Abstract
Background and Objective
Teriflunomide is a once-daily oral immunomodulatory agent approved in 80 countries for the treatment of patients with relapsing multiple sclerosis (RMS). The study objective was to estimate the cost effectiveness of teriflunomide (14 mg tablet, daily) versus interferon beta-1b (250 mcg subcutaneous injection, every other day) among RMS patients from the Chinese healthcare system perspective.
Methods
A Markov model with annual cycles and a lifetime horizon was utilized to assess cost-effectiveness of teriflunomide in comparison with interferon beta-1b in RMS patients. Treatment effects, including 3-month confirmed disability worsening and annualized relapse rate, were derived from a network meta-analysis. Cost inputs included costs related to treatment acquisition, administration, monitoring, natural disease management through Expanded Disability Status Scale states, relapse treatment, and adverse event management. These costs were calculated as the product between unit costs from published sources and healthcare resource utilization patterns identified in a survey conducted among 11 neurologists across different areas in China. Health effects were expressed as quality-adjusted life years (QALYs) with costs in local currency (¥) and US dollars (US$), 2018.
Results
Teriflunomide dominated interferon beta-1b and was associated with lower total costs (teriflunomide ¥1,887,144 vs interferon beta-1b ¥2,061,393) and higher QALYs (teriflunomide 9.60 QALYs vs interferon beta-1b 8.88 QALYs). In probabilistic sensitivity analysis, teriflunomide was dominant in 62.2% of model runs.
Conclusion
Teriflunomide is a cost-effective therapy over a lifetime time horizon compared to interferon beta-1b in the treatment of RMS patients in China. Results should be interpreted with caution as head-to-head comparisons are not available.
Electronic supplementary material
The online version of this article (10.1007/s40261-019-00750-3) contains supplementary material, which is available to authorized users.
Key Points
| Our modelling, comparative study generates evidence regarding the efficacy and costs of resource use burden among relapsing multiple sclerosis (RMS) patients in China treated with teriflunomide and interferon beta-1b. |
| Teriflunomide was associated with lower total cost and higher quality-adjusted life years (QALYs) compared to interferon beta-1b. |
| The results of this study can provide scientific reference for decision making on the allocation of healthcare resources for the treatment of RMS in China. |
Introduction
Multiple sclerosis (MS) is a chronic immune-mediated demyelinating disease of the central nervous system (CNS), which generally affects persons aged between 20 and 50 years [1, 2]. MS is one of the leading causes of disability in young adults in the developed world, affecting approximately 2–2.5 million people globally [3]. There are various types of MS, with relapsing multiple sclerosis (RMS) representing more than 85% of patients [4]. This subgroup is characterized by periods of remission and relapse. Relapses occur when the patient experiences symptoms due to demyelination and axonal loss of the white and grey matter of the CNS. Relapses can vary in severity and duration, lasting anywhere from hours to weeks. Relapse symptoms may include problems with bladder and bowel, vision, tremors, speech, swallowing, balance, dizziness, stiffness, and spasms.
While several regional studies have examined MS prevalence in China and have indicated increasing prevalence over time, no comprehensive national prevalence studies are available [5–7]. A study by Cheng et al. [8] reports prevalence of 1.39 cases per 100,00 people [95% confidence interval (CI) 1.16–1.66] for year 2004–2005 while Liu et al. estimates the prevalence of MS to be 3.7 (95% CI 1.65–5.8) and 6.7 (95% CI 2.7–9.56) cases per 100,000 people for males and females, respectively, using hospital data from 2013 the Shandong Province [9]. The mean age at onset of MS was 36 for males and 33.7 for females [9]. Estimates for RMS prevalence are also limited with no burden of illness estimates of MS among Chinese patients available from review of publicly available literature [10].
Chinese expert consensus on MS diagnosis and treatment [11] suggest management of MS with the use of corticosteroids, immunosuppressants, immunoglobulin, and monoclonal antibodies, as well as interferon beta-1b 250 mcg (Betaferon®), which is the only marketed disease-modifying therapy (DMT) approved in China and was added to the National Reimbursement Drug List in 2017. Recently, teriflunomide (14 mg tablet, daily) (Aubagio®) was approved as the only oral DMT indicated for the treatment of RMS in China, following global approval in more than 80 other countries.
In clinical trials, teriflunomide reduced the risk of relapse compared to placebo [12]. Long-term data over 9 years have shown that teriflunomide is well tolerated, and efficacy is maintained in a similar manner to that observed in the registrational TEMSO and TOWER Phase 3 clinical trials [12]. From an analysis of pooled safety and tolerability data from four placebo-controlled teriflunomide studies and their extensions (the Phase 2 study NCT01487096, and the Phase 3 studies TEMSO, TOWER, and TOPIC), no unexpected safety signals were detected over a treatment duration exceeding 12 years with a cumulative exposure exceeding 6800 patient-years [13].
Teriflunomide has been evaluated as cost effective compared to other therapies when studied internationally [14, 15], but cost-effectiveness evidence is limited on how this new therapy compares to other DMTs from the Chinese healthcare perspective. The objective of this study was to evaluate the cost effectiveness of teriflunomide compared to interferon beta-1b among patients in China with RMS.
Methods
A Markov cost-effectiveness model with annual periods was developed, similar to the School of Health and Related Research (SchARR) model published previously (Fig. 1) [16], incorporating RMS disability states as measured using the Kurtzke Expanded Disability Status Scale (EDSS) [17]. Transition probabilities were based on a natural history model based on the prognosis of patients receiving best supportive care (BSC). Relative treatment effects for teriflunomide and interferon beta-1b used in the model were estimated from a mixed treatment comparison used previously for teriflunomide’s technology assessment by NICE [18]. The model was from a Chinese healthcare system perspective and a lifetime horizon. Utility model inputs were obtained from the literature. Cost inputs were derived using unit costs from the literature and a healthcare utilization survey among key opinion leaders (KOLs) on the management on RMS in China. An annual discount rate of 3% was applied for costs and health outcomes. Costs were inflated to ¥2018 values as needed using the Chinese Consumer Price index of medical care.
Fig. 1.
Schematic of patient flow in the model across Expanded Disability Status Scale (EDSS) states in relapsing multiple sclerosis (RMS)
A description of the methods used to conduct the survey and a detailed description of the model structure are presented below.
Description of KOL Survey on Healthcare Utilization Model Inputs
Data on healthcare resource utilization and the costs associated with RMS in China are limited [10]. While a national claims database China Health Insurance Association (CHIRA) exists and may be available to researchers on a case-by-case basis [19, 20], our literature review did not identify studies using that database to define economic inputs in MS or RMS populations.
A total of 11 KOLs from various regions of China specializing in MS were identified to be surveyed for collection of healthcare resource utilization and costs. KOLs had to meet the following inclusion criteria to participate in the survey: (1) attending neurologist or higher level (e.g. assistant director physician, director physician), (2) have at least 10 years of experience in managing of MS patients, and (3) currently manage more than 15 MS patients a year.
The survey was developed and shared with the KOLs prior to the interview. The survey included 3 main components: (1) characteristics of RMS patients and treatment patterns, (2) resource use (i.e. drugs, lab tests, office and emergency visits, and hospitalizations) associated with RMS management during remission and relapse, by disease severity based on mild, moderate, and severe EDSS score, and (3) resource use associated with the management of adverse events (AEs). Two face-to-face interviews and 9 phone interviews were conducted across the 11 KOLs in various regions of China including the north region, middle region, south region, and west region between January and March 2018. Inputs derived from the physician questionnaire are comprehensively detailed in the Supplementary Material section.
Model Structure and Inputs
The disability states in the model are defined using EDSS, which has scores ranging from 0 (normal) to 9 (patient confined to bed and unable to communicate effectively or eat/swallow). At each model cycle, patients can stay in the same disability state, progress to a higher (worse) disability state, regress to a lower (better) disability state, or die (Fig. 1).
Patients enter the model on active treatment with a DMT and move on to BSC when they withdraw from the DMT. Treatment impacts the natural history of the disease (i.e. the natural progression of a patient who is not being treated). The effects of treatment in the model aim to delay disability worsening, reduce the frequency of relapse, and reduce relapses leading to hospitalization. Patients may also experience treatment-related AEs.
The probability of progressing to a higher/lower disability state depends on the patient’s current EDSS health state. The probability of dying in any state depends on the current EDSS state and is modelled indirectly using mortality ratios for each EDSS state multiplied by age- and gender-specific background mortality.
Costs and quality-adjusted life years (QALYs) per patient are calculated based on the time spent in each EDSS state, incidence of relapses, treatment-related AEs, the treatment received, and the time spent on treatment.
Population Characteristics
The model population represented pooled RMS patients (treatment-naïve and treatment-experienced) as obtained from the TEMSO and TOWER Phase III studies [21, 22]; at baseline, the cohort was characterized by a median age of 38 years, a female to male ratio of 2.5, and a mean EDSS of 2.6.
Natural History Data Sources for Disability Transitions and Annualized Relapses
Data on natural disease history provide information on how RMS patients progress in disease severity in the absence of treatment. To model disability worsening as a probability of being in any of the defined health states of the Markov model, we used the transition matrix of the British Columbia Multiple Sclerosis (BCMS) database, which has been identified by the Scientific advisory group of the UK MS Risk Sharing Scheme as the most appropriate natural history data in prior cost-effectiveness analyses [23, 24].
For the annualized number of relapses by EDSS level in the absence of DMT treatment, we used EDSS state-dependent natural history relapse rates sourced from the Sylvia Lawry Centre for Multiple Sclerosis Research (SLCMSR) database [25].
The model stratified relapse events by relapses leading to hospitalization or not, and assumed the proportion of relapses leading to hospitalization or not to be constant across all EDSS states. Relapses that require hospitalization incur greater costs and quality of life (QoL) losses than those that do not lead to hospitalization. Based on the Chinese KOL survey, the percentage of relapses leading to hospitalization was 82%, and 18% for those not leading to hospitalization.
Efficacy Inputs
The beneficial effects of DMTs are to delay disability progression and to reduce relapse rates. Both efficacy inputs were modelled by applying the DMT-specific relative efficacy ratios to the natural history rates of disability and annualized incident relapse rates. The results of a mixed treatment comparison were used to populate the relative treatment efficacy of teriflunomide and interferon beta-1b as estimated previously from a mixed treatment comparison used for teriflunomide’s technology assessment by NICE (Table 1) [18].
Table 1.
Comparative treatment efficacy of disease-modifying therapies for relapsing multiple sclerosis
| Treatment | Confirmed disability worsening, HR (95% CI) 3 months | Annualized relapse rate, RR (95% CI) |
|---|---|---|
| Teriflunomide 14 mg | 0.69 (0.53, 0.89) | 0.67 (0.58, 0.76) |
| Interferon beta-1b 250 mcg | 0.94 (0.65, 1.32) | 0.70 (0.60, 0.80) |
| Placebo (BSC) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) |
BSC best supportive care, CI confidence interval, HR hazard ratio, RR relative risk
In a sensitivity analysis, the model was also run using relative efficacy of teriflunomide versus placebo from Chinese subgroup of patients in the TOWER study with hazard ratio (HR) = 0.32 (0.07, 1.51) for confirmed disability worsening and risk ratio (RR) = 0.29 (0.15, 0.57) relative annualized relapse rates [26]. The treatment efficacy for interferon beta-1b was assumed to be similar to that of the pooled RMS population in Table 1, as no similar data source was found regarding interferon beta-1b efficacy in Chinese patients.
The model also assumed that treatment efficacy may diminish over time and therefore incorporated treatment waning multipliers to bring the relative efficacy parameters closer to null in the later years of the lifetime horizon. The treatment effect for teriflunomide and interferon beta-1b was assumed to be 100% for the first 2 years, after which it decreased to 75% of the original treatment effect for the third, fourth, and 5 years of treatment; thereafter, the waning effect was 50% for each year [27, 28].
Treatment Withdrawal
The model assumes that the annual probability of withdrawal for each treatment does not persist over the lifetime horizon. Patients are assumed to withdraw from teriflunomide or interferon beta-1b at a constant rate of 10% in the first 2 years of the time horizon, followed by 3% every year thereafter, similar to assumed rates in the literature [18, 29, 30]. A similar treatment withdrawal assumption for teriflunomide and interferon beta-1b was used for this analysis [31–33].
Additionally, while prior economic models have assumed that patients receiving DMT would not experience a treatment effect in more severe stages of the disease, and patients were assumed to withdraw from treatment [34], no clinical trials have directly addressed this concern and therapy continuation is recommended as long as the patient’s underlying disease is stable [35].
Mortality
The model calculated a weighted average mortality rate based indirectly on the age and sex of the modelled population, using the background all-cause mortality rates for Chinese men and women in 2015 as obtained from the World Health Organization [36], and multiplied by a mortality factor [37] corresponding to each EDSS health state [29].
Cost Inputs
Total costs per patient were calculated by summing costs related to treating relapses and disability worsening, drug acquisition costs associated with DMT treatment, and monitoring costs and management of treatment-related AEs. With the exception of drug acquisition costs, individual component costs were derived based on healthcare resource utilization patterns identified by the Chinese KOL survey for each of the three categories by EDSS severity (mild EDSS 0–3.5, moderate EDSS 4–6, severe EDSS 6.5–9.5). Unit cost in China for each resource item based on public sources was applied to calculate the cost by EDSS severity. Direct medical cost by EDSS state were derived after the use of linear interpolation techniques [38] and are shown in (Table 2). Further details are provided in Supplementary Fig. 1.
Table 2.
Annual cost inputs used in the study [by Chinese yuan (¥) and USD ($)]
| RMS management cost by EDSS score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Direct medical cost | ¥0 ($0) |
¥2019 ($304) |
¥9736 ($1465) |
¥17,453 ($2625) |
¥25,169 ($3786) |
¥32,886 ($4947) |
¥39,187 ($5895) |
¥45,015 ($6772) |
¥50,844 ($7648) |
¥56,672 ($8525) |
| Cost of managing relapse | |||
|---|---|---|---|
| Leading to hospitalization | ¥16,865 ($2537) | ||
| Not leading to hospitalization | ¥3685 ($554) | ||
| Treatment | Treatment acquisition cost | Administration cost | Monitoring costs | |
|---|---|---|---|---|
| Year 1 | Year 2+ | |||
| Teriflunomide 14 mg | ¥93,816 ($14,113) | ¥0 ($0) | ¥1603 ($241) | ¥1101 ($166) |
| Interferon beta-1b 250 mcg | ¥106,200 ($15,976) | ¥4.5 ($0.7) | ¥1501 ($226) | ¥1101 ($166) |
Average exchange rate for 2018 of US$1 = ¥6.6476 was used for conversion
In this table, unit cost in China was based on the China Health Insurance Research Association (CHIRA) database, resource use estimates were obtained from the Chinese key opinion leaders survey
Please refer to Supplemental Material for a detailed list of references
EDSS expanded disability status scale, RMS relapsing multiple sclerosis
To calculate the costs associated with relapses in the model, resource use estimates obtained from the Chinese KOLs and unit cost in China based on the China Health Insurance Research Association (CHIRA) database were used (Table 2).
The annual treatment acquisition cost of interferon beta-1b was sourced from the 2017 national reimbursed drug negotiation [39], while the annual acquisition cost for teriflunomide was based on the cost patients pay after enrolling in a patient assistant program (PAP).
Treatment monitoring costs were calculated based on resource use obtained from the Chinese package insert of teriflunomide and from the KOLs for interferon beta-1b. Unit costs associated with the monitoring resources were based on published sources in China.
All cost inputs are comprehensively detailed and referenced in the Supplementary Material section.
Utility Inputs for EDSS States and Relapse
Utility weights for each EDSS were based on a published evaluation by UK MS Risk Sharing Scheme [24]. The annual utility loss per relapse was − 0.03 for relapses leading to hospitalization and − 0.009 for relapses not leading to hospitalization, assuming a 1.5-month duration of relapse, as recommended previously [23, 40], multiplied by utility loss values for relapse from the UK MS Survey [41].
AE Inputs
The selection of AEs was based on a scientifically rigorous set of criteria without necessarily restricting the analysis to grade 3/4 AEs, as severe AEs may be rare and may not significantly contribute to cumulative costs and benefits. The selection of AEs followed a systematic approach, as used previously in RMS [29]. First, AEs listed on the package insert with at least a 5% overall incidence and/or with at least 3% higher incidence in the drug arm than in the placebo arm of the clinical trials were considered. The list of AEs was then sorted first by the magnitude of the difference between the drug and placebo arms in each comparator trial, and then sorted by the absolute AE incidence in the drug arm. The top AEs were then selected for inclusion in the model and were validated by Chinese KOLs. Resource use associated with each AE was obtained from the Chinese KOLs and unit cost for each resource item was applied to calculate the cost of managing the selected AEs. To estimate the disutility of AEs over one annual cycle, the utility loss per AE was multiplied by the duration of the AE, as sourced from the published literature (Table 3).
Table 3.
Annual incidence of AEs by treatment and associated management cost [Chinese yuan (¥) and USD ($)] and disutilities
| Treatment | Adverse events | Annual probability | Annual cost | Annual disutility |
|---|---|---|---|---|
| Teriflunomide 14 mg | Nausea | 7.4% | ¥140 ($21) | − 0.00013 |
| Neutrophil count (≥ 1.5 × 109/L and < LLN) | 13.9% | ¥463 ($70) | 0 | |
| Hair thinning | 6.7% | ¥51 ($8) | 0 | |
| Lymphocyte count (≥ 0.8 x 109/L and < LLN) | 6.8% | ¥463 ($70) | 0 | |
| ALT/AST increased | 7.8% | ¥157 ($24) | 0 | |
| Diarrhea | 7.3% | ¥155 ($23) | − 0.0003 | |
| Interferon beta-1b 250 mcg | Injection site reaction | 45.43% | ¥76 ($11) | − 0.00003 |
| Lymphocytes count decreased (< 1500/mm3) | 54.45% | ¥463 ($70) | 0 | |
| Influenza-like symptoms | 28.65% | ¥45 ($7) | − 0.01225 | |
| Fever/chills | 13.79% | ¥34 ($5) | − 0.00029 |
Average exchange rate for 2018 of US$1 = ¥6.6476 was used for conversion
The top AEs were validated by Chinese key opinion leaders and resource use associated with each AE was obtained by the same way. Please refer to Supplemental Material for a detailed list of references
AEs adverse events, ALT alanine aminotransferase, AST aspartate aminotransferase, LLN lower limit of normal
Sensitivity and Scenario Analysis
Several data parameter sensitivity analyses were conducted by setting teriflunomide disability worsening and relapse rate efficacy ratios at the upper and lower 95% CI values, varying treatment acquisition costs (± 10%), assessing the sensitivity around the cost of managing relapse (± 20%), and varying the model time horizon and treatment withdrawal rate for the comparators. Probabilistic sensitivity analysis (PSA) to assess the uncertainty around all model parameters simultaneously was also carried out.
Results
Teriflunomide dominated interferon beta-1b and was associated with lower total costs (teriflunomide ¥1,887,144 vs interferon beta-1b ¥2,061,393) and higher QALYs (teriflunomide 9.60 QALYs vs interferon beta-1b 8.88 QALYs) (Table 4). Cost savings were generated predominantly by savings in treatment acquisitions costs (− ¥136,104) and disease-related costs (− ¥36,144).
Table 4.
Base-case results [Chinese yuan (¥) and USD ($)] by treatment
| Treatment | Total costs | Total life-years | Total QALYs | Incremental costs | Incremental QALYs | ICER |
|---|---|---|---|---|---|---|
| Teriflunomide 14 mg | ¥1,887,144 ($283,884) | 20.28 | 9.60 | − ¥174,249 (− $26,212) | 0.73 | Dominant |
| Disease-relateda | ¥758,765 ($114,141) | NA | 9.60 | − ¥36,144 (− $5,437) | 0.68 | |
| Treatment-relatedb | ¥1,126,796 ($169,504) | NA | NA | − ¥136,104 (− $20,474) | NA | |
| AEs | ¥1583 ($238) | NA | − 0.00005 | − ¥2001 (− $301) | 0.04185 | |
| Interferon beta-1b | ¥2,061,393 ($310,096) | 20.11 | 8.88 | Reference | Reference | Reference |
| Disease-relateda | ¥794,909 ($119,578) | NA | 8.92 | |||
| Treatment-relatedb | ¥1,262,900 ($189,978) | NA | NA | |||
| AEs | ¥3584 ($539) | NA | − 0.0419 |
Average exchange rate for 2018 of US$1 = ¥6.6476 was used for conversion
ICER incremental cost-effectiveness ratio, Incr. incremental, NA not applicable, QALY quality-adjusted life-year
aCosts and QALYs due to disability and relapses
bTreatment acquisition cost, administration cost, and monitoring cost
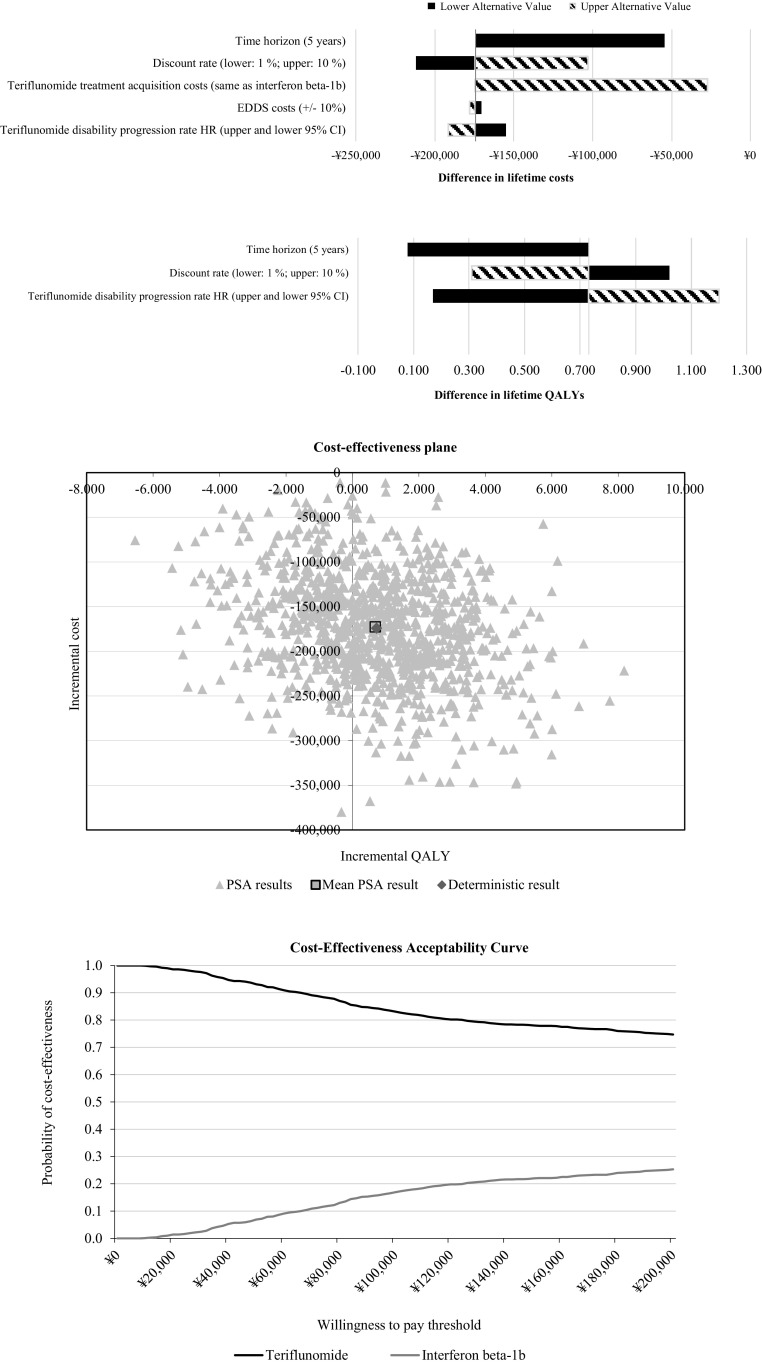
In univariate analysis, the discount rate, length of time horizon, and treatment acquisition cost had the biggest impact on cost differences. Relative treatment efficacy assumptions with respect to slowing progression had the biggest impact on differences in QALYs (Fig. 2). Furthermore, PSA indicated that the cloud of bootstrap iterations primarily occupied the lower cost/higher QALY quadrant, with the probability of teriflunomide being cost effective as 78% at ¥162,000/QALY threshold (3 times GDP per capita in China) and being dominant in 62.2% of model runs.
Fig. 2.
Tornado diagram of univariate analyses (top); probabilistic incremental cost-effectiveness ratio (ICER) estimates based on 1000 iterations for the pooled relapsing multiple sclerosis (RMS) population (middle); cost-effectiveness acceptability curve for the pooled RMS population (bottom)
It should be noted that about one-third of bootstrapped cost-effectiveness estimates ended up in the south-west quadrant of the cost-effectiveness plane (lower incremental cost and lower incremental efficacy), which may have a counterintuitive interpretation. When estimates fall in that quadrant, relinquishing one additional unit of effectiveness gain valued at high willingness to pay can be preferred rather than the savings associated with the lower cost of treatment. In those cases, interferon beta-1b will be the cost-effective therapy of choice and this possibility is reflected in the downward sloping cost-effectiveness acceptability curve with higher willingness to pay thresholds, which, however, still result in probabilities of cost effectiveness greater than 50% for teriflunomide.
In a scenario where teriflunomide was assumed to be of the same acquisition cost as interferon beta-1b, teriflunomide continued to be dominant with lower total costs ∆ = − ¥27,293 and higher QALYs ∆ = 0.73.
In the scenario sensitivity analysis incorporating efficacy data from the Chinese patient subgroup from teriflunomide’s TOWER study, teriflunomide was associated with even lower total costs (¥1,807,930), higher QALYs (10.73), and a probability of teriflunomide being cost effective of 89% at ¥162,000/QALY threshold.
Discussion
Our study provides new information on resource use and burden among RMS patients treated with teriflunomide and interferon beta-1b. Teriflunomide was a cost-effective treatment compared to interferon beta-1b. This was due largely to lower acquisition costs and lower disease-related costs.
While the prevalence of diagnosed RMS is estimated to be lower in the Asia Pacific region as compared to Western countries, it should be considered that China has a population of more than 1 billion people and even a relatively rare disease may affect a large absolute number of patients and impose a significant burden [7]. It is well documented that caring for patients with RMS poses economic, psychological, emotional, and quality-of-life burdens on patients, caregivers, and relatives [42] and leads to negative spill-over effects in society [43]. As such, treatment with effective DMT may reduce social and family burden by reducing productivity loss and indirect cost related to RMS. When the model was run from a societal perspective, incorporating costs due to short- and long-term productivity losses as well as for indirect care (inputs described in Supplementary Table 3), the benefit of using teriflunomide was even higher with lower costs (teriflunomide ¥2,731,728 vs interferon beta-1b ¥2,966,990) and higher QALYs (teriflunomide 8.99 QALYs vs interferon beta-1b 8.19 QALYs).
As is common with cost-effectiveness analyses, we recognize the following limitations and potential future improvements that must be considered when interpreting the data. While RMS transition probabilities and health preference data were used as referenced from Western and high-income countries, the data are from well-known and utilized sources. A recent systematic literature review of 23 publications from 10 countries concludes that the model structure has converged over time [44] and other international studies, such as our work, rely on the abovementioned model inputs [45–48].
Additionally, while further study is needed to verify the estimates derived from the KOL survey, it should be noted that in sensitivity analyses, the estimates of EDSS costs we used did not substantially impact study results. For clarity and transparency, in Supplementary Table 1 and 2 we have added a detailed summary of resources use during the relapse phase and by EDSS category, as provided by KOLs. For validity check, we compared the derived estimates from KOL to recently published data on MS costs in Russia [49] where costs are expected to be more similar to those in China than another high-income Western country. The Russia study reports direct costs (excluding DMT) of approximately US$1670 for mild severity patients EDDS 0–3; US$3220 for moderate severity patients EDSS 4–6.5, and US$7800 for severe patients of EDSS 7–9 [49]. These costs are comparable to those derived from the Chinese KOL survey with US$884 average for the management of mild patients, US$5237 for moderate severity patients, and US$7648 for severe patients in our study.
Second, other sources of natural history data could be considered in the model as they become available. Testing the impact of these matrices against the current model could inform decision-makers about the applicability of current data to the decision problem of interest. Use of separate network meta-analysis estimates in all different subgroups considered in the model could better inform the efficacy parameters in the model. It is recommended that a new meta-analysis is conducted for all population options in the model in order to ensure a consistent set of evidence for treatment effect.
Additionally, in the absence of published data, the inputs regarding the administration, monitoring, withdrawal, and resolution of AEs associated with each treatment were obtained from the KOL survey, which reflect the clinical practice, combining with the package inserts of these two treatments. Clinical experts have validated the numbers applied in the model, but further studies are needed to verify these estimates.
The model did not incorporate any additional utilities due to the ease of use of an oral versus injectable administration. Currently, there is no head-to-head or indirect comparison study being conducted to demonstrate the administration benefit of teriflunomide compared with interferon beta-1b. In a comparison to another injectable (interferon beta-1a subcutaneous injection), a randomized Phase 3 clinical trial demonstrated that average compliance was high and not different between teriflunomide 14 mg (98.4%) and interferon beta-1a (97.6%) [33]. Given this evidence, for this analysis we assumed that compliance was similar between teriflunomide and interferon beta-1b. It should be added, however, that patients receiving teriflunomide expressed significantly better treatment satisfaction than those treated with interferon beta-1a [33].
Due to worsening of symptoms or occurrence of AEs, patients are likely to discontinue their treatment or move to an alternative treatment regimen; however, our model may not fully represent MS management in clinical practice. One of the main limitations of this study is related to the model assumption that patients discontinuing treatment were not allowed to switch to a different treatment or reinitiate treatment at a later time. However, definitive real-world evidence on the adherence, withdrawal, combination therapy, gaps of care, as well as switching patterns among DMT agents in RMS is not yet available, and future research should incorporate that evidence when it becomes available.
Conclusion
From a Chinese healthcare system perspective, our analysis suggests that teriflunomide is a cost-effective disease-modifying therapy for RMS compared to interferon beta-1b. Results should be interpreted with caution as head-to-head comparisons between teriflunomide and interferon beta-1b are not yet available. The results of this study can provide scientific reference for decision making on the healthcare resources allocation for the treatment of RMS in China.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
This study was funded by Sanofi China. However, publication of the study results was not contingent on sponsor’s approval. All authors contributed to the development of the publication and maintained control over the final content.
Conflict of interest
Viktor Chirikov, Fen Du, Yu-Chen Yeh, and Xin Gao are employees of Pharmerit International, which received funding from Sanofi China to conduct this research. Li Liu and Ruiqi Liu are employees of Sanofi China. Yan Xu and Ningying Mao report no conflict of interest.
References
- 1.Campbell JD, Ghushchyan V, Brett McQueen R, et al. Burden of multiple sclerosis on direct, indirect costs and quality of life: national US estimates. Mult Scler Relat Disord. 2014;3:227–236. doi: 10.1016/j.msard.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Gold R, Wolinsky JS, Amato MP, Comi G. Evolving expectations around early management of multiple sclerosis. Ther Adv Neurol Disord. 2010;3:351–367. doi: 10.1177/1756285610385608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev. 2010;9:A387–A394. doi: 10.1016/j.autrev.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 4.National Multiple Sclerosis Society. Types of MS. 2012. at http://www.nationalmssociety.org/What-is-MS/Types-of-MS).
- 5.Makhani N, Morrow S, Fisk J, et al. MS incidence and prevalence in Africa, Asia, Australia and New Zealand: a systematic review. Mult Scler Relat Disord. 2014;3:48–60. doi: 10.1016/j.msard.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Eskandarieh S, Heydarpour P, Minagar A, Pourmand S, Sahraian MA. Multiple sclerosis epidemiology in east Asia, south east Asia and south Asia: a systematic review. Neuroepidemiology. 2016;46:209–221. doi: 10.1159/000444019. [DOI] [PubMed] [Google Scholar]
- 7.Cheong WL, Mohan D, Warren N, Reidpath DD. Multiple sclerosis in the Asia Pacific region: a systematic review of a neglected neurological disease. Frontiers in neurology 2018;9. [DOI] [PMC free article] [PubMed]
- 8.Cheng Q, Miao L, Zhang J, et al. A population-based survey of multiple sclerosis in Shanghai, China. Neurology. 2007;68:1495–1500. doi: 10.1212/01.wnl.0000260695.72980.b7. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Cui Y, Han J. Estimating epidemiological data of Multiple sclerosis using hospitalized data in Shandong Province, China. Orphanet J Rare Dis. 2016;11:73. doi: 10.1186/s13023-016-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu S, Donga P, Zhu G, Pan F, Wang C. The burden of multiple sclerosis in China: A literature review. Neurology Asia 2011;16.
- 11.Neuroimmunology group of neurology branch for Chinese medical association and Neuroimmunology branch of China society of immunology. China expert consensus on Multiple sclerosis diagnosis and treatment (2014)[J]. Chin J Neurol, 2015, 48(5):362-367.
- 12.O’connor P, Comi G, Freedman MS, et al. Long-term safety and efficacy of teriflunomide Nine-year follow-up of the randomized TEMSO study. Neurology. 2016;86:920–930. doi: 10.1212/WNL.0000000000002441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comi G, Freedman MS, Kappos L, et al. Pooled safety and tolerability data from four placebo-controlled teriflunomide studies and extensions. Mult Scler Relat Disord. 2016;5:97–104. doi: 10.1016/j.msard.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Hay JW, Niu X. Cost effectiveness of fingolimod, teriflunomide, dimethyl fumarate and intramuscular interferon-beta1a in relapsing-remitting multiple sclerosis. CNS Drugs. 2015;29:71–81. doi: 10.1007/s40263-014-0207-x. [DOI] [PubMed] [Google Scholar]
- 15.Soini E, Joutseno J, Sumelahti ML. Cost-utility of First-line disease-modifying treatments for relapsing-remitting multiple sclerosis. Clin Ther. 2017;39(537–57):e10. doi: 10.1016/j.clinthera.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 16.Chilcott J, McCabe C, Tappenden P, et al. Modelling the cost effectiveness of interferon beta and glatiramer acetate in the management of multiple sclerosis Commentary evaluating disease modifying treatments in multiple sclerosis. BMJ. 2003;326:522. doi: 10.1136/bmj.326.7388.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 18.Rose EB, Wheatley A, Langley G, Gerber S, Haynes A. Respiratory syncytial virus seasonality—United States, 2014–2017. Morb Mortal Wkly Rep. 2018;67:71. doi: 10.15585/mmwr.mm6702a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia L, Li J-H, Zhao K, Wu H-Y. Incidence and in-hospital mortality of acute aortic dissection in China: analysis of China Health Insurance Research (CHIRA) data 2011. JGC. 2015;12:502. doi: 10.11909/j.issn.1671-5411.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Zhang J, Du F, Montgomery W, Li H, Flynn J. Real world evidence in mainland China: experience with the use of health care claims data. Value Health. 2014;17:A728. doi: 10.1016/j.jval.2014.08.066. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365:1293–1303. doi: 10.1056/NEJMoa1014656. [DOI] [PubMed] [Google Scholar]
- 22.Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:247–256. doi: 10.1016/S1474-4422(13)70308-9. [DOI] [PubMed] [Google Scholar]
- 23.Palace J, Bregenzer T, Tremlett H, et al. UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model. BMJ Open. 2014;4:e004073. doi: 10.1136/bmjopen-2013-004073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palace J, Duddy M, Bregenzer T, et al. Effectiveness and cost-effectiveness of interferon beta and glatiramer acetate in the UK Multiple Sclerosis Risk Sharing Scheme at 6 years: a clinical cohort study with natural history comparator. Lancet Neurol. 2015;14:497–505. doi: 10.1016/S1474-4422(15)00018-6. [DOI] [PubMed] [Google Scholar]
- 25.Held U, Heigenhauser L, Shang C, Kappos L, Polman C. Sylvia Lawry Centre for MSR Predictors of relapse rate in MS clinical trials. Neurology. 2005;65:1769–1773. doi: 10.1212/01.wnl.0000187122.71735.1f. [DOI] [PubMed] [Google Scholar]
- 26.Macdonell R, Freedman M, Xu X, et al. Efficacy and safety of teriflunomide in chinese patients with relapsing forms of ms: a subgroup analysis of the phase 3 tower study. J Neurol Sci. 2017;381:135. doi: 10.1016/j.jns.2017.08.403. [DOI] [Google Scholar]
- 27.TA303: Teriflunomide for treating relapsing-remitting multiple sclerosis. 2014. at https://www.nice.org.uk/Guidance/ta303.).
- 28.TA312: Alemtuzumab for treating relapsing-remitting multiple sclerosis. 2014. at https://www.nice.org.uk/guidance/ta312?unlid=408428645201631253649.).
- 29.Mauskopf J, Fay M, Iyer R, Sarda S, Livingston T. Cost-effectiveness of delayed-release dimethyl fumarate for the treatment of relapsing forms of multiple sclerosis in the United States. J Med Econ. 2016;19:432–442. doi: 10.3111/13696998.2015.1135805. [DOI] [PubMed] [Google Scholar]
- 30.Tappenden P, McCabe C, Chilcott J, et al. Cost-Effectiveness of disease-modifying therapies in the management of multiple sclerosis for the medicare population. Value Health. 2009;12:657–665. doi: 10.1111/j.1524-4733.2008.00485.x. [DOI] [PubMed] [Google Scholar]
- 31.Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. CEOR. 2017;9:251. doi: 10.2147/CEOR.S130334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen K, Schüssel K, Kieble M, et al. Adherence to disease modifying drugs among patients with multiple sclerosis in Germany: a retrospective cohort study. PLoS One. 2015;10:e0133279. doi: 10.1371/journal.pone.0133279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vermersch P, Czlonkowska A, Grimaldi LM, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler J. 2014;20:705–716. doi: 10.1177/1352458513507821. [DOI] [PubMed] [Google Scholar]
- 34.Park P, Hall N. Disease-Modifying Therapies for Relapsing-Remitting and Primary Progressive Multiple Sclerosis: Effectiveness and Value. 2017.
- 35.Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24:96–120. doi: 10.1177/1352458517751049. [DOI] [PubMed] [Google Scholar]
- 36.Life tables by country—China. World Health Organization. http://apps.who.int/gho/data/?theme=main&vid=60340. Accessed 7 Apr 2018.
- 37.Pokorski RJ. Long-term survival experience of patients with multiple sclerosis. J Insurance Med N Y. 1997;29:101–106. [PubMed] [Google Scholar]
- 38.Tappenden P, McCabe C, Simpson E, Chilcott J, Nixon R, Madan J. The clinical effectiveness and cost-effectiveness of interferon-beta and glatiramer acetate in the management of relapsing/remitting and secondary-progressive multiple sclerosis. Sheffield: The University of Sheffield; 2006. [Google Scholar]
- 39.Notice of the Ministry of Human Resources and Social Security on the inclusion of 36 drugs in the Category B of the National Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug List. 2017. Website:http://www.mohrss.gov.cn/yiliaobxs/YILIAOBXSzhengcewenjian/201707/t20170718_274153.html. Accessed 29 Jul 2018.
- 40.Guo S, Pelligra C, Thibault CS, Hernandez L, Kansal A. Cost-effectiveness analyses in multiple sclerosis a review of modelling approaches. Pharmacol Econ. 2014;32:559–572. doi: 10.1007/s40273-014-0150-1. [DOI] [PubMed] [Google Scholar]
- 41.Gani R, Giovannoni G, Bates D, Kemball B, Hughes S, Kerrigan J. Cost-effectiveness analyses of natalizumab (Tysabri) compared with other disease-modifying therapies for people with highly active relapsing-remitting multiple sclerosis in the UK. Pharmacoeconomics. 2008;26:617–627. doi: 10.2165/00019053-200826070-00008. [DOI] [PubMed] [Google Scholar]
- 42.Oleen-Burkey M, Castelli-Haley J, Lage MJ, Johnson KP. Burden of a multiple sclerosis relapse. Patient-Patient-Centered Outcomes Res. 2012;5:57–69. doi: 10.2165/11592160-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 43.Lavelle TA, Wittenberg E, Lamarand K, Prosser LA. Variation in the spillover effects of illness on parents, spouses, and children of the chronically ill. Appl Health Econ Health Policy. 2014;12:117–124. doi: 10.1007/s40258-014-0079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernandez L, O’Donnell M, Postma M. Modeling approaches in cost-effectiveness analysis of disease-modifying therapies for relapsing–remitting multiple sclerosis: an updated systematic review and recommendations for future economic evaluations. Pharmacol Econ. 2018;5:1–30. doi: 10.1007/s40273-018-0683-9. [DOI] [PubMed] [Google Scholar]
- 45.Ordóñez J, Serafini P, Machado M. Cost-utility analysis of dimethyl fumarate versus fingolimod and teriflunomide in patients with relapsing-remitting multiple sclerosis in Colombia. Value Health. 2016;19:A434. doi: 10.1016/j.jval.2016.09.506. [DOI] [Google Scholar]
- 46.Taheri S, Yousefi N, Sahraian M, Mehralian G. Cost-effectiveness analysis of alemtuzumab in comparison with natalizumab, intramuscular interferon beta-1a, subcutaneous interferon beta-1b, and fingolimod for the treatment of relapsing-remitting multiple sclerosis in Iran. Value in Health. 2017;20:A723. doi: 10.1016/j.jval.2017.08.1946. [DOI] [Google Scholar]
- 47.Lee J, Ko S. The cost effectiveness of fingolimod for the treatment multiple sclerosis in Korea. Value Health. 2017;20:A724. doi: 10.1016/j.jval.2017.08.1951. [DOI] [Google Scholar]
- 48.Alskaabi MF. Cost-effectiveness of oral agents in relapsing-remitting multiple sclerosis compared to interferon-based therapy in Saudi Arabia: University of Toledo; 2017. [DOI] [PMC free article] [PubMed]
- 49.Boyko A, Kobelt G, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe: results for Russia. Mult Scler J. 2017;23:155–165. doi: 10.1177/1352458517708668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.