Abstract
Introduction
We reviewed the literature for preoperative computed tomography carotid angiography and/or carotid duplex to determine their respective sensitivity and specificity in assessing the degree of carotid stenosis. We aimed to identify whether one imaging modality can accurately identify critical stenosis in patients presenting with transient ischaemic attack or symptoms of a cerebrovascular accident requiring carotid endarterectomy.
Methods
Systematic search of MEDLINE, Embase, Cochrane database of systematic reviews, all Evidence-Based Medicine Reviews (Cochrane Database of Systematic Reviews, ACP Journal club, Database of Abstracts of Reviews of Effects, Cochrane Clinical Answers, Cochrane Controlled Trials Register, Cochrane Methodology Register, Health Technology Assessment and NHS Economic Evaluation Database) for primary studies relating to computed tomography carotid angiography (CTA) and/or carotid duplex ultrasound (CDU). Studies included were published between 1990 and 2018 and focused on practice in the UK, Europe and North America.
Results
The sensitivity and specificity of CTA and CDU are comparable. CDU is safe and readily available in the clinical environment hence its use in the initial preoperative assessment of carotid stenosis. CDU is an adequate imaging modality for determining stenosis greater than 70%; sensitivity and specificity are improved when the criteria for determining greater than 70% stenosis are adjusted. Vascular laboratories opting to use duplex as their sole imaging modality should assess the sensitivity and specificity of their own duplex procedure before altering practice to preoperative single imaging for patients.
Conclusions
The sensitivity and specificity of CTA (90.6% and 93%, respectively) and CDU (92.3% and 89%, respectively) are comparable. Both are dependent on criteria used in vascular laboratories. CDU sensitivity and specificity was improved to 98.7% and 94.1%, respectively, where peak systolic velocity and end diastolic velocity were assessed. Either modality can be used to determine greater than 70% stenosis, although a secondary imaging modality may be required for cases of greater than 50% stenosis.
Keywords: Ultrasonography, doppler, duplex; computed tomography angiography; endarterectomy, carotid; sensitivity and specificity
Introduction
Carotid endarterectomy, according to the North American Symptomatic Carotid Endarterectomy Trial, (NASCET) benefits patients presenting with transient ischaemic attack or stroke found to have 70–99% stenosis of the internal carotid artery (ICA).1 The European Carotid Surgery Trial (ECST) found that the risk of major stroke increased dependent on the degree of stenosis and patients with ECST stenosis greater than 80% benefited more from having surgery to decrease the long term risk of stroke.2 NASCET and ECST criteria are not equivalent, and it is suggested that an 82% ECST stenosis corresponds to a 70% NASCET stenosis.3 The relationship between NASCET and ECST criteria was defined based on regression analysis as:3
![]()
The criteria are summarised in the following equations:3,4
Current clinical practice for patients presenting with symptoms of transient ischaemic attack or stroke suggestive of carotid artery stenosis is that they undergo carotid duplex ultrasound (CDU) initially, followed by computed tomography carotid angiography (CTA) to identify the extent of stenosis, if present. The main objective of this review was to identify whether duplex ultrasound and/or CTA should be used preoperatively to screen patients for the extent and character of their carotid artery stenosis, before undergoing carotid endarterectomy, where required.
CDU is a cost effective, non-invasive and safe imaging modality that provides expedited imaging of the carotid arteries (carotid bifurcation and ICA).5 CDU combines ultrasound technology with doppler, allowing the operator to assess the calibre of the carotid arteries in addition to blood flow and velocities.6 NASCET and/or ECST criteria can be used for the determination of carotid stenosis however in clinical practice vascular laboratories base the degree of stenosis on a combination of peak systolic velocity (PSV), end diastolic velocity (EDV) or ratios of the two.5
CTA uses ionising radiation and administration of contrast to the patient, creating one-dimensional images which are subsequently reformed into a two-dimensional digital image of the intra- and extracranial parenchyma and vasculature.5,6 These images can either be helical/spiral CTA or use new multidetector computed tomography, which provide lower radiation doses and require less contrast media to be given to patients.5 The recreation of images can be further enhanced via computer software allowing for presentation via multiplanar reconstruction, volume-rendering or other means.6
The gold standard imaging modality used for determining degree of carotid stenosis is intra-arterial angiography or digital subtraction angiography (DSA);5 however, the incidence of angiography-related stroke led to the use of non-invasive imaging modalities discussed here.7
Methods
To conduct this review, literature was critically appraised using PRISMA guidelines and focused on the use of preoperative CTA and/or CDU to assess the extent of carotid artery stenosis. The following databases were searched: MEDLINE, Embase, Cochrane Database of Systematic Reviews and all Evidence-Based Medicine Reviews (encompassing Cochrane Database of Systematic Reviews, ACP Journal club, Database of Abstracts of Reviews of Effects, Cochrane Clinical Answers, Cochrane Controlled Trials Register, Cochrane Methodology Register, Health Technology Assessment and NHS Economic Evaluation Database) using the Ovid platform. Limitations were placed on the search to identify only English literature. The timeline of interest was set from 1990 to May 2018. Only studies published in this time were considered. Two searches were conducted using Ovid, and 309 papers were identified. A manual search through titles and abstracts identified papers for further detailed review.
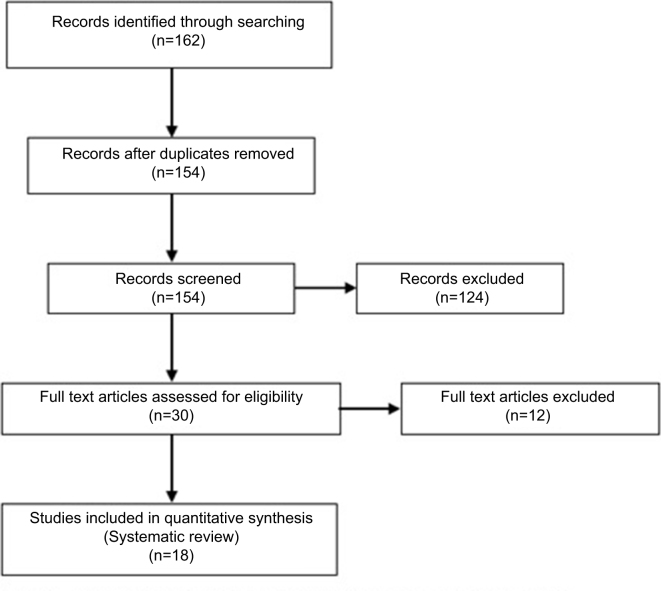
Search terms used to identify studies which assessed the accuracy of CTA were: ‘tomography’, ‘x-ray’ ‘computed AND sensitivity and specificity AND carotid stenosis’. This search identified a total of 177 results. When the search was limited to the English language, a total of 162 papers were retained, of which 8 were excluded as duplicates (Fig 1).
Figure 1.
Studies assessing the sensitivity and specificity of computed tomography carotid angiography
Search terms used for the assessment of the accuracy of CDU were: carotid stenosis AND ultrasonography, doppler, duplex AND sensitivity and specificity. This search identified a total of 158 results. When the search was limited to the English language, a total of 147 papers were retained, although 7 were duplicates so only 140 were reviewed further (Fig 2).
Figure 2.
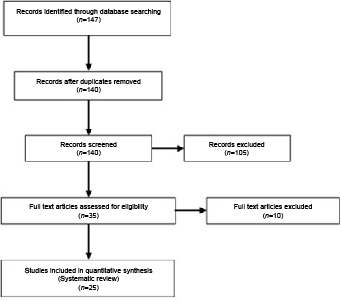
Studies assessing the sensitivity and specificity of carotid duplex ultrasound
The identified papers were assessed individually for their relevance to the systematic review. Exclusion criteria used to eliminate further studies during the review were:
studies conducted outside of the area of interest (North America, Europe and the UK)
studies where the full-text article was unavailable to the reviewer
studies not including both sensitivity and specificity data
review articles or meta-analyses.
Results
The database search produced a total of 43 primary studies.
Computed tomography carotid angiography
The data were separated based on the use of NASCET and ECST criteria to determine the degree of stenosis, as shown in Tables 1 and 2. Weighted means for all the studies using NASCET criteria was then calculated (Table 3). The overall sensitivity and specificity of a greater than 70% NASCET stenosis was 90.6% and 93%, respectively, from a total of nine studies, which assessed a total of 1245 carotid arteries, compared with DSA.
Table 1.
Sensitivity and specificity of computed tomography carotid angiography based on North American Symptomatic Carotid Endarterectomy Trial criteria
| Stenosis (%) | CT type | Carotid arteries (n) | Sensitivity (%) | Specificity (%) | Reference |
| > 70 | Helical/spiral | 56 | 100 | 100 | Link et al12 |
| Dual source | 80 | 80 | 91 | Biermann et al13 | |
| Dual source | 80 | 100 | 84 | ||
| Axial | 44 | 67 | 96 | Leclerc et al14 | |
| Axial | 236 | 98.3 | 100 | Moll et al15 | |
| Helical/spiral | 44 | 100 | 100 | Randoux et al16 | |
| Helical/spiral | 59 | 65 | 100 | Patel et al17 | |
| Multi-detector | 129 | 90.9 | 54.9 | Bucek et al18 | |
| Multi-detector | 268 | 88.2 | 92.4 | Bartlett et al19 | |
| Helical/spiral | 73 | 75 | 96 | Silvennoin et al20 | |
| Axial | 45 | 90 | 87 | Howard et al21 | |
| Multi-detector | 336 | 95 | 99 | Anzidei et al22 | |
| Not known | 180 | 78 | 93 | Marquering et al23 | |
| Dual source | 80 | 80 | 100 | Tsiflikas et al24 | |
| Dual source | 80 | 91 | 83 | ||
| Axial | 48 | 81.8 | 100 | Gupta et al25 | |
| > 60 | Axial | 96 | 87 | 90 | Magarelli et al26 |
| Occlusion | Axial | 236 | 100 | 100 | Moll et al15 |
| > 50 | Multi-detector | 129 | 95.8 | 59.6 | Bucek et al18 |
| Multi-detector | 268 | 75 | 93.8 | Bartlett et al19 | |
| Helical/spiral | 73 | 88 | 82 | Silvennoin et al20 | |
| Not known | 180 | 75 | 98 | Marquering et al23 | |
| Axial | 48 | 91.7 | 72.7 | Gupta et al27 | |
| Axial | 72 | 85 | 83 | ||
| < 50 | Multi-detector | 268 | 95.2 | 93.2 | Bartlett et al19 |
CT, computed tomography.
Table 2.
Sensitivity and Specificity of computed tomography carotid angiography based on European Carotid Surgery Trial criteria
| Stenosis (%) | CT type | Carotid arteries (n) | Sensitivity (%) | Specificity (%) | Reference |
| > 70 | Multi-detector | 129 | 94.7 | 46.3 | Bucek et al18 |
| > 50 | Multi-detector | 129 | 96.4 | 42.5 | Bucek et al18 |
CT, computed tomography.
Table 3.
Weighted mean analysis of computed tomography carotid angiography sensitivity and specificity results
| Stenosis (%) CTA NASCET | Weighted mean sensitivity (%) | Weighted mean specificity (%) | Studies (n) | Carotids (n) |
| > 70 | 88.9 | 92.4 | 17 | 1882 |
| > 70a | 90.6 | 93 | 9 | 1245 |
| > 50 | 81.7 | 85.6 | 6 | 770 |
aGold standard.
CTA, computed tomography carotid angiography; NASCET, North American Symptomatic Carotid Endarterectomy Trial.
Carotid duplex ultrasound
The sensitivity and specificity of the different methods for determining carotid stenosis via CDU were collated (Table 4) and, based on these data, a weighted mean was calculated (Table 5). One study looking at power doppler was excluded, from the analysis as it was the only study using this method and the sample size was too small for inclusion independently.8
Table 4.
Sensitivity and specificity of duplex ultrasound
| Degree of stenosis (%) | Method of measurement | Carotid arteries (n) | Sensitivity (%) | Specificity (%) | Reference |
| > 80 | PSV + EDV | 67 | 85 | 71 | Patel et al17 |
| PSV | 2186 | 87 | 90 | Shaalan et al28 | |
| EDV | 2186 | 84 | 91 | ||
| > 70 | PSV | 120 | 96 | 86 | Neale et al29 |
| EDV | 120 | 91 | 93 | ||
| PSV + EDV | 120 | 96 | 91 | ||
| Not known | 137 | 93 | 92 | Young et al30 | |
| PSV | 156 | 79 | 86 | Patel et al31 | |
| Adjusted PSV | 156 | 94 | 83 | ||
| PSV | 56 | 87 | 98 | Link et al12 | |
| PSV | 174 | 93 | 86 | Alexandrov et al32 | |
| PSV | 154 | 96.2 | 67.4 | Curley et al33 | |
| PSV | 154 | 37 | 96 | ||
| PSV + EDV | 92 | 79 | 96.3 | Belsky et al34 | |
| PSV | 236 | 87.7 | 99.2 | Moll et al15 | |
| ECST – PSV | 134 | 88 | 86 | Jogestrand et al35 | |
| PSV | 313 | 87.5 | 75.7 | Nederkoorn et al36 | |
| PSV | 65 | 94 | 86 | Knudsen et al37 | |
| PSV | 326 | 81.5 | 95.6 | Konstantinos et al38 | |
| NASCET – PSV | 158 | 88 | 67 | Staikov et al39 | |
| NASCET – EDV | 158 | 88 | 63 | ||
| ECST –PSV | 158 | 97 | 65 | ||
| ECST – EDV | 158 | 96 | 65 | ||
| PSV | 71 | 92.9 | 81.9 | Borisch et al40 | |
| PSV + EDV | 978 | 98.8 | 95.5 | Leonardo et al41 | |
| NASCET – PSV | 52 | 86.4 | 100 | Clevert et al8 | |
| PSV | 68 | 100 | 78 | Korteweg et al42 | |
| PSV + EDV | 147 | 100 | 87.1 | Shakhnovich et al43 | |
| PSV | 397 | 99 | 86 | AbuRahma et al10 | |
| NASCET – PSV | 45 | 94 | 67 | Howard et al21 | |
| NASCET – PSV | 336 | 67 | 87 | Anzidei et al22 | |
| > 50 | PSV | 18 | 95 | 50 | Boyle et al44 |
| Not known | 328 | 90 | 76 | Srinivasan et al45 | |
| PSV | 326 | 88 | 97.2 | Konstantinos et al38 | |
| PSV | 2186 | 82 | 88 | Shaalan et al28 | |
| PSV + EDV | 147 | 100 | 87.8 | Shakhnovich et al43 | |
| PSV | 397 | 93 | 68 | AbuRahma et al10 | |
| PSV | 65 | 80 | 100 | Preiss et al46 | |
| EDV | 65 | 100 | 71 | ||
| PSV + EDV | 65 | 80 | 100 | ||
| >60% | PSV | 210 | 96 | 86 | Carpenter et al47 |
| EDV | 210 | 97 | 52 | ||
| PSV + EDV | 210 | 100 | 100 |
ECST, European Carotid Surgery Trial; EDV, end diastolic velocity; NASCET, North American Symptomatic Carotid Endarterectomy Trial; PSV, peak systolic velocity.
Table 5.
Weighted mean analysis of duplex ultrasound
| Criteria | Weighted mean | Studies (n) | Carotids (n) | |
| Sensitivity (%) | Specificity (%) | |||
| Duplex – PSV > 50% stenosis | 85.1 | 86.4 | 8 | 3271 |
| Duplex – PSV >70% stenosis | 85.8 | 84.5 | 20 | 3329 |
| Duplex – PSV >70% stenosis (Consensus ONLY) | 92.4 | 87.9 | 5 | 902 |
| Duplex – PSV > 70% stenosis (narrowed consensus ONLY) | 82.8 | 87 | 8 | 1366 |
| Duplex – EDV > 70% stenosis | 91.7 | 72 | 3 | 436 |
| Duplex – EDV + PSV > 70% stenosis | 97.3 | 94.2 | 4 | 1337 |
| Duplex – > 60% stenosis | 97.7 | 79.3 | 3 | 630 |
| Duplex – >80% stenosis | 85.5 | 90.2 | 3 | 4439 |
EDV, end diastolic velocity; PSV, peak systolic velocity.
Discussion
Overall, our review established a sensitivity and specificity of CTA compared with CDU for identifying greater than 70% stenosis as 90.6% and 93%, and 92.3% and 89% respectively (Table 6), compared with DSA This suggests that CTA and CDU are comparable in their ability to identify patients with greater than 70% stenosis and exclude patients with less than 70% stenosis who may not benefit from carotid endarterectomy. The sensitivity and specificity of CDU improved in three studies when the criteria for reporting the degree of stenosis was altered. When CDU criteria included both EDV and PSV to identify stenosis greater than 70%, the sensitivity and specificity increased to 98.7% and 94.1%, respectively, greater than that found with CTA.
Table 6.
Comparison of computed tomography carotid angiography compared with duplex ultrasound – sensitivity and specificity
| Criteria | Weighted mean (%) | Studies (n) | Carotids (n) | |
| Sensitivity | Specificity | |||
| CTA – NASCET > 70% stenosis | 88.9 | 92.4 | 17 | 1882 |
| CTA – NASCET > 70% stenosisa | 90.6 | 93 | 9 | 1245 |
| CTA – NASCET > 50% stenosis | 81.7 | 85.6 | 6 | 770 |
| Duplex – PSV > 50% stenosis | 85.1 | 86.4 | 8 | 3271 |
| Duplex – PSV > 70% stenosis | 85.8 | 84.5 | 20 | 3329 |
| Duplex – PSV > 70% stenosisb | 86.5 | 87.8 | 12 | 2223 |
| Duplex – PSV > 70% stenosisa,c | 92.3 | 89 | 4 | 857 |
| Duplex – EDV > 70% stenosis | 91.7 | 72 | 3 | 436 |
| Duplex – EDV + PSV > 70% stenosis | 97.3 | 94.2 | 4 | 1337 |
| Duplex – EDV + PSV > 70% stenosisa | 98.7 | 94.1 | 3 | 1245 |
a Gold standard.
b Non-consensus and consensus.
c Consensus only.
EDV, end diastolic velocity; NASCET, North American Symptomatic Carotid Endarterectomy Trial; PSV, peak systolic velocity.
After identifying CDU sensitivities and specificities, we further categorised the data as consensus compliant and non-compliant. Focusing on those studies that adhered to the consensus PSV criteria (greater than 230 cm/s) a weighted mean analysis identified a sensitivity and specificity of 92.3% and 89%, respectively. Inclusion of studies using narrowed consensus criteria (range from greater than 250 cm/s to 285 cm/s) decreased the sensitivity and specificity to 86.5% and 87.8%, respectively. All studies analysed used DSA as the gold standard comparator.
The sensitivity and specificity of both imaging modalities decreased when the degree of stenosis was greater than 50%; the results were 81.7% and 85.6% for CTA respectively, and 85.1% and 86.4% for CDU, respectively. This decrease could prove problematic where vascular centres begin to consider patients with a greater than 50% stenosis, as per 2018 European Society for Vascular Surgery (ESVS) guidelines.7 Where stenosis is measured as less than 70%, a second imaging modality may be used to ensure the accuracy of the first investigation. In this situation, another CDU scan may be ordered or an alternative non-invasive imaging modality such as CTA or magnetic resonance angiography may be used to determine whether the extent of the stenosis is less than that required for surgical intervention (greater than 70%).7
CDU is recognised for its ability to identify plaque morphology more easily than CTA.5 However, this ability is hindered where the plaque is significantly calcified, there is operator inexperience or a lack of strict reporting criteria.6 It is suggested that CTA may be better suited for analysing heavily calcified plaques, in addition to providing a more objective and reproducible assessment of plaque morphology than CDU, as images can be reviewed.5 CTA is limited by factors such as the use of ionising radiation and intravenous iodinated contrast media, which are contraindicated for certain patients, such as those with renal dysfunction or those taking metformin. Iodinated contrast agents carry the rare risk of reactions in patients (1/3000). Patients may suffer from circulatory collapse, arrhythmias or bronchospasm, in addition to the risk of nephropathy as a direct result of contrast administration.6 Thus, the use of CTA for imaging of the carotid arteries is not without its risks and limitations compared with CDU.
Standardisation of CDU reporting criteria was not addressed until 2002 when a panel of experts published standard criteria to determine the degree of carotid stenosis.9 The consensus report criteria suggestive of 70–99% stenosis in the ICA are as follows:9
PSV >230 cm/s
EDV >100 cm/s
ICA/common carotid artery (CCA) PSV ratio > 4.0
PSV of > 125–230 cm/s is consistent with > 50–69% stenosis.
Although these criteria were introduced over 10 years ago, they have not necessarily been followed and we can see from our data that different vascular laboratories have developed their own criteria.10 Reporting of CDU is heterogeneous, which the analysis in this paper considered, therefore the weighted mean is categorised based on the conformance to the consensus criteria. Operator variability is one of the biggest weaknesses of CDU as an imaging modality, as a range of factors can affect the result, such as the angle of probe approach, or even the gain set on the scanner.6,8
UK Health Technology Assessment determined that CDU should remain the preferred imaging modality for identifying patients with 70–99% stenosis, primarily due to the cost effectiveness and the sensitivity of the test in detecting stenosis greater than 70%.11 However, the decreased accuracy in diagnosing stenosis of less than 70% is a cause for concern and a criticism acknowledged by the report,11 a point that our data supports.
Our analysis suggests that the imaging modality most able to adapt its criteria to improve the sensitivity and specificity is CDU. On this basis, our findings correlate with the recommendations of the UK working group4 and ESVS guidelines.7 They suggest the following criteria to determine stenosis greater than 70%:
ICA PSV > 230 cm/s
ICA EDV > 100 cm/s
Ratio of ICA PSV in centimetres/second to CCA PSV – (ICAPSV : CCAPSV) > 4.0
Operators must record the PSV and EDV in both the ICA and CCA, used to calculate the above ratios.
Our data suggest that a combination of the PSV and EDV consensus criteria should be used for CDU assessment of stenosis to sufficiently provide diagnostic confidence.4 It is therefore suggested that, where vascular centres use both CDU and CTA to assess patients, only CDU may be used provided criteria are audited to ensure its accuracy in determining stenosis greater than 70%.
Limitations
The search method for this study is stated clearly, allowing the reader to recreate the search carried out to identify the papers included and excluded from this review. We have indexed the details of each study collected and the results of these studies as reported by the authors; the data are collated where possible to determine the overall sensitivity and specificities of the two imaging modalities focused on as part of the review.
This study did however have its limitations. The literature search identified studies to May 2018 and it therefore may fail to include studies published after this date, as pending abstracts were not part of the database search. Reliance was placed on the original paper’s authors reporting of their results and their methodology, and thus the original raw data could not be accessed to assess the quality of statistical calculation and the data extraction methods. The weighted means were restricted to studies which reported the sensitivity and specificity data, and thus excluded other studies which failed to report this data.
We sought to diminish publication bias where possible by including all the data in the original article rather than selecting the optimal criteria and data points set out by the author.
References
- 1.Barnett HJM, Taylor DW et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991; (7): 445–453. [DOI] [PubMed] [Google Scholar]
- 2.Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; (9113): 1,379–1,387. [PubMed] [Google Scholar]
- 3.Rothwell PM, Gibson RJ, Slattery J et al. Equivalence of measurements of carotid stenosis: a comparison of three methods on 1001 angiograms. European Carotid Surgery Trialists’ Collaborative Group. Stroke 1994; (12): 2,435–2,439. [DOI] [PubMed] [Google Scholar]
- 4.Oates CP, Naylor AR, Hartshorne T et al. Joint Recommendations for Reporting Carotid Ultrasound Investigations in the United Kingdom. Eur J Vasc Endovasc Surg 2009; (3): 251–261. [DOI] [PubMed] [Google Scholar]
- 5.Maldonado TS. What are current preprocedure imaging requirements for carotid artery stenting and carotid endarterectomy: have magnetic resonance angiography and computed tomographic angiography made a difference? Semin Vasc Surg 2007; (4): 205–215. [DOI] [PubMed] [Google Scholar]
- 6.Weale AR, Urriza-Rodriguez D. Imaging in vascular disease. Surgery 2015; (7): 308–314. [Google Scholar]
- 7.Naylor AR, Ricco JB, de Borst GJ et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018; (1): 3–81. [DOI] [PubMed] [Google Scholar]
- 8.Clevert DA, Johnson T, Michaely H et al. High-grade stenoses of the internal carotid artery: Comparison of high-resolution contrast enhanced 3D MRA, duplex sonography and power Doppler imaging. Eur J Radiol 2006; (3): 379–386. [DOI] [PubMed] [Google Scholar]
- 9.Grant EG, Benson CB, Moneta GL et al. Carotid artery stenosis: grayscale and Doppler ultrasound diagnosis: Society of Radiologists in Ultrasound consensus conference. Ultrasound Q 2003; (4): 190–198. [DOI] [PubMed] [Google Scholar]
- 10.AbuRahma AF, Srivastava M, Stone PA et al. Critical appraisal of the carotid duplex consensus criteria in the diagnosis of carotid artery stenosis. J Vasc Surg 2011; (1): 53–60. [DOI] [PubMed] [Google Scholar]
- 11.Wardlaw J, Chappell F, Stevenson M et al. Accurate, practical and cost-effective assessment of carotid stenosis in the UK. Health Technol Assess 2006; (30): iii-iv, ix-x, 1–182. [DOI] [PubMed] [Google Scholar]
- 12.Link J, Brossmann J, Penselin V et al. Common carotid artery bifurcation: preliminary results of CT angiography and color-coded duplex sonography compared with digital subtraction angiography. AJR Am J Roentgenol 1997; (2): 361–365. [DOI] [PubMed] [Google Scholar]
- 13.Biermann C, Tsiflikas I, Thomas C et al. Evaluation of computer-assisted quantification of carotid artery stenosis. J Digit Imaging 2011; (2): 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leclerc X, Godefroy O, Lucas C et al. Internal carotid arterial stenosis: CT angiography with volume rendering. Radiology 1999; (3): 673–682. [DOI] [PubMed] [Google Scholar]
- 15.Moll R, Dinkel HP. Value of the CT angiography in the diagnosis of common carotid artery bifurcation disease: CT angiography versus digital subtraction angiography and color flow Doppler. Eur J Radiol 2001; (3): 155–162. [DOI] [PubMed] [Google Scholar]
- 16.Randoux B, Marro B, Koskas F et al. Carotid artery stenosis: prospective comparison of CT, three-dimensional gadolinium-enhanced MR, and conventional angiography. Radiology 2001; (1): 179–185. [DOI] [PubMed] [Google Scholar]
- 17.Patel SG. Outcome, observer reliability, and patient preferences if CTA, MRA, or Doppler ultrasound were used, individually or together, instead of digital subtraction angiography before carotid endarterectomy. J Neurol Neurosurg Psychiatry 2002; (1): 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucek RA, Puchner S, Haumer M et al. Grading of internal carotid artery stenosis: can CTA overcome the confusion? J Endovasc Ther 2006; (4): 443–450. [DOI] [PubMed] [Google Scholar]
- 19.Bartlett ES, Walters TD, Symons SP, Fox AJ. Quantification of carotid stenosis on CT angiography. Am J Neuroradiol 2006; (1): 13–19. [PMC free article] [PubMed] [Google Scholar]
- 20.Silvennoinen HM, Ikonen S, Soinne L et al. CT angiographic analysis of carotid artery stenosis: comparison of manual assessment, semiautomatic vessel analysis, and digital subtraction angiography. Am J Neuroradiol 2007; (1): 97–103. [PMC free article] [PubMed] [Google Scholar]
- 21.Howard P, Bartlett ES, Symons SP et al. Measurement of carotid stenosis on computed tomographic angiography: reliability depends on postprocessing technique. Can Assoc Radiol J 2010; (3): 127–132. [DOI] [PubMed] [Google Scholar]
- 22.Anzidei M, Napoli A, Zaccagna F et al. Diagnostic accuracy of colour Doppler ultrasonography, CT angiography and blood-pool-enhanced MR angiography in assessing carotid stenosis: a comparative study with DSA in 170 patients. Radiol Med 2012; (1): 54–71. [DOI] [PubMed] [Google Scholar]
- 23.Marquering HA, Nederkoorn PJ, Smagge L et al. Performance of semiautomatic assessment of carotid artery stenosis on CT angiography: clarification of differences with manual assessment. AJNR Am J Neuroradiol 2012; (4): 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsiflikas I, Biermann C, Thomas C et al. Carotid artery stenosis: performance of advanced vessel analysis software in evaluating CTA. Eur J Radiol 2012; (9): 2,255–2,259. [DOI] [PubMed] [Google Scholar]
- 25.Gupta A, Baradaran H, Mtui EE et al. Detection of symptomatic carotid plaque using source data from MR and CT angiography: a correlative study. Cerebrovasc Dis 2015; (3–4): 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magarelli N, Scarabino T, Simeone AL et al. Carotid stenosis: a comparison between MR and spiral CT angiography. Neuroradiology 1998; (6): 367–373. [DOI] [PubMed] [Google Scholar]
- 27.Gupta A, Mtui EE, Baradaran H et al. CT angiographic features of symptom-producing plaque in moderate-grade carotid artery stenosis. AJNR Am J Neuroradiol 2015; (2): 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaalan WE, Wahlgren CM, Desai T et al. Reappraisal of velocity criteria for carotid bulb/internal carotid artery stenosis utilizing high-resolution B-mode ultrasound validated with computed tomography angiography. J Vasc Surg 2008; (1): 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neale ML, Chambers JL, Kelly AT et al. Reappraisal of duplex criteria to assess significant carotid stenosis with special reference to reports from the North American Symptomatic Carotid Endarterectomy Trial and the European Carotid Surgery Trial. J Vasc Surg 1994; (4): 642–649. [DOI] [PubMed] [Google Scholar]
- 30.Young GR, Humphrey PR, Shaw MD et al. Comparison of magnetic resonance angiography, duplex ultrasound, and digital subtraction angiography in assessment of extracranial internal carotid artery stenosis. J Neurol Neurosurg Psychiatry 1994; (12): 1,466–1,478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel MR, Kuntz KM, Klufas RA et al. Preoperative assessment of the carotid bifurcation. Can magnetic resonance angiography and duplex ultrasonography replace contrast arteriography? Stroke 1995; (10): 1,753–1,758. [DOI] [PubMed] [Google Scholar]
- 32.Alexandrov AV, Vital D, Brodie DS et al. Grading carotid stenosis with ultrasound : an interlaboratory comparison. Stroke 1997; (6): 1,208–1,210. [DOI] [PubMed] [Google Scholar]
- 33.Curley PJ, Norrie L, Nicholson A et al. Accuracy of carotid duplex is laboratory specific and must be determined by internal audit. Eur J Vasc Endovasc Surg 1998; (6): 511–514. [DOI] [PubMed] [Google Scholar]
- 34.Belsky M, Gaitini D, Goldsher D et al. Color-coded duplex ultrasound compared to CT angiography for detection and quantification of carotid artery stenosis. Eur J Ultrasound 2000; (1): 49–60. [DOI] [PubMed] [Google Scholar]
- 35.Jogestrand T, Lindqvist M, Nowak J. diagnostic performance of duplex ultrasonography in the detection of high grade internal carotid artery stenosis. Eur J Vasc Endovasc Surg 2002; (6): 510–518. [DOI] [PubMed] [Google Scholar]
- 36.Nederkoorn PJ, Mali WPTM, Eikelboom BC et al. Preoperative diagnosis of carotid artery stenosis: accuracy of noninvasive testing. Stroke 2002; (8): 2,003–2,008. [DOI] [PubMed] [Google Scholar]
- 37.Knudsen L, Johansen A, Justesen P, Jørgensen HB. Accuracy of duplex scan of internal carotid arteries. Eur J Vasc Endovasc Surg 2002; (1): 86–87. [DOI] [PubMed] [Google Scholar]
- 38.Filis KA, Arko FR, Johnson BL et al. Duplex Ultrasound Criteria for Defining the Severity of Carotid Stenosis. Ann Vasc Surg 2002; (4): 413–421. [DOI] [PubMed] [Google Scholar]
- 39.Staikov IN, Nedeltchev K, Arnold M et al. Duplex sonographic criteria for measuring carotid stenoses. J Clin Ultrasound 2002; (5): 275–281. [DOI] [PubMed] [Google Scholar]
- 40.Borisch I, Horn M, Butz B et al. Preoperative evaluation of carotid artery stenosis: comparison of contrast-enhanced MR angiography and duplex sonography with digital subtraction angiography. AJNR Am J Neuroradiol 2003; (6): 1,117–1,122. [PMC free article] [PubMed] [Google Scholar]
- 41.Leonardo G, Crescenzi B, Cotrufo R et al. Improvement in accuracy of diagnosis of carotid artery stenosis with duplex ultrasound scanning with combined use of linear array 7.5 MHz and convex array 3.5 MHz probes: validation versus 489 arteriographic procedures. J Vasc Surg 2003; (6): 1,240–1,247. [DOI] [PubMed] [Google Scholar]
- 42.Korteweg MA, Kerkhoff H, Bakker J, Elgersma OEH. Efficacy of patient selection strategies for carotid endarterectomy by contrast-enhanced MRA on a 1 T machine and duplex ultrasound in a regional hospital. Clin Radiol 2008; (2): 174–183. [DOI] [PubMed] [Google Scholar]
- 43.Shakhnovich I, Kiser D, Satiani B. Importance of validation of accuracy of duplex ultrasonography in identifying moderate and severe carotid artery stenosis. Vasc Endovasc Surg 2010; (6): 483–488. [DOI] [PubMed] [Google Scholar]
- 44.Boyle MJ, Wolinski AP, Grimley RP. Accuracy of duplex versus angiography in patients undergoing carotid surgery. J R Soc Med 1995; (1): 20–23. [PMC free article] [PubMed] [Google Scholar]
- 45.Srinivasan J, Mayberg MR, Weiss DG, Eskridge J. Duplex accuracy compared with angiography in the Veterans Affairs Cooperative Studies Trial for Symptomatic Carotid Stenosis. Neurosurgery 1995; (4): 648–655. [DOI] [PubMed] [Google Scholar]
- 46.Preiss JE, Itum DS, Reeves JG et al. Carotid duplex criteria for patients with contralateral occlusion. J Surg Res 2015; (1): 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carpenter JP, Lexa FJ, Davis JT. Determination of sixty percent or greater carotid artery stenosis by duplex doppler ultrasonography. J Vasc Surg 1995; (6): 697–705. [DOI] [PubMed] [Google Scholar]