Abstract
Skull base defects following endonasal surgery for pituitary macroadenoma need to be addressed during the surgery to prevent serious postoperative complications like cerebrospinal fluid (CSF) leak. The objective of this study is to assess the incidence of CSF leak following pituitary surgery and the methods of effective skull base repair. This is a retrospective observational study conducted in a tertiary care hospital after obtaining due clearance from the Institutional ethics committee. The charts of patients who underwent endonasal pituitary surgery between 2013 and 2018 were studied and details noted. Patients undergoing revision surgery or with history of preoperative radiotherapy were excluded from the study. 52 patients were included in the study. Based on the type of CSF leak, the patients were grouped into four. 19 patients (36.5%) had an intraoperative CSF leak. 3 patients developed a postoperative CSF leak. Based on the histopathology, 4 patients had ACTH secreting tumor. 8 patients had growth hormone secreting tumor, 22 had gonadotropin secreting tumor, 9 patients had a non-functioning tumour and 9 patients had prolactinoma. The type of skull base repair performed in these patients were grouped into 4.18 patients underwent type I repair, 21 patients underwent type II repair, 8 patients underwent type III repair and 5 patients underwent type IV repair. We have observed that the pedicled nasoseptal flap is particularly advantageous over other repair techniques, especially in low pressure leaks. The strategy for skull base repair should be tailored to suit each patient to minimise the occurrence of morbidity and the duration of hospital stay.
Keywords: Pituitary adenoma, Duragen, Surgicel, CSF leak, Nasoseptal flap, Fibrin glue
Introduction
The endonasal trans-sphenoidal route of excision of pituitary macroadenoma is considered a safe and effective procedure. The post-operative complications associated with this surgery includes Cerebrospinal fluid (CSF) fistula, meningitis, pneumocephalus and subdural hematoma [1]. The most common complication is however CSF fistula [2]. It is absolutely necessary to repair the skullbase following excision of a pituitary adenoma to avoid a CSF leak and the repair strategy needs to be individualised to each patient [3]. This study analysed the incidence of CSF leak and methods of surgical repair of skull base defects following pituitary adenoma excision done by the endonasal trans-sphenoidal route.
Method
This is a retrospective chart study conducted in a tertiary care hospital. Patients who underwent trans-sphenoidal excision of pituitary adenomas were included in the study. Clearance from Institutional ethics committee was obtained. Medical records of patients who had undergone either a complete endoscopic or a combined endoscopic and microscopic approach of trans-sphenoidal excision between the period of 2013–2018 was analysed. Adjunct use of operating microscope in addition to nasal endoscopes was based on the preference of the neurosurgeon. The age, sex and surgical details were noted. The operative notes were studied for the incidence of CSF leak, size of CSF leak and the surgical strategy used for repairing the skullbase defect. The postoperative notes were studied for the incidence of postoperative infection and postoperative CSF leak. The type of tumor in each case following histopathological examination and immunohistochemistry was recorded. Patients undergoing revision surgeries and patients with history of previous radiation therapy were excluded from the study group.
The entire surgical procedure was done as a combined procedure by an ENT surgeon and a neurosurgeon. While the ENT surgeon gave access to the tumour by the endonasal route using a rigid 4 mm wide-angled nasal endoscope, the neurosurgeon excised the tumour under the vision of the endoscope and/or operating microscope.
In cases where a large skull base defect was expected, a pedicled nasoseptal flap [4] was harvested upfront and secured safely in the nasopharynx for the duration of the surgery. In 4 patients, where the expected size of skull base defect could not be assessed preoperatively, a nasoseptal rescue flap was harvested [5].
Intraoperatively, following surgical excision, an examination for identification of CSF leak was carried out with the anaesthetist performing a Valsalva manoeuvre on the patient.
Based on the size of CSF leak, the patients were grouped into 4 [6].
Type I: No intraoperative cerebrospinal fluid leak observed
Type II: Minor leak. No significant diaphragmatic openings
Type III: Moderate/severe persistent leak. Unexpected opening of the arachnoid of diaphragma sellae
Type IV: Large skull base exposure or high-flow leak predicted to occur as part of planned expanded approach or tumor larger than 3 cm.
Based on the materials used for skullbase repair, as noted from the operative notes, the patients were grouped into as follows.
Type I: Surgicel
Type II: Surgicel + Nasoseptal flap ± Fibrin glue
Type III: Surgicel + Abdominal Fat + Fascia lata ± Fibrin glue (Figs. 1, 2)
Fig. 1.
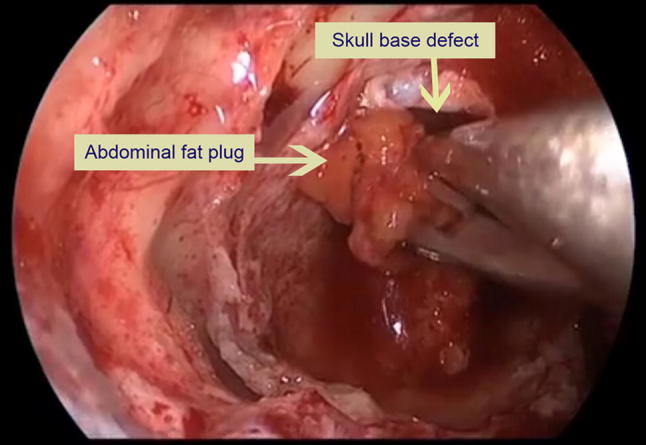
Plugging the skull base defect with abdominal fat
Fig. 2.
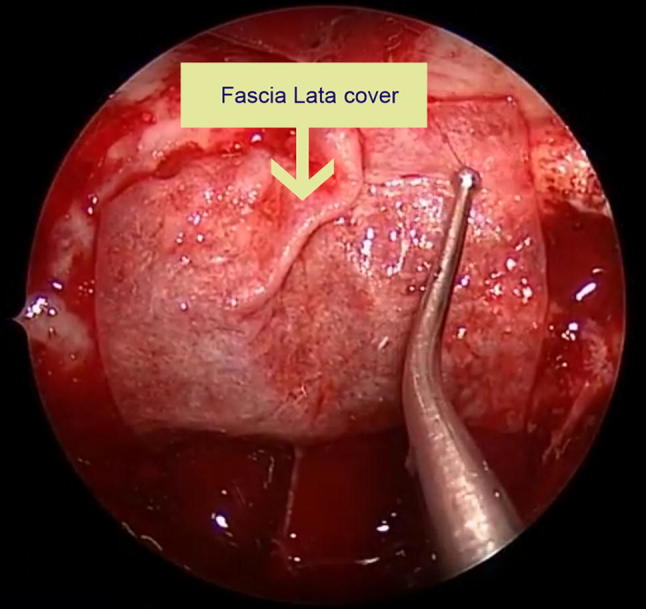
Applying Fascia Lata over fat plug
Type IV: Duragen + Nasoseptal flap ± Fibrin glue (Figs. 3, 4)
Fig. 3.
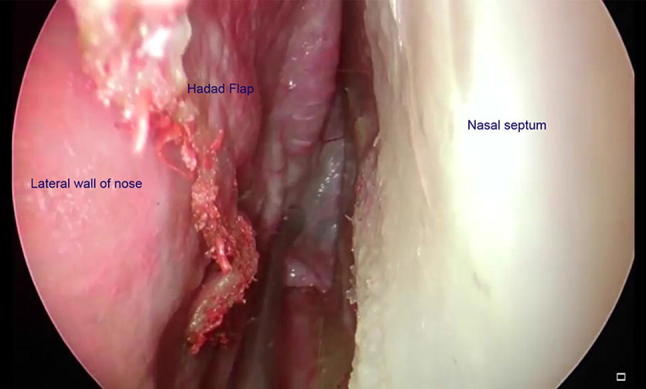
Harvesting a pedicled nasoseptal flap
Fig. 4.
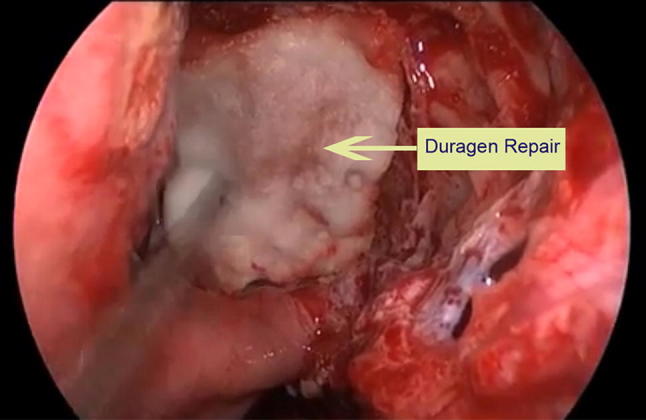
Application of Duragen to repair skull base defect
Following the surgery, the nasal cavities were packed with polyvinyl acetal sponge (Ivalon). The nasal pack was removed on 2nd postoperative day.
Results
52 patients were included in the study. The age ranged from 23 to 73 with a mean age of 49.25. The study group had 26 men and 26 women.
22 of them underwent pituitary adenoma excision by trans-sphenoidal route with the use of an endoscope and a microscope. 30 of them underwent excision by transnasal endoscopic technique only.
19 of the 52 patients had an intraoperative CSF leak (36.5%). 5 of them underwent endoscopic assisted microscopic excision. 14 of them underwent endoscopic excision.
3 (5.8%) of these patients had postoperative CSF leak which was managed conservatively and resolved in 8–12 days.
10 patients had a type II leak. 7 patients had type III leak.
2 patients had type IV leak (Table 1).
Table 1.
Type of CSF leak and the respective repair done in each group
| Type of leak | Number of patients | Type of repair | Number of patients |
|---|---|---|---|
| I | 33 | I | 18 |
| II | 15 | ||
| II | 10 | II | 6 |
| III | 3 | ||
| IV | 1 | ||
| III | 7 | III | 5 |
| IV | 2 | ||
| IV | 2 | IV | 2 |
Based on the histopathology, 4 patients had ACTH secreting tumor. 8 patients had growth hormone secreting tumor, 22 had gonadotropin secreting tumor, 9 patients had a non-functioning tumour and 9 patients had prolactinoma (Fig. 5).
Fig. 5.
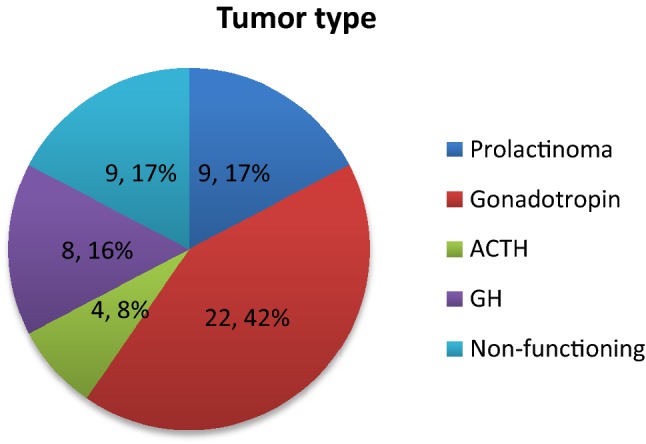
Incidence of various types of pituitary adenoma
Discussion
The incidence of intraoperative CSF leak is 36.5% in our study. This is comparable to other studies and falls within the range of 18.1–53.2% as quoted by Shiley et al. [7].
Intraoperative CSF Leak
14 patients who underwent endoscopic transsphenoidal excision of pituitary adenoma developed intraoperative CSF leak (46.6%). The frequency of intraoperative CSF leak in patients undergoing combined microscopic and endoscopic excision was 22.7%. This is in contrast to the meta-analysis by Yu et al. [8] which noted that the incidence of intraoperative CSF leak was lesser in patients undergoing endoscopic excision. However there was no statistical difference in the incidence of CSF leak in patients undergoing endoscopic and microscopic excision of pituitary adenoma. In our study, Chi Square test was applied and there was no statistical significance between the incidence of intraoperative CSF leak between both the surgeries (p = 0.077).
CSF Leak Repair And Postoperative CSF Leak
Based on the above mentioned classification, we found that 18 of the 33 patients with no intraoperative CSF leak had undergone type I repair. 15 patients had undergone type II repair in spite of no intraoperative leak. A nasoseptal rescue flap [5] had been harvested in two of these patients. Zhang et al. [3] observed that 53.8% patients developed postoperative leak without showing intraoperative CSF leak. In our study, we found that patients who did not exhibit intraoperative CSF leak did not develop a postoperative leak also. This is in contrast to a significant 2% of patients undergoing type II repair developing postoperative leak in a study conducted by Dehdashti et al. [6]. One important difference in the method of type II repair between the two studies is the additional use of a nasoseptal flap in our study. This is found to significantly decrease the incidence of a postoperative CSF leak.
6 patients with type II CSF leak underwent a type II repair. Fibrin glue was used in all 6 of them. Fibrin glue helps in holding the graft together and enhances the strength of the repair. 3 patients with type II leak had undergone a type III repair. 2 of these patients had undergone a combined microscopic and endoscopic approach, 1 of them developed a postoperative leak. 1 patient underwent an endoscopic excision and this patient developed a postoperative CSF leak. 1 patient with a type II leak was expected to have a high pressure leak postoperatively and hence underwent a type IV repair with duragen and pedicled nasoseptal flap.
5 of the 7 patients with type III CSF leak underwent type III repair. One of them developed a postoperative leak. This patient had undergone a combined endoscopic and microscopic approach to pituitary adenoma.
In our study, we have observed that the three patients who developed a postoperative leak had undergone a type III repair. The type III leak repair involved the use of surgicel, fat, fascia lata with or without fibrin glue. None of our patients undergoing a type II or type IV repair developed a postoperative leak.
We note that the use of a pedicled nasoseptal flap (as in our type II or type IV repair) decreases the chances of a postoperative CSF leak. It also avoids the need for an additional incision and postoperative wound care as in harvesting a abdominal fat or a fascia lata graft. Reconstruction with a nasoseptal flap is rapid and effective. It is readily available and does not interfere with the imaging done to detect recurrence [9].
Patients who developed a postoperative CSF leak were managed conservatively with strict bed rest, diuretics and laxatives to avoid straining.
The use of a lumbar drain for CSF leak in pituitary adenomas has been described in literature, however none of the patients included in our study needed a lumbar drain following the surgery. A low flow CSF leak does not need a lumbar drain following pituitary adenoma surgery, irrespective of the size of the skull base defect [10].
Tumour Type and Skull Base Defect
Gonadotropin secreting tumour was the most common type observed in our study. 2 of the 22 patients developed a postoperative leak. 1 out of the 9 patients with prolactinoma developed a postoperative CSF leak. In the study by Tamašauskas et al. [2], the most common tumor type was a non-functioning adenoma. Intraoperative CSF leak occurred more frequently in Growth hormone secreting tumours in their study. But, intraoperative leak was most common in gonadotropin secreting tumors in our study. However, there is no statistically significant difference between the incidences of CSF leak in various tumour types.
Conclusion
We have observed that transsphenoidal microsurgery does not give a significant advantage over transsphenoidal endoscopic surgery. The advantages of endoscopic excision includes better visualisation, better tumor resection and the lack of need for the use of a nasal speculum [3]. It has an advantage over the microscope in the detection of small CSF leaks.
Use of a pedicled nasoseptal flap is helpful in managing CSF leaks in the perioperative period. In low pressure leaks, the use of a nasoseptal flap alone is adequate in management instead of a multilayer closure that has been described in literature thus far. Surgical reconstruction protocols of skull base defects have been described in previous studies. However, the extent of repair and the material used for repair varies based on the size of the defect and surgeon’s preference. We emphasize the need for a skull base repair strategy tailored to each patient to mitigate and minimise associated morbidity and reduction in hospital day.
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
This article does not contain any studies with animals performed by any of the authors.
Contributor Information
Preethi Umamaheswaran, Email: upreethi@gmail.com.
Visvanathan Krishnaswamy, Email: visvanathan@outlook.com.
Ganesh Krishnamurthy, Email: drkrishganesh@gmail.com.
Sanjeev Mohanty, Email: drsanjeevmohanty@gmail.com.
References
- 1.Hegazy HM, Carrau RL, Snyderman CH, Kassam A, Zweig J. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea: a meta-analysis. Laryngoscope. 2000;110:1166–1172. doi: 10.1097/00005537-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Tamašauskas A, Šinkūnas K, Draf W, et al. Management of cerebrospinal fluid leak after surgical removal of pituitary adenomas. Medicina. 2008;44:302–307. doi: 10.3390/medicina44040039. [DOI] [PubMed] [Google Scholar]
- 3.Zhang C, Ding X, Lu Y, Hu L, Hu G. Cerebrospinal fluid rhinorrhoea following transsphenoidal surgery for pituitary adenoma: experience in a Chinese centre. Acta Otorhinolaryngol Ital. 2017;37(4):303–307. doi: 10.14639/0392-100X-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Sayed IH, Roediger FC, Goldberg AN, Parsa AT, McDermott MW. Endoscopic reconstruction of skull base defects with the nasal septal flap. Skull Base. 2008;18(6):385–394. doi: 10.1055/s-0028-1096202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivera-Serrano CM, Snyderman CH, Gardner P, et al. Nasoseptal “rescue” flap: a novel modification of the nasoseptal flap technique for pituitary surgery. Laryngoscope. 2011;121:990–993. doi: 10.1002/lary.21419. [DOI] [PubMed] [Google Scholar]
- 6.Dehdashti AR, Stofko D, Okun J, Obourn C, Kennedy T. Endoscopic endonasal reconstruction of skull base: repair protocol. J Neurol Surg Part B Skull Base. 2016;77(3):271–278. doi: 10.1055/s-0035-1568871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiley SG, Limonadi F, Delashaw JB, et al. Incidence, etiology, and management of cerebrospinal fluid leaks following trans-sphenoidal surgery. Laryngoscope. 2003;113:1283–1288. doi: 10.1097/00005537-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Yu S, Du Q, Yao S, et al. Outcomes of endoscopic and microscopic transsphenoidal surgery on non- functioning pituitary adenomas: a systematic review and meta- analysis. J Cell Mol Med. 2018;22(3):2023–2027. doi: 10.1111/jcmm.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang MD, Escott E, Thomas AJ, et al. The MR imaging appearance of the vascular pedicle nasoseptal flap. AJNR Am J Neuroradiol. 2009;30(4):781–786. doi: 10.3174/ajnr.A1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhan R, Chen S, Xu S, Liu JK, Li X. Postoperative low-flow cerebrospinal fluid leak of endoscopic endonasal transsphenoidal surgery for pituitary adenoma: wait and see, or lumbar drain? J Craniofacial Surg. 2015;26(4):1261–1264. doi: 10.1097/SCS.0000000000001691. [DOI] [PMC free article] [PubMed] [Google Scholar]


