Abstract
Alzheimer’s disease (AD) is a complex neurodegenerative disorder with multiple dysfunctional pathways. Therefore, a sophisticated treatment strategy that simultaneously targets multiple brain cell types and disease pathways could be advantageous for effective intervention. To elucidate an effective treatment, we developed an in vitro high-throughput screening (HTS) assay to evaluate candidate drugs for their ability to enhance the integrity of the blood-brain barrier (BBB) and improve clearance of amyloid-β (Aβ) using a cell-based BBB model. Results from HTS identified etodolac and α-tocopherol as promising drugs for further investigation. Both drugs were tested separately and in combination for the purpose of targeting multiple pathways including neuroinflammation and oxidative stress. In vitro studies assessed the effects of etodolac and α-tocopherol individually and collectively for BBB integrity and Aβ transport, synaptic markers and Aβ production in APP-transfected neuronal cells, as well as effects on inflammation and oxidative stress in astrocytes. Transgenic 5XFAD mice were used to translate in vitro results of etodolac and α-tocopherol independently and with concurrent administration. Compared to either drug alone, the combination significantly enhanced the BBB function, decreased total Aβ load correlated with increased expression of major transport proteins, promoted APP processing towards the neuroprotective and non-amyloidogenic pathway, induced synaptic markers expression, and significantly reduced neuroinflammation and oxidative stress both in vitro and in vivo. Collective findings demonstrated the combination produced mixed interaction showing additive, less than additive or synergistic effects on the evaluated markers. In conclusion, this study highlights the significance of combination therapy to simultaneously target multiple disease pathways, and suggest the repurposing and combination of etodolac and α-tocopherol as a novel therapeutic strategy against AD.
Keywords: Alzheimer’s Disease, Blood-brain barrier, Amyloid-β, Neuroinflammation, Oxidative stress, Combination therapy
1. Introduction
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disorder and the most common form of dementia. While the precise etiology of AD remains unknown, it is suggested that a combination of environmental, lifestyle, and genetic factors affect the brain over time, ultimately leading to AD (Selkoe, 2001; Thomas and Fenech, 2007). AD is a complex disease involving multiple distinct dysfunctional pathways, and as such, pharmacological interventions that target a single pathway generally possess limited efficacy (Agatonovic-Kustrin et al., 2018). Therefore, a sophisticated treatment strategy which is capable of targeting multiple disease pathways simultaneously is required for effective intervention.
The hallmarks of AD include neuronal and synaptic loss, compromised blood-brain barrier (BBB) integrity and function, extracellular depositions of amyloid-β (Aβ), and the presence of neurofibrillary tangles (NFTs) (Masters et al., 1985; Grundke-Iqbal et al., 1986; DeKosky and Scheff, 1990; Bowman et al., 2007; Knopman et al., 2018). Furthermore, strong evidence suggests that neuroinflammation and oxidative stress significantly contribute to AD pathology and disease progression (Agostinho et al., 2010; De Felice and Lourenco, 2015). Microglia and astrocytes are the major cellular components of innate immunity in the central nervous system, contributing to neuroinflammation in AD (Hensely, 2010). Upon exposure to Aβ and NFTs, microglia and astrocytes release proinflammatory cytokines, chemokines, and interleukins including TNFα, IL-1, IL-6, and IL-18, resulting in neuroinflammation that ultimately leads to neuronal damage (Akiyama et al., 2000). Epidemiological studies have established a correlation between long-term non-steroidal anti-inflammatory drug (NSAID) use with a decreased risk of AD (Vlad et al., 2008). The proposed mechanism underlying the neuroprotective ability of NSAIDs is their effect on the regulation of cyclooxygenase enzymes (COX-1 and COX-2), whose levels are elevated in AD pathology (McGeer and McGeer, 2007). Oxidative stress has also been linked to AD progression through an imbalance between reactive oxygen and nitrogen species production, scavenging, and neutralization by endogenous antioxidant defense mechanisms (Zhao and Zhao, 2013).
The BBB is part of the neurovascular unit consisting of a thick continuous, non-fenestrated endothelial cell membrane connected by tight junction proteins, basement membranes, pericytes, astrocytic end-feet and neurons (Hawkins and Davis, 2005). The BBB endothelium has several transporters and receptors such as P-glycoprotein (P-gp) and low density lipoprotein receptor-related protein 1 (LRP1) that regulate traffic across the BBB and play a key role in brain Aβ homeostasis (Shibata et al., 2000, Sagare et al., 2012, Qosa Mohamed, Alqahtani et al., 2016). Compromised BBB integrity and function is a characteristic of AD pathology and is associated with altered protein expression and cellular secretions, inflammatory activation, oxidative stress, and neuronal damage (Bowman et al., 2007). Dysfunction in the BBB has further been shown to reduce Aβ transport-mediated clearance across the BBB and the formation of Aβ plaques (Erickson and Banks, 2013).
The complex nature of AD necessitates a strategy that is capable of simultaneous targeting of brain cells and multiple disease pathways to efficiently slow, hold, or reverse AD pathology. Therefore, the greatest therapeutic benefit is likely to be achieved with synergistic combination regimens that address multiple aspects of AD pathology and capable of targeting individual brain cell types including endothelial cells of the BBB, astrocytes and neurons. Several studies reported combination therapies to reduce neurodegeneration and AD pathology (Hu et al., 2018; Mori et al., 2017; Skovgard et al., 2018). For example, Mori and colleagues evaluated the effect of octyl gallate and ferulic acid combination in a mouse model of AD, and reported the combination significantly attenuated neuroinflammation, oxidative stress, and synaptotoxicity (Mori et al., 2017). In addition, others investigated the effect of combined ondansetron, a 5-HT3 receptor antagonist, with donepezil on hippocampal oscillations during electrical stimulation of the brainstem pedunculopontine tegmental nucleus in rats, and found that ondansetron potentiated donepezil effects (Skovgard et al., 2018). Collectively, these findings suggest the combination therapy could provide an efficient strategy to treat AD.
To this end, we previously developed an in vitro high-throughput screening (HTS) assay to evaluate compounds based on their ability to improve barrier integrity and function in a cell-based BBB model (Qosa, Mohamed, Al Rihani et al., 2016; Elfakhri et al., 2018). Screening results indicated etodolac and α-tocopherol as strong candidate drugs for further evaluation. Etodolac is an FDA approved NSAID, and α-tocopherol is a naturally occurring lipid of vitamin E with potent antioxidant activity (Traber and Atkinson, 2007). The aim of this study was to investigate and compare the effect of combined etodolac and α-tocopherol on Aβ-related pathology to either drug alone in individual brain cell types and on multiple pathways involved in AD pathology using in vitro and in vivo models.
2. Experimental method
2.1. Materials
Fibronectin from bovine plasma, etodolac and α-tocopherol were purchased from Sigma-Aldrich (St. Louis, MO). HTS 3μm polycarbonate membrane transwell 96-well plates were purchased from Corning Inc. (Corning, NY). Dulbecco’s modified Eagle’s medium (DMEM), sterile PBS, and penicillin/streptomycin antibiotics were obtained from Gibco (Grand Island, NY). Fetal bovine serum (FBS) was purchased from Atlanta biologicals (Flowery Branch, GA). 14C-inulin ([carboxyl-14C]-inulin, M.W. 5000 Da) was purchased from American Radiolabeled Chemicals (St. Louis, MO). Synthetic mono-iodinated and nonoxidized Aβ40 (125I-Aβ40; human, 2200 Ci/mmol) was purchased from PerkinElmer (Boston, MA). Total protein measurement’s reagents with the bicinchoninic acid (BCA) method were obtained from Pierce (Rockford, IL). Antibodies used were mouse monoclonal antibody against light chain LRP1 (Abcam, Cambridge, MA), mouse monoclonal antibody C-219 against P-gp from BioLegend (San Diego, CA), mouse monoclonal antibody against GAPDH from (Santa Cruz Biotechnology, TX). Monoclonal antibodies for claudin-5 and ZO-1 were purchased from Invitrogen (Carlsbad, CA), goat polyclonal antibodies against actin (C-11) and HRP-labeled secondary antibodies were purchased from Santa Cruz Biotechnology. Antibodies for pre- and post-synaptic proteins SNAP-25 and PSD-95, respectively, were purchased from GeneTex (Irvine, CA), antibodies for APP (4G8), soluble APPα (sAPPα), soluble APPβ (sAPPβ) were purchased from Immuno-Biological Laboratories (Minneapolis, MN. All other chemicals, reagents and supplies were purchased from VWR (West Chester, PA) and Fisher Scientific (Hampton, NH).
2.2. Cell culture
Immortalized mouse brain endothelial cell line bEnd3 was obtained from ATCC (Manassas, VA). bEnd3 cells (passage 25–35) were cultured in DMEM supplemented with 10% FBS, and penicillin G (100 IU/ml)/streptomycin (100 g/ml). Astrocyte cultures were prepared from CCF-STTG1 human astrocytoma cell line (ATCC). Astrocyte cells were maintained in RPMI-1640 medium containing 10% FBS and antibiotics. Neuronal cultures were prepared from SH-SY5Y cell line transfected with APP695 (hereafter SH-SY5Y-APP). Neuronal cells were maintained in DMEM media, supplemented with 10% FBS containing geneticin (Gibco) added at 400 μg/ml. Cultures were maintained in a humidified atmosphere (5% CO2/95% air) at 37°C and media was changed every other day.
2.2. Concentration-dependent studies for treatments effect on the cell-based BBB model intactness and 125I-Aβ40 transport
bEnd3 cells seeding was performed as reported previously (Qosa, Mohamed, Al Rihani et al., 2016). In brief, 96 well plate inserts were coated with 50 μl of fibronectin solution (30 μg/ml in PBS). Two hours later, bEnd3 cells were loaded to the upper side (A) at a density of 50,000 cells/cm2, and 200 μl of fresh media were added to the lower side (B). Cells were incubated and cultured at 37°C, 5% CO2/95% air for five days to achieve optimal barrier integrity of bEnd3 cells. On day five, bEnd3 cells were treated with 50 μl media containing less than 0.1% DMSO (as vehicle), etodolac, or α-tocopherol added to the upper side in the concentration range of 1–10 μM. For the combination, α-tocopherol concentration was fixed at 5 μM with increasing concentration of etodolac (1–10 μM). Twenty-four hours later, the effect of treatments on monolayer integrity and Aβ40 transport across the monolayer were evaluated by monitoring 14C-inulin (0.5 mM) permeability and 125I-Aβ40 (0.1 nM) transport added to B side (Qosa, Abuasal et al., 2016). Thirty minutes later, aliquots from both sides were separately collected for radioactivity analysis using Wallac 1470 Wizard Gamma and Wallac 1414 WinSpectral Liquid Scintillation Beta Counters (PerkinElmer; Waltham, MA). The transport quotients (TQ) of (125I-Aβ40 CQB→A) was determined as previously reported (Qosa, Abuasal et al., 2016). Three independent experiments were carried out for each treatment group.
2.4. Animal experiments
All animal experiments and procedures were approved by the Institutional Animal Care and Use Committee of the University of Louisiana at Monroe according to the National Institutes of Health guidelines. Surgical and treatment procedures were consistent with the IACUC policies and procedures. In vivo evaluations of etodolac, α-tocopherol, and combination for their effects on BBB integrity, Aβ brain load, brain synapses, Aβ production, neuroinflammation, and oxidative stress were performed in 5XFAD mice as a model of AD. 5XFAD female mice (Jackson laboratory, Bar Harbor, ME) were housed in plastic containers under the conditions of 12 h light/dark cycle, 22°C, 35% relative humidity, and ad libitum access to water and food (Standard chow from Teklad Laboratory diets, Harlan Laboratories, Madison, WI, USA). 5XFAD is a well-known transgenic mouse model for AD with five mutations including APP KM670/671NL (Swedish), APP I716V (Florida), APP V717I (London), PSEN1 M146L, PSEN1 L286V, leading to early and aggressive Aβ accumulation (specially Aβ42) associated with inflammatory astrocytes activation, gliosis developing in parallel with plaque deposition, synapse degeneration, neuronal loss, and deficits in spatial learning (Oakley et al., 2006).
Mice were divided into three groups, and each group has its own control as follows (n=5 mice per treatment). Mice were housed in groups with each cage contained 2–3 mice. At 4 months of age, mice received the treatments for one month and then euthanized for brains collection at the age of 5 months. For etodolac treatment, control and treatment groups (n=5 mice each) received daily intraperitoneal (IP) injection of vehicle and 10 mg/kg, respectively, for one month. Etodolac was dissolved in 40% DMSO in water. For α-tocopherol treatment, instead of providing the mice with municipal tap water, they were supplemented with Aqua-Jel (Ancare Corporation; Bellmore, NY), which is a non-wetting sterile water gel for animal hydration (Moccia et al., 2010). Control subgroup was supplemented with Aqua-Jel, and treatment group was provided with 10 mg/kg/day of α-tocopherol mixed with Aqua-Jel for one month. This method of administration was selected because α-tocopherol has limited solubility that necessitates high DMSO or ethanol addition for complete solubilization if combined with etodolac; this in addition to avoid repeated IP injections for individual administration for the combination group that could affect the results. α-Tocopherol dose (10 mg/kg/day) in Aqua-Jel was prepared based on the average daily water intake for a 25 g adult mouse that is 4 ml (4 g), and by mixing 0.25 mg α-tocopherol with 4 g Aqua-Jel. As each cage housed 2–3 mice, one jar containing 20 g Aqua-Jel (containing 1.25 mg α-tocopherol) was placed in each cage. While α-tocopherol administration by its addition to Aqua-Jel could introduce variability in the administered dose, a concern which is similar to that of adding drugs to food or drinking water, these methods have been used and showed acceptable results (Batarseh et al., 2018; Evangelista et al., 2018; Elfakhri et al., 2018; Qosa et al., 2012). The combination group received 10 mg/kg/day etodolac (IP) and supplemented with 10 mg/kg/day of α-tocopherol mixed with Aqua-Jel for one month. Control subgroup received etodolac vehicle and Aqua-Jel. Aqua-Jel containers were changed every day to maintain freshness. During the treatment period, animals’ body weights were measured every 2 weeks and health status and normal behavior were checked daily. Mice body weights were not significantly different between the treatment and control subgroups, ranging from 22.1 ± 2.6 to 25.3 ± 2.3 g, 24.5 ± 1.1 to 26.6 ± 0.4 g, and 23.7 ± 1.9 to 24.8 ± 0.9 g, for etodolac, α-tocopherol, and combination groups each with its control subgroup respectively. At the end of the treatment period, mice were euthanized by an overdose of ketamine/xylazine anesthetic followed by decapitation to collect brains for analyses. Mice brains were divided into hemispheres where right hemispheres were homogenized and used for microvessels extraction and biochemical analysis (n=5 mice per treatment), and left hemispheres were used for immunostaining analysis (n=5 mice per treatment).
2.5. Brain microvessels isolation
Brain microvessels were isolated as described previously (Elfakhri et al., 2018). To brain homogenate, one volume of 30% Ficoll 400 (Sigma-Aldrich) was added. Homogenates were centrifuged at 8000 g for 10 min, and the resulting pellets were suspended in ice-cold DPBS (2.7 mM KCl, 1.46 mM KH2PO4, 136.9 mM NaCl, 8.1 mM Na2HPO4, 0.9 mM CaCl2, and 0.5 mM MgCl2 supplemented with 5 mM D-glucose, 1 mM sodium pyruvate, pH 7.4) containing 1% BSA and passed over a glass bead column to collect microvessels adhering to the glass beads. Isolated microvessels were used to determine ZO-1, claudin-5, LRP1, and P-gp expressions by Western blot.
2.6. Western blot analysis
For the in vitro studies, bEnd3 and SH-SY5Y-APP cells were seeded in 10 mm cell culture dishes (Corning) at a density of 1×106 cells per dish and allowed to grow in a humidified incubator (5%CO2/ 95% air) at 37°C. At 80–90% confluence, cells were treated with media containing 0.1% DMSO as control or 10 μM of etodolac, α-tocopherol, or combination (10 μM each) for 24 h. At the end of treatment period, cells were washed with ice-cold PBS twice, scraped, collected, and lysed in RIPA buffer containing 1% protease inhibitors cocktail on ice then centrifuged at 21,000 g for 10 min at 4°C. Samples were stored at −80°C until time of analysis. For Western blotting, total protein for each sample was determined using the BCA protein assay. Protein samples (25μg) were loaded and resolved using 7.5% SDS-polyacrylamide gel, then transferred electrophoretically onto nitrocellulose membranes. Membranes were incubated in 2% BSA blocking solution followed by overnight incubation at 4°C with primary antibodies; analyzed bEnd3 proteins include claudin-5, LRP1, P-gp, ZO-1, and housekeeping protein β-actin was used for normalization of the samples; neuronal proteins include PSD-95, SNAP-25, APP and housekeeping protein GAPDH was used for normalization. sAPPα and sAPPβ were also analyzed in the media of treated neuronal cells. Secondary antibodies used were: anti-mouse IgG antibody (1:2000 dilutions) for P-gp, PSD-95, APP, sAPPα, and GAPDH; anti-rabbit IgG antibody (1:2000 dilutions) for LRP1, claudin-5, sAPPβ, SNAP-25, and ZO-1; and anti-goat IgG antibody (1:1500 dilutions) for β-actin, all labeled with horseradish peroxidase (HRP). Protein blots were developed using a chemiluminescence detection kit (Thermo Fisher Scientific). Bands were visualized using ChemiDoc system (Bio Rad; Hercules, CA), and bands intensities were measured by densitometric analysis. Three independent Western blotting experiments were carried out for each treatment group.
For the in vivo Western blotting experiments, protein extracts were prepared from brain microvessels and brain tissues homogenate of the 5XFAD mice groups treated with etodolac, α-tocopherol, combination or their corresponding control groups. Isolated microvessels and brain homogenates were homogenized in RIPA buffer containing 1% protease inhibitor cocktail and centrifuged at 15,000 g for 10 min at 4°C. Supernatants were stored at −80°C for subsequent Western blot analysis. Twenty-five micrograms of protein samples were loaded and resolved using 7.5% SDS-polyacrylamide gel, and then transferred electrophoretically onto nitrocellulose membranes as described above. Similar antibodies used above were used to immunoblot proteins in mice brains’ homogenates and microvessels.
2.7. Immunohistochemical analysis
All cryostat brain slices (15 μm) were acetone-fixed then blocked for one hour with 10% normal donkey serum in PBS. Double immunostaining of capillaries with Aβ was performed using rabbit polyclonal collagen IV antibody (Millipore; Temecula, CA) at 1:200 dilution for capillaries detection followed by IgG-CFL 594 conjugated donkey anti-rabbit (Santa Cruz Biotechnology) as a secondary antibody, and Alexa Fluor-488 conjugated anti-Aβ antibody (6E10; BioLegend) for Aβ detection at 1:200 dilution. To assess IgG extravasation from brain microvessels, brain sections were fixed and blocked, as described above, then probed by dual immunohistochemical staining for collagen-IV and mouse IgG using rabbit anti-collagen-IV and fluorescein-conjugated donkey anti-IgG (Santa Cruz Biotechnology), respectively, both at 1:200 dilution. To evaluate astrogliosis induced by Aβ accumulation, brain sections were fixed and prepared as above. Double immunostaining was performed using GFAP antibody (Santa Cruz Biotechnology) for astrocytes at 1:100 dilution and with Alexa-fluor 488 labeled 6E10 for total Aβ at 1:200 dilution. All images were captured using Nikon Eclipse Ti-S inverted fluorescence microscope (Melville, NY) at a total magnification of 20X. Quantification of total Aβ load in the hippocampus and cortex was performed using ImageJ version 1.44 software (National Institutes of Health, Bethesda, MD) after adjusting for threshold.
2.8. Human Aβ40 and Aβ42 specific ELISA
Aβ levels in the brain homogenates of 5XFAD mice treated with etodolac, α-tocopherol, combination or controls were determined using sandwich ELISA. For Aβ detection Sensolyte Anti-human Aβ40 Quantitative ELISA Kit (AnaSpec, Inc., Fremont, CA), and Ultrasensitive anti-human Aβ42 ELISA kit (Invitrogen) were used, according to the manufacturers’ instructions. All antibodies used were provided by the kits. All samples were run at least in duplicate and corrected to the total protein amount in each sample using BCA assay.
2.9. Treatments effect on inflammatory and oxidative stress markers in CCF-STTG1 cells and brain homogenate
Effect of treatments on COX-2, prostaglandin E2 (PGE2), and total nitric oxide (NO) levels in in CCF-STTG1 cells was evaluated. CCF-STTG1 cells were seeded in culture dishes at a density of 1×106 cells per dish and allowed to grow in a humidified incubator (5%CO2/ 95% air) at 37°C. At 70% confluence, cells were treated with media containing 0.1% DMSO as control or 10 ng/ml interlukin-1 beta (IL-1β). Twenty-four hours later, media was replaced with RPMI media containing vehicle, 10 μM etodolac, 10 μM α-tocopherol, or combination (10 μM each) for 24 h. At the end of treatment period, media was collected and stored for analysis; cells were washed with ice-cold PBS twice, scraped, collected, and lysed in RIPA buffer containing 1% protease inhibitors cocktail on ice then centrifuged at 21,000 g for 10 min at 4°C. The supernatant of the samples was collected and stored at −80°C until time of analysis. COX-2 levels were determined using sandwich ELISA kit (R&D Systems, MN), PGE2 ELISA kit (Cayman Chemical; Ann Arbor, MI), and total nitric oxide assay kit (Fisher Scientific). All assays were performed according to the manufacturer’s instructions.
To prepare mice brain homogenates, brain hemispheres were homogenized in ice-cold DPBS containing 1% protease inhibitors cocktail. Mice brain homogenates were used to evaluate treatments effect on COX-2, and oxidative stress markers nitrate and nitrite, protein carbonyl and superoxide dismutase (SOD) levels using colorimetric assay kits (Cayman chemical) according to the manufacturer’s instructions.
2.10. Evaluation of drug interaction by CDI
The coefficient of drug interaction (CDI) was used to analyze the in vitro and in vivo effects of drugs combination. For reduced (i.e. decreased function) effect, the equation used was: CDI=AB/(A×B); and for enhanced (i.e. gain of function) effect, the equation used was: CDI=(A×B)/AB, where AB is the ratio of the combination group to its control group; A or B is the ratio of the single drug to its control group. In this study, combination index scale was defined as: CDI <0.9 synergistic, CDI = 0.9–1.1 additive, CDI > 1.1 antagonistic.
2.11. Statistical analysis
In vitro data were expressed as mean ± SD for 3–4 independent experiments. In vivo data were expressed as mean ± SEM. Statistical analysis for difference between the three control groups (40% DMSO in water as IP vehicle, Aqua-Jel, and their combination) demonstrated no significant difference in all immunostaining assays. Thus, data obtained from control groups were combined and in the figures we present n=9 mice for immunostaining results that combined n=3 mice from each control group, and n=6 mice for remaining assays combining n=2 from each control group). All comparisons and statistical analyses were performed between etodolac or α-tocopherol vs. controls or COMB groups. The experimental results were statistically analyzed for significant difference using two-tailed unpaired Student’s t-test for two groups and using a one-way ANOVA with Dunnett’s post hoc test for more than two groups. A p-value of less than 0.05 was considered statistically significant. Data analyses were done using GraphPad Prism version 7.03.
3. Results
3.1. Improved BBB integrity following etodolac, α-tocopherol, and combination treatments
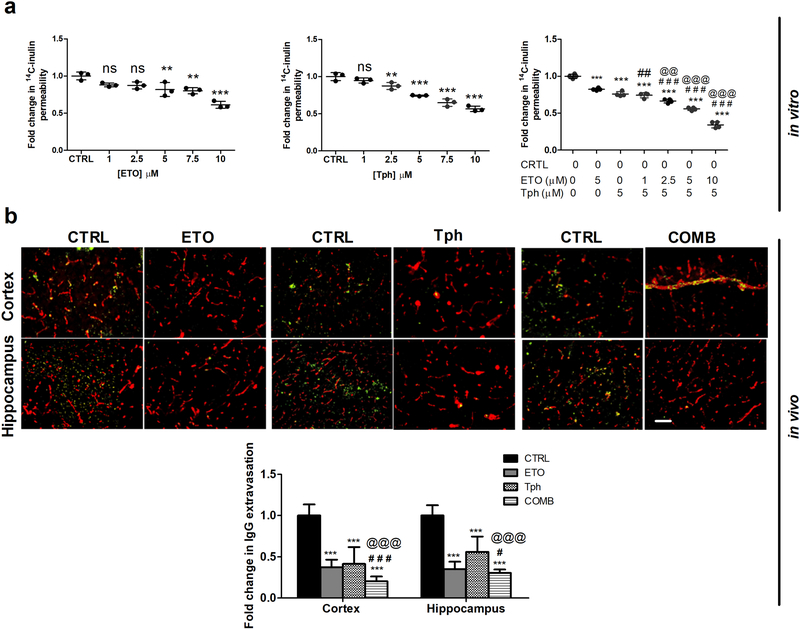
A bEnd3 cell-based BBB model was utilized to specifically evaluate and compare effect of the combination to individual drugs, etodolac and α-tocopherol, treatments on the BBB integrity. As a proof-of-concept for the drugs effect on BBB tightness, the treatments were evaluated in the absence of a stressor, e.g. Aβ oligomers, to disrupt the monolayer. In addition, recent findings from our lab demonstrated etodolac to enhance the monolayer integrity in the absence and presence of Aβ oligomers (Qosa, Mohamed, Al Rihani et al., 2016); thus in this work, this experiment was performed in the absence of the stressor. Consistent with our previously published HTS studies (Qosa, Mohamed, Al Rihani et al., 2016; Elfakhri et al., 2018), etodolac and α-tocopherol each significantly enhanced tightness of the in vitro cell-based BBB model in a concentration dependent manner as demonstrated by reduction of the paracellular permeability marker 14C-inulin across the bEnd3 monolayer when compared to the control (Fig. 1a). As expected, etodolac-α-tocopherol combination showed significantly higher effect on the intactness of bEnd3 monolayer than either drug alone, and the effect was observed at lower concentrations of etodolac. The combination of 10μM etodolac and 5μM α-tocopherol produced the most significant effect, with a 65.7% reduction in 14C-inulin permeability.
Figure 1.
Combination (COMB) treatment significantly enhanced BBB intactness compared to vehicle (control = CTRL), and etodolac (ETO) and/or α-tocopherol (Tph) treated groups.
(a) In vitro concentration-dependent effect of ETO, Tph, and COMB on permeation of the paracellular permeability marker 14C-inulin across a bEnd3 cell-based BBB model. Data represented as mean ± SD of 3–4 independent experiments with n=4 wells/treatment/experiment. (b) Representative brain sections of 5XFAD mice treated with ETO, Tph, and COMB stained with anti-IgG to quantify endogenous IgG extravasation (green) and anti-collagen antibody to resolve microvessels (red) in cortex and hippocampus regions with quantitative analysis of IgG optical density. Scale bar, 50 μm. Data represented as mean ± SEM of n=5 mice per treatment and n=9 mice for control group. ns = not significant; ** p < 0.01, *** p < 0.001 compared to CTRL; # p < 0.05, ## p < 0.01, ### p < 0.001represent COMB compared to ETO alone; @@ p < 0.01, @@@ p < 0.001represent COMB compared to Tph alone.
The effect of combination compared to etodolac and α-tocopherol on the BBB tightness was then investigated in vivo by evaluating the levels of IgG extravasation following one month of treatment. Immunostaining results demonstrated etodolac and α-tocopherol treatments significantly reduced IgG extravasation by ~65 in cortices, and by 74 and 47% in hippocampi of 5XFAD mice brains, respectively (Fig. 1b), and the combination further and significantly enhanced tightness as demonstrated by reduced IgG extravasation by >80% when compared to either drug alone and in both regions. It was also observed that IgG was retained in the capillary lumens of combination treated mice when compared to either treatment alone, providing further support that the treatment enhanced the tightness of leaky microvessels (Fig. 1b).
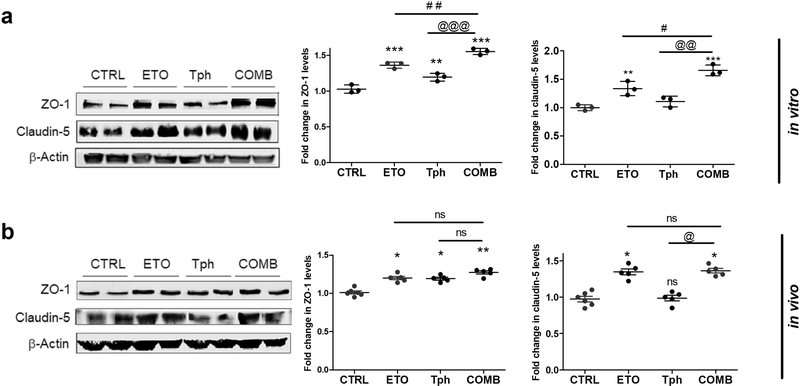
Claudin-5 and ZO-1 expressions were quantified by Western blot to compare effect of combination with vehicle, etodolac and α-tocopherol treatments on selected tight junction proteins responsible for monolayer intactness. As shown in Fig. 2a, etodolac, α-tocopherol, and combination, each tested at 10 μM concentrations, significantly induced the expression of claudin-5 and/or ZO-1 with the combination demonstrating the highest effect. Etodolac enhanced ZO-1 and claudin-5 expressions by ~35% compared to control. α-Tocopherol did not significantly enhance claudin-5 expression but increased ZO-1 by 20%. The combined treatment provided further increase in the expressions of ZO-1 and claudin-5 when compared to etodolac and α-tocopherol alone.
Figure 2.
Combination (COMB) treatment significantly increased tight junction proteins expression in vitro in bEnd3 cells and in vivo in isolated mice brain microvessels compared to vehicle treated groups (control = CTRL), and etodolac (ETO) and/or α-tocopherol (Tph).
(a) Representative Western blot and densitometry analysis of ZO-1 and claudin-5 expression in bEnd3 cells. Cells were treated with 10μM of each compound for 24 h. Data represented as mean ± SD of 3 independent experiments with n=3 dishes/treatment/experiment. (b) Representative Western blot and densitometry analysis of ZO-1 and claudin-5 in vivo from microvessels isolated from 5XFAD mice brains. Microvessels were isolated from the homogenate of the right hemisphere of mice brains (n=5–6 mice). Mice were treated with ETO, Tph or COMB at 10 mg/kg/day each for 30 days. Data represented as mean ± SEM of n=5–6 mice per group. ns = not significant; * p < 0.05, ** p < 0.01, *** p < 0.001 compared to CTRL; # p < 0.05, ## p < 0.01 represent COMB compared to ETO alone; @ p < 0.05, @@ p < 0.01, @@@ p < 0.001represent COMB compared to Tph alone.
Densitometry analyses of tight junction proteins in isolated microvessels showed a significant comparable increase in ZO-1 by 20–27% by each of the 3 treatments (Fig. 2b). Significant effects on claudin-5 expression was observed only in etodolac (34%) and combination (36%) groups but not α-tocopherol, which is consistent with the in vitro data (Fig. 2).
3.2. Increased expression of major Aβ transport proteins and 125I-Aβ40 transport across the cell-based BBB model
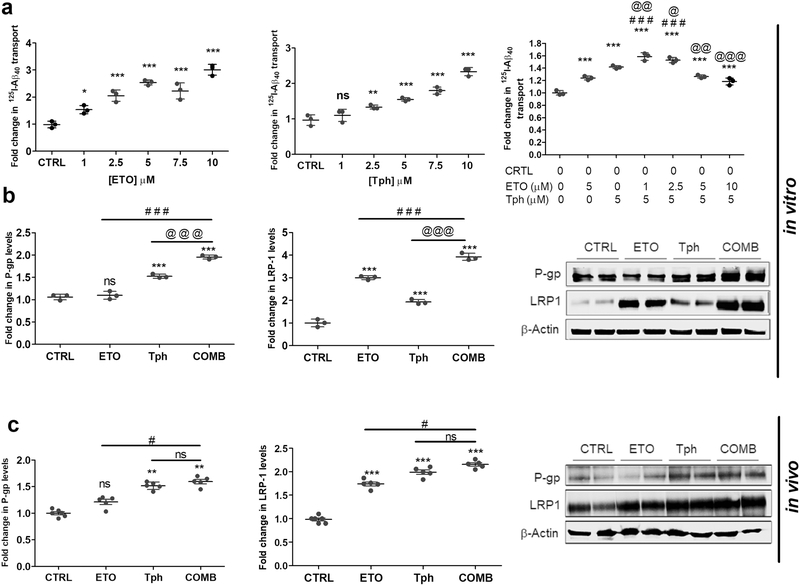
To determine the effect of etodolac, α-tocopherol, and combination on Aβ transport across the monolayer, transport study of 125I-Aβ40 (TQB→A) was performed as reported previously (Qosa, Abuasal et al. 2014; Elfakhri et al, 2018). Each of the three treatments increased the transport of 125I-Aβ40 in a concentration-dependent manner (Fig. 3a). Combination mixture of 5 μM α-tocopherol with 1μM etodolac showed the highest transport effect when compared to other mixtures and the control. Interestingly, however, increasing etodolac concentration (above 2.5 μM) resulted in a concentration-dependent decrease in the transport of 125I-Aβ40. While the reason is not clear, it could be related to the competitive inhibitory effect on P-gp and/or LRP1 transport activity when both drugs were combined. Next, effects of etodolac, α-tocopherol, and combination treatment on expression of Aβ major transport proteins in vitro were assessed by Western blot. Etodolac treatment did not significantly contribute to P-gp expression while α-tocopherol significantly increased P-gp by 95%, and LRP1 expression was significantly upregulated by both etodolac and α-tocopherol by more than 2-fold. The combination significantly induced both proteins (Fig. 3b; p>0.001).
Figure 3.
Combination (COMB) treatment differentially and significantly altered Aβ transport across the cell-based BBB model associated with increased expression of Aβ major transport proteins in vitro in bEnd3 cells, and in vivo in isolated mice brain microvessels compared to control (CTRL), and etodolac (ETO) and/or α-tocopherol (Tph).
(a) Concentration-dependent effects of ETO, Tph and COMB on the transport of 125I-Aβ40 across bEnd3 cells-based BBB model. Data represented as mean ± SD of 3 independent experiments with n=4 wells/treatment/experiment. (b) Representative Western blot and densitometry analysis of P-gp and LRP1 expressions in bEnd3 cells. Cells were treated with 10μM of each compound for 24 h. Data represented as mean ± SD of 3 independent experiments with n=3 dishes/treatment group/experiment. (c) Representative Western blot and densitometry analysis of P-gp and LRP1 expressions in vivo from microvessels isolated from 5XFAD mice brains. Microvessels were isolated from the homogenate of the right hemisphere of mice brains (n=5–6 mice). Mice were treated with ETO, Tph or COMB at 10 mg/kg/day each for 30 days. Data represented as mean ± SEM of n=5–6 mice per group. ns = not significant; * p < 0.05, ** p < 0.01, *** p < 0.001 compared to CTRL; # p < 0.05, ### p < 0.001represent COMB compared to ETO alone; @ p < 0.05, @@ p < 0.01, @@@ p < 0.001represent COMB compared to Tph alone.
Western blot analyses were also used to assess P-gp and LRP1 expression in isolated microvessels from mice brains treated with etodolac, α-tocopherol, and combination (Fig. 3c). Densitometry quantification showed no significant effect on P-gp expression with etodolac treatment, consistent with the in vitro data. α-Tocopherol treatment resulted in a 52% increase in P-gp that was not significantly altered by combination. Expression of LRP1 increased 74% with etodolac, 100% with α-tocopherol, and 117% with the combined treatment.
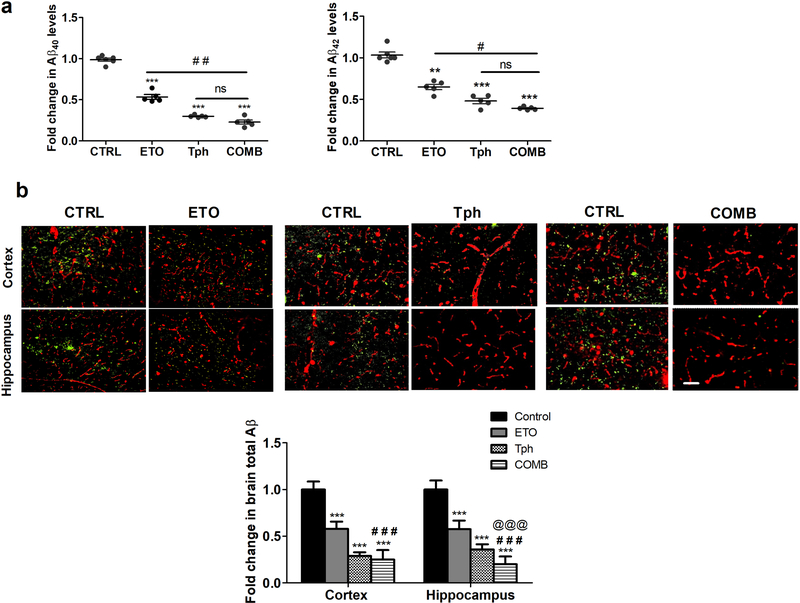
3.3. Reduced levels of Aβ40, Aβ42 and total Aβ in 5XFAD mice brains
Brain levels of Aβ40 and Aβ42 were quantified by ELISA. One-month treatment with etodolac, α-tocopherol, and combination significantly reduced Aβ40 by 46, 70, and 77% (Fig. 4a) and Aβ42 by 35, 51, and 61% (Fig. 4b), respectively, compared to control groups. Total Aβ levels were also determined semi-quantitatively by immunohistochemical analysis of total Aβ, with collagen-IV as a marker of microvessels, in the brains of 5XFAD mice. Findings demonstrated a significant reduction in total Aβ (as detected by 6E10 antibody) in all treated groups but the effect of combination was significantly higher when compared to etodolac in cortex and hippocampus regions, and α-tocopherol in the hippocampus. Semi-quantitative analysis for total Aβ showed a significant reduction by 75 and 83% in brains cortices and hippocampi, respectively, when etodolac and α-tocopherol were given in combination (Fig. 4b).
Figure 4.
Combination (COMB) treatment significantly reduced brain Aβ levels.
(a) Quantitative analysis of Aβ40 and Aβ42 levels in mice brains homogenate as measured by ELISA (n=5–6 mice per group). (b) Representative brain sections of 5XFAD mice treated with etodolac (ETO), α-tocopherol (Tph), and COMB stained with 6E10 antibody to quantify total Aβ load (green) and anti-collagen antibody to detect microvessels (red), and their optical density quantitation in both brain regions, cortex and hippocampus (n=9 mice for control group and n=5 mice for treatment groups). Scale bar, 50 μm. Mice were treated with ETO, Tph or COMB at 10 mg/kg/day each for 30 days. Data are presented as mean ± SEM. ns = not significant; ** p < 0.01, *** p < 0.001 compared to CTRL; # p < 0.05, ## p < 0.01, ### p < 0.001represent COMB compared to ETO alone; @@@ p < 0.001represent COMB compared to Tph alone.
3.4. Attenuated neuroinflammatory and oxidative stress markers following treatments
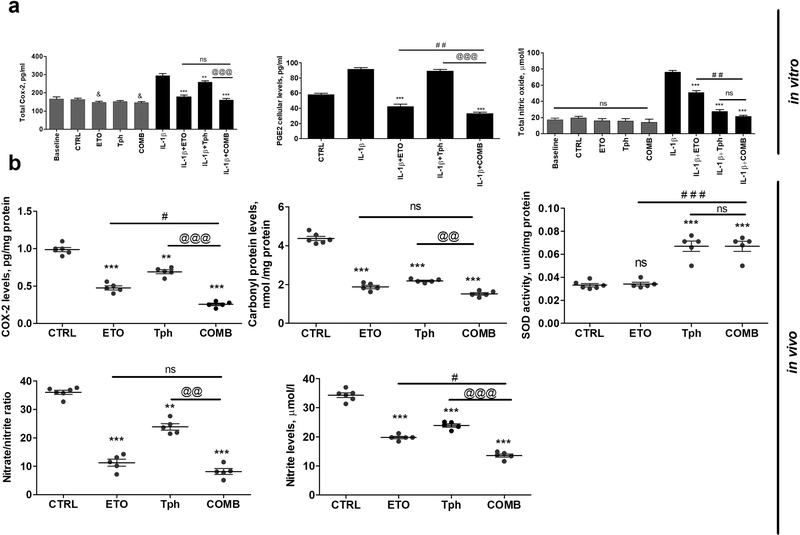
Sandwich ELISA was performed to study the effect of treatments on COX2 enzyme levels in the presence or absence of IL-1β. COX2 levels were significantly increased following CCF-STTG1 cells induction with IL-1β for 24 h (Fig. 5a). COX2 and PGE2, a downstream product of COX2, levels were significantly reduced nearly to baseline levels with etodolac and thus combination (p<0.001). α-Tocopherol was also able to significantly reduce COX2 levels, however to a lesser extent than etodolac, but has no effect on PGE2. α-Tocopherol showed no effect on COX2 levels in CCF-STTG1 cells that were not induced by IL-1β; etodolac and combination, however, showed a mild but significant effect, when compared to control (p<0.05). In addition, the effect of each treatment on total NO with and without IL-1β induction was determined and shown in Fig. 5a. In the absence of IL-1β induction, cells treated with etodolac, α-tocopherol, and combination for 24 h showed no effect on NO total level. However, upon stimulation by IL-1β for 24 h, α-tocopherol and to a lesser extent etodolac significantly reduced NO in comparison with IL-1β treated cells that was further reduced by combination.
Figure 5.
Combination (COMB) treatment significantly reduced neuroinflammation and oxidative stress biomarkers.
(a) Effect of etodolac (ETO, 10 μM), α-tocopherol (Tph, 10 μM), and COMB treatments on COX2, PGE2, and NO levels in CCF-STTG1 astrocyte cells treated or not with IL-1β (10 ng/ml) for 24 h. Baseline represents cells without treatment, while control (CTRL) indicates cells treated with 0.1% DMSO as vehicle. Cells were treated with ETO and Tph, and COMB for 24 h. Data represented as mean ± SD of 3 independent experiments with n=3 dishes/treatment group/experiment. (b) Effect of ETO, Tph and COMB treatments on COX2, carbonyl protein, SOD activity, nitrate/nitrite and nitrite in 5XFAD mice brains homogenate. Mice were treated with ETO, Tph or COMB at 10 mg/kg/day each for one month. Data are presented as mean ± SEM for n=5–6 mice in each group. ns = not significant; & p < 0.05 compared to CTRL; * p < 0.05, ** p < 0.01, *** p < 0.001 compared to CTRL; ## p < 0.01, ### p < 0.001represent COMB compared to ETO alone; @@ p < 0.01, @@@ p < 0.001represent COMB compared to Tph alone.
To examine the in vivo impact of combination compared to etodolac and α-tocopherol treatments on the neuroinflammation and oxidative stress biomarkers in 5XFAD mice brain homogenates, COX2 activity and carbonyl protein, nitrate and nitrite levels, and SOD activity were determined (Fig. 5b). Etodolac and α-tocopherol treated groups demonstrated a substantial reduction in the level and activities of their corresponding markers, which were further reduced by combination treatment when compared to α-tocopherol (p<0.01 or p<0.001) and to a lesser extent with etodolac (p<0.05 or not significant). SOD activity was significantly increased by α-tocopherol and as expected by combination (p<0.001), but not etodolac (Fig. 5b).
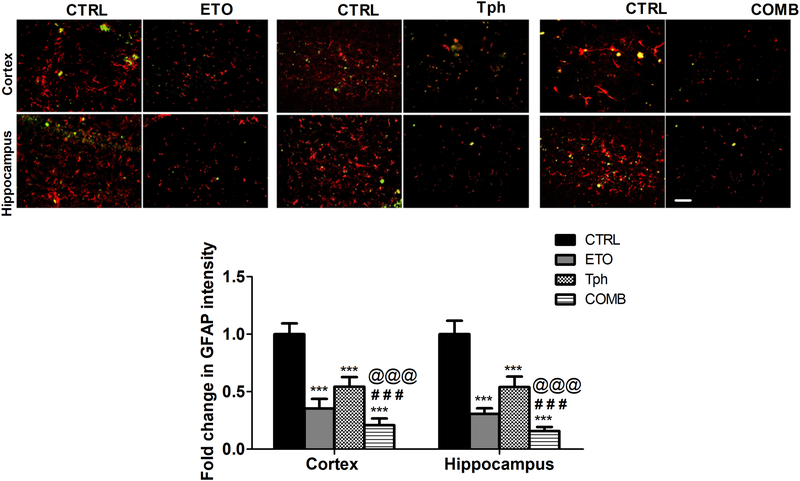
Astrogliosis is a feature of AD pathology associated with reactive astrocytes. To evaluate either drug or their combination effects on reducing astrocytes reactivity, we assessed GFAP levels and astrocytes shape/size by immunostaining of sections prepared from mice brains. Results demonstrated a significant reduction in the number and intensity of cells immunolabeled for the astrocytic marker GFAP associated with decrease cells branching in both brain regions, cortex and hippocampus. Etodolac reduced GFAP levels by 70–75%, α-tocopherol treatment by 40% and combination treatment by 80–85% in both regions that was significantly different from either drug alone (p<0.001; Fig. 6).
Figure 6.
Combination (COMB) treatment significantly reduced astrogliosis marker GFAP.
Representative brain sections of 5XFAD mice treated with etodolac (ETO), α-tocopherol (Tph), and COMB stained with anti-GFAP antibody (red) to detect activated astrocytes and 6E10 antibody (green) to detect total Aβ, and GFAP optical density quantitation in both brain regions, cortex and hippocampus. Scale bar, 50 μm. Mice were treated with ETO, Tph or COMB at 10 mg/kg/day each for 30 days. Data are presented as mean ± SEM for n=9 mice for control group and n=5 mice for treatment groups. ns = not significant; *** p < 0.001 compared to CTRL; ### p < 0.001represents COMB compared to ETO alone; @@@ p < 0.001represents COMB compared to Tph alone.
3.5. Differential treatment effects on sAPPα, sAPPβ, and total APP expressions
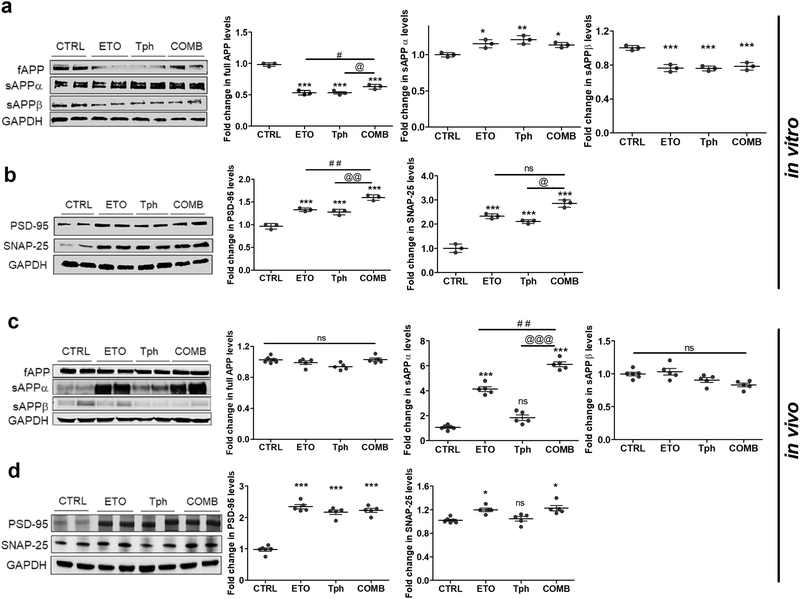
The effect of etodolac, α-tocopherol, and combination treatments on APP processing and Aβ production in vitro and in vivo was assessed by monitoring levels of full-length APP, sAPPα and sAPPβ levels. SH-SY5Y transfected with APP695 (SH-SY5Y-APP) neuronal cells treated with etodolac, α-tocopherol or combination demonstrated a significant decrease in the expression of total APP by ~50% (Fig. 7a). In addition, the treatments increased the expression of sAPPα by 15–21% and significantly reduced the expression of sAPPβ by 22–24% (Fig. 7a).
Figure 7.
Combination (COMB) treatment significantly increased sAPPα production and synaptic markers PSD-95 and SNAP-25.
Representative Western blot and densitometry analysis of (a) full length APP (fAPP), sAPPα and sAPPβ, and (b) PSD-95 and SNAP-25 in SH-SY5Y-APP cells treated with etodolac (ETO), α-tocopherol (Tph), and COMB 10μM each for 24 h. Data represented as mean ± SD of 3 independent experiments with n=3 dishes/treatment group/experiment. (c) Representative Western blot and densitometry analysis of fAPP, sAPPα and sAPPβ, and (d) PSD-95 and SNAP-25 expressions in the brain homogenates of 5XFAD mice. Mice were treated with ETO, Tph or COMB at 10 mg/kg/day each for 30 days. Data are presented as mean ± SEM for n=5–6 mice in each group. ns = not significant; * p < 0.05, ** p < 0.01, *** p < 0.001 compared to CTRL; # p < 0.05, ## p < 0.01 represent COMB compared to ETO alone; @ p < 0.05, @@ p < 0.01, @@@ p < 0.001represent COMB compared to Tph alone.
In vivo, etodolac, α-tocopherol, and combination effect on Aβ production was also evaluated in 5XFAD mice brain homogenates, and the results were different from the in vitro observed effect. The results showed none of the treatments had significant effect on total APP or sAPPβ (Fig. 7c). However, etodolac significantly increased sAPPα levels by 320%, α-tocopherol increased it by 109% but was not significant (p>0.05), with combination demonstrated a greater increase by 510% (p<0.01).
3.6. Increased expressions of synaptic markers PSD-95 and SNAP-25 following treatments
Western blot analysis was performed to study the effect of etodolac, α-tocopherol, and their combination treatments on expression levels of synaptic markers, PSD-95 and SNAP-25 (Fig. 7b and d). The in vitro results showed statistically significant increases of 33% for etodolac and 28% for α-tocopherol independently on PSD-95 expression. Etodolac and α-tocopherol combined therapy, however, showed a 60% significant increase in PSD-95 expression over control, an effect that was also significantly higher than either drug alone (p<0.01). For their effect on SNAP-25, etodolac and α-tocopherol significantly increased SNAP-25 expression by 132 and 110%, respectively, and the combination further increased the expression by 185% (Fig. 7b). In vivo, PSD-95 expression was greatly increased over control with etodolac, α-tocopherol, and their combination inducing the expression by approximately 2.3-fold. The treatments effect on SNAP-25, although mild, but significant, that was attributed to etodolac but not α-tocopherol (Fig. 7d).
3.7. In vitro and in vivo CDI for the combination
To explore the type of interaction for the combination, the coefficient of drugs interaction (CDI) was calculated for evaluated in vitro and in vivo effects. The results are listed in Table 1 demonstrating mixed effects with additive, synergistic and antagonistic effects.
Table 1.
Nature of interaction between etodolac and α-tocopherol as determined by CDI.
| In vitro studies | CDI | Effect of combination |
|---|---|---|
| Inulin permeability α-Tocopherol (5μM) + Etodolac 1.0 μM | 1.1 | Additive |
| 2.5 | 1.0 | Additive |
| 5.0 | 0.9 | Additive |
| 10.0 | 0.7 | Synergistic |
| Aβ Transport α-Tocopherol (5μM) + Etodolac 1.0 μM | 1.3 | Antagonistic |
| 2.5 | 1.9 | Antagonistic |
| 5.0 | 2.8 | Antagonistic |
| 10.0 | 3.6 | Antagonistic |
| Tight junctions | ||
| ZO1 | 1.1 | Additive |
| Claudin-5 | 0.9 | Additive |
| Transport proteins | ||
| LRP1 | 1.5 | Antagonistic |
| P-gp | 0.9 | Additive |
| Inflammatory and OS | ||
| COX2 | 1.0 | Additive |
| Nitrate | 1.1 | Additive |
| PGE2 | 0.8 | Synergistic |
| Aβ production | ||
| APP | 2.2 | Antagonistic |
| sAPPα | 1.2 | Antagonistic |
| sAPPβ | 1.4 | Antagonistic |
| PSD95 | 1.1 | Additive |
| SNAP25 | 1.7 | Antagonistic |
| In vivo studies | CDI | Effect of combination |
|---|---|---|
| IgG Extravasation | ||
| Cortex | 1.3 | Antagonistic |
| Hippocampus | 1.6 | Antagonistic |
| Aβ load (IHC) | ||
| Cortex | 1.5 | Antagonistic |
| Hippocampus | 1.0 | Additive |
| ELISA | ||
| Aβ40 | 1.2 | Antagonistic |
| Aβ42 | 1.2 | Antagonistic |
| Tight junctions | ||
| ZO1 | 1.1 | Additive |
| Claudin-5 | 1.0 | Additive |
| Transport proteins | ||
| LRP1 | 1.6 | Antagonistic |
| P-gp | 1.2 | Antagonistic |
| GFAP | ||
| Cortex | 1.1 | Additive |
| Hippocampus | 1.0 | Additive |
| Inflammatory and OS | ||
| COX2 | 0.8 | Synergistic |
| Nitrate | 0.9 | Additive |
| Carbonyl | 1.6 | Antagonistic |
| Nitrate/Nitrite | 1.1 | Additive |
| SOD | 1.0 | Additive |
| Aβ production | ||
| APP | 1.0 | Additive |
| sAPPα | 1.1 | Additive |
| sAPPβ | 0.9 | Additive |
| PSD95 | 2.2 | Antagonistic |
| SNAP25 | 1.1 | Additive |
4. Discussion
In this work, experiments were designed to evaluate the effect of etodolac, α-tocopherol and their combination on individual brain cells and on multiple AD involved pathological processes. In vitro experiments were used to assess effects of each drug and their combination on tight-junctions and transporters in brain endothelial cells, APP expression and processing and synaptic markers in neurons, and inflammation and oxidative stress markers in astrocytes. These results were complimented with in vivo studies in 5XFAD transgenic mice to assess the effect of each drug and combined therapy on a complex multi-cellular system model of AD. Using this methodology, we were able to comprehensively demonstrate the potential benefits of this novel combination therapy. Our findings show etodolac and α-tocopherol combination significantly reduced BBB leakiness, shifted APP processing to favor the neuroprotective and non-amyloidogenic sAPPα, improved expression of synaptic markers, reduced inflammation and oxidative stress biomarkers. We report that some effects are due primarily to one drug independently, while others are the result of combined effects from both drugs.
As demonstrated by our in vivo experiments, each drug separately and, to a larger extent the combination treatment, significantly reduced AD pathology. This observed effect is attributed to drugs’ direct effect on the endothelial cell of the BBB, astrocytes and neurons, as well as their collective effect by reducing brain Aβ that is expected to reduce neuroinflammation and BBB damage (Qosa, LeVine et al., 2014; Choi et al., 2018), and reducing astrogliosis and oxidative stress that could positively impact neuronal and BBB functions (Batarseh et al., 2016). Targeting multiple brain cellular and mechanistic pathways could provide a novel approach to stop and/or slow progression of AD.
Consistent with the in vitro data, in vivo findings demonstrated the combination therapy significantly enhanced BBB integrity and functionality as determined by increased expression of tight junction and significant reduction in IgG extravasation, and increased Aβ major transport proteins in isolated microvessels, which collectively reduced Aβ brain load. Besides increased clearance of Aβ across the BBB, reduced brain Aβ levels could be explained by reduced Aβ production which was observed in vitro measured by reduced sAPPβ, an effect that was very mild in vivo caused by α-tocopherol and thus the combination, but didn’t reach significant level. This disagreement in expression between in vitro and in vivo may be due to differences in the genetic predisposition of APP production between the cells (wild type APP) and transgenic mouse model (mutated APP) used for this study. Alternatively, it may suggest a more complex expression control system on the organismal level that cannot be replicated by a single cell type alone in culture. Interestingly, however, both in vitro and in vivo results showed a significant increase in sAPPα with highest effect observed with the combination, an effect that is additive and mainly caused by etodolac. sAPPα possesses neurotrophic and neuroprotective activities (Plummer et al., 2016), which is expected to positively impact spatial and cognitive memory (Turner et al., 2003).
Based on the in vitro data, etodolac and α-tocopherol directly reduced IL-1β-induced inflammation and oxidative stress. Neuroinflammation involves activation of microglia and astrocytes, which under physiological conditions have a phagocytic function (Streit et al., 2004; Erickson et al., 2012; Morales et al., 2014). In AD, these cells become activated and secrete proinflammatory cytokines, reactive oxygen species, prostaglandins, and nitric oxide. Prolonged release of these factors may eventually lead to neuronal death (Gomez-Nicola and Boche, 2015). The inflammatory mediator COX2 is induced by Aβ, resulting in downstream elevation of prostaglandins, such as PGE2 (Calder, 2006). PGE2 is a major arachidonic acid metabolite of the cyclooxygenase pathway in activated glial cells and has been proposed to contribute to neurotoxicity in AD (Prasad et al., 1998). Therefore, the effects of etodolac, α-tocopherol, and their combination on COX2 and PGE2 levels was investigated. As expected, etodolac significantly reduced COX2 and PGE2 levels, an effect that was synergized by α-tocopherol addition as demonstrated by the CDI calculations. The combination also reduced the neuroinflammation and oxidative stress markers NO, nitrate/nitrite levels, and increased SOD activity, effects that were additive caused by both drugs for the former and by α-tocopherol for the latter, which further signifies the beneficial effect of the combination. Taken together, etodolac was shown to primarily reduce neuroinflammation, while α-tocopherol reduced multiple markers of oxidative stress. By combining the two, both neuroinflammation and oxidative stress were reduced simultaneously and to a greater extent. Additionally, besides their direct effects on both process, etodolac and α-tocopherol could indirectly reduce neuroinflammation and oxidative stress markers by reducing brain levels of toxic Aβ.
Astrocytes under a neuroinflammatory state undergo morphological changes (Sun and Jakobs, 2012). 5XFAD mice exhibit neuroinflammation demonstrated by astrogliosis characterized by the astrocyte activation marker GFAP, an activation that is proportional to Aβ levels in the mice brains (Oakley et al., 2006). GFAP is an intermediate filament protein expressed in the cytoskeleton of mature astrocytes throughout the nervous system and its expression changes during development, aging, and in various diseases including AD (Kamphuis et al., 2014). 5XFAD brains show GFAP-positive astrocytes beginning at 2 months of age demonstrating neuroinflammation (Oakley et al., 2006), compared to wild-type mice that exhibit significantly lower GFAP levels in their brains (Giannoni et al., 2016; Maiti et al., 2018). Thus, in the current work, 5XFAD mice brain sections were evaluated for GFAP expression to assess effect of treatments on astrogliosis. Compared to vehicle treated mice, all treated groups significantly reduced GFAP levels, with the combined therapy revealing an additive effect caused by both drugs, thus resulting in the most significant effect. It is likely that collective improvements in BBB function and reduced neuroinflammation and oxidative stress in addition to decreased Aβ accumulation is responsible for the reduction in GFAP suggesting improved neurovascular health and neuroprotection. Besides their direct effect to induce synaptic markers expressions in neuronal cells as demonstrated by the in vitro data, reduced brain Aβ load, neuroinflammation and oxidative stress by etodolac, α-tocopherol and combination could also contributed to restoring post- and pre-synaptic markers expression.
To explore the type of interactions for the combination, CDI was calculated for in vitro and in vivo studies. The nature of interaction for the combination was mixed showing additive, synergistic and antagonistic (less than additive) effects (Table 1). CDI similarities between in vitro and in vivo were observed for the combination effect on amyloid levels, tight junction proteins, LRP1, and nitrate; however, the effect differed in the remaining evaluated proteins. Several factors could explain the inconsistent effect of the combination between the in vitro and in vivo studies including the complexity of in vivo biological systems compared to relatively simple single cell type systems, and concentrations used in the in vitro studies compared to drugs levels in mice brains following the administration of etodolac and α-tocopherol. Although the effect against Aβ-related pathology in 5XFAD mice was significantly enhanced by the combination compared to either drug alone, the effect was not always synergistic or additive. Moreover, the additive effect, in some cases, was primarily caused by one drug but not the other, which support the beneficial effect of combination therapy. For example, the increased levels of claudin-5 and SNAP25 was caused by etodolac, while α-tocopherol increased SOD (Fig. 2, 5 and 7). CDI findings from the in vivo data demonstrated the combination effect was synergistic only on COX2 levels; additive on tight junction proteins, GFAP, nitrate, SOD, APP, sAPPβ, sAPPα and SNAP25; and antagonistic on IgG extravasation, amyloid load, transport proteins, carbonyl and PSD95.
While CDI determination could provide valuable information on the nature of drugs interaction when combined, it should cautiously looked at especially in the absence of dose dependent studies for the combination where in the current work etodolac and α-tocopherol were examined at a single dose that is 10 mg/kg each. Additionally, an important point to consider is that the effect could be capacity limited where a synergistic effect could not simply be reached, which produce an antagonistic or less than additive effect. For example, the combination effect on IgG extravasation was antagonistic, although the effect was significantly higher than either drug alone. Similarly, the combination effect on amyloid load in 5XFAD mice brains, a model that aggressively produce and accumulate brain Aβ, was antagonistic. In the absence of dose dependent studies, one can speculate the observed effect with combination could reached maximum capacity to increase tightness or decrease amyloid load in transgenic mice overexpressing APP.
Collectively, our results show that combined etodolac and α-tocopherol therapy significantly reduced Aβ-related pathology in the brains of 5XFAD mice, a mouse model of AD. Etodolac primarily reduced neuroinflammation, while α-tocopherol was effective in reducing oxidative stress. Used in combination, however, each of the pathways tested was significantly enhanced and often the two drugs were shown to interact less than additive or synergistic to produce significant differences beyond simple additive effects.
In summary, the results of this study provide comprehensive and compelling evidence of combined etodolac and α-tocopherol administration as a potential therapeutic strategy for the treatment of AD. Further studies are required to evaluate dose-dependent effect of the combination and collective effect on memory function.
Highlights.
A combination therapy to simultaneously target multiple disease pathways provides effective strategy against AD.
Etodolac and α-tocopherol combination effectively restored the BBB function in vitro and in 5XFAD mice brains.
The combination conferred more effective anti-amyloid, anti-inflammatory, anti-oxidant and neuroprotective effect than either drug alone.
The combination demonstrated variable interactions indicating additive, synergistic, and antagonistic effects.
Etodolac, an NSAID, and α-tocopherol, an antioxidant, combination could have the potential to prevent, slow or treat AD.
Acknowledgements
This research work was funded by the National Institute of Neurological Disorders and Stroke (NIH/NINDS) under grant number R15NS091934 (A.K). We thank Dr. Elizabeth E. Eckman, Biomedical Research Institute of New Jersey, NJ, USA for providing the APP transfected SHSY5Y cell line.
Footnotes
Conflicts of interest
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agatonovic-Kustrin S, Kettle C, Morton DW, 2018. A molecular approach in drug development for Alzheimer’s disease. Biomed Pharmacother 106:553–565. [DOI] [PubMed] [Google Scholar]
- Agostinho P, Cunha RA, Oliveira C, 2010. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr Pharm Des. 16:2766–2778. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T, 2000. Inflammation and Alzheimer’s disease. Neurobiol Aging 21:383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batarseh YS, Duong QV, Mousa YM, Al Rihani SB, Elfakhri K, Kaddoumi A, 2016. Amyloid-β and Astrocytes Interplay in Amyloid-β Related Disorders. Int J Mol Sci. 17:338. doi: 10.3390/ijms17030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batarseh YS, Kaddoumi A, 2018. Oleocanthal-rich extra-virgin olive oil enhances donepezil effect by reducing amyloid-β load and related toxicity in a mouse model of Alzheimer’s disease. J Nutr Biochem. 55:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, Quinn JF, 2007. Blood-brain barrier impairment in Alzheimer’s diease: stability and functional significance. Neurology 68: 1809–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC, 2006. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids 75:197–202. [DOI] [PubMed] [Google Scholar]
- Choi JY, Yeo IJ, Kim KC, Choi WR, Jung JK, Han SB, Hong JT, 2018. K284–6111 prevents the amyloid beta-induced neuroinflammation and impairment of recognition memory through inhibition of NF-κB-mediated CHI3L1 expression. J Neuroinflammation 15:224. doi: 10.1186/s12974-018-1269-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- De Felice FG, Lourenco MV, 2015. Brain metabolic stress and neuroinflammation at the basis of cognitive impairment in Alzheimer’s disease. Front Aging Neurosci. 7:94. doi: 10.3389/fnagi.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW,1990. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 27:457–464. [DOI] [PubMed] [Google Scholar]
- Elfakhri KH, Duong QV, Langley C, Depaula A, Mousa YM, Lebeouf T, Cain C, Kaddoumi A, 2018. Characterization of Hit Compounds Identified from High-throughput Screening for their Effect on Blood-brain Barrier Integrity and Amyloid-β Clearance: In Vitro and In Vivo Studies. Neuroscience 379:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Banks WA, 2013. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J Cereb Blood Flow Metab 33:1500–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Dohi K, Banks WA, 2012. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation 19:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista Vaz R, Draganov DI, Rapp C, Avenel F, Steiner G, Arras M, Bergadano A, 2018. Preliminary pharmacokinetics of tramadol hydrochloride after administration via different routes in male and female B6 mice. Vet Anaesth Analg. 45:111–122. [DOI] [PubMed] [Google Scholar]
- Giannoni P, Arango-Lievano M, Neves ID, Rousset MC, Baranger K, Rivera S, Jeanneteau F, Claeysen S, Marchi N, 2016. Cerebrovascular pathology during the progression of experimental Alzheimer’s disease. Neurobiol Dis 88:107–117. [DOI] [PubMed] [Google Scholar]
- Gomez-Nicola D, Boche D, 2015. Post-mortem analysis of neuroinflammatory changes in human Alzheimer’s disease. Alzheimers Res Ther 7:42. doi: 10.1186/s13195-015-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI, 1986. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 83:4913–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP, 2005. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57:173–185. [DOI] [PubMed] [Google Scholar]
- Hensely K, 2010. Neuroinflammation in Alzheimer’s disease: mechanisms, pathologic consequences and potential for therapeutic manipulation. J Alzheimer’s Dis 21:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Wang L, Ma S, Kirisci L, Feng Z, Xue Y, Klunk WE, Kamboh MI, Sweet RA, Becker J, Lv Q, Lopez OL, Xie XQ 2018. Synergism of antihypertensives and cholinesterase inhibitors in Alzheimer’s disease. Alzheimers Dement (N Y) 4:542–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis W, Middeldorp J, Kooijman L, Sluijs JA, Kooi EJ, Moeton M, Freriks M, Mizee MR, Hol EM, 2014. Glial fibrillary acidic protein isoform expression in plaque related astrogliosis in Alzheimer’s disease. Neurobiol Aging 35:492–510. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Haeberlein SB, Carrillo MC, Hendrix JA, Kerchner G, Margolin R, Maruff P, Miller DS, Tong G, Tome MB, Murray ME, Nelson PT, Sano M, Mattsson N, Sultzer DL, Montine TJ, Jack CR Jr, Kolb H, Petersen RC, Vemuri P, Canniere MZ, Schneider JA, Resnick SM, Romano G, van Harten AC, Wolk DA, Bain LJ, Siemers E, 2018. The National Institute on Aging and the Alzheimer’s Association Research Framework for Alzheimer’s disease: Perspectives from the Research Roundtable. Alzheimers Dement. 4:563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K, 1985. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA 82:4245–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG, 2007. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging 28:639–647. [DOI] [PubMed] [Google Scholar]
- Maiti P, Paladugu L, Dunbar GL, 2018. Solid lipid curcumin particles provide greater anti-amyloid, anti-inflammatory and neuroprotective effects than curcumin in the 5xFAD mouse model of Alzheimer’s disease. BMC Neurosci 19(1):7. doi: 10.1186/s12868-018-0406-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Moccia KD, Olsen CH, Mitchell JM, Landauer MR, 2010. Evaluation of Hydration and Nutritional Gels as Supportive Care after Total-Body Irradiation in Mice (Mus musculus). J Am Assoc Lab Anim Sci. 49:323–328. [PMC free article] [PubMed] [Google Scholar]
- Morales I, Guzmán-Martínez L, Cerda-Troncoso C, Farías GA, Maccioni RB, 2014. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci 8:112. doi: 10.3389/fncel.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Koyama N, Tan J, Segawa T, Maeda M, Town T, 2017. Combination therapy with octyl gallate and ferulic acid improves cognition and neurodegeneration in a transgenic mouse model of Alzheimer’s disease. J Biol Chem 292:11310–11325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R, 2006. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 26:10129–10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer S, Van den Heuvel C, Thornton E, Corrigan F, Cappai R, 2016. The neuroprotective properties of the amyloid precursor protein following traumatic brain injury. Aging Dis 7:163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad NK, Hovland AR, La Rosa FG, Hovland PG, 1998. Prostaglandins as putative neurotoxins in Alzheimer’s disease. Proc Soc Exp Biol Med 219:120–125. [DOI] [PubMed] [Google Scholar]
- Qosa H, Abuasal BS, Romero IA, Weksler B, Couraud PO, Keller JN, Kaddoumi A, 2014. Differences in amyloid-β clearance across mouse and human blood–brain barrier models: kinetic analysis and mechanistic modeling. Neuropharmacology 79:668–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, Abuznait AH, Hill RA, Kaddoumi A, 2012. Enhanced brain amyloid-β clearance by rifampicin and caffeine as a possible protective mechanism against Alzheimer’s disease. J Alzheimers Dis. 31:151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, LeVine H 3rd, Keller JN, Kaddoumi A, 2014. Mixed oligomers and monomeric amyloid-β disrupts endothelial cells integrity and reduces monomeric amyloid-β transport across hCMEC/D3 cell line as an in vitro blood-brain barrier model. Biochim Biophys Acta 1842:1806–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, Mohamed LA, Al Rihani SB, Batarseh YS, Duong QV, Keller JN, Kaddoumi A, 2016. High-Throughput Screening for Identification of Blood-Brain Barrier Integrity Enhancers: A Drug Repurposing Opportunity to Rectify Vascular Amyloid Toxicity. J Alzheimers Dis 53:1499–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, Mohamed LA, Alqahtani S, Abuasal BS, Hill RA, Kaddoumi A, 2016. Transporters as Drug Targets in Neurological Diseases. Clin Pharmacol Ther 100:441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare AP, Bell RD, Zlokovic BV, 2012. Neurovascular dysfunction and faulty amyloid β-peptide clearance in Alzheimer disease. Cold Spring Harb Perspect Med pii: a011452. doi: 10.1101/cshperspect.a011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, 2001. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 81:741–766. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV, 2000. Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood–brain barrier. J Clin Invest. 106:1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovgard K, Agerskov C, Kohlmeier KA, Herrik KF, 2018. The 5-HT3 receptor antagonist ondansetron potentiates the effects of the acetylcholinesterase inhibitor donepezil on neuronal network oscillations in the rat dorsal hippocampus. Neuropharmacology 143:130–142. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Mrak RE, Griffin WS, 2004. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation 1:14 DOI: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Jakobs TC, 2012. Structural Remodeling of Astrocytes in the Injured CNS. The Neuroscientist 18:567–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Fenech M, 2007. A review of genome mutation and Alzheimer’s disease. Mutagenesis 22: 15–33. [DOI] [PubMed] [Google Scholar]
- Traber MG, Atkinson J, 2007. Vitamin E, Antioxidant and Nothing More. Free Radic Biol Med. 43:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PR, O’Connor K, Tate WP, Abraham WC, 2003. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol. 70:1–32. [DOI] [PubMed] [Google Scholar]
- Vlad SC, Miller DR, Kowall NW, Felson DT, 2008. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 70:1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Zhao B, 2013. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid Med ell Longev. 2013:316523. doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]