Abstract
Bananas are usually ripened with calcium carbide (CaC2), a dangerous substance that can cause food poisoning. The objective was to test the empirical ripening banana method using Bowdichia virgilioides leaves compared to carbide. Ripening tests were carried out using ‘Pacovan’ banana fruits with B. virgilioides leaves and carbide following the empirical method used by Borborema farmers, Paraíba, Brazil. Bowdichia virgilioides leaves induced increased respiration and ascorbic acid production and reduced acidity, chlorophyll and pH in banana fruits like CaC2. Leaves of B. virgilioides induce ripening of ‘Pacovan’ banana with safer and same results than with CaC2.
Introduction
Musa sp. has global economic importance as one of the most important basic food sources along with rice, maize and wheat1. About 150 countries produce this fruit, totaling more than 100,000 t year−1. India (25,000 t year−1), China (10,000 t year−1), Philippines (8,900 t year−1), Ecuador (6,770 t year−1) and Brazil are the main banana producers in the world2–4. These countries have socioeconomic similarities highlighting the importance of this culture for the economy and regional development5, mainly for small producers6. Simplified cultivation processes, high demand and acceptance of bananas in the domestic market of these countries, enable their production, albeit often with low quality and/or productivity7. In addition, cooperatives and associations are important channels to organize and support banana farming activities8.
Banana fruit ripening depends on intrinsic factors such as respiration and ethylene production/sensitivity9 and market requirements10. Locally marketed bananas may be harvested at a later maturation stages, but bananas for export should be harvested the day before or the day of shipment11. In this case, maturation standardization is induced by air conditioning12 to plan banana commercialization and industrialization13. Acetylene and traces of this compound, produced by calcium carbide (CaC2), accelerate and standardize ripening (color uniformity) without losses to quality or taste14. These products cannot be used in organic or agroecological production systems15, but have no restrictions in countries such as Bangladesh, India, Nepal and Pakistan16.
CaC2 can cause adverse effects to human health, such as choking, motor coordination problems, headaches, respiratory tract inflammation, respiratory system irritation, mucous membrane and skin burns and reduction of the oxygen supply to the brain due to the chemical reaction of this product with water11. Effective, low-cost and natural methods can avoid the harmful health effects of ripening chemical inducers.
Merchant producers of the Borborema polo, Paraíba state, Brazil mature bananas with Bowdichia virgilioides Kunth (Fabaceae) leaves with results like those obtained with CaC2 but at lower cost. The ripening process with leaves of this plant includes the collection of B. virgilioides leaves at the coolest time of day, avoiding dew and excessive humidity during subsequent stages. The leaves of this plant are placed on the ground and the banana fruits are placed over them, but the proportion of leaves and fruits is not precise. The bananas and the leaves are then covered with plastic sheeting without air exchange between the external and internal environments. The tarp is left for 24 hours or longer depending on the fruit quantity. Masonry tanks of 1 m2 are also used with this method (personal farmer communication, 2017).
The sustainable management of B. virgilioides plants can facilitate the use of its leaves to induce banana ripening more economically. The objective was to test the empirical method of banana maturation using B. virgilioides leaves compared to the conventional method with CaC2.
Results
Ethylene concentration was higher in atmosphere of the treatments with 4, 6, 8, and 10 g of leaves B. virguloides, followed by treatment with CaC2 in the laboratory ripening trial. In the field ripening trial, the ethylene concentration was higher in atmosphere of the treatment with leaves B. virguloides, followed by treatment with CaC2. Acetylene (C2H2) was only detected on treatment with CaC2 in the laboratory and field ripening trial. Respiratory rate, ascorbic acid content, malic acid, pH, and chlorophyll in ‘Pacovan’ bananas matured with B. virgilioides leaves and CaC2 do not differed (p < 0.05) in the laboratory (6, 8, and 10 g) and field ripening trial. The respiratory rate and ascorbic acid content of matured ‘Pacovan’ bananas was higher with B. virgilioides leaves and CaC2 than control in the laboratory (6, 8, and 10 g) and field ripening trial. The malic acid, pH, and total chlorophyll concentration of ripened ‘Pacovan’ bananas of B. virgilioides leaves and CaC2 was lower than control in the laboratory (6, 8, and 10 g) and field ripening trial (Table 1).
Table 1.
Ethylene = C2H4 (ppm), Acetylene = C2H2 (ppm), Respiratory rate = RR (mg of CO2 Kg−1 h−1), ascorbic acid = AsA (mg 100 g−1), malic acid = MA (%), pH, and chlorophyll = Chlo (mg 100 g−1) in bananas matured with 2, 4, 6, 8 e 10 g of Bowdichia virgilioides leaves (T1, T2, T3, T4 and T5), carbide (g 50 Kg−1 carbide) (T6) and, uncoated with plastic film (T7) fruits covered with plastic film (T8) in the laboratory ripening trial and LBv = B.
| Treat. | C2H4 | C2H2 | RR | AsA | MA | pH | Chlo |
|---|---|---|---|---|---|---|---|
| Laboratory ripening trial | |||||||
| T1 | 27.9 ± 2.1c | nd | 17.6 ± 1.6b | 16.1 ± 2.1b | 45.0 ± 2.5a | 5.6 ± 0.2a | 10.3 ± 0.7a |
| T2 | 66.7 ± 4.9a | nd | 48.6 ± 2.1a | 35.3 ± 3.6a | 38.8 ± 2.8a | 4.9 ± 0.2b | 10.8 ± 0.9a |
| T3 | 69.7 ± 5.8a | nd | 52.4 ± 3.0a | 40.9 ± 3.0a | 33.7 ± 2.1b | 5.0 ± 0.3b | 1.2 ± 0.1b |
| T4 | 68.4 ± 6.4a | nd | 54.6 ± 1.9a | 33.8 ± 2.9a | 32.6 ± 3.0b | 4.6 ± 0.5b | 1.2 ± 0.1b |
| T5 | 71.9 ± 7.4a | nd | 59.7 ± 3.1a | 42.6 ± 3.1a | 35.3 ± 3.0b | 4.5 ± 0.1b | 1.0 ± 0.1b |
| T6 | 32.7 ± 3.5b | 387.2 ± 12.2 | 45.3 ± 3.6a | 45.7 ± 2.6a | 38.0 ± 2.9b | 4.5 ± 0.1b | 2.3 ± 0.1b |
| T7 | — | — | 18.3 ± 1.0b | 12.9 ± 0.9b | 42.1 ± 3.0a | 5.5 ± 0.1a | 11.1 ± 0.1a |
| T8 | 19,8 ± 1,4d | nd | 17.4 ± 0.9b | 13.2 ± 0.6b | 42.3 ± 2.4a | 5.9 ± 0.1a | 10.5 ± 0.1a |
| CV % | 11.78 | 10.1 | 13.12 | 17.98 | 8.43 | 4.37 | 3.14 |
| Field ripening trial | |||||||
| LBv | 65.6 ± 5.0a | nd | 76.4 ± 9.2a | 44.7 ± 4.1a | 35.2 ± 3.9b | 4.5 ± 0.1b | 1,9 ± 0.1b |
| CaC2 | 61.9 ± 5.2a | 317.1 ± 22.2 | 85.4 ± 9.8a | 42.9 ± 5.1a | 31.4 ± 4.9b | 4,5 ± 0.1b | 2.1 ± 0.1b |
| Control | 34.1 ± 2.2b | nd | 54.1 ± 8.7b | 16.4 ± 3.1b | 51.7 ± 7.9a | 5.1 ± 0.1a | 11.4 ± 0.5a |
| CV (%) | 10.12 | 9.7 | 12.14 | 16.15 | 9.12 | 4.87 | 4.10 |
virgilioides leaves and CaC2=calcium carbide in the field laboratory ripening trial.
nd = not detected. *Means followed by the same letter per column do not differ by Tukey Test at 5% probability.
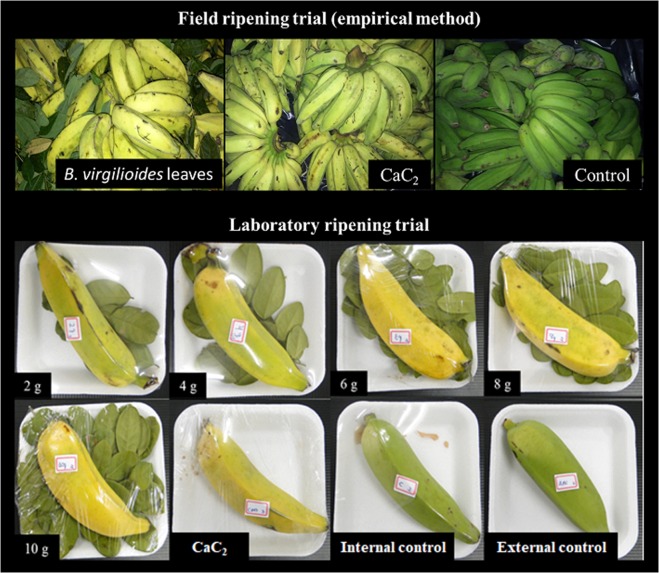
The ‘Pacovan’ banana peel ripened with B. virgilioides leaves and CaC2 changed color than control in laboratory (6, 8, and 10 g) and field ripening trial (Fig. 1).
Figure 1.
General appearance of bananas ripened with Bowdichia virgilioides leaves and carbide.
Discussion
The highest ethylene concentrations in the treatments with 4, 6, 8, and 10 g of B. virgilioides leaves are due to a cumulative effect of the gas produced by fruits and leaves. Ethylene concentration increases under atmospheric conditions modified by gas exchange limitation and autocatalysis17. The ethylene detected in the treatment with CaC2 was the autocatalytic produced by the banana fruits and induced by the calcium carbide. CaC2 may increase respiration rate, ethylene autocatalytic, chlorophyll degradation, carotenoid synthesis, starch conversion to sugar, increased activity of cell wall enzymes degradation, color change, texture, fruit aroma, and taste18. Acetylene is an ethylene analog used to initiate fruit ripening19. However, acetylene has lower biological activity than ethylene and higher concentrations for the same exposure period and for the same responses are needed20. In bananas, 0.01 ml L−1 of ethylene at 18 °C for 24 h began to ripen, while 1.0 ml L−1 of acetylene was required for a similar effect in several Florida hose cultivars19. The C2H2 was only detected on treatment with CaC2 due to the presence of this compound which is industrially produced and only releases C2H2 when reacted with water21.
The increase in the respiratory rate of ‘Pacovan’ bananas ripened with 6, 8, and 10 g of B. virgilioides leaves and CaC2 is due to the climacteric induction of respiration by the ethylene and acetylene emanated by B. virgilioides leaves and CaC2, respectively. Phosphofructokinase activity, which regulates this pathway13, produces energy (ATP) from starch degradation and hexose oxidation resulting in climacteric respiration22. In addition, the fruit exposure to ethylene and acetylene produced by B. virgilioides leaves and CaC2, respectively, may have increased the activity of the enzymes synthase and oxidase of ACC23 inducing climacteric respiration and accelerating maturation.
The highest ascorbic acid content (AsA) in bananas ripened with 6, 8, and 10 g of B. virgilioides leaves and CaC2 is due to the higher demand for AsA24,25 in these fruits, presumably by the most oxidized redox cell state26,27. The early fruit ripen, induced by ethylene and acetylene, produces reactive oxygen species28 increasing the demand for AsA reacting with superoxide, hydroxyl and peroxyl radicals, hydrogen peroxide, hypochlorite and singlet oxygen29. However, the biosynthesis of AsA is an antioxidant response by the D-glucosone, D-galacturonate, myo-inositol and D-mannose/L-galactose30–32. The AsA accumulation in these fruits may also be associated with the low oxidation of this pH-dependent molecule, with maximum at pH 5 and 11.5, being faster in alkaline media33 pathways but not necessarily related to its accumulation34.
The reduction of ripen ‘Pacovan’ banana acidity (malic acid) with 6, 8, and 10 g of B. virgilioides leaves and CaC2 is due to the oxidation of organic acids during fruit ripening by the increase in tricarboxylic acid cycle activity35. These acids were more rapidly and extensively degraded during the climacteric respiration36 induced by ethylene and acetylene, emanating from the B. virgilioides leaves and CaC2, respectively.
The pH reduction in ‘Pacovan’ bananas can be explained by the increase in the ascorbic acid content exceeding the titratable acidity reduction in the fruits matured with 6, 8, and 10 g of B. virgilioides leaves and CaC2. The AsA accumulation reduced the pH of these fruits due to the acidic character of this molecule attributed to the enodiol group (-HOC=COH-). The hydrogens of the enodiol group can dissociate, resulting in the strong ascorbic acid acidity and therefore are potential reducing agents27.
The lowest concentration of total chlorophyll in the ‘Pacovan’ banana peel ripe fruit induced with 6, 8 and 10 g of B. virgilioides leaves is due to the structural decomposition of chlorophyll by chlorophyllases, stimulated by ethylene37 and acetylene emanated from leaves and CaC2, respectively. The increase in the activity of these enzymes in these treatments coincides with the climacteric increase in fruit respiration38, which was also induced by ethylene and acetylene. Ethylene and acetylene its analogues accelerate the chlorophyll losses39 and regulates the yellowing of banana peels40. The drop of total chlorophyll concentration in banana fruits induced by CaC2 was similar due lower biological activity than ethylene and higher concentrations for the same exposure period and for the same responses are needed20,41.
Conclusion
The method used by Borborema producers in the Paraíba state, Brazil to ripen ‘Pacovan’ bananas with Bowdichia virgilioides leaves is safer and has the same results than those obtained with carbide.
Material and Methods
Location and raw material
Banana bunches of the ‘Pacovan’ variety, from agroecological production, were harvested in the early hours in the morning. Banana fruits were selected and standardized according to size, absence of physiological defects and infections, at the maturation stage 3 with yellowish green color42. Part of the harvested fruits were transported to the laboratory and part remained in the field. Banana ripening was evaluated with B. virgilioides leaves (harvested according to the producers orientation) and calcium carbide (CaC2) in the field and laboratory.
Laboratory ripening trial
‘Pacovan’ banana bites were scrapped with a stainless-steel knife and the fruits were, individualized in trays of expanded polystyrene for 30 min, to reduce the ethylene effect produced in their wound. The treatments were 2.0 g of B. virgilioides leaves + plastic film coating (T1); 4.0 g of B. virgilioides leaves + plastic film coating (T2); 6.0 g of B. virgilioides leaves + plastic film coating (T3); 8.0 g of B. virgilioides leaves + plastic film coating (T4); 10.0 g of B. virgilioides leaves + plastic film coating (T5); CaC2 (g 50 kg−1) + coating with plastic film (T6); internal control (only coated with plastic film) (T7) and external control (without coating with plastic film) (T8) per banana. Treatments were stored at 27 ± 2 °C and relative humidity of 87 ± 5%. The fruits remained under these conditions for 48 hours and were then evaluated. The experiment was developed in triplicate.
Field ripening trial (empirical method)
Two and a half kilograms of Bowdichia virgilioides leaves were placed covering the entire soil and 100 kg of banana fruits are placed over them. The bananas and the leaves were then covered with tarp without air exchange between the external and internal environments. The tarp was left for 24 hours (personal farmer communication, 2017). The same procedure was performed by replacing the leaves with CaC2 (0,5 g kg−1 of fruit). The control consisted only of the fruits covered by the tarp.
Ethylene and acetylene quantification
Ten air samples were withdrawn with syringes from the atmosphere beneath the tarp. The syringes needle tip were sealed with rubber and immediately taken to the laboratory where were injected into a GC-14B (ShimadzuCrop Kyoto Japan), with Porapak-Q packaged column and flame ionization detector for ethylene and acetylene analysis.
Respiratory rate
Banana fruits were placed in hermetically sealed containers with 10 mL of 0.5 N NaOH. The CO2, produced by the fruits, was measured by titration and after 24 h, the NaOH was titrated with 1 N HCl with results expressed as mg of CO2 kg−1 h−1. The respiratory rate was estimated by the equation: mgCO2 Kg−1 fresh matter = (B − L) × C/MF where B = volume in mL spent for titration of the “control” (container without fruit, only with NaOH); L = volume spent to neutralize NaOH; C = correction factor (0.98); MF = fresh fruit mass. The hourly respiratory rate was determined with the formula mg CO2 Kg−1 h−1 = mg CO2 g−1 fresh matter × 1000/IT; IT = time interval between titrations (24 h).
Ascorbic acid
ascorbic acid content was determined by titration with a 0.02% 2,6-diclophenol-indophenol (DFI) indicator solution in 5.0 g of fresh banana mass diluted in 30 ml of oxalic acid at 0.5%43.
Titratable acidity
The malic acid content (acidity) of the banana was determined by titrametry in five-gram pulp samples of the fruit diluted in 50 mL of distilled water. Then, 4–5 phenolphthalein indicator droplets were added and titration performed with 0.1 N NaOH44.
pH
The pH was determined in digital bench pH meter in samples, 30 min after the dilution of five grams of banana pulp in 50 ml of distilled water.
Total chlorophyll
Two grams of the banana peel were macerated with 7 mL of 80% (v/v) acetone and the extract filtered and the volume then filled with 80% (v/v) acetone. The absorbance was read at wavelengths at 646.8 and 663.2 nm, and calculated by the equation: total chlorophyll (T) = 7.15 × A663.2 + 18.71 × A646.8 45.
Experimental design and data analysis
The experiment was carried out in a completely randomized design with eight treatments and four replications. Each experimental unit had one banana per tray. The results were submitted to variance analysis by the F test and the means compared by the Tukey test (P < 0.05) with the program Assistat version 7.7.
Acknowledgements
To Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do estado de Minas Gerais (FAPEMIG) and “Programa Cooperativo sobre Proteção Florestal/PROTEF do Instituto de Pesquisas e Estudos Florestais/IPEF” for financial support. Dr. Phillip John Villani (University of Melbourne, Australia) revised and corrected the English language used in this manuscript.
Author Contributions
W.S.R., R.C.N. and O.O.F. designed the research. R.C.N., O.O.F. and L.S.R. performed the experiments. W.S.R., F.L.F., J.C.Z., L.S.R. and M.B.A. wrote the manuscript. All authors approved the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rivaildo da Costa Nascimento and Wellington Souto Ribeiro contributed equally.
References
- 1.Food and Agriculture Organization of the United Nations (FAO), http://faostat.fao.org/default.aspx?lang=en (2013). [PubMed]
- 2.Fairtrade Foundation. Unpeeling the banana trade, http://www.fairtrade.org.uk/includes/documents/cm_docs/2009/f/1_ft_banana_reportweb.pdf (2009).
- 3.Fairtrade Foundation. Fairtrade bananas case study, http://www.fairtrade.org.uk/includes/documents/cm_docs/2012/W/WINFA (2012).
- 4.Smith J. Culturing development: Bananas, petri dishes and ‘mad science’. Journal of Eastern African Studies. 2007;1:212–233. doi: 10.1080/17531050701452424. [DOI] [Google Scholar]
- 5.FAO - Food and Agriculture Organization of the United Nations. FAOSTAT: statistics database, http://faostat3.fao.org/home/index.html#DOWNLOAD (2014).
- 6.The State of Sustainability Initiatives Review 2014: Standards and the Green Economy, http://www.iisd.org/library/state-sustainability-initiatives-review-2014-standards-and-green-economy (2014).
- 7.UN Conference on Trade and Development. Infocomm commodity profile: Banana, http://www.unctad.info/en/Infocomm/AACP-Products/COMMODITY-PROFILE–Banana (2012).
- 8.Banana Industry Advisory Committee. Banana strategic investment plan 2012–2014, http://cms2live.horticulture.com.au/admin/assets/library/strategic_plans/pdfs/ PDF_File_62.pdf (2011).
- 9.Ishemo A, Bushell B. Farming Cooperatives: Opportunities and Challenges for Women Farmers in Jamaica. Journal of International Women’s Studies. 2017;18:13–29. [Google Scholar]
- 10.Dash PK, Rai R. Translating the “Banana genome” to delineate stress resistance, dwarfing, parthenocarpy and mechanisms of fruit ripening. Frontiers of Plant Science. 2016;7:e1543. doi: 10.3389/fpls.2016.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islam MN, et al. A legislative aspect of artificial fruit ripening in a developing country like Bangladesh. Chemical Engineering Research Bulletin. 2016;18:30–37. doi: 10.3329/cerb.v18i1.26219. [DOI] [Google Scholar]
- 12.Ogazi, P. O. Plantain: Production, processing and utilization. Paman and Associates Publishers, Okigwe, Nigeria, (1996).
- 13.Cancian AJ, Carvalho VD. Manejo pós-colheita da banana. Informe Agropecuário. 1980;6:47–53. [Google Scholar]
- 14.Seymour, G. B., Taylor, J. E. & Tucker, G. A. Biochemistry of fruit ripening. London: Chapman and Hall (1993).
- 15.Zeitschriften W. Effects of calcium carbide and 2-chloroethylphosphonic acid on fruit quality of thai mangoes under various postharvest ripening regimes. European Journal of Horticultural Science. 2009;36:411–418. [Google Scholar]
- 16.Altieri MA, Nicholls CI. Agroecology scaling up for food sovereignty and resiliency. Sustainable agriculture reviews. Springer, Dordrecht. 2012;11:1–29. doi: 10.1007/978-94-007-5449-2_1. [DOI] [Google Scholar]
- 17.Paul V, Pandey R. Role of internal atmosphere on fruit ripening and storability—a review. Journal of Food Science and Technology. 2014;51:1223–1250. doi: 10.1007/s13197-011-0583-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Islam, M. N., Mursalat, M. & Khan, M. S. A review on the legislative aspect of artificial fruit ripening. Agriculture & Food Security5 (2016).
- 19.Burg SP, Burg EA. Molecular requirements for the biological activity of ethylene. Plant Physiology. 1967;42:144–152 9. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medlicott AP, Sigrist JMM, Sy O. Ripening of mangos following low-temperature storage. Journal of the American Society for Horticultural Science. 1990;115:430–434. doi: 10.21273/JASHS.115.3.430. [DOI] [Google Scholar]
- 21.Trotus IT, Zimmermann T, Schüth F. Catalytic reactions of acetylene: a feedstock for the chemical industry revisited. Chemical Reviews. 2014;114:1761–1782. doi: 10.1021/cr400357r. [DOI] [PubMed] [Google Scholar]
- 22.Harmutk L. Chlorophyls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzimology. 1987;148:350–382. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- 23.Stanley, J. K. Postharvest physiology of perishable plant products. CSB Publ. Distrib., New Delhi, India, 143–256 (1998).
- 24.Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annual Review of Plant Biology. 1984;35:155–189. doi: 10.1146/annurev.pp.35.060184.001103. [DOI] [Google Scholar]
- 25.Loewus MW, Bedgar DL, Saito K, Loewus FA. Conversion of L-sorbosone to L-ascorbic acid by a NADP-dependent dehydrogenase in bean and spinach leaf. Plant Physiology. 1990;94:1492–1495. doi: 10.1104/pp.94.3.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:e365. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- 27.Cunha CP, et al. Ethylene-induced transcriptional and hormonal responses at the onset of sugarcane ripening. Scientific Reports. 2017;7:e43364. doi: 10.1038/srep43364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar V, et al. Fruit ripening mutants reveal cell metabolism and redox state during ripening. Protoplasma. 2016;253:581–594. doi: 10.1007/s00709-015-0836-z. [DOI] [PubMed] [Google Scholar]
- 29.Yamauchi T, Shimamura S, Nakazono M, Mochizuki T. Aerenchyma formation in crop species: a review. Field Crops Research. 2013;152:8–16. doi: 10.1016/j.fcr.2012.12.008. [DOI] [Google Scholar]
- 30.Kehrer JP, Robertson JD, Smith CV. Free radicals and reactive oxygen species. Comprehensive Toxicology. 2010;2:277–307. doi: 10.1016/B978-0-08-046884-6.00114-7. [DOI] [Google Scholar]
- 31.Agius F, et al. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nature Biotechnology. 2003;21:177. doi: 10.1038/nbt777. [DOI] [PubMed] [Google Scholar]
- 32.Bohu T, et al. Characterization of pH dependent Mn (II) oxidation strategies and formation of a bixbyite-like phase by Mesorhizobium australicum T-G1. Frontiers in Microbiology. 2015;6:734. doi: 10.3389/fmicb.2015.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Tang M, Viikari L. Xylans inhibit enzymatic hydrolysis of lignocellulosic materials by cellulases. Bioresource Technology. 2012;121:8–12. doi: 10.1016/j.biortech.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 34.González-Agüero M, et al. The unusual acid-accumulating behavior during ripening of cherimoya (Annona cherimola Mill.) is linked to changes in transcription and enzyme activity related to citric and malic acid metabolism. Molecules. 2016;21:e398. doi: 10.3390/molecules21050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mellidou I, Kanellis AK. Genetic control of ascorbic acid biosynthesis and recycling in horticultural crops. Frontiers in Chemistry. 2017;5:1–8. doi: 10.3389/fchem.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerreiro AC, Gago CML, Miguel MGC, Faleiro ML, Antunes MDC. The influence of edible coating enriches with citral and eugenol on the raspberry storage ability, nutritional and sensory quality. Food Packaging and Shelf Life. 2016;9:20–28. doi: 10.1016/j.fpsl.2016.05.004. [DOI] [Google Scholar]
- 37.Mellidou I, Kanellis AK. Genetic control of ascorbic acid biosynthesis and recycling in horticultural crops. Frontiers in Chemistry. 2017;5:50. doi: 10.3389/fchem.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhillon WS, Mahajan BVC. Ethylene and ethephon induced fruit ripening in pear. Journal of Stored Products and Postharvest Research. 2011;2:45–51. [Google Scholar]
- 39.Thomas P, Janave MT. Effect of temperature on chlorophyllase activity, chlorophyll degradation and carotenoids of Cavendish bananas during ripening. International Journal of Food Science & Technology. 2007;27:57–63. doi: 10.1111/j.1365-2621.1992.tb01178.x. [DOI] [Google Scholar]
- 40.Dominguez M, Vendrell M. Ethylene biosynthesis in banana fruit: Evolution of EFE activity and ACC levels in peel and pulp during ripening. Journal of Horticultural Science and Biotechnology. 1993;68:63–70. doi: 10.1080/00221589.1993.11516329. [DOI] [Google Scholar]
- 41.Jones B, et al. Ethylene and developmentally - regulated processes in ripening climacteric fruit. Acta Horticuturae. 2001;553:133–138. doi: 10.17660/ActaHortic.2001.553.25. [DOI] [Google Scholar]
- 42.White Martins, http://www.whitemartins.com.br (2017).
- 43.Soto, M. Bananos: cultivo e comercializacion (No. 338.174772 S686B.). Litografía e imprenta LIL (1992).
- 44.Strohecker, R., & Henning, H. M. Análisis de vitaminas, métodos comprobados (No. QP801. V5 S7e) (1967).
- 45.Normas Analíticas do Instituto Adolfo Lutz. Métodos químicos e físicos para análise de alimentos, São Paulo: Instituto Adolfo Lutz. 533 (1985).