Abstract
Interleukin(IL)-27 regulates immune responses in inflammation. The underlying mechanism of IL-27 functions has long been attributed to its ability to induce IL-10 production in activated CD4 T cells. Here, we report that Foxp3+ regulatory T cells (Tregs) are the main target cells of IL-27 mediating its immunoregulatory functions in vivo. Systemically delivered IL-27 efficiently prevents the development of experimental autoimmune encephalomyelitis (EAE), an autoimmune inflammation in the central nervous system. However, it failed to do so upon Treg depletion. IL-27 signaling in Tregs was necessary, as transferring Tregs deficient in IL-27Rα or Lag3, a downstream molecule induced by IL-27, was unable to protect mice from EAE. IL-27 efficiently induced IL-10 expression in CD4 T cells in vitro; however, we found no evidence supporting IL-27-induced IL-10 induction in CD4 T cells in vivo. Taken together, our results uncover an irreplaceable contribution of Tregs during IL-27-mediated control of inflammation.
Introduction
IL-27 is an immunoregulatory cytokine produced by activated antigen presenting cells and is best known for its anti-inflammatory functions capable of attenuating various types of inflammatory responses (1). Earlier studies found that IL-27 is a Th1-promoting cytokine inducing IFNγ production in activated T cells by elevating expression of the T-bet and IL-12Rβ2, key components for Th1 differentiation (2, 3). However, the IL-27 action on CD4 T cells has received greater attention for its anti-inflammatory effects during Th17 immunity. In the model of Th17 type autoimmune inflammation, experimental autoimmune encephalomyelitis (EAE), systemic IL-27 administration prevents EAE induction and attenuates ongoing disease (4). Concordantly, Il27ra−/− mice develop severe autoimmune inflammation with augmented Th17 immunity (5). Direct impact of IL-27 on conventional CD4 T cells, antagonizing Th17 differentiation and inducing IL-10 production in effector T cells, has been considered the primary pathway underpinning anti-inflammatory functions of IL-27 (5). However, multiple cell types express the functional IL-27 receptor and can respond to the cytokine. Foxp3+ regulatory CD4 T cells (Tregs) playing a key role in immune tolerance and inflammation express high level of the IL-27 receptors. We recently reported that IL-27 signaling in Tregs is indispensable for Tregs to control EAE pathogenesis, as Treg-specific Il27ra−/− mice develop progressive EAE and fail to recover from the disease (6). While IL-27-induced IL-10 has been proposed as a major cellular factor mediating IL-27 functions (7), IL-10 expression by CNS infiltrating CD4 T cells in Treg-specific Il27ra−/− mice was instead elevated despite the severe EAE. Therefore, the precise contribution of Tregs and IL-10 during IL-27-induced treatment remains unclear.
Here, we utilized adoptive Treg transfer approach together with systemic IL-27 administration to investigate the mechanisms by which IL-27 attenuates EAE. Depleting Tregs during EAE induction results in lethal disease, and it is rescued by adoptive transfer of in vitro generated Foxp3+ iTregs. While systemic IL-27 treatment attenuates EAE development, it completely fails to do so when Tregs are absent. To determine the cellular mechanisms underlying Treg-mediated protection during IL-27 treatments, Tregs deficient in IL-27Rα or Lag3, a surface molecule induced by IL-27 stimulation in Tregs (8), were transferred into Treg-depleted mice with ongoing EAE. Unlike wild type Tregs, neither Tregs rescued the recipients from lethal disease even with systemic IL-27 administration. Although IL-27 increases IL-10 mRNA expression in activated CD4 T cells in vitro, we observed that in vivo IL-27 treatment significantly reduces IL-10 protein secretion by antigen specific CD4 T cells or in the circulation. Taken together, our results demonstrate that Foxp3+ Tregs serve the primary target cells of IL-27 to mediate its anti-inflammatory roles during autoimmune neuroinflammation irrespective of IL-10+ CD4 T cells.
Materials and Methods
Mice
C57BL/6 (B6), B6 Foxp3DTR, and B6 Il27ra−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). B6 Foxp3GFP and B6 Lag3−/− mice were obtained from Drs. Yasmine Belkaid and Christoph Benoist, respectively.
All animals were bred and maintained under specific pathogen-free facility at the Lerner Research Institute, and all experiments were performed in accordance with the guidelines of the Lerner Research Institute Animal Care and Use Committee.
EAE induction
EAE was induced by subcutaneous immunization with MOG35–55 peptide (300 or 150 μg) emulsified in IFA supplemented with 5 mg/ml Mycobacterium tuberculosis extract H37Ra (Difco, Detroit, MI). The animals also received 200 ng pertussis toxin (Sigma, St. Louis, MO) i.p. on days 0 and 2. The mice were daily scored for EAE as before (6). In some experiments, FACS sorted WT and Ly5.1+ Lag3−/− Tregs were labeled with Cell Trace dye (Thermo Fisher, Waltham, MA) and transferred into mice with ongoing EAE. Treg trafficking and Foxp3 expression were subsequently determined.
Subcutaneous implantation of osmotic pumps
On day 7 post immunization, mice were anesthetized and an incision was made on the back. A miniosmotic pump (Alzet, Durect, Cupertino, CA) containing 400 ng rIL-27 (R&D system, Minneapolis, MN) were inserted. Sham surgery was made as control groups.
In vitro T cell differentiation and adoptive transfer
FACS sorted naïve CD4+Foxp3−CD44low T cells were stimulated with immobilized anti-CD3 (2C11) and soluble anti-CD28 mAb (37.51), 100 U/ml rhIL-2 (obtained from the BRB, NCI), and 5 ng/ml TGFβ (Peprotech, Rocky Hill, NJ). After 3 days, iTreg cells (CD4 Foxp3+) cells were cell sorted using a FACSAria (BD, San Jose, CA). 5 × 106 cells were intravenously transferred 7 days after EAE induction. For Th1 and Th2 cells, IL-12 (10ng/ml)/anti-IL-4 (10μg/ml) and IL-4 (1000U/ml)/anti-IFNγ (10μg/ml) were added, respectively. For Th17 cells, IL-6 (10ng/ml)/TGFβ (2.5ng/ml) and anti-IL-4/anti-IFNγ (10μg/ml) were added. Cytokines and antibodies were purchased from Peprotech and eBioscience, respectively.
Quantitative real-time PCR analysis
RNA was isolated from the CNS tissue using a GeneJET RNA isolation kit (Thermo Fisher), and cDNA was synthesized using a M-MLV Reverse Transcriptase (Promega, Madison, WI). qPCR analysis was performed using a QuantStudio 3 Real-Time PCR System (Applied Biosystems, Waltham, MA) using TaqMan reagents (Applied Biosystems) or SYBR green master mixes (Applied Biosystems). The gene expression was normalized to the housekeeping gene gapdh.
Flow cytometry
CNS infiltrating CD4 T cells were enumerated using a FACSLSR II (BD Biosciences) and analyzed with a FlowJo software (TreeStar, Ashland, OR). BrdU incorporation was determined according to manufacturer (BD PharMingen).
Cytokine quantification
The serum cytokine level was measured using a cytometric bead array (CBA kit, BD Biosciences) according to the manufacturer’s instructions. The data were analyzed using the CBA software, and the standard curve for each cytokine was generated using the mixed cytokine standard. Draining lymph node and the CNS cells isolated from mice with ongoing EAE were in vitro stimulated with 20 μg/ml MOG35–55 peptide for 48h. IL-10 and IFNγ secretion in the culture supernatant was determined by ELISA (R&D Systems) according to the manufacturer’s instructions.
Statistical analysis
Statistical significance was determined by the Student’s t-test or one-way ANOVA using the Prism 5 software (GraphPad, La Jolla, CA). A p value of <0.05 was considered statistically significant.
Results and Discussion
Treg depletion at EAE onset results in lethal disease, and adoptive Treg transfer protects mice from the lethality
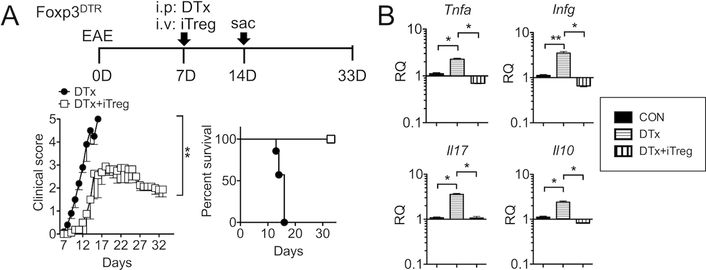
The primary goal of this study is to investigate the role of Tregs during IL-27-mediated control of autoimmune inflammation. We utilized the Foxp3DTR mice that express the diphtheria toxin (DTx) receptor under the control of the Foxp3 promoter for acute Treg depletion (9). At the disease onset (day 7 post induction), mice were injected with DTx. Treg-depleted mice rapidly developed severe EAE, resulting in 100% mortality by day 15 post induction (Fig 1A). High mortality upon Treg depletion was similarly observed during mild form of EAE induced by suboptimal dose of 150μg MOG peptide immunization, which induced mild EAE that only peaked at the clinical score of <2 (Supplementary Fig S1). These results demonstrate the key involvement of Tregs during active EAE induced by both optimal and suboptimal doses of antigen. Quantitative PCR analysis of the CNS tissues demonstrate that the expression of proinflammatory cytokines including TNFα, IFNγ, and IL-17 dramatically increased following Treg depletion (Fig 1B). Interestingly, IL-10 expression in the CNS also increased upon Treg depletion (Fig 1B). In vitro generated Foxp3+ iTreg transfer into Treg-depleted recipients completely rescued the recipients from lethal disease and those mice displayed typical EAE severity peaking at score of 3 around day 19 post induction (Fig 1A). Likewise, CNS expression of inflammatory cytokines including IL-10 significantly diminished following Treg transfer (Fig 1B). These results demonstrate that Foxp3+ Tregs play an instrumental role in controlling autoimmune inflammation in the CNS especially at the time of disease onset.
Figure 1. Treg depletion at EAE onset results in lethal disease, which is rescued by iTreg transfer.
(A) Experimental design of EAE induction and Treg adoptive transfer. Groups (n=5~8) of Foxp3DTR mice were induced for EAE as described in Materials and Methods. At disease onset (7 days post induction), mice were treated with 1μg DTx alone (filled circle). Some mice received 5 × 106 Foxp3+ iTregs together with DTx (open square). The mice were daily monitored and scored for clinical diseases and survival. (B) RNA was isolated from the CNS on day 14 post induction and evaluated for gene expression by qPCR. Expression of the indicated genes was normalized to the gapdh gene, and relative quantification (RQ) value was calculated. Data are mean ± SD of three independent experiments. * p<0.05; ** p<0.01.
Systemic IL-27 administration protects mice only in the presence of Foxp3+ Tregs
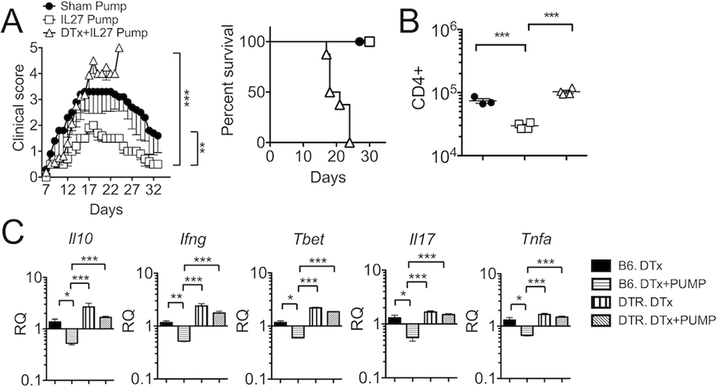
As systemic delivery of IL-27 prevents EAE induction and attenuates ongoing disease (4), we next examined whether IL-27 administered may reverse lethal EAE developed in Treg-depleted mice. As anticipated, IL-27 delivered via an osmotic pump significantly inhibited the severity of EAE as compared to sham controls (Fig 2A). To our surprise, the treatment effect of IL-27 was completely abolished when Tregs were depleted. Although slightly delayed compared to DTx alone group (Fig 1A), all IL-27/DTx treated group succumbed to death by day 25 post induction (Fig 2A). Consistent with the clinical manifestation, the number of CNS-infiltrating CD4 T cells, while reduced by IL-27 treatment, remained high in IL-27/DTx treated groups (Fig 2B). Expression of inflammatory cytokines (IFNγ, IL-17, TNFα and IL-10) as well as T-bet transcription factor was compared. DTx treatment in wild type B6 had no effect in disease progression (data not shown). Cotreatment of IL-27 significantly reduced their expression in B6 recipients (Fig 2C). Expression of the listed cytokines was greatly elevated upon Treg depletion, and IL-27 treatment had no impact on the expression without Tregs (Fig 2C). Therefore, the presence of Tregs is essential for systemically delivered IL-27 to downregulate pathogenic responses.
Figure 2. In vivo IL27 treatment fail to protect mice from lethal EAE without Tregs.
(A) EAE induction and Treg depletion were performed as described in Figure 1. A miniosmotic pump containing rIL-27 was subcutaneously implanted 7 days post induction: sham (n = 5), IL-27 pump (n = 5), and DTx with IL-27 pump (n = 6). Mice were daily monitored for clinical disease and survival. (B) Total number of CNS infiltrating CD4 T cells was enumerated 14 days post induction. (C) B6 wild type or B6 Foxp3DTR mice induced for EAE were treated with DTx with or without IL-27 pump (n = 5~10) and sacrificed 14 days post EAE induction. The CNS tissues were harvested and the indicated cytokine gene expression was measured by qPCR. Each symbol represents individually tested animal. Data are mean ± SD of three independent experiments. * p< 0.05; ** p< 0.01; *** p< 0.001.
IL-27 signaling in Foxp3+ Tregs is required for transferred Tregs to mediate protection from lethal EAE
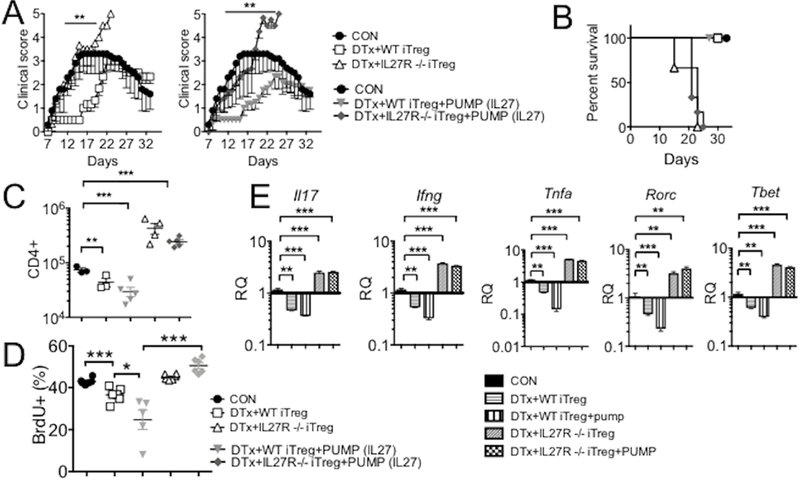
To ensure that IL-27 signaling in Tregs is instrumental for Treg-dependent protection during systemic IL-27 administration, we generated Foxp3+ iTregs from Il27ra−/− naive CD4 T cells (Il27ra−/− Foxp3GFP mice). iTreg differentiation of Il27ra−/− naive CD4 T cells was comparable to that of wild type T cells (data not shown). FACS sorted GFP+ Tregs were transferred into DTx-treated Foxp3DTR recipients at the disease onset as described in Fig 1A. Unlike wild type iTregs, Il27ra−/− iTregs transferred were incapable of rescuing the recipients from the lethal EAE, resulting in 100% mortality by day 23 post induction (Fig 3A and 3B). Even more striking is that systemic IL-27 treatment in Il27ra−/− Treg recipients had no impact on the disease progression and lethality, and they all succumbed to death by day 25 post induction (Fig 3A and 3B), which was in stark contrast to wild type iTreg recipients where exogenous IL-27 further attenuated the disease severity. Therefore, IL-27 signaling in Tregs appears to be a vital pathway conferring Tregs the ability to inhibit the pathogenic processes. Consistent with the disease severity, IL-27 significantly diminished CD4 T cell infiltration and cytokine expression in the CNS when Tregs were able to respond do it, whereas it had no effect in doing so when Tregs were unable to respond to it (Fig 3C and 3E). In vivo BrdU incorporation experiments demonstrated that the IL-27 acting on Tregs may suppress inflammation by inhibiting effector T cell proliferation (Fig 3D). Although IL-27 may influence effector functions of Th1 and Th17 cells by directly suppressing key transcription factors (3, 10), IL-27-mediated inhibition of the transcription factors and cytokine was completely lost in the absence of Tregs or of IL-27 signaling in Tregs (Fig 2C and3E). Therefore, IL-27 signaling in Foxp3+ Tregs seems essential for the control of autoimmune inflammation in the CNS.
Figure 3. IL27 signaling in Treg is necessary for protection of EAE.
(A and B) Groups of Foxp3DTR mice were induced for EAE. Treg depletion, IL-27 pump implantation, and Treg transfer were performed as described in Figures 1 and 2. WT or Il27ra−/− Tregs were used. Mice were daily monitored for clinical diseases and survival. (C) CNS infiltrating CD4+ T cell number was calculated 14 days post induction. (D) In vivo BrdU incorporation was determined as described in the Materials and Methods. (E) qPCR analysis was performed to assess TNFα, IFNγ, IL17, Rorc, and T-bet gene expression in the CNS. Each symbol represents individually tested animal. Data are mean ± SD of three independent experiments. * p< 0.05; ** p< 0.01; *** p< 0.001.
Lag3 mediates IL-27-induced protection from lethal EAE via Tregs
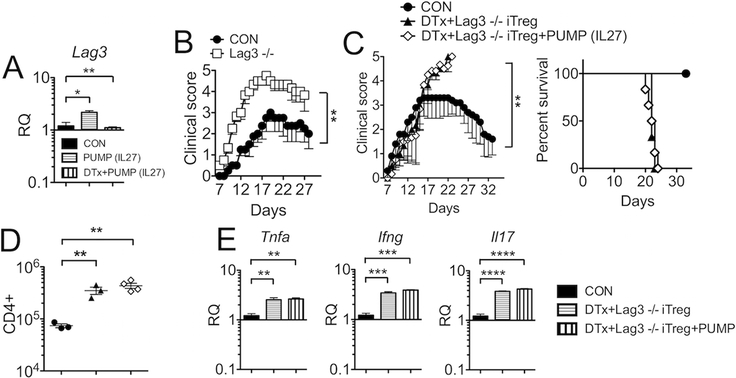
IL-27 induces expression of the lymphocyte-activating gene 3 (Lag3), a negative regulator of activated T cells, in Tregs (11). Consistent with this, IL-27 administration significantly induced Lag3 expression in the CNS during EAE, whereas the induction was lost when Tregs were absent (Fig 4A). We investigated whether Lag3 expression in adoptively transferred Tregs is necessary during IL-27-mediated protection of lethal EAE in endogenous Treg-depleted mice. Given the inhibitory roles of Lag3, it is expected that Lag3 deficiency results in elevated immune activation. Indeed, Lag3−/− mice develop severe EAE (Fig 4B), although they did not succumb to the disease (data not shown). To test if Lag3 expression in Tregs is necessary for Treg functions, we repeated adoptive Treg transfer experiment following endogenous Treg depletion utilizing Lag3−/− iTregs. iTreg differentiation of Lag3−/− naive CD4 T cells (from Lag3−/− Foxp3GFP mice) was comparable to wild type cells (data not shown). As shown in Fig 4C, Lag3−/− iTregs transferred into DTx treated mice with ongoing EAE failed to rescue mice from lethal disease. Furthermore, systemic IL-27 treatment in these animals was still incapable of protecting mice from the lethal EAE (Fig 4C). Inability of Lag3−/− Tregs to prevent lethal disease irrespective of systemic IL-27 delivery was also exemplified in the number of CNS infiltrating CD4 T cells (Fig 4D) and in inflammatory cytokine expression in the CNS (Fig 4E). Importantly, CNS trafficking and Foxp3 expression were comparable between Lag3−/− and WT iTregs transferred (data not shown, and Supplementary Fig S2), strongly suggesting that impaired suppressive function of Lag3−/− Tregs is not due to defects in trafficking or in Foxp3 expression. Therefore, Lag3 expression in Tregs appears to be the main downstream pathway through which Tregs control inflammatory responses in response to IL-27.
Figure 4. Lag3 expression on Tregs is critical during IL-27 treatment of EAE.
(A) EAE-induced Foxp3DTR mice treated with IL-27 pump alone or DTx plus IL-27 pump were sacrificed and Lag3 expression in the CNS tissue was determined by qPCR analysis. (B) Groups of B6 (n = 4) and Lag3−/− (n = 6) mice were induced for EAE. (C) Groups of Foxp3DTR mice were induced for EAE. Treg depletion, IL-27 pump implantation, and Lag3−/− iTreg transfer were performed as described in Figures 1 and 2. Mice were daily monitored for clinical diseases and survival. (D) CNS infiltrating CD4+ T cell number was calculated 14 days post induction. (E) qPCR analysis was performed to assess TNFα, IFNγ, and IL17 gene expression in the CNS. Each symbol represents individually tested animal. Data are mean ± SD of three independent experiments. * p< 0.05; ** p< 0.01; *** p< 0.001; ****, p<0.0001.
IL-27 does not induce IL-10 production in CD4 T cells in vivo
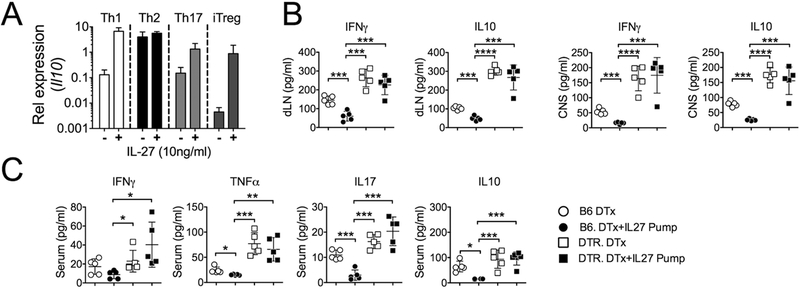
Induction of IL-10-producing Foxp3− CD4 T cells (i.e. Tr1 cells) by IL-27 has been regarded a major mechanism underpinning IL-27-mediated immune regulation (12). Since we found that IL-10 expression in the CNS was reduced after IL-27 administration and increased after Treg depletion (Fig 2C), we reasoned that IL-27-mediated IL-10 induction in activated CD4 T cells may not occur in vivo. Addition of IL-27 during in vitro stimulation significantly upregulated IL-10 mRNA expression during Th1, Th17, and iTreg differentiation (Fig 5A). However, MOG peptide specific IFNγ and IL-10 secretion from the draining LN or CNS cells from mice with ongoing EAE was significantly reduced only when IL-27 was given (Fig 5B). Furthermore, such IL-27-induced reduction was completely abolished without Tregs (Fig 5B). To further corroborate the cytokine responses, we measured serum cytokine levels in mice described above using a cytokine bead array (CBA). Consistent with ex vivo antigen-induced cytokine production, serum levels of IFNγ, TNFα, IL-17A, and IL-10 were significantly reduced by IL-27 administration in wild type mice (Fig 5C). Treg depletion elevated serum cytokine levels, and the level remained unchanged in the absence of Tregs even with systemic IL-27 administration (Fig 5C).
Fig 5. IL27 induces IL-10 expression in vitro but not in vivo.
(A) Naive CD4 T cells were stimulated in conditions promoting Th1, Th2, Th17, and iTreg differentiation. IL-27 was added during the stimulation. Il10 mRNA expression at the end of 3-day culture was determined by qPCR analysis. (B and C) Groups of B6 or Foxp3DTR mice were induced for EAE and treated with DTx alone or together with IL-27 pump as described in Figure 2. Upon sacrifice at 14 days post induction, (B) CNS and dLN cells were isolated and ex vivo restimulated with MOG peptide for 48h. IL-10 and IFNγ secretion in the culture supernatant was determined by ELISA. (C) In addition, serum levels of IFNγ, TNFα, IL-17 and IL-10 were measured using a CBA assay. Each symbol represents individually tested animal. Experiments were repeated at least twice with similar results. * p< 0.05; ** p< 0.01; *** p< 0.001; **** p<0.0001.
Earlier studies reported that IL-27 promotes generation of Foxp3− IL-10+ CD4 T cells that are capable of alleviating inflammation in autoimmunity and infection (13-15). The key evidence that had led to the conclusion mostly came from in vitro assays where IL-27 was added during T cell stimulation (13-15). Fitzgerald et al. utilized a passive EAE model in which antigen primed lymph node cells were restimulated in the presence of IL-27 prior to transfer, from which they found that these cells induce less severe disease in an IL-10-dependent mechanism (4, 13, 16). Our results offer the evidence that IL-27 has different capacity to induce IL-10 expression depending on how it is introduced to activated T cells. In vitro, IL-27 induces IL-10 production in developing Th1, Th17, and iTregs, consistent with those previous studies. In vivo, however, systemically delivered IL-27 (at low yet possibly physiologic level) may preferentially target Tregs to improve their regulatory functions, which in turn inhibit IL-10 production from effector T cells. In the absence of Tregs, the expression of inflammatory cytokines including IL-10 becomes uncontrolled, and most importantly, IL-27 administered in vivo has little impact on the cytokine expression and disease progression. Probably the most compelling evidence comes from the finding that antigen specific cytokine production measured following restimulation of lymph node or CNS infiltrating cells with the antigens. Those T cells, if isolated from mice given systemic IL-27, secreted significantly less IL-10, while they produced dramatically elevated IL-10 if isolated from Treg depleted animals, irrespective of systemic IL-27 treatment. Therefore, anti-inflammatory function of IL-27 may occur through action on Tregs and IL-27-induced IL-10 production by conventional Foxp3− T cells may not be a major pathway of IL-27 in vivo. Notably, high dose (~1μg) IL-27 delivered by gene therapy for more than 2 weeks depletes Tregs and enhances effector T cell functions (17). On the other hand, our study with an osmotic pump delivers ~2ng per hour over 7-day period, resulting in reduced inflammation and diminished Treg accumulation in the CNS. The amount of IL-27 may thus have distinct impact on Treg homeostasis. IL-10-independent regulation of inflammatory responses by IL-27 has previously been reported. Moon et al. showed that IL-27-Fc fusion protein delivered into mice with collagen-induced arthritis protects mice from the disease and that IL-10 production level remains unchanged regardless of protection (18). Rostami and colleagues reported utilizing a passive EAE model that encephalitogenic T cells restimulated in vitro with IL-27 together with IL-23 induce less severe EAE, and that the IL-27-induced suppression was IL-10-independent (16).
IL-27 activates both Stat1 and Stat3, and they are required to generate IL-10+ cells by IL-27 in vitro (15). IL-27 is also able to activate negative feedback mechanism that prevents excessive IL-10 production in T cells by inducing the enzyme cholesterol 25-hydroxylase (Ch25h) that synthesizes the oxysterol 25-hydroxycholesterol (25-OHC), a negative regulator of IL-10 secretion (19). It is thus possible that molecular factors mediating IL-27 signaling pathway may differ between in vitro and in vivo stimulation. Determining the precise pathways through which IL-27 mediates immunoregulatory functions in vitro versus in vivo will be a subject of future investigation. In sum, we would argue that the experimental modality chosen for IL-27 delivery may have different influences on the development of adaptive immunity. This is especially important since IL-27 is considered as a therapeutic tool to modulate ongoing inflammation.
Supplementary Material
Acknowledgement
The authors thank Ms. Jennifer Powers for cell sorting.
Supported by grants from National Multiple Sclerosis Society (RG1411-02051) and NIH (AI125247 and AI121524) to B.M.
Abbreviations used in this article:
- DTx
diphtheria toxin
- EAE
experimental autoimmune encephalomyelitis
- Lag3
lymphocyte activation gene 3
- Treg
regulatory T cells
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Yoshida H, and Hunter CA. 2015. The immunobiology of interleukin-27. Annu Rev Immunol 33: 417–443. [DOI] [PubMed] [Google Scholar]
- 2.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, and Yoshida H. 2003. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol 170: 4886–4890. [DOI] [PubMed] [Google Scholar]
- 3.Kamiya S, Owaki T, Morishima N, Fukai F, Mizuguchi J, and Yoshimoto T. 2004. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J Immunol 173: 3871–3877. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, and Rostami A. 2007. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol 179: 3268–3275. [DOI] [PubMed] [Google Scholar]
- 5.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, and Ghilardi N. 2006. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol 7: 929–936. [DOI] [PubMed] [Google Scholar]
- 6.Do J, Kim D, Kim S, Valentin-Torres A, Dvorina N, Jang E, Nagarajavel V, DeSilva TM, Li X, Ting AH, Vignali DAA, Stohlman SA, Baldwin WM 3rd, and Min B. 2017. Treg-specific IL-27Ralpha deletion uncovers a key role for IL-27 in Treg function to control autoimmunity. Proc Natl Acad Sci U S A 114: 10190–10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pot C, Apetoh L, Awasthi A, and Kuchroo VK. 2011. Induction of regulatory Tr1 cells and inhibition of T(H)17 cells by IL-27. Semin Immunol 23: 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Do JS, Visperas A, Sanogo YO, Bechtel JJ, Dvorina N, Kim S, Jang E, Stohlman SA, Shen B, Fairchild RL, Baldwin Iii WM, Vignali DA, and Min B. 2016. An IL-27/Lag3 axis enhances Foxp3+ regulatory T cell-suppressive function and therapeutic efficacy. Mucosal immunology 9: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JM, Rasmussen JP, and Rudensky AY. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol 8: 191–197. [DOI] [PubMed] [Google Scholar]
- 10.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, and Kastelein RA. 2009. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol 182: 5748–5756. [DOI] [PubMed] [Google Scholar]
- 11.Do JS, Visperas A, Sanogo YO, Bechtel JJ, Dvorina N, Kim S, Jang E, Stohlman SA, Shen B, Fairchild RL, Baldwin WM III, Vignali DA, and Min B. 2016. An IL-27/Lag3 axis enhances Foxp3+ regulatory T cell-suppressive function and therapeutic efficacy. Mucosal Immunol 9: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pot C, Apetoh L, and Kuchroo VK. 2011. Type 1 regulatory T cells (Tr1) in autoimmunity. Semin Immunol 23: 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, and Rostami A. 2007. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol 8: 1372–1379. [DOI] [PubMed] [Google Scholar]
- 14.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, and Weiner HL. 2007. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol 8: 1380–1389. [DOI] [PubMed] [Google Scholar]
- 15.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, and Hunter CA. 2007. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol 8: 1363–1371. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald DC, Fonseca-Kelly Z, Cullimore ML, Safabakhsh P, Saris CJ, Zhang GX, and Rostami A. 2013. Independent and interdependent immunoregulatory effects of IL-27, IFN-beta, and IL-10 in the suppression of human Th17 cells and murine experimental autoimmune encephalomyelitis. J Immunol 190: 3225–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J, Liu JQ, Shi M, Cheng X, Ding M, Zhang JC, Davis JP, Varikuti S, Satoskar AR, Lu L, Pan X, Zheng P, Liu Y, and Bai XF. 2018. IL-27 gene therapy induces depletion of Tregs and enhances the efficacy of cancer immunotherapy. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moon SJ, Park JS, Heo YJ, Kang CM, Kim EK, Lim MA, Ryu JG, Park SJ, Park KS, Sung YC, Park SH, Kim HY, Min JK, and Cho ML. 2013. In vivo action of IL-27: reciprocal regulation of Th17 and Treg cells in collagen-induced arthritis. Exp Mol Med 45: e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vigne S, Chalmin F, Duc D, Clottu AS, Apetoh L, Lobaccaro JA, Christen I, Zhang J, and Pot C. 2017. IL-27-Induced Type 1 Regulatory T-Cells Produce Oxysterols that Constrain IL-10 Production. Front Immunol 8: 1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.