Abstract
Mouse and human pluripotent stem cells have been widely used to study the development of the hematopoietic and immune systems. While not all cells can be derived with the same efficiency, immune cells such as natural killer (NK) cells and macrophages can be easily produced from pluripotent stem cells to enable development of new cell-based therapies. NK cells and macrophages are part of the innate immune system, the first line of defense against malignancies and infectious disease. Human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs)-derived NK cells can be produced at a clinical scale suitable for translation into clinical trials. Additionally, pluripotent stem cells can be genetically modified to produce hESC and iPSC-derived human NK cells with enhanced anti-tumor activity. These engineered NK cells can express a stabilized version of the high-affinity Fc receptor CD16, chimeric antigen receptors (CARs), or other strategies to enable more potent and targeted cellular immunotherapies. Moreover, macrophages can also be routinely and efficiently produced from hESCs and iPSCs as a tool to expand our knowledge of the basic biology of these cells. hESC/iPSC-derived macrophages can also be employed as a novel approach for cancer immunotherapy, as well as a strategy to repair or regenerate diseased/damaged tissues and organs.
Background:
Mouse and human pluripotent stem cells have been now used for decades in key studies to better understand mammalian hematopoiesis. Studies with mouse ESCs to create genetic knock-out mice have been instrumental to define key genetic mechanisms that mediate development of different hematopoietic lineages (1, 2). In an effort to move this work into a human system, our group was the first to derive blood cells (mainly myeloid and erythroid cells) from hESCs (3) and later the first to produce lymphocytes (4). These initial studies on hESC-derived lymphocytes produced NK cells with phenotype and function similar to NK cells isolated from peripheral blood, including the ability to kill diverse tumor cells in vitro and in vivo (4, 5). We have subsequently used human iPSCs to produce NK cells and also demonstrated the ability of hESC/hiPSC-derived NK cells to kill virally-infected cells, most notably HIV (6, 7).
As discussed in this review, these hESC/iPSC-derived NK cells can now be translated into clinical therapies to better treat relapsed/refractory cancers. Additionally, hESCs/iPSCs provide a stable and standardized platform for genetic modification to produce NK cells with improved anti-tumor activity. In particular, we have produced iPSC-derived NK cells that express CARs to improve killing of typically NK cell-resistant tumors (8). While not covered in this review, other innate lymphoid cells (ILCs) can also be derived from hESCs and hiPSCs (9).
There is obviously considerable interest to also produce cells of the adaptive immune system (T cells and B cells) from hESCs/hiPSCs; however, this has been a much more challenging endeavor and is covered elsewhere in this review series. While several groups have produced T cells from hESCs (10–12) this is typically markedly less efficient than T cell development from hematopoietic stem/progenitor cells (HSPCs) isolated from umbilical cord blood (UCB), or NK cell development from hESCs and iPSCs. Indeed, we previously identified key transcriptional regulators that are differentially expressed in hESC-derived CD34+CD45+ HPC compared to UCB CD34/45+ cells. Specifically, upregulation of ID genes in hESC-derived cells may skew lymphocyte development toward NK cells and inhibit T and B cell lineages (13). Notably, production of T cells from iPSCs that were reprogrammed from T cells (termed T-hiPSCs) is markedly more efficient than T cell development from hESCs or non-T cell-derived hiPSCs (14–16). This finding suggests that epigenetic memory (17) and/or T cell receptor (TcR) rearrangement in T cells alleviates a block in T cell development. Also notable is the relative paucity of evidence of mature B cell production from hESCs/hiPSCs, though some systems have been successful at B cell development (18, 19). On the other hand, macrophages are another major effector cell type of innate immunity that play an important role in tissue homeostasis, including recognition and elimination of pathogens and malignant cells. However, macrophage research has been mostly conveyed by using blood monocyte-derived macrophages which require large amounts of blood from donors (donor-specific variation) and are relatively hard to genetically manipulate. Therefore, hESC/iPSC-derived macrophages provide an attractive alternative system for deriving terminally differentiated and consistent human macrophages. Therefore, this review will focus on production and potential clinical translation of NK cells and myeloid cells (monocytes) from hESCs and hiPSCs.
NK cells
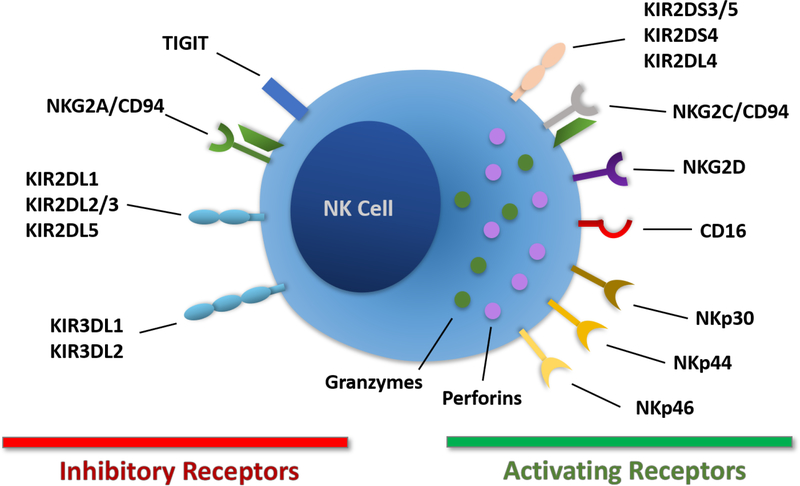
NK cells were identified in 1975 by two different groups as immune cells able to kill tumor cells without human leukocyte antigen (HLA) restriction and without prior sensitization (20, 21). These properties contrast to T cells that require recognition of self-HLA molecules to mediate their activity. NK cells are recognized to play a central role in the early response to viral infection and tumors and are also involved in organ transplant rejection (22–24). NK cells are considered innate immune cells because of their early response to infections without prior sensitization and absence of antigen-specific cell surface receptors, though they do express a repertoire of activating and inhibitory receptors that regulate their activity (25) (Figure 1). NK cells utilize these receptors to recognize key elements on tumor cells and virally-infected cells and mediate potent cytolytic activity and cytokine production (26, 27). As NK cells function as allogeneic effector cells, they do not need to be isolated from individual patients (as with autologous T cell-based therapies) for cellular immunotherapies.
Figure 1. Expression of key NK cell surface receptors.
NK cells express inhibitory and activating receptors that regulate their effector functions.
NK cells originate from CD34+ HSPCs in the bone marrow (BM) and represent 5–15% of circulating lymphocytes in most healthy individuals (28). Under normal physiological conditions they are present in a broad range of tissues, including but not limited to the skin, liver, gut, lungs and kidneys (29, 30). As noted, NK cells express a combination of activating and inhibitory receptors that control cell stimulation and their effector functions. Normal healthy cells express HLA class I molecules on their surface that engage killer immunoglobulin-like receptors (KIRs) that play a key role in self-tolerance of NK cells. In contrast, tumor cells or virally-infected cells often downregulate HLA class I expression to escape T cell cytotoxicity, leading to less inhibitory signaling and increased cytolytic activity by NK cells. Ligands for NK cell activating receptors are often upregulated by cellular stress associated with viral infection or tumor development (26, 27) and receptor engagement can lead to the initiation of NK cell-mediated cytotoxicity, secretion of pro-inflammatory cytokines and elimination of target cells. NK cell activating receptors include NKG2D, NKp30, NKp44 and NKp46 (Figure 2). Additionally, most NK cells express the low-affinity activating receptor FcγRIIIa (CD16a) which binds the Fc domain of immunoglobulins to mediate antibody-dependent cellular cytotoxicity (ADCC) (23, 28).
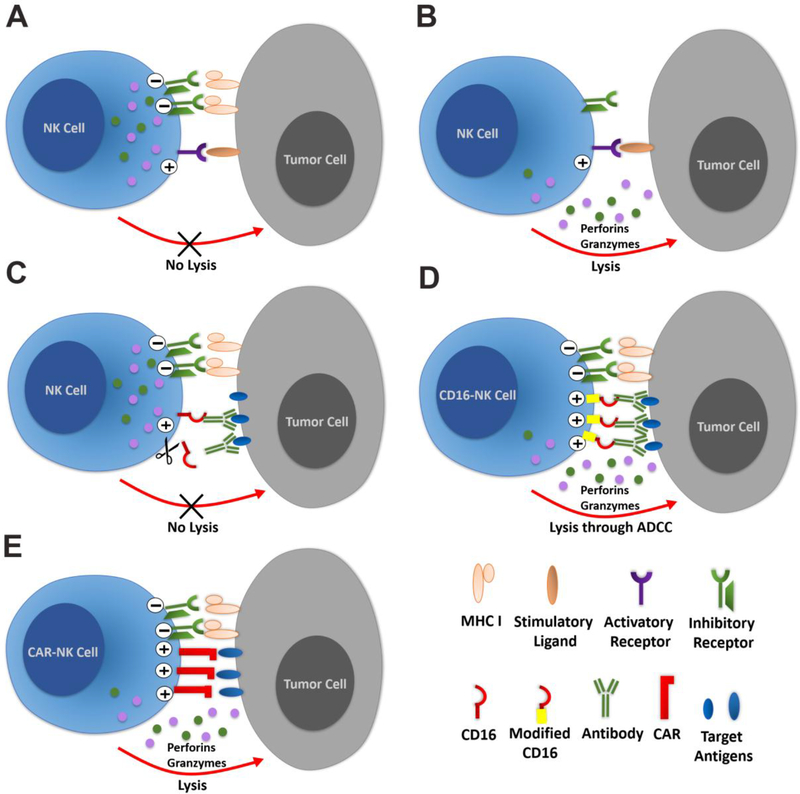
Figure 2. Regulation of NK cell activity.
NK cells are a key meditator of anti-tumor and antiviral activity. (A) Normally, NK cell activity is balanced by engagement of activating and inhibitory receptors to prevent killing of normal (non-tumor and non-infected) cells. (B) Tumor cells may down-regulate expression of major histocompatibility complex (MHC) class I molecules to avoid T cell-mediated killing that requires MHC Class I expression. MHC class I molecules bind KIRs that mediate inhibitory signals for NK cells. Therefore, the loss of MHC class I on tumor cells can shift the balance towards activating receptors leading to secretion of granules containing granzymes and perforins to kill the tumor cells. (C) Another mechanism of action for NK cells is the recognition of specific antigens through the binding of CD16 with antigen-specific antibodies even if the overexpression of inhibitory ligands by tumor cells and the cleavage of CD16 on NK cells may inhibit their activation. (D) Overexpression of modified non-cleavable CD16 on hESC or hiPSC-derived NK cells can increase the activating signal to overcome the inhibitory signals from tumor cells. (E) CAR-expressing NK cells, such as those that can be derived from hESCs or hiPSCs can mediate anti-tumor activity by providing additional activating signals through the specific binding of the CARs to their antigens expressed on tumor cells.
Adoptive NK cell immunotherapy
The ability of allogeneic NK cells to kill tumor cells has led to many clinical trials for over a decade (31). Initial studies by Miller and colleagues demonstrated the efficacy of allogeneic NK cells in treatment of hematological malignancies, most notably finding that 30–50% of patients with refractory or relapsed acute myeloid leukemia (AML) could achieve complete remission after receiving allogeneic peripheral blood (PB) NK cells (32–36). Many clinical trials using NK cell-based immunotherapy are now recruiting patients for treatment of hematologic or solid tumors (31, 37, 38). NK cells isolated from PB have been the most common source for clinical trials involving NK cell-based immunotherapies (31) but alternative sources include expanded UCB-derived NK cells (UCB-NK) (39–41) and cell lines such as NK-92 (42). Although NK-92 cells demonstrate anti-tumor activity and a good safety profile in clinical trials (42), they are aneuploid and therefore need to be irradiated prior to administration. This irradiation prevents expansion of the cells post-infusion and limits their survival in patients (43). As expansion of NK cells post-adoptive transfer clearly correlates to improved activity (33), this need to irradiate NK92 cells limits their anti-tumor activity. There are also limitations in using allogeneic PB as the source of NK cells, because the yield of cells can be strongly donor-dependent and the cell population remains quite heterogeneous, leading to activity that varies from donor to donor (33). Different NK cell populations isolated or derived from UCB have also been used in clinical trials to treat hematological malignancies (31, 39, 40, 44). However, like PB NK cells, UCB-NK cells differ between donors and need to be expanded prior to treatment.
NK cells derived from human pluripotent stem cells
hESC-derived NK cells were first produced by co-culturing hESCs with murine stromal cells (S17 and C166 cells) that had previously demonstrated the ability to sustain expansion of human HSPCs. This co-culture lead to production of hESC-derived clusters of CD34+CD45+ HPCs that were subsequently cultured on a second stromal cell population (AFT024 cells) to obtain CD45+CD56+ NK cells (4) (Table 1). These hESC-derived NK cells were able to lyse human tumor cells by both direct cell-mediated cytotoxicity and ADCC while also producing NK cell-specific cytokines. hESC-NK cells were shown to more effectively mediate tumor (leukemia) clearance in an in vivo mouse xenograft model than UCB-NK cells (5). Subsequent studies aimed to use more defined conditions for hESC/hiPSC-derived NK cell production leading to a novel 3-step protocol using stromal-free and serum-free conditions (45). Here, we use a “spin EB” (embryoid bodies) protocol in serum-free media to derive CD34+CD45+ hematopoietic progenitor cells from hESC or hiPSC (45–47) (Table 1). The EBs are then transferred to gelatincoated plates and cultured in media containing interleukin (IL)-3, IL-15, IL-7, stem cell factor (SCF) and fms-like tyrosine kinase 3 ligand (FLT3L) where they differentiate into both autologous stromal cells and CD45+CD56+ NK cells. Finally, hESC/hiPSC-derived NK cells can be expanded to clinical scale using a membrane bound IL-21 expressing cell line (45). Like PBNK and UCB-NK, hESC/iPSC-derived NK cells express activating and inhibitory receptors such as CD16a, NKp44, NKp46, NKG2D, TRAIL and KIRs (45) (Figure 3). Likewise, when cultured under these conditions hESC/hiPSC-NK cells demonstrate potent cytotoxic activity against both hematologic and solid tumors through both direct cell-mediated activity and ADCC while producing cytokines such as interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) (4, 48). Notably, studies that inject millions of hESC/hiPSC-NK cells into immunodeficient mice have never demonstrated development of teratomas (5, 48).
Table 1-.
In vitro generation of NK cells and Macrophages
| Cell production |
Serum and feeder status |
Hematopoietic differentiation protocols |
NK cells or macrophages differentiation protocols | Yield | Refs. |
|---|---|---|---|---|---|
| hESC-NK cells |
Feeder-free, serum-free (APEL media) or with BSA (BPEL) |
Spin EB, 8–11 days; (BMP4, SCF, VEGF) |
EB on coated plates, 28–35 days; (IL-15, IL-7, IL-3, FLT3L) |
1–2 log expansion of the hESC- derived CD56+ CD45+ NK cells. 2–3 log expansion using artificial APCs in long term culture |
(45, 47) |
| hESC/hiPSC -NK cells |
Serum and feeder- dependent |
PSC plated on S17 or M210 stromal cells for 17–21 days |
Sorted CD34+ hematopoietic progenitors plated on EL08–1D2 feeder cells, 21–35 days; (IL-15, IL-7, IL-3, FLT3L) |
1–2 log expansion of the hESC- derived CD56+ CD45+ NK cells. 2–3 log expansion using artificial APCs in long term culture |
(4, 5) |
| hESC/hiPSC -NK cells |
Serum- dependent and partially feeder- dependent |
Spin EB, 8–11 days; (BMP4, SCF, VEGF) |
EB plated on EL08–1D2 feeder cells 28–35 days; (IL-15, IL-7, IL-3, FLT3L) |
1–2 log expansion of the hESC- derived CD56+ CD45+ NK cells. 2–3 log expansion using artificial APCs in long term culture |
(88) |
| hESC/hiPSC -Mφ |
Serum-free, feeder-free |
Spin EB, 4 days; (BMP4, SCF, VEGF) |
IL-3, M-CSF (Mφ progenitor cells) and M-CSF (mature Mφ). First harvest after 21 days and multiple harvests with reducing yields |
Around 2.5 × 106 cells over 4 weeks from 1 well of 6- well plate |
(68) |
| hiPSC-Mφ | Serum-free, feeder- dependent |
Spin EB, 5 days; (Mouse feeder layer, bFGF) |
IL-3, M-CSF (Mφ progenitor cells) and M-CSF. First harvest after 21 days and multiple harvests with reducing yields |
Around 5 × 105 to 1 × 106 cells/week from 1 well of 6- well plate |
(89) |
| hiPSC-Mφ | Serum-free, feeder-free |
Spin EB, 6 days; (BMP4, SCF, VEGF) |
IL-3, M-CSF (Mφ progenitor cells) and M-CSF mature Mφ). 7–10 days for first harvest, multiple harvests with reducing yields) |
Around 4 × 106 Macrophages over 4 weeks from 1 Well of 6-well plate |
Unpublished (Kaufman lab) |
BMP4: Bone morphogenetic protein 4; VEGF: Vascular endothelial growth factor; bFGF: Basic fibroblast growth factor; BSA: Bovine serum albumin; APCs: Antigen presenting cells; APEL: Albumin polyvinylalcohol essential lipids; BPEL: BSA polyvinylalchohol essential lipids.
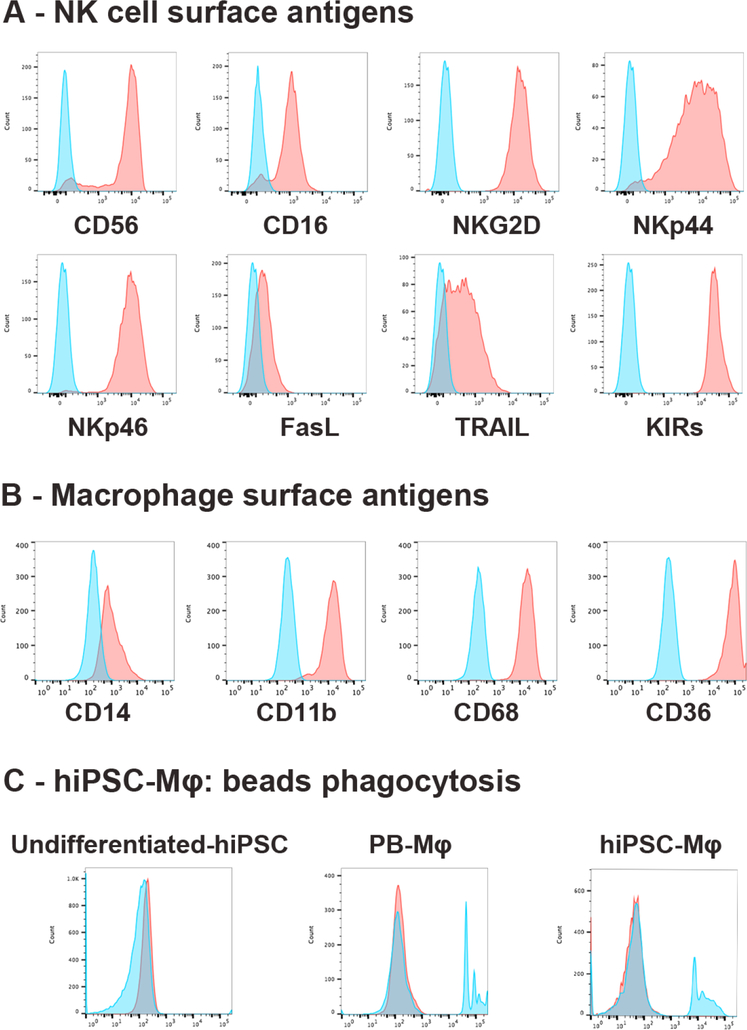
Figure 3 – Phenotype and activity of hiPSC-derived NK cells and macrophages.
(A) Phenotype of hiPSC-derived NK cells and (B) phenotype of hiPSC-derived macrophages expressing typical surface antigens. Each of these demonstrate expression of surface antigens typical of NK cells and macrophages isolated from PB. (C) Phagocytic activity of hiPSC-Mφ is demonstrated by analysis of undifferentiated hiPSCs (negative control), PB-Mφ and hiPSCs-Mφ as analyzed by flow cytometry (blue filled: cells treated with 2 μm beads; red filled: untreated control).
Human pluripotent stem cells as a resource to produce NK cells with improved antitumor activity.
The unique biology of NK cells allows them to serve as a safe and effective allogeneic immunotherapeutic strategy. NK cells can be genetically-modified to express CARs or to adapt their receptor repertoire such as through deletion of inhibitory receptors or expression of cytokines (Figure 2). To overcome the reduced efficiency of genetic modification of mature NK cell through transfection (49), hESCs and hiPSCs can be modified in an undifferentiated state utilizing either viral (retroviral or lentiviral vectors) or non-viral transposon systems (Piggybac or Sleeping Beauty) and then differentiated to produce genetically modified NK cells (8, 50).
Initial studies to enhance the activity of hESC/hiPSC-derived NK cells used a CD4/CD3ζ CAR (6, 7). In this study, the CD4/ζ hESC and hiPSC-derived NK cells were able to suppress the replication of HIV in vitro and in vivo, while the viral load in the PB of treated mice was significantly reduced. For anti-tumor CAR-NK cells, instead of using CAR constructs that were originally designed for T cell-based therapies, subsequent studies used novel NK cell-specific CARs utilizing NK cell-specific transmembrane and co-stimulatory domains. Specifically, after screening 10 different NK-CAR constructs, we demonstrated that iPSCs engineered with a CAR consisting of the transmembrane domain of NKG2D, the 2B4 co-stimulatory domain and the CD3ζ signaling domain (NKG2D-2B4ζ) mediated strong antigen-specific NK cell signaling. This NKG2D-2B4ζ CAR (termed CAR4) was linked to a ScFv specific for the tumor antigen mesothelin. When these iPSC-derived CAR4-NK cells were assessed in a xenograft model of ovarian cancer they showed enhanced anti-tumor activity compared to a third generation T cell-CAR expressed in NK cells. Additionally, direct comparison between conventional CAR-T cells and the iPSC-derived CAR4-NK cells demonstrated improved survival and less toxicity from the hiPSC-CAR-NK cell therapy compared to conventional CAR-T cells in an ovarian carcinoma xenograft model (8).
NK cells also express CD16a, an Fc receptor that mediates ADCC, a key mechanism behind the efficacy of monoclonal antibody-based therapies against cancer (for example rituximab against non-Hodgkin’s lymphoma and trastuzumab against breast cancer) (51). CD16a presents two different polymorphisms, one with a higher affinity for the Fc domain of antibodies and one with lower affinity (52). CD16a expression has been shown to decrease on NK cells due to a metalloprotease, ADAM17, which cleaves CD16a from the cell surface after receiving activating signals coming from the same receptor (53). ADAM17 is expressed by both tumor cells and activated NK cells (54). ADAM17-mediated cleavage of CD16a reduces their cytolytic activity (Figure 2). To overcome this mechanism, iPSCs were engineered to express a non-cleavable version of CD16a and iPSC-NK cells with this CD16a/S197P (54) demonstrated improved ADCC (53). These studies illustrate the important ability to be able to routinely genetically modify hESCs/iPSCs, as well as produce clinical-scale quantities of NK cells that now enable these human pluripotent stem cells to serve as a valuable platform to generate NK cells suitable for allogeneic, “off-the-shelf” cellular immunotherapy against cancers that are refractory to conventional therapy, as well as for possible treatment of chronic viral infections. Phase 1 clinical trials using iPSC-derived NK cells are planned to start in early 2019 to treat relapsed/refractory cancers, the first human pluripotent stem cell-derived blood product to enter the clinical arena.
Use of uman pluripotent stem cells to derive therapeutic macrophages
Initial studies to demonstrate hematopoietic differentiation of hESCs produced hematopoietic progenitor cells (HPCs) that made stereotypic myeloid colonies when plated in semi-solid methylcellulose-based media containing hematopoietic cytokines (3). Importantly, these hematopoietic colonies demonstrated differentiation of hESC into myeloid, erythroid and megakaryocytic cells that were essentially the same as those produced by HPCs isolated from BM or UCB. Sorting for CD34+ cells and quantification of these hematopoietic colony-forming cells (CFCs) further illustrated that hESC-derived hematopoietic cells were similar to “normal” blood cells. Additionally, these studies found that monocytes/macrophages were relatively straight-forward to produce from hESCs using the stromal based differentiation system described above.
Macrophages are myeloid cells that contribute to numerous functions including tissue development, homeostasis, tissue repair and innate immunity against invading pathogens and cancer (55). Distinct types of macrophages develop from different developmental hematopoietic sites: the embryonic yolk sac (YS), the fetal liver (FL), and the post-natal BM (56). Macrophages that arise from the YS are independent from HSC precursors and populate in the brain as microglia (57). The FL is populated with YS-derived macrophages and constitutes the major source for other tissue-resident macrophage (TRM) populations including those found in the liver, lung, spleen, pancreas and kidney. Those macrophages derived from the YS and the FL reside in the tissues during embryonic development, persist into adulthood and self-renew during adult life through a Myb-independent proliferation mechanism (independent from HSCs) (58–60).
BM-derived macrophages arise from post-natal HSCs that originate in the aorta-gonadmesonephros (AGM) of the embryo and are dependent on the transcription factor c-myb in their development (61, 62). In contrast to YS/FL-derived macrophages, BM-derived macrophages have a short life and populate tissues only under inflammatory conditions (63). Studies of TRMs involve the isolation of these cells from different tissues with mechanical dissociation techniques (64–66). While effective, this strategy is not optimal due to the impact on gene expression patterns upon isolation procedures, possible contamination of RNA from phagocytosis of host tissue cells, and the lack of access to sufficient numbers of appropriate cells. Alternatively, macrophages can be generated in vitro from human pluripotent stem cells. Numerous studies (Table 1) have utilized mouse ESC and iPSC systems to efficiently produce macrophages in vitro (58, 67, 68). In addition to stromal-based methods, EB formation combined with colony-stimulating factor 1 (CSF-1), macrophage-CSF (M-CSF) and IL-3 supplementation has been shown to produce macrophages from hESCs (67). Additionally, human iPSCs have also been used to generate a homogeneous population of non-adherent monocytes (>90% CD14+) from the culture supernatant. These cells can be continuously harvested for several months while maintaining the ability to differentiate into mature macrophages. These iPSC-derived macrophages demonstrate typical characteristics including the ability to endocytose lipoprotein, phagocytose opsonized yeast particles, secrete specific cytokines in response to lipopolysaccharide (LPS), and be activated differentially with IFN-γ and IL-4 (68). More recently, our group has developed a method to rapidly generate large numbers of macrophage progenitor cells and mature macrophages (~4×106 per well in a 6-well plate format over a course of 4 weeks) from human iPSCs using defined feeder and serum-free culture conditions that yield a highly consistent and pure population of macrophages (CD14+, CD11b+, CD68+, CD36+) which are able to phagocytose latex beads (Figure 3). Notably, despite a similar phenotype and function, hESC/iPSC-derived macrophage have some key differences with adult BM-derived or PB monocyte-derived macrophages, likely related to their development via a Myb independent mechanism, similar to TRMs. Therefore, care should be taken when interpreting the result of studies relying on such methods.
Use of hESC/iPSC-derived macrophages for infectious disease modeling
Recent studies using iPSC-derived macrophages (iPSC-Mφ) have been used to better understand pathogenesis of Zika virus (ZIKV) and Dengue virus (DENV) infections, two closely related mosquito-transmitted flaviviruses that lead to various clinical outcomes (69). Specifically, while both viruses were able to infect iPSC-Mφ, only DENV and not ZIKA activated secretion of migration inhibitory factor (MIF) and diminished macrophage migration. Conversely, ZIKAinfected iPSC-Mφ demonstrated enhanced migration and decreased pro-inflammatory responses. These results are consistent with previous observations that after recognition of pathogen-associated molecular patterns, such as double stranded RNA, macrophages become activated and secrete a broad array of pro-inflammatory cytokines (e.g., IL-1B, TNF-α, and IL-6) and chemokines (e.g., CCL4 and CXCL8/IL-8) that enhance host immune responses (70).
Of note, the use of PSC-Mφ also enables novel studies on host-pathogen interactions and the role of human genetics in the outcome of infections. This is especially relevant for infectious agents requiring macrophages as a source of persistence and replication, such as Salmonella (71), Chlamydia (72) and HIV (73), which had remained largely unexplored due to the lack of an appropriate model system. Interestingly, iPS-Mφ infected with Bacillus Calmette-Guérin (BCG) resulted in cell apoptosis as well as nitric oxide (NO) and TNF-α secretion similar to macrophages generated in vitro from a human monocyte cell line (74). These studies suggest that PSC-Mφ could be utilized to unravel the distinct impact of infectious agents on disease pathogenesis.
Regenerative cell-based therapy for macrophage-related diseases
IPSC-Mφ also provide a highly attractive source for cell and gene therapy. Specifically, this approach has led to the development of novel therapeutic targets for diseases involving macrophages such as pulmonary alveolar proteinosis and liver fibrosis. Mucci et al recently evaluated the airway homing, plasticity and therapeutic efficacy of iPSC-Mφ in a murine model of hereditary pulmonary alveolar proteinosis. They found that a single administration of 2.54×106 iPSC-Mφ resulted in efficient airway homing and conversion to an alveolar macrophage phenotype within two months, while alleviating disease symptoms, displaying significant plasticity and the therapeutic potential of iPSC-Mφ (75). The same group applied TALEN-mediated integration of the corrected gene; granulocyte M-CSF (GM-CSF) receptor alpha-chain (CD116) into patient-specific iPSCs and demonstrated functionally corrected macrophages with typical morphology, surface phenotype, phagocytic and secretory activity as well as functional gene expression suggesting a novel source of cell-based gene therapy for treatment of genetic diseases (76). The regenerative capacity of murine iPSC-Mφ for liver fibrosis has been evaluated in vivo. Although murine iPSC-Mφ showed comparable morphology and cell surface markers to BM-derived macrophages, they displayed less phagocytic activity, reduced response to classic stimulators, for instance LPS and IFN-γ, and increased response to IL-4 compared to BM-derived macrophages. Therapeutic application of murine iPSC-Mφ significantly diminished the amount of hepatic fibrosis to 50% of control groups and repopulation of Kupffer cells, highlighting the beneficial effect of in vitro PSC-Mφ to repair a model of liver injury (77).
Cell-based therapy of cancer using macrophages
Distinct macrophage populations reside in different tissues of the body and constitute around 520% of the cells in every tissue (78) and contribute to a variety of conditions including the tumor microenvironment (79). The role of macrophages in the tumor microenvironment seems to be a double-edged sword, with some studies suggesting they contribute to disease progression (80, 81). However, increasing evidence suggests that targeting of macrophages can be used to contribute to tumor elimination and a subsequent cure (82–84). Based on these findings, several groups have attempted to use hiPSC-Mφ as a novel cell-based therapy approach for cancer therapy. Koba, et al generated an iPSC-derived myeloid/macrophage cell line (iPS-ML) with enhanced proliferation capacity that grew continuously in an M-CSF-dependent manner. A large supply of cells could be readily obtained using this technology for at least several months. Intraperitoneal administration of iPS-ML into SCID mice with pre-established peritoneal gastric cancer resulted in massive accumulation and infiltration into the tumor tissues and dramatically suppressed tumor growth. Furthermore, iPS-ML/IFN-β inhibited the intra-peritoneal growth of gastric and pancreatic cancers suggesting that iPS-ML are a promising treatment strategy for disseminated cancers for which no standard treatment is available (85). The same group has also applied the enhanced proliferation approach to the mouse ESC, ES-ML cell line. ES-ML expressing an anti-HER2 mAb reduced the number of co-cultured mouse Colon-26 cancer cells expressing HER-2. Injection of ES-ML with IFN-γ and LPS into mice inhibited cancer progression in the mouse peritoneal cavity and prolonged survival of the treated animals. Interestingly, transporter associated with antigen processing (TAP)-deficient ES-ML conferred a therapeutic benefit to MHC-mismatched allogeneic recipient mice demonstrating the possible use of TAP-deficient iPSC-myeloid cell lines in cancer therapy (86). iPSC-Mφ have also been developed for adoptive cell-based therapy of advanced melanoma to overcome the difficulties relating to application on a broad scale and poor infiltration of immune cells in solid tumors. iPSML genetically modified to express type I IFNs (iPS-ML-IFN) exhibited significant inhibition of metastatic human melanoma in immunodeficient mice, with histological examination confirming the presence of macrophages in the tumor nests. These macrophages expressed M1 polarizing markers, influencing the environment toward inflammatory and anti-tumor conditions (87). To achieve successful macrophage-based immunotherapeutic, repetitive administration of large numbers of cells might be necessary. Given the fact that the hiPSC-Mφ platform is able to produce cells on a broad scale, ample number of cells for cancer therapy can certainly be obtained by this approach.
Overall conclusions about hESC/hiPSC-derived NK cells and macrophages
Human pluripotent stem cells-derived blood cells, including NK cells and macrophages are valuable tools to study immune cell biology, disease pathogenesis and immune cell-based therapeutics. hESCs and iPSCs not only provide a platform to develop clinical grade cell products but also to better understand the developmental biology of innate immune cells during the differentiation process from HsPC to mature cells. NK cells expressing the repertoire of activating and inhibitory receptors commonly expressed on PB-NK can be differentiated from both hESC and hiPSC on a clinical scale when cultured with APCs. ESC/iPSCs can be easily transfected and used to differentiate NK cells to investigate and modify the function of their receptors. Moreover, specific CARs for anti-cancer therapies can be engineered into hESC/hiPSC derived-NK cells to generate more potent NK cells. Well-defined protocols facilitate large populations of macrophages derived from hESCs/iPSCs that are highly phagocytic and resemble PB monocyte-derived macrophages (CD14+, CD11b+, CD68+, CD36+). This ability to produce a large number of homogeneous NK cells and macrophages facilitates their clinical translation for novel “off-the-shelf” cell-based therapies.
Highlights:
Large clinical-scale number of NK cells and macrophages can be routinely derived from human pluripotent stem cells
hESCs and iiPSCs can be used to produce targeted chimeric antigen receptor (CAR)-expressing NK cells
Preclinical studies showed enhanced anti-tumor activity for hESC/iPSC-derived NK cells
hESC/iPSC-derived macrophages can be used for disease modeling, cancer therapy, regenerative medicine
hESCs and iPSCs are a valuable platform to make “off-the-shelf” therapeutic products
Acknowledgements:
We thank all members of the Kaufman lab for helpful discussions regarding this work. Funding support for research in the Kaufman lab includes NIH/NCI R01 CA203348, U01 CA217885, CIRM DISC2–09615, CIRM TRAN1–10587, the Takeda-Sanford Innovation Alliance, and sponsored research support from Fate Therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112(9):3543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98(19):10716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woll PS, Martin CH, Miller JS, Kaufman DS. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. Journal of immunology (Baltimore, Md : 1950). 2005;175(8):5095–103. [DOI] [PubMed] [Google Scholar]
- 5.Woll PS, Grzywacz B, Tian X, Marcus RK, Knorr DA, Verneris MR, et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113(24):6094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ni Z, Knorr DA, Bendzick L, Allred J, Kaufman DS. Expression of chimeric receptor CD4zeta by natural killer cells derived from human pluripotent stem cells improves in vitro activity but does not enhance suppression of HIV infection in vivo. Stem cells (Dayton, Ohio). 2014;32(4):1021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ni Z, Knorr DA, Clouser CL, Hexum MK, Southern P, Mansky LM, et al. Human pluripotent stem cells produce natural killer cells that mediate anti-HIV-1 activity by utilizing diverse cellular mechanisms. Journal of virology. 2011;85(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell stem cell. 2018;23(2):181–92.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelos MG, Ruh PN, Webber BR, Blum RH, Ryan CD, Bendzick L, et al. Aryl hydrocarbon receptor inhibition promotes hematolymphoid development from human pluripotent stem cells. Blood. 2017;129(26):3428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galic Z, Kitchen SG, Kacena A, Subramanian A, Burke B, Cortado R, et al. T lineage differentiation from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103(31):11742–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slukvin II. Hematopoietic specification from human pluripotent stem cells: current advances and challenges toward de novo generation of hematopoietic stem cells. Blood. 2013;122(25):4035–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seet CS, He C, Bethune MT, Li S, Chick B, Gschweng EH, et al. Generation of mature T cells from human hematopoietic stem and progenitor cells in artificial thymic organoids. Nature methods. 2017;14(5):521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin CH, Woll PS, Ni Z, Zuniga-Pflucker JC, Kaufman DS. Differences in lymphocyte developmental potential between human embryonic stem cell and umbilical cord blood-derived hematopoietic progenitor cells. Blood. 2008;112(7):2730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vizcardo R, Masuda K, Yamada D, Ikawa T, Shimizu K, Fujii S, et al. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell stem cell. 2013;12(1):31–6. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura T, Kaneko S, Kawana-Tachikawa A, Tajima Y, Goto H, Zhu D, et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell stem cell. 2013;12(1):114–26. [DOI] [PubMed] [Google Scholar]
- 16.Themeli M, Riviere I, Sadelain M. New cell sources for T cell engineering and adoptive immunotherapy. Cell stem cell. 2015;16(4):357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467(7313):285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carpenter L, Malladi R, Yang CT, French A, Pilkington KJ, Forsey RW, et al. Human induced pluripotent stem cells are capable of B-cell lymphopoiesis. Blood. 2011;117(15):4008–11. [DOI] [PubMed] [Google Scholar]
- 19.Cho SK, Webber TD, Carlyle JR, Nakano T, Lewis SM, Zuniga-Pflucker JC. Functional characterization of B lymphocytes generated in vitro from embryonic stem cells. Proc Natl Acad Sci U S A. 1999;96(17):9797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. International journal of cancer. 1975;16(2):216–29. [DOI] [PubMed] [Google Scholar]
- 21.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. European journal of immunology. 1975;5(2):112–7. [DOI] [PubMed] [Google Scholar]
- 22.Paul S, Lal G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Frontiers in immunology. 2017;8:1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nature immunology. 2016;17(9):1025–36. [DOI] [PubMed] [Google Scholar]
- 24.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nature reviews Cancer. 2016;16(1):7–19. [DOI] [PubMed] [Google Scholar]
- 26.Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nature reviews Immunology. 2018. [DOI] [PubMed] [Google Scholar]
- 27.Brandstadter JD, Yang Y. Natural killer cell responses to viral infection. Journal of innate immunity. 2011;3(3):274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nature immunology. 2008;9(5):495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freud AG, Mundy-Bosse BL, Yu J, Caligiuri MA. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity. 2017;47(5):820–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrega P, Ferlazzo G. Natural killer cell distribution and trafficking in human tissues. Frontiers in Immunology. 2012;3:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veluchamy JP, Kok N, van der Vliet HJ, Verheul HMW, de Gruijl TD, Spanholtz J. The Rise of Allogeneic Natural Killer Cells As a Platform for Cancer Immunotherapy: Recent Innovations and Future Developments. Frontiers in immunology. 2017;8:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–7. [DOI] [PubMed] [Google Scholar]
- 34.Handgretinger R, Lang P, Andre MC. Exploitation of natural killer cells for the treatment of acute leukemia. Blood. 2016;127(26):3341–9. [DOI] [PubMed] [Google Scholar]
- 35.Pfeiffer MM, Schumm M, Müller I, Handgretinger R, Lang P. IL-15-stimulated CD3/CD19depleted stem-cell boosts in relapsed pediatric patients after haploidentical SCT. Leukemia. 2012;26:2435. [DOI] [PubMed] [Google Scholar]
- 36.Koehl U, Kalberer C, Spanholtz J, Lee DA, Miller JS, Cooley S, et al. Advances in clinical NK cell studies: Donor selection, manufacturing and quality control. Oncoimmunology. 2016;5(4):e1115178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dianat-Moghadam H, Rokni M, Marofi F, Panahi Y, Yousefi M. Natural killer cell-based immunotherapy: From transplantation toward targeting cancer stem cells. Journal of cellular physiology. 2018. [DOI] [PubMed] [Google Scholar]
- 38.Martin-Antonio B, Sune G, Perez-Amill L, Castella M, Urbano-Ispizua A. Natural Killer Cells: Angels and Devils for Immunotherapy. International journal of molecular sciences. 2017;18(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarvaria A, Jawdat D, Madrigal JA, Saudemont A. Umbilical Cord Blood Natural Killer Cells, Their Characteristics, and Potential Clinical Applications. Frontiers in immunology. 2017;8:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rezvani K, Rouce R, Liu E, Shpall E. Engineering Natural Killer Cells for Cancer Immunotherapy. Molecular therapy : the journal of the American Society of Gene Therapy. 2017;25(8):1769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spanholtz J, Preijers F, Tordoir M, Trilsbeek C, Paardekooper J, de Witte T, et al. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PloS one. 2011;6(6):e20740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suck G, Odendahl M, Nowakowska P, Seidl C, Wels WS, Klingemann HG, et al. NK-92: an ‘off-theshelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer immunology, immunotherapy : CII. 2016;65(4):485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15(12):1563–70. [DOI] [PubMed] [Google Scholar]
- 44.Dolstra H, Roeven MWH, Spanholtz J, Hangalapura BN, Tordoir M, Maas F, et al. Successful Transfer of Umbilical Cord Blood CD34(+) Hematopoietic Stem and Progenitor-derived NK Cells in Older Acute Myeloid Leukemia Patients. Clin Cancer Res. 2017;23(15):4107–18. [DOI] [PubMed] [Google Scholar]
- 45.Knorr DA, Ni Z, Hermanson D, Hexum MK, Bendzick L, Cooper LJ, et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem cells translational medicine. 2013;2(4):274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng ES, Davis R, Stanley EG, Elefanty AG. A protocol describing the use of a recombinant proteinbased, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nature protocols. 2008;3(5):768–76. [DOI] [PubMed] [Google Scholar]
- 47.Bock AM, Knorr D, Kaufman DS. Development, expansion, and in vivo monitoring of human NK cells from human embryonic stem cells (hESCs) and and induced pluripotent stem cells (iPSCs). Journal of visualized experiments : JoVE. 2013(74):e50337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hermanson DL, Bendzick L, Pribyl L, McCullar V, Vogel RI, Miller JS, et al. Induced Pluripotent Stem Cell-Derived Natural Killer Cells for Treatment of Ovarian Cancer. Stem cells (Dayton, Ohio). 2016;34(1):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boissel L, Betancur M, Lu W, Wels WS, Marino T, Van Etten RA, et al. Comparison of mRNA and lentiviral based transfection of natural killer cells with chimeric antigen receptors recognizing lymphoid antigens. Leukemia & lymphoma. 2012;53(5):958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moriarity BS, Rahrmann EP, Keng VW, Manlove LS, Beckmann DA, Wolf NK, et al. Modular assembly of transposon integratable multigene vectors using RecWay assembly. Nucleic acids research. 2013;41(8):e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Exley MA, Orange JS. Overview: NK-cell-based Immunotherapies: Toward & Into Clinical Trials. Clinical immunology (Orlando, Fla). 2017;177:1–2. [DOI] [PubMed] [Google Scholar]
- 52.Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy. Frontiers in immunology. 2015;6:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu H, Lai YS, Li Y, Blum RH, Kaufman DS. Concise Review: Human Pluripotent Stem Cells to Produce Cell-Based Cancer Immunotherapy. Stem cells (Dayton, Ohio). 2018;36(2):134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jing Y, Ni Z, Wu J, Higgins L, Markowski TW, Kaufman DS, et al. Identification of an ADAM17 cleavage region in human CD16 (FcgammaRIII) and the engineering of a non-cleavable version of the receptor in NK cells. PloS one. 2015;10(3):e0121788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guerriero JL. Macrophages: The Road Less Traveled, Changing Anticancer Therapy. Trends in molecular medicine. 2018;24(5):472–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science (New York, NY). 2010;330(6005):841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Senju S, Haruta M, Matsunaga Y, Fukushima S, Ikeda T, Takahashi K, et al. Characterization of dendritic cells and macrophages generated by directed differentiation from mouse induced pluripotent stem cells. Stem cells (Dayton, Ohio). 2009;27(5):1021–31. [DOI] [PubMed] [Google Scholar]
- 59.Ginhoux F, Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016;44(3):439–49. [DOI] [PubMed] [Google Scholar]
- 60.Buchrieser J, James W, Moore MD. Human Induced Pluripotent Stem Cell-Derived Macrophages Share Ontogeny with MYB-Independent Tissue-Resident Macrophages. Stem cell reports. 2017;8(2):33445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science (New York, NY). 2012;336(6077):86–90. [DOI] [PubMed] [Google Scholar]
- 62.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science (New York, NY). 2010;327(5966):656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annual review of immunology. 2008;26:421–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159(6):1327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37(6):1076–90. [DOI] [PubMed] [Google Scholar]
- 66.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nature immunology. 2012;13(11):1118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karlsson KR, Cowley S, Martinez FO, Shaw M, Minger SL, James W. Homogeneous monocytes and macrophages from human embryonic stem cells following coculture-free differentiation in M-CSF and IL-3. Experimental hematology. 2008;36(9):1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Wilgenburg B, Browne C, Vowles J, Cowley SA. Efficient, long term production of monocytederived macrophages from human pluripotent stem cells under partly-defined and fully-defined conditions. PloS one. 2013;8(8):e71098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lang J, Cheng Y, Rolfe A, Hammack C, Vera D, Kyle K, et al. An hPSC-Derived Tissue-Resident Macrophage Model Reveals Differential Responses of Macrophages to ZIKV and DENV Infection. Stem cell reports. 2018;11(2):348–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hale C, Yeung A, Goulding D, Pickard D, Alasoo K, Powrie F, et al. Induced pluripotent stem cell derived macrophages as a cellular system to study salmonella and other pathogens. PloS one. 2015;10(5):e0124307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yeung ATY, Hale C, Lee AH, Gill EE, Bushell W, Parry-Smith D, et al. Exploiting induced pluripotent stem cell-derived macrophages to unravel host factors influencing Chlamydia trachomatis pathogenesis. Nature communications. 2017;8:15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson JS, Bandi S, Kaufman DS, Akkina R. Derivation of normal macrophages from human embryonic stem (hES) cells for applications in HIV gene therapy. Retrovirology. 2006;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hong D, Ding J, Li O, He Q, Ke M, Zhu M, et al. Human-induced pluripotent stem cell-derived macrophages and their immunological function in response to tuberculosis infection. Stem cell research & therapy. 2018;9(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mucci A, Lopez-Rodriguez E, Hetzel M, Liu S, Suzuki T, Happle C, et al. iPSC-Derived Macrophages Effectively Treat Pulmonary Alveolar Proteinosis in Csf2rb-Deficient Mice. Stem cell reports. 2018;11(3):696–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuhn A, Ackermann M, Mussolino C, Cathomen T, Lachmann N, Moritz T. TALEN-mediated functional correction of human iPSC-derived macrophages in context of hereditary pulmonary alveolar proteinosis. Scientific reports. 2017;7(1):15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haideri SS, McKinnon AC, Taylor AH, Kirkwood P, Starkey Lewis PJ, O’Duibhir E, et al. Injection of embryonic stem cell derived macrophages ameliorates fibrosis in a murine model of liver injury. NPJ Regenerative medicine. 2017;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hume DA, Gordon S. Mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Identification of resident macrophages in renal medullary and cortical interstitium and the juxtaglomerular complex. The Journal of experimental medicine. 1983;157(5):1704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41(1):21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caux C, Ramos RN, Prendergast GC, Bendriss-Vermare N, Menetrier-Caux C. A Milestone Review on How Macrophages Affect Tumor Growth. Cancer research. 2016;76(22):6439–42. [DOI] [PubMed] [Google Scholar]
- 82.Jaiswal S, Chao MP, Majeti R, Weissman IL. Macrophages as mediators of tumor immunosurveillance. Trends in immunology. 2010;31(6):212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ring NG, Herndler-Brandstetter D, Weiskopf K, Shan L, Volkmer JP, George BM, et al. AntiSIRPalpha antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci U S A. 2017;114(49):E10578–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weiskopf K, Jahchan NS, Schnorr PJ, Cristea S, Ring AM, Maute RL, et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. The Journal of clinical investigation. 2016;126(7):2610–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koba C, Haruta M, Matsunaga Y, Matsumura K, Haga E, Sasaki Y, et al. Therapeutic effect of human iPS-cell-derived myeloid cells expressing IFN-beta against peritoneally disseminated cancer in xenograft models. PloS one. 2013;8(6):e67567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haga E, Endo Y, Haruta M, Koba C, Matsumura K, Takamatsu K, et al. Therapy of peritoneally disseminated colon cancer by TAP-deficient embryonic stem cell-derived macrophages in allogeneic recipients. Journal of immunology (Baltimore, Md : 1950). 2014;193(4):2024–33. [DOI] [PubMed] [Google Scholar]
- 87.Miyashita A, Fukushima S, Nakahara S, Kubo Y, Tokuzumi A, Yamashita J, et al. Immunotherapy against Metastatic Melanoma with Human iPS Cell-Derived Myeloid Cell Lines Producing Type I Interferons. Cancer immunology research. 2016;4(3):248–58. [DOI] [PubMed] [Google Scholar]
- 88.Ni Z, Knorr DA, Kaufman DS. Hematopoietic and nature killer cell development from human pluripotent stem cells. Methods Mol Biol. 2013;1029:33–41. [DOI] [PubMed] [Google Scholar]
- 89.Lachmann N, Ackermann M, Frenzel E, Liebhaber S, Brennig S, Happle C, et al. Large-scale hematopoietic differentiation of human induced pluripotent stem cells provides granulocytes or macrophages for cell replacement therapies. Stem cell reports. 2015;4(2):282–96. [DOI] [PMC free article] [PubMed] [Google Scholar]