Abstract
Sleep is essential to all animals with a nervous system. Nevertheless, the core cellular function of sleep is unknown, and there is no conserved molecular marker to define sleep across phylogeny. Time-lapse imaging of chromosomal markers in single cells of live zebrafish revealed that sleep increases chromosome dynamics in individual neurons but not in two other cell types. Manipulation of sleep, chromosome dynamics, neuronal activity, and DNA double-strand breaks (DSBs) showed that chromosome dynamics are low and the number of DSBs accumulates during wakefulness. In turn, sleep increases chromosome dynamics, which are necessary to reduce the amount of DSBs. These results establish chromosome dynamics as a potential marker to define single sleeping cells, and propose that the restorative function of sleep is nuclear maintenance.
Do single neurons require sleep and what is the conserved cellular function of sleep? In this paper, the authors use real-time imaging of chromosomes in individual cells within live zebrafish to show that sleep increases chromosome dynamics, which are necessary to reduce DNA damage that is accumulated during wakefulness.
Introduction
Sleep is vital to animal life and is found in all studied animals, ranging from jellyfish to worm, fly, zebrafish, rodents, and humans1–5. Prolonged sleep deprivation can be lethal, and sleep disturbances are associated with various deficiencies in brain performance6. Sleep is regulated by circadian and homeostatic processes7, and is coupled with reduced awareness of the environment and a high risk for survival. Several mechanisms can explain the roles of sleep, ranging from macromolecule biosynthesis, energy conservation, and metabolite clearance, to synaptic plasticity and memory consolidation8–12. However, why sleep has evolved and which fundamental ancestral functions it regulates, remain enigmatic.
In mammals and birds, sleep is defined by behavioral criteria and cycles of electroencephalographic (EEG) patterns, which differentiate between wakefulness and sleep states. In non-mammalian animals, including zebrafish, sleep is solely defined by behavioral criteria, such as periods of immobility associated with a species-specific posture and an increased threshold of arousal to external stimuli13–16. In all animals, including animals with simple neuronal networks2,3, circuits of sleep- and wake-promoting neurons orchestrate the behavioral states17. Evidence across multiple animals supports the notion that sleep can occur locally in the brain18 or perhaps even in a small number of cells19. Nevertheless, although sleep significantly contributes to the overall temporal organization of the transcriptome20,21, there are no molecular markers that can be reliably used across phylogeny to define sleep in a single cell22.
The nuclear architecture and the dynamic changes in chromatin organization regulate vital cellular processes, including epigenetics, genomic stability, transcription, cell cycle, and DNA replication and repair23,24. Chromatin dynamics, such as chromosome movements and structural genomic arrangements, are regulated by proteins that interact with the nuclear lamina and envelope in dividing cells25–27. In mature and non-dividing neurons, the role of chromatin dynamics is less understood28. Accumulating evidence showed that chromatin remodeling is implicated in circadian function. The changes in chromatin organization and epigenetic landscape shape the expression profile of a large number of rhythmic genes29. However, the effect of sleep on chromatin dynamics in neurons is unknown.
Recent works showed that sleep can be induced by cellular stress in Caenorhabditis elegans and mammals30,31. Moreover, sleep has been associated with the faster repair of DNA double-strand breaks (DSBs) in mice and fruit flies32. The causes of DSBs are diverse and include reactive oxygen species (ROS), ionizing radiation, and inadvertent action of nuclear enzymes33. Notably, neuronal activity can also induce DSBs. In specific mouse neurons, DSBs can be generated by physiological brain activity during natural exploration of the environment34. Furthermore, activity-induced DSBs facilitate the expression of immediate early genes in mouse and cell cultures, possibly because they resolve topological constraints in the genome35.
We hypothesized that sleep has evolved in order to enable single neurons to perform nuclear maintenance. To test which nuclear process favors sleep time, real-time imaging of chromosome dynamics, neuronal activity as well as quantification of DSBs and sleep, were coupled with genetic and pharmacological manipulations in live zebrafish. The findings propose a definition for a single sleeping neuron; i.e., increased chromosome dynamics, and suggest a role for sleep; i.e., nuclear maintenance.
Results
Imaging chromosome dynamics in live larvae
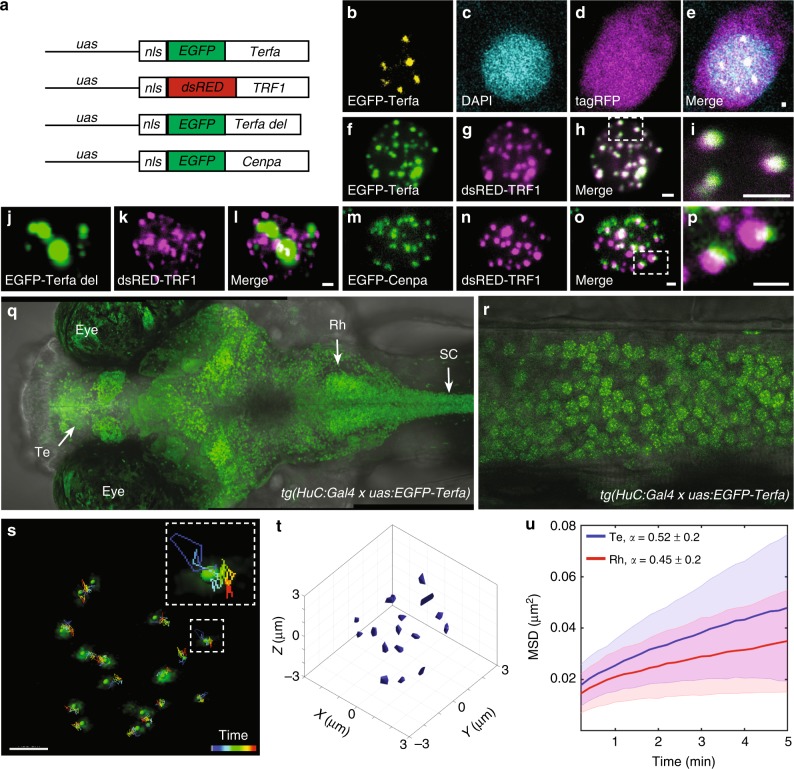
Chromatin dynamics constitute a fundamental component of genome regulation and cell function36. In order to visualize and quantify chromosome dynamics in live zebrafish, the zebrafish telomeric repeat binding factor a (terfa) was cloned, and the telomere marker EGFP-Terfa was expressed in zebrafish neurons, resulting in a nucleus-specific punctum pattern (Fig. 1a–e). To verify that EGFP-Terfa marks chromosomes, the human telomere marker uas:dsRED-TRF137 was co-injected with either uas:EGFP-Terfa or the DNA binding-site-deleted construct uas:EGFP-Terfa del into one-cell-stage tg(HuC:Gal4) embryos. While zebrafish and human telomeric markers co-localized (Fig. 1f–i), deletion of the Terfa DNA binding site resulted in non-specific protein aggregates in the nucleus (Fig. 1j–l). To further validate that the puncta mark chromosomes, the zebrafish centromere protein a (cenpa) was cloned, and the EGFP-Cenpa was used as a centromere marker. Two-color imaging showed that EGFP-Cenpa and dsRED-TRF1 puncta were expressed adjacently on the chromosome, but not co-localized, as expected from telomeric and centromeric markers (Fig. 1m–p). To continuously image chromosome dynamics in all neurons of live fish, a stable tg(uas:EGFP-Terfa) transgenic line was generated and crossed with tg(HuC:Gal4) zebrafish (Fig. 1q, r). Neurons in the telencephalon (Te), rhombencephalon (Rh), spinal cord (SC), and habenula (Hb), of 6-day post-fertilization (dpf) larvae were imaged during 9.5 min. Single-particle tracking (SPT) analysis38 was used to detect and quantify the motility of telomere trajectories (Supplementary Movie 1, Fig. 1s, t). While telomeres in the Rh, SC, and Hb neurons showed a similar volume of motion of 0.0069 ± 0.0002, 0.0066 ± 0.0003, and 0.0063 ± 0.0004 µm3, respectively, telomeres in Te neurons showed increased volume of motion (0.01 ± 0.0004 µm3, Supplementary Fig. 1a). Calculation of the mean square displacement (MSD) for single trajectories of all Te and Rh neurons demonstrated anomalous subdiffusion of the puncta (Fig. 1u), which is typical to chromosome diffusion38. Indeed, analysis of both telomeric and centromeric markers that are expressed in the same SC neurons (Fig. 1m–p, Supplementary Movie 2) showed similar dynamics (Supplementary Fig. 1b), verifying that the puncta mark chromosomes. Altogether, these results transfer in vitro chromatin experiments to whole organisms, and demonstrate the capability of monitoring chromosome dynamics in live larvae.
Fig. 1.
Imaging single chromosome dynamics in live larvae. a The DNA constructs were used to express telomere, centromere, and truncated telomere markers. b–e One-plane view of a representative neuron that expresses cellular tagRFP (magenta) and nuclear EGFP-Terfa (yellow) telomeric markers in live larvae. f–i Co-localization of zebrafish (f) and human (g) telomeric markers in the nucleus of a zebrafish neuron. j–l Co-expression of truncated zebrafish (j) and human (k) telomeric markers. m–p Co-expression of centromere (m) and telomere (n) markers. Dorsal (q) and lateral (spinal cord, r) views of 6-dpf tg(HuC:Gal4)/tg(uas:EGFP-Terfa) larvae (Te telencephalon, Rh rhombencephalon, SC spinal cord). s Live time-lapse imaging of a single neuron nucleus shows the movement of chromosomes measured over 9.5 min. Dashed box shows high magnification of a single trajectory. t 3D single-particle tracking (SPT) shows the volume of motion of chromosomes in a single nucleus. u In all Te and Rh neurons, the time-averaged mean square displacement (MSD) for telomeres trajectory (Te: n = 340 chromosomes, Rh: n = 475 chromosomes). Lines represent the means, and the shaded area represents the standard deviation (SD). Scale bar = 1 µm
Sleep increases chromosome dynamics in neurons
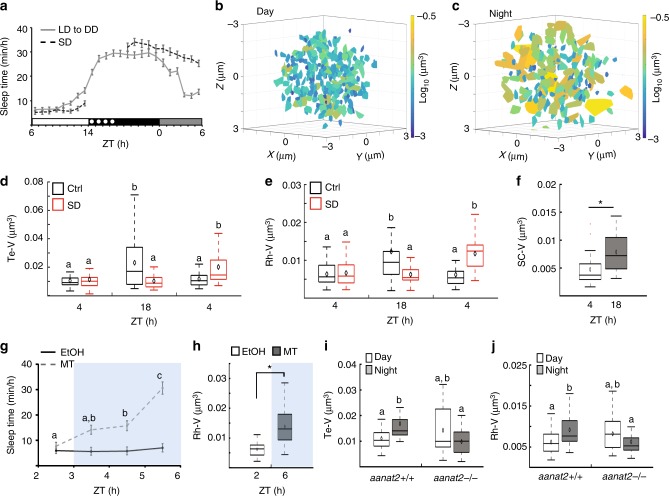
In the diurnal zebrafish larvae, sleep is regulated by circadian and homeostatic processes and is defined as at least 1 min of immobility accompanied by an increased arousal threshold15,39. Continuous video tracking of behavior showed that, as is the case in WT larvae40, 6 dpf tg(HuC:Gal4)/tg(uas:EGFP-Terfa) larvae sleep more during the night, under a 14 h light/10 h dark to constant darkness (LD to DD) cycle (day: 8.6 ± 0.7 min/h; night: 28.05 ± 1.2 min/h; subjective day: 17.8 ± 1.01 min/h; Fig. 2a). Moreover, analysis of larval activity specifically during the wake bouts showed that the average time of activity is reduced during the night compared to the day (day: 5.6 ± 0.25 s/min; night: 1.6 ± 0.06 s/min, Supplementary Fig. 2). The behavioral immobility and reduced sensory input during sleep may favor behavioral-state-dependent cellular processes in neurons. Alterations in chromatin structure can affect a variety of nuclear processes, including genome stability, transcription, DNA repair, chromosome segregation, and condensation24,37. To test whether chromosome dynamics change between day and night, single nuclei were imaged in Te and Rh neurons of 6 dpf tg(HuC:Gal4)/tg(uas:EGFP-Terfa) larvae during the day [zeitgeber time 4 (ZT4)], night (ZT18), and the following subjective day (ZT4, Fig. 2a). Rh and Te neurons were selected because these regions regulate locomotor activity and cognition, and because these neurons exhibit standard and high levels of chromosome dynamics, respectively (Supplementary Fig. 1a). Remarkably, the time-lapse imaging of telomere markers showed that chromosome dynamics increased by approximately two-fold during nighttime sleep in both brain regions (average volume of motion, day: Rh—0.006 ± 0.0006 µm3, Te—0.01 ± 0.0008 µm3; night: Rh—0.012 ± 0.002 µm3, Te—0.023 ± 0.003 µm3; subjective day: Rh—0.006 ± 0.0005 µm3, Te—0.011 ± 0.001 µm3, Fig. 2b–e, Supplementary Movie 3, 4). Using centromeric markers, similar changes in chromosome dynamics were visualized in SC neurons (day: 0.0047 ± 0.0004 µm3; night: 0.0078 ± 0.0006 µm3, Fig. 2f, Supplementary Movie 2). In order to differentiate between sleep and circadian effect, the 6 dpf tg(HuC:Gal4)/tg(uas:EGFP-Terfa) larvae were sleep-deprived for 4 h during the night, and behavioral sleep rebound was observed during the following subjective day (ZT4; LD to DD: 12.4 ± 0.8 min/h; SD: 27.5 ± 1.3 min/h, Fig. 2a). Immediately following SD, chromosome dynamics were reduced by approximately two-fold (Rh—0.0062 ± 0.0005 µm3, Te—0.01 ± 0.0009 µm3, Fig. 2d, e) compared with the levels observed during the night in the control group, and were similar to the levels observed during the day. On the following day, after 10 h of recovery, when the sleep-deprived larvae demonstrated sleep rebound (Fig. 2a), chromosome dynamics increased by approximately two-fold (Rh—0.011 ± 0.0008 µm3, Te—0.02 ± 0.002 µm3, Fig. 2d, e) in the sleep-deprived larvae, and reached the levels observed during nighttime sleep in the sibling control larvae. These results show that sleep increases chromosome dynamics in a homeostatic-dependent manner.
Fig. 2.
Sleep increases chromosome dynamics in neurons. a Recording of sleep was performed in 6 dpf control or sleep-deprived larvae under an 14 h light/10 h dark cycle following by constant darkness (LD to DD, control: n = 119 larvae, sleep deprivation (SD): n = 96 larvae). b, c The area scanned by all chromosomes from all imaged Te neurons during 9.5 min (day: n = 26 cells; night: n = 29 cells). Color was coded according to the levels of volume of motion. d–f Volume of chromosome dynamics over 9.5 min per cell (EGFP-Terfa in d and e, EGFP-Cenpa in f). d Te neurons, ctrl: day (n = 26 cells), night (n = 29 cells), subjective day (n = 25 cells). SD: day (n = 26 cells), night (n = 35 cells), subjective day (n = 25 cells). P = 1.3 × 10−7, F = 17.54, degrees of freedom = 2. e Rh neurons, ctrl: day (n = 23 cells), night (n = 30 cells), subjective day (n = 35 cells). SD: day (n = 27 cells), night (n = 25 cells), subjective day (n = 26 cells). P = 7 × 10−6, F = 12.82, degrees of freedom = 2, determined by two-way ANOVA followed by Tukey test. f SC neurons: day (n = 33 cells), night (n = 33 cells). *P = 0.0001, determined by two-tailed t-test: two samples assuming unequal variance. Red crosses indicate outliers. g, h Monitoring sleep and chromosome dynamics under melatonin (MT) treatment. Blue background represents time under treatment. g Pretreated and EtOH/MT-treated larvae (EtOH: n = 48; MT: n = 48 larvae). P = 7.4 × 10−4, F = 14.3, degrees of freedom = 3, determined by two-way ANOVA followed by Tukey test. Values are presented as means ± SEM. h Pre-treated cells (n = 22) and MT-treated cells (n = 25). *P = 7 × 10−6, determined by two-tailed t-test: two samples assuming unequal variance. i Te neurons, aanat2 +/+ : day (n = 16 cells), night (n = 21 cells). aanat2−/−: day (n = 14 cells), night (n = 32 cells). P = 1.7 × 10−3, F = 12.26, degrees of freedom = 1. j Rh neurons, aanat2+/+ : day (n = 24 cells), night (n = 24 cells). aanat2−/−: day (n = 19 cells), night (n = 30 cells). P = 7.6 × 10−4, F = 10.42, degrees of freedom = 1, determined by two-way ANOVA followed by Tukey test. Boxplots: black diamond represents the mean, boxes indicate the median and the 25th-to-75th percentiles, whiskers extend to the most extreme data points. Letters or asterisks indicate significant differences. ZT zeitgeber time
To further validate the regulation of chromosome dynamics by the sleep state, the tg(HuC:Gal4)/tg(uas:EGFP-Terfa) larvae were treated during the day with melatonin, which is a strong sleep-promoting hormone in the diurnal zebrafish14,41. Sleep time and chromosome dynamics were monitored prior to and during melatonin treatment. As expected, while ethanol (EtOH) administration did not affect sleep time, under 3 h of melatonin treatment, sleep increased in melatonin-treated larvae (prior to melatonin treatment: 7.7 ± 1.5 min/h; 3 h following melatonin treatment: 30.7 ± 2.4 min/h, Fig. 2g). In accordance, melatonin-derived sleep increases chromosome dynamics (prior to melatonin treatment: 0.006 ± 0.0005 µm3; during melatonin treatment: 0.013 ± 0.0013 µm3, Fig. 2h). Thus, sleep is sufficient to increase chromosome dynamics. In order to understand if sleep is not only sufficient but also necessary to increase chromosome dynamics, we crossed the tg(HuC:Gal4)/tg(uas:EGFP-Terfa) zebrafish with arylalkylamine N-acetyltransferase-242 mutant zebrafish (aanat2−/−), which lack melatonin signaling. The aanat2−/− larvae exhibit reduced sleep time during the night, although their intrinsic molecular circadian clock is intact43. Imaging single neurons during day and night revealed that while chromosome dynamics increased in aanat2+/+ larvae during the night (day: Rh—0.006 ± 0.0006 µm3, Te—0.01 ± 0.001 µm3; night: Rh—0.009 ± 0.0009 µm3, Te—0.014 ± 0.0008 µm3, Fig. 2i, j), as was the case in WT larvae (Fig. 2d, e), it was reduced during the night in aanat2−/− (night: Rh—0.006 ± 0.0006 µm3, Te—0.009 ± 0.0009 µm3) compared with aanat2+/+ larvae. Thus, although the molecular circadian clock is intact, chromosome dynamics were similar in both day and night, which is in accordance with the reduced nighttime sleep in aanat2−/− larvae (Fig. 2i, j). These results show that chromosome dynamics in neurons are regulated by the behavioral sleep/wake state.
Sleep-dependent changes in chromosome dynamics may not be specific to neurons. To test whether these changes are also present in other cell types, we monitored chromosome dynamics in peripheral endothelial and Schwann cells. Chromosome dynamics were imaged during day and night in endothelial and Schwann cells using tg(fli:Gal4)/tg(uas:EGFP-Terfa) and mbp:Gal4-injected tg(uas:EGFP-Terfa) 6 dpf larvae, respectively (Supplementary Fig. 3a, b). Chromosome dynamics did not differ between day and night in both cell types (Supplementary Fig. 3c). These results show that sleep-dependent changes in chromosome dynamics that were observed in neurons, do not occur in endothelial or Schwann cells.
Sleep reduces DSBs that are accumulated during wakefulness
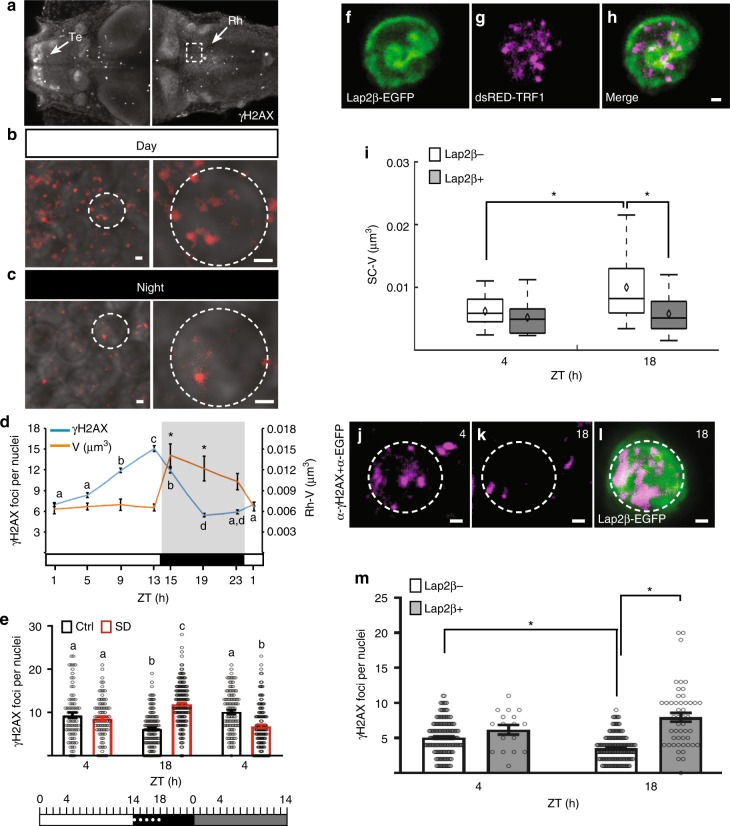
What is the physiological benefit of sleep-dependent chromosome dynamics? Since the genome can be hit by dozens of DSBs per day33,44, we speculated that sleep and increased chromosome dynamics are essential for the recovery from wakefulness-derived DNA damage. To test our hypothesis, the levels of DSBs and chromosome dynamics were monitored in Rh neurons during the 24-h sleep/wake cycle. The γH2AX marker, which is activated as part of the DNA damage response system45, was used to quantify DSBs during day and night in single cells (Fig. 3a–e). Whole head staining showed increased localization of γH2AX in the Te compared to other brain areas, such as the Rh (Fig. 3a). This DSB enrichment is correlated with the increased neuronal activity detected in the Te (Supplementary Fig. 4). In the 24-h experiment, we quantified DSBs in the Rh because it better represents the distribution of γH2AX in the entire CNS. During the day, the number of DSBs consistently increased, and peaked 1 h before darkness (15.1 ± 0.46 γH2AX foci, Fig. 3d). During the night, the number of DSBs dramatically decreased, reached the minimum levels at ZT19 (5.4 ± 0.24 γH2AX foci, Fig. 3d), and remained low until the beginning of the day. Concurrently, during the day, chromosome dynamics remained at similar low levels (ZT1: 0.0063 ± 0.0007; ZT5: 0.0067 ± 0.0005; ZT9: 0.007 ± 0.0008; ZT13: 0.0065 ± 0.0005 µm3, Fig. 3d). In contrast, following 1 h of darkness, chromosome dynamics increased by two-fold, and the high levels were maintained during the entire night (ZT15: 0.014 ± 0.0016; ZT19: 0.012 ± 0.0018; ZT23: 0.01 ± 0.0011 µm3, Fig. 3d). These results show that while chromosome dynamics keep constant low levels, DSB number accumulates during the day. During the night, following robust increase in chromosome dynamics, the number of DSBs was gradually reduced, showing that chromosome dynamics correlate with efficient reduction of DSBs during the night.
Fig. 3.
Chromosome dynamics are essential for reducing the number of accumulating DSBs. a–c Dorsal view of 6 dpf larvae stained with γH2AX. a Arrows indicate the telencephalon (Te) and rhombencephalon (Rh). Dashed box showing the Rh represents the area analyzed in d and e. Representative images from the Rh region during day (b) and night (c) are shown. Dashed circle indicates a single neuron. d The number of DSBs (P = 1 × 10−16, F = 118, degrees of freedom = 7) and chromosome dynamics (*P = 8 × 10−7, F = 7, degrees of freedom = 7) in single nuclei over 24 h, in 14 h light/10 h dark cycle. Determined by one-way ANOVA followed by a Tukey test. Lower case letters indicate significant changes between γH2AX groups. Asterisks indicate significant changes between chromosome dynamics groups. White and black horizontal bars represent light and dark periods, respectively. e Numbers of DSBs (dot plot, means ± SEM). Ctrl: day (n = 83 cells), night (n = 131 cells), subjective day (n = 105 cells). SD: day (n = 86 cells), night (n = 170 cells), subjective day (n = 135 cells). P = 1 × 10−6, F = 62.04, degrees of freedom = 2, determined by two-way ANOVA followed by a Tukey test. White, black, and gray rectangles represent day, night, and subjective day, respectively. Dotted white line represents the sleep deprivation (SD) period. f–h Co-expression of Lap2β-EGFP (f) and human telomeric markers (g) in SC-neuronal nucleus. i Volume of chromosome dynamics in control (Lap2β−, day: n = 25; night: n = 21 cells) and Lap2β-overexpressing cells (Lap2β+, day: n = 25; night: n = 19 cells). *P = 0.026, F = 5.1, degrees of freedom = 1, determined by two-way ANOVA followed by a Tukey test. j–l Representative images of double immunohistochemistry using α-γH2AX (magenta) and α-EGFP (green) in single SC neurons during day (j) and night (k, l). Dashed circle indicates a single nucleus. m The number of DSBs (dot plot, means ± SEM) in control (Lap2β−, day: n = 177; night: n = 177 cells) and Lap2β-overexpressing cells (Lap2β+, day: n = 17; night: n = 49 cells). *P = 1 × 10−6, F = 18.8, degrees of freedom = 1, determined by two-way ANOVA followed by a Tukey test. Zeitgeber time (ZT4)-day, ZT18-night. Boxplots: black diamond represents the mean, boxes indicate the median and the 25th-to-75th percentiles, whiskers extend to the most extreme data points. Letters or asterisks indicate significant differences. Scale bar = 1 µm
In order to distinguish between circadian and sleep effect on DSB levels, we quantified the number of DSBs in sleep-deprived larvae. In the control group, the number of γH2AX foci per cell in the Rh neurons decreased during the night and increased back to daytime levels on the following subjective day (day: 9.3 ± 0.61; night: 6.1 ± 0.36, subjective day: 10.16 ± 0.46, Fig. 3e). In contrast, following SD, the γH2AX foci number increased during the night (11.9 ± 0.37), and then, post-sleep recovery, was reduced on the following subjective day (6.76 ± 0.44, Fig. 3e). These experiments show that DSBs increased during wakefulness and decreased during sleep in neurons.
In order to test whether the amount of DSBs changed between day and night in other cell types, we performed immunostaining assays using both anti-γH2AX and anti-EGFP in tg(fli:EGFP) and tg(mbp:EGFP) 6 dpf larvae (Supplementary Fig. 3d-i). In contrast to the day/night changes observed in neurons, the number of DSBs was constantly low during day and night in endothelial and Schwann cells (Supplementary Fig. 3j), suggesting that wakefulness and sleep do not affect the levels of DSBs in these cells.
Chromosome dynamics are necessary to reduce DSBs
In order to causally link chromosome dynamics and the efficient reduction in DNA damage, we manipulated chromosome dynamics by overexpressing the zebrafish Lamina-associated polypeptide 2 (Lap2β) in specific neurons. This protein physically interacts with lamins and anchors chromatin to the nuclear lamina in mammals and zebrafish46,47. In order to monitor chromosome dynamics and DSBs in Lap2β-overexpressing and control neurons, pT2-uas:Lap2β-EGFP and pT2-uas:dsRED-TRF1 constructs were co-injected into tg(HuC:Gal4) embryos, and neurons that express either both Lap2β-EGFP and dsRED-TRF1 (Fig. 3f–h) or only dsRED-TRF1 in the SC were analyzed during day and night in 6 dpf larvae. As expected, chromosome dynamics increased during the night in the control dsRED-TRF1-expressing neurons (day: 0.006 ± 0.0004; night: 0.009 ± 0.001 µm3, Fig. 3i). In contrast, overexpression of Lap2β inhibited chromosome dynamics, specifically during the night, and the levels were similar during both day and night (day: 0.005 ± 0.0005; night: 0.005 ± 0.0007 µm3, Fig. 3i). Thus, the overexpression of Lap2β impedes the sleep-dependent increase of chromosome dynamics. Next, we measured the number of γH2AX foci in the control and in Lap2β-overexpressing neurons. As was found in the Rh (Fig. 3b–e), the number of γH2AX foci per neuron in the SC decreased by approximately 30% during the night (day: 5.1 ± 0.18; night: 3.5 ± 0.14, Fig. 3j, k, m). Remarkably, the number of γH2AX foci increased by 120% in Lap2β-overexpressing neurons compared with the control neurons during the night (7.95 ± 0.63 γH2AX foci, Fig. 3l, m). These results show that genetic inhibition of chromosome dynamics increases the number of DSBs specifically during nighttime sleep. Furthermore, these results suggest that sleep-dependent increase in chromosome dynamics is necessary to reduce DNA damage.
Neuronal activity can reduce chromosome dynamics
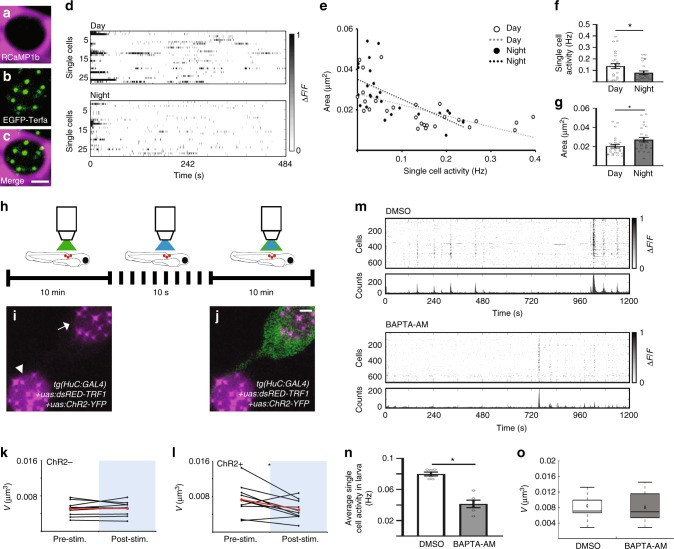
Which cellular processes induce DSBs during wakefulness, and how do they affect chromosome dynamics? In mammals, neuronal activity promotes the formation of DSBs34,35. In zebrafish, we found sleep-dependent changes in the levels of chromosome dynamics and DSBs in neurons (Figs. 2 and 3) but not in two other non-excitable cell types (Supplementary Fig. 3), and showed correlation between increased spontaneous neuronal activity and enriched expression of γH2AX in the Te (Fig. 3a, Supplementary Fig. 4). Therefore, since chromosome dynamics is required to reduce the number of DSBs we rationalized that the intensity and frequency of neuronal activity should affect chromosome dynamics within individual neurons. To study activity-dependent chromosome dynamics, a tg(uas:RCaMP1b) transgenic fish was generated and crossed with the tg(HuC:Gal4)/tg(uas:EGFP-Terfa) line. Then, spontaneous chromosome dynamics and neuronal activity were simultaneously imaged in one plane in single neurons within the Rh during day and night (Fig. 4a–c). The results showed moderate negative correlation between neuronal activity and chromosome dynamics (Fig. 4d–g). While the average frequency of single cell activity was reduced (day: 0.138 ± 0.022 Hz; night: 0.079 ± 0.015 Hz, Fig. 4d–f), the average chromosome dynamics increased during the night in Rh neurons (day: 0.02 ± 0.002 µm2; night: 0.027 ± 0.002 µm2, Fig. 4e, g). Taking into account that neurons in the Rh and SC regulate tail movement and locomotor activity48, which are markedly reduced during sleep, these results show that, at the single-cell level, chromosome dynamics increased in resting cells during the night.
Fig. 4.
Neuronal excitation reduces chromosome dynamics in the Rh and SC. a–c Simultaneous 2D imaging of neuronal activity (magenta) and chromosome dynamics (green) in the Rh neurons. d Raster plot of ΔF/F of 27 and 26 RCaMP1b-expressing single cells during day and night, respectively. Grayscale: ΔF/F amplitude. e A moderate negative correlation between chromosome dynamics and neuronal activity during day and night (RDay = −0.62, RNight = −0.64, RTotal = −0.64), determined by Pearson correlation coefficient. The average single-cell activity (f, *Pactivity = 0.035) and chromosome dynamics (g, *Pdynamics = 0.03) during day and night. Determined by two-tailed t-test: two samples assuming unequal variance. h–l Optogenetic stimulation of neuronal activity reduces chromosome dynamics. h–j The neurons expressed both dsRED-TRF1 and ChR2-YFP (arrow) or only dsRED-TRF1 (arrowhead). k, l Chromosome dynamics before and following the light stimuli (ChR2−, n = 9 cells; ChR2+, n = 10 cells). *P = 0.031, determined by two-tailed t-test: two-paired samples for means. Average change is marked by red line. m–o Inhibition of neuronal activity during the night does not affect chromosome dynamics. m Representative raster plots of ΔF/F in the Rh of 6 dpf tg(HuC:GCaMP5) larvae under DMSO (n = 6 larvae) or BAPTA-AM (n = 6 larvae) treatments. Grayscale: ΔF/F amplitude. Bottom: histogram of the Ca2+ transients of all cells. n Average spontaneous neuronal activity in single neurons of the Rh under either DMSO (n = 6 larvae) or BAPTA-AM (n = 6 larvae) treatment (ZT23). *P = 2 × 10−4, t = 2.3, degrees of freedom = 7, determined by two-tailed t-test: two samples assuming unequal variance. o Volume of chromosome dynamics during nighttime (ZT23) in DMSO (n = 13 cells) and BAPTA-AM (n = 11 cells)-treated larvae. Values are presented as boxplots and means (black diamonds) or as dot plots and means ± SEM. Boxplots indicate the median and the 25th-to-75th percentiles. The whiskers extend to the most extreme data points. ZT zeitgeber time. Scale bar = 2 µm
In order to causally test the effect of neuronal activity on chromosome dynamics, we used optogenetics and transiently expressed the neural-activating cation channel channelrhodopsin-2 (ChR2-YFP)49 and the telomere marker dsRED-TRF1 in neurons of the SC (Fig. 4h–j), which showed similar chromosome dynamics and spontaneous neuronal activity as in Rh neurons (Supplementary Fig. 1a, Supplementary Fig. 4). Chromosome dynamics were quantified in single cells that did or did not express the ChR2-YFP before and following blue light stimulation during daytime wakefulness (Fig. 4h–j). While chromosome dynamics did not change post-stimulation in dsRED-TRF1-expressing cells (Fig. 4k), they were reduced in stimulated dsRED-TRF1+ChR2-YFP-expressing cells (pre-stimulation: 0.0073 ± 0.001 µm3; post-stimulation: 0.005 ± 0.0007 µm3, Fig. 4l). Thus, neuronal activity reduces chromosome dynamics in a single active cell under spontaneous conditions and optogenetic manipulation.
Neuronal activity could affect chromosome dynamics via the formation of DSBs. In order to uncouple neuronal activity from DNA damage, neuronal activity was inhibited specifically during the night, when the DSB level is low. The Ca+2 chelator BAPTA-AM, which lowers intracellular Ca+2 levels, was used. To validate the efficiency of BAPTA-AM in Rh neurons, we quantified spontaneous neuronal activity in BAPTA-AM-treated and control 6 dpf tg(HuC:GCaMP5) larvae. As expected, the average activity of a single Rh neuron was reduced by two-fold in BAPTA-AM-treated larvae compared with the control group (DMSO: 0.08 ± 0.002 Hz; BAPTA-AM: 0.04 ± 0.004 Hz, Fig. 4m, n). In these neurons, chromosome dynamics were monitored at the end of the night (ZT23), when the DNA damage is low (Fig. 3d). Inhibition of neuronal activity did not affect chromosome dynamics, which remain high in both BAPTA-AM-treated and control larvae (DMSO—0.0084 ± 0.001, BAPTA-AM—0.008 ± 0.001 µm3, Fig. 4o). These results indicate that neuronal activity is not an essential regulator of chromosome dynamics; however, it can reduce chromosome dynamics, possibly via the induction of DSBs.
Sleep and chromosome dynamics increased following induction of DSBs
Neuronal activity is only one of several processes that can induce DSBs during wakefulness. The causes for DNA damage are diverse and include intrinsic and extrinsic factors33. In order to simulate the effect of these different factors, we used etoposide (ETO), which induces DSBs45, and the number of γH2AX foci, chromosome dynamics, and sleep time were monitored during the day (Fig. 5a–d). As expected, after 2 h under ETO treatment (ZT2–4), the γH2AX foci number increased (DMSO: 10.44 ± 0.37; ETO: 14.01 ± 0.47, Fig. 5b). Furthermore, sleep time (Fig. 5c) and chromosome dynamics (Fig. 5d) did not change under ETO treatment. However, 1 h following ETO withdrawal, sleep time increased in ETO-treated larvae (DMSO: 6.95 ± 1.1 min/h; ETO: 13.28 ± 1.8 min/h, Fig. 5c), while DSB levels remained high (DMSO: 10.87 ± 0.35; ETO: 13.7 ± 0.46 γH2AX foci, Fig. 5b) and chromosome dynamics remained low (DMSO: 0.0065 ± 0.0004; ETO: 0.0094 ± 0.001 µm3, Fig. 5d). Notably, following 2 h of recovery from the ETO treatment, sleep time remained high (DMSO: 6.71 ± 1 min/h; ETO: 11.4 ± 1.8 min/h, Fig. 5c), and chromosome dynamics increased by approximately two-fold (DMSO: 0.0062 ± 0.0005; ETO: 0.015 ± 0.0017 µm3, Fig. 5d), accompanied by a reduction of the number of DSBs (9.65 ± 0.42 γH2AX foci, Fig. 5b). In order to examine how the formation of DSBs will affect sleep time and chromosome dynamics during the night, we exposed the larvae to ETO for 2 h (ZT16–18). Similar to the results obtained during the day, under ETO treatment, the γH2AX foci number increased by 60% (DMSO: 5.4 ± 0.33; ETO: 8.66 ± 0.4, Fig. 5e) and sleep time did not change (Fig. 5f). However, chromosome dynamics decreased by approximately two-fold (DMSO: 0.0126 ± 0.003; ETO: 0.0049 ± 0.0004 µm3, Fig. 5g). These results suggest that while chromosome dynamics are low during the formation of DSBs, the accumulation of DSBs during wakefulness triggers sleep, which increases chromosome dynamics and eventually reduces the number of DSBs.
Fig. 5.
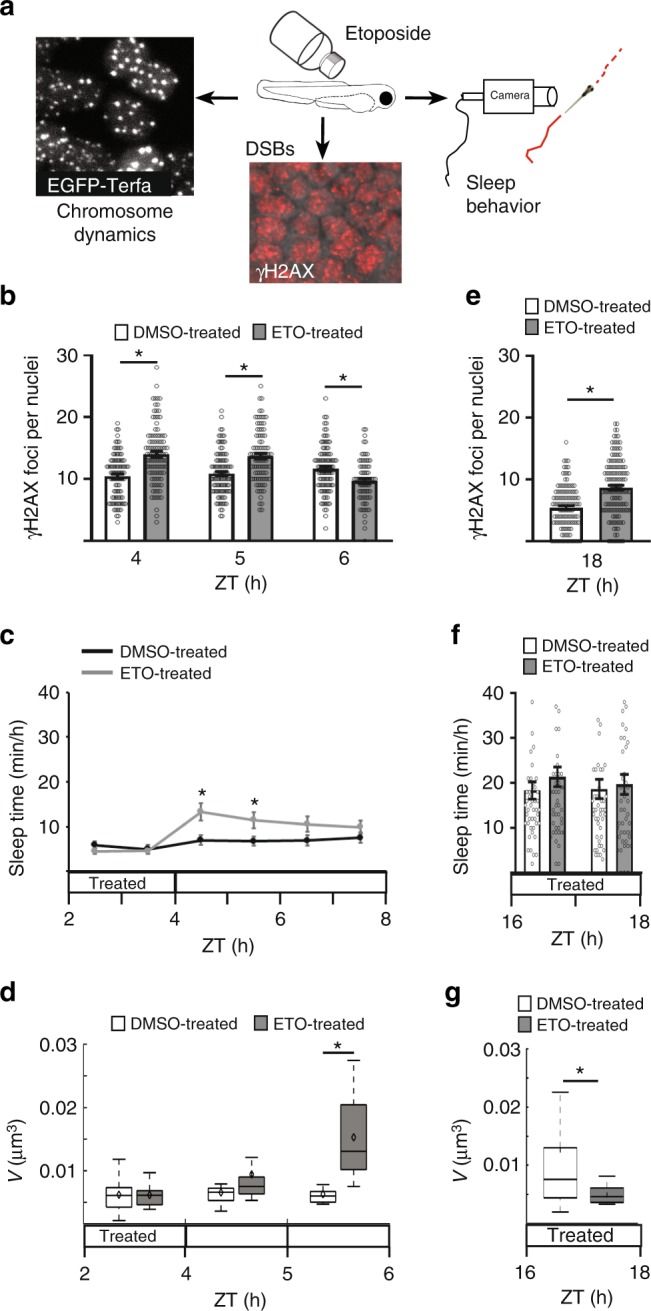
Sleep and chromosome dynamics increased following induction of DSBs. a Larvae were treated with etoposide (ETO) during the day (ZT2–4, b–d) and night (ZT16–18, e–g), and DSBs, chromosome dynamics, and sleep were quantified. b Number of γH2AX foci in single nuclei. During treatment (DMSO: n = 93 cells; ETO: n = 99 cells, *P = 2.2 × 10−8), 1 h following treatment (DMSO: n = 102 cells; ETO: n = 89 cells, *P = 2.2 × 10−6), 2 h following treatment (DMSO: n = 98 cells; ETO: n = 73 cells, *P = 6.8 × 10−4). c Total sleep time. DMSO-treated (n = 84 larvae), ETO-treated (n = 78 larvae), PZT2–3 = 0.18, PZT3–4 = 0.71. Post-treatment: DMSO-treated (n = 84 larvae), ETO-treated (n = 78 larvae), *PZT4–5 = 0.0035, *PZT5–6 = 0.02, PZT6–7 = 0.07, PZT7–8 = 0.2. d Chromosome dynamics in neurons. During treatment (DMSO: n = 22 cells; ETO: n = 11 cells), 1 h following treatment (DMSO: n = 17 cells; ETO: n = 13 cells), 2 h following treatment (DMSO: n = 11 cells; ETO: n = 16 cells, *P = 3.8 × 10−5). Red crosses indicate outliers. e–g Number of γH2AX foci in single neuronal nuclei (e, DMSO: n = 118; ETO: n = 138 cells, *P = 1.65 × 10−9), total sleep time (f, DMSO: n = 48; ETO: n = 48 larvae) and chromosome dynamics (g, DMSO: n = 22; ETO: n = 14 cells, *P = 0.02) in DMSO- and ETO-treated larvae during the night. Significant differences of all experiments were determined by two-tailed t-test: two samples assuming unequal variance. Values are presented as boxplots and means (black diamonds) or as dot plots and means ± SEM. Boxplots indicate the median and the 25th-to-75th percentiles. The whiskers extend to the most extreme data points. ZT zeitgeber time. Scale bar = 1 µm
Discussion
The beneficial role of sleep in simple neuronal networks and in the complex brain is a mystery50,51, and it is unclear if and why a single neuron requires sleep. The methods developed in this work enable the study of sleep-dependent nuclear processes in single cells of live animals. We visualized 3D motions of individual chromosomes, DSBs, neuronal activity and behavior in live zebrafish, and directly linked the physiology of single neurons to the entire organism during sleep and wakefulness. Genetic and pharmacological manipulation of the melatonin signaling, as well as SD experiments, revealed that chromosome dynamics increased about two-fold during sleep in neurons. These sleep-dependent changes were not observed in two other non-excitable cell types. In neurons, DSBs accumulate during wakefulness and SD, when chromosome dynamics are low. Imaging of spontaneous neuronal activity, single-cell optogenetic and pharmacological experiments indicated that DSB formation by neuronal activity and other factors, can reduce chromosome dynamics. In turn, sleep benefits the brain because it increases chromosome dynamics, which are essential for the efficient reduction of the number of DSBs (Fig. 6).
Fig. 6.
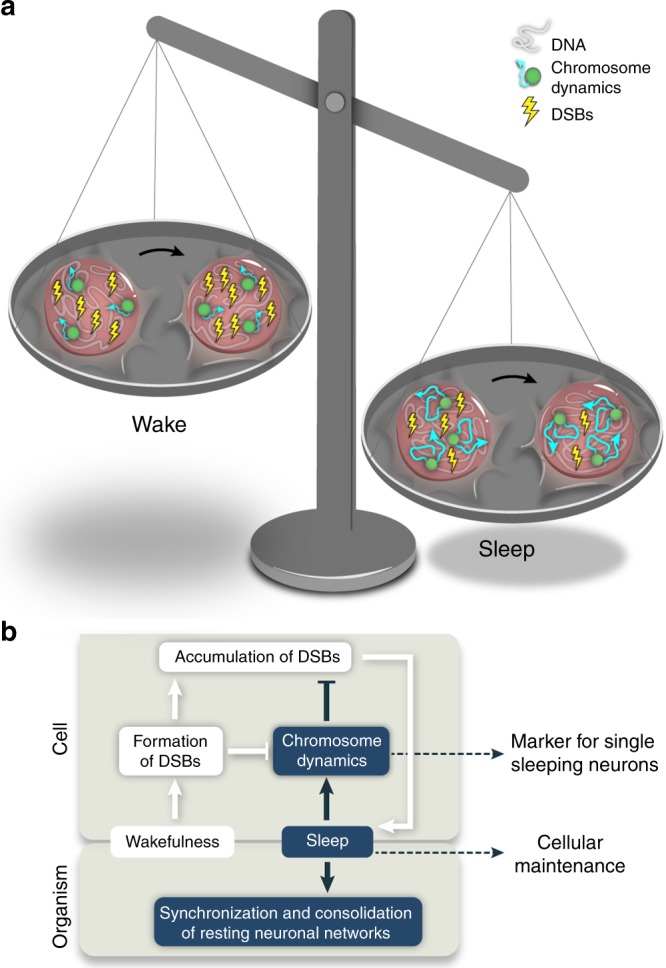
A proposed function for sleep. a During wakefulness, chromosome dynamics are low and the number of DSBs is accumulated in neurons. The beneficial role of sleep is to increase the chromosome dynamics that are essential for the efficient reduction of the number of DSBs in single neurons. b DSBs are increased in the nucleus during wakefulness and are formed by intrinsic and extrinsic factors, such as neuronal activity, irradiation, and oxidative stress, when chromosome dynamics are low. At a given threshold, accumulations of DSBs in multiple neuronal networks can trigger sleep, which increases chromosome dynamics that are necessary for the reduction of DNA damage. This mechanism suggests that chromosome dynamics can define single sleeping neurons, and that one of the functions of sleep is nuclear maintenance
Why do chromosome dynamics increase during sleep? The dynamic organization of the genome is often altered in diseases52, and changes in chromatin dynamics have been shown to regulate key nuclear processes, including epigenetics, DNA damage and repair, transcription, and enhancer–promoter interaction. For example, transcriptional activation by enhancers and promoters is associated with an increase in their loci mobility in live embryonic stem cells53. However, although across the sleep–wake cycle global gene expression in the brain is dynamically regulated, the expression of the majority of genes peaks during wakefulness9,54, whereas chromosome dynamics increase during sleep. Thus, regulation of the expression of specific genes by chromosome dynamics should be explored. Alternatively, we propose that increased chromosome dynamics enhance the efficiency of DSB elimination during sleep. Notably, although chromosome mobility increases following induction of DSBs, the increase appears to require a threshold level of DNA damage in yeast55. In agreement with that, we show that continuous wakefulness inhibits chromosome dynamics, thus preventing the efficient repair of DSBs. We propose that at a given threshold level of DNA damage, sleep is triggered in order to induce chromosome dynamics, which enable the cell to balance between DNA damage and repair (Fig. 6a). Inhibition of a sleep-dependent increase in chromosome dynamics, as demonstrated in the Lap2β-overexpressing neurons, can lead to accumulation of DSBs and potentially to cell death. This mechanism can perhaps explain why prolonged sleep deprivation can be lethal6, and why aging and neurodegenerative disorders are associated with both abnormal sleep56 and increased mutations in the genome of human neurons57.
Sleep is typically monitored at the network and whole-organism levels. However, by overcoming a technology barrier, this work determined the effect of sleep on the physiology of single neurons in the context of a live organism. At the cellular level, our results show that a single DNA damaged neuron, but not an endothelial or Schwann cell, requires relatively long periods of sleep in order to increase chromosome dynamics and eliminate the accumulation of DSBs. Indeed, in mammalian cells and embryonic zebrafish cells, clearance of γH2AX-stained foci typically takes several hours58–60. These nuclear processes are not efficient during prolonged wakefulness, possibly because all cell resources and energy are involved in neuronal function61. In non-neuronal cell types, such as endothelial and Schwann cells, repair of DSBs may occur in a cell-autonomous manner, which does not affect the behavior of the whole organism. The molecular mechanisms that regulate chromosome dynamics in neurons during sleep and wake require further investigation. Lap2β and lamin A are two potential regulators38; however, the involvement of other factors, such as proteins of the linker of the nucleoskeleton and cytoskeleton (LINC) complex26, cannot be excluded.
At the whole-organism level, an important question is how wake and sleep states affect these cellular processes. We propose that sleep behavior synchronizes and consolidates local sleep in neuronal networks in order to perform essential nuclear maintenance that requires prolonged periods of reduced body movement and responsiveness to external stimuli (Fig. 6b). This is essential because desynchronized networks, where individual neurons reach the rest threshold at various time points, would have disabled the behavioral performance of the animals. Nevertheless, this explanation may not settle with the relatively high global brain activity during sleep that is only slightly lower than during wakefulness in zebrafish62. Likewise, in mammals, based on EEG measurements in the cortex, global activity during rapid eye movement (REM) sleep is almost similar to the activity during wakefulness63. Yet, these studies and experimental approaches focused on average neuronal activity across large brain regions, such as the cortex, while here, we determined the effect of sleep on single neurons, and can differentiate single wake- versus sleep-active neurons. Similarly, in mice, microendoscopic calcium imaging of single cells in the dorsal pons showed that wake-active GABAergic neurons are significantly more active during wakefulness than REM and non-rapid eye movement (NREM) sleep64. In the zebrafish rhombencephalon, we identified mainly wake-active neurons, suggesting that, at least in the hindbrain but also likely in additional deep brain regions, neurons reduce their activity during sleep. Since, to date, different sleep stages were not detected in fish, future works in mammals are expected to enhance understanding of the correlation between EEG, neuronal activity, and chromosome dynamics in specific neuronal populations during wakefulness, REM, and NREM sleep.
What is the conserved function of sleep? How is it regulated? And how can sleep be defined at the cellular level? Studying sleep across phylogeny can provide key insights into these fundamental questions50. Using non-mammalian models, several sleep genes were identified1,65, and sleep-dependent structural synaptic plasticity was visualized in worms, flies, and zebrafish66–68. However, conserved functions and cellular markers were not yet identified. We suggest that in zebrafish, sleep is beneficial to the brain because single neurons can increase chromosome dynamics and perform nuclear maintenance during a consolidated offline period. Future studies on additional vertebrate and invertebrate multicellular animals, ranging from cnidarians to mammals, can establish chromosome dynamics as an evolutionarily conserved cellular sleep marker. This marker may be used to define sleeping single neurons in a live mounted animal even under the microscope, where behavioral criteria are insufficient. Altogether, we postulate that sleep behavior has evolved in order to regulate rest bouts of functionally linked single neurons, thus enabling coordinated nuclear maintenance.
Methods
Zebrafish husbandry and transgenic lines
Adult zebrafish (male and female) were raised and maintained in fully automated zebrafish housing systems (Aquazone, Israel; temperature 28 ± 0.5 °C, pH 7.0, conductivity 500 μS) under 14 h light/10 h dark cycles, and fed twice a day. Embryos were produced by natural spawning and raised in egg-water containing methylene blue (0.3 ppm) in a light-controlled incubator at 28 ± 0.5 °C and under 14 h light/10 h dark cycle. All experiments were done at the larval stages and specific age is indicated at the manuscript. The transgenic lines tg(uas:EGFP-Terfa) and tg(uas:RCaMP1b) were generated using the Tol2 system69. The transgenic lines tg(mbp:EGFP), tg(HuC:Gal4), tg(fli:Gal4) and tg(fli:EGFP), as well as tg(HuC:GCaMP5) were kindly provided by Cheol-Hee Kim (Chungnam National University Daejeon, Korea), Bettina Schmid (Ludwig Maximilian University of Munich, Germany), Karina Yaniv (Weizmann Institute of Science, Israel), and German Sumbre (Institute de Biologie-Ecole Normale Superieure, France), respectively. The aanat2−/− mutant was kindly provided by David Prober (California Institute of Technology, CA, USA). All animal protocols were reviewed and approved by the Bar-Ilan University Bioethics Committee.
DNA constructs
In order to prepare constructs of chromosome markers, the coding sequences of terfa (NM_173243.2) and cenpa (NM_001164240.1) were amplified using the following primers: Terfa: 5′-ccaaccggtatgagcgacaaaccctgc-3′ and 5′-ccaatcgattcagaccatcttgagcttgac-3′; Cenpa: 5′-ccaaccggtatgcctcgccatacatcg-3′ and 5′-ccaatcgatttacatgtgttcaacgcctctg-3′. The EGFP coding sequence was amplified with 5′-accgaattcaccatggctccaaagaagaagcgtaaggtaatggtgagcaagggcgaggagc-3′ and 5′-ccaaccggtcttgtacagctcgtccatgcc-3′ primers, the forward primer includes nls sequence (underlined). Triple ligation of EcoRI/ClaI-digested pT2-uas:MCS vector, EcoRI/AgeI-digested egfp, and AgeI/ClaI-digested terfa or AgeI/ClaI-digested cenpa were performed. pT2-uas:dsRED-TRF1 was generated by amplifying dsRED-TRF1 flanked by EcoRI/ClaI from cmv:dsRED-TRF1 vector (primers: 5′-accgaattcatgaagcttgcctcctccgag-3′ and 5′-ccaatcgattcagtcttcgctgtctgagga-3′), and cloning into EcoRI/ClaI-digested pT2-uas:MCS vector.
The human TRF1 DNA binding site contains three helices located at the C-terminus of the protein70. BLAST analysis of the human TRF1 and zebrafish Terfa revealed homology (68%) of these motifs. To delete the DNA binding site of Terfa, pT2-uas:EGFP-Terfa del construct was generated. The pT2-uas:EGFP-Terfa construct was amplified using the primers 5′-ggtgtactttttagctggagctgtagaagt-3′ and 5′-tgaatcgatgatgatccagacatgataaga-3′, and ligated using T4-ligase (NEB, Ipswich, MA, USA), which resulted in the deletion of 162 bp in the C-terminus and truncated protein.
In order to prepare the pT2-mbp:Gal4 construct, approximately 1.9 Kb of the mbp promoter (AY860977) was amplified using the primers 5′-ccggggcccataataacaatcccaactc-3′ and 5′-ccgaccggtgtagtccttctccgctca-3′. The PCR product was digested with ApaI and AgeI restriction enzymes and cloned into ApaI- and AgeI-digested pT2-hcrt:Gal4 vector replacing the hcrt promoter.
In order to prepare the pT2-uas:RCaMP1b construct, RCaMP1b coding sequence was amplified from pGP-CMV-NES-jRCaMP1b (gift from Douglas Kim, Addgene plasmid # 63136)71, using the 5′-ctcagatctcgccaccatgctgcaga-3′ and 5′-ttactcgaggcggccgcctacttcgctgtc-3′ primers. The PCR product was digested with BglII and XhoI restriction enzymes and cloned into BglII and XhoI digested pT2-uas:MCS vector. The uas:ChR2-YFP construct was kindly provided by Prof. Philippe Mourrain (Stanford University, USA).
In order to prepare the pT2-uas:Lap2β-EGFP construct, the coding sequences of lap2β (NM_001141787.1) were amplified using the primers 5′-acacgaattcaccatggctccaaagaagaagcgtaaggtaatgtcggaatttctggaga-3′ and 5′-gttgaattcttgctggtactgtcatctgtgccgctc-3′; the forward primer includes the nls sequence (underlined). The PCR product was digested by EcoRI restriction enzyme and cloned into EcoRI-digested pT2-uas:EGFP vector.
Imaging
All imaging experiments were conducted on 6–7 dpf larvae. Larvae were mounted with low-melting-point agarose 2.0%, and paralyzed in 0.3 mg/ml pancuronium bromide (P1918, Sigma-Aldrich, St. Louis, MO, USA). Imaging was performed using a Zeiss LSM710 upright confocal/two-photon microscope (Zeiss, Oberkochen, Germany) with ×63, 1.0 NA objective. In order to standardize the optimal conditions for single chromosome imaging, we imaged the motion of single chromosomes using either 2-photon or confocal microscopes. Using either microscope, we observed changes in chromosome dynamics during day and night (2-photon: day—0.013 ± 0.002, night—0.025 ± 0.01; confocal: day—0.009 ± 0.002, night—0.027 ± 0.008; n = 5 Te neurons for each group). Imaging chromosome dynamics using Mai-Tai 2-photon laser (Spectra-Physics, Santa Clara, CA, USA), tuned to 920 nm excitation wavelength, resulted in relatively low signal-to-noise ratio. In contrast, imaging of chromosomes using the confocal microscope equipped with argon laser (488 nm) resulted in an efficient signal-to-noise ratio. This is because high-magnification confocal imaging capture larger focal plan that limits loss of particle trajectories in the z-axis. Therefore, in chromosome-dynamic experiments, we used a confocal microscope with the following parameters: image resolution of 256 × 256, 50 cycles of 30 planes, speed of 242.04 ms per plane. One plane size was typically 16.8 × 16.8 × 1.3 µm, with intervals of 0.3 µm between planes in all Z stacks.
In simultaneous confocal imaging of RCaMP1b and EGFP-Terfa (lasers: argon 488 nm, DPSS 561 nm), the following parameters were used: image resolution of 256 × 256, scanning time of 8 min at 4.13 Hz on 16.8 × 16.8 μm single plane. In HuC-driven GCaMP5 imaging, 6 dpf tg(HuC:GCaMP5) larvae were mounted, and GCaMP5 signal was monitored using a Mai-Tai 2-photon laser, tuned to 920 nm, with a ×20 1.0 NA objective. Scanning was performed at a single plane for 20 min at 4.13 Hz on 140 × 140 μm in Te and SC neurons, and 170 × 170 μm in Rh neurons.
Transient expression assays
Transient expression assays of the DNA constructs pT2-uas:EGFP-Terfa-del, pT2-uas:dsRED-TRF1, pT2-uas:EGFP-Cenpa, uas:ChR2-YFP, pT2-uas:Lap2β-EGFP, and pT2-mbp:Gal4 were performed by microinjection of approximately 2 nl plasmid into one-cell-stage embryos, at a concentration of 30 ng/µl each, using a micromanipulator and PV830 Pneumatic PicoPump (World Precision Instruments, Sarasota, FL). Imaging of all transient experiments was performed in 6 dpf larvae.
Optogenetic experiments
The expression vectors pT2-uas:dsRED-TRF1 and pT2-uas:ChR2-YFP were co-injected into one-cell-stage tg(HuC:Gal4) embryos, and positive embryos were raised under 14 h light/10 h dark cycles. At 6 dpf (ZT4), dsRED-TRF1- or dsRED-TRF1+ChR2-YFP-positive neurons were imaged for 10 min with DPSS laser (561 nm). Then, we stimulated the neurons ten times with blue light pulses in 1-s intervals. Following the stimulation, imaging of the same individual neurons was repeated using both DPSS (561 nm) and argon (488 nm) lasers.
Melatonin experiments
Individual 6 dpf tg(HuC:Gal4)/tg(uas:EGFP-Terfa) live larvae were treated with either 10 µM melatonin (M5250, Sigma-Aldrich, St. Louis, MO, USA) or 0.7% EtOH during daytime. Sleep behavior and chromosome dynamics were monitored in Rh neurons of the same larvae before and during treatment with each compound.
BAPTA-AM experiments
Individual 6 dpf tg(HuC:Gal4)/tg(uas:EGFP-Terfa) and tg(HuC:GCaMP5) live larvae were treated with either 5 mM BAPTA-AM (A1076, Sigma-Aldrich, St. Louis, MO, USA) or 0.1% DMSO diluted in embryo water for 4 h during the night (ZT19–23). During the 4th hour of treatment, chromosome dynamics and neuronal activity were monitored in Rh neurons.
Etoposide experiments
6 dpf tg(HuC:Gal4)/tg(uas:EGFP-Terfa) larvae were treated for 2 h with either 10 µM etoposide (E1383, Sigma-Aldrich, St. Louis, MO, USA) or 0.01% DMSO diluted in embryo water during day or night. Following treatment, the compound was removed, and the larvae recovered in fresh water. Three parameters were monitored: number of DSBs, chromosome dynamics, and sleep behavior. To quantify the number of DSBs and chromosome dynamics in Rh neurons, larvae were either fixed (for immunohistochemistry) or live-imaged, respectively, following the compound treatment and during the recovery period. In behavioral assays, sleep was monitored as described below.
Behavioral assays
In order to monitor sleep, larvae were individually placed in 48-well plates containing either embryo water, melatonin, ETO, EtOH, or DMSO compound. Larva-containing plates were placed in the Noldus DanioVision tracking system (Noldus Information Technology, Wageningen, Netherlands) under either light or dark. Live video-tracking were conducted using the EthoVision XT 12 software (Noldus Information Technology, Wageningen, Netherlands) with the following parameters for detection: dynamic subtraction, subject is darker than background, dark contrast 16–60, current frame weight 1, subject size of minimum 1 pixel and maximum 125,000 pixels, subject contour turned off, video sample rate of 12.5 frames per second, and no pixel smoothing. Data analyses of sleep time were performed according to the threshold parameters: distance, 0.3 cm; time, stop velocity 0.59 cm/s, start velocity 0.6 cm/s40. SD was performed on freely swimming larvae in Petri dishes by 4 h of gentle, random, and unsynchronized manual, mechanical tapping11. Sleep was monitored continuously prior to and 1 h following SD.
Immunohistochemistry assays
Larvae were fixed for 2 h in 4% paraformaldehyde (PFA) in PBS at 4 °C, and washed in PBS + 0.5% Triton. The larvae were then blocked with 10% fetal bovine serum diluted in PBS for 1 h at room temperature. After blocking, the larvae were incubated in primary antibody: rabbit anti-EGFP (SC-8334, Santa Cruz Biotechnology, Santa Cruz, CA), 1:250 dilution; mouse anti-γH2AX (GTX127342, GeneTex), 1:100 dilution, in blocking buffer overnight at 4 °C. On the following day, larvae were washed in PBS + 0.5% Triton and blocked for 1 h. Anti-GFP was detected with a donkey polyclonal secondary anti-rabbit IgG H&L (Alexa Fluor® 488, 2 mg/ml, ab150061, Abcam), and anti-γH2AX was detected with donkey anti-mouse IgG H&L (Alexa Fluor® 594) (2 mg/ml, ab150064, Abcam), in blocking buffer overnight at 4 °C. Next, larvae were washed in PBS + 0.5% Triton, fixed for 30 min in 4% PFA in PBS, and washed in PBS + 0.5% Triton.
Data analysis
3D SPT analysis of a time-lapse image sequence was performed in two stages. First, images were analyzed using Imaris software (Bitplane AG, Zurich, Switzerland), and punctum coordinates were located in each frame using the following parameters: detected ellipsoids with XY diameter of 0.3–0.6 µm and Z diameter of 1.3 µm, “quality” filter type to detect as much puncta as possible, and the “autoregressive motion” algorithm, with maximum distance of 1.25 µm without filling gaps. We verified that there was no concatenation between different puncta, and only punctum trajectories that were continuously trackable during the entire imaging session were quantified. In cases where the entire nucleus, and consequently all chromosomes, rotated significantly in a similar direction, the cells were excluded from the analysis. Then, only a nucleus with at least four reliable tracks was analyzed using custom-made MATLAB software (Mathworks, Natick, MA, USA) for nucleus drift and rotation correction38. In single-plane imaging, analysis was performed in the 1st minute in order limit loss of punctum trajectory in the z-axis.
In calcium imaging analysis (RCaMP1b and GCaMP5), imaging movies were registered to remove drifts using the Template Matching plugin in ImageJ. Then, using the custom-made MATLAB (Mathworks, Natick, MA, USA) programs FindROI and ProcessCalciumData72, regions of interest (ROI) were selected, and DeltaFoF and raster plot were determined.
To quantify the number of DSBs, snapshot images of 18 × 18 µm were acquired and the number of γH2AX foci per single cell (~15 cells per image) was counted manually using Cell Counter plugin in ImageJ.
Statistical information
All imaging experiments were performed independently at least three times, whereas at least three larvae were imaged in each independent imaging experiment. In all experiments, larvae were selected randomly. Number of larvae, cells or chromosomes, statistical tests and P values are stated in each figure legend. Differences between two categorical groups (ctrl/SD and day/night/subjective day, EtOH/melatonin and pre-/post-treatment, day/night and aanat2+/+/aanat2−/− or day/night and Lap2β+/Lap2β−) on continuous variables (chromosome dynamics, sleep, or γH2AX foci number) were determined by two-way ANOVA using Statistica software (TIBCO). Differences between more than two groups were determined using one-way ANOVA using Statistica software (TIBCO). Differences between two groups were determined by two-tailed t-test: two samples assuming unequal variance using Analysis ToolPac in Excel. Changes between paired samples in the optogenetic experiments were determined by two-tailed t-test: paired two samples for means using Analysis ToolPac in Excel. Chromosome dynamics and neuronal activity correlation were determined by Pearson correlation coefficient using the social science statistics calculator.
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The authors thank the members of the Appelbaum lab for technical assistance, Noa Alon for assistance in designing the schematic illustrations, and Luis de Lecea, David Klein, German Sumbre, Eran Meshorer, and Ofer Yizhar for helpful discussions and comments. The authors also thank Ms. Sharon Victor for assistance in editing the manuscript. This work was supported by the Israel Science Foundation (Grant no. 690/15 to L.A.) and by the US–Israel Binational Science Foundation (BSF, Grant no. 2017105 to L.A.).
Author contributions
D.Z. and L.A. conceived and designed the experiments. D.Z. performed molecular, imaging, and behavioral experiments. T.L.-G. performed immunohistochemistry experiments. D.Z. and L.A. analyzed data. Y.G. and I.B. analyzed chromosome dynamic experiments and supplied reagents. D.Z. and L.A. prepared the figures and wrote the paper, with feedback from all authors.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks Roberto Peeza and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-08806-w.
References
- 1.Levitas-Djerbi T, Appelbaum L. Modeling sleep and neuropsychiatric disorders in zebrafish. Curr. Opin. Neurobiol. 2017;44:89–93. doi: 10.1016/j.conb.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Trojanowski NF, Raizen DM. Call it worm sleep. Trends Neurosci. 2016;39:54–62. doi: 10.1016/j.tins.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nath RD, et al. The jellyfish Cassiopea exhibits a sleep-like state. Curr. Biol. 2017;27:2984–2990. doi: 10.1016/j.cub.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmerman JE, Naidoo N, Raizen DM, Pack AI. Conservation of sleep: insights from non-mammalian model systems. Trends Neurosci. 2008;31:371–376. doi: 10.1016/j.tins.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011;146:194–207. doi: 10.1016/j.cell.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krause AJ, et al. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017;18:404–418. doi: 10.1038/nrn.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eban-Rothschild A, Appelbaum L, de Lecea L. Neuronal mechanisms for sleep/wake regulation and modulatory drive. Neuropsychopharmacology. 2017;43:937–952. doi: 10.1038/npp.2017.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Mackiewicz M, et al. Macromolecule biosynthesis: a key function of sleep. Physiol. Genomics. 2007;31:441–457. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- 10.Scharf MT, Naidoo N, Zimmerman JE, Pack AI. The energy hypothesis of sleep revisited. Prog. Neurobiol. 2008;86:264–280. doi: 10.1016/j.pneurobio.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appelbaum L, et al. Circadian and homeostatic regulation of structural synaptic plasticity in hypocretin neurons. Neuron. 2010;68:87–98. doi: 10.1016/j.neuron.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie L, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allada R, Siegel JM. Unearthing the phylogenetic roots of sleep. Curr. Biol. 2008;18:R670–R679. doi: 10.1016/j.cub.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhdanova IV, Wang SY, Leclair OU, Danilova NP. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001;903:263–268. doi: 10.1016/S0006-8993(01)02444-1. [DOI] [PubMed] [Google Scholar]
- 15.Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J. Neurosci. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokogawa T, et al. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol. 2007;5:e277. doi: 10.1371/journal.pbio.0050277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saper CB, Fuller PM. Wake–sleep circuitry: an overview. Curr. Opin. Neurobiol. 2017;44:186–192. doi: 10.1016/j.conb.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rattenborg NC, Amlaner CJ, Lima SL. Behavioral, neurophysiological and evolutionary perspectives on unihemispheric sleep. Neurosci. Biobehav. Rev. 2000;24:817–842. doi: 10.1016/S0149-7634(00)00039-7. [DOI] [PubMed] [Google Scholar]
- 19.Vyazovskiy VV, Harris KD. Sleep and the single neuron: the role of global slow oscillations in individual cell rest. Nat. Rev. Neurosci. 2013;14:443–451. doi: 10.1038/nrn3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cirelli C. Cellular consequences of sleep deprivation in the brain. Sleep Med. Rev. 2006;10:307–321. doi: 10.1016/j.smrv.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Allebrandt KV, et al. Identifying pathways modulating sleep duration: from genomics to transcriptomics. Sci. Rep. 2017;7:4555. doi: 10.1038/s41598-017-04027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thimgan MS, Duntley SP, Shaw PJ. Changes in gene expression with sleep. J. Clin. Sleep Med. 2011;7:S26–S27. doi: 10.5664/JCSM.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauer MH, Gasser SM. Chromatin and nucleosome dynamics in DNA damage and repair. Genes Dev. 2017;31:2204–2221. doi: 10.1101/gad.307702.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soutoglou E, Misteli T. Mobility and immobility of chromatin in transcription and genome stability. Curr. Opin. Genet. Dev. 2007;17:435–442. doi: 10.1016/j.gde.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christophorou N, et al. Microtubule-driven nuclear rotations promote meiotic chromosome dynamics. Nat. Cell Biol. 2015;17:1388–1400. doi: 10.1038/ncb3249. [DOI] [PubMed] [Google Scholar]
- 26.Chang W, Worman HJ, Gundersen GG. Accessorizing and anchoring the LINC complex for multifunctionality. J. Cell Biol. 2015;208:11–22. doi: 10.1083/jcb.201409047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibuya H, Ishiguro K, Watanabe Y. The TRF1-binding protein TERB1 promotes chromosome movement and telomere rigidity in meiosis. Nat. Cell Biol. 2014;16:145–156. doi: 10.1038/ncb2896. [DOI] [PubMed] [Google Scholar]
- 28.Takizawa T, Meshorer E. Chromatin and nuclear architecture in the nervous system. Trends Neurosci. 2008;31:343–352. doi: 10.1016/j.tins.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Koike N, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill AJ, Mansfield R, Lopez JMNG, Raizen DM, Van Buskirk C. Cellular stress induces a protective sleep-like state in C. elegans. Curr. Biol. 2014;24:2399–2405. doi: 10.1016/j.cub.2014.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villafuerte G, et al. Sleep deprivation and oxidative stress in animal models: a systematic review. Oxid. Med. Cell. Longev. 2015;2015:234952. doi: 10.1155/2015/234952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellesi M, Bushey D, Chini M, Tononi G, Cirelli C. Contribution of sleep to the repair of neuronal DNA double-strand breaks: evidence from flies and mice. Sci. Rep. 2016;6:36804. doi: 10.1038/srep36804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suberbielle E, et al. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β. Nat. Neurosci. 2013;16:613–621. doi: 10.1038/nn.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madabhushi R, et al. Activity-induced DNA breaks govern the expression of neuronal early-response genes. Cell. 2015;161:1592–1605. doi: 10.1016/j.cell.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yadav T, Quivy JP, Almouzni G. Chromatin plasticity: a versatile landscape that underlies cell fate and identity. Science. 2018;361:1332–1336. doi: 10.1126/science.aat8950. [DOI] [PubMed] [Google Scholar]
- 37.Bronshtein I, et al. Exploring chromatin organization mechanisms through its dynamic properties. Nucleus. 2016;7:27–33. doi: 10.1080/19491034.2016.1139272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bronshtein I, et al. Loss of lamin A function increases chromatin dynamics in the nuclear interior. Nat. Commun. 2015;6:8044. doi: 10.1038/ncomms9044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elbaz I, Foulkes NS, Gothilf Y, Appelbaum L. Circadian clocks, rhythmic synaptic plasticity and the sleep–wake cycle in zebrafish. Front. Neural Circuits. 2013;7:9. doi: 10.3389/fncir.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elbaz I, Yelin-Bekerman L, Nicenboim J, Vatine G, Appelbaum L. Genetic ablation of hypocretin neurons alters behavioral state transitions in zebrafish. J. Neurosci. 2012;32:12961–12972. doi: 10.1523/JNEUROSCI.1284-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Appelbaum L, et al. Sleep–wake regulation and hypocretin–melatonin interaction in zebrafish. Proc. Natl. Acad. Sci. U.S.A. 2009;106:21942–21947. doi: 10.1073/pnas.906637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gothilf Y, et al. Zebrafish serotonin N-acetyltransferase-2: marker for development of pineal photoreceptors and circadian clock function. Endocrinology. 1999;140:4895–4903. doi: 10.1210/endo.140.10.6975. [DOI] [PubMed] [Google Scholar]
- 43.Gandhi AV, Mosser EA, Oikonomou G, Prober DA. Melatonin is required for the circadian regulation of sleep. Neuron. 2015;85:1193–1199. doi: 10.1016/j.neuron.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vilenchik MM, Knudson AG. Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc. Natl. Acad. Sci. U.S.A. 2003;100:12871–12876. doi: 10.1073/pnas.2135498100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smart DJ, et al. Assessment of DNA double-strand breaks and gammaH2AX induced by the topoisomerase II poisons etoposide and mitoxantrone. Mutat. Res. 2008;641:43–47. doi: 10.1016/j.mrfmmm.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dechat T, Vlcek S, Foisner R. Review: lamina-associated polypeptide 2 isoforms and related proteins in cell cycle-dependent nuclear structure dynamics. J. Struct. Biol. 2000;129:335–345. doi: 10.1006/jsbi.2000.4212. [DOI] [PubMed] [Google Scholar]
- 47.Schoft VK, et al. The lamina-associated polypeptide 2 (LAP2) isoforms beta, gamma and omega of zebrafish: developmental expression and behavior during the cell cycle. J. Cell Sci. 2003;116:2505–2517. doi: 10.1242/jcs.00450. [DOI] [PubMed] [Google Scholar]
- 48.Ahrens MB, et al. Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature. 2012;485:471–477. doi: 10.1038/nature11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 50.Joiner WJ. Unraveling the evolutionary determinants of sleep. Curr. Biol. 2016;26:R1073–R1087. doi: 10.1016/j.cub.2016.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Misteli T. Higher-order genome organization in human disease. Cold Spring Harb. Perspect. Biol. 2010;2:a000794. doi: 10.1101/cshperspect.a000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu B, et al. Transcription-coupled changes in nuclear mobility of mammalian cis-regulatory elements. Science. 2018;359:1050–1055. doi: 10.1126/science.aao3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellesi M, de Vivo L, Tononi G, Cirelli C. Effects of sleep and wake on astrocytes: clues from molecular and ultrastructural studies. BMC Biol. 2015;13:66. doi: 10.1186/s12915-015-0176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seeber A, Dion V, Gasser SM. Checkpoint kinases and the INO80 nucleosome remodeling complex enhance global chromatin mobility in response to DNA damage. Genes Dev. 2013;27:1999–2008. doi: 10.1101/gad.222992.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grigg-Damberger MM. Sleep in aging and neurodegenerative diseases. Suppl. Clin. Neurophysiol. 2004;57:508–520. doi: 10.1016/S1567-424X(09)70390-3. [DOI] [PubMed] [Google Scholar]
- 57.Lodato MA, et al. Aging and neurodegeneration are associated with increased mutations in single human neurons. Science. 2018;359:555–559. doi: 10.1126/science.aao4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leatherbarrow EL, Harper JV, Cucinotta FA, O’Neill P. Induction and quantification of gamma-H2AX foci following low and high LET-irradiation. Int. J. Radiat. Biol. 2006;82:111–118. doi: 10.1080/09553000600599783. [DOI] [PubMed] [Google Scholar]
- 59.Shibata A, et al. Factors determining DNA double-strand break repair pathway choice in G2 phase. EMBO J. 2011;30:1079–1092. doi: 10.1038/emboj.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pereira S, et al. Low doses of gamma-irradiation induce an early bystander effect in zebrafish cells which is sufficient to radioprotect cells. PLoS One. 2014;9:e92974. doi: 10.1371/journal.pone.0092974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shetty PK, Galeffi F, Turner DA. Cellular links between neuronal activity and energy homeostasis. Front. Pharmacol. 2012;3:43. doi: 10.3389/fphar.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee DA, et al. Genetic and neuronal regulation of sleep by neuropeptide VF. eLife. 2017;6:e25727. doi: 10.7554/eLife.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol. Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cox J, Pinto L, Dan Y. Calcium imaging of sleep–wake related neuronal activity in the dorsal pons. Nat. Commun. 2016;7:10763. doi: 10.1038/ncomms10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raizen DM, Zimmerman JE. Non-mammalian genetic model systems in sleep research. Sleep Med. Clin. 2011;6:131–139. doi: 10.1016/j.jsmc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang G, Grone B, Colas D, Appelbaum L, Mourrain P. Synaptic plasticity in sleep: learning, homeostasis and disease. Trends Neurosci. 2011;34:452–463. doi: 10.1016/j.tins.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dabbish NS, Raizen DM. GABAergic synaptic plasticity during a developmentally regulated sleep-like state in C. elegans. J. Neurosci. 2011;31:15932–15943. doi: 10.1523/JNEUROSCI.0742-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324:105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawakami K, et al. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell. 2004;7:133–144. doi: 10.1016/j.devcel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 70.Hanaoka S, Nagadoi A, Nishimura Y. Comparison between TRF2 and TRF1 of their telomeric DNA-bound structures and DNA-binding activities. Protein Sci. 2005;14:119–130. doi: 10.1110/ps.04983705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dana H, et al. Sensitive red protein calcium indicators for imaging neural activity. eLife. 2016;5:e12727. doi: 10.7554/eLife.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Romano SA, et al. An integrated calcium imaging processing toolbox for the analysis of neuronal population dynamics. PLoS Comput. Biol. 2017;13:e1005526. doi: 10.1371/journal.pcbi.1005526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.