ‐16.98 [‐187.54, 153.59] |
Comparison 3. Psychological therapies + pulmonary rehabilitation (PR) versus PR alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in depressive symptoms | 2 | 114 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.00, 0.74] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
de Godoy 2003.
| Methods |
Study design: blind randomised controlled trial Country: Brazil |
|
| Participants |
Sample size: 30 participants randomised to the intervention Mean (SD) age (years): intervention group: 62.1 (SD 14.9), control group: 58.8 (SD 11.8) Gender: intervention group: male 12 (SD 85.7), control group: male 10 (SD 62.5) Inclusion criteria: patients with diagnosed COPD Exclusion criteria: physical incapacity to perform the proposed protocol, refusal to participate in the pulmonary rehabilitation programme, and lack of adherence to the pulmonary rehabilitation programme due to illness of more than 2 weeks’ duration |
|
| Interventions |
Intervention: pulmonary rehabilitation programme with psychotherapy sessions 24 physiotherapy sessions, 24 physical exercise sessions, 12 psychotherapy sessions, and 3 educational sessions Control: pulmonary rehabilitation programme without psychotherapy sessions 24 physiotherapy sessions, 24 physical exercise sessions, and 3 educational sessions Duration of the intervention: 12 weeks Number of participants randomised to the intervention group: N = 14 Number of participants randomised to the control group: N = 16 |
|
| Outcomes |
Primary outcomes reported: depressive symptoms screened with BDI, exercise capacity measured by 6MWT Time points measured: baseline and at 12 weeks |
|
| Notes | In the intervention group at baseline: 58% had very low depression, 20% low depression, 22% moderate depression | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No description of methods of randomisation provided |
| Allocation concealment (selection bias) | High risk | No description of methods of allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention group was blinded in relation to the activities of control group and vice versa; however, blinding methods of participants and personnel were not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants completed the trial |
| Selective reporting (reporting bias) | Unclear risk | The authors state that a protocol was approved by an institutional ethics committee; however, the word 'protocol' seems to be used synonymously with the word 'procedure' of the intervention. No further details regarding a published protocol were provided. |
| Other bias | Low risk | None |
Doyle 2017.
| Methods |
Study design: pragmatic randomised controlled trial Country: Australia Aims of the study: to investigate the impact of cognitive behavioural therapy (CBT) and active social control (befriending) on depression and anxiety symptoms in people with chronic obstructive pulmonary disease |
|
| Participants |
Sample size: N = 120 participants were eligible to enter the study; N = 110 were randomised to either intervention or control groups; N = 54 intervention group, N = 56 control group. N = 95 completed the trial and N = 92 provided data at the second follow‐up Mean (SD) age (years): 68.5 (SD 9.4) years in the intervention group; 67.0 (SD 9.1) years in the control group Gender: 64.8% female in the intervention group; 66.1% female in the control group Inclusion criteria: patients with diagnosis of COPD: living in the community, aged 45 or older, with access to a phone and can hear over the phone, speak English, if on psychotropic medication have been on stable dose for at least 3 months, score 8 or higher on the HADS or 10 or higher on PHQ‐9 Exclusion criteria: not reported |
|
| Interventions |
Intervention: following an introductory session, eight telephone administered CBT sessions were offered. Each phone call occurred weekly – approximately 30 minutes in duration. Intervention was delivered by a registered or provisionally registered psychologist, experienced in the delivery of phone CBT and with knowledge of COPD Control: active social control – befriending: one introductory session followed by eight scheduled weekly telephone calls by volunteers who had been provided with training and supervision. Each phone call lasted approximately 30 minutes Duration of the intervention: 8 weeks Number of participants randomised to the intervention group: N = 54 Number of participants randomised to the control group: N = 56 |
|
| Outcomes |
Primary outcomes reported: depression measured by Patient Health Questionnaire‐9 (PHQ‐9), anxiety measured by Beck Anxiety Inventory (BAI) Time points measured: baseline, 8 weeks post‐treatment assessment, 16 weeks follow‐up assessment |
|
| Notes | 70% of the study participants had attended, or were attending pulmonary rehabilitation programmes and 19%, at the time of the trial, were receiving other psychological or psychiatric services | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer generated random sequence available only to the statistician not involved in the study, will be used to produce randomised allocation slips." |
| Allocation concealment (selection bias) | Unclear risk | The relevant information reported regards only the researchers undertaking assessments and data analysis, confirming that they were blind to group allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Owing to the nature of the intervention and control conditions, the therapists and volunteers delivering the interventions and participants are not able to be blind to the conditions." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "The study is blind to the researchers undertaking the baseline and follow‐up assessments and the data analysis." However, the primary outcomes were self‐rated by participants who were not blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data analysed based on the intention‐to‐treat design with the last observation carried forward (LOCF). Prior to imputation it was assessed whether or not the data were missing at random. Sensitivity analysis was performed and results were in line with LOCF imputation |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was published (Doyle 2017) |
| Other bias | High risk | The study received funding from Beyondblue and Bruce Wall Trust (Australia) |
Farver‐Vestergaard 2018.
| Methods |
Study design: cluster‐randomised controlled trial Country: Denmark Aims of the study: to test the efficacy of mindfulness‐based cognitive therapy (MBCT) as an add‐on to a standard pulmonary rehabilitation (PR) programme in improving psychological distress and physical health status impairment in COPD |
|
| Participants |
Sample size: N = 84 randomised Mean (SD) age (years): intervention arm: 66.7 (SD 8.03) years; control arm: 67.7 (SD 7.54) years Gender: N = 48 women, N = 36 men; intervention arm: N = 24 women/15 men; control arm: N = 24 women/21 men Inclusion criteria: 1) a spirometry‐confirmed (FEV1 < 50%) COPD diagnosis, together with a Medical Research Council dyspnoea score of > or equal to 3 and 2; 2) physical capability to attend the exercise component of PR Exclusion criteria: a comorbid diagnosis of stroke, dementia, unstable coronary heart disease, and inability to speak or understand Danish |
|
| Interventions |
Intervention: group‐based MBCT intervention included 30 to 60 min individual phone interview followed by eight weekly 105 min group sessions of meditation and educational cognitive exercises. Treatment manual was developed and piloted with four patients with COPD. The programme was conducted by a clinical psychologist. Weekly handouts and an audio compact disc with meditation exercises were provided to each patient for between‐session practice. The MBCT was added to the PR programme after each of the eight weekly 90 min sessions Control: eight weeks of PR programme with two sessions per week. One session per week included physical exercise only (for 90 min) and the second session included physical exercise and COPD education (for 150 min). The programme followed the guidelines of the American Thoracic Society and the European Respiratory Society Duration of the intervention: 8 weeks (follow‐up assessments: 2 months, 3 months, and 6 months) Number of participants randomised to the intervention group: N = 39 Number of participants randomised to the control group: N = 45 |
|
| Outcomes |
Primary outcomes reported: psychological distress (HADS); total scores range from 0 to 42; with higher scores representing higher levels of depression and/or anxiety COPD assessment test (CAT); total scores range from 0 to 40; with higher scores representing higher levels of physical health impairment Time points measured: data were collected pre‐treatment (at baseline), mid‐treatment (at 4 weeks), post‐treatment (at 2 months, 3 months, and 6 months) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients referred to pulmonary rehabilitation programme were cluster randomised to either MBCT + PR or PR only. A random allocation sequence of 12 units was generated by an independent researcher. |
| Allocation concealment (selection bias) | Low risk | Researchers and clinicians involved in patient recruitment were blind to the allocation sequence, which was kept secure |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to the nature of psychosocial interventions, patient blinding could not be maintained throughout the intervention; information about study arm allocation was provided to patients, research assistants, and clinicians 2 to 7 days before the intervention commenced |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Researchers and clinicians involved in patient recruitment were blinded to sequence allocation, however, due to the nature of this intervention, details about allocation of participants were provided to patients and research personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported. Intention‐to‐treat analysis applied |
| Selective reporting (reporting bias) | Low risk | The trial was registered at ClincialTrials.gov: NCT02042976 |
| Other bias | Low risk | None |
Howard 2014.
| Methods |
Study design: prospective, randomised, single‐blind, parallel‐group trial Country: UK Aims of the study: to compare the effectiveness of the COPD breathlessness manual versus British Lung Foundation information booklets (CM vs IB) on health service use, mood, and health status |
|
| Participants |
Sample size: N = 222 randomised Mean (SD) age (years): intervention arm: 71.2 (SD 10.4) years; control arm: 73.2 (SD 11.4) years Gender: intervention arm: N = 44 males/56 females; control arm: N = 41 males/59 females Inclusion criteria: a diagnosis of COPD; a self‐rating breathlessness score on the Medical Research Council (MRC) dyspnoea scale of 3 or more; willingness to participate; ability to provide informed consent; and ability to read and write in English or with assistance Exclusion criteria: known psychosis and personality disorders; receiving psychological therapy; participating in PR, or having had PR within the previous six months; cognitive impairment; dementia; and verbal or written communication problems (or both) |
|
| Interventions |
Intervention: participants received self‐help manual based on CBT. The main theme was around breaking the cognitive behavioural maintenance cycle of breathlessness, panic, frustration, and depression with a specific focus on ways to manage distress. 5 weeks of self‐help manual with a phone session at weeks 3 and 6 Control: participants received a series of British Lung Foundation COPD booklets and were encouraged to work through them over 5 weeks, plus two 30‐minute phone call booster sessions at weeks 3 and 6 Duration of the intervention: 6 weeks (follow‐up assessments: 6‐month for depression and 12‐month for hospital utilisation) Number of participants randomised to the intervention group: N = 112 Number of participants randomised to the control group: N = 110 |
|
| Outcomes |
Outcomes reported: use of health service (frequency of hospital admissions) was a primary outcome for this study Secondary outcomes reported: anxiety (HADS), depression (HADS), CRQ (dyspnoea, fatigue, emotional functioning, mastery) Time points measured: baseline, 6 weeks, 6 months, and 12 months (only primary outcome) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple blocked random sampling was undertaken. Computerised random blocks of six at a time were randomised, three in each group |
| Allocation concealment (selection bias) | Low risk | Participants were blind to group allocation. Primary and secondary care staff were aware of patients' participation in the trial, but were unaware of group allocation and the trial was separate to any clinical care provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was a single‐blind trial; no detail provided on blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates and reasons reported. Intention‐to‐treat approach applied |
| Selective reporting (reporting bias) | Unclear risk | No information available |
| Other bias | Low risk | None |
Hynninen 2010.
| Methods |
Study design: randomised controlled trial Country: Norway Aims of the study: 1) To examine the effect of CBT on clinically significant symptoms of anxiety and depression compared with enhanced standard care 2) To investigate the eventual effects of sex and age on change in symptoms of anxiety and depression |
|
| Participants |
Sample size: N = 78 participants were eligible to enter the study; N = 51 were randomised to either intervention or control groups; N = 25 intervention group, N = 26 control group. N = 41 completed the trial and provided data at the second follow‐up. However, data from randomised sample of N = 51 were used in the analyses Mean (SD) age (years): 59.3 (SD 7.6) years in the intervention group; 62.6 (SD 9.9) years in the control group Gender: 11 female/14 male in the intervention group; 15 female/11 male in the control group Inclusion criteria: 40 years or older, COPD diagnosis confirmed by FEV1 of less than 80% predicted, scores greater than 13 on BDI‐II, and greater than 15 on BAI, not participating in other comprehensive psychosocial interventions (Pulmonary Rehabilitation), no signs of cognitive impairment (23 or higher on Mini‐Mental State Examination), no other severe psychiatric disorders Exclusion criteria: not reported |
|
| Interventions |
Intervention: cognitive behavioural therapy was based on a manualised group approach previously developed and tested by Stanley 2005 and Kunik 2008. The original manual was modified with the aim to help patients modify beliefs and change behavioural patterns to reduce psychological or somatic symptoms. There were seven weekly two‐hour group sessions; in each of the groups there were 4 to 6 participants. Participants were called one month and three months after post‐treatment assessment. The key components of the CBT intervention included: psychoeducation, relaxation, cognitive therapy, behavioural activation, fear‐based exposure, sleep management skills Control: standard care for COPD plus a phone call every two weeks during the seven‐week intervention period Duration of the intervention: 8 weeks (with follow‐up assessments at 6 months) Number of participants randomised to the intervention group: N = 25 Number of participants randomised to the control group: N = 26 |
|
| Outcomes |
Primary outcomes reported: anxiety (BAI), depression (BDI‐II) Secondary outcomes reported: quality of life (SGRQ), spirometry, Client Satisfaction Questionnaire Time points measured: baseline, 8‐week post‐treatment assessment, 8‐month follow‐up assessment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were divided into matched pairs according to their lung function test, and from each pair one participant was randomly assigned to CBT or enhanced standard care |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was implemented using numbered containers that were identical in appearance for the two groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention, neither participants nor therapists were blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20% attrition reported with reasons described; intention‐to‐treat analysis applied |
| Selective reporting (reporting bias) | Unclear risk | No published protocol or trial registration reported |
| Other bias | Low risk | None |
Jiang 2012.
| Methods |
Study design: randomised controlled trial Country: China Aims of the study: to explore the effects of an uncertainty management intervention on uncertainty, anxiety, depression, and quality of life of COPD outpatients in China. |
|
| Participants |
Sample size: N = 100 were randomised to two arms Mean (SD) age (years): intervention arm: 65.2 (SD 8.96), control arm: 64.7 (SD 8.05) Gender: intervention arm: N = 35 males (SD 71.4), control arm: N = 32 males (SD 68.1) Inclusion criteria: moderate or severe COPD, disease duration: less than 2 years since COPD diagnosis, at least one COPD exacerbation with a duration of at least 3 days Exclusion criteria: unable to communicate clearly and give informed consent, concurrent oncologic or psychiatric diseases, drug or alcohol abuse history |
|
| Interventions |
Intervention: self‐help manual and phone‐guided uncertainty management intervention Control: standard care Duration of the intervention: 10 months Number of participants randomised to the intervention group: N = 50, completed N = 49 Number of participants randomised to the control group: N = 50, completed N = 47 |
|
| Outcomes |
Primary outcomes reported: depressive symptoms measured with HADS, health‐related quality of life measured with SF‐36 Time points measured: baseline and at 10 months |
|
| Notes | The participants at baseline scored lower than 8 as measured by HADS‐D | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to an intervention group or a control group using block randomisation |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention nurses were blinded to the group assignment and they collected data at patients’ homes; however, participants were not blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very low attrition rates reported |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | None |
Kunik 2001.
| Methods |
Study design: single‐blind randomised controlled trial Country: USA Aims of the study: the hypothesis: compared to an educational intervention, a single 2 h session of group CBT, with a 6‐week follow‐up, would reduce anxiety and depression, improve physical and mental functioning, and lead to a better QoL and greater satisfaction with treatment in older patients with COPD |
|
| Participants |
Sample size: N = 53 participants were randomised, N = 48 completed the trial Mean (SD) age (years): 71.3 (SD 5.9) years reported for the whole study sample Gender: 83% male in the whole study sample Inclusion criteria: COPD patients, aged 60 or older with diagnosis confirmed by reviewing pulmonary function tests in the medical records or portable spirometry with FEV < 0.75. Patients were recruited and included regardless of the presence or absence of depressive or anxious symptoms Exclusion criteria: patients with the following chronic conditions were excluded: uncontrolled malignancy, myocardial infarction within the prior six months, coronary artery disease requiring surgical intervention or angioplasty, end‐stage renal disease on dialysis, delirium, and dementia |
|
| Interventions |
Intervention: cognitive behavioural therapy The CBT was administered to small groups of patients (N = 6 to 10) during a single session, lasting approximately 2 hours, by a board‐certified geropsychiatrist. The intervention addressed education, relaxation, cognitive interventions, and graduated practice. During the first hour, participants learned about the role of anxiety and depression in chronic medical illness. During the second hour, participants were instructed in the use of three types of coping skills to aid in the control of anxiety or stress symptoms: relaxation strategies, thought stopping, and self‐instructional training. At the end of the session, participants were given workbooks and audiotapes reviewing the coping skills and providing practice exercises. Participants were asked to use the skills daily. For 6 weeks, participants received weekly calls to monitor compliance. Control: COPD education group Participants attended one group session with a board‐certified internist who discussed COPD process, aetiology, and treatment options. Participants were called weekly during the subsequent six weeks after the session, allowing an opportunity to ask questions about COPD. Duration of the intervention: a single session, lasting approximately 2 hours, plus a weekly phone call for 6 weeks. After the session, the participants were contacted by phone on a weekly basis, allowing opportunity to ask questions, monitor compliance, and increase the probability of continued practice. Number of participants randomised to the intervention group: N = 24; N = 21 completed Number of participants randomised to the control group: N = 29; N = 27 completed |
|
| Outcomes |
Primary outcomes reported: baseline assessment with self‐report scales GDS, BAI, SF‐36, 6MWD Time points measured: baseline and post‐treatment assessment, conducted at the end of 6 weeks |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned using a random number table to either CBT or COPD education group |
| Allocation concealment (selection bias) | High risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind study; however, no specific information provided on who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Single‐blind study; no specific information provided on who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition reported. Participants who dropped out were excluded from the analysis. No reasons for dropouts provided |
| Selective reporting (reporting bias) | Unclear risk | No prespecified protocol available to determine selective reporting. No trial registration reported |
| Other bias | Low risk | None |
Kunik 2008.
| Methods |
Study design: prospective parallel randomised controlled trial Country: USA Aims of the study: to compare the effects of CBT group treatment and COPD education on disease‐specific and generic quality of life (primary outcome), depressive and anxiety symptoms, six‐minute walk distance and use of health services (secondary outcomes) |
|
| Participants |
Sample size: N = 238 randomised, N = 108 completed at 12 months Mean (SD) age (years): 66.1 (SD 10.1) years in the intervention group; 66.5 (SD 10.5) years in the control group Gender: 112 male (96.6%) in the intervention group; 114 male (95.8%) in the control group Inclusion criteria: diagnosis of COPD (FEV1/FVC < 70% and FEV1 < 70% predicted) confirmed with spirometry; moderate anxiety, moderate depression, or both; treatment by a primary care provider or pulmonologist to maximise comparability of usual care across groups Exclusion criteria: cognitive disorder evidenced by a score of 23 or less on the Mini‐Mental State Examination, a psychotic disorder, or current non‐nicotine substance abuse or dependence |
|
| Interventions |
Intervention: eight one‐hour sessions of group cognitive behavioural therapy were developed, based on previous studies (Kunik 2001), and integrated with interventions for anxiety and depression. The intervention included education and awareness training focused on anxiety, depression, and associated physiological, cognitive, and behavioural symptoms (session 1), relaxation training (session 2), increasing pleasurable activity and decreasing anxiety‐related avoidance (sessions 2 & 3), cognitive therapy (alternative thoughts, encouraging self‐statements and thought‐stopping; sessions 4 & 5), problem‐solving techniques (session 6), sleep‐management skills (session 7), and skills review and planning for maintenance of gains (session 8). There were additional home‐practice exercises assigned. Control: eight COPD education sessions, designed to control for contact time and group social support were developed by a co‐investigator. Topics included: breathing strategies and airway management, pathophysiology of lung disease, medications, use of oxygen, avoidance of environmental irritants, nutrition, exercise, smoking cessation, and end‐of‐life planning. The same psychology interns and post‐doctoral fellows who conducted the CBT treatment also provided these education sessions. Duration of the intervention: eight one‐hour sessions of group CBT Number of participants randomised to the intervention group: N = 118; N = 52 completed the trial at 12 months; 60 participants included in the analyses Number of participants randomised to the control group: N = 120; N = 56 completed the trial at 12 months; 63 participants included in the analyses |
|
| Outcomes |
Primary outcomes reported: COPD‐specific quality of life was measured using the Chronic Respiratory Questionnaire (CRQ). General quality of life was measured using the Medical Outcomes Survey Short Form‐36 (SF‐36) Secondary outcomes: BDI‐II, BAI, 6MWD Time points measured: baseline, follow‐up assessments: 1 month, 2, 4, 8, and 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Statistical Analysis Systems PLAN procedure was used to create the randomisation list. A statistician provided randomisation numbers and treatment codes to the study co‐ordinator when sufficient patients for two classes had completed the baseline assessment and consented to participate." |
| Allocation concealment (selection bias) | Unclear risk | "The instructor assigned the treatment to the code initially by flipping a coin." No specific method for allocation concealment was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study personnel performing assessments were blinded to treatment condition; however, the participants, due to the nature of the trial wouldn't have been blinded – there was no information provided in the paper |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study personnel performing assessments were blinded to treatment condition; however, the primary outcomes were self‐rated by participants who were not blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants with missing data were excluded. Reasons for dropout reported and intention‐to‐treat analysis conducted |
| Selective reporting (reporting bias) | Unclear risk | No specific protocol for the study was referenced; however, the trial is a continuation of previously published studies |
| Other bias | Low risk | None |
Lamers 2010.
| Methods |
Study design: randomised controlled trial Country: the Netherlands Aims of the study: the hypothesis: minimal psychological intervention would reduce symptoms of depression and anxiety and would also improve disease‐specific quality of life |
|
| Participants |
Sample size: N = 187 randomised participants; N = 119 completed after the third follow‐up assessment at 9 months Mean (SD) age (years): 70.5 (SD 6.3) years in the intervention group; 71.5 (SD 7.1) years in the control group Gender: 59 male (61.5%), 37 female (38.5%) in the intervention group; 53 male (58.2%), 38 female (41.8%) in the control group Inclusion criteria: all patients aged 60 years and older who had COPD according to their GP were mailed a depression screening questionnaire (Patient Health Questionnaire‐9 – PHQ‐9). After the PHQ‐9 screening, all patients who reported having at least two symptoms of depression, one being loss of interest or depressed mood, were invited to a diagnostic interview, consisting of the Mini International Neuropsychiatric Interview (MINI) and the Hamilton Depression Rating Scale (HDRS). Patients with minor depression, mild major depression, moderate major depression, or dysthymia were included in the study. Exclusion criteria: the patients' General Practitioners (GPs) first excluded from the initial, broad selection, patients without COPD, those who were bedridden, on a waiting list for a nursing home, had major depression, used antidepressants, had major psychiatric conditions, or were currently receiving psychosocial or psychiatric treatment, had serious cognitive problems, lost their spouse, or were not fluent in Dutch. All remaining patients, aged 60 years and older, who had COPD according to their GP, were mailed a depression screening questionnaire (PHQ‐9). After the PHQ‐9 screening, patients with severe major depression, according to the Hamilton Depression Rating Scale (HDRS > 18) and patients with suicidal risk were excluded and referred back to their GP. |
|
| Interventions |
Intervention: nurse‐administered minimal psychological intervention (MPI), based on principles of CBT and self‐management. The intervention was previously evaluated in the Depression in Elderly with Long‐Term Afflictions (DELTA) study in a population of elderly patients with diabetes or COPD. Nurses were trained by a GP, psychologist, and psychiatrist. The intervention was tailored to individual patients. Depending on progress, patients received two to ten visits over a period of at most three months. Duration of the intervention: two to ten visits at home, over at most three months. Patients had, on average, four intervention contacts, each lasting approximately one hour. Number of participants randomised to the intervention group: N = 96; analysed at final follow‐up assessment N = 58 Number of participants randomised to the control group: N = 91; analysed at final follow‐up assessment N = 61 |
|
| Outcomes |
Primary outcomes reported: self‐administered questionnaires: depression – Beck Depression Inventory (BDI), anxiety – Symptom Checklist‐90 (SCL), disease specific QoL – Saint George's Respiratory Questionnaire (SGRQ). Time points measured: baseline, one week, 3 months, 9 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator was used. A block randomisation scheme was used, stratified for general practice. Block size was set at 2, as we expected to include only a small number of patients per practice |
| Allocation concealment (selection bias) | Low risk | Data entry was performed by researchers blinded to the allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rates reported; slightly higher in the intervention group (40%) compared to the controls (33%). Procedures on missing item scores and relevant data imputation reported. Analyses based on the intention‐to‐treat principle. Analyses in which missing observations were imputed were also performed |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was published (Lamers 2010) |
| Other bias | Low risk | None |
Lee 2015.
| Methods |
Study design: randomised controlled trial Country: South Korea Aims of the study: to examine the effects of nurse‐led, problem‐solving therapy (PST) on coping, self‐efficacy, and depressive symptoms for patients with chronic obstructive pulmonary disease (COPD), using a randomised controlled trial |
|
| Participants |
Sample size: N = 254 randomised; N = 151 completed the trial Mean (SD) age (years): mean age for all participants included was 66.1 (SD 8.2) years Gender: male 91% of the total study sample Inclusion criteria: patients who were 40 to 80 years of age, diagnosed with COPD by a physician based on a pulmonary function test, in stable condition, and expected to live ≥ 6 months, as determined by a physician who specialised in respiratory medicine. The forced expiratory volume (FEV) 1% predicted was used to assess the severity of COPD. Exclusion criteria: patients who had severe comorbid conditions that interfered with walking, communication difficulties due to hearing loss, or illiteracy. |
|
| Interventions |
Intervention: telephone‐based problem solving therapy (PST). The design included: 1) telephone‐based counselling, considering the physical condition of the participant with COPD and the cost‐effectiveness of intervention, 2) PST led by a trained nurse as the primary interventionist. The nurse provided the individualised intervention through 12 telephone sessions per patient, with an interval of every 2 weeks over 6 months. Control: usual care Duration of the intervention: 6 months in total; 12 fortnightly telephone sessions per person. Initial session was no longer than 60 minutes, and consecutive sessions were no longer than 30 minutes Number of participants randomised to the intervention group: N = 129; N = 78 completed Number of participants randomised to the control group: N = 125; N = 73 completed |
|
| Outcomes |
Primary outcomes reported: coping (Jalowiec Coping Scale), self‐efficacy (COPD Self‐Efficacy Scale), depressive symptoms (Center for Epidemiologic Studies – Depression scale (CES‐D) – Korean version with 20 items) Time points measured: baseline, and 6 months post‐intervention |
|
| Notes | The mean of the baseline score for depressive symptoms was 14.2 out of 60. The cutoff score for CES‐D for the Korean population > 24. However, 25/151 participants had scores above the cutoff; post‐hoc analysis was performed with data reported for this subset of the sample. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Screening yielded 254 recruited patients who were randomly assigned to two groups." The methods of randomisation were not described. |
| Allocation concealment (selection bias) | High risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "To ensure that usual care was delivered, the physicians taking care of these study participants were not informed of which patients were in the intervention group or comparison group." Assuming by the nature of the intervention, the participants wouldn't have been blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rates (41%) and general reasons for dropout reported. Detailed information on dropouts in this study not reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol information provided |
| Other bias | Low risk | None |
Perkins‐Porras 2018.
| Methods |
Study design: randomised controlled trial Country: UK Aims of the study: to assess feasibility of conduction a larger trial investigating the immediate and short‐term effects of a 10‐minute mindfulness‐based body scan in reducing dyspnoea, anxiety, and depression among hospital inpatients, admitted with an acute COPD exacerbation. |
|
| Participants |
Sample size: N = 80 randomised but N = 50 included in the analyses Mean (SD) age (years): mean age for the intervention group was 70.0 (10.3) years; for the control group, 72.6 (9.8) years Gender: at baseline, female group was 50% of the intervention participants and 47.5% of the control participants Inclusion criteria: clinical diagnosis of COPD, stable condition following admission to the acute ward Exclusion criteria: lack of English comprehension, younger than 40 years, respiratory failure, signs of confusion, dementia (assessed by mini‐mental), or diagnosis of a pre‐existing psychiatric disorder |
|
| Interventions |
Intervention: a 10‐minute audio recording of a mindfulness‐based body scan Control: active control condition: a 10‐minute audio recording of a natural history text Duration of the intervention: 10 minutes (assessments 3 days) Number of participants randomised to the intervention group: N = 40; N = 26 completed Number of participants randomised to the control group: N = 40; N = 24 completed |
|
| Outcomes |
Primary outcomes reported: dyspnoea (Borg scale), depression, anxiety (HADS), Time points measured: day 1 pre‐intervention, and day 3 post‐intervention |
|
| Notes | The study was not blinded. The results cannot be generalised to the COPD population, due to the specific acute respiratory condition of the recruited participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by a computer generated list of random numbers, using block randomisation to either intervention or control group |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates reported, but no information on missing or incomplete data procedures provided |
| Selective reporting (reporting bias) | Unclear risk | No information about a published protocol or trial registration was referenced |
| Other bias | Low risk | None |
Rosser 1983.
| Methods |
Study design: randomised controlled trial Country: UK Aims of the study: the principal hypothesis was that a combination of psychotherapy and medical treatment is more effective than medical treatment alone in relieving the dyspnoea of patients with Chronic Obstructive Airways Disease. |
|
| Participants |
Sample size: N = 65 randomised into two arms Mean (SD) age (years): 66 (SD 9) years for both arms Gender: 68% of males in total for both arms Inclusion criteria: not specified Exclusion criteria: the presence of another severe illness or a cause of dyspnoea other than COPD, living too far away to take part in the study, inability to understand English, dementia detected clinically or by screening questionnaire (Hoch 1964), and other conditions that were a contra‐indication to psychotherapy |
|
| Interventions |
Intervention: psychotherapy consisted of 8 weekly, 45‐minute sessions. There were three groups: analytic group received treatment from one of the two psychoanalysts; supportive group received treatment from the same two psychoanalysts, but this group controlled for the effect of specific psychoanalytical intervention; nurse group received treatment from an experienced medical nurse without psychotherapeutic training Control: usual care Duration of the intervention: 8 weeks (with a 6‐month follow‐up assessment) Number of participants randomised to the intervention group: N = 32 Number of participants randomised to the control group: N = 33 |
|
| Outcomes |
Primary outcomes reported: lung function (FEV1), dyspnoea (Fletcher grading), exercise tolerance (bicycle test or walk test), psychiatric morbidity (GHQ, VAMD for depression, VAMA for anxiety) Time points measured: baseline, 8 weeks post‐intervention |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported, with method of randomisation described |
| Allocation concealment (selection bias) | Low risk | Reported, and method described in detail |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants were not blinded; however, the research personnel (assessors) were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The assessors were blind to the allocation of participants between therapy groups; assessment was completely blind during the interview and partially blind during assessment of outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates reported inconsistently across the paper |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | None |
Schüz 2015.
| Methods |
Study design: cluster‐randomised controlled trial Country: Australia Aims of the study: to explore the influence of anxiety and depression on the effects of a trial intervention aimed at increasing self‐management skills and subsequent self‐management behaviour, for increased physical activity among COPD patients |
|
| Participants |
Sample size: N = 182 randomised, N = 154 included in analysis Mean (SD) age (years): intervention arm: 68.2 (SD 7.9) years; control arm: 67.3 (SD 7.6) years Gender: intervention arm: N = 49 (SD 54) females; control arm: 47 (SD 51) females Inclusion criteria: smoking history > 10 pack‐years, post bronchodilator FEV1/FVC ratio < 0.7, and FEV1 30% to 80% Exclusion criteria: unable to participate in self‐care activities due to mental or physical incapacity, end‐stage cancer, poor English language skills, and nursing home resident |
|
| Interventions |
Intervention: telephone‐based health mentoring programme with a cognitive behavioural basis; up to 16 x 30 min phone calls by trained community nurse Control: usual care plus regular monthly phone calls from a research nurse, to avoid confounding by difference in periodic contact. The phone calls did not provide psychological advice Duration of the intervention: 12 months Number of participants randomised to the intervention group: N = 90; included in final analysis N = 74 Number of participants randomised to the control group: N = 92; included in final analysis N = 80 |
|
| Outcomes | Physical activity, self‐management knowledge, anxiety, and depression Time points measured: baseline, 6 months, 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participating practices were randomised using a code generated from a random numbers table, stratified in blocks of four by the Rural, Remote and Metropolitan Areas (RRMA) classifications appropriate to Tasmania. Randomisation occurred at general practice level to avoid contamination between the intervention and control groups |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation was provided by sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants or research officers was not possible, given the nature of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments were conducted by research officers not directly involved in delivering the intervention or control phone calls, and undertaken at study offices or at GPs' practices, according to patient preference |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates reported, but no information on missing or incomplete data procedures provided |
| Selective reporting (reporting bias) | Low risk | Trial registered and sufficient information provided (Schüz 2015) |
| Other bias | Low risk | None |
BAI: Beck Anxiety Inventory
BDI: Beck Depression Inventory
CAT: Chronic obstructive pulmonary disease Assessment Test
CBT: cognitive‐behavioural therapy
CES‐D: Center for Epidemiologic Studies Depression
COPD: chronic obstructive pulmonary disease
CRQ: Chronic Respiratory Questionnaire
FEV: forced expiratory volume
FEV1: forced expiratory volume in 1 second
FVC: forced vital capacity
GDS: Geriatric Depression Scale
GHQ: General Health Questionnaire
GP: general practitioner
HDRS: Hamilton Depression Rating Scale
HADS: Hospital Anxiety and Depression Scale
LOCF: last observation carried forward
MBCT: mindfulness‐based cognitive therapy
MINI: Mini International Neuropsychiatric Interview
MPI: minimal psychological intervention
N: number
PHQ‐9: Patient Health Questionnaire‐9
PST: problem‐solving therapy
QoL: quality of life
SCL: Symptom Checklist
SD: standard deviation
SF‐36: Medical Outcomes Survey Short‐Form‐36
SGRQ: Saint George's Respiratory Questionnaire
6MWD: 6‐minute walk distance – the distance walked during the 6‐min walk test (6MWT)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alexopoulos 2016 | There was no appropriate comparator intervention in the study |
| Blumenthal 2006 | Participants with COPD comprised only 40% of the study population; in the protocol, we specified that a subset of eligible participants needed to be at least 60% |
| Bove 2016 | The study's main focus was on COPD's psychological comorbidity: anxiety |
| de Godoy 2005 | A study by de Godoy 2005 uses the study sample from de Godoy 2003 and was excluded because participation rates between intervention groups were overlapping |
| Eiser 1997 | Participants were not randomised; the participants in the control group were matched to demographic characteristics of the participants in the intervention group |
| Emery 1998 | There was no appropriate comparator intervention in the study |
| Heslop‐Marshal 2015 | The study's main focus was on COPD's psychological comorbidity: anxiety |
| Kapella 2011 | The study's main focus was on insomnia and behavioural sleep medicine |
| Kheirabadi 2008 | The experimental intervention used educational package on lifestyle modification rather than a psychological therapy |
| Livermore 2010 | The study's main focus was on COPD's psychological comorbidity: anxiety, panic, and dyspnoea |
| Livermore 2015 | The study's main focus was on COPD's psychological comorbidity: anxiety, panic, and dyspnoea |
| Luk 2017 | It is a prospective controlled trial; participants were not randomised |
| Stanley 2005 | Comprehensive outcome data were not available in this publication; it's a case study. |
| Stoop 2015 | The subset of eligible participants with COPD was less than 60% |
COPD: chronic obstructive pulmonary disease
Characteristics of ongoing studies [ordered by study ID]
ISRCTN59537391.
| Trial name or title | A tailored, psychological intervention for mild to moderate anxiety or depression in people with chronic obstructive pulmonary disease (COPD) |
| Methods | A randomised controlled trial |
| Participants | Adult patients with COPD who have mild anxiety, depression, or both, and their carers |
| Interventions |
Intervention arm: "cognitive behavioural approach (CBA) programme which enhances the benefits of PR, with the aim of reducing mild or moderate anxiety or depression (or both) in people with moderate to severe COPD. CBA treatment programme will involve between five and eight 30‐ to 40‐minute weekly sessions with trained respiratory healthcare professionals. 10‐ to 15‐minute weekly telephone follow‐ups will be scheduled with patients if they decide to attend the PR programme after completion of the CBA intervention. In addition, carers of patient participants will be invited to join the study (with patient permission) and will be interviewed about their experiences of being a carer and their mental well‐being. Control arm: standard care with British Lung Foundation booklet about COPD provided and a DVD about self‐management in COPD |
| Outcomes |
Primary: depression (HADS‐D), anxiety (HADS‐A), with 6‐ and 12‐month follow‐up assessment. Secondary outcome measures used at 6 at 12 months: BDI‐II, BAI, Illness Perception Questionnaire, dyspnoea, health‐related QoL (CRQ‐SRS), pulmonary function, social engagement, use of health and social care services, smoking status |
| Starting date | 1 May 2017 (pilot) |
| Contact information | s.j.c.taylor@qmul.ac.uk |
| Notes |
NCT02499653.
| Trial name or title | A psychological intervention to promote acceptance and adherence to non‐invasive ventilation in people with chronic obstructive pulmonary disease: study protocol of a randomised controlled trial |
| Methods | A randomised controlled trial |
| Participants | Patients with COPD for whom non‐invasive ventilation (NIV) is recommended |
| Interventions |
Intervention arm: psychological support including counselling, relaxation, and mindfulness‐based exercises, at home or at the Clinical and Research Centre, HD Respiratory Rehabilitation Unit (Milan, Italy), or via telemedicine. Control arm: standard care and education video material related to the management of disease |
| Outcomes | "The primary outcome will be a latent variable related to indices of use of NIV equipment and adherence to treatment regime; while survival rates and psychological variables will constitute the secondary outcomes" |
| Starting date | 14 July 2015 ‐ trial registration |
| Contact information | eleonora.volapto@unicatt.it Department of Psychology, Università Cattolica del Sacro Cuore, Largo A. Gemelli, 1, 20123 Milan, Italy |
| Notes |
BAI: Beck Anxiety Inventory
BDI: Beck Depression Inventory
COPD: chronic obstructive pulmonary disease
CRQ: Chronic Respiratory Questionnaire
HADS: Hospital Anxiety Depression Scale
Differences between protocol and review
As per protocol, studies were eligible for inclusion in this review if the participants were diagnosed with COPD (FEV1/FVC less than 70% predicted) and a recognised depressive disorder at the time of recruitment to the trial, assessed using a standardised diagnostic criteria or a formal, validated instrument (i.e. a questionnaire), or both. However, more often than not, trials testing effectiveness of psychological therapies apply screening criteria for depressive symptoms at baseline assessment, therefore, we allowed inclusion of such studies.
Furthermore, in the protocol, we stated that a sensitivity analysis would be conducted only for primary outcomes. However, we decided to conduct a sensitivity analysis for a secondary outcome, namely the change in quality of life, as measured by the SGRQ (Analysis 1.4).
Contributions of authors
Justyna Pollok:
Designing the review; co‐ordinating the review; data collection for the review; undertaking searches; screening search results; organising retrieval of papers; screening papers against eligibility criteria; appraising quality of papers; extracting data from papers; writing to authors of papers for additional information; data management for the review; entering data into RevMan 5; analysis of data; interpretation of data; writing the review.
Joep van Agteren:
Screening search results; screening papers against eligibility criteria; extracting data from papers; appraising quality of papers; general feedback on final version of the review.
Adrian Esterman:
Providing general advice on methodology; analysis and interpretation of data.
Kristin Carson‐Chahhoud:
Screening papers against eligibility criteria; extracting data from papers.
Sources of support
Internal sources
No sources of support supplied
External sources
Respiratory Medicine Department at The Queen Elizabeth Hospital, Australia.
Declarations of interest
All authors: None known
Edited (no change to conclusions)
References
References to studies included in this review
de Godoy 2003 {published data only}
- Godoy DV, Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Archives of Physical Medicine and Rehabilitation 2003;84:1154‐8. [DOI] [PubMed] [Google Scholar]
Doyle 2017 {published data only}
- Doyle C, Bhar S, Fearn M, Ames D, Osborne D, You E, et al. The impact of telephone‐delivered cognitive behaviour therapy and befriending on mood disorders in people with chronic obstructive pulmonary disease: a randomized controlled trial. British Journal of Health Psychology 2017;22:542‐56. [DOI] [PubMed] [Google Scholar]
- Doyle C, Dunt D, Ames D, Fearn M, You E, Bhar S. Study protocol for a randomized controlled trial of telephone‐delivered cognitive behavior therapy compared with befriending for treating depression and anxiety in older adults with COPD. International Journal of COPD 2016;11:327‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farver‐Vestergaard 2018 {published data only}
- Farver‐Vestergaard I, O'Toole MS, O'Connor M, Lokke A, Bendstrup E, Basdeo SA, et al. Mindfulness‐based cognitive therapy in COPD: a cluster randomised controlled trial. European Respiratory Journal 2018;51:1702082. [DOI] [PubMed] [Google Scholar]
- NCT02042976. Mindfulness‐based cognitive therapy for chronic obstructive pulmonary disease [A randomised controlled trial of mindfulness‐based cognitive therapy (MBCT) for patients with chronic obstructive pulmonary disease (COPD)]. https://clinicaltrials.gov/ct2/show/NCT02042976 (first received 23 January 2014).
Howard 2014 {published data only}
- Howard C, Dupont S. 'The COPD breathlessness manual': a randomised controlled trial to test a cognitive‐behavioural manual versus information booklets on health service use, mood and health status, in patients with chronic obstructive pulmonary disease. Primary Care Respiratory Medicine 2014;24:14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hynninen 2010 {published data only}
- Hynninen MJ, Bjerke N, Pallesen S, Bakke PS, Nordhus IH. A randomized controlled trial of cognitive behavioral therapy for anxiety and depression in COPD. Respiratory Medicine 2010;104:986‐94. [DOI] [PubMed] [Google Scholar]
Jiang 2012 {published data only}
- Jiang X, He G. Effects of an uncertainty management intervention on uncertainty, anxiety, depression, and quality of life of chronic obstructive pulmonary disease outpatients. Research in Nursing and Health 2012;35:409‐18. [DOI] [PubMed] [Google Scholar]
Kunik 2001 {published data only}
- Kunik ME, Braun U, Stanley MA, Wristers K, Molinari V, Stoebner D, et al. One session cognitive behavioural therapy for elderly patients with chronic obstructive pulmonary disease. Psychological Medicine 2001;31:717‐23. [DOI] [PubMed] [Google Scholar]
Kunik 2008 {published data only}
- Kunik ME, Veazey C, Cully JA, Souchek J, Graham DP, Hopko D, et al. COPD education and cognitive behavioral therapy group treatment for clinically significant symptoms of depression and anxiety in COPD patients: a randomized controlled trial. Psychological Medicine 2008;38:385‐96. [DOI] [PubMed] [Google Scholar]
Lamers 2010 {published data only}
- Lamers F, Jonkers CCM, Bosma H, Chavannes NH, Knottnerus JA, Eijk JTM. Improving quality of life in depressed COPD patients: effectiveness of a minimal psychological intervention. COPD: Journal of Chronic Obstructive Pulmonary Disease 2010;7(5):315‐22. [DOI] [PubMed] [Google Scholar]
- Lamers F, Jonkers CCM, Bosma H, Diederiks JPM, Eijk JThM. Effectiveness and cost‐effectiveness of a minimal psychological intervention to reduce non‐severe depression in chronically ill elderly patients: the design of a randomised controlled trial [ISRCTN92331982]. BioMed Central (BMC) Publish Health 2006;6:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Lee H, Yoon Y, Lim Y, Jung HY, Kim S, Yoo Y, et al. The effect of nurse‐led problem‐solving therapy on coping, self‐efficacy and depressive symptoms for patients with chronic obstructive pulmonary disease: a randomised controlled trial. Age and Ageing 2015;44:397‐403. [DOI] [PubMed] [Google Scholar]
Perkins‐Porras 2018 {published data only}
- Perkins‐Porras L, Riaz M, Okekunle A, Zhelezna S, Chakravorty I, Ussher M. Feasibility study to assess the effect of a brief mindfulness intervention for patients with chronic obstructive pulmonary disease: a randomised controlled trial. Chronic Respiratory Disease 2018;15(4):400‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosser 1983 {published data only}
- Rosser R, Denford J, Heslop A, Kinston W, Macklin D, Minty K, et al. Breathlessness and psychiatric morbidity in chronic bronchitis and emphysema: a study of psychotherapeutic management. Psychological Medicine 1983;13:93‐110. [DOI] [PubMed] [Google Scholar]
Schüz 2015 {published data only}
- ACTRN12608000112369. Pathways to Lung Health: a comprehensive self‐management programme for chronic obstructive pulmonary disease in the community [The effect of telephone delivered health‐mentoring by community and practice nurses on quality of life in chronic obstructive pulmonary disease compared to usual care in the community]. the Australian and New Zealand Clinical Trails Research/ACTRN12608000112369 (first received 26 February 2008).
- Schüz N, Walters JAE, Cameron‐Tucker H, Scott J, Wood‐Baker R, Walters EH. Patient anxiety and depression moderate the effects of increased self‐management knowledge on physical activity: a secondary analysis of a randomised controlled trial on health‐mentoring in COPD. COPD: Journal of Chronic Obstructive Pulmonary Disease 2015;12(5):502‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alexopoulos 2016 {published data only}
- Alexopoulos GS, Sirey JA, Banerjee S, Kiosses D, Pollari C, Novitch RS, et al. Two behavioral interventions for patients with major depression and severe COPD. American Journal of Geriatric Psychiatry 2016;24:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blumenthal 2006 {published data only}
- Blumenthal JA, Babyak MA, Carney RM, Keefe FJ, Davis RD, LaCaille RA, et al. Telephone‐based coping skills training for patients awaiting lung transplantation. Journal of Consulting and Clinical Psychology 2006;74(3):535‐44. [DOI] [PubMed] [Google Scholar]
Bove 2016 {published data only}
- Bove DG, Lomborg K, Jensen AK, Overgaard D, Lindhardt BO, Midtgaard J. Efficacy of a minimal home‐based psycho‐educative intervention in patients with advanced COPD: a randomised controlled trial. Respiratory Medicine 2016;121:109‐16. [DOI] [PubMed] [Google Scholar]
de Godoy 2005 {published data only}
- Godoy DV, Godoy RF, Junior BB, Vaccari Pf, Michelli M, Teixeira PJZ, et al. The effect of psychotherapy provided as part of a pulmonary rehabilitation program for the treatment of patients with chronic obstructive pulmonary disease. The Journal Brasileiro de Pneumologia 2005;31(6):499‐505. [Google Scholar]
Eiser 1997 {published data only}
- Eiser N, West C, Evans S, Jeffers A, Quirk F. Effects of psychotherapy in moderately severe COPD: a pilot study. European Respiratory Journal 1997;10:1581‐4. [DOI] [PubMed] [Google Scholar]
Emery 1998 {published data only}
- Emery CF, Schein RL, Hauck ER, MacIntrye NR. Psychological and cognitive outcomes of a randomized trial of exercise among patients with chronic obstructive pulmonary disease. Health Psychology 1998;17(3):232‐40. [DOI] [PubMed] [Google Scholar]
Heslop‐Marshal 2015 {published data only}
- Heslop‐Marshal K, Baker C, Carick‐Sen D, Stenton SC, Newton J, Burns GP, et al. Prevalence of anxiety and patient characteristics from a randomised controlled trial (RCT) to identify if cognitive behavioural therapy (CBT) by respiratory nurses reduces anxiety in COPD. Thorax 2015;70(3;M24):A237. [Google Scholar]
Kapella 2011 {published data only}
- Kapella MC, Herdegen JJ, Perlis ML, Shaver JL, Larson JL, Law JA, et al. Cognitive behavioural therapy for insomnia comorbid with COPD is feasible with preliminary evidence of positive sleep and fatigue effects. International Journal of COPD 2011;6:625‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kheirabadi 2008 {published data only}
- Kheirabadi GR, Keypour M, Attaran N, Bagherian R, Maracy MR. Effect of add‐on 'self management and behaviour modification' education on severity of COPD. Tanaffos 2008;7(3):23‐30. [Google Scholar]
Livermore 2010 {published data only}
- Livermore N, Sharpe L, McKenzie D. Prevention of panic attacks and panic disorder in COPD. European Respiratory Journal 2010;35:557‐63. [DOI] [PubMed] [Google Scholar]
Livermore 2015 {published data only}
- Livermore N, Dimitri A, Sharpe L, McKenzie DK, Gandevia SC, Butler JE. Cognitive behaviour therapy reduces dyspnoea ratings in patients with chronic obstructive pulmonary disease (COPD). Respiratory Physiology & Nuerobiology 2015;216:35‐42. [DOI] [PubMed] [Google Scholar]
Luk 2017 {published data only}
- Luk EK, Gorelik A, Irving L, Khan F. Effectiveness of cognitive behavioural therapy in a community‐based pulmonary rehabilitation programme: a controlled clinical trial. Journal of Rehabilitation Medicine 2017;49:264‐9. [DOI] [PubMed] [Google Scholar]
Stanley 2005 {published data only}
- Stanley MA, Veazey C, Hopko D, Diefenbach G, Kunik ME. Anxiety and depression in chronic obstructive pulmonary disease: a new intervention and case report. Cognitive and Behavioural Practice 2005;12:424‐36. [Google Scholar]
Stoop 2015 {published data only}
- Stoop CH, Nefs G, Pommer AM, Pop VJM, Pouwer F. Effectiveness of a stepped care intervention for anxiety and depression in people with diabetes, asthma or COPD in primary care: a randomised controlled trial. Journal of Affective Disorders 2015;184:269‐79. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN59537391 {published data only}
- ISRCTN59537391. A tailored, psychological intervention for mild to moderate anxiety or depression in people with chronic obstructive pulmonary disease (COPD) [Working together against COPD]. www.isrctn.com/ISRCTN59537391 (first received 9 February 2017).
NCT02499653 {published data only}
- NCT02499653. A psychological intervention to promote acceptance and adherence to non‐invasive ventilation in people with chronic obstructive pulmonary disease: study protocol of a randomised controlled trial. clinicaltrials.gov/ct2/show/NCT02499653 (first received 16 July 2015). [DOI] [PMC free article] [PubMed]
- Volpato E, Banfi P, Pagnini F. A psychological intervention to promote acceptance and adherence to non‐invasive ventilation in people with chronic obstructive pulmonary disease: study protocol of a randomised controlled trial. Trials 2017;18(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abrams 2011
- Abrams TE, Vaughan‐Sarrazin M, Vander Weg MW. Acute exacerbations of chronic obstructive pulmonary disease and the effect of existing psychiatric comorbidity on subsequent mortality. Psychosomatics 2011;52(5):441‐9. [DOI] [PubMed] [Google Scholar]
Almagro 2002
- Almagro P, Calbo E, Ochoa de Echagüen A, Barreiro B, Quintana S, Heredia JL, et al. Mortality after hospitalization for COPD. Chest 2002;121(5):1441‐8. [DOI] [PubMed] [Google Scholar]
Altemus 2014
- Altemus M, Sarvaiya N, Epperson CN. Sex differences in anxiety and depression clinical perspectives. Frontiers in Neuroendocrinology 2014;35(3):320‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 1997
- Altman DG, Bland JM. Statistics notes. Units of analysis. BMJ 1997;314:1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Antony 2003
- Antony MM, Roemer E. Behavior therapy. In: Gurman AS, Messer SB editor(s). Essential Psychotherapies. 2nd Edition. New York: Guilford, 2003:182‐223. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington DC: American Psychiatric Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Washington DC: American Psychiatric Publishing, 2013. [Google Scholar]
Aryal 2013
- Aryal S, Diaz‐Guzman E, Mannino DM. COPD and gender differences: an update. Translational Research 2013;162(4):208‐18. [DOI] [PubMed] [Google Scholar]
Atlantis 2013
- Atlantis E, Fahey P, Cochrane M, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD. Chest 2013;144(3):766‐77. [DOI] [PubMed] [Google Scholar]
ATS 2002
- American Thoracic Society. ATS statement: guidelines for the six‐minute walk test. American Journal of Respiratory and Critical Care Medicine 2002;166:111‐7. [DOI] [PubMed] [Google Scholar]
Baraniak 2011
- Baraniak A, Sheffield D. The efficacy of psychologically based interventions to improve anxiety, depression and quality of life in COPD: a systematic review and meta‐analysis. Patient Education and Counseling 2011;83:29‐36. [DOI: 10.1016/j.pec.2010.04.010] [DOI] [PubMed] [Google Scholar]
Barnes 2016
- Barnes PJ. Sex differences in chronic obstructive pulmonary disease mechanisms. American Journal of Respiratory and Critical Care Medicine 2016;193(8):813‐4. [DOI] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561‐71. [DOI] [PubMed] [Google Scholar]
Bentsen 2013
- Bentsen SB, Miaskowski C, Rustoen T. Demographic and clinical characteristics associated with quality of life in patients with chronic obstructive pulmonary disease. Quality of Life Research 2014;23:991‐8. [DOI] [PubMed] [Google Scholar]
Borg 1982
- Borg GA. Psychophysical basis of perceived exertion. Medicine and Science in Sports and Exercise 1982;14:377‐81. [PubMed] [Google Scholar]
Buist 2007
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD study): a population‐based prevalence study. Lancet 2007;370:741‐50. [DOI] [PubMed] [Google Scholar]
Butland 1982
- Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two‐, six‐, and 12‐minute walking tests in respiratory disease. British Medical Journal 1982;284(6329):1607‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cafarella 2012
- Cafarella PA, Effing TW, Usmani ZA, Frith PA. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology 2012;17:627‐38. [DOI] [PubMed] [Google Scholar]
Camp 2009
- Camp PG, O'Donnell DE, Postma DS. Chronic obstructive pulmonary disease in men and women. Proceedings of the American Thoracic Society 2009;6:535‐38. [DOI] [PubMed] [Google Scholar]
Campbell 2000
- Campbell M, Grimshaw J, Steen N. Sample size calculations for cluster randomised trials. Changing Professional Practice in Europe Group (EU BIOMED II Concerted Action). Journal of Health Services Research and Policy 2000;5(1):12‐6. [DOI] [PubMed] [Google Scholar]
Carr 2009
- Carr A. The effectiveness of family therapy and systemic interventions for adult‐focused problems. Journal of Family Therapy 2009;31:45‐74. [Google Scholar]
Coventry 2007
- Coventry P, Hind D. Comprehensive pulmonary rehabilitation for anxiety and depression in adults with chronic obstructive pulmonary disease: a systematic review and meta‐analysis. Journal of Psychosomatic Research 2007;63:551‐65. [DOI] [PubMed] [Google Scholar]
Coventry 2011
- Coventry PA, Gemmell I, Todd CJ. Psychosocial risk factors for hospital readmission in COPD patients on early discharge services: a cohort study. BMC Pulmonary Medicine 2011;11:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coventry 2013
- Coventry PA, Bower P, Keyworth C, Kenning C, Knopp J, Garrett C, et al. The effect of complex interventions on depression and anxiety in chronic obstructive pulmonary disease: systematic review and meta‐analysis. PLoS One 2013;8(4):e60532. [DOI: 10.1371/journal.pone.0060532] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coventry 2014
- Coventry PA, Hudson JL, Kontopantelis E, Archer J, Richards DA, Gilbody S, et al. Characteristics of effective collaborative care for treatment of depression: a systematic review and meta‐regression of 74 randomised controlled trial. PloS One 2014;9(9):e108114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cristea 2017
- Cristea IA, Hollon SD, David D, Karyotaki E, Cuijpers P, Stefan SI. The effects of cognitive behavioral therapy are not systematically falling: a revision of Johnsen & Friborg. Psychological Bulletin 2017;143(3):326‐40. [DOI] [PubMed] [Google Scholar]
Cuijpers 2010
- Cuijpers P, Donker T, Straten A, Li J, Andersson G. Is guided self‐help as effective as face‐to‐face psychotherapy for depression and anxiety disorders? A systematic review and meta‐analysis of comparative outcome studies. Psychological Medicine 2010;40:1943‐57. [DOI] [PubMed] [Google Scholar]
Dalal 2011
- Dalal AA, Shah M, Lunacsek O, Hanania NA. Clinical an economic burden of depression/anxiety in chronic obstructive pulmonary disease patients within a managed care population. Respiratory Medicine 2011;105(10):1516‐22. [Google Scholar]
David 2018
- David D, Cristea I, Hofmann SG. Why cognitive behavioral therapy is the current gold standard of psychotherapy. Frontiers in Psychiatry 2018;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davison 2003
- Davison GC, Neale JM. Abnormal Psychology. New York: John Wiley & Sons, 2003. [Google Scholar]
de Mello 2005
- Mello MF, Jesus Mari J, Bacaltchuk J, Verdeli H, Neugebauer R. A systematic review of research findings on the efficacy of interpersonal therapy for depressive disorders. European Archives of Psychiatry and Clinical Neuroscience 2005;255(2):75‐82. [DOI] [PubMed] [Google Scholar]
de Voogd 2009
- Voogd JN, Wempe JB, Koëter GH, Postema K, Sonderen E, Ranchor AV, et al. Depressive symptoms as predictors of mortality in patients with COPD. Chest 2009;135(3):619‐25. [DOI] [PubMed] [Google Scholar]
DeJean 2013
- Jean D, Giacomini M, Vanstone M, Brundisini F. Patient experiences of depression and anxiety with chronic disease: a systematic review and qualitative meta‐syntheiss. Health Quality Ontario 2013;13(16):1‐33. [PMC free article] [PubMed] [Google Scholar]
Devine 1996
- Devine EC, Pearcy J. Meta‐analysis of the effects of psycho‐educational care in adults with chronic obstructive pulmonary disease. Patient Education and Counselling 1996;29:167‐78. [DOI] [PubMed] [Google Scholar]
Di Marco 2006
- Marco F, Verga M, Reggenta M, Maria Casanova F, Santus P, Blasi F, et al. Anxiety and depression in COPD patients: the roles of gender and disease severity. Respiratory Medicine 2006;100(10):1767‐74. [DOI] [PubMed] [Google Scholar]
DiNicola 2013
- DiNicola G, Julian L, Gregorich SE, Blanc PD, Katz PP. The role of social support in anxiety for persons with COPD. Journal of Psychosomatic Research 2013;74(2):110‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 2013a
- Doyle T, Palmer S, Johnson J, Babyak M, Smith P, Mabe S, et al. Association of anxiety and depression with pulmonary‐specific symptoms in chronic obstructive pulmonary disease. International Journal of Psychiatry in Medicine 2013;45(2):189‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duggan 2014
- Duggan C, Parry G, McMurran M, Davidson K, Dennis J. The recording of adverse events from psychological treatments in clinical trials: evidence from a review of NIHR‐funded trials. Trials 2014;15(1):335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Effing 2007
- Effing T, Monninkhof EM, Valk PDLP, Palen J, Herwaarden CLA. Self‐management education for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD002990.pub2] [DOI] [PubMed] [Google Scholar]
Fan 2007
- Fan VS, Ramsey SD, Giardino ND, Make BJ, Emery CF, Diaz PT, et al. National Emphysema Treatment Trial (NETT) Research Group. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Archives of Internal Medicine 2007;167(21):2345‐53. [DOI] [PubMed] [Google Scholar]
Felker 2010
- Felker B, Bush KR, Harel O, Shofer JB, Shores MM, Au DH. Added burden of mental disorders on health status among patients with chronic obstructive pulmonary disease. Primary Care Companion to the Journal of Clinical Psychiatry 2010;12(4:):e1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleehart 2014
- Fleehart S, Fan VS, Nguyen HQ, Lee J, Kohen R, Herting JR, et al. Prevalence and correlates of suicide ideation in patients with COPD: a mixed methods study. International Journal of COPD 2014;9:1321‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 2012
- Foster N, Little P. Methodological issues in pragmatic trials of complex interventions in primary care. British Journal of General Practice 2012;62(594):10‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fritzsche 2011
- Fritzsche A, Clamor A, Leupoldt A. Effects of medical and psychological treatment of depression in patients with COPD ‐ a review. Respiratory Medicine 2011;105:1422‐33. [DOI] [PubMed] [Google Scholar]
GOLD update 2018
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Global Initiative for Chronic Obstructive Pulmonary Disease Revised 2018.
Goodwin 2012
- Goodwin RD, Lavoie KL, Lemeshow AR, Jenkins E, Brown ES, Fedoronko DA. Depression, anxiety, and COPD: the unexamined role of nicotine dependence. Nicotine and Tobacco Research 2012;14(2):176‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 6 August 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Greening 2006
- Greening T. Five basic postulates of humanistic psychology. Journal of Humanistic Psychology 2006;46(3):239‐40. [Google Scholar]
Groenwegen 2003
- Groenwegen KH, Schols AM, Wouters EF. Mortality and mortality‐related factors after hospitalization for acute exacerbation of COPD. Chest 2003;124(2):459‐67. [DOI] [PubMed] [Google Scholar]
Gudmundsson 2005
- Gudmundsson G, Gislason T, Janson C, Lindberg E, Hallin R, Ulrik CS, et al. Risk factors for rehospitalisation in COPD: role of health status, anxiety and depression. European Respiratory Journal 2005;26(3):414‐9. [DOI] [PubMed] [Google Scholar]
Guyatt 1987
- Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987;42(10):773‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. Rating depressive patients. Journal of Clinical Psychiatry 1960;41:21‐4. [PubMed] [Google Scholar]
Hanania 2011
- Hanania NA, Müllerova H, Locantore NW, Vestbo J, Watkins ML, Wouters EF, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study investigators. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. American Journal of Respiratory and Critical Care Medicine 2011;183:604‐11. [DOI] [PubMed] [Google Scholar]
Hayes 2005
- Hayes AM, Beevers CG, Feldman GC, Laurenceau JP, Perlman C. Avoidance and processing as predictors of symptom change and positive growth in integrative therapy for depression. International Journal of Behavioural Medicine 2005;12(2):111‐22. [DOI] [PubMed] [Google Scholar]
Hegerl 2013
- Hegerl U, Mergl R. Depression and suicidality in COPD. European Respiratory Journal 2014;44:734‐43. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoch 1964
- Hoch PH, Zubin J. The evaluation of psychiatric treatment: the proceedings of the Fifty‐second Annual Meeting of the American Psychopathological Association held in New York City, February 1962. New York: Grune & Stratton, 1964. [Google Scholar]
Hofmann 2012
- Hofmann SG, Asnaani A, Vonk IJJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta‐analyses. Cognitive Therapy and Research 2012;36(5):427‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunot 2013
- Hunot V, Moore THM, Caldwell DM, Furukawa TA, Davis P, Jones H, Honyashiki M, Chen P, Lewis G, Churchill R. 'Third wave' cognitive and behavioural therapies versus other psychological therapies for depression. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD008704.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutter 2010
- Hutter N, Schnurr A, Baumeister H. Health care costs in patients with diabetes mellitus and comorbid mental disorders ‐ a systematic review. Diabetologia 2010;53(12):2470‐9. [DOI] [PubMed] [Google Scholar]
Jang 2018
- Jang SM, Kim KU, Na HJ, Song SE, Lee SH, Lee H, et al. Depression is a major determinant of both disease‐specific and generic health‐related quality of life in people with severe COPD. Chronic Respiratory Disease 2018 May 9 [Epub ahead of print]; Vol. 16. [DOI: 10.1177/1479972318775422] [DOI] [PMC free article] [PubMed]
Johnsen 2015
- Johnsen TJ, Friborg O. The effects of cognitive behavioral therapy as an anti‐depressive treatment is falling: a meta‐analysis. Psychological Bulletin 2015;141(4):747. [DOI] [PubMed] [Google Scholar]
Jones 1991
- Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respiratory Medicine 1991;85(Suppl B):25‐31. [DOI] [PubMed] [Google Scholar]
Jones 2009
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. European Respiratory Journal 2009;34:648‐54. [DOI] [PubMed] [Google Scholar]
Kandel 1998
- Kandel ER. A new intellectual framework for psychiatry. American Journal of Psychiatry 1998;155:457‐69. [DOI] [PubMed] [Google Scholar]
Kaplan 2009
- Kaplan BJ, Sadock VA, Ruiz P. Kaplan and Sadock's Comprehensive Textbook of Psychiatry. 10th Edition. Philadelphia (US): Wolters Kluwer Health/Lippincott Williams & Wilkins (LWW), 2017. [Google Scholar]
Karajgi 1990
- Karajgi B, Rifkin A, Doddi S, Kolli R. The prevalence of anxiety disorders in patients with chronic obstructive pulmonary disease. American Journal of Psychiatry 1990;147:200‐1. [DOI] [PubMed] [Google Scholar]
Kazdin 2000
- Kazdin AE. Encyclopedia of Psychology. Oxford: American Psychological Association; Oxford University Press, 2000. [Google Scholar]
Kim 2000
- Kim HF, Kuic ME, Molinari VA, Hillman SL, Lalani S, Orengo CA, et al. Functional impairment in COPD patients: the impact of anxiety and depression. Psychosomatics 2000;41(6):465‐71. [DOI] [PubMed] [Google Scholar]
Kim 2014
- Kim KU, Park HK, Jung HY, Ahn JJ, Moon E, Kim YS, et al. Association of depression with disease severity in patients with chronic obstructive pulmonary disease. Lung 2014;192:243‐9. [DOI] [PubMed] [Google Scholar]
Kring 2007
- Kring AM, Davison GC, Neale JM, Johnson SL. Abnormal Psychology. 10th Edition. Hoboken, New Jersey (US): John Wiley and Sons Inc, 2007. [Google Scholar]
Lamprecht 2011
- Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska‐Mogielnicka E. COPD in never smokers. Results from the population‐based burden of obstructive lung disease study. Chest 2011;139(4):752‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lecheler 2017
- Lecheler L, Richter M, Franzen DP, Rampini SK, Cheetham M, Jenewein J, et al. The frequent and under‐recognised co‐occurrence of acute exacerbated COPD and depression warrants screening: a systematic review. European Respiratory Review 2017;26(144):170026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linden 2006
- Linden DEJ. How psychotherapy changes the brain ‐ the contribution of functional neuroimaging. Molecular Psychiatry 2006;11:528‐38. [DOI] [PubMed] [Google Scholar]
Lopez 2006
- Lopez AD, Mathers CD. Measuring the global burden of disease and epidemiological transitions: 2002‐2030. Annals of Tropical Medicine and Parasitology 2006;100:481‐99. [DOI] [PubMed] [Google Scholar]
Lovibond 1995
- Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2nd Edition. Sydney: The Psychology Foundation, University of New South Wales, 1995. [Google Scholar]
MacGillivray 2003
- MacGillivray S, Arroll B, Hatcher S, Ogston S, Reid I, Sullivan F, et al. Efficacy and tolerability of selective serotonin reuptake inhibitors compared with tricyclic antidepressants in depression treated in primary care: a systematic review and meta‐analysis. BMJ 2003;326:1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathers 2006
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine 2006;3(11):e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maurer 2008
- Maurer J, Rebbapragada V, Borson S, Goldstein R, Kunik ME, Yohannes AM, et al. Anxiety and depression in COPD. Chest 2008;134:43S‐53S. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKenzie 2003
- McKenzie DK, Frith PA. The COPDX Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease. The Medical Journal of Australia 2003;178(6):1. [DOI] [PubMed] [Google Scholar]
Meader 2011
- Meader N, Mitchell A, Chew‐Graham C, Goldberg D, Rizzo M, Kessler D, et al. Case identification of depression in patients with chronic physical health problems: a diagnostic accuracy meta‐analysis of 113 studies. British Journal of General Practice 2011;61(593):e808‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mikkelsen 2004
- Mikkelsen RL, Middelboe T, Pisinger C, Stage KB. Anxiety and depression in patients with chronic obstructive pulmonary disease (COPD): a review. Nordic Journal of Psychiatry 2004;58:65‐70. [DOI] [PubMed] [Google Scholar]
Morrow 2015
- Morrow EH. The evolution of sex differences in disease. Biology of Sex Differences 2015;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naylor 2012
- Naylor C, Parsonage M, McDaid D, Knapp M, Fossey M, Galea A. Long‐term conditions and mental health. The cost of co‐morbidities. The Kings Fund, London, UK 2012.
NCT00105911
- NCT00105911. A cognitive‐behavioural intervention for depression and anxiety in COPD. clinicaltrials.gov/ct2/show/NCT00105911 (first received 18 March 2005).
NCT02042976
- NCT02042976. Mindfulness‐based cognitive therapy for chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT02042976 (first received 23 January 2014).
Ng 2007
- Ng TP, Niti M, Tan WC, Cao Z, Ong KC, Eng P. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Archives of Internal Medicine 2007;167(1):60‐7. [DOI] [PubMed] [Google Scholar]
NHPA 2015
- National Health Performance Authority. Healthy communities: potentially preventable hospitalisations in 2013–14. www.myhealthycommunities.gov.au/our‐reports/potentially‐preventable‐hospitalisations/december‐2015 December 2015.
NICE 2009
- National Institute for Health and Care Excellence. Depression in adults with a chronic physical health problem: recognition and management. Clinical guideline [CG91]. National Collaborating Centre for Mental Health. London, UK October 2009.
NICE 2010
- National Collaborating Centre for Mental Health (UK). Depression: The treatment and management of depression in adults (updated edition). National Clinical Practice Guideline 90. The British Psychological Society and The Royal College of Psychiatrists 2010. [PubMed]
Norcross 2005
- Norcross JC, Goldfried MR. Handbook of Psychotherapy Integration. 2nd Edition. New York: Oxford, 2005. [Google Scholar]
Norton 2013
- Norton S, Cosco T, Doyle F, Done J, Sacker A. The Hospital Anxiety and Depression Scale: a meta confirmatory factor analysis. Journal of Psychosomatic Research 2013;74(1):74‐81. [DOI] [PubMed] [Google Scholar]
Norweg 2005
- Norweg AM, Whiteson J, Malgady R, Mola A, Rey M. The effectiveness of different combinations of pulmonary rehabilitation program components: a randomized controlled trial. Chest 2005;128:663‐72. [DOI] [PubMed] [Google Scholar]
O'Connor 2009
- O’Connor EA, Whitlock EP, Beil TL, Gaynes BN. Screening for depression in adult patients in primary care settings: a systematic evidence review. Annals of Internal Medicine 2009;151:793‐803. [DOI] [PubMed] [Google Scholar]
Omachi 2009
- Omachi TA, Katz PP, Yelin EH, Gregorich SE, Iribarren C, Blanc PD, et al. Depression and health‐related quality of life in chronic obstructive pulmonary disease. American Journal of Medicine 2009;122(8):778.e9‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Panagioti 2014
- Panagioti M, Scott C, Blakemore A, Coventry PA. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. International Journal of COPD 2014;9:1289‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patsopoulos 2011
- Patsopoulos NA. A pragmatic view on pragmatic trials. Dialogues in Clinical Neuroscience 2011;13(2):217‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petty 1998
- Petty TL. Supportive therapy in COPD. Chest 1998;113:256S‐62S. [DOI] [PubMed] [Google Scholar]
Pignone 2002
- Pignone MP, Gaynes BN, Rushton JL, Burchell CM, Orleans CT, Mulrow CD, et al. Screening for depression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2002;136(10):765‐76. [DOI] [PubMed] [Google Scholar]
Pildal 2007
- Pildal J, Hróbjartsson A, Jørgensen KJ, Hilden J, Altman DG, Gøtzsche PC. Impact of allocation concealment on conclusions drawn from meta‐analyses of randomized trials. International Journal of Epidemiology 2007;36(4):847‐57. [DOI] [PubMed] [Google Scholar]
Pollok 2016
- Pollok J, Agteren J, Carson K, Esterman A, Smith B, Licinio J. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD012347] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pollok 2018
- Pollok J, Agteren J, Carson‐Chahhoud K. Pharmacological interventions for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD012346.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pooler 2014
- Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. International Journal of Chronic Obstructive Pulmonary Disease 2014;9(3):15‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pumar 2014
- Pumar MI, Gray CR, Walsh JR, Yang IA, Rolls TA, Ward DL. Anxiety and depression ‐ important psychological comorbidities of COPD. Journal of Thoracic Disease 2014;6(11):1615‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabe 2007
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine 2007;176(6):532‐55. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ries 1995
- Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation of physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Annals of Internal Medicine 1995;122:823‐32. [DOI] [PubMed] [Google Scholar]
Roche 2007
- Roche N. Where current pharmacological therapies fall short in COPD: symptom control is not enough. European Respiratory Review 2007;16:98‐104. [Google Scholar]
Rose 2002
- Rose C, Wallace L, Dickson R, Ayres J, Lehman R, Searle Y, et al. The most effective psychologically based treatments to reduce anxiety and panic in patients with chronic obstructive pulmonary disease (COPD): a systematic review. Patient Education and Counseling 2002;47:311‐8. [PUBMED: 12135822] [DOI] [PubMed] [Google Scholar]
Schulz 2002
- Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet 2002;359:781‐5. [DOI] [PubMed] [Google Scholar]
Shedler 2010
- Shedler J. The efficacy of psychodynamic psychotherapy. American Psychologist Association 2010;65(2):98‐109. [DOI: 10.1037/a0018378] [DOI] [PubMed] [Google Scholar]
Shinohara 2013
- Shinohara K, Honyashiki M, Imai H, Hunot V, Caldwell DM, Davis P, Moore THM, Furukawa TA, Churchill R. Behavioural therapies versus other psychological therapies for depression. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD008696.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2014
- Smith SM, Sonego S, Ketcheson L, Larson JL. A review of the effectiveness of psychological interventions used for anxiety and depression in chronic obstructive pulmonary disease. BMJ Open Respiratory Research 2014;1:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solano 2006
- Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. Journal of Pain and Symptom Management 2006;31(1):58‐69. [DOI] [PubMed] [Google Scholar]
Spritzer 1999
- Spritzer RL, Kroenke K, Williams JBW, Patient Health Questionnaire Study Group. Validity and utility of a self‐report version of PRIME‐MD: the PHQ Primary Care Study. The Journal of the Americal Medical Association 1999;282:1737‐44. [DOI] [PubMed] [Google Scholar]
Spruit 2013
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. ATS/ERS Task Force on Pulmonary Rehabilitation. An Official Thoracic Society/ European Respiratory Society Statement: key concepts and advances in pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2013;188(8):e13‐64. [DOI] [PubMed] [Google Scholar]
Stiles‐Shields 2014
- Stiles‐Shields C, Kwasny MJ, Cai X, Mohr DC. Therapeutic alliance in face‐to‐face and telephone‐administered cognitive behavioral therapy. Journal of Consulting and Clinical Psychology 2014;82:349‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tolin 2010
- Tolin DF. Is cognitive‐behavioural therapy more effective than other therapies? A meta‐analytic review. Clincal Psychology Review 2010;30:710‐20. [DOI] [PubMed] [Google Scholar]
Torabipour 2016
- Torabipour A, Hakim A, Angali KA, Dolatshah M, Yusofzadeh M. Cost analysis of hospitalized patients with chronic obstructive pulmonary disease: a state‐level cross‐sectional study. Journal of Respiratory Diseases, Thoracic Surgery, Intensive Care and Tuberculosis 2016;15(2):75‐82. [PMC free article] [PubMed] [Google Scholar]
Tselebis 2013
- Tselebis A, Bratis D, Pachi A, Moussas G, Ilias I, Harikiopoulou M, et al. A pulmonary rehabilitation program reduces levels of anxiety and depression in COPD patients. Multidisciplinary Respiratory Medicine 2013;8(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tselebis 2016
- Tselebis A, Pachi A, Ilias I, Kosmas E, Bratis D, Moussas G, et al. Strategies to improve anxiety and depression in patients with COPD: a mental health perspective. Neuropsychiatric Disease and Treatment 2016;12:297‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tyrer 2014
- das Nair R, Anderson P, Clarke S, Leighton P, Lincoln NB, Mhizha‐Murira JR, et al. Clinical and cost effectiveness of cognitive behaviour therapy for health anxiety in medical patients: a multi‐centre randomised controlled trial. Lancet 2014;383:219‐25. [DOI] [PubMed] [Google Scholar]
Usmani 2011
- Usmani ZA, Carson KV, Cheng JN, Esterman AJ, Smith BJ. Pharmacological interventions for the treatment of anxiety disorders in chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD008483.pub2] [DOI] [PubMed] [Google Scholar]
Usmani 2013
- Usmani ZA, Carson KV, Heslop K, Esterman AJ, Soyza A, Smith BJ. Psychological therapies for the treatment of anxiety disorders in chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010673] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van de Velde 2010
- Velde S, Bracke P, Levecque K. Gender differences in depression in 23 European countries. Cross‐national variation in the gender gap in depression. Social Science and Medicine 2010;71(2):305‐13. [DOI] [PubMed] [Google Scholar]
van Manen 2002
- Manen JG, Bindels PJ, Dekker FW, IJzermans CJ, Zee JS, Schadé E. Risk of depression in patients with chronic obstructive pulmonary disease and its determinants. Thorax 2002;57(5):412‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Viinamaki 1998
- Viinamaki H, Kuikka J, Tiihonen J, Lehtonen J. Change in monoamine transporter density related to clinical recovery: a case control study. Nordic Journal of Psychiatry 1998;52:39‐44. [Google Scholar]
von Leupoldt 2012
- Leupoldt A, Fritzsche A, Trueba AF. Behavioral medicine approaches to chronic obstructive pulmonary disease. Annals of Behavioral Medicine 2012;44:52‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vozoris 2018
- Vozoris NT, Wang X, Austin PC, Stephenson AL, O'Donnell DE, Gershon AS, et al. Serotonergic antidepressant use and morbidity and mortality among older adults with COPD. European Respiratory Journal 2018;52:1800475. [DOI] [PubMed] [Google Scholar]
Waltman 2016
- Waltman SH, Creed TA, Beck AT. Are the effects of cognitive behavior therapy for depression falling? Review and critique of the evidence. Clinical Psychology Science and Practice 2016;23(2):113‐22. [Google Scholar]
Ware 1993
- Ware JE, Snow KK, Kosinski M, Gandek B. SF‐36 Health Survey: manual and interpretation guide. Boston (MA): Health Institute, New England Medical Center, 1993. [Google Scholar]
Watson 2013
- Watson M, White C, Davolls S, Mohammed A, Lynch A, Mohammed K. Problem‐focussed interactive telephone therapy for cancer patients: a phase II feasibility trial. Psychooncology 2013;22:1485‐91. [DOI] [PubMed] [Google Scholar]
WHO 2017
- World Health Organization. COPD predicted to be third leading cause of death in 2030. www.who.int/respiratory/copd/World_Health_Statistics_2008/en/ (accessed prior to 2 November 2018).
WHO 2018
- World Health Organization. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease ‐ 2018 report. www.goldcopd.org/wp‐content/uploads/2017/11/GOLD‐2018‐v6.0‐FINAL‐revised‐20‐Nov_WMS.pdf (accessed prior to 2 November 2018):pp 1‐117.
Wittchen 2011
- Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. European Neuropsychopharmacology 2011;21(9):655‐79. [DOI] [PubMed] [Google Scholar]
Wood‐Baker 2012
- Wood‐Baker R, Reid D, Robinson A, Walters EH. Clinical trial of community nurse mentoring to improve self‐management in patients with chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:407‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2018
- Yang IA, Brown JL, George J, Jenkins S, McDonald CF, McDonald V. The COPD‐X Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease 2018. Version 2.53. Lung Foundation Australia March 2018.
Yesavage 1982
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research 1982‐3;17(1):37‐49. [DOI] [PubMed] [Google Scholar]
Yohannes 2000
- Yohannes AM, Baldwin RC, Connolly MJ. Depression and anxiety in elderly outpatients with chronic obstructive pulmonary disease: prevalence, and validation of the BASDEC screening questionnaire. International Journal of Geriatric Psychiatry 2000;15(12):1090‐6. [DOI] [PubMed] [Google Scholar]
Yohannes 2001
- Yohannes AM, Connolly MJ, Baldwin RC. A feasibility study of antidepressant drug therapy in depressed elderly patients with chronic obstructive pulmonary disease. International Journal of Geriatric Psychiatry 2001;16(5):451‐4. [DOI] [PubMed] [Google Scholar]
Yohannes 2006
- Yohannes A, Baldwin R, Connoly M. Depression and anxiety in elderly patients with chronic obstructive pulmonary disease. Age Ageing 2006;35:457‐9. [DOI] [PubMed] [Google Scholar]
Yohannes 2008
- Yohannes AM. Management of anxiety and depression in patients with COPD. Espert Review of Respiratory Medicine 2008;2(3):337‐47. [DOI] [PubMed] [Google Scholar]
Zhang 2011
- Zhang MW, Ho RC, Cheung MW, Fu E, Mak A. Prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: a systematic review, meta‐analysis and meta‐regression. General Hospital Psychiatry Journal 2011;33(3):217‐23. [DOI] [PubMed] [Google Scholar]


