Abstract
The mesolimbic hypothesis has been a central dogma of schizophrenia for decades, positing that aberrant functioning of midbrain dopamine projections to limbic regions causes psychotic symptoms. Recently, however, advances in neuroimaging techniques have led to the unanticipated finding that dopaminergic dysfunction in schizophrenia is greatest within nigrostriatal pathways, implicating the dorsal striatum in the pathophysiology and calling into question the mesolimbic theory. At the same time our knowledge of striatal anatomy and function has progressed, suggesting new mechanisms via which striatal dysfunction may contribute to the symptoms of schizophrenia. This Review draws together these developments, to explore what they mean for our understanding of the pathophysiology, clinical manifestations, and treatment of the disorder.
Keywords: nigrostriatal, psychosis, antipsychotic, positron emission tomography, magnetic resonance imaging, neuroimaging
Highlights
Techniques for characterising the mesostriatal dopamine system, both in humans and animal models, have advanced significantly over the past decade.
In vivo imaging studies in schizophrenia patients demonstrate that dopaminergic dysfunction in schizophrenia is greatest in nigrostriatal as opposed to mesolimbic pathways.
Better understanding of striatal structure and function has enhanced our insight into the neurobiological basis of psychotic symptoms.
The role of other neurotransmitters in modulating striatal dopamine function merits further exploration, and modulating these neurotransmitter systems has potential to offer new therapeutic strategies.
Schizophrenia and the Striatum
Schizophrenia is a syndrome consisting of positive symptoms (such as delusions and hallucinations), negative ones (including flattened affect and lack of motivation), and cognitive ones. Dysregulated dopaminergic modulation of striatal function is fundamental to many models that seek to explain the mechanisms underlying the symptoms of schizophrenia 1, 2, 3, 4. Furthermore, all licensed pharmacological treatments of schizophrenia affect the dopamine system, and while several atypical antipsychotics have been proposed to act via alternative nondopaminergic mechanisms, such as the serotonergic system, it is still the case that they all bind to dopamine receptors, and there is no clear relationship between efficacy and serotonergic effects [5].
In this paper, we review how advances in neuroscientific methods have improved our understanding of striatal structure and function. We then examine the evidence regarding striatal dysfunction in schizophrenia, and discuss how recent findings suggest a re-evaluation of prior hypotheses may be required. Finally, we ask what these developments mean for our understanding of the clinical manifestations and treatment of the disorder.
Striatal Structure and Function
Striatal Connectivity
The striatum is an integral part of the corticobasal ganglia circuitry. Extensive work mapping its pathways, as summarised below, suggests that it acts as an integrative hub for information processing in the brain.
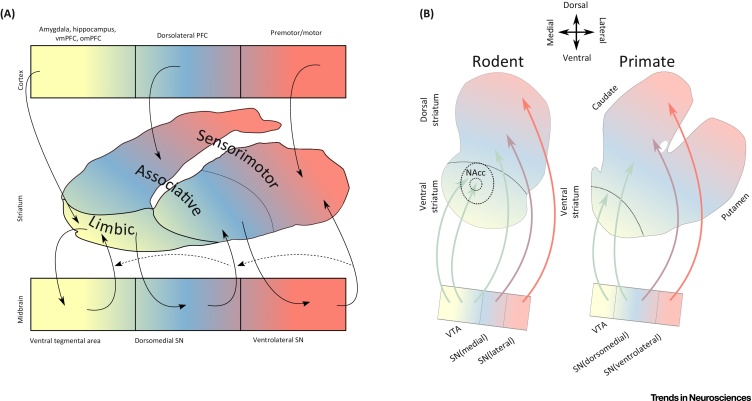
Initial primate research aimed at mapping striatal connections involved lesioning cortical areas and recording the location of subsequent striatal degeneration. Later work used retrograde tracers injected into the striatum to determine both cortical and midbrain connections [6]. Corticostriatal connections were shown to run in three parallel, and well-segregated pathways, that effectively parcellated the striatum into limbic (see Glossary), associative, and sensorimotor functional subdivisions based on their specific inputs and outputs (Figure 1A) [7]. At the time, it was thought that these corticostriatal loops operated in parallel with minimal crosstalk; an idea referred to as the parallel processing model. Subsequent studies, however, suggested that in addition to these parallel loops, there are projections from one loop to another [8], which promote information funnelling from the ventral to dorsal striatum (Figure 1A).
Figure 1.
Striatal Connectivity. (A) Summary of primate tracing studies mapping connections between cortex (top row), striatum (middle), and midbrain (bottom). The primate striatum can be divided into NAcc, olfactory tubercle, caudate nucleus, and putamen (for simplicity, not indicated in the figure). The division between caudate and putamen, however, has little biological relevance [6]. Tract tracing studies showed that striatocortical connections run in three parallel pathways. Motor areas project to the caudal putamen [117]; dorsolateral prefrontal cortex to caudate and rostral putamen [118]; and limbic areas to the ventral striatum [119]. These subdivisions were termed the sensorimotor, associative, and limbic striatum. Subsequent research used retrograde tracers injected into striatum to determine midbrain connections 6, 8. This showed that ventral tegmental area and medial SN project primarily to limbic striatum, while central/ventrolateral parts of the SN project to the associative and sensorimotor striatum. The striatum in turn has efferents projecting back to the midbrain. In addition to these reciprocal connections, feedforward striato-nigro-striatal connections allow information to pass along the striatum from limbic to motor regions via the associative striatum 8, 112, 120. (B) Summary of rodent–primate differences in mesostriatal connectivity. In rodents, the ventral striatum is proportionally larger than in primates. The NAcc shell is innervated by the medial VTA, and the NAcc core by the central VTA, whereas the lateral VTA innervates a region homologous to the associative striatum; the SN also has some connections to the associative region in addition to the more dorsal regions of the striatum. In primates, the VTA is proportionally smaller; it innervates the ventral striatum, whereas the dorsal tier SN innervates the associative striatum, and the ventral tier innervates the sensorimotor striatum (for a more detailed review of differences between primates and rodents see 14, 121). Abbreviations: NAcc, nucleus accumbens; omPFC, orbitomedial PFC; PFC, prefrontal cortex; SN, substantia nigra; vmPFC, ventromedial PFC; VTA, ventral tegmental area.
Recent methodological advances have further refined our understanding of corticostriatal architecture 9, 10. These advances underscore the lack of a strict 1:1 topographic mapping, and indicate that corticostriatal pathways overlap. Based on its exceptional degree of input heterogeneity, the associative striatum has been highlighted as an information processing hub [11]. Furthermore, cluster analysis of corticostriatal input patterns has shown that in addition to the three subdivisions discussed above, a fourth subdivision is apparent in the tail of the striatum; its most caudal part [11]. This region receives cortical input from a range of areas including limbic and auditory cortex, and is distinct in its composition, consisting almost exclusively of D1 expressing medium spiny neurons (MSNs) [12].
In addition to its widespread cortical connectivity, the striatum has extensive bidirectional connections to the midbrain (Figure 1A). The development of the CLARITY tissue preparation method (which enables lipid removal, while preserving tissue structure), has allowed for in-depth examination of mesostriatal connectivity, and has identified projections from the dorsolateral to dorsomedial projecting dopamine neurons, suggesting a novel pathway for lateral to medial information flow, in addition to previously identified medial to lateral routes (Figure 1A) [13].
Many of these more recent findings on striatal connectivity have been demonstrated only in rodents to date, and interspecies differences must be borne in mind when seeking to draw parallels with primate and human anatomy (Figure 1B) [14]. Human neuroimaging studies have, however, produced findings that are in keeping with some of the pathways discussed above. Resting state functional magnetic resonance imaging (fMRI) [15] and diffusion tensor imaging [16] studies support the division of the striatum into functional subdivisions. Moreover, compared to anatomical divisions, these functional subdivisions display greater homogeneity in terms of dopamine release [16], highlighting their relevance for understanding striatal function. Likewise, the preclinical finding that the associative striatum acts as an integrative hub via the convergence of multiple distal cortical inputs, is consistent with human studies 17, 18, 19.
In summary, it appears that, in addition to well-established parallel pathways, there also exists, across species, a high degree of pathway crossing and information funnelling, with regions in the associative striatum acting as integrative hubs. Furthermore, there appear to be a variety of pathways allowing for bidirectional information transfer across the striatum.
Striatal Neurochemistry
Recent advances have refined understanding of striatal neurochemistry. In this section, we consider these findings and their implications for mechanisms by which abnormalities of nondopaminergic neurotransmitter systems may contribute to dopaminergic dysfunction. We also discuss their relevance for developing new treatment approaches to normalise striatal dopamine function and possibly treat schizophrenia without requiring dopamine D2/3 receptor blockade.
Most dopamine neurons have the potential to release GABA as a cotransmitter, and a smaller proportion corelease glutamate. This cotransmission varies across the striatum, and can moderate the reciprocal relationship between dopaminergic and cholinergic neurons [20]. Specifically, in the dorsal striatum, dopamine neurons do not corelease glutamate, and the firing of dopamine neurons in this region is accompanied by pauses in cholinergic interneuron firing secondary to dopamine D2 receptor and GABA signalling. In the ventral striatum, by contrast, a burst-pause occurs secondary to glutamate cotransmission [20]. In addition to these functional differences across the striatum, dopamine receptors themselves show fundamentally different responses to dopamine, dependent on their striatal location. D2 receptors in the accumbens show both greater sensitivity to dopamine and a slower postsynaptic current compared to those in dorsal regions [21]. This is not secondary to differences in D2/3 ratios, but rather appears to result from differences in Gα subunits [21].
Corticostriatal neurons synapse upon cholinergic interneurons, which in turn modulate dopamine neurons via nicotinic receptors situated on these dopamine neurons, thereby mediating the corticostriatal control of striatal dopamine release [22]. It is also these cholinergic interneurons that drive GABA release from dopaminergic neurons [23]. Muscarinic regulation of striatal dopamine function has also been shown, although there is no evidence that dopamine neurons in the striatum display muscarinic receptors [24], and it appears this modulation occurs secondary to a variety of mechanisms, including autoreceptors on cholinergic terminals that inhibit acetylcholine release [25], endocannabinoid signalling pathways [26], and modulation of MSN projections to the substantia nigra [27]. In the dorsal striatum both M2 and M4 receptors are needed for this muscarinic modulation of dopamine release, whereas in the ventral striatum only M4 receptors are required [24].
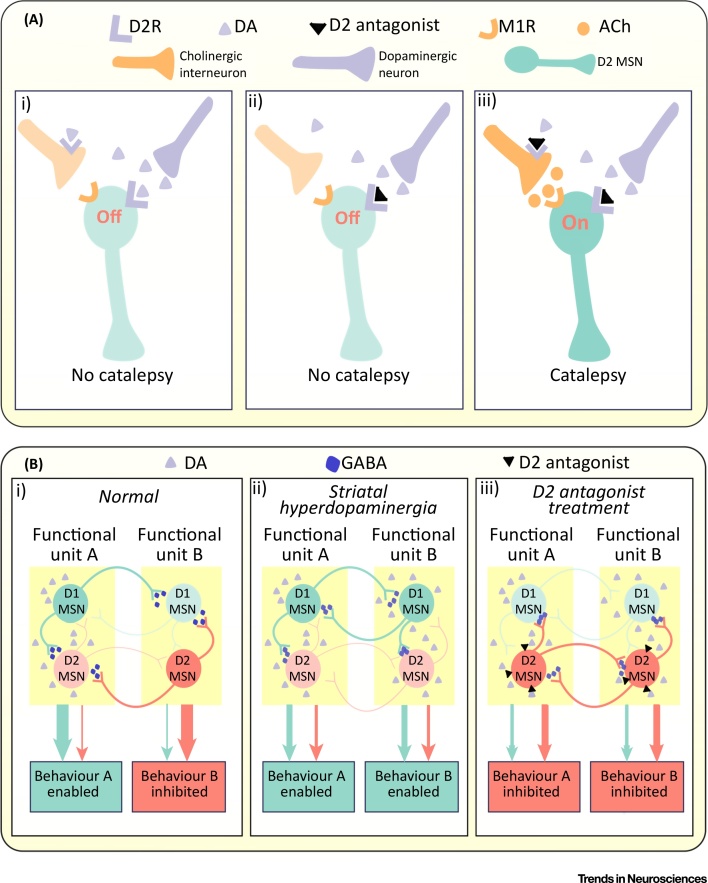
Of relevance to the treatment of schizophrenia, Kharkwal et al. demonstrated that the extrapyramidal side effects of antipsychotic medications may primarily result from the blockade of D2 receptors on cholinergic interneurons [28]. Blockade of D2 receptors was shown to increase the firing of D2 expressing indirect pathway MSNs, both due to the direct effect on these neurons, and as a result of increased acetylcholine-mediated activation of M1 receptors on the MSNs, occurring as a result of D2 blockade on cholinergic interneurons (Figure 2A) [28]. The authors suggest that this mechanism may have the potential to minimise movement side effects, and this is supported by the finding that the antipsychotic with the lowest risk of these is clozapine. While the favourable profile of clozapine in this domain may also result from its low affinity for the D2 receptor and low D2 occupancy at clinically therapeutic doses, it also displays significant antagonism at the M1 receptor, which these findings suggest contributes significantly to its lack of significant movement side effects.
Figure 2.
Striatal Neurochemistry and Neurotransmission. (A) The role of cholinergic interneurons in mediating extrapyramidal side effects [28]. (i) In wild-type mice, D2Rs mediate inhibitory actions both by directly reducing firing of the indirect pathway MSN, and by reducing firing of cholinergic interneurons. (ii) In knockout mice without D2Rs on cholinergic interneurons, D2 antagonism of the MSN by itself is insufficient to induce catalepsy. (iii) Activating, in addition, M1Rs, results in catalepsy, which occurs due to increased firing of the cholinergic interneuron secondary to D2 antagonism. (B) The relationship between striatal dopamine, lateral inhibition, and behavioural selection [35]. The figure illustrates three scenarios, based on a hypothetical pair of functional units (A and B) each controlling a specific behavioural outcome (behaviour A and B, respectively). (i) Localized dopaminergic signalling in functional unit A activates D1 direct pathway MSNs, while suppressing D2 indirect pathway MSNs, enabling the execution of desired behaviour A. GABAergic lateral inhibition suppresses competing behaviour coded for by functional unit B. (ii) Spatially disorganised dopaminergic signalling means that there is nonspecific activation of multiple functional units, and undesirable behaviours are no longer suppressed. (iii) Dopamine antagonists enhances the activity of indirect pathway neurons, but without regional specificity, meaning that desirable behaviours are also suppressed. Abbreviations: Ach, acetylcholine; DA, dopamine; D2R, dopamine D2 receptor; M1R, M1 muscarinic receptor; MSN, medium spiny neuron.
Striatal Function
New findings regarding striatal anatomy and neurochemistry have also refined our understanding of the functional architecture of the striatum. We now discuss how recent neurobiological advances relate to our understanding of the role of the striatum in behavioural selection, salience processing, and habit formation.
The output pathways of the striatum include the direct pathway and indirect pathway, where D1 expressing MSNs project directly to the output nuclei of the basal ganglia, and D2 type MSNs project indirectly via the pallidum (Figure 3). Recent findings suggest, however, that these pathways are less distinct than previously thought, with D1 and D2 MSNs of the ventral striatum often not adhering to the direct and indirect pathways, respectively 29, 30. While these classical pathways better describe the architecture of the dorsal striatum, interpathway communication has also been demonstrated here. Lateral projections from indirect pathway MSNs inhibit direct pathway MSNs via GABA release, and striatal dopamine release reduces this GABA release thereby supressing this lateral inhibition [31]. In the opposite direction, direct pathway neurons show collaterals that bridge across to the indirect pathway [32]. Moreover, recent findings indicate that neural activity in both pathways increases when animals initiate behaviour, in contrast to the classical accelerator/brake model 33, 34. One interpretation of this finding is that the activation observed in the indirect pathway corresponds to the suppression of alternative, undesirable behaviour [35], and that this suppression may occur via collaterals between direct and indirect pathway MSNs [35] (Figure 2B).
Figure 3.
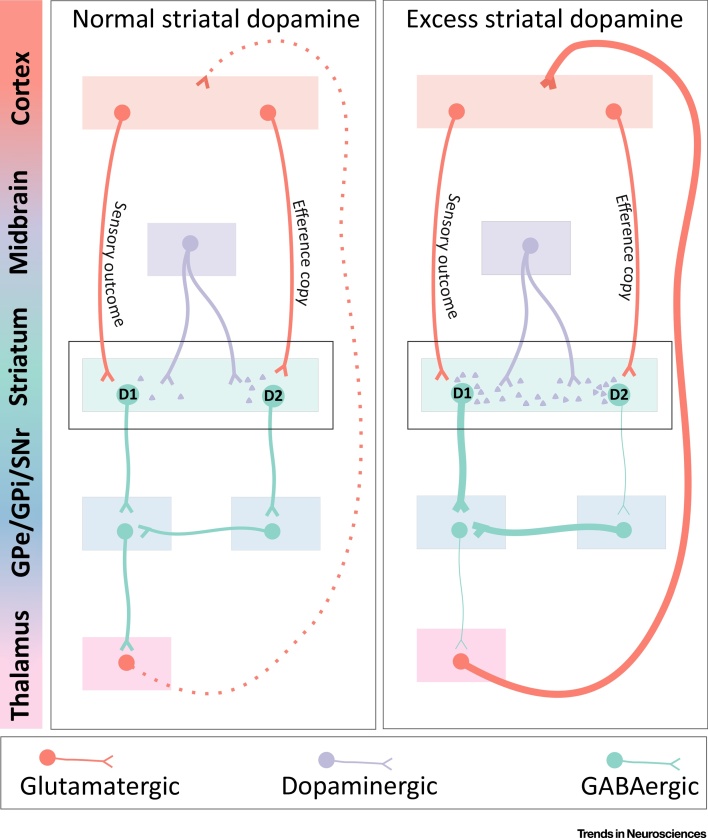
Mechanisms via Which Excess Striatal Dopamine May Impair Efference Copy Transmission. With normal striatal dopamine signalling (left), neurons carrying the efference copy signal preferentially synapse onto dopamine-D2-expressing GABAergic medium spiny neurons of the indirect pathway. Increased dopamine release within the striatum (right) inhibits these D2-expressing neurons. Excess striatal dopamine may therefore interfere with appropriate transmission of the efference copy signal. In the auditory areas of the dorsal striatum this could result in inner speech being mischaracterised as externally generated.
The activity of mesostriatal dopamine neurons has been shown to signify the discrepancy between expected and actual rewards, which has been termed the reward prediction error [36]. In addition to reward processing, some models have emphasised the role of mesostriatal dopamine neurons in assigning salience to environmental stimuli, and their potential relevance to the development of psychotic symptoms [1]. It has been debated whether mesostriatal dopaminergic neurons carry information regarding salience that goes beyond reward related information 37, 38. Recent studies suggest that while dopamine signalling within the ventral striatum is strongly linked to stimulus value, dopamine signalling in more dorsal regions does not track value, but rather the novelty and intensity of stimuli, and in particular threat-related information 13, 39, 40. It has also been recently demonstrated that optogenetic stimulation of dopamine neurons is sufficient to imbue unremarkable environmental stimuli with motivational properties, and that these stimuli are subsequently able to evoke dopaminergic activity, despite never having possessed any intrinsic salience [41]. Recent human positron emission tomography (PET)–fMRI studies also provide evidence that striatal dopamine has a broader role than simply encoding reward prediction errors, and is associated with the function of cortical salience networks [42], and making more general inferences about the state of the environment [43].
Striatal function along a ventral–dorsal axis is also relevant to habit formation. During the learning of action–outcome pairings, performance is goal directed and sensitive to changes in outcome values. After extensive training, however, performance moves to a stimulus–response mode that is inflexible, and no longer responds to changes in outcome. This evolution of behaviour from contingency-dependent learning to habitual responding has been associated with a shift from ventral to dorsal striatal processing [44]. Supporting this, lesions to nigrostriatal pathways and dorsal striatum disrupt habit formation, amphetamine sensitisation encourages habit formation, and it is the dorsal striatum that is implicated in the habitual responses to drug cues experienced by addicts [45]. Recent work has focused more specifically upon the role of the striatal tail, and is consistent with these earlier findings in that this region appears to be involved in storing stable values while the ventral region acts as a flexible coder of information [46].
Striatal Dopamine and Schizophrenia
The Mesolimbic Dogma
Early models of schizophrenia proposed that dysfunction of the mesolimbic pathway underlay positive psychotic symptoms (Box 1) [4]. Related to that, it was also proposed that newer antipsychotics benefit from mesolimbic selectivity when compared to older agents [47]. While questions were raised regarding the precise locus of striatal dysfunction [48], a focus on mesolimbic pathways persisted, likely due to the absence of robust evidence to refute it. As a result, the mesolimbic hypothesis became a central dogma of schizophrenia, featured in many textbooks, and frequently invoked in discussions regarding the pathophysiology of the illness 1, 2, 49, 50. As it was not possible to measure limbic dopamine function in vivo, the theory was based on indirect evidence. Moreover, it originated when the dorsal striatum was thought to be solely involved in motor function, and unlikely to be involved in psychosis. Subsequent advances suggest it may in fact be these dorsal regions that play a central role in the pathophysiology of the disorder.
Figure I.
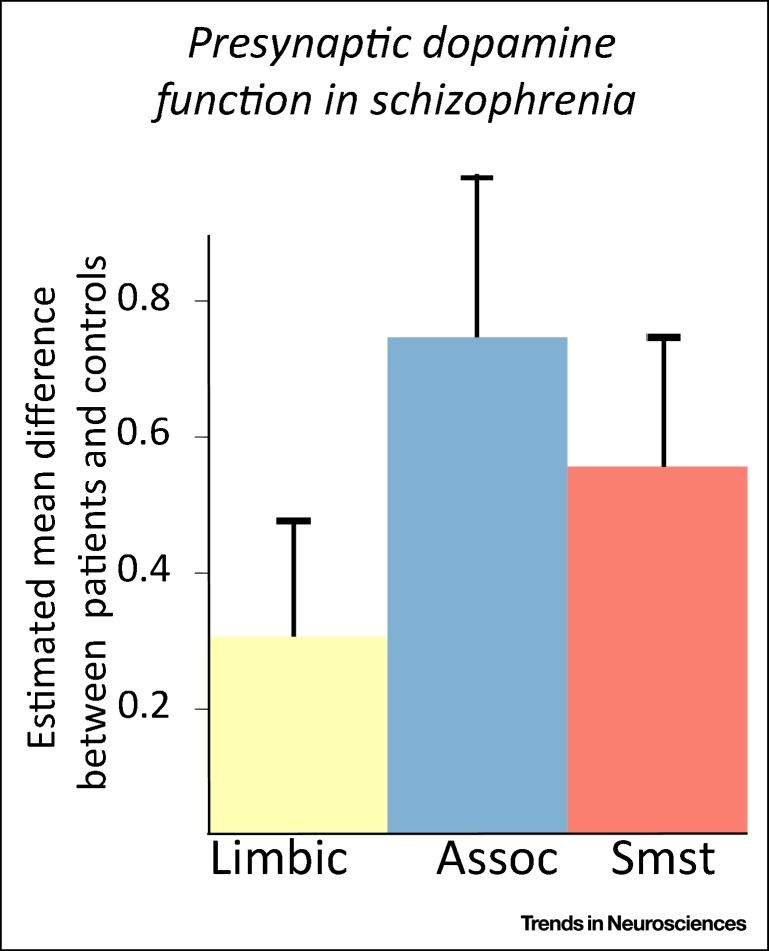
Results of a Meta-analysis Examining Positron Emission Tomography Measures of Presynaptic Dopamine in Schizophrenia Patients and Controls [61]. Abbreviations: Assoc, associative striatum; Limbic, limbic striatum; Smst, sensorimotor striatum. *Statistically significant difference between patients and controls (P<0.05 in a random effects meta-analysis).
Box 1. Mesolimbic Hypothesis of Schizophrenia.
Clinical Findings
The origins of the mesolimbic hypothesis date back to observations that symptoms, displayed during epileptic seizures localised to limbic areas, were similar to the symptoms of schizophrenia [122]. It was also noted that individuals with tumours in limbic areas were likely to be diagnosed with schizophrenia [123]. Further support came from research using electrodes implanted in individuals with schizophrenia, which demonstrated increased activity in limbic regions during periods of active psychosis [124].
Amphetamine Models
The above-mentioned clinical findings were not neurotransmitter specific. The link to dopamine was based on three complementary findings. First, that high doses of amphetamines were able to induce a florid psychotic state [125]; second, that in rodents amphetamine induced dopamine release appeared greatest in the NAcc [126]; and third, that amphetamine-induced stereotypy was specific to increased dopaminergic transmission in the NAcc [127].
Antipsychotics
Injections of antipsychotics into the NAcc abolished amphetamine-induced behaviour, but injections into caudate had no effect [128]. Furthermore, some antipsychotics specifically upregulated dopamine turnover in the NAcc, supporting the hypothesis that dopamine blockade of this region was central to the antipsychotic effect of antipsychotics. This was in keeping with findings that while typical antipsychotic drugs increased c-fos and neurotensin expression in the NAcc and dorsal striatum, atypicals affected expression solely in the NAcc, suggesting that the NAcc was central to antipsychotic effects whereas dorsal actions might be solely related to motor side effects [129].
Together these findings led to the hypothesis that psychosis is due to overactivity in the mesolimbic dopamine pathway 2, 49, 50. Modern neurochemical imaging findings, however, suggest that it is within dorsal regions of the striatum that dopaminergic aberrations are greatest [61] (Figure I).
Alt-text: Box 1
Postmortem Studies of Striatal Dopamine Function
Early postmortem studies investigating striatal dopaminergic abnormalities produced inconsistent results. Initial studies measured concentrations of dopamine, and while some reported an association between schizophrenia and increased concentrations specifically in the nucleus accumbens (NAcc) [51], others found dopamine concentrations elevated specifically in the dorsal striatum [52]. Moreover, tyrosine hydroxylase activity, the rate-limiting enzyme in dopamine synthesis, was shown to be elevated throughout the striatum [53], as was dopamine receptor density [54].
More recently, no differences between patients and controls in terms of either density of dopaminergic terminals [55], or levels of tyrosine hydroxylase have been found in the NAcc 56, 57. Inferences from postmortem studies, however, are limited by the fact that most patients have received antipsychotic drug treatment, which may upregulate dopamine receptors [58], and alter presynaptic dopamine function [59], and furthermore dopamine and its metabolites are greatly affected by death [60].
In vivo Neuroimaging of the Striatum
PET allows quantification of the dopamine system in vivo. Radiolabelling of the dopamine precursor l-dihydroxyphenylalanine enables the measurement of its uptake and conversion in dopamine neurons to give an index of dopamine synthesis capacity. Alternatively, imaging the competition between endogenous dopamine and radioligands specific to postsynaptic dopamine receptors can determine baseline levels of dopamine (following depletion of endogenous dopamine using compounds such as α-methylparatyrosine), as well as the magnitude of dopamine release (following a pharmacological or psychological challenge).
Meta-analysis of studies using these PET and single photon emission computed tomography (SPECT) techniques shows that there is a robust increase in striatal dopamine synthesis and release in psychosis [61]. There is no evidence of major alterations in dopamine D2/3 receptors, although it is possible that increased receptor occupancy by raised endogenous dopamine levels masks this, or alternatively that differences between groups in the affinity state of the receptor are not detected with the antagonist ligands generally used 62, 63, 64.
Early PET and SPECT studies of presynaptic dopamine function could distinguish anatomical subdivisions of the striatum but lacked sufficient resolution to accurately distinguish between functional subdivisions. Accordingly, these studies reported outcomes for the whole striatum or anatomical subdivisions. However, the subsequent development of higher resolution PET scanners has enabled the assessment of striatal functional subdivisions as well. The first studies to report on these subdivisions in patients with schizophrenia found that the greatest differences in dopamine function were within the associative striatum, with differences in the limbic subdivision not reaching statistical significance 63, 65. The finding that dopaminergic dysfunction is greatest in the associative region has been replicated in multiple studies since then 63, 66, 67, 68. Meta-analysis of these studies found that dopaminergic function was significantly elevated in patients relative to controls in associative (Hedges’ g = 0.73) and sensorimotor regions (g = 0.54), but was not significantly altered in the limbic subdivision (Box 1) [61].
Studies investigating presynaptic dopamine function in individuals at clinical high risk (CHR) of psychosis also found the greatest abnormality to be in the associative striatum 65, 69, and that conversion from CHR to psychosis is associated with a progressive increase in dopamine synthesis capacity in the dorsal (predominantly sensorimotor) striatum, while no significant change was observed in the limbic subdivision [70].
In a multimodal study, frontal activation during a working memory task was shown to correlate inversely with associative striatum dopamine synthesis capacity in a CHR group [71]. In the same sample, dopamine synthesis capacity in the associative striatum correlated with greater activation in the left inferior frontal region during a verbal fluency task in the CHR group, but not in the control group [72]. In both studies, no correlations were seen for limbic or sensorimotor subdivisions. fMRI only studies have shown hypoconnectivity between cortex and dorsal striatum in individuals with schizophrenia 73, 74, 75, 76, CHR individuals [77], and individuals at genetic risk [73]. Greater activity within the dorsal striatum as measured during resting-state MRI has also been shown to correlate with psychotic symptoms [78], and treatment response correlates with increased functional connectivity between the associative striatum and prefrontal cortex [79]. Meanwhile, diffusion tensor imaging has found reduced anatomical connectivity between the dorsolateral prefrontal cortex and the associative striatum in schizophrenia [80]. It is important to recognise, however, that some fMRI studies have also shown alterations in ventral striatal function in patients with psychotic disorders relative to controls [81].
In summary, in contrast to the mesolimbic theory, in vivo neuroimaging studies have provided evidence that dopaminergic dysfunction in schizophrenia is greatest within dorsal, as opposed to ventral regions of the striatum.
How Could Striatal Dysfunction Lead to Symptoms of Schizophrenia?
An ongoing question is how the neurobiological abnormalities identified in patients translate to the diverse psychopathology with which they present. We now suggest several mechanisms whereby striatal dysfunction could contribute to the clinical manifestations of the disorder. It is important to note, however, that many of these proposals are speculative at this stage, and furthermore, that schizophrenia is a heterogeneous disorder and no single brain region or neurotransmitter is likely to be able to account for all symptoms in all patients.
Aberrant Salience and Delusional Form
Theoretical models linking biological substrates to phenomenological experience in psychosis have frequently built upon the finding that mesostriatal dopamine signalling is involved in marking the salience of environmental stimuli [1]. Excessive spontaneous dopamine transients are proposed to lend irrelevant external or internal stimuli significance due to the temporal association of the stimuli with striatal signalling 1, 82. Although interpretations have often focused upon the mesolimbic pathway, it is apparent that nigrostriatal pathways are also involved in signalling salience 13, 39, 40, 83, and the finding that dopaminergic activity within dorsal regions of the striatum is tied to signalling threat-related information [39], may have relevance to the fact that delusions are frequently persecutory in nature.
Existing models have proposed that delusions then develop secondary to cognitive processes attempting to construct a coherent explanation for these unusual experiences. Historically, however, discussions regarding the phenomenology of delusions have emphasised form of thought over content. Delusional form is characterised by a certainty of conviction, that is accompanied by an inability to shift perspective, and an imperviousness to counterargument [84]. Given the role of the dorsal striatum in habit formation and the coding of stable values 44, 46, one can speculate that the dopaminergic dysfunction of the dorsal striatum that accompanies the onset of psychosis could lead to a more habit-oriented mode of cognition, contributing to the rigid form of thought as well as its unusual content [70].
Another finding of relevance to theories of aberrant salience attribution in schizophrenia is the following one. A stimulus, even if initially lacking inherent salience, once paired with dopaminergic activity, maintains the ability to evoke dopaminergic activity over time [41]. This suggests that in psychosis, once an environmental stimulus has been highlighted by aberrant dopamine signalling, it may maintain its ability to trigger dopaminergic activity, potentially cementing its position in a delusional framework, even if the system subsequently returns to normal function.
The Dorsal Striatum as an Integrative Hub
The striatum, and specifically areas of the associative striatum, can be viewed as an integrative hub. Regions of the caudate receive inputs from nearly the entire cortex 11, 17; and, additionally, via various connections with the midbrain [13], the associative striatum acts as a moderator of communication between limbic and motor regions.
Given that the striatum performs an integrative role, functional disruption secondary to aberrant dopamine signalling may lead to the associative impairments observed in schizophrenia. Based on preclinical models, it has been proposed that the underlying pathophysiology in schizophrenia represents a combination of increased aberrant spontaneous phasic dopamine release, and a reduction in adaptive phasic release in response to relevant stimuli [82]. This would lead to increased noise in dopamine signalling in the associative striatum, which could explain findings of reduced functional connectivity between the associative striatum and cortex [73], and could disrupt integration of cortical inputs from emotional, cognitive, and motor areas. This provides a potential neurobiological correlate for Bleuler’s original description of the syndrome as principally resulting from a loss of association between thought processes, emotion, and behaviour [85], and could underlie symptoms such as inappropriate affect. However, this has yet to be definitively tested.
Regionally targeted dopamine signalling enables the precise selection of specific behaviours over others, and collaterals between direct and indirect pathway MSNs [35] mediate the appropriate integration of multiple signals (Figure 2B). Undirected dopamine transmission impairs this mechanism, leading to disorganised behaviour. In contrast, in the context of pharmacological approaches, D2 antagonism has the ability to supress overactivity within these systems, but potentially impairs the execution of desired behaviour (Figure 2B).
Abnormal Perceptions and Efference Copies
An efference copy is an internally generated replica of an outgoing motor signal, that has the effect of dampening sensory perceptions occurring as a result of the motor act, encouraging it to be perceived as self-authored and avoiding attribution to an external agent. The possibility, therefore, that disruption of efference copy mechanisms could contribute to passivity phenomena has long been suggested [86].
Efference copies accompanying internally generated motor cortex activity travel via pyramidal tract neurons to the dorsal striatum 87, 88. There is some evidence that the glutamatergic corticostriatal neurons thought to encode the efference copy tend to synapse upon the GABAergic D2 striatal MSNs of the indirect pathway (Figure 3) 87, 89, 90. One might speculate therefore that excessive dopaminergic signalling within the striatum inhibits D2 expressing neurons, thereby reducing activity of the indirect pathway and potentially impeding the appropriate transmission of the efference copy signal, meaning that internally generated phenomena may not be coded as such. However, this hypothesis remains to be tested, including in humans, and is only one possible mechanism of disrupted efference copy signalling.
In addition to motor passivity phenomena, a similar mechanism may contribute to auditory hallucinations related to inner speech, such as thought echo. Inner speech is in certain respects a motor act, in that it is thought to result from motor plans for speech that are subsequently aborted [91]. Recent research suggests that efference copy mechanisms account for the fact that it is typically easily distinguished from external speech [92]. The neurobiological correlate of inner speech includes neural activation in cortical areas involved in the perception of external speech, such as the secondary auditory cortex [93], and these cortical areas project to the dorsal striatum [18]. Abnormalities of the dorsal striatum have been associated with auditory hallucinations in studies of brain structure, metabolic rate, and perfusion, and it is possible that the mechanism discussed above may contribute to the relationship between these symptoms and striatal dysfunction 94, 95, 96.
Efference-copy mechanisms, however, are less likely to account for auditory hallucinations that are phenomenologically unrelated to inner speech, and a predictive coding framework, which is a more generalisable model, seems more relevant in this context. Predictive coding refers to the idea that the brain compares prior expectations with new sensory evidence, and uses the discrepancy between the two (the prediction error) to update its model of the world. The certainty regarding one’s prior expectation is described as the precision of that prediction. Both the extent of the difference between prior and sensory data, and the precision of these determine the magnitude of the prediction error [97]. Recent research has demonstrated that auditory hallucinations are related to a greater ability of priors to influence perception [98]. It has also been demonstrated that amphetamine-induced dopamine release in the associative striatum is associated with this increased weighting of priors [99]. These findings are complemented by recent preclinical research showing that neurons in the tail of the striatum code prior beliefs regarding the value of auditory stimuli [100].
Cognitive and Negative Symptoms
It addition to the role that striatal dopamine signalling plays in the development of positive symptoms, several mechanisms have been suggested for its contribution to the cognitive and negative symptoms of the disorder as well.
Cognitive impairments in schizophrenia have been suggested to result from cortical hypodomaminergia; an idea supported by the importance of cortical dopamine signalling for prefrontal related cognition 101, 102. Recent work in rodents has extended this framework to include striatal involvement, by showing that the relationship between cortical and striatal dopamine is bidirectional, and that increased dorsal striatal dopaminergic signalling can reduce mesocortical dopamine release and produce cognitive deficits 103, 104. While in vivo imaging evidence for a deficit in cortical dopamine transmission has emerged [105], and the multimodal studies discussed above have suggested that striatal dysfunction may be functionally linked to cortical hypofunction, the direction of causality remains unclear and has yet to be determined, including in human studies 71, 72.
Striatal hyperdopaminergia could conceivably result in cognitive impairments either by disrupting signalling between the frontal cortex and associative striatum, or by potentially driving cortical dopamine dysregulation [103]. Of relevance to this question is an animal model of striatal D2 receptor overexpression, designed to mimic the increased striatal dopamine signalling observed in schizophrenia 103, 106. Studies using this model found that striatal D2 overexpression led to both reductions in cortical dopamine turnover and cognitive deficits [106]. These abnormalities persisted even following the normalisation of striatal dopamine transmission, suggesting that while increased striatal dopamine signalling leads to cognitive impairments, subsequent adaptive changes may underlie their persistence [106]. This could contribute to the finding that the attenuation of striatal dopamine transmission with antipsychotics is of limited benefit in treating cognitive symptoms. This potential role of associative striatum, is also supported by in vivo studies showing that reduced connectivity of the associative striatum and substantia nigra is related to the severity of cognitive deficits in schizophrenia 75, 107.
There is also evidence that striatal dysfunction may contribute directly to negative symptoms. Studies using probabilistic learning tasks have shown that negative symptoms in individuals with schizophrenia may be related to impaired reward-based learning 108, 109, 110, 111. Multiple studies have demonstrated that the striatum plays a key role in the orchestration of this type of behaviour [112], and it has been proposed that excessive aberrant dopamine release may mask adaptive striatal dopamine release, thereby contributing to these behavioural deficits in schizophrenia [111]. This is consistent with neuroimaging studies showing reduced midbrain and striatal activation during reward processing in patients 81, 113.
Therapeutic Implications
In this section we consider the implications of the evidence discussed earlier for the development of new treatments for schizophrenia that are not D2 receptor blockers and that might address the striatal dopamine dysfunction seen in the disorder.
As previously discussed, studies have shown that the magnitude of dopaminergic abnormalities in schizophrenia varies across the striatum, with the most marked dysfunction seen in the associative striatum. These findings suggest that anatomically selective modulation of dopamine function is preferable [61]. This could have the potential benefit of reducing adverse effects that may occur secondary to dopamine antagonism in regions such as the cortex, where PET imaging evidence indicates dopamine signalling may be unaltered or even reduced in schizophrenia.
The existing mechanistic understanding of striatal circuitry points at some possible therapeutic approaches that could allow more anatomically precise modulation of striatal dopamine function. One such mechanism, as discussed earlier, is muscarinic modulation of striatal dopamine release. Preclinical studies have shown that M4 positive allosteric modulators (PAMs) act on striatal MSNs to specifically inhibit dorsal striatum dopamine release via endocannabinoid signalling 114, 115. Other preclinical studies have shown that activation of group 1 metabotropic glutamate receptors may also selectively reduce dorsal striatum dopamine transmission via interaction with M4 receptors, but that unlike M4 activation, mGlu1 PAMs appear to have the advantage of not reducing motivational responding [115]. Encouragingly, a PAM of the M1/M4 receptor has shown efficacy in treating schizophrenia, although tolerability issues have prevented further clinical trials [116].
Concluding Remarks and Future Perspectives
Recent in vivo imaging evidence consistently suggests that the major abnormality in dopamine function in schizophrenia is located within the dorsal rather than the limbic striatum. Increasing knowledge regarding the structure, function, and neurochemistry of the striatum has improved our understanding of how these dopaminergic abnormalities may lead to symptoms. While these developments highlight potential pathways for the development of new treatments, the translation of these advances to meaningful clinical interventions, remains a significant challenge (See Outstanding Questions).
Outstanding Questions.
Is anatomically precise modulation of dopamine signalling within the striatum possible? The variation across the striatum both in terms of dopamine receptor distributions, and of the mechanisms that control striatal dopamine and striatal function, suggests that it may be, but this remains to be tested.
Do endocannabinoids, GABAgeric, cholinergic, and glutamatergic interventions have therapeutic potential given their role in modulating striatal function?
Do primary striatal abnormalities exist in schizophrenia, or is dysfunction entirely secondary to upstream pathology? Genes associated with schizophrenia, overlap significantly with genes expressed by MSNs but not with those expressed by dopamine neurons. Abnormalities such as patch-matrix differentiation, or ones associated with cholinergic interneurons might also contribute to striatal dysfunction in schizophrenia. Recently, greater densities of afferent excitatory synapses have been found in the NAcc of individuals with schizophrenia, this could, via the feedforward mechanisms discussed (Figure 1A), drive increased dopamine release in the dorsal striatum.
Are specific symptoms associated with dopamine dysregulation in specific striatal loci? For instance, are verbal hallucinations associated with dopaminergic abnormalities in striatal regions displaying connectivity to the secondary auditory cortex? Are motor symptoms associated with the motor striatum?
What is the optimal striatal parcellation? Preclinical findings suggest current atlases may oversimplify the picture, and point at the importance of improving our understanding of striatal connectivity. Could we improve our ability to characterise striatal dopamine dysfunction by using individual participant functional or anatomical connectivity to parcellate the striatum in multimodal studies of schizophrenia?
Disclaimer Statement
RM declares no financial conflicts of interest. AA-D has received research support from Pierre Fabre, Otsuka, Forest, Pfizer, and Neurocrine; served on advisory boards of Roche, Otsuka, Lundbeck; and given lectures sponsored by Otsuka. She is an advisor and holds shares in System 1 Biosciences and in Storm Biosciences. ODH has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organised by Astra–Zeneca, Autifony, BMS, Eli Lilly, Heptares, Jansenn, Lundbeck, Lyden-Delta, Otsuka, Servier, Sunovion, Rand and Roche. Neither Dr Howes nor his family have been employed by or have holdings/a financial stake in any biomedical company.
Acknowledgements
RM’s work is supported by a clinical research training fellowship grant from the Wellcome trust (no. 200102/Z/15/Z). OH’s work is supported by Medical Research Council-UK (no. MC-A656-5QD30), Maudsley Charity (no. 666), Brain and Behavior Research Foundation, and Wellcome Trust (no. 094849/Z/10/Z) grants, and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Glossary
- Amphetamine sensitisation
repetitive administration of amphetamine leading to progressively greater amphetamine induced dopamine release.
- Associative striatum
precommissural dorsal caudate, postcommissural caudate, and precommissural dorsal putamen. Receives afferent connections from the dorsolateral prefrontal cortex. Homologous to the rodent dorsomedial striatum.
- Clinical high risk (CHR)
individuals experiencing intermittent or attenuated psychotic symptoms, below the level at which a psychotic disorder would be diagnosed.
- Diffusion tensor imaging
measurement of water diffusion patterns to infer the white matter structure of the brain and map anatomical connectivity between regions.
- Direct pathway
striatal output pathway, in which D1 MSNs project directly from the striatum to the output nuclei of the basal ganglia.
- Efference copy
internally generated replica of an outgoing motor signal, that signals that the subsequent motor act is self-generated and thereby dampens sensory perceptions occurring as a result of that act.
- Inappropriate affect
emotional expression not in keeping with the circumstances that provoked it.
- Indirect pathway
striatal output pathway, in which D2 MSNs project from the striatum to the output nuclei of the basal ganglia indirectly via the pallidum.
- Limbic striatum
equivalent to the ventral striatum. Receives afferent projections from limbic areas: ventromedial prefrontal cortext, orbitofrontal cortex, dorsal anterior cingulate and medial temporal lobe.
- Medium spiny neuron (MSN)
GABAergic projection neurons of the striatum. Typically classified as either D1 type of the direct pathway, and D2 type of the indirect pathway.
- Nucleus accumbens (NAcc)
main component of the ventral striatum.
- Passivity phenomenon
symptom of schizophrenia in which one feels under external influence and no longer in control of one’s movements or thoughts.
- Positive psychotic symptoms
symptoms such as hallucinations, delusions, and disorganised thought and behaviour.
- Positron emission tomography (PET)
technique in which a radioactive atom is attached to a biologically active molecule. Positrons emitted by this molecule produce gamma rays that are detected to allow visualisation of its anatomical distribution.
- Resting state functional magnetic resonance imaging (fMRI)
brain activity is measured in the absence of any explicit task, and correlations between spontaneous fluctuations allow for the inference of functional connectivity between regions.
- Salience
quality of an item that causes it to stand out from its environment.
- Sensorimotor striatum
postcommissural putamen. Receives afferent projections from motor and premotor cortical areas. Homologous to dorsolateral striatum in rodents.
- Thought echo
form of auditory hallucination in which an individual perceives their thoughts being spoken aloud as an external stimulus.
References
- 1.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 2.Davis K.L. Dopamine in schizophrenia: a review and reconceptualization. Am. J. Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger D. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry. 1987;45:1055. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 4.Meltzer H.Y., Stahl S.M. The dopamine hypothesis of schizophrenia: a review. Schizophr. Bull. 1976;2:19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]
- 5.Kapur S., Seeman P. Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics?: A new hypothesis. Am. J. Psychiatry. 2001;158:360–369. doi: 10.1176/appi.ajp.158.3.360. [DOI] [PubMed] [Google Scholar]
- 6.Haber S.N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2016;18:7–21. doi: 10.31887/DCNS.2016.18.1/shaber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander G., Crutcher M. Functional architecture of basal ganglia circuits: neural substrates of parrallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 8.Haber S.N. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Oh S.W. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung K., Deisseroth K. CLARITY for mapping the nervous system. Nat. Methods. 2013;10:508–513. doi: 10.1038/nmeth.2481. [DOI] [PubMed] [Google Scholar]
- 11.Hunnicutt B.J. A comprehensive excitatory input map of the striatum reveals novel functional organization. eLife. 2016;5:1–32. doi: 10.7554/eLife.19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gangarossa G. Spatial distribution of D1R- and D2R-expressing medium-sized spiny neurons differs along the rostro-caudal axis of the mouse dorsal striatum. Front. Neural Circuits. 2013;7:1–16. doi: 10.3389/fncir.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lerner T.N. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell. 2015;162:635–647. doi: 10.1016/j.cell.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Düzel E. Functional imaging of the human dopaminergic midbrain. Trends Neurosci. 2009;32:321–328. doi: 10.1016/j.tins.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Marquand A.F. Functional corticostriatal connection topographies predict goal-directed behaviour in humans. Nat. Hum. Behav. 2017;1:0146. doi: 10.1038/s41562-017-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tziortzi A.C. Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography. Cereb. Cortex. 2014;24:1165–1177. doi: 10.1093/cercor/bhs397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Averbeck B.B. Estimates of projection overlap and zones of convergence within frontal–striatal circuits. J. Neurosci. 2014;34:9497–9505. doi: 10.1523/JNEUROSCI.5806-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi E.Y. Convergence of prefrontal and parietal anatomical projections in a connectional hub in the striatum. Neuroimage. 2017;146:821–832. doi: 10.1016/j.neuroimage.2016.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarbo K., Verstynen T.D. Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. J. Neurosci. 2015;35:3865–3878. doi: 10.1523/JNEUROSCI.2636-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuhma N. Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling. Neuron. 2014;81:901–912. doi: 10.1016/j.neuron.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcott P.F. Regional heterogeneity of D2-receptor signaling in the dorsal striatum and nucleus accumbens. Neuron. 2018;98 doi: 10.1016/j.neuron.2018.03.038. P575–P587.E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosillo P. Cortical control of striatal dopamine transmission via striatal cholinergic interneurons. Cereb. Cortex. 2016;26:4160–4169. doi: 10.1093/cercor/bhw252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson A.B. Striatal cholinergic interneurons drive GABA release from dopamine terminals. Neuron. 2014;82:63–70. doi: 10.1016/j.neuron.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Threlfell S., Cragg S.J. Dopamine signaling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons. Front. Syst. Neurosci. 2011;5:11. doi: 10.3389/fnsys.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin J.H. Muscarinic regulation of dopamine and glutamate transmission in the nucleus accumbens. Proc. Natl. Acad. Sci. U. S. A. 2015;112:8124–8129. doi: 10.1073/pnas.1508846112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster D.J. Antipsychotic-like effects of M4 positive allosteric modulators are mediated by CB2 receptor-dependent inhibition of dopamine release. Neuron. 2016;91:1244–1252. doi: 10.1016/j.neuron.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moehle M.S. Cholinergic projections to the substantia nigra pars reticulata inhibit dopamine modulation of basal ganglia through the M4 muscarinic receptor. Neuron. 2017;96:1358–1372.e4. doi: 10.1016/j.neuron.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kharkwal G. Parkinsonism driven by antipsychotics originates from dopaminergic control of striatal cholinergic interneurons. Neuron. 2016;91:67–78. doi: 10.1016/j.neuron.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kupchik Y.M. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat. Neurosci. 2015;18:1230–1232. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soares-Cunha C. Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat. Commun. 2016;7:1–11. doi: 10.1038/ncomms11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobbs L.K.K. Dopamine regulation of lateral inhibition between striatal neurons gates the stimulant actions of cocaine. Neuron. 2016;90:1100–1113. doi: 10.1016/j.neuron.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cazorla M. Dopamine d2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron. 2014;81:153–164. doi: 10.1016/j.neuron.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui G. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbera G. Spatially compact neural clusters in the dorsal striatum encode locomotion relevant information. Neuron. 2016;92:202–213. doi: 10.1016/j.neuron.2016.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burke D.A. Striatal local circuitry: a new framework for lateral inhibition. Neuron. 2017;96:267–284. doi: 10.1016/j.neuron.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schultz W. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 37.Fiorillo C.D. Two dimensions of value: dopamine neurons represent reward but not aversiveness. Science. 2013;341:546–549. doi: 10.1126/science.1238699. [DOI] [PubMed] [Google Scholar]
- 38.Horvitz J.C. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 39.Menegas, W. et al. Dopamine neurons projecting to the posterior striatum reinforce avoidance of theratening stimuli. Nat. Neurosci. (in press) [DOI] [PMC free article] [PubMed]
- 40.Menegas W. Opposite initialization to novel cues in dopamine signaling in ventral and posterior striatum in mice. eLife. 2017;6:1–26. doi: 10.7554/eLife.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunders B.T. Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat. Neurosci. 2018;21:1072–1083. doi: 10.1038/s41593-018-0191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCutcheon R. Mesolimbic dopamine function is related to salience network connectivity: an integrative PET and MR study. Biol. Psychiatry. 2018 doi: 10.1016/j.biopsych.2018.09.010. Published online September 28, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nour, M.M. et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc. Natl. Acad. Sci. U. S. A. (in press) [DOI] [PMC free article] [PubMed]
- 44.Everitt B.J., Robbins T.W. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci. Biobehav. Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Balleine B.W. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H.F., Hikosaka O. Distinct basal ganglia circuits controlling behaviors guided by flexible and stable values. Neuron. 2013;79:1001–1010. doi: 10.1016/j.neuron.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinon B.J., Lieberman J.A. Mechanisms of action of atypical antipsychotic drugs: a critical analysis. Psychopharmacology (Berl) 1996;124:2–34. doi: 10.1007/BF02245602. [DOI] [PubMed] [Google Scholar]
- 48.Lidsky T.I. Reevaluation of the mesolimbic hypothesis of antipsychotic drug action. Schizophr. Bull. 1995;21:67–74. doi: 10.1093/schbul/21.1.67. [DOI] [PubMed] [Google Scholar]
- 49.Stahl S. Cambridge University Press; 2013. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. [Google Scholar]
- 50.Sadock B.J., Sadock V.A. Lippincott Williams & Wilkins; 2008. Kaplan & Sadock’s Concise Textbook of Clinical Psychiatry. [Google Scholar]
- 51.Bird E.D. Increased brain dopamine and reduced glutamic acid decarboxylase and choline acetyl transferase activity in schizophrenia and related psychoses. Lancet. 1977;310:1157–1159. doi: 10.1016/s0140-6736(77)91542-2. [DOI] [PubMed] [Google Scholar]
- 52.Crow T.J. Monoamine mechanisms in chronic schizophrenia: post-mortem neurochemical findings. Br. J. Psychiatry. 1979;134:249–256. doi: 10.1192/bjp.134.3.249. [DOI] [PubMed] [Google Scholar]
- 53.Toru M. Neurotransmitters, receptors and neuropeptides in post-mortem brains of chronic schizophrenic patients. Acta Psychiatr. Scand. 1988;78:121–137. doi: 10.1111/j.1600-0447.1988.tb06312.x. [DOI] [PubMed] [Google Scholar]
- 54.Owen F. Increased dopamine-receptor sensitivity in schizophrenia. Lancet. 1978;312:29–32. doi: 10.1016/s0140-6736(78)91740-3. [DOI] [PubMed] [Google Scholar]
- 55.McCollum L.A. Elevated excitatory input to the nucleus accumbens in schizophrenia: a postmortem ultrastructural study. Schizophr. Bull. 2015;41:1123–1132. doi: 10.1093/schbul/sbv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCollum L.A., Roberts R.C. Uncovering the role of the nucleus accumbens in schizophrenia: a postmortem analysis of tyrosine hydroxylase and vesicular glutamate transporters. Schizophr. Res. 2015;169:369–373. doi: 10.1016/j.schres.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCollum L.A. Tyrosine hydroxylase localization in the nucleus accumbens in schizophrenia. Brain Struct. Funct. 2016;221:4451–4458. doi: 10.1007/s00429-015-1174-9. [DOI] [PubMed] [Google Scholar]
- 58.Mizrahi R. Effects of antipsychotics on D3 receptors: a clinical PET study in first episode antipsychotic naive patients with schizophrenia using [11C]-(+)-PHNO. Schizophr. Res. 2011;131:63–68. doi: 10.1016/j.schres.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Vernaleken I. Modulation of [18F]fluorodopa (FDOPA) kinetics in the brain of healthy volunteers after acute haloperidol challenge. Neuroimage. 2006;30:1332–1339. doi: 10.1016/j.neuroimage.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 60.Vulto A.G. Rapid postmortem increase in extracellular dopamine in the rat brain as assessed by brain microdialysis. J. Neurochem. 1988;51:746–749. doi: 10.1111/j.1471-4159.1988.tb01808.x. [DOI] [PubMed] [Google Scholar]
- 61.McCutcheon R. Defining the locus of dopaminergic dysfunction in schizophrenia: a meta-analysis and test of the mesolimbic hypothesis. Schizophr. Bull. 2018;44:1301–1311. doi: 10.1093/schbul/sbx180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howes O.D. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch. Gen. Psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kegeles L.S. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch. Gen. Psychiatry. 2010;67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 64.Slifstein M., Abi-Dargham A. Is it pre- or postsynaptic? Imaging striatal dopamine excess in schizophrenia. Biol. Psychiatry. 2018;83:635–637. doi: 10.1016/j.biopsych.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 65.Howes O.D. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch. Gen. Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 66.Mizrahi R. Increased stress-induced dopamine release in psychosis. Biol. Psychiatry. 2012;71:561–567. doi: 10.1016/j.biopsych.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 67.Egerton A. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol. Psychiatry. 2013;74:106–112. doi: 10.1016/j.biopsych.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 68.Howes O.O.D. Midbrain dopamine function in schizophrenia and depression: a post-mortem and positron emission tomographic imaging study. Brain. 2013;136:3242–3251. doi: 10.1093/brain/awt264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mizrahi R. Stress-induced dopamine response in subjects at clinical high risk for schizophrenia with and without concurrent cannabis use. Neuropsychopharmacology. 2013;39:1479–1489. doi: 10.1038/npp.2013.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Howes O. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol. Psychiatry. 2011;16:885–886. doi: 10.1038/mp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fusar-poli P. Abnormal frontostriatal interactions in people with prodromal signs of psychosis. Arch. Gen. Psychiatry. 2010;67:683–691. doi: 10.1001/archgenpsychiatry.2010.77. [DOI] [PubMed] [Google Scholar]
- 72.Fusar-Poli P. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol. Psychiatry. 2011;16:67–75. doi: 10.1038/mp.2009.108. [DOI] [PubMed] [Google Scholar]
- 73.Fornito A. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. 2013;70:1143–1151. doi: 10.1001/jamapsychiatry.2013.1976. [DOI] [PubMed] [Google Scholar]
- 74.Yoon J.H. Impaired prefrontal-basal ganglia functional connectivity and substantia nigra hyperactivity in schizophrenia. Biol. Psychiatry. 2013;74:122–129. doi: 10.1016/j.biopsych.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoon J.H. Task-evoked substantia nigra hyperactivity associated with prefrontal hypofunction, prefrontonigral disconnectivity and nigrostriatal connectivity predicting psychosis severity in medication naïve first episode schizophrenia. Schizophr. Res. 2014;159:521–526. doi: 10.1016/j.schres.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horga G. Dopamine-related disruption of functional topography of striatal connections in unmedicated patients with schizophrenia. JAMA Psychiatry. 2016;73:862. doi: 10.1001/jamapsychiatry.2016.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dandash O. Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophr. Bull. 2014;40:904–913. doi: 10.1093/schbul/sbt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sorg C. Increased intrinsic brain activity in the striatum reflects symptom dimensions in schizophrenia. Schizophr. Bull. 2013;39:387–395. doi: 10.1093/schbul/sbr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sarpal D.K. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 2015;72:5–13. doi: 10.1001/jamapsychiatry.2014.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Levitt J.J. Reduced structural connectivity in frontostriatal white matter tracts in the associative loop in schizophrenia. Am. J. Psychiatry. 2017;174:1102–1111. doi: 10.1176/appi.ajp.2017.16091046. [DOI] [PubMed] [Google Scholar]
- 81.Radua J. ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis. JAMA Psychiatry. 2016;72:1243–1251. doi: 10.1001/jamapsychiatry.2015.2196. [DOI] [PubMed] [Google Scholar]
- 82.Maia T.V., Frank M.J. An integrative perspective on the role of dopamine in schizophrenia. Biol. Psychiatry. 2017;81:52–66. doi: 10.1016/j.biopsych.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oyama K. Discrete coding of stimulus value, reward expectation, and reward prediction error in the dorsal striatum. J. Neurophysiol. 2015;114:2600–2615. doi: 10.1152/jn.00097.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jaspers K. Springer; 1913. Allgemeine Psychopathologie. [Google Scholar]
- 85.Bleuler E. International Universities Press; 1950. Dementia Praecox or the Group of Schizophrenias. [Google Scholar]
- 86.Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr. Bull. 1978;4:636–640. doi: 10.1093/schbul/4.4.636. [DOI] [PubMed] [Google Scholar]
- 87.Shipp S. The functional logic of corticostriatal connections. Brain Struct. Funct. 2017;222:669–706. doi: 10.1007/s00429-016-1250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fee M.S. The role of efference copy in striatal learning. Curr. Opin. Neurobiol. 2014;25:194–200. doi: 10.1016/j.conb.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deng Y. Differential organization of cortical inputs to striatal projection neurons of the matrix compartment in rats. Front. Syst. Neurosci. 2015;9:1–14. doi: 10.3389/fnsys.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reiner A. Corticostriatal projection neurons – dichotomous types and dichotomous functions. Front. Neuroanat. 2010;4:1–15. doi: 10.3389/fnana.2010.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sokolov A.N. Plenum Press; 1972. Inner Speech and Thought. [Google Scholar]
- 92.Whitford T.J. Neurophysiological evidence of efference copies to inner speech. eLife. 2017;6:1–23. doi: 10.7554/eLife.28197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alderson-Day B. The brain’s conversation with itself: neural substrates of dialogic inner speech. Soc. Cogn. Affect. Neurosci. 2015;11:110–120. doi: 10.1093/scan/nsv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhuo C. Cerebral blood flow alterations specific to auditory verbal hallucinations in schizophrenia. Br. J. Psychiatry. 2017;210:209–215. doi: 10.1192/bjp.bp.115.174961. [DOI] [PubMed] [Google Scholar]
- 95.Horga G. Differential brain glucose metabolic patterns in antipsychotic-naive first-episode schizophrenia with and without auditory verbal hallucinations. J. Psychiatry Neurosci. 2011;36:312–321. doi: 10.1503/jpn.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O’Daly O.G. Brain structural changes in schizophrenia patients with persistent hallucinations. Psychiatry Res. Neuroimaging. 2007;156:15–21. doi: 10.1016/j.pscychresns.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 97.Sterzer P. The predictive coding account of psychosis. Biol. Psychiatry. 2018;84:634–643. doi: 10.1016/j.biopsych.2018.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Powers A.R. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science. 2017;357:596–600. doi: 10.1126/science.aan3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cassidy C.M. A perceptual inference mechanism for hallucinations linked to striatal dopamine. Curr. Biol. 2018;28 doi: 10.1016/j.cub.2017.12.059. P503–P514.E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo L. Stable representation of sounds in the posterior striatum during flexible auditory decisions. Nat. Commun. 2018;9:1534. doi: 10.1038/s41467-018-03994-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brozoski T.J. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 102.Goldman-Rakic P.S. Working memory dysfunction in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 103.Simpson E.H. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krabbe S. Increased dopamine D2 receptor activity in the striatum alters the firing pattern of dopamine neurons in the ventral tegmental area. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E1498–E1506. doi: 10.1073/pnas.1500450112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Slifstein M. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia. JAMA Psychiatry. 2015;72:316–324. doi: 10.1001/jamapsychiatry.2014.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kellendonk C. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 107.Yoon J.H. Impaired prefrontal–basal ganglia functional connectivity and substantia nigra hyperactivity in schizophrenia. Biol. Psychiatry. 2013;74:122–129. doi: 10.1016/j.biopsych.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gold J.M. Negative symptoms in schizophrenia result from a failure to represent the expected value of rewards: behavioral and computational modeling evidence HHS Public Access. Arch. Gen. Psychiatry. 2012;69:129–138. doi: 10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Strauss G.P. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol. Psychiatry. 2011;69:424–431. doi: 10.1016/j.biopsych.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morris R.W. Corticostriatal control of goal-directed action is impaired in schizophrenia. Biol. Psychiatry. 2015;77:187–195. doi: 10.1016/j.biopsych.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 111.Maia T.V., Frank M.J. an integrative perspective on the role of dopamine in schizophrenia. Biol. Psychiatry. 2017;81:52–66. doi: 10.1016/j.biopsych.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2009;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murray G.K. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol. Psychiatry. 2008;13 doi: 10.1038/sj.mp.4002058. 239, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Foster D.J. Antipsychotic-like effects of M4 positive allosteric modulators are mediated by CB2 receptor-dependent inhibition of dopamine release. Neuron. 2016;91:1244–1252. doi: 10.1016/j.neuron.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yohn S.E. Activation of the mGlu1metabotropic glutamate receptor has antipsychotic-like effects and is required for efficacy of M4muscarinic receptor allosteric modulators. Mol. Psychiatry. 2018 doi: 10.1038/s41380-018-0206-2. Published online August 16, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shekhar A. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry. 2008;165:1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- 117.Kunzle H. An autoradiographic analysis of the efferent connections from premotor and adjacent prefrontal regions (areas 6 and 9) in Macaca fascicularis. Brain Behav. Evol. 1978;15:185–234. doi: 10.1159/000123779. [DOI] [PubMed] [Google Scholar]
- 118.Goldman-Rakic P.S., Selemon L.D. Springer US; 1986. Topography of Corticostriatal Projections in Nonhuman Primates and Implications for Functional Parcellation of the Neostriatum. [Google Scholar]
- 119.Kunishio K., Haber S.N. Primate cingulostriatal projection: limbic striatal versus sensorimotor striatal input. J. Comp. Neurol. 1994;350:337–356. doi: 10.1002/cne.903500302. [DOI] [PubMed] [Google Scholar]
- 120.Haber S.N. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Joel D., Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- 122.Gibbs F.A. Ictal and non-ictal psychiatric disorders in temporal lobe epilepsy. J. Nerv. Ment. Dis. 1951;113:522–528. [PubMed] [Google Scholar]
- 123.Malamud N., Mania S. Psychiatric disorder with intracranial tumors of limbic system. Arch. Neurol. 1967;17:113–123. doi: 10.1001/archneur.1967.00470260003001. [DOI] [PubMed] [Google Scholar]
- 124.Heath R.G. Common characteristics of epilepsy and schizophrenia: clinical observation and depth electrode studies. Am. J. Psychiatry. 1962;118:1013–1026. doi: 10.1176/ajp.118.11.1013. [DOI] [PubMed] [Google Scholar]
- 125.Connell P. Amphetamine psychosis. Br. Med. J. 1957;5018:582. [Google Scholar]
- 126.Di Chiara G., Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U. S. A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yehuda S., Wurtman R.J. Dopaminergic neurons in the nitro-striatal and mesolimbic pathways: mediation of specific effects of d-amphetamine. Eur. J. Pharmacol. 1975;30:154–158. doi: 10.1016/0014-2999(75)90094-1. [DOI] [PubMed] [Google Scholar]
- 128.Pijnenburg A.J. Inhibition of d-amphetamine-induced locomotor activity by injection of haloperidol into the nucleus accumbens of the rat. Psychopharmacologia. 1975;41:87–95. doi: 10.1007/BF00421062. [DOI] [PubMed] [Google Scholar]
- 129.Deutch A.Y. Regionally specific effects of atypical antipsychotic drugs on striatal Fos expression: the nucleus accumbens shell as a locus of antipsychotic action. Mol. Cell. Neurosci. 1992;3:332–341. doi: 10.1016/1044-7431(92)90030-6. [DOI] [PubMed] [Google Scholar]