Abstract
Active propagation of electrical signals in C. elegans neurons requires ion channels capable of regenerating membrane potentials. Here we report regenerative depolarization of a major gustatory sensory neuron, ASEL. Whole-cell patch-clamp recordings in vivo showed supralinear depolarization of ASEL upon current injection. Furthermore, stimulation of animal’s nose with NaCl evoked all-or-none membrane depolarization in ASEL. Mutant analysis showed that EGL-19, the α1 subunit of L-type voltage-gated Ca2+ channels, is essential for regenerative depolarization of ASEL. ASEL-specific knock-down of EGL-19 by RNAi demonstrated that EGL-19 functions in C. elegans chemotaxis along an NaCl gradient. These results demonstrate that a natural substance induces regenerative all-or-none electrical signals in dendrites, and that these signals are essential for activation of sensory neurons for chemotaxis. As in other vertebrate and invertebrate nervous systems, active information processing in dendrites occurs in C. elegans, and is necessary for adaptive behavior.
Introduction
Sensory information is commonly coded by the frequency and timing of action potentials1,2, whereas many sensory neurons, including crustacean stretch receptors, insect and vertebrate retinal photoreceptors and mammalian hair cells, use graded potentials3,4. High-impedance neurons with short processes such as C. elegans neurons are also thought to transmit electrical signals by passive propagation. Indeed, a lack of channels necessary for Na+-dependent action potentials5,6 has previously led to an emphasis on passive current spread as a mechanism for electrical signal propagation in C. elegans neurons7–12. Graded electrical potentials and graded synaptic transmission in motor neurons are also observed in C. elegans13 and the parasitic nematode, Ascaris suum14,15. Upon current injection, however, non-linear depolarization is observed in a gustatory sensory neuron, ASER, in C. elegans16. Furthermore, when depolarizing current is artificially injected, regenerative potentials with all-or-none properties have been reported in C. elegans RMD neurons17.
Calcium-dependent action potentials have been recorded in C. elegans pharyngeal muscles18,19 and body-wall muscles20,21. Voltage-gated Ca2+ channels (VGCCs) can also generate spikes in dendrites of rodent neocortical pyramidal neurons22,23. VGCCs of vertebrates have been classified into three subtypes, based on structural, functional, kinetic, and pharmacological differences24. In particular, the α1 subunits of Cav1, Cav2, and Cav3 calcium channels are key determinants of the three calcium channel subtypes L-type, R,N,P/Q-type and T-type, respectively. In C. elegans, three genes, egl-19, unc-2 and cca-1, encode pore-forming L-type, R,N,P/Q-type and T-type α subunits of VGCCs, respectively19,25,26. In C. elegans body-wall muscles, the L-type VGCC in particular, and extracellular Ca2+ are essential to generate all-or-none action potentials20,21.
The bilaterally symmetric pair of bipolar head neurons, ASEL and ASER, are major gustatory chemosensory neurons in C. elegans, and terminate with ciliated endings in the animal’s nose, which is exposed to the environment27,28. Various chemicals, including Na+, K+, Cl−, Mg2+, Li+, alkaline pH, cAMP, and biotin, are sensed by the ASEL and ASER neuron pair29–32. Increased or decreased NaCl concentrations are sensed, respectively, by ASEL and ASER31,32. An increase in environmental NaCl concentration is detected partly by the receptor guanylyl cyclase GCY-14 in ASEL, which produces cGMP30. Upon stimulation of ASEL with NaCl, Ca2+ transients observed in the cell body are defective in tax-2 or tax-4 animals, suggesting that ASEL is activated by Ca2+ influx through TAX-2/TAX-4 cGMP-gated cation channels13,30,33,34. In previous electrophysiological studies, ASER was reported to be nearly isopotential and unable to generate classical Na+ action potentials16. However, it is still not known whether ASEL is also isopotential in response to environmental increases in NaCl concentration.
Here, we show that an environmental increase in NaCl concentration induces Ca2+ influx through TAX-2/TAX-4 cGMP-gated channels into ASEL sensory endings. The resulting membrane depolarization activates EGL-19 VGCC for regeneration of all-or-none electrical signals in ASEL dendrites. It demonstrates that the small, simple nervous system of C. elegans uses mechanisms of information processing similar to those of other invertebrate and vertebrate brains, in which both active and passive electrical signal transmission are used35,36.
Results
Supralinear depolarization of ASE sensory neurons upon current injection
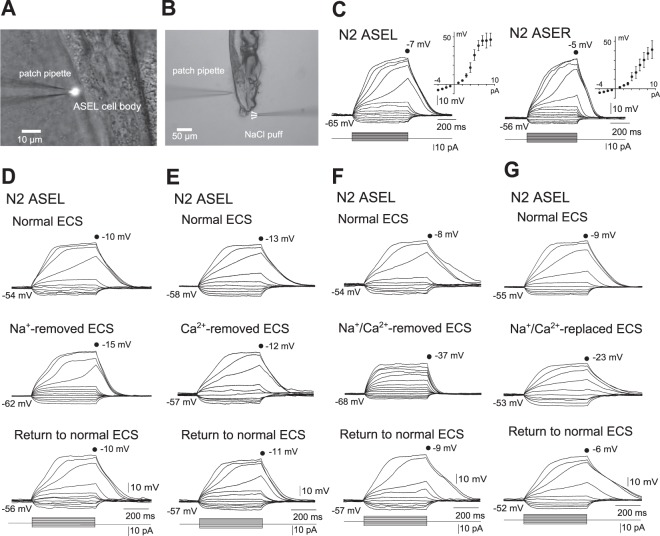
We first tested whether regenerative membrane depolarization occurs in ASE neurons in response to current steps, using in vivo whole-cell patch-clamp recordings (Fig. 1A) of a slit-worm preparation16. ASEL and ASER had resting membrane potentials of −61.7 ± 1.1 mV (n = 22) and −56.6 ± 1.0 mV (n = 7), and input resistances 2.2 ± 0.2 GΩ (n = 22) and 1.6 ± 0.2 GΩ (n = 7), respectively (Fig. 1C). These resting potentials were comparable to those observed previously in ASER16 and RMD17. In response to depolarizing current steps, membrane potentials of both ASEL and ASER neurons depolarized linearly at lower current intensities. Importantly, the voltage trajectories of both cell types became supralinear at higher currents, and reached a plateau during the depolarization phase as shown in steady-state current-voltage (I-V) plots (Fig. 1C). Such non-linear depolarization has also previously observed in ASER16, but has not previously been reported in ASEL.
Figure 1.
Whole-cell, current-clamp recordings in vivo of ASE neurons in wild-type N2 animals. (A) Image of a patch-clamp recording. gcy-7p::GFP-transfected N2 specifically producing GFP in ASEL was glued to a cover glass, and was transferred to a recording chamber. Under a microscope, a small piece of cuticle and body wall was dissected to exteriorize ASEL cell bodies. (B) Image of an animal under stimulation with NaCl-containing buffer. Animals were treated with the same procedures as in (A), and their nose tips were stimulated with NaCl solutions released from a puffing capillary. (C) Membrane voltage changes in response to current steps and averaged I-V curves in wild-type ASEL (left, n = 22) and ASER (right, n = 7). Error bars, s.e.m. (D–G) Membrane voltage changes in response to current steps in wild-type ASEL. Current-clamp recordings of an animal were carried out three times consecutively, first and third in normal ECS (top and bottom), and the second in ECS, from which Na+ was removed (Na+-removed ECS) (D), Ca2+ of which was removed (Ca2+-removed ECS) (E), Na+ and Ca2+ of which were removed (Na+/Ca2+-removed ECS. In this experiment, current was injected until the membrane potential exceeded −40 mV) (F), or Na+ and Ca2+ of which were replaced with NMDG+ and Mg2+ (Na+/Ca2+-replaced ECS) (G). These are representatives of at least three independent recordings.
In RMD neurons, the amplitude of nonlinear depolarization was reduced when the chamber solution was replaced with Na+-free, or Na+- and Ca2+-free extracellular saline (ECS)17. To identify ion species required for the supralinear depolarization of ASEL, we removed Na+ and/or Ca2+ from the ECS (Fig. 1D–G). When either Na+ or Ca2+ alone was removed from ECS, supralinear depolarization was still observed in response to steps of depolarizing current (Fig. 1D,E). When Na+ or Ca2+ was replaced with NMDG+ or Mg2+, respectively, in ECS, supralinear depolarization still remained (Supplementary Fig. S1). When both Na+ and Ca2+ were removed from ECS, in contrast, supralinear depolarization disappeared (Fig. 1F). In addition, when both Na+ and Ca2+ were replaced with NMDG+ and Mg2+ in ECS, supralinearity was markedly reduced (Fig. 1G). Complete recovery from all these changes was observed following a return to normal ECS. These results suggest that either Na+ or Ca2+ is required for supralinear depolarization of ASEL upon current injection, as previously observed in RMD neurons17.
All-or-none membrane depolarization evoked in ASEL by NaCl
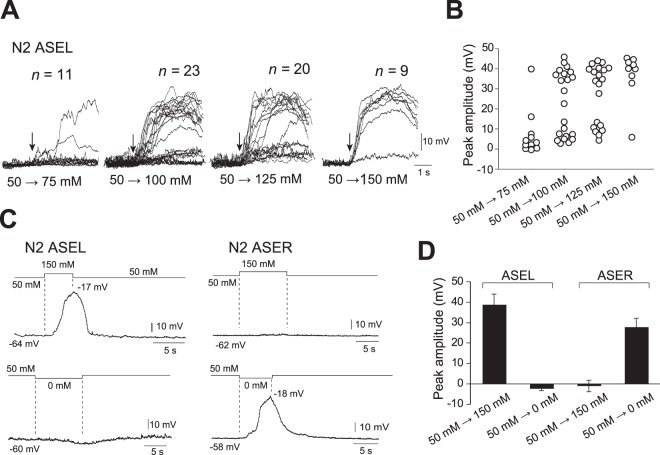
Whole-cell recordings from wild-type N2 animals (Fig. 1B) showed that ASEL depolarized in response to a puff of various concentrations of NaCl to animal’s nose in ECS containing 50 mM NaCl (Fig. 2A). This depolarization continued during the application of NaCl, and after the cessation of NaCl application, the membrane potential repolarized to resting levels (Fig. 2C, left). Conversely, a puff of NaCl-free buffer induced depolarization of ASER membrane potentials (Fig. 2C, right). The ECS was perfused from the animal’s tail to its nose in the chamber in order to prevent stimulation of any other parts of the body than its nose by NaCl. Furthermore, Ca2+ transients in ASE neurons are observed in unc-13 and unc-31 mutants32, which lack neurotransmitter and neuropeptide release, respectively, indicating that depolarization of ASE neurons was directly evoked by stimulation of ASE neurons with NaCl. Therefore, the ASE responses were solely due to stimulation of their sensory endings in the tip of the nose.
Figure 2.
Membrane depolarization evoked by application of various concentrations of NaCl to the nose tips of animals. (A) Membrane voltage traces of ASEL in response to 75 mM, 100 mM, 125 mM, or 150 mM NaCl. An experimental setting is shown in Fig. 1B. Vertical arrows indicate onset times of NaCl-evoked depolarization. Voltage traces from different animals were superimposed by synchronizing onset times at the same position. (B) Peak amplitudes, which are shown by circles, of membrane voltage traces shown in (A). (C) Membrane voltage traces of ASEL and ASER in response to application of 150 mM NaCl or NaCl-free buffer, respectively. Note that ASEL and ASER responded to increases and decreases of NaCl concentration, respectively. (D) Mean amplitudes of membrane voltage peaks in response to 150 mM NaCl or NaCl-free buffer (ASEL: n = 7 up-steps, n = 3 down-steps; ASER: n = 4 up-steps, n = 6 down-steps). Error bars, s.e.m.
While the depolarization amplitudes of wild-type ASEL evoked by various concentrations of NaCl, which ranged from 75 mM to 150 mM, were at similar amplitudes, higher NaCl concentrations showed higher probabilities of evoking depolarization (Fig. 2A,B), indicating that membrane depolarization is an all-or-none phenomenon. Thereafter, therefore, we used a 100 mM up-step and a 50 mM down-step of NaCl concentration for stimulation of ASEL and ASER, respectively. On average, ASEL and ASER peak amplitudes induced by application of up-steps and down-steps were 38.8 ± 5.1 mV and 27.8 ± 4.3 mV, respectively (Fig. 2D). These results demonstrate that increases in NaCl concentration induce all-or-none electrical signals in ASEL.
EGL-19, an L-type VGCC, participates in active electrical signal propagation in ASEL, but not in ASER
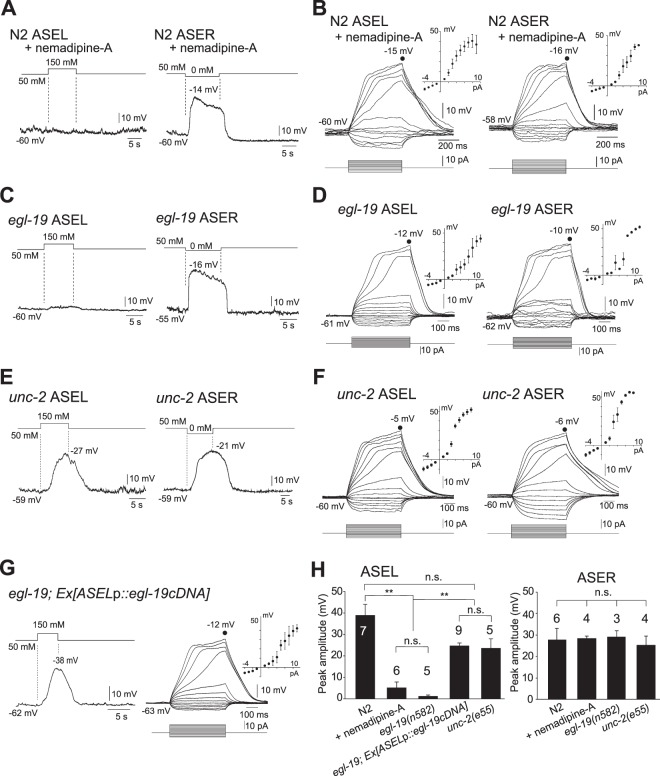
As described above, depolarization was observed in ASEL upon an increase in environmental NaCl concentration. Bath application of nemadipine-A, an antagonist of the α1 subunit of L-type VGCCs37, blocked NaCl-induced depolarization of ASEL (Fig. 3A,H, left). In contrast, the response of ASER to a puff of NaCl-free buffer was not blocked by nemadipine-A (Fig. 3A,H, right). Upon current injection above a threshold level, however, supralinear depolarization was observed in both ASEL and ASER in the presence of nemadipine-A (Fig. 3B), suggesting the existence of other depolarizing voltage-gated channels than those inhibited by the drug.
Figure 3.
EGL-19 plays an essential role in active propagation of electrical signals in ASEL. (A,B) Membrane voltage traces of wild-type ASEL and ASER in the presence of nemadipine-A, 1.0 μM, upon stimulation with 150 mM NaCl and NaCl-free buffer, respectively, to the nose tips of animals (A), or upon current steps with mean I-V curves (ASEL: n = 6, ASER: n = 4) (B). (C,D) Membrane voltage traces of ASEL and ASER in egl-19 mutants upon stimulation with 150 mM NaCl and NaCl-free buffer, respectively (C), or upon current injection with mean I-V curves (ASEL: n = 5, ASER: n = 3) (D). (E,F) Membrane voltage traces of ASEL and ASER in unc-2 mutants upon stimulation with 150 mM NaCl and NaCl-free buffer, respectively (E), or upon current injection with mean I-V curves (ASEL: n = 5, ASER: n = 4) (F). (G) Membrane voltage traces of ASEL in egl-19 transgenic animals specifically producing wild-type EGL-19 in ASEL upon stimulation with 150 mM NaCl buffer (left), or upon current injection with mean I-V curves (right, n = 9). (H) Mean amplitudes of membrane voltage peaks of the traces shown above in (A,C,E,G). Numbers above bars indicate numbers of animals measured. N2 data are derived from Fig. 2D. **p < 0.01, n.s., not significant by one-way ANOVA with Dunn’s test. Error bars, s.e.m.
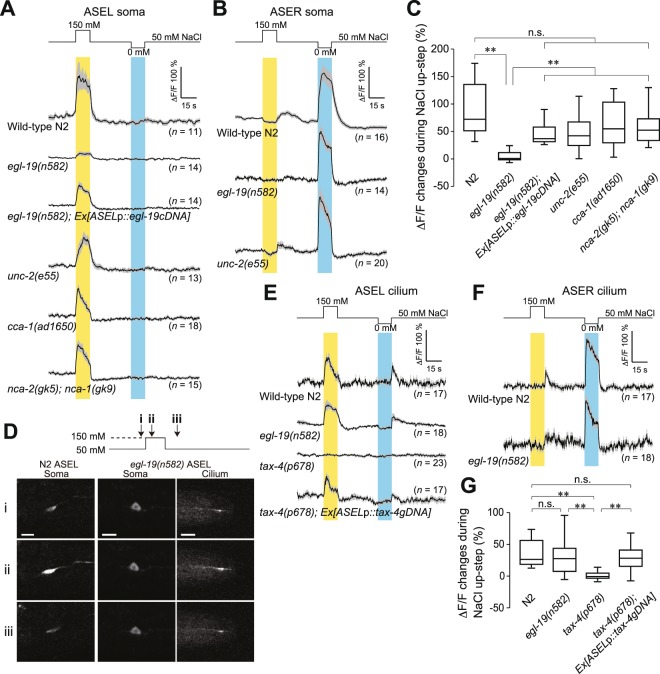
The blockade by nemadipine-A of NaCl-induced depolarization suggests that an L-type VGCC contributes to depolarization of ASEL. Since EGL-19 is the sole target of nemadipine-A among C. elegans VGCCs37, we examined whether ASEL depolarization in egl-19(n582), a loss-of-function allele, is observed following a puff of 150 mM NaCl to its nose. As expected, NaCl-induced depolarization was not observed in ASEL of egl-19, like wild-type animals treated with nemadipine-A (Fig. 3C, left). In contrast, membrane depolarization was observed in egl-19 ASER in a manner similar to that of wild type upon application of NaCl-free buffer (Fig. 3C, right). NaCl step-evoked membrane depolarizations of ASE neurons were correlated with Ca2+ transients induced by the same NaCl concentration steps (Fig. 4A,B). Consistent with the lack of NaCl-induced membrane depolarization in egl-19 ASEL, Ca2+ imaging of cell bodies also demonstrated that egl-19 ASEL failed to respond to stimulation with 150 mM NaCl (Fig. 4A,C), whereas egl-19 ASER showed similar level of Ca2+ transients to those of wild type (Fig. 4B).
Figure 4.
Ca2+ transients in cell body and sensory cilium of ASE neurons of animals immobilized in microfluidic chambers. (A) Ca2+ transients in ASEL cell bodies of wild type, egl-19, ASEL-specifically rescued egl-19, unc-2, cca-1, and a double mutant, nca-2; nca-1, in response to NaCl concentration changes for 15 s, which are shown on top. Grey bands, s.e.m. (B) Ca2+ transients in ASER cell bodies of wild-type, egl-19, and unc-2 animals in response to NaCl concentration changes for 15 s. (C) Mean ΔF/F changes in ASEL cell bodies measured in (A) during 15-s stimulation with 150 mM NaCl buffers. Horizontal lines in boxes indicate 25th, 50th, and 75th percentiles, and whiskers represent 5th and 95th percentiles. (D) Upon stimulation of ASEL cilia with 150 mM NaCl buffer for 15 s, Ca2+ transients of ASEL cilia of egl-19(n582) were monitored 3 s before the stimulation (i), 5 s after the NaCl up-step (ii), and 8 s after cessation of the stimulation (iii). Note that Ca2+ influx into the cilia, but not to the cell body, of egl-19 ASEL was detected. Ca2+ transients in the ASEL cell body of wild-type N2 are also shown as references at the left. (E) Ca2+ transients in ASEL cilia of wild type, egl-19, tax-4, and tax-4 rescued by ASEL-specific expression of tax-4 genomic DNA, in response to NaCl concentration changes for 15 s as shown on top. (F) Ca2+ transients in ASER cilia of wild-type and egl-19 animals in response to NaCl concentration changes for 15 s. (G) Mean ΔF/F changes in ASEL cilium in (E) during 15-s stimulation with 150 mM NaCl buffers. Horizontal lines in boxes indicate 25th, 50th, and 75th percentiles, and whiskers represent 5th and 95th percentiles. **p < 0.01, n.s., not significant by Steel-Dwass test.
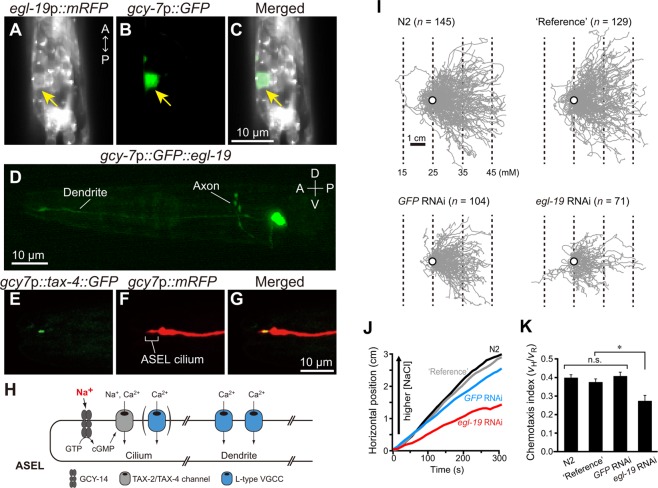
As described above, upon an increase in environmental NaCl concentration, EGL-19 is required for depolarization of ASEL, but not of ASER. Therefore, we examined the possibility that EGL-19 is produced in ASEL but not in ASER by observing expression of a transcriptional fusion, egl-19p::mRFP, which expresses mRFP under control of the egl-19 promoter. mRFP was widely produced in most neurons, as well as body-wall and pharyngeal muscles in the head of wild-type animals (Supplementary Fig. S2A). Since mRFP co-localized with GFP produced specifically in ASEL and ASER, furthermore, both ASE neurons express egl-19 (Fig. 5A–C and Supplementary Fig. S2B–D), indicating that asymmetry of the depolarizing activity is not due to expression of egl-19.
Figure 5.
EGL-19 produced in ASEL is required for chemotaxis. (A–D) Expression patterns of egl-19. (A) mRFP production under control of the egl-19 promoter. Note mRFP produced in ASEL as indicated by arrow. (B,C) GFP production under control of the gcy-7 promoter. Note specific production of GFP in ASEL. (D) Subcellular localization of the GFP::EGL-19 fusion protein produced under control of the gcy-7 promoter. Note specific localization of the fusion in ASEL dendrite, axon and cell soma. (E–G) Subcellular localization of the TAX-4 protein in ASEL. (E) TAX-4::GFP produced under control of the gcy-7 promoter. (F) mRFP under control of the gcy-7 promoter. (G) Merged image of (E,F). Note that TAX-4::GFP located in the chemosensory cilium, but not in dendrite, of ASEL. (H) Subcellular localization of membrane proteins involved in NaCl-induced signal propagation in the ASEL cilium and dendrite. (I) Trajectories of animals, wild-type N2, N2 producing GFP in ASEL and coelomocytes (‘Reference’), ‘Reference’ animals treated with GFP RNAi and ‘Reference’ animals treated with egl-19 RNAi, on chemotaxis assay plates with an NaCl linear gradient. Open circles indicate the starting position for all trajectories of individual animals during the initial 250 s. Dotted lines indicate NaCl concentrations calculated by assuming that the gradient is linear (5 mM NaCl/cm). (J) Mean horizontal positions of the four strains during the NaCl chemotaxis. (K) Effect of knock-down of EGL-19 specifically in ASEL by RNAi on chemotactic efficiency of animals toward preferred NaCl concentrations. Chemotaxis index vH/vR, which is horizontal velocity divided by velocity along the trajectory, was computed for each strain (see Materials and methods ‘Behavioral analysis’). Each data point represents the mean ± s.e.m. One-way ANOVA with Dunn’s post-hoc test was used for statistical analysis of the data. *p < 0.05. n.s., not significant.
When wild-type egl-19 cDNA was expressed specifically in ASEL of egl-19 animals, NaCl-induced depolarization recovered in egl-19 ASEL (Fig. 3G). Consistently, a significant increase in Ca2+ transients, compared to that in egl-19, was observed in egl-19, which produced wild-type EGL-19 specifically in ASEL, by stimulation with 150 mM NaCl buffer (Fig. 4A,C). Taken together, these results demonstrate that EGL-19 plays an indispensable role in electrical signal propagation in ASEL, but not in ASER, even though egl-19 is expressed in both.
Other channels are not required for ASEL dendritic signal propagation
Current injection induced supralinear depolarization in both ASEL and ASER of egl-19 animals (Fig. 3D), suggesting the existence of other channels in the supralinear depolarization. In unc-2(e55) animals, furthermore, smaller amplitudes and slower onsets of depolarization were previously observed in RMD neurons17. Therefore, we also examined whether other VGCCs participate in electrical signal propagation. unc-2 and cca-1 genes encode α1 subunits of the R,N,P/Q- and T-type VGCCs, respectively19,26,38,39. ASE neurons of unc-2 mutants showed supralinear depolarization upon depolarizing current injection in a similar manner to that of wild type (Fig. 3F), indicating that neuronal excitability of ASEL and ASER is normal in unc-2 mutants. NaCl-induced membrane depolarization was also observed both in ASEL and ASER of unc-2 (Fig. 3E). Upon an increase in environmental NaCl concentration, consistently, an increase in Ca2+ concentration was observed in the ASEL cell body of unc-2 (Fig. 4A,C). ASEL of the cca-1(ad1650) mutant with a 2.5-kb genomic deletion showed a response to increased environmental NaCl concentration that was indistinguishable from that of wild type (Fig. 4A,C), indicating that T-type VGCC does not play a role in signal propagation of ASEL.
In C. elegans, nca-1 and nca-2 encode channels homologous to vertebrate NALCN, a voltage-independent, nonselective cation channel that acts as a Na+ leak channel regulating resting potentials40,41. In HSN neurons, NCA-1 and NCA-2 are enriched in the non-synaptic region of axons and affect synaptic transmission42. Therefore, we also examined whether NCA-1 and NCA-2 contribute to signal propagation in ASEL. Upon an increase in environmental NaCl concentration, normal Ca2+ transients were observed in ASEL cell bodies of nca-2(gk5); nca-1(gk9) double mutants (Fig. 4A,C), indicating that NCA-1 and NCA-2 are not essential for signal propagation in ASEL.
Ca2+ influx is normal in cilia, but does not occur in cell body, of ASEL in egl-19
It is known that an increase in environmental NaCl concentration activates a receptor guanylyl cyclase, GCY-14, which induces Ca2+ influx into ASEL cilia through activation of TAX-2/TAX-4 cGMP-gated channels30 by increasing cGMP concentration. This Ca2+ influx is likely to induce depolarization of the cilium membrane potential, since NaCl application to the nose tip of tax-4 mutants failed to evoke membrane depolarization of ASEL (Supplementary Fig. S3B,C). This is consistent with specific subcellular localization of TAX-4 to ASEL cilia (Fig. 5E–G). The cilium membrane depolarization may in turn activate VGCCs to propagate the electrical signal from the cilium to the cell body of ASEL, since NaCl application to the nose tip of egl-19 mutants failed to evoke membrane depolarization of ASEL (Fig. 3C, left). Consistently, the NaCl application also failed to cause Ca2+ influx into the cell body of egl-19 mutants (Fig. 4A,C).
To test the scenario that EGL-19 plays an essential role in active propagation of dendritic electric signals in ASEL, we measured Ca2+ transients of ASEL and ASER cilia upon increases and decreases in environmental NaCl concentration. Ca2+ transients were clearly observed in ASEL and ASER cilia of both wild type and egl-19 upon an increase and a decrease in environmental NaCl concentration, respectively (Fig. 4D–G), demonstrating that Ca2+ influx into cilia occurs in egl-19 mutants.
In contrast, no Ca2+ influx into ASEL cilia nor depolarization of ASEL was observed in tax-4 mutants (Fig. 4E,G and Supplementary Fig. S3). ASEL-specific expression of the wild-type tax-4 gene rescued the defect of Ca2+ influx into the cilia in tax-4 (Fig. 4E,G), consistent with mediation of Ca2+ influx by TAX-2/TAX-4 cGMP-gated channels.
Unlike wild type, however, an increase in Ca2+ concentration in the cell body could not be observed in egl-19 (Fig. 4A,C,D), suggesting that Ca2+ influx through TAX-2/TAX-4 channels induces depolarization of the ASEL cilium membrane, which in turn activates the EGL-19 VGCC for ASEL dendritic electrical signal propagation from the cilia to the cell body. Consistent with this finding, TAX-4 was specifically localized to the ASEL cilium, and EGL-19 was found located in the dendrites, axons and cell body of ASEL (Fig. 5D–H). Since supralinear depolarization of egl-19 ASEL was observed upon depolarizing current injection, VGCCs other than EGL-19 are likely to function in ASEL.
EGL-19 in ASEL is required for chemotaxis along NaCl gradients
The results described above suggest that EGL-19 plays a role in C. elegans chemotaxis along NaCl gradients. ASEL activation increases the probability of forward locomotion upon an increase in environmental NaCl concentration, while ASER activation increases probability of backward locomotion followed by a pirouette, a large-angle turn, upon a decrease in environmental NaCl concentration32,43.
As described above, an increase in environmental NaCl concentration failed to activate ASEL of egl-19 mutants due to the lack of dendritic electrical signal propagation. Therefore, egl-19 mutants are likely to be inefficient in chemotaxis toward preferred NaCl concentrations, although functional ASER less efficiently brings animals to preferred NaCl concentrations than wild type. To test this possibility, we analyzed chemotactic speeds and chemotaxis indices of wild-type animals in which EGL-19 was ASEL-specifically knocked down using RNA interference (RNAi) (Fig. 5I–K).
The ‘run’ speeds of animals treated with ASEL-specific RNAi against egl-19 or GFP were statistically significantly slower than those of wild type and ‘reference’ animals untreated with RNAi (Supplementary Fig. S4A; see Materials and Methods for the definition of ‘run’ and ‘reference’). This slow locomotion may be due to the small body size of animals treated with ASEL-specific RNAi (Supplementary Fig. S4B), because ASE neurons release hormones required for normal body size44,45. The chemotaxis index of animals with EGL-19 knocked down by ASEL-specific RNAi was statistically significantly lower than that of wild-type and ‘reference’ animals, including one in which GFP was knocked down with ASEL-specific RNAi (Fig. 5K). These results are consistent with vital roles for both ASEL and ASER in chemotaxis of animals along NaCl gradients, and non-functionality of ASEL due to the lack of dendritic electrical signal propagation significantly affects chemotactic behavior.
Discussion
Active propagation of dendritic electrical signals in ASEL
The ASE neuron pairs, ASEL and ASER, are required for C. elegans chemotaxis toward preferred concentrations of NaCl, and are activated by increases and decreases in NaCl concentration, respectively. In the present study, we analyzed activity of ASE neurons using in vivo whole-cell patch-clamp recordings and Ca2+ imaging of their somata and cilia. We found that: (1) In response to depolarizing current steps, supralinear depolarization of both ASEL and ASER occurred (Fig. 1C). This non-linear depolarization was blocked when both Na+ and Ca2+ ions were removed from the bath solution (Fig. 1F). (2) Upon stimulation of an animal’s nose with various concentrations of NaCl, all-or-none membrane depolarization was observed in ASEL (Fig. 2A,B). (3) The EGL-19 VGCC plays an essential role in the all-or-none depolarization of ASEL, but not in ASER (Fig. 3A,C,G,H). (4) Upon an increase in environmental NaCl concentration, Ca2+ influx into the ASEL cilium through the TAX-2/TAX-4 cGMP-dependent channel is normal in egl-19 mutants (Fig. 4D,E,G). (5) TAX-4 exists only in the cilia of ASEL, and EGL-19 exists in the dendrite, cell body and axon of ASEL (Fig. 5D–G). (6) Wild-type animals in which EGL-19 was cell-specifically knocked down in ASEL with RNAi were partially deficient in chemotaxis toward preferred concentrations of NaCl (Fig. 5I–K).
These results are consistent with the following scenario (Fig. 5H): The receptor guanylyl cyclase GCY-14 of ASEL cilia is one of the sensor molecules that detects an increase in environmental Na+ ion concentrations and produces cGMP30,31. Upon activation of the cGMP-gated TAX-2/TAX-4 channel through an increase in cGMP concentration produced by GCY-14, Ca2+ influx occurs in ASEL cilia, resulting in ciliary membrane depolarization. Consistently, membrane depolarization was not observed in tax-4 mutants when the nose tip was stimulated with NaCl (Supplementary Fig. S3B,C). The cilium membrane depolarization is likely to open EGL-19 VGCCs in ASEL dendrites, since NaCl-induced membrane depolarization was not observed in ASEL of wild-type animals treated with nemadipine-A, nor in egl-19 mutants (Fig. 3A,C). Thus, electric signals actively propagate to the ASEL cell body from its cilium through its dendrite.
When stimulated with NaCl-free buffer, in contrast, membrane depolarization of ASER was clearly observed in wild-type animals treated with nemadipine-A and in egl-19 mutants (Fig. 3A,C, right), suggesting that the EGL-19 VGCC is not required for dendritic signal propagation in ASER. This is consistent with the previous observation that ASER is isopotential and depolarization can spread passively16. Upon depolarizing current injection, supralinear depolarization was observed in ASEL and ASER of wild-type animals treated with nemadipine-A, egl-19 and unc-2 mutants (Fig. 3B,D,F), suggesting that EGL-19, UNC-2, and other VGCCs act redundantly in electric signal propagation in ASEL and ASER.
The lack of active propagation of dendritic electrical signals in ASEL negatively affected chemotaxis toward preferred concentrations of NaCl, and animals were less effectively attracted to preferred concentrations than were wild-type animals (Fig. 5I–K). As described above, increased environmental NaCl concentration activates ASEL for forward locomotion, while decreased NaCl concentration activates ASER for reverse locomotion. While animals can locate preferred concentrations of NaCl with only one of these two sensory neurons, activation of both neurons enables animals to find preferred concentrations more effectively.
All-or-none regenerative propagation of sensory signals in dendrites
ASEL detects various water-soluble ions in the environment, including Na+, Li+, Mg2+ and alkaline pH30,31,45, and ASER senses nearly all salts including K+, Br−, I− and the amino acid, methionine31,46,47. A receptor guanylyl cyclase, GCY-14, of ASEL cilia senses both Na+ and alkaline pH30. Therefore, multiple chemicals are sensed by a single sensor molecule, and many chemicals are detected by a single sensory neuron in C. elegans. How does C. elegans respond to only significant environmental changes mixed with sensory noise from other ions? By setting the threshold that evokes regenerative depolarization in dendrites of sensory neurons, C. elegans may be able to respond to only significant environmental changes among many others.
While a 100-mM increase in NaCl concentration reliably evoked membrane depolarization in ASEL, NaCl concentration changes ranging from 75 mM to 25 mM evoked depolarization less consistently (Fig. 2A,B). However, C. elegans could detect shallower NaCl concentration gradients during chemotaxis (Fig. 5I). Sensitivity of the GCY-14 receptor guanylyl cyclase to NaCl may change during chemotaxis along NaCl gradients. Guanylyl cyclase activity is regulated by upstream kinase homology domains (KHD), the stability of which is modified by phosphorylation45,48–50. Therefore, GCY-14 sensitivity to NaCl may be dependent upon the phosphorylation states of the receptor KHD. Furthermore, sensory activity of ASEL is also regulated by downstream effector proteins of GCY-1430, which include EGL-4, a cGMP-dependent protein kinase (PKG)51, phosphodiesterases52,53, TAX-6, a homolog of calcineurin A, and NCS-1, a neuronal calcium sensor54–56. These downstream effectors may also modulate ASEL activity during the chemotaxis.
C. elegans neurons send electrical signals both actively and passively
As described above, both ASEL and ASER were supralinearly depolarized in response to depolarizing current steps. This non-linear depolarization was blocked when both Na+ and Ca2+ ions were removed from the bath solution. By injecting current pulses, similarly, RMD neurons were nonlinearly depolarized, and showed bistability following cessation of the current step17. Furthermore, olfactory AWA neurons seem to actively send electrical signals, since an increase in Ca2+ concentration in the AWA cell body upon diacetyl application is abolished by nemadipine-A treatment and egl-19 mutation57,58.
Upon current injection, supralinear depolarization was observed in both ASEL and RMD of egl-19, which encodes an L-type VGCC, and unc-2, which encodes an R,N,P/Q-type VGCC. Furthermore, normal increases in Ca2+ concentration were also observed in cca-1, which encodes a T-type VGCC, or in nca-2; nca-1 double mutants, both of which encode channels homologous to vertebrate NALCN, a voltage-insensitive, non-selective cation channel40,41. These results suggest that multiple classes of Ca2+ channels, which include yet unidentified channels, contribute redundantly to the supralinear depolarization.
It is now apparent that upon stimulation with NaCl, the C. elegans nervous system actively propagates electrical signals. In addition to ASER, motor neurons and AVA interneurons propagate electrical signals passively9,17. As in mammalian brain, therefore, both active and passive propagation of electric signals occur in the C. elegans nervous system.
Materials and Methods
Strains
C. elegans strains used in this study were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN). Animals were grown to adulthood on nematode growth medium (NGM) (50 mM NaCl, 2% agar, 2.5% peptone, 5 mg/L cholesterol, 1.0 mM CaCl2, 1.0 mM MgSO4, 25 mM potassium phosphate, pH 6.0) seeded with Escherichia coli OP50 at 20 °C using standard methods59. Strains used in this work are listed in Supplementary Table S1.
Electrophysiology
After hatching, Day-4 hermaphrodite animals were immobilized on a circular (12 mm in diameter) cover glass, which was coated with Sylgard 184 (Dow Corning, Midland, MI), with a glue (Histoacryl Blue, B. Braun, Melsungen, Germany). The cover glass was transferred to a recording chamber placed on the stage of an upright microscope with a 60x water-immersion objective (model BX50WI; Olympus), and was perfused (2–3 mL/min) with ECS (see below). A small piece of a cuticle and body wall of an animal’s head was cut out with a sharp glass needle, and ASE cell bodies producing GFP were brought out from the head. Borosilicate glass pipettes (GC150TF-10; Harvard Apparatus, UK) with a tip resistance of 6–8 MΩ were used as electrodes for current-clamp recordings in a whole-cell patch- clamp configuration.
Patch pipettes were pulled on a DMZ universal puller (Zeitz Instruments, Munich, Germany). Recordings were performed with a Multiclamp 700B amplifier (Molecular Devices, Foster City, CA) and the Clampex software (Molecular Devices). Recordings were corrected for junction potentials, and series resistances were compensated by 50%. Recordings with 1–15 GΩ seal resistances were normally observed, and recordings with seal resistances less than 1.0 GΩ were discarded. Before the compensation, recordings with 20–70 MΩ series resistances were normally observed, and recordings with higher than 100 MΩ were discarded. Data were acquired at 15 kHz and filtered at 2.4 kHz.
NaCl stimulation of living animals was carried out by puffing a designated concentration of NaCl from a pipette (tip pore size: <10 µm), using Pico Pump (model PV830; World Precision Instruments, Sarasota, FL). The puff capillary tip was placed within the range of 50 µm from animal’s nose, and NaCl buffer was puffed for at least 5 s as shown in Fig. 1B.
The following solutions were used for electrophysiological recordings: ECS consisted of 50 mM NaCl, 150 mM sucrose, 10 mM glucose, 15 mM HEPES, 5 mM CaCl2, 1.0 mM MgCl2, and 5 mM KCl (pH 7.2, ~330 mOsm). For Na+- or Ca2+-removed ECS, 50 mM NaCl or 5 mM CaCl2 were removed, respectively, from normal ECS. For Na+- or Ca2+-replaced ECS, 50 mM NaCl or 5 mM CaCl2 was replaced with 50 mM NMDG or 5 mM MgCl2, respectively. For Na+/Ca2+-removed ECS, 50 mM NaCl and 5 mM CaCl2 were removed from normal ECS. For Na+/Ca2+ replaced-solution, both of 50 mM NaCl and 5 mM CaCl2 were replaced with 50 mM NMDG and 5 mM MgCl2. To Ca2+-removed or Ca2+-replaced ECS, 5 mM EGTA was added. For NaCl stimulation, ECS containing 0 mM, 75 mM, 100 mM, 125 mM, or 150 mM NaCl was puffed to the animal’s nose. To apply nemadipine-A to animals, ECS containing 1.0 μM nemadipine-A was perfused. Osmolarity of all the ECS described above was adjusted to ~330 mOsm with sucrose dissolved in doubly deionized H2O.
The recording pipette solution contained 115 mM K-gluconate, 15 mM KCl, 5 mM MgCl2, 0.25 mM CaCl2, 10 mM HEPES, 20 mM sucrose, 5 mM EGTA, 5 mM Mg2ATP, 0.5 mM Na2GTP, and was adjusted to pH 7.2 and 315 mOsm. To visually observe neuronal cell damage, 10 μM sulforhodamine was included in pipette solution. Chemical reagents were obtained from either Sigma Aldrich (St. Louis, MO) or Wako Chemical (Osaka, Japan).
Chemotaxis assay
One day before assay, Day-4 animals (4 days after hatching) were selected and transferred to new NGM plates seeded with OP50. The following animals were used for chemotaxis assay: Wild-type N2; OF1201 (‘Reference’), which cell-specifically produces GFP in ASEL and coelomocytes; OF1202 (‘GFP RNAi’), which cell-specifically produces GFP in coelomocytes, but not in ASEL, and mRFP in the tail; and OF1203 (‘egl-19 RNAi’), which cell-specifically produces GFP in coelomocytes, but not in ASEL, and mRFP in the tail.
Immediately before assay, 5–10 animals were transferred to a chemotaxis (CTX) agar plate, containing 1.5% agar, 1.0 mM CaCl2, 1.0 mM MgSO4, 5 mM potassium phosphate, pH 6.0, with a worm picker, and allowed to run freely on the agar surface in order to remove attached bacteria. After ~30 s, animals were transferred to the center of an NaCl gradient plate using a worm picker. Movements of the animals were recorded at 5 frames/s with a CCD camera (INFINITY3-6URM; Lumenera, Canada) for 10 min. The chemotaxis assay was performed at 20 °C.
NaCl gradient plates were made in square dishes (10 cm × 10 cm; Thermo Fisher Scientific) in a slanted position (with one side ~5-mm higher than the other) by pouring 25 mL of melted CTX agar containing 50 mM NaCl, 1.5% agar, 1.0 mM CaCl2, 1.0 mM MgSO4, 5 mM potassium phosphate, pH 6.0 in the plate. After solidifying, the dish was laid flat and filled with 25 mL melted CTX agar containing 1.5% agar, 1.0 mM CaCl2, 1.0 mM MgSO4, 5 mM potassium phosphate, pH 6.0. The dish was kept open without a lid for 1.0 hr at room temperature for drying. The plate was used for chemotaxis assay 16–22 hrs after casting.
Behavioral analysis
Animal trajectories on CTX agar plates were captured with an ImageJ plug-in (MosaicSuite; MOSAIC Group, Dresden, Germany), and analyzed using a custom R script (https://www.r-project.org/). To quantify chemotactic performance of each strain, we calculated a non-dimensional index for every trajectory: the mean horizontal velocity of animals along the NaCl gradient, <vH>, divided by the mean ‘run’ speed along the trajectory, <vR>, as described previously60. We defined ‘run’ as continuous locomotion for more than 3 s without interruption by a turn in which a directional change larger than 60° occurs for over 1.2 s. Trajectories between 150 s and 250 s were used for the calculation of chemotaxis index since ‘run’ speeds became relatively constant at 150 s after placing animals on CTX plates (Supplementary Fig. S5). At 250 s, animals started to reach the wall of the plate.
Statistical analysis
Statistical analysis was performed using Sigma Plot v13 (Systat Software Inc.) or R v3.4.3. For data analysis of electrophysiology and behavioral assays, one-way ANOVA with Dunn’s post-hoc test was used. For analysis of Ca2+ imaging data, the Steel-Dwass test was used for multiple comparisons. All data points are means ± s.e.m., unless otherwise stated.
Note added
During revision of the manuscript, all-or-none action potentials evoked by current injection was observed in the AWA olfactory neuron61.
Supplementary information
Acknowledgements
We thank Hiroshi Kagoshima for a plasmid, the Caenorhabditis Genetic Center for strains, and laboratory members for their comments on the manuscript. This work was supported by funding from OIST, Japan.
Author Contributions
T.S., M.O.-S. and Y.M. carried out electrophysiology, and analyzed data. T.S. and M.O.-S. wrote the first draft of the manuscript. T.M. generated the strains, designed and performed Ca2+ imaging, and modified the second draft of the manuscript. E.S. designed, and conducted chemotaxis assay, followed by trajectory and data analyses. J.R.W. supervised the electrophysiology and edited the manuscript. I.N.M. designed the entire experiments, analyzed data, and wrote the final version of the manuscript.
Data Availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tomomi Shindou, Mayumi Ochi-Shindou and Takashi Murayama contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-40158-9.
References
- 1.Brette R. Philosophy of the Spike: Rate-Based vs. Spike-Based Theories of the Brain. Front. Syst. Neurosci. 2015;9:151. doi: 10.3389/fnsys.2015.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Rullen R, Thorpe SJ. Rate coding versus temporal order coding: what the retinal ganglion cells tell the visual cortex. Neural. Comput. 2001;13:1255–1283. doi: 10.1162/08997660152002852. [DOI] [PubMed] [Google Scholar]
- 3.Baden T, Euler T, Weckstrom M, Lagnado L. Spikes and ribbon synapses in early vision. Trends Neurosci. 2013;36:480–488. doi: 10.1016/j.tins.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Rydqvist B, Lin JH, Sand P, Swerup C. Mechanotransduction and the crayfish stretch receptor. Physiol. Behav. 2007;92:21–28. doi: 10.1016/j.physbeh.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 5.Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- 6.Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacological Reviews. 2005;57:387–395. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- 7.Geffeney SL, et al. DEG/ENaC but not TRP channels are the major mechanoelectrical transduction channels in a C. elegans nociceptor. Neuron. 2011;71:845–857. doi: 10.1016/j.neuron.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindsay TH, Thiele TR, Lockery SR. Optogenetic analysis of synaptic transmission in the central nervous system of the nematode Caenorhabditis elegans. Nat. Commun. 2011;2:306. doi: 10.1038/ncomms1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu P, Chen B, Wang ZW. SLO-2 potassium channel is an important regulator of neurotransmitter release in Caenorhabditis elegans. Nat. Commun. 2014;5:5155. doi: 10.1038/ncomms6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu P, Chen B, Mailler R, Wang ZW. Antidromic-rectifying gap junctions amplify chemical transmission at functionally mixed electrical-chemical synapses. Nat. Commun. 2017;8:14818. doi: 10.1038/ncomms14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- 12.Ramot D, MacInnis BL, Goodman MB. Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat. Neurosci. 2008;11:908–915. doi: 10.1038/nn.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Q, Hollopeter G, Jorgensen EM. Graded synaptic transmission at the Caenorhabditis elegans neuromuscular junction. Proc. Natl. Acad. Sci. USA. 2009;106:10823–10828. doi: 10.1073/pnas.0903570106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis RE, Stretton AOW. Passive membrane properties of motorneurons and their role in long-distance signaling in the nematode Ascaris. J. Neurosci. 1989;9:403–414. doi: 10.1523/JNEUROSCI.09-02-00403.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis RE, Stretton AOW. Signaling properties of Ascaris motorneurons: graded active responses, graded synaptic transmission, and tonic transmitter release. J. Neurosci. 1989;9:415–425. doi: 10.1523/JNEUROSCI.09-02-00415.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman MB, Hall DH, Avery L, Lockery SR. Active currents regulate sensitivity and dynamic range in C. elegans neurons. Neuron. 1998;20:763–772. doi: 10.1016/S0896-6273(00)81014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mellem JE, Brockie PJ, Madsen DM, Maricq AV. Action potentials contribute to neuronal signaling in C. elegans. Nat. Neurosci. 2008;11:865–867. doi: 10.1038/nn.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raizen DM, Avery L. Electrical activity and behavior in the pharynx of Caenorhabditis elegans. Neuron. 1994;12:483–495. doi: 10.1016/0896-6273(94)90207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shtonda B, Avery L. CCA-1, EGL-19 and EXP-2 currents shape action potentials in the Caenorhabditis elegans pharynx. J. Exp. Biol. 2005;208:2177–2190. doi: 10.1242/jeb.01615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao S, Zhen M. Action potentials drive body wall muscle contractions in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2011;108:2557–2562. doi: 10.1073/pnas.1012346108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu P, et al. Genetic dissection of ion currents underlying all-or-none action potentials in C. elegans body-wall muscle cells. J. Physiol. 2011;589:101–117. doi: 10.1113/jphysiol.2010.200683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larkum ME, Kaiser KMM, Sakmann B. Calcium electrogenesis in distal apical dendrites of layer 5 pyramidal cells at a critical frequency of back-propagating action potentials. Proc. Natl. Acad. Sci. USA. 1999;96:14600–14604. doi: 10.1073/pnas.96.25.14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérez-Garci E, Larkum ME, Nevian T. Inhibition of dendritic Ca2+ spikes by GABAB receptors in cortical pyramidal neurons is mediated by a direct Gi/o-beta-subunit interaction with Cav1 channels. J. Physiol. 2013;591:1599–1612. doi: 10.1113/jphysiol.2012.245464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catterall W, Perez-Reyes E, Snutch T, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 25.Frokjaer-Jensen C, et al. Effects of voltage-gated calcium channel subunit genes on calcium influx in cultured C. elegans mechanosensory neurons. J. Neurobiol. 2006;66:1125–1139. doi: 10.1002/neu.20261. [DOI] [PubMed] [Google Scholar]
- 26.Mathews EA, et al. Critical residues of the Caenorhabditis elegans unc-2 voltage-gated calcium channel that affect behavioral and physiological properties. J. Neurosci. 2003;23:6537–6545. doi: 10.1523/JNEUROSCI.23-16-06537.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. Comp. Neurol. 1975;160:313–337. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 28.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 29.Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–742. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- 30.Murayama T, Takayama J, Fujiwara M, Maruyama IN. Environmental alkalinity sensing mediated by the transmembrane guanylyl cyclase GCY-14 in C. elegans. Curr. Biol. 2013;23:1007–1012. doi: 10.1016/j.cub.2013.04.052. [DOI] [PubMed] [Google Scholar]
- 31.Ortiz CO, et al. Lateralized gustatory behavior of C. elegans is controlled by specific receptor-type guanylyl cyclases. Curr. Biol. 2009;19:996–1004. doi: 10.1016/j.cub.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki H, et al. Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature. 2008;454:114–117. doi: 10.1038/nature06927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komatsu H, et al. Functional reconstitution of a heteromeric cyclic nucleotide-gated channel of Caenorhabditis elegans in cultured cells. Brain Res. 1999;821:160–168. doi: 10.1016/S0006-8993(99)01111-7. [DOI] [PubMed] [Google Scholar]
- 34.Ward A, Liu J, Feng Z, Xu XZ. Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nat. Neurosci. 2008;11:916–922. doi: 10.1038/nn.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grienberger C, Chen X, Konnerth A. Dendritic function in vivo. Trends. Neurosci. 2015;38:45–54. doi: 10.1016/j.tins.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Stuart GJ, Spruston N. Dendritic integration: 60 years of progress. Nat. Neurosci. 2015;18:1713–1721. doi: 10.1038/nn.4157. [DOI] [PubMed] [Google Scholar]
- 37.Kwok TCY, et al. A small-molecule screen in C. elegans yields a new calcium channel antagonist. Nature. 2006;441:91–95. doi: 10.1038/nature04657. [DOI] [PubMed] [Google Scholar]
- 38.Schafer WR, Kenyon CJ. A calcium-channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature. 1995;375:73–78. doi: 10.1038/375073a0. [DOI] [PubMed] [Google Scholar]
- 39.Steger KA, Shtonda BB, Thacker C, Snutch T, Avery L. The C. elegans T-type calcium channel CCA-1 boosts neuromuscular transmission. J. Exp. Biol. 2005;208:2191–2203. doi: 10.1242/jeb.01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Humphrey JA, et al. A putative cation channel and its novel regulator: cross-species conservation of effects on general anesthesia. Curr. Biol. 2007;17:624–629. doi: 10.1016/j.cub.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 41.Lu B, et al. The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell. 2007;129:371–383. doi: 10.1016/j.cell.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 42.Yeh E, et al. A putative cation channel, NCA-1, and a novel protein, UNC-80, transmit neuronal activity in C. elegans. PLoS Biol. 2008;6:e55. doi: 10.1371/journal.pbio.0060055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iino Y, Yoshida K. Parallel use of two behavioral mechanisms for chemotaxis in Caenorhabditis elegans. J. Neurosci. 2009;29:5370–5380. doi: 10.1523/JNEUROSCI.3633-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujiwara M, et al. The Importance of cGMP Signaling in Sensory Cilia for Body Size Regulation in Caenorhabditis elegans. Genetics. 2015;201:1497–1510. doi: 10.1534/genetics.115.177543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maruyama, I. N. Receptor Guanylyl Cyclases in Sensory Processing. Front. Endocrinol (2017). [DOI] [PMC free article] [PubMed]
- 46.Adachi T, et al. Reversal of salt preference is directed by the insulin/PI3K and Gq/PKC signaling in Caenorhabditis elegans. Genetics. 2010;186:1309–1319. doi: 10.1534/genetics.110.119768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith HK, et al. Defining specificity determinants of cGMP mediated gustatory sensory transduction in Caenorhabditis elegans. Genetics. 2013;194:885–901. doi: 10.1534/genetics.113.152660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joubert SJL, De Lean A. Reduced activity of the NPR-A kinase triggers dephosphorylation and homologous desensitization of the receptor. Biochemistry. 2001;40:11096–11105. doi: 10.1021/bi010580s. [DOI] [PubMed] [Google Scholar]
- 49.Potter LR, Garbers DL. Dephosphorylation of the guanylyl cyclase-A receptor causes desensitization. J. Biol. Chem. 1992;267:14531–14534. [PubMed] [Google Scholar]
- 50.Schroter J, et al. Homologous desensitization of guanylyl cyclase A, the receptor for atrial natriuretic peptide, is associated with a complex phosphorylation pattern. FEBS J. 2010;277:2440–2453. doi: 10.1111/j.1742-4658.2010.07658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daniels SA, Ailion M, Thomas JH, Sengupta P. egl-4 acts through a transforming growth factor-beta/SMAD pathway in Caenorhabditis elegans to regulate multiple neuronal circuits in response to sensory cues. Genetics. 2000;156:123–141. doi: 10.1093/genetics/156.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J, et al. C. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog. Nat. Neurosci. 2010;13:715–722. doi: 10.1038/nn.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Halloran DM, Hamilton OS, Lee JI, Gallegos M, L’Etoile ND. Changes in cGMP Levels Affect the Localization of EGL-4 in AWC in Caenorhabditis elegans. PLoS One. 2012;7:e31614. doi: 10.1371/journal.pone.0031614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomez M, et al. Ca2+ signaling via the neuronal calcium sensor-1 regulates associative learning and memory in C. elegans. Neuron. 2001;30:241–248. doi: 10.1016/S0896-6273(01)00276-8. [DOI] [PubMed] [Google Scholar]
- 55.Hukema RK, Rademakers S, Dekkers MPJ, Burghoorn J, Jansen G. Antagonistic sensory cues generate gustatory plasticity in Caenorhabditis elegans. EMBO J. 2006;25:312–322. doi: 10.1038/sj.emboj.7600940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuhara A, Inada H, Katsura I, Mori I. Negative regulation and gain control of sensory neurons by the C. elegans calcineurin TAX-6. Neuron. 2002;33:751–763. doi: 10.1016/S0896-6273(02)00607-4. [DOI] [PubMed] [Google Scholar]
- 57.Larsch J, Ventimiglia D, Bargmann CI, Albrecht DR. High-throughput imaging of neuronal activity in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2013;110:E4266–4273. doi: 10.1073/pnas.1318325110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larsch J, et al. A Circuit for Gradient Climbing in C. elegans Chemotaxis. Cell Rep. 2015;12:1748–1760. doi: 10.1016/j.celrep.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo L, et al. Dynamic encoding of perception, memory, and movement in a C. elegans chemotaxis circuit. Neuron. 2014;82:1115–1128. doi: 10.1016/j.neuron.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Q, Kidd PB, Dobosiewicz M, Bargmann CI. C. elegans AWA olfactory neurons fire calcium-mediated all-or-none action potentials. Cell. 2018;175:57–70. doi: 10.1016/j.cell.2018.08.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.